Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Laboratory | Microscopic (histologic) description | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: von Stillfried S, Boor P. Renal disease-general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneyrenaldisease.html. Accessed March 31st, 2025.
Definition / general
- Renal disease: basic terminology in nontumor kidney pathology and kidney diseases
Essential features
- Each of the 3 anatomical kidney compartments should be assessed and reported (Am J Kidney Dis 2022;80:119)
- Glomeruli (count overall number of glomeruli and number of globally sclerotic glomeruli)
- Tubulointerstitium (semiquantitatively estimate the extent of interstitial fibrosis and tubular atrophy)
- Vasculature (focusing on arteries and arterioles, estimate degree of narrowing of the vascular lumen)
- Injuries should be categorized as active versus chronic lesions (Am J Kidney Dis 2022;80:119)
- Lesions and nomenclature should be reported according to an international standards (Kidney Int 2020;98:1120)
- Focal (< 50% of the glomeruli or the tubulointerstitium)
- Diffuse (> 50% of the glomeruli or the tubulointerstitium)
- Segmental (< 50% of a glomerulus)
- Global (> 50% of a glomerulus, special case: global glomerulosclerosis denotes almost fully sclerotic glomeruli)
ICD coding
Epidemiology
- Congenital abnormalities of the kidney and urinary tract (CAKUT) occur in ~1 in 500 live births and are responsible for 40 - 50% of childhood end stage renal disease (StatPearls: Embryology, Kidney, Bladder, and Ureter [Accessed 7 August 2023])
- Acute kidney injury (AKI) can be seen in up to 7% of hospital admissions and 30% of intensive care unit (ICU) admissions (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Glomerulonephritis constitutes 25 - 30% of all end stage renal disease cases (StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Chronic kidney disease (CKD) affects ~13% of the world's population and is the 16th leading cause of years of life lost worldwide (Adv Ther 2022;39:33, JAMA 2019;322:1294)
Sites
- Kidney
Etiology
- Causes of CAKUT (StatPearls: Embryology, Kidney, Bladder, and Ureter [Accessed 7 August 2023])
- Disruptions to the signaling pathways of LIM1, PAX2 / PAX8 and ODD1, GDNF-RET, bone morphogenic protein 4 (BMP4) and gremlin
