Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Gupta RK. Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease - general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneyparaproteingeneral.html. Accessed April 2nd, 2025.
Definition / general
- Monoclonal gammopathy of renal significance (MGRS) is a broad term that includes all kidney diseases caused by a nephrotoxic monoclonal immunoglobulin (mostly immunoglobulin light chains, also known as M protein) secreted by any clonal low grade / smoldering plasma cell disease or low grade B cell lymphoproliferative disease
- Underlying hematologic disorder typically does not cause tumor complications or meet any specific criteria for any immediate specific therapy
- References: Nat Rev Nephrol 2019;15:45, Neth J Med 2019;77:243, N Engl J Med 2021;384:1931
Essential features
- MGRS includes all kidney diseases caused by any plasma cell or B cell clonal disorder (leukemia / lymphoma) that does not meet criteria for neoplasm but produces monoclonal immunoglobulins or light chains
- Kidney biopsy is the gold standard for diagnosis of MGRS, with the light chain restriction exhibited by the clonal deposit in the kidney being the same as the paraprotein present in blood, bone marrow or lymph node tissue
- Paraprotein deposits either in a single compartment (glomeruli, tubules, vessels or interstitium) or synchronously in multiple compartments resulting in MGRS associated kidney symptoms
- Some cases, due to indirect mechanism, do not show deposition of the paraprotein in the kidney
- Clone directed therapy (bortezomib for plasma cell clones and rituximab for B cell clones that express CD20 receptors) is the current standard of care
- References: N Engl J Med 2021;384:1931, Blood 2012;120:4292
Terminology
- Kidney disease associated with monoclonal gammopathy of undetermined significance (MGUS)
ICD coding
Epidemiology
- MGRS has an overall prevalence of 6% in individuals diagnosed with MGUS in the U.S. (Neth J Med 2019;77:243)
- MGRS related diseases are present in 40 - 45% of patients with monoclonal gammopathy who undergo kidney biopsy (N Engl J Med 2021;384:1931, J Am Soc Nephrol 2020;31:2400)
- Age: mostly adults (50 and beyond); frequency is 3% in people aged > 50 years, 5% in > 70 years and up to 8% in > 80 years (Nat Rev Nephrol 2019;15:45)
- M:F exact ratio not known but incidence likely higher in men
- 2 - 3 times higher incidence of MGUS in African Americans than in Caucasians (Nat Rev Nephrol 2019;15:45)
- No geographic bias known
- Associated in 100% of cases with a covert or overt B cell or a plasma cell clonal disease that does not meet current criteria for immediate treatment and simultaneously causes symptomatic renal disease (N Engl J Med 2021;384:1931, Nat Rev Nephrol 2019;15:45)
Sites
- Kidney, involvement of one or more compartments (glomerulus, tubule, blood vessel and interstitium)
Pathophysiology
- Excess of the monoclonal protein (entire immunoglobulin or only the light chain, rarely heavy chain) is present within the kidney or systemic microcirculation and may form misfolded proteins and deposit as fibrils, may resist normal proteolytic degradation due to unusual variable domains (kappa light chain mostly) and deposit as crystals or may activate complement pathways (classical or alternative pathway)
- Immunoglobulins or complement may be deposited in the glomerulus; this is in addition to the monoclonal light chain or sometimes even without the light chain (as in C3 glomerulopathy)