- Genes associated with bilateral renal agenesis, including ANOS1, EYA1 and RET
- Multicystic dysplastic kidney disease (MCDK) is thought to be caused by a defect in the genes involved in the GDNF-RET signaling pathway between the ureteric bud and the metanephric blastema
- Autosomal dominant polycystic kidney disease (PKD) caused by heterogeneous mutations in PKD1 in 85% of cases, with the remaining 15% due to PKD2 mutations; autosomal recessive PKD caused by mutations in PKHD1
- Causes of AKI (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Prerenal
- Reduced blood flow to the kidney (caused by hypotension, hypovolemia, renal vasoconstriction or glomerular efferent arteriolar vasodilation)
- Renal
- Acute tubular necrosis, acute interstitial nephritis, glomerulonephritis, intratubular obstruction
- Postrenal
- Obstruction of the urinary flow by renal / ureteral calculi, tumors, blood clots or any urethral obstruction
- Prerenal
- Causes of glomerulonephritis (GN) (StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Immune complex GN
- Pauci-immune GN
- Antiglomerular basement membrane (GBM) GN
- Monoclonal Ig GN
- C3 glomerulopathy
- Causes of CKD (StatPearls: Chronic Renal Failure [Accessed 7 August 2023], Lancet 2017;389:1238)
- Diabetes mellitus type 2 (30 - 50%)
- Diabetes mellitus type 1 (3.9%)
- Hypertension (27.2%)
- Primary glomerulonephritis (8.2%)
- Chronic tubulointerstitial nephritis (3.6%)
- Hereditary or cystic diseases (3.1%)
- Secondary glomerulonephritis or vasculitis (2.1%)
- Plasma cell dyscrasias or neoplasm (2.1)
- Sickle cell nephropathy (SCN)
Clinical features
- Clinical indications for renal biopsy (typical presentations) (adapted from BMJ Open 2019;9:e023479)
- Microscopic or macroscopic hematuria (StatPearls: Hematuria [Accessed 7 August 2023])
- Alport syndrome
- Immune complex GN: IgA nephropathy, IgA vasculitis, infection related GN, lupus nephritis and fibrillary GN with polyclonal Ig deposits
- Membranoproliferative glomerulonephritis
- Antiglomerular basement membrane (GBM) GN (Goodpasture syndrome)
- Pauci-immune GN: PR3 antineutrophil cytoplasmic antibodies (ANCA) GN, myeloperoxidase (MPO) ANCA GN and ANCA negative GN with systemic or renal vasculitis
- C3 glomerulopathy: C3 glomerulonephritis, dense deposit disease