- Glomerulus with monoclonal light chain or sole complement deposition often shows influx of inflammatory cells in addition to the proliferation of resident glomerular cells or visceral epithelial cells (forming crescents)
- Kappa light chain clone is more common in MGRS lesions, except in amyloid light chain (AL) amyloidosis wherein lambda is more common
- Some cases result from paraprotein disruption of complement pathway without direct kidney paraprotein deposition
- References: Nat Rev Nephrol 2019;15:45, Neth J Med 2019;77:243, Mod Pathol 2011;24:1462
Etiology
- Low grade plasma cell disorder (MGUS) and smoldering plasma cell disease (smoldering multiple myeloma)
- Smoldering Waldenström macroglobulinemia
- Low grade or early stage chronic lymphocytic leukemia (CLL)
- Monoclonal B cell lymphocytosis (MBL), a recently recognized B cell clonal equivalent of MGUS
- Low grade B cell non-Hodgkin lymphomas such as marginal zone lymphoma, mantle cell lymphoma and mucosa associated lymphoid tissue (MALT) lymphoma
- All lesions listed above result in a nephrotoxic monoclonal protein (mostly a light chain, rarely a heavy chain) that deposits in single or multiple compartments of the kidney (glomeruli, tubules, vessels or interstitium)
- Small dangerous B cell or plasma cell clones that are below the detection threshold for conventional hematopathologic work up
- Reference: Nat Rev Nephrol 2019;15:45
Clinical features
- Acute renal failure
- Acute on chronic renal failure
- Proteinuria
- Edema
- Anemia
- Fanconi syndrome
- Arthralgia
- Dizziness
- Nausea and vomiting
- Peripheral neuropathy
- Lymphadenopathy (for certain low grade B cell lymphomas)
- References: Clin J Am Soc Nephrol 2018;13:128, Clin J Am Soc Nephrol 2016;11:2260
Diagnosis
- Urinalysis
- 24 hour urine collection
- Detection of monoclonal free light chains (kappa or lambda, never both) in blood or urine
- Kidney biopsy examination by light microscopy, immunofluorescence and electron microscopy
- Bone marrow examination
- Flow cytometry of blood or bone marrow
- Cytogenetics and FISH studies
- Immunoelectron microscopy (where available)
- Laser dissection tandem mass spectrometry (LDTMS), where available
- References: Neth J Med 2019;77:243, Blood 2012;120:4292, Nat Rev Nephrol 2019;15:45
Laboratory
- Increased urinary protein (subnephrotic or nephrotic)
- Microscopic hematuria
- Increased serum creatinine
- Abnormal serum calcium, bicarbonate, chloride, phosphate and uric acid levels
- Renal tubular acidosis
- Bence-Jones protein in urine
- Serum / urine protein electrophoresis (SPEP / UPEP) showing an abnormal M spike protein
- Serum or urine immunofixation showing an abnormal kappa or lambda light chain (never both) with or without a complete monoclonal immunoglobulin (IgG, IgM, IgA)
- Elevated free kappa (K) or lambda (L) light chain with elevated K/L or L/K ratio
- Low serum complement levels (not always)
- Positive serum cryoglobulin level
- Blood or bone marrow flow cytometry showing light chain restriction, either for K or L
- Cytogenetics and FISH studies may reveal MYD88 mutation, cyclin D1 mutation, IGHV mutation, del(17p) mutation, hyperdiploidy, trisomy 21 mutation, etc.
- LDTMS shows K or L restriction in biopsy tissue
- References: Neth J Med 2019;77:243, N Engl J Med 2021;384:1931
Prognostic factors