- Nephrotic syndrome (proteinuria > 3.5 g/24 h or 40 mg/m2 per hour, edema, hypoalbuminemia < 30 g/L, hyperlipidemia, hyperlipiduria, hypercoagulability and thrombophilia) (StatPearls: Glomerulonephritis [Accessed 7 August 2023])
- Minimal change disease
- Focal segmental glomerulosclerosis
- Membranoproliferative glomerulonephritis
- Membranous nephropathy
- Human immunodeficiency virus (HIV) associated nephropathy
- Diabetic nephropathy
- Amyloidosis
- Nephritic syndrome (proteinuria < 3.5 g/24 h or 40 mg/m2 per hour, rapid decrease in glomerular filtration rate [GFR], oliguria, hematuria, systemic hypertension, decrease in serum complement levels) (StatPearls: Nephritic Syndome [Accessed 7 August 2023])
- Antiglomerular basement membrane (GBM) GN (Goodpasture syndrome)
- Immune complex GN: IgA nephropathy, IgA vasculitis, infection related GN, lupus nephritis and fibrillary GN with polyclonal Ig deposits
- Pauci-immune GN: PR3 ANCA GN, MPO ANCA GN and ANCA negative GN with systemic or renal vasculitis
- Impaired kidney function (acute or chronic decrease of estimated GFR [eGFR])
- Acute decrease of eGFR / acute kidney injury (AKI) (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Hypovolemia: hemorrhage, severe burns and gastrointestinal fluid losses, such as diarrhea, vomiting, high ostomy output
- Hypotension from decreased cardiac output: cardiogenic shock, massive pulmonary embolism, acute coronary syndrome
- Hypotension from systemic vasodilation: septic shock, anaphylaxis, anesthetics, hepatorenal syndrome
- Renal vasoconstriction: NSAIDs, iodinated contrast, amphotericin B, calcineurin inhibitors, hepatorenal syndrome
- Glomerular efferent arteriolar vasodilation: ACE inhibitors, angiotensin receptor blockers
- Chronic decrease of eGFR (StatPearls: Chronic Renal Failure [Accessed 7 August 2023], Lancet 2017;389:1238)
- Possible renal involvement in systemic disease in
- Diabetes mellitus type 2 and type 1
- Hypertension
- Hereditary or cystic diseases
- Plasma cell dyscrasias or neoplasm
- Secondary glomerulonephritis or vasculitis
- Sickle cell nephropathy (SCN)
- Possible renal involvement in systemic disease in
- Acute decrease of eGFR / acute kidney injury (AKI) (StatPearls: Acute Kidney Injury [Accessed 7 August 2023])
- Microscopic or macroscopic hematuria (StatPearls: Hematuria [Accessed 7 August 2023])
Diagnosis
- Clinical, laboratory, imaging and biopsy findings
Laboratory
- Chronic kidney disease (CKD)
- Glomerular filtration rate: GFR < 60 mL/min/1.73 m2 (GFR categories G3a - G5) (Kidney Int Suppl 2013;3:19)
- Decreased GFR can be detected by current estimating equations for GFR based on serum creatinine or cystatin C but not by serum creatinine or cystatin C alone
- Albuminuria as a marker of kidney damage (increased glomerular permeability), urine albumin excretion rate (AER) ≥ 30 mg/24 hours, approximately equivalent to urine albumin to creatinine ratio (ACR) ≥ 30 mg/g (≥ 3 mg/mmol)
GFR categories in CKD GFR category GFR (mL/min/1.73 m2) Terms G1 ≥ 90 Normal or high G2 60 - 89 Mildly decreased* G3a 45 - 59 Mildly to moderately decreased G3b 30 - 44 Moderately to severely decreased G4 15 - 29 Severely decreased G5 < 15 Kidney failure Abbreviations: chronic kidney disease (CKD), glomerular filtration rate (GFR)
*Relative to young adult level
In the absence of evidence of kidney damage, neither GFR category G1 nor G2 fulfill the criteria for CKD
Albuminuria categories in CKD
(KDIGO: Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease [Accessed 28 July 2023])ACR (approximate equivalent) Category AER (mg/24 hours) (mg/mmol) (mg/g) Terms A1 < 30 < 3 < 30 Normal to mildly increased A2 30 - 100 3 - 30 30 - 300 Moderately increased* A3 > 300 > 30 > 300 Severely increased** Abbreviations: albumin excretion rate (AER), albumin to creatinine ratio (ACR), chronic kidney disease (CKD)
*Relative to young adult level
**Including nephrotic syndrome (albumin excretion usually 42200 mg/24 hours [ACR 42220 mg/g; 4220 mg/mmol])
- Urinary sediment abnormalities as markers of kidney damage (Kidney Int Suppl 2013;3:19)
- Isolated, nonvisible (microscopic) hematuria with abnormal red blood cell (RBC) morphology (anisocytosis) in GBM disorders
- RBC casts in proliferative glomerulonephritis
- White blood cell (WBC) casts in pyelonephritis or interstitial nephritis
- Oval fat bodies or fatty casts in diseases with proteinuria
- Granular casts and renal tubular epithelial cells in many parenchymal diseases (nonspecific)
- Renal tubular disorders (Kidney Int Suppl 2013;3:19)
- Renal tubular acidosis
- Nephrogenic diabetes insipidus
- Renal potassium wasting
- Renal magnesium wasting
- Fanconi syndrome
- Nonalbumin proteinuria
- Cystinuria
- Glomerular filtration rate: GFR < 60 mL/min/1.73 m2 (GFR categories G3a - G5) (Kidney Int Suppl 2013;3:19)