- In response to treatment, a greater hematologic response of the patient's primary hematolymphoid disease translates to a greater probability of recovery of kidney function
- Favorable factors for renal recovery include no minimal residual disease at all (reduction of involved light chain level at < 2 mg/dL) or at least, a good partial hematologic response (reduction of the involved free light chain at < 4 mg/dL)
- In patients with MGRS who did not receive appropriate hematologic treatment prior to kidney transplantation, recurrence in transplant kidney is common
- References: N Engl J Med 2021;384:1931, Am J Kidney Dis 2022;79:202
Case reports
- 44 year old woman with nephrotic syndrome and decreased renal function (Rom J Morphol Embryol 2017;58:1065)
- 54 year old man with progressive bone pain and renal dysfunction (Medicine (Baltimore) 2018;97:e12027)
- 55 year old hypertensive woman presenting with generalized edema, proteinuria and recent history of seizure (Indian J Nephrol 2014;24:376)
- 57 year old man with recent diagnosis of hypercalcemia and presenting with lethargy, anorexia and right flank pain (Pathology 2020;52:283)
- 75 year old man with known chronic kidney disease who presents with rapid decline in renal function and proteinuria (BMC Nephrol 2018;19:129)
Treatment
- Aim of MGRS treatment is to preserve or improve kidney function by targeting the B cell or plasma cell clone
- Current consensus supports clone directed therapy over general immunosuppressive therapy
- Patients with plasma cell clones and MGRS: treatment with bortezomib is indicated
- Patients with B cell clones (expressing CD20) and MGRS: rituximab based therapy is indicated
- Supportive care for hypertension, proteinuria and impairment of mineral metabolism
- References: N Engl J Med 2021;384:1931, Neth J Med 2019;77:243
Microscopic (histologic) description
- Various morphologic subtypes, depending on location within kidney and physical structure
- Deposits are mostly monoclonal and composed of light chains (in most MGRS subtypes)
- Deposits may occasionally be nonmonoclonal (C3 glomerulopathy with monoclonal gammopathy)
- Deposits may rarely be absent (thrombotic microangiopathy, monoclonal gammopathy associated)
- Deposits may be organized (deposits with substructure - fibrils, microtubules, crystals) or nonorganized (solid amorphous or powder-like)
- Common morphologic subtypes include
- AL or AH or AHL amyloidosis (L for light chain, H for heavy chain)
- Fibrillary glomerulonephritis (FGN), monoclonal type
- Immunotactoid glomerulonephritis (ITGN), monoclonal subtype
- Cryoglobulinemic glomerulonephritis, type I and type II (type 1 and 2 CryoGN)
- Light chain proximal tubulopathy (LCPT), crystalline and noncrystalline type
- Monoclonal immunogloubulin deposition disease (MIDD), presenting either as light chain deposition disease (LCDD, common) or heavy chain deposition disease (HCDD, rare)
- Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID)
- C3 glomerulopathy with monoclonal gammopathy (C3G)
- Crystal storing histiocytosis (CSH)
- Crystalglobulin induced nephropathy (CIN) / crystalglobulinemic glomerulonephritis
- Thrombotic microangiopathy, monoclonal gammopathy associated (TMA)
- Patterns of glomerular pathology (glomerulonephritis / glomerulopathy) include
- Membranoproliferative glomerulonephritis (PGNMID, ITGN, C3G, CryoGN, FGN)
- Mesangioproliferative glomerulonephritis (PGNMID, FGN, ITGN)