- Acute kidney injury (AKI) (PLoS One 2014;9:e114369)
- Increased serum creatinine
Diagnosis and staging criteria for AKI of RIFLE, AKIN, KDIGO definitions based on serum creatinine Classification Definition for AKI Stage Serum creatinine criteria for AKI staging* RIFLE Increase in SCr ≥ 50% within 7 days Risk To ≥ 1.5 times baseline Injury To ≥ 2 times baseline Failure To ≥ 3 times baseline or ≥ 44 μmol/L increase to at least 354 μmol/L AKIN Increase in SCr ≥ 26.5 μmol/L or ≥ 50% within 48 hours 1 Increase of ≥ 26.5 μmol/L or to 1.5 - 2 times baseline 2 To 2 - 3 times baseline 3 To ≥ 3 times baseline or ≥ 26.5 μmol/L increase to at least 354 μmol/L or initiation of RRT KDIGO Increase in SCr ≥ 26.5 μmol/L within 48 hours
or ≥ 50% within 7 days1 Increase in SCr ≥ 26.5 μmol/L within 48 hours or to 1.5 - 2 times baseline 2 To 2 - 3 times baseline 3 To ≥ 3 times baseline or to at least 354 μmol/L or initiation of RRT For patients meeting diagnosis criteria for AKI according to RIFLE, AKIN or KDIGO, the stage based on percentage increase were determined by the ratio of peak SCr value obtained during hospitalization to baseline
Abbreviations: acute kidney injury (AKI); risk, injury, failure, loss, end stage renal failure (RIFLE); Acute Kidney Injury Network (AKIN); Kidney Disease: Improving Global Outcomes (KDIGO); serum creatinine (SCr); renal replacement therapy (RRT)
*Urine output was not used because records of hourly urine output were not available in the majority of patients
- Markers such as NGAL, KIM1 and IL18 are surrogates for renal damage; their role as biomarkers to diagnose AKI, predict risk for AKI or predict progression of AKI to CKD is still under exploration
- Increased serum creatinine
Microscopic (histologic) description
- Developmental and cystic diseases
- Large group of hereditary and nonhereditary kidney diseases of variable etiology (Adv Anat Pathol 2006;13:26)
- Glomerular disease (including monoclonal gammopathy of renal significance [MGRS] / paraprotein related kidney disease)
| Glomerular lesion | PAS / silver stain | IF / IHC | EM | Differential diagnoses | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thick appearance of GBM | Relatively acellular mesangial expansion / no double contour, feathery spikes on silver stain, Congo red positive | Negative for IgG, IgA, IgM, C3 and C1q positive for 1 light chain, with smudgy mesangial and capillary wall pattern, often also in vessels (most often λ but κ can also form amyloid; rare cases of heavy chain amyloid exist) | Randomly arranged, nonbranching fibrils of 9 - 11 nm diameter |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Double contour | Negative |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Irregular or undetectable by LM | Negative | Basketweaving, thin GBM |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Membranous (thick appearance of GBM), spikes by silver stain | Granular capillary loop IgG polyclonal and C3 staining; in specific etiology positive for PLA2R, THSD7A, EXT1 / EXT2, NELL1 and others (Kidney Int 2023;103:469) | Subepithelial deposits |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying double contours by silver stain, negative Congo red | Polyclonal IgG with lesser C3; positive DNAJB9 | Randomly arranged fibrillary substructure 11 - 24 nm |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mesangial hypercellularity / mesangial expansion | Varying mesangial expansion with nodules, thick GBM, arteriolar hyalinosis / no double contour | Negative | No deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying mesangial hypercellularity with nodules, thick GBM / no double contour | Monoclonal light chain staining of GBM and tubular basement membrane | Corresponding amorphous powdery deposits |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Varying mesangial hypercellularity without nodules, thick GBM / no double contour | Polyclonal IgG with full house staining (i.e., all immunoglobulins [IgG, IgA, IgM], C3 and C1q) | Subendothelial deposits; may show fingerprint-like substructure; reticular aggregates in endothelial cells |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IgA mesangial deposits | Mesangial deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IgG (starry sky appearance by IF) or IgM predominant deposits | Hump shaped deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C3 positive deposits | Hump shaped deposits |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Tubulointerstitial disease (including infectious disease)
| Tubulointerstitial lesion | PAS / silver stain | Differential diagnoses | ||||||||||||||||||||||||||||||||||