- Endocapillary proliferative glomerulonephritis (PGNMID)
- Membranous glomerulonephritis (rare, PGNMID)
- Nodular glomerulosclerosis pattern (AL or AH amyloid, MIDD)
- Variable nonnodular mesangial or paramesangial expansion (AL amyloid, FGN)
- Mesangiolysis, capillary double contours and intracapillary microthrombi (TMA)
- Crescentic glomerulonephritis (AH amyloid, PGNMID, FGN)
- Capillary wall spikes or eyelash sign (AL amyloid)
- Crystals occluding glomerular capillary loops (CIN)
- Cryoplugs occluding glomerular capillary loops (CryoGN)
- Crystal laden histiocytes occluding glomerular capillary loops (CSH, glomerular type, rare)
- Patterns of tubular pathology include
- Acute tubular injury (LCPT)
- Crystals within proximal tubular epithelium (LCPT)
- Pale material in tubular basement membranes (AL or AH amyloid)
- Amyloid cast within lumina (rare)
- Patterns of vascular pathology include
- Pale waxy material in vessel wall (AL or AH amyloid)
- Thrombus in vessel lumina (TMA)
- Crystal within vessel lumina (CIN)
- Patterns of interstitial pathology include
- Interstitial amyloid (AL or AH amyloid)
- Monoclonal plasma cell or B cell infiltrate (as in chronic lymphocytic leukemia, for example)
- Infiltrate by crystal laden histiocytes (CSH, interstitial type, more common)
- Reference: Nat Rev Nephrol 2019;15:45
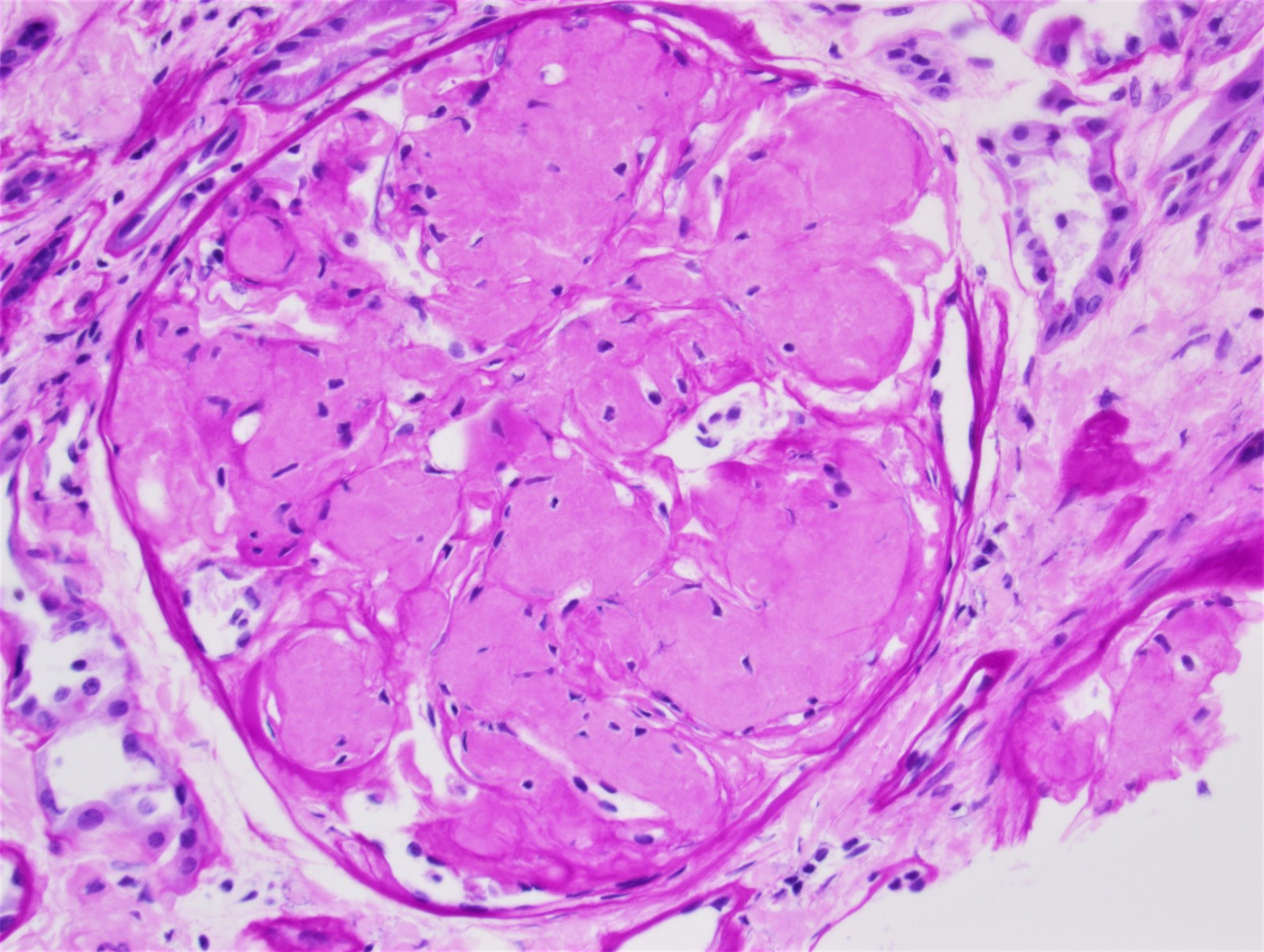
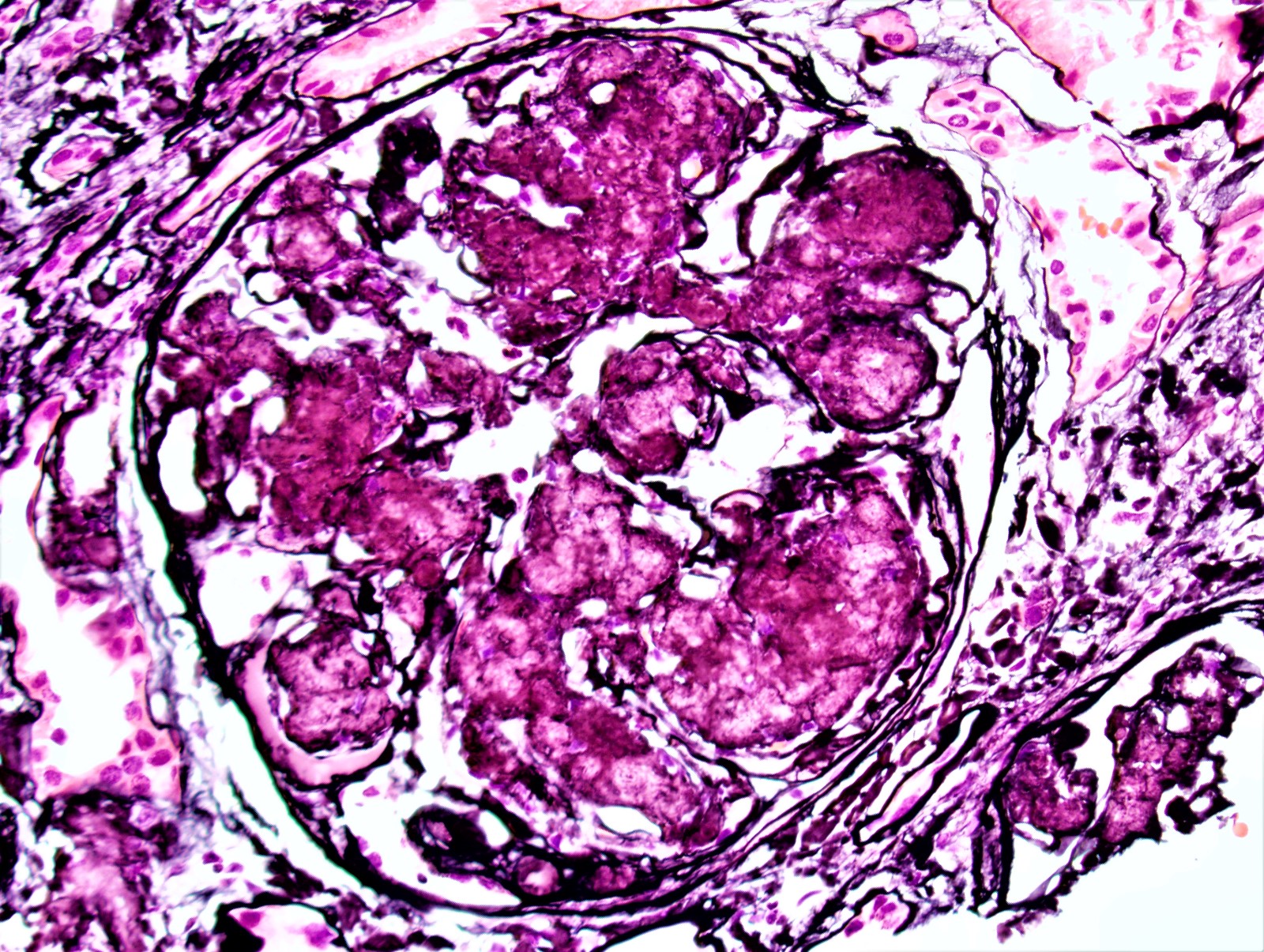
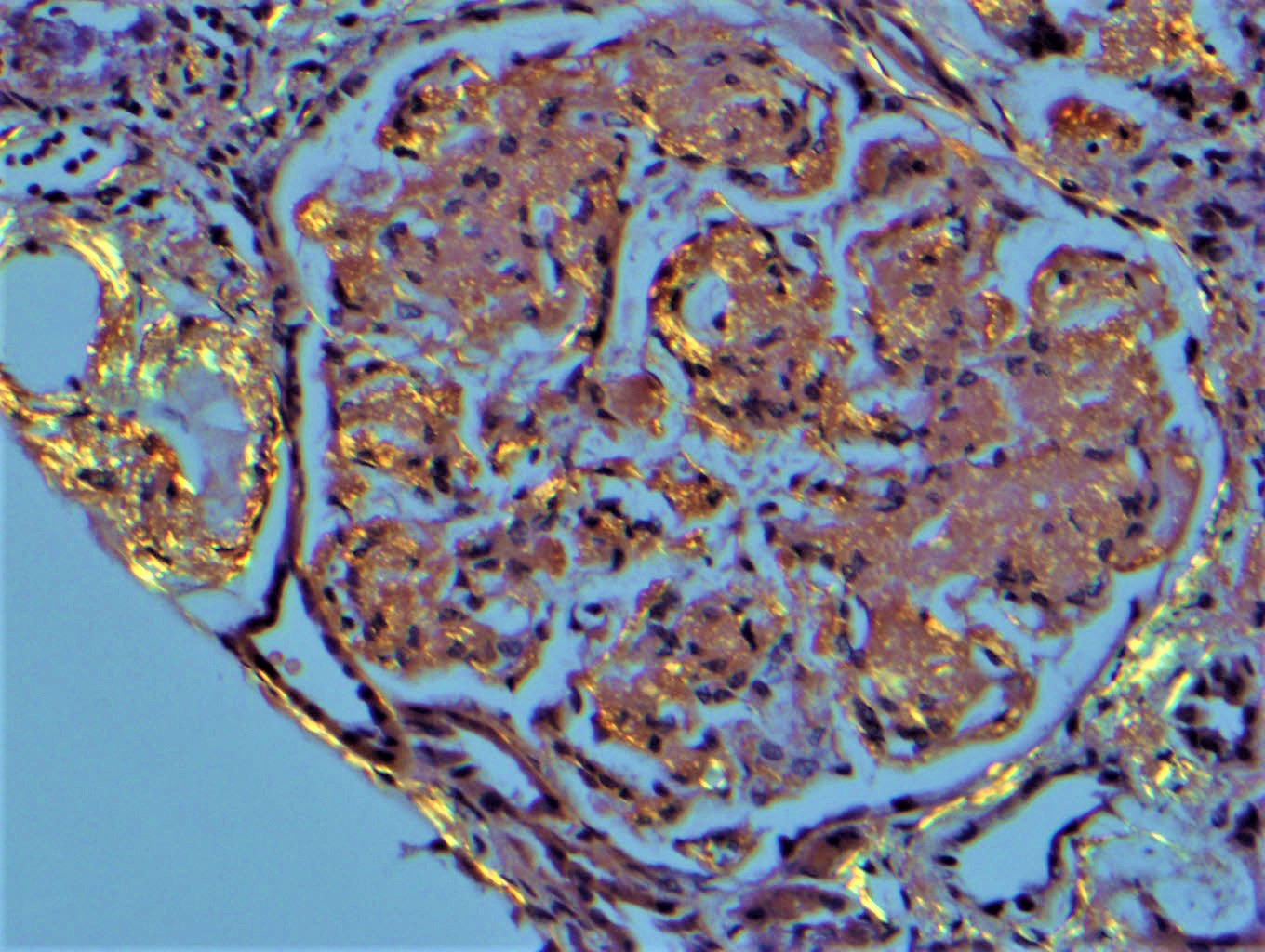
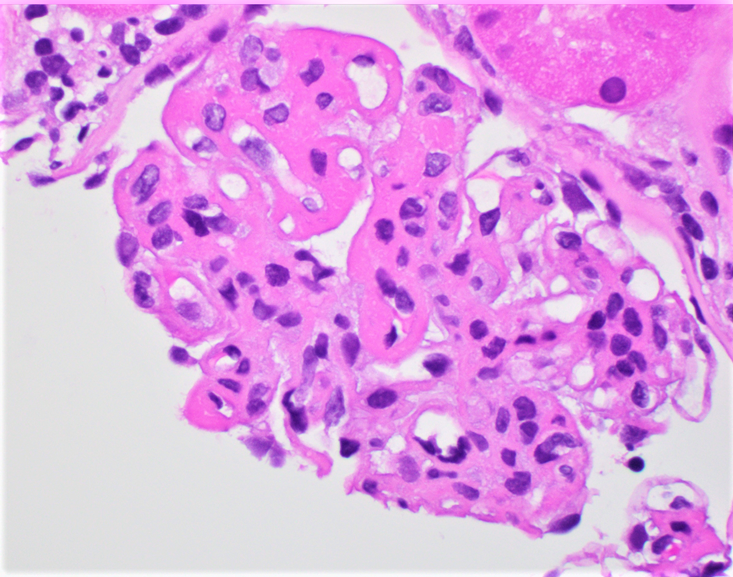
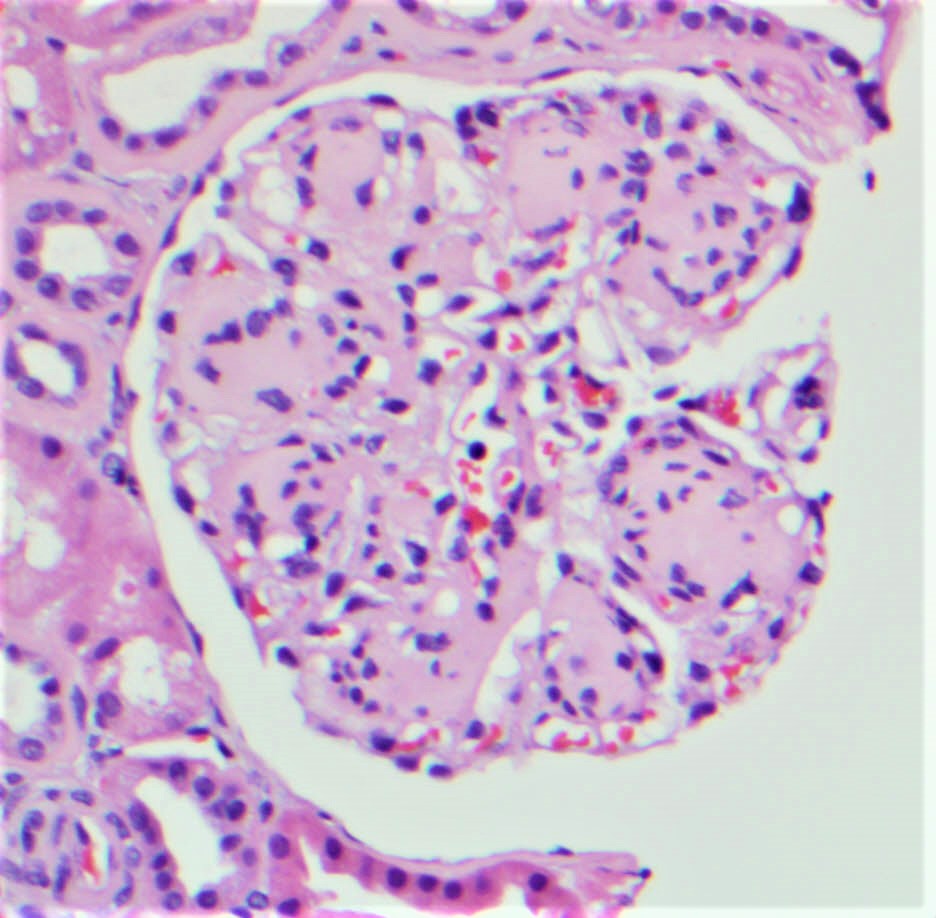
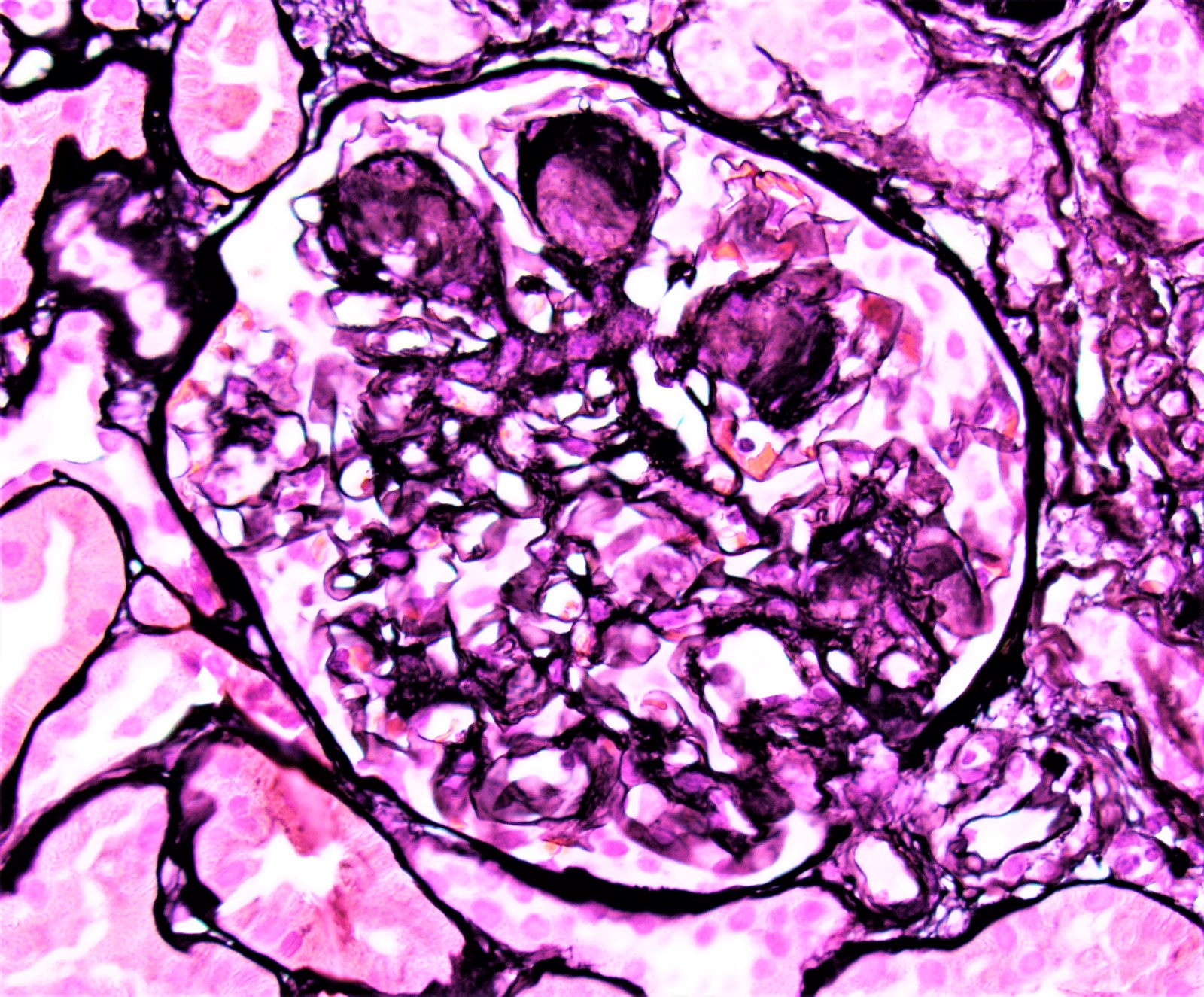
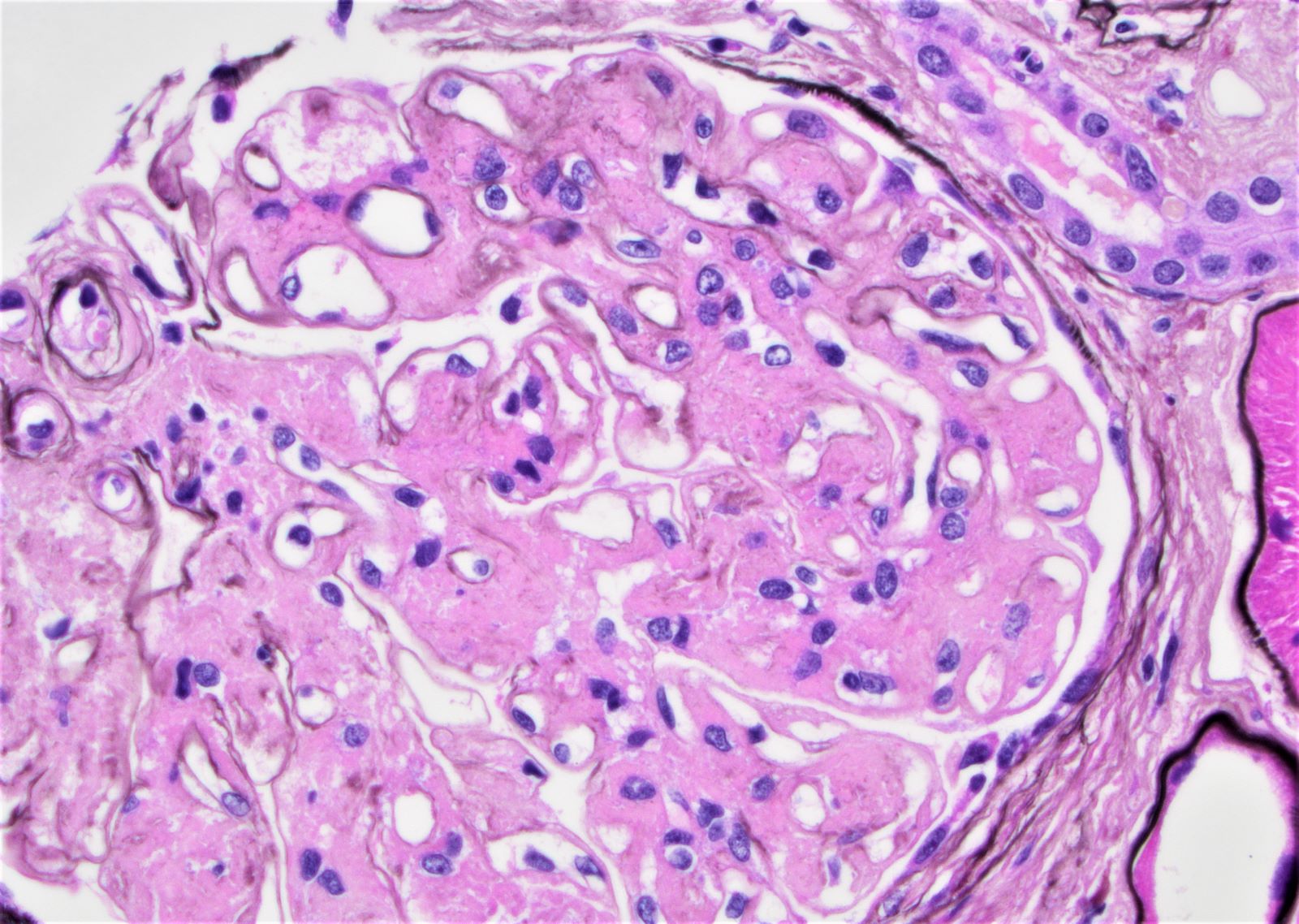
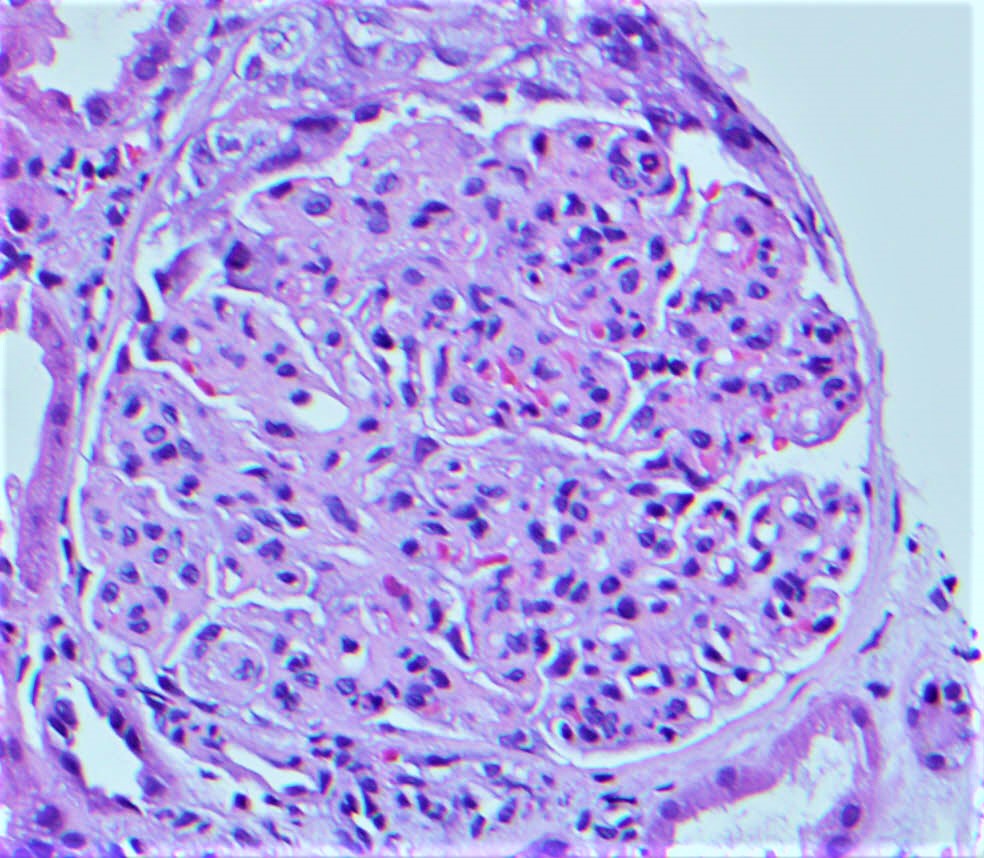
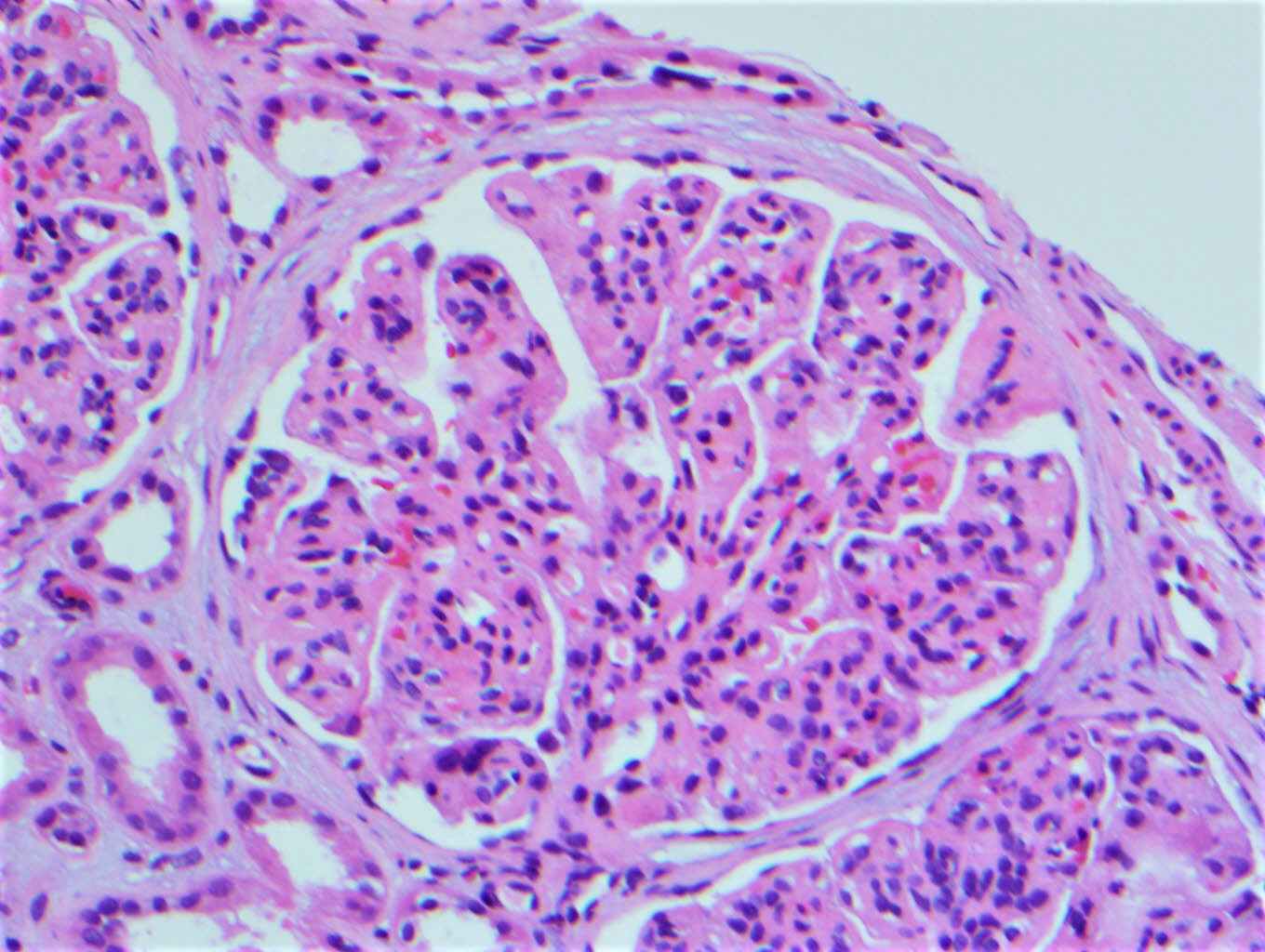
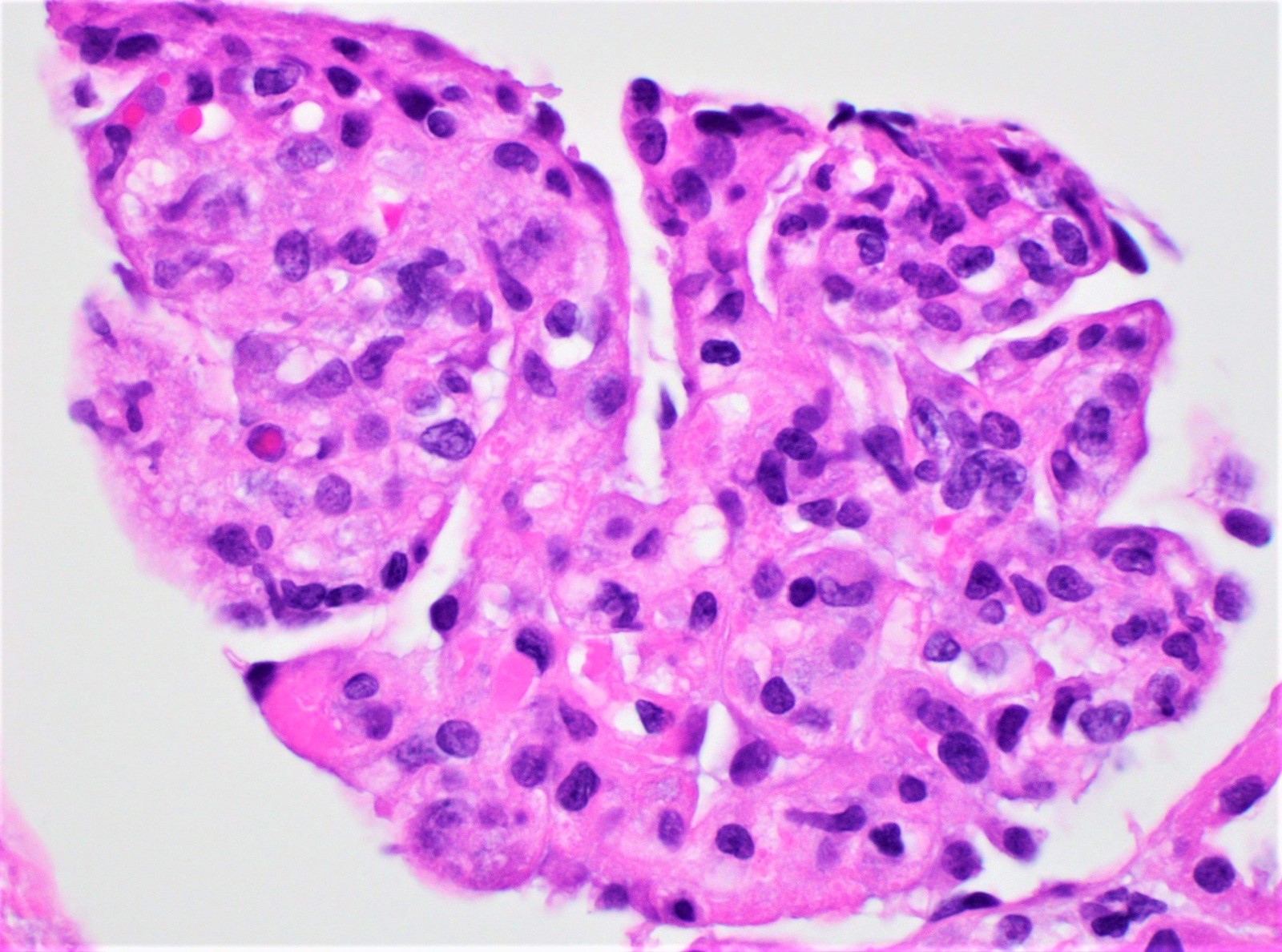