| Tubular injury | Cell detachment, individual cell injury / blebbing / degeneration / apoptosis |
| ||||||||||||||||||||||||||||||||||
| Cell detachment in isolation or with associated glomerular necrosis |
| |||||||||||||||||||||||||||||||||||
| Cell detachment in zones of injury |
| |||||||||||||||||||||||||||||||||||
| With immunglobulin deposition | Tubulitis, TBMs stain strongly for IgG, variable C3 staining |
| ||||||||||||||||||||||||||||||||||
| Interstitial fibrosis and tubular atrophy | Diffuse pattern | Nonspecific | ||||||||||||||||||||||||||||||||||
| Striped pattern along medullary rays |
| |||||||||||||||||||||||||||||||||||
| Patchy / geographic, jigsaw puzzle pattern |
| |||||||||||||||||||||||||||||||||||
| Interstitial edema | Increased interstitial space with loose appearance and normal thickness TBMs |
| ||||||||||||||||||||||||||||||||||
| Interstitial inflammation | Medullary angiitis with increased neutrophils in vasa recta |
| ||||||||||||||||||||||||||||||||||
| Intratubular neutrophils forming plugs |
| |||||||||||||||||||||||||||||||||||
| Interstitial lymphocytic infiltrate |
| |||||||||||||||||||||||||||||||||||
| Lymphocytes and interstitial fibrosis |
| |||||||||||||||||||||||||||||||||||
- Vascular disease
| Vascular lesion | PAS / silver stain | Differential diagnoses |
| Sclerosis | Intimal fibrosis / medial hypertrophy |
|
| Thrombosis, necrosis and red blood cell fragments within vascular wall, mucoid intima expansion | Arteriolar / glomerular predominance |
|
| Arteriolar / interlobular artery predominance, fibrinoid necrosis |
| |
| Emboli | Clear, needle shaped spaces with surrounding foreign body reaction in interlobular arteries, sometimes in smaller vessels, rarely in glomeruli |
|
| Vasculitis | Inflammation with lymphocytes / PMNs; may be transmural or intimal |
|
| Endotheliosis | Swollen endothelial cells |
|
| Abbreviations: antineutrophil cytoplasmic antibodies (ANCA), electron microscopy (EM), hematoxylin eosin staining (H&E), immunohistochemistry (IHC), International Society of Nephrology / Renal Pathology Society (ISN / RPS), tubular basement membrane (TBM), thrombotic microangiopathy (TMA) | ||
| Adapted from Am J Kidney Dis 2022;80:119 | ||
- Renal allograft (Transplantation 2018;102:1795, Am J Transplant 2020;20:2318)
- 2019 Banff classification for antibody mediated rejection (ABMR), borderline changes, T cell mediated rejection (TCMR) and polyomavirus nephropathy (Am J Transplant 2020;20:2318)
- Category 1: normal biopsy or nonspecific changes
- Category 2: antibody mediated changes
- Active ABMR
- Chronic active ABMR; all 3 criteria must be met for diagnosis
- Chronic (inactive) ABMR
- Category 3: borderline (suspicious) for acute TCMR
- Category 4: TCMR
- Acute TCMR
- Chronic active TCMR
- Category 5: polyomavirus nephropathy
- Infectious
- Cytomegalovirus infection (Am J Kidney Dis 2016;68:e35)
- Polyomavirus nephropathy (Am J Kidney Dis 2016;68:e37)
- Calcineurin inhibitor nephrotoxicity (Am J Kidney Dis 2017;69:e21)
- 2019 Banff classification for antibody mediated rejection (ABMR), borderline changes, T cell mediated rejection (TCMR) and polyomavirus nephropathy (Am J Transplant 2020;20:2318)
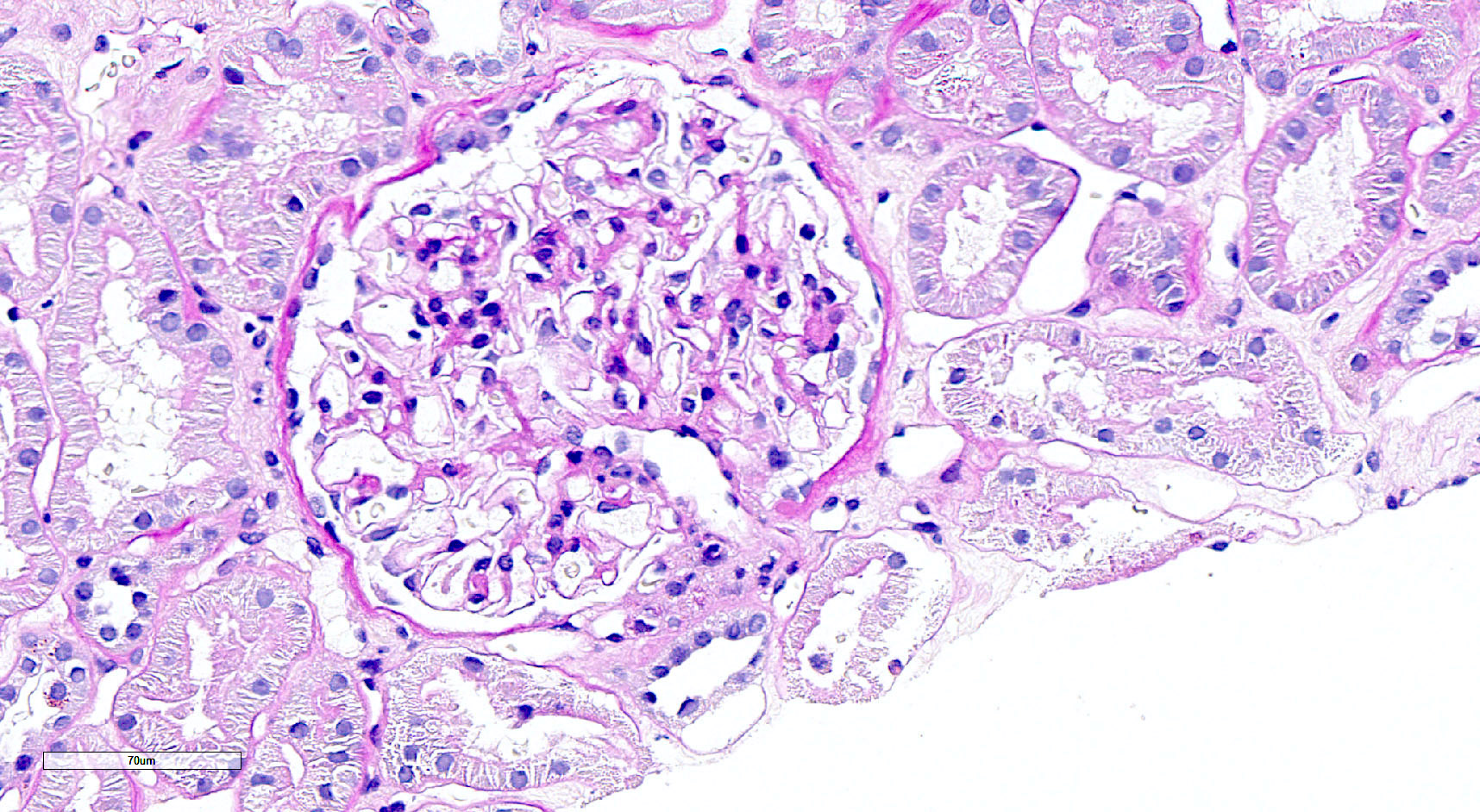
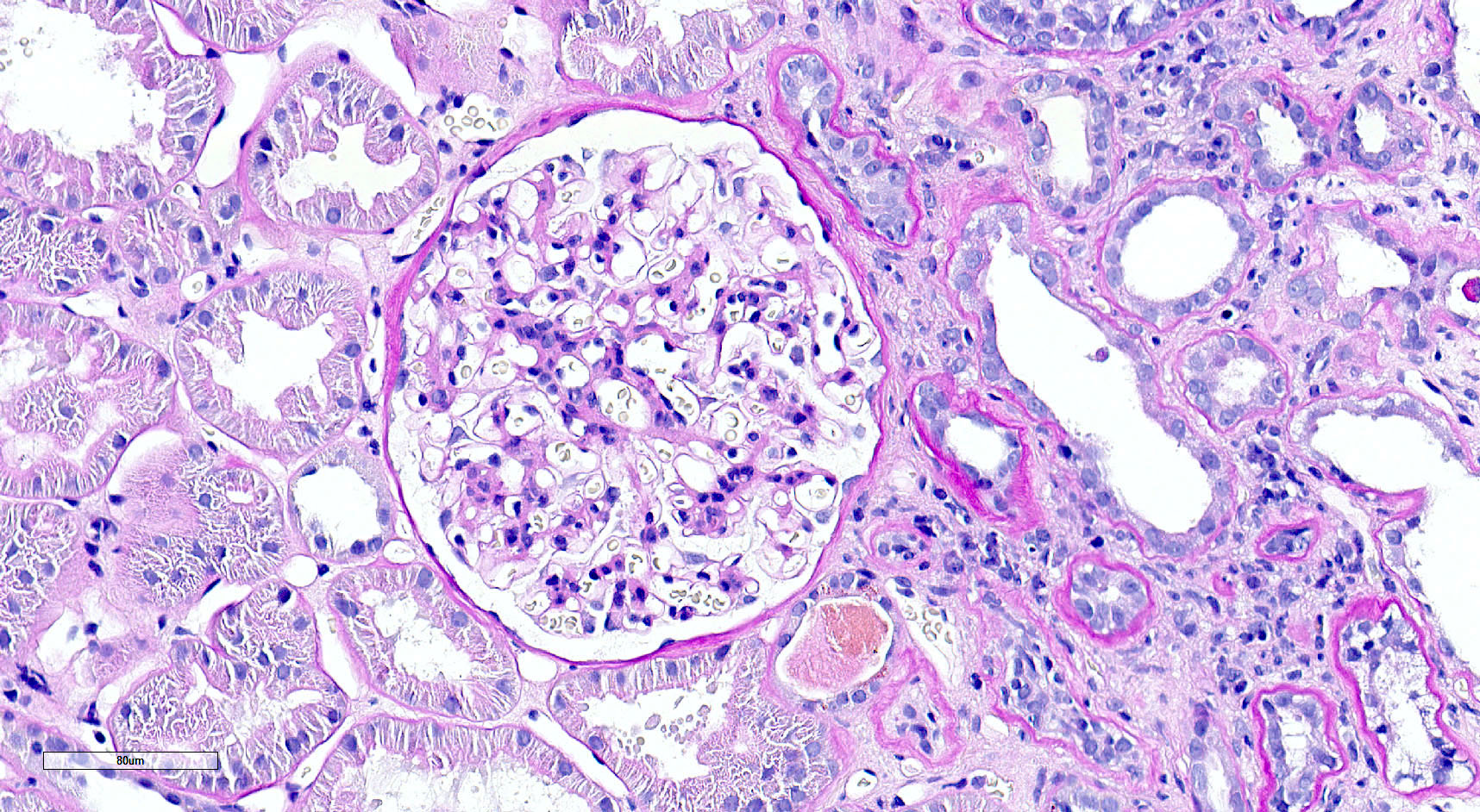
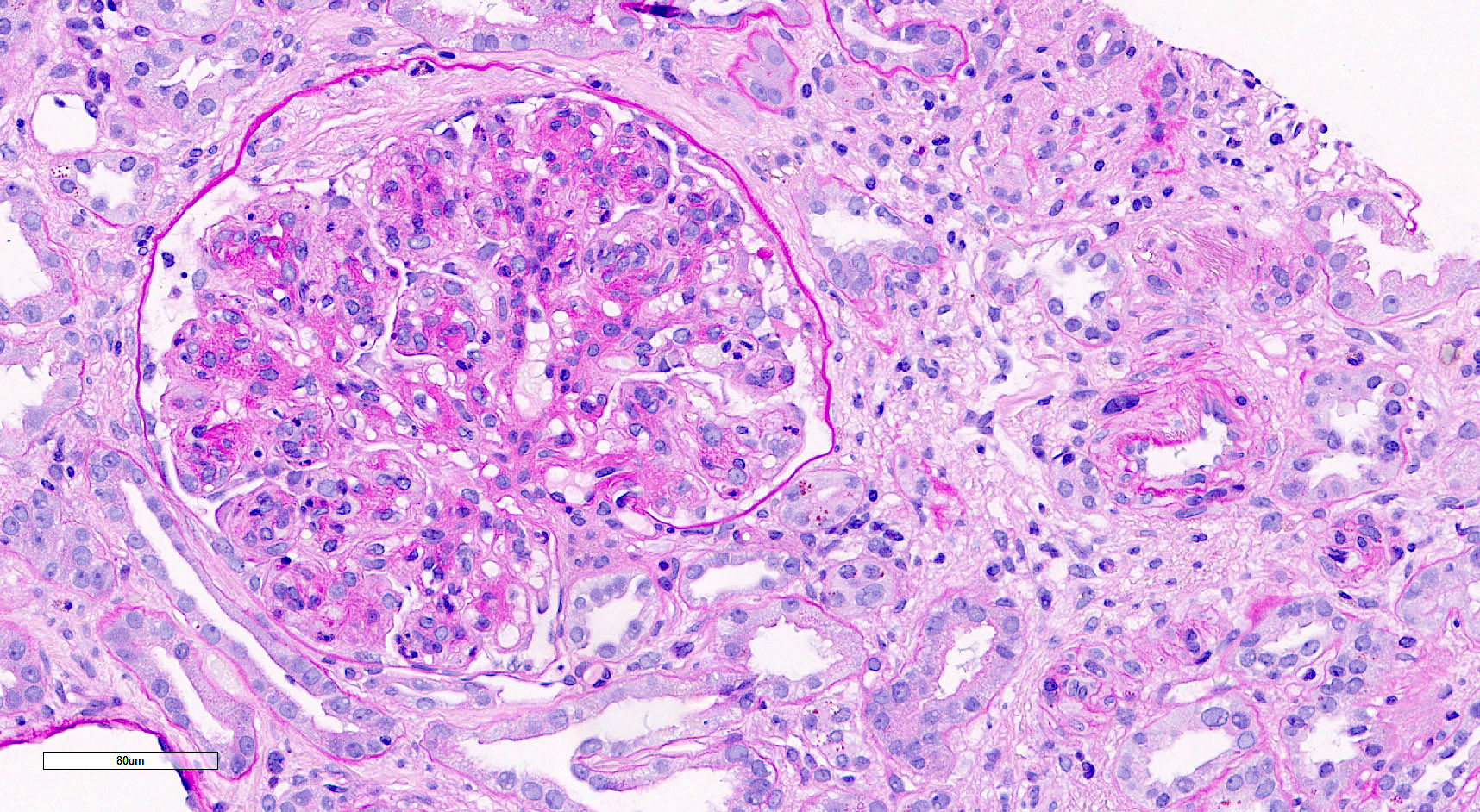
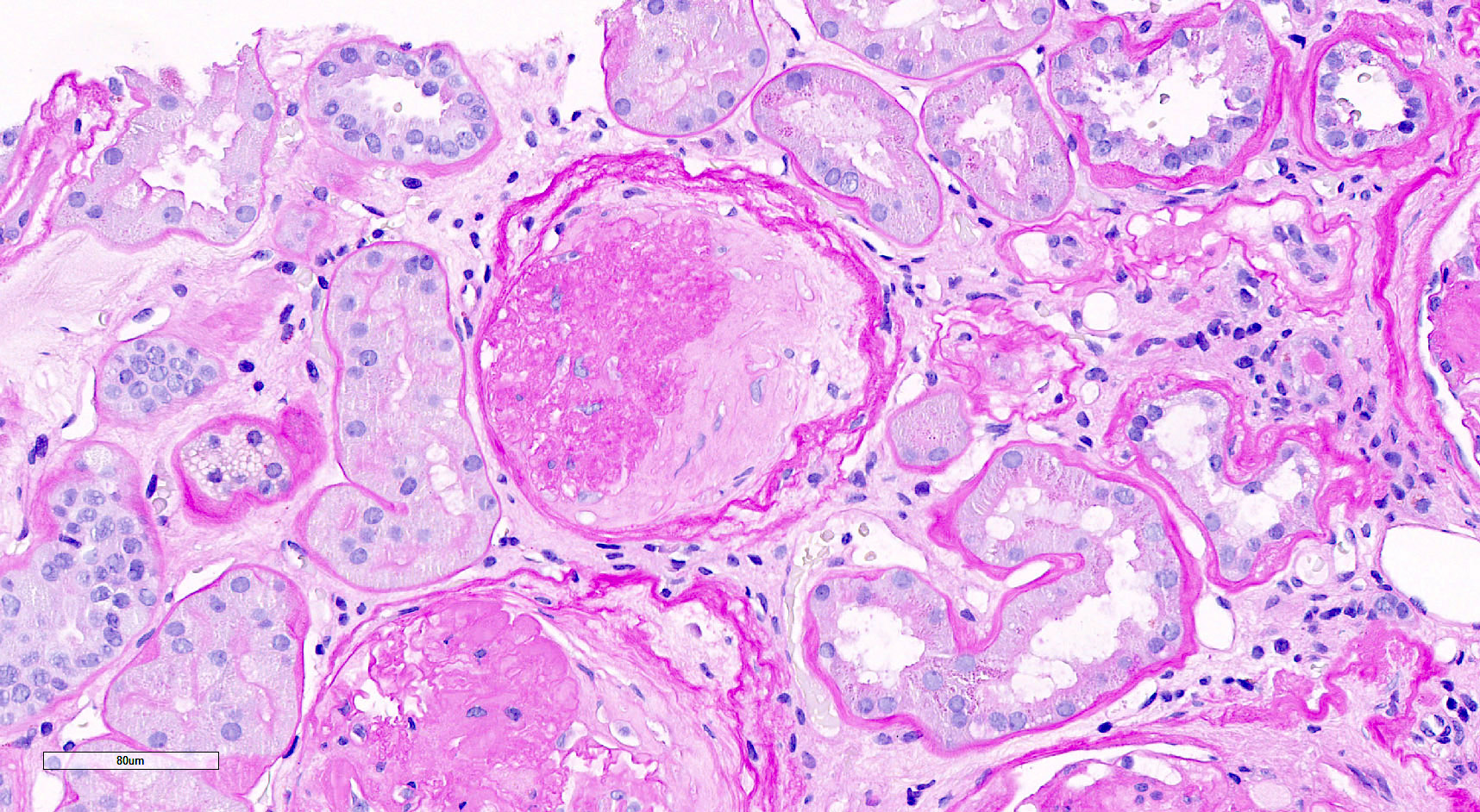
Microscopic (histologic) images
Contributed by Saskia von Stillfried, M.D.
Additional references
Board review style question #1
Board review style answer #1
D. Mesangial hypercellularity. There are > 4 mesangial cells per mesangial area. Answers A and B are incorrect because the glomerular tuft shows no endocapillary hypercellularity and no extracapillary proliferation is visible. Answers C and E are incorrect because the image shows mesangial hypercellularity but no basement membrane thickening as in membranoproliferative glomerulonephritis.
Comment Here
Reference: Renal disease-general
Comment Here
Reference: Renal disease-general
Board review style question #2
Board review style answer #2
C. Global glomerulosclerosis. Global glomerulosclerosis is characterized by shrunken and retracted glomerular tuft with PAS positive glomerular basement membrane remaining and faintly PAS positive fibrous matrix replacing Bowman space. Answers A, B and D are incorrect because the glomerulus is completely sclerotic and not diagnostic for extracapillary proliferation (including fibrocellular crescent) or segmental glomerulosclerosis. Answer E is incorrect because the Bowman space is filled not with Tamm-Horsfall protein but with sclerotic matrix.
Comment Here
Reference: Renal disease-general
Comment Here
Reference: Renal disease-general