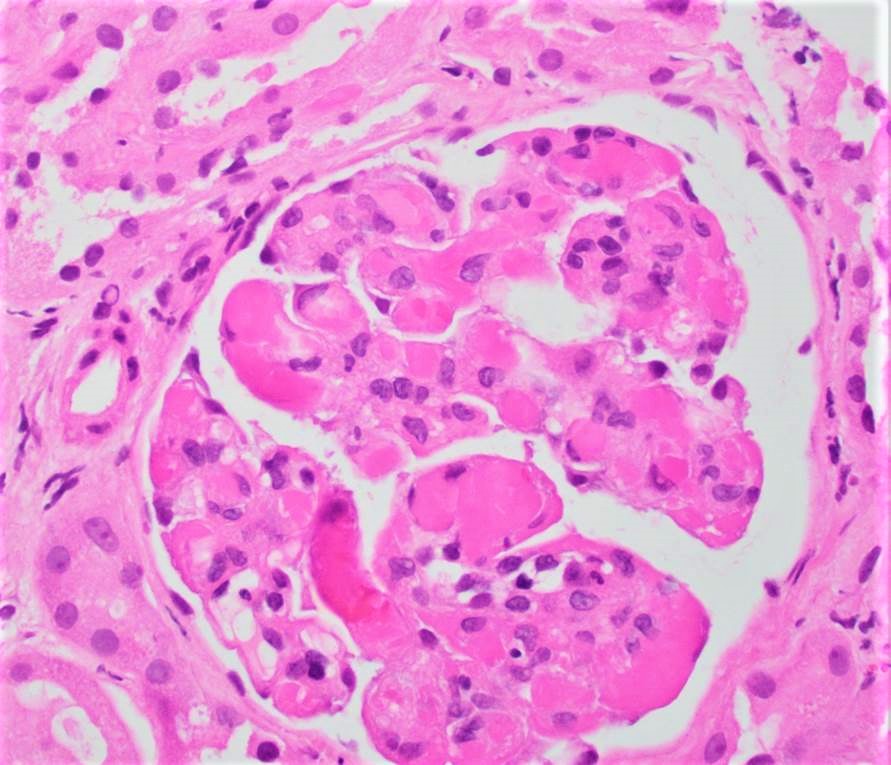
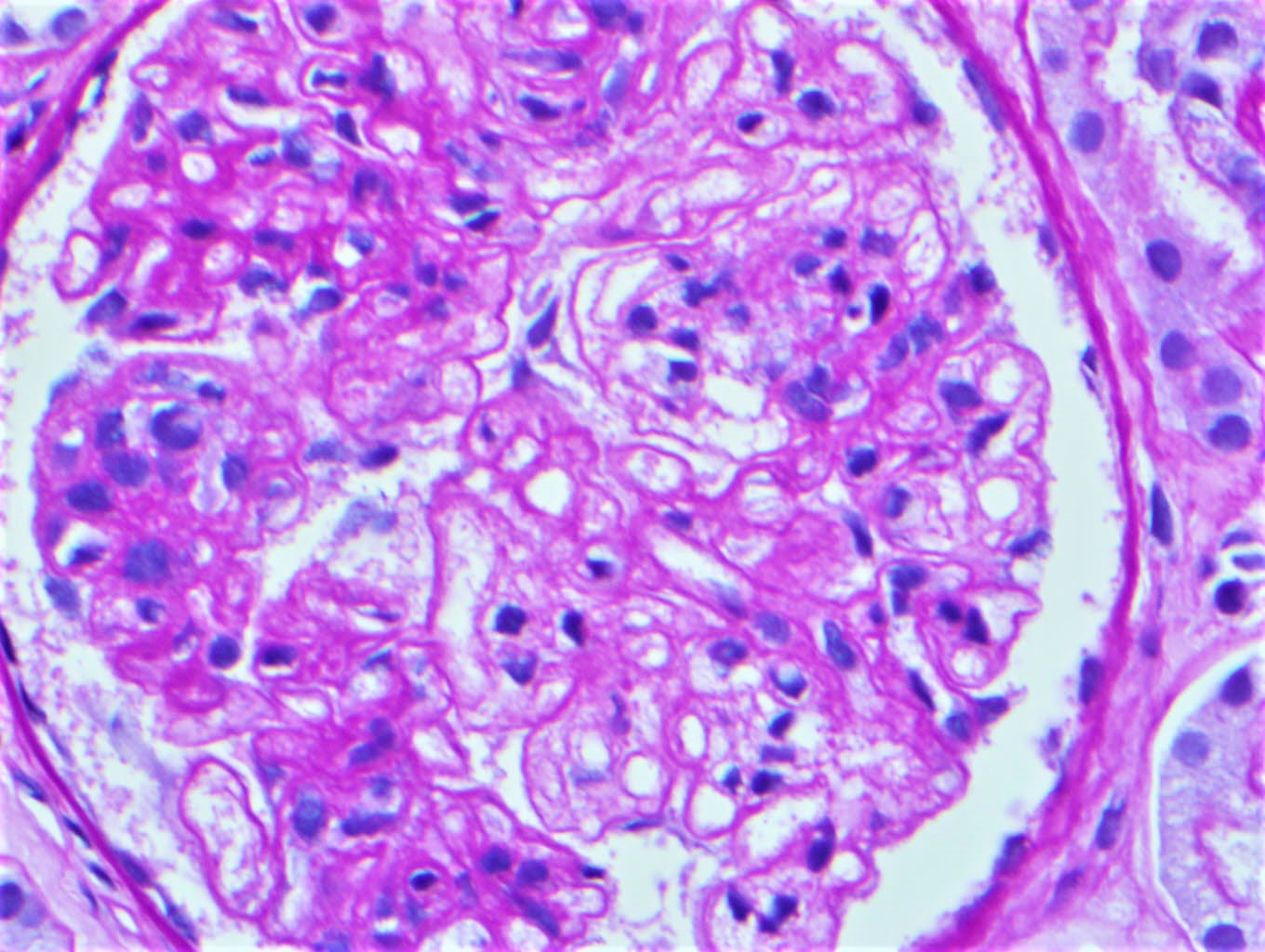
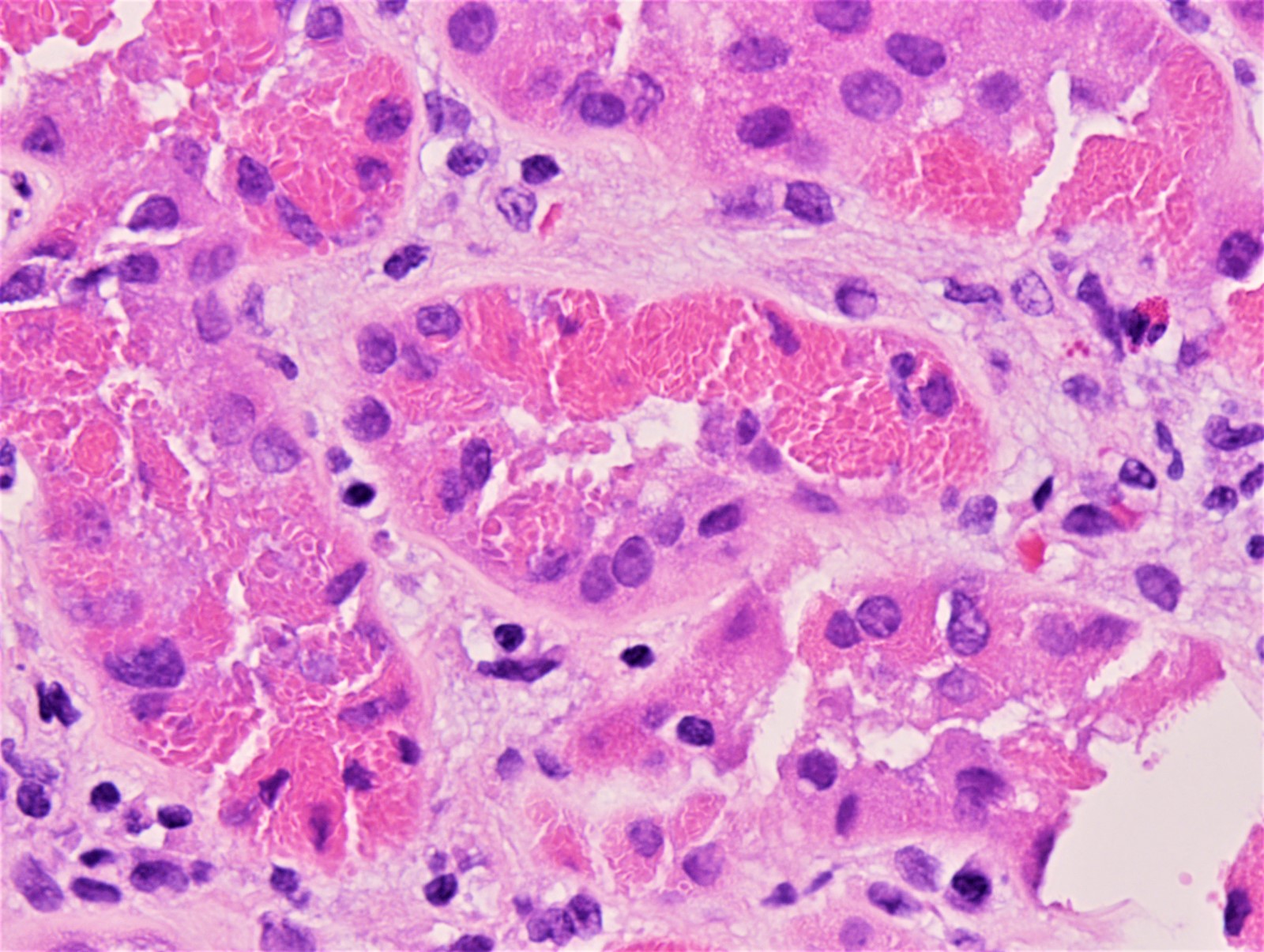
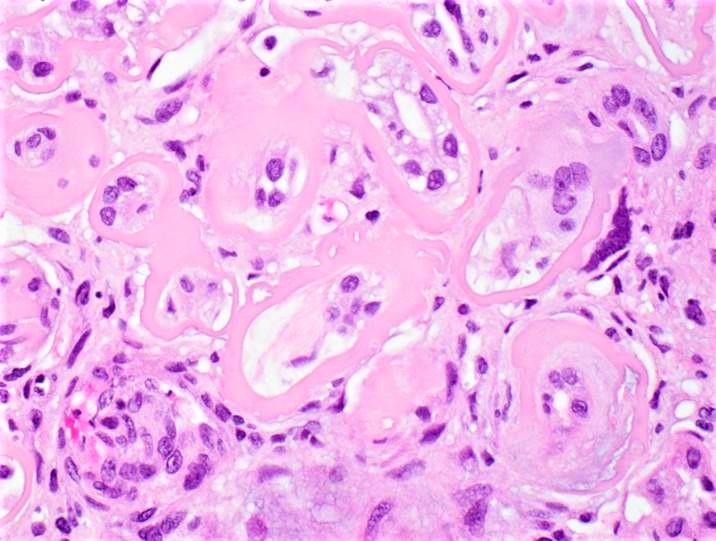
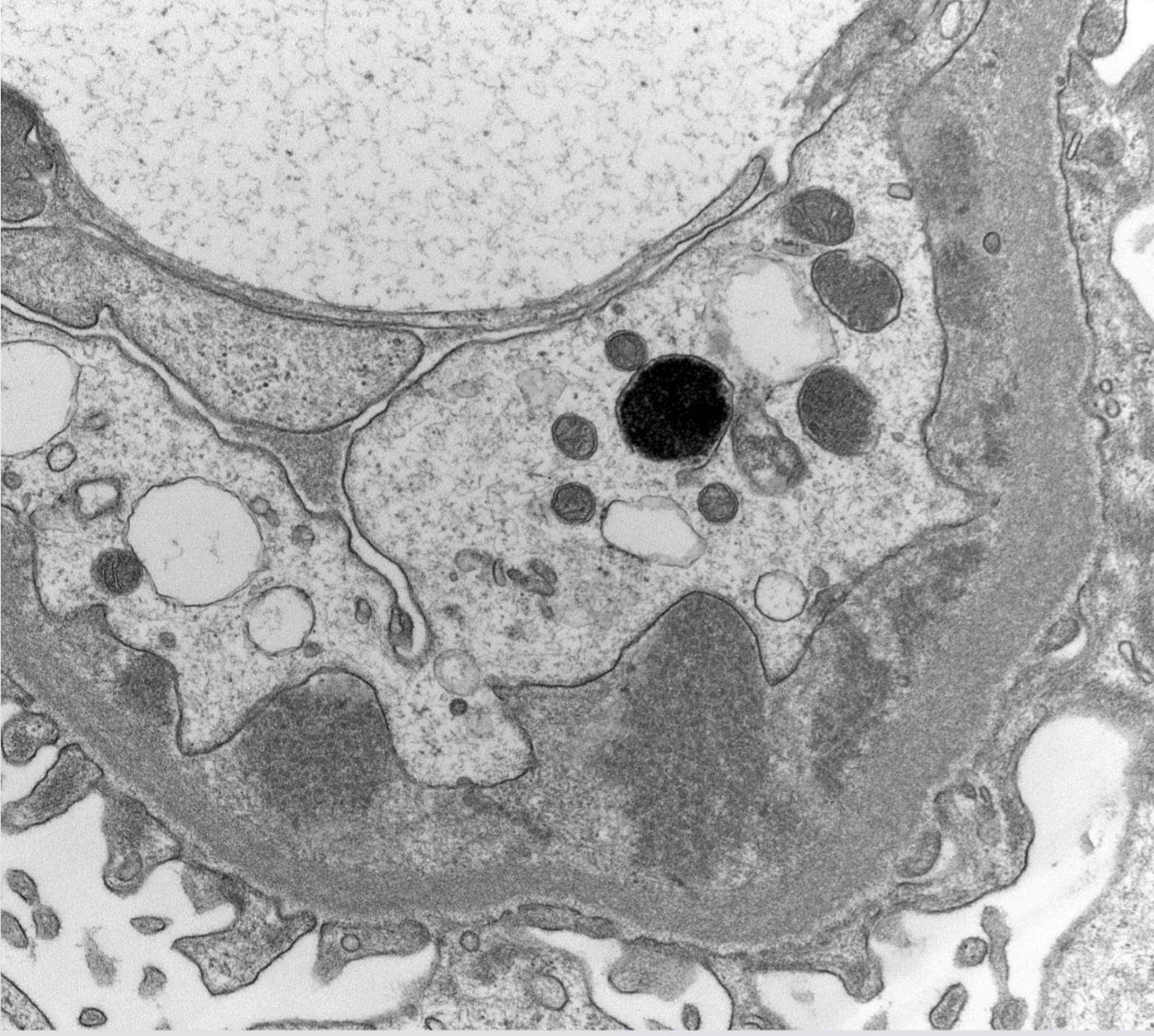
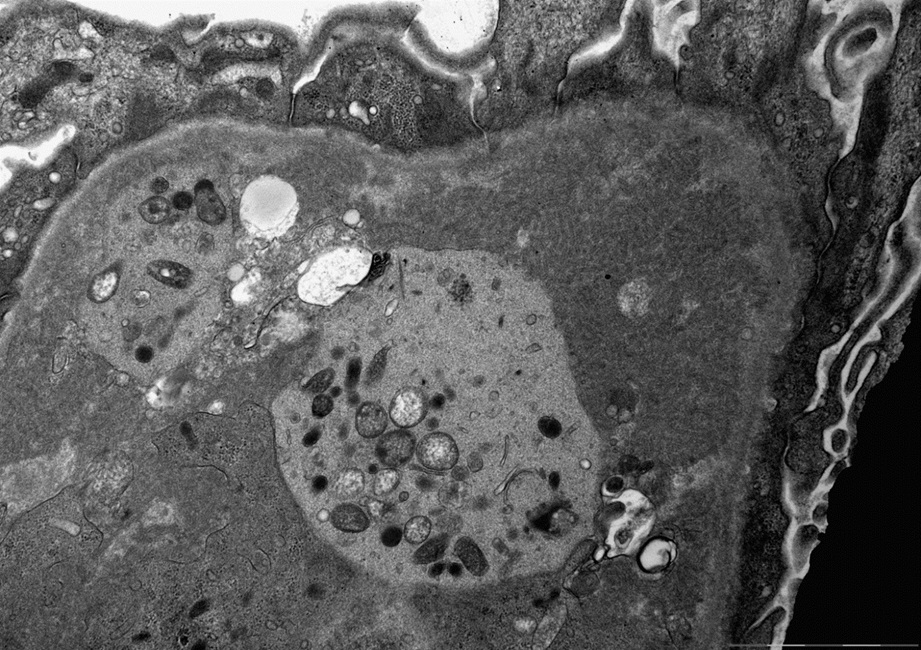
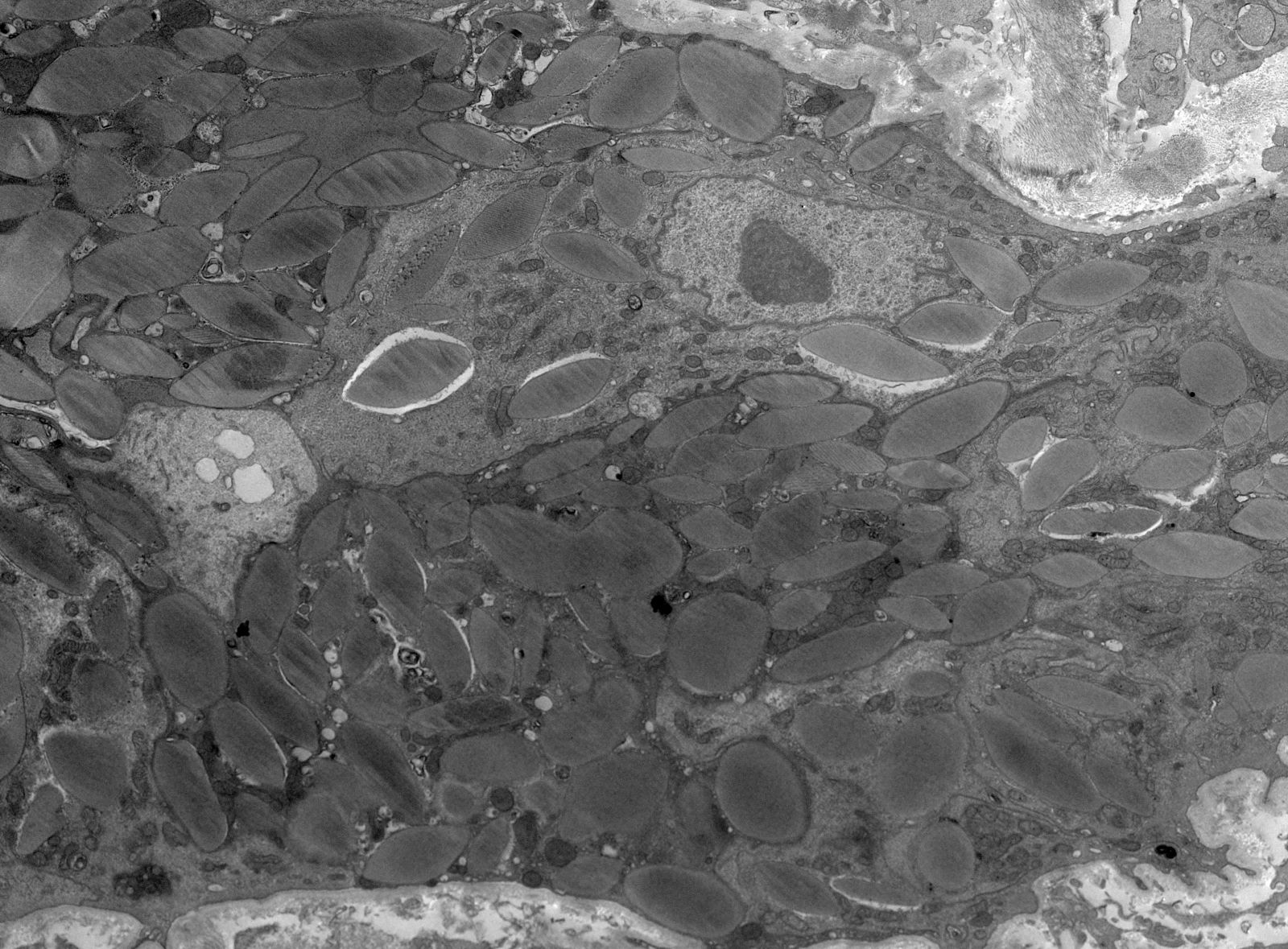
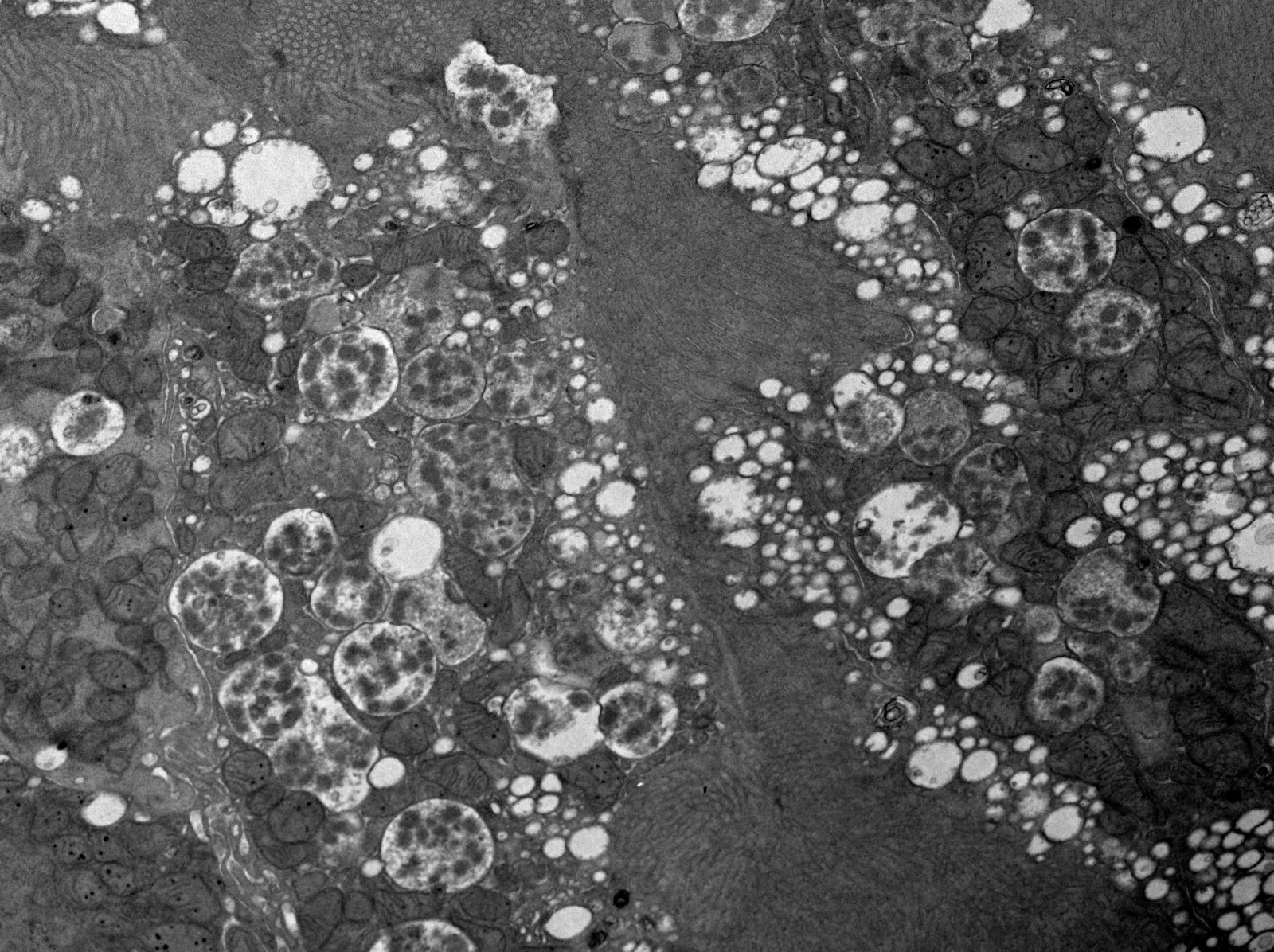
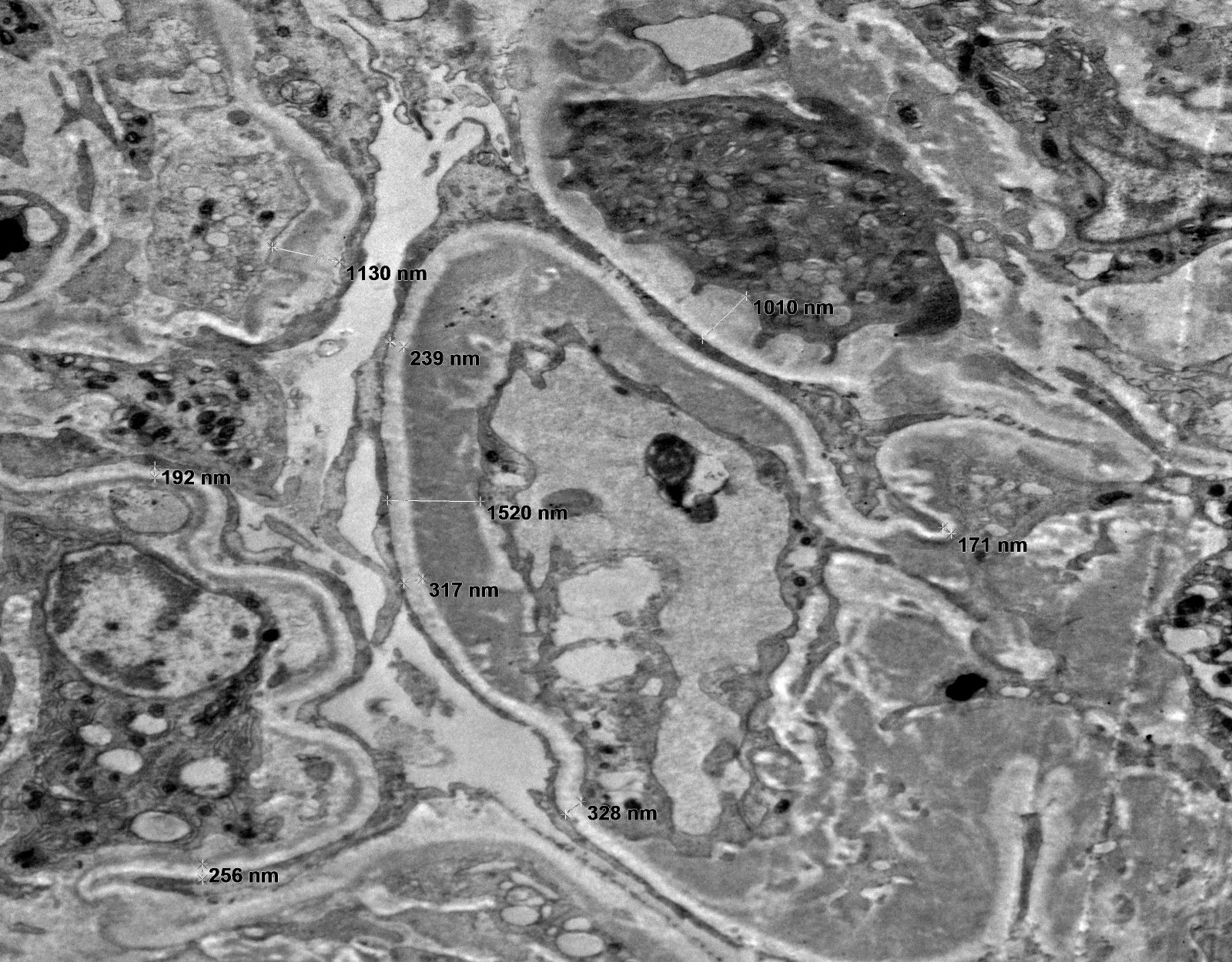
Microscopic (histologic) images
Contributed by Rajib K. Gupta, M.D., Ashley Flowers, M.D. and Lois J. Arend, M.D., Ph.D.
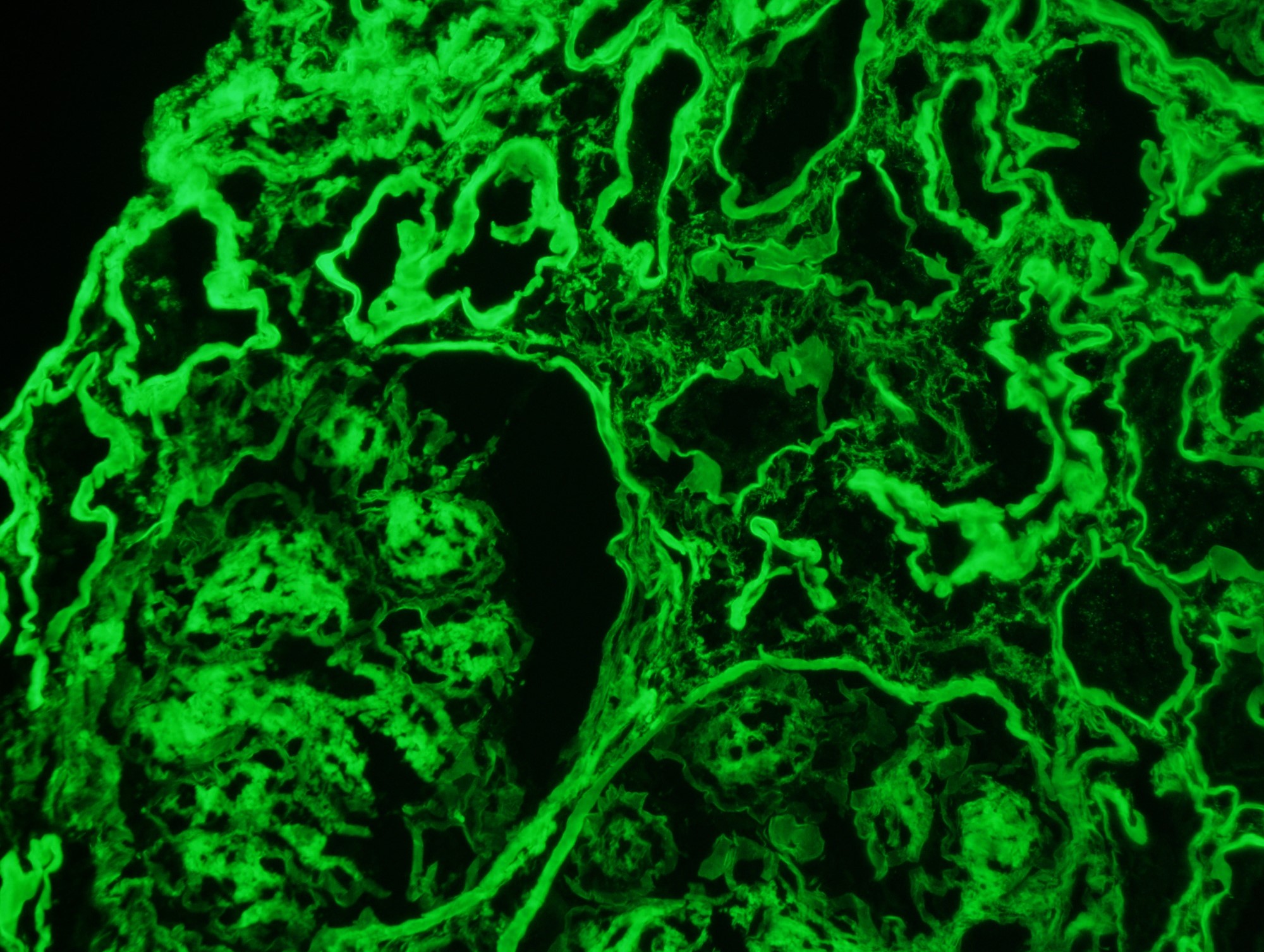
Immunofluorescence description
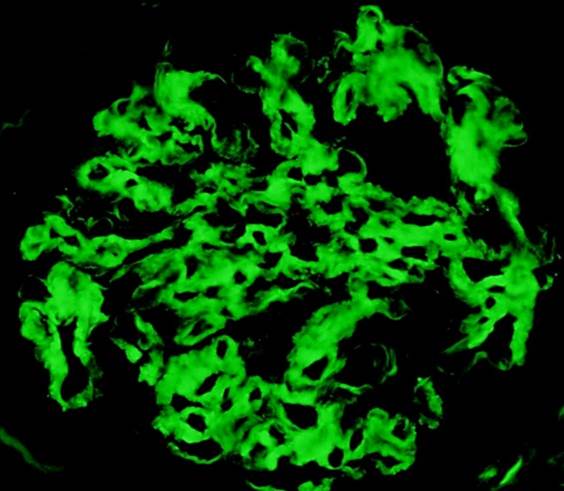
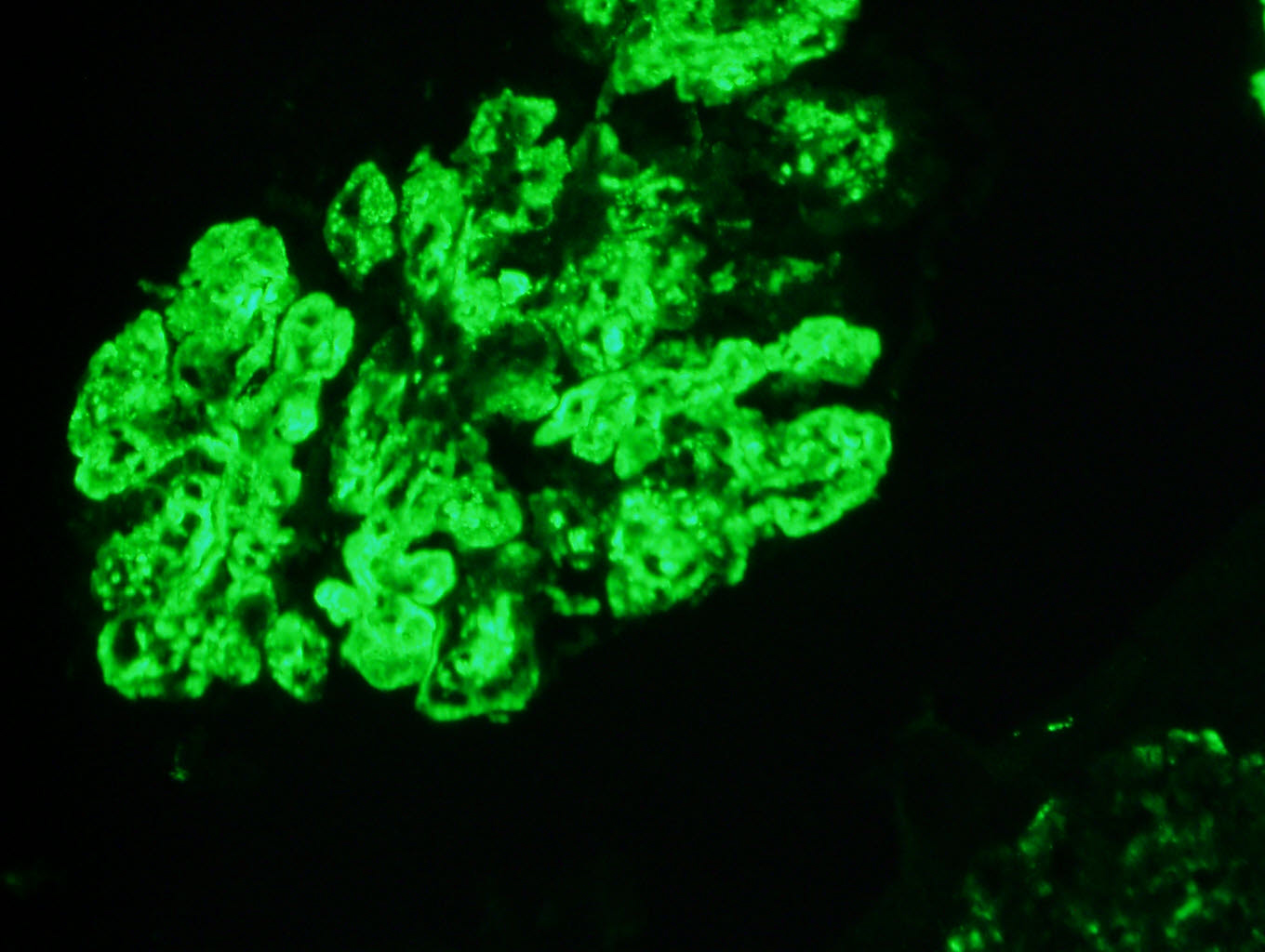
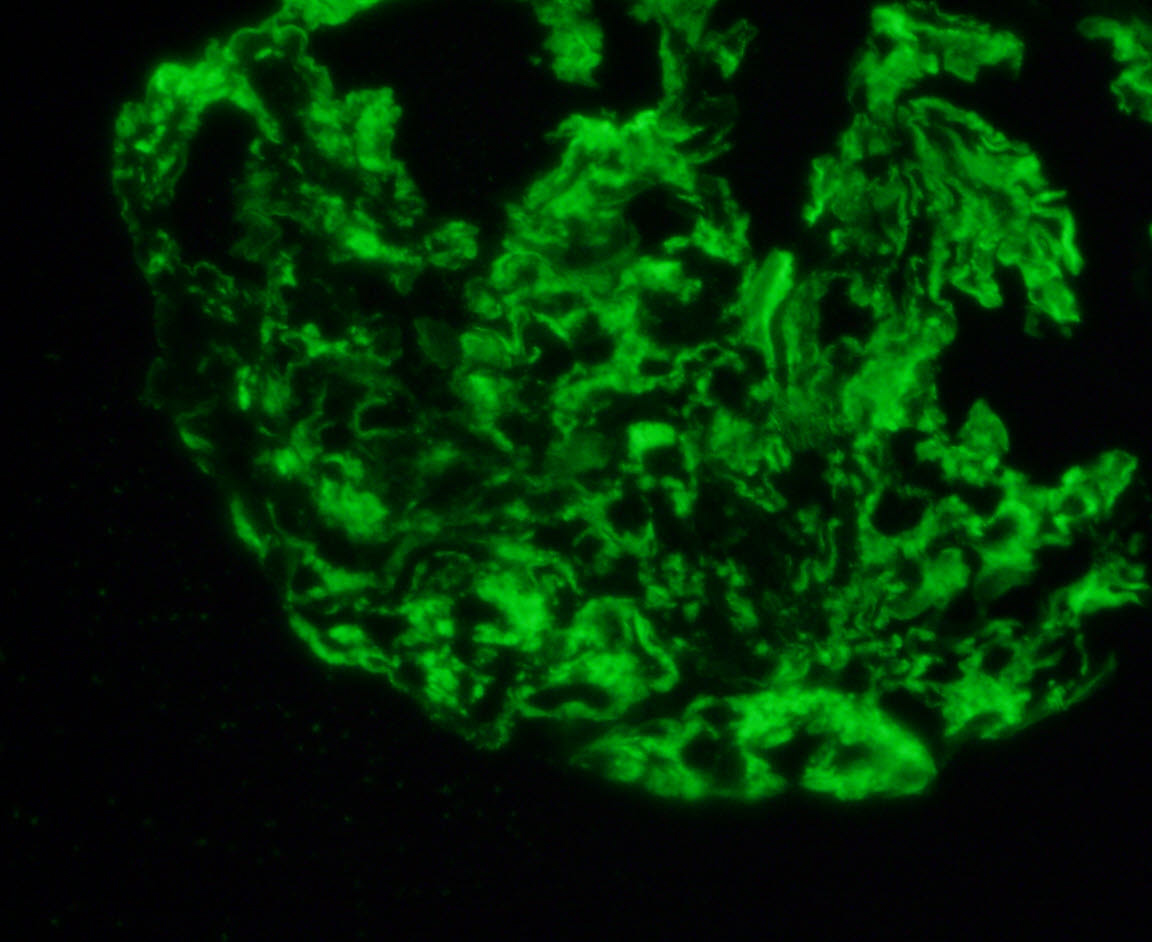
- Frozen section immunofluorescence (IF) confirms light chain restriction (K or L, never both)
- Immunofluorescence after pronase digestion (pronase IF) should also be performed if available to confirm light or heavy chain restriction
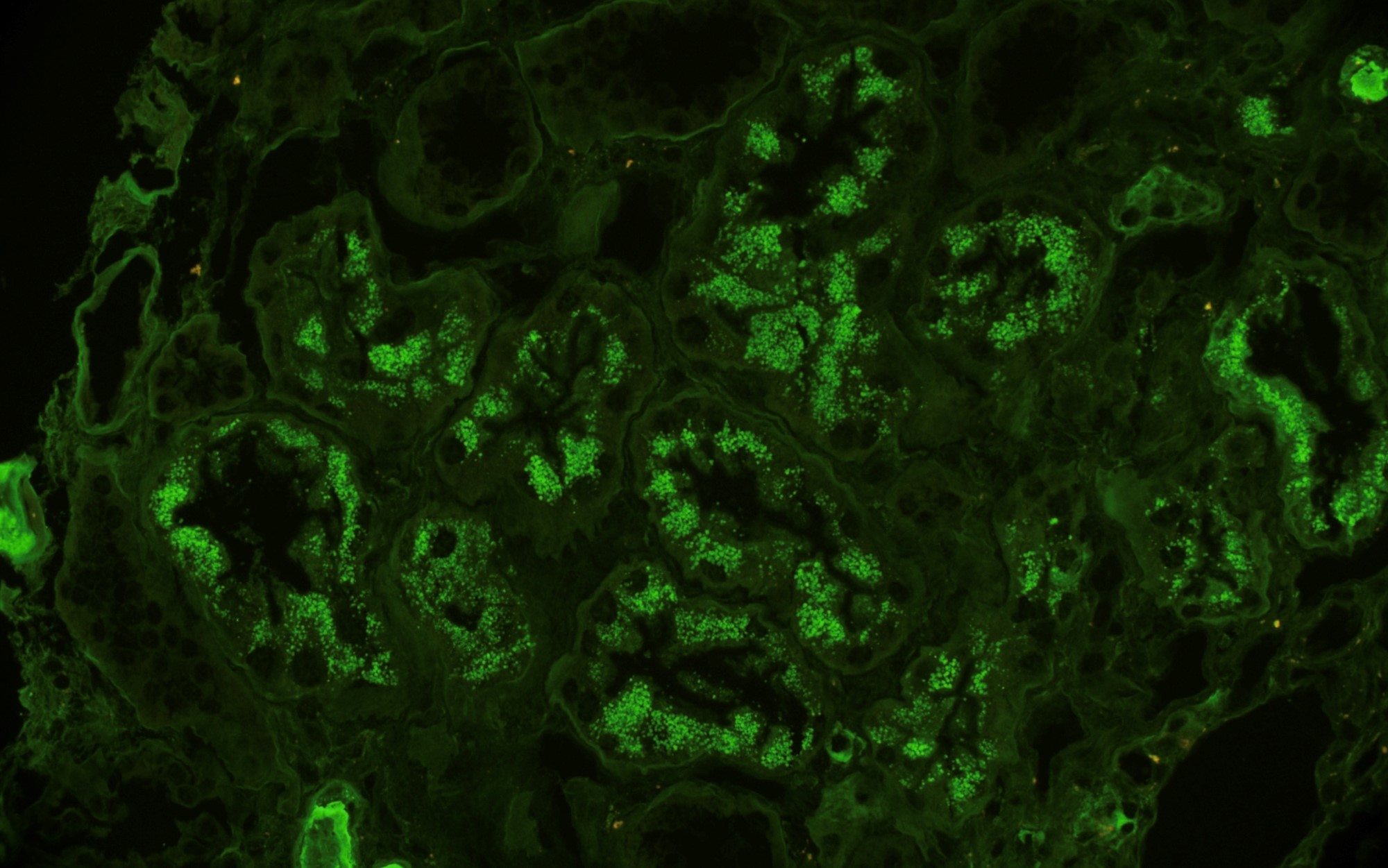
- Light chain restriction on IF may be seen in the form of granular or smudgy staining for K or L in the glomerular mesangium or capillary walls, linear staining of K or L in the tubular basement membranes, granular staining for K or L in tubular cytoplasmic inclusions or intraluminal K or L staining of cryoplug or crystal
- In C3G, there will be solitary IF staining for C3 only or dominant staining for C3 but no light chain staining
- IgG subclass (IgG1, IgG2, IgG3 and IgG4) staining should be performed in all cases with IgG dominant deposits (e.g., PGNMID; HCDD; HLCDD, Type 1 Cryogobulenemic GN)
- Heavy light chain antibody staining may be useful but still in validation phase
- References: Nat Rev Nephrol 2019;15:45, Kidney Int 2021;100:155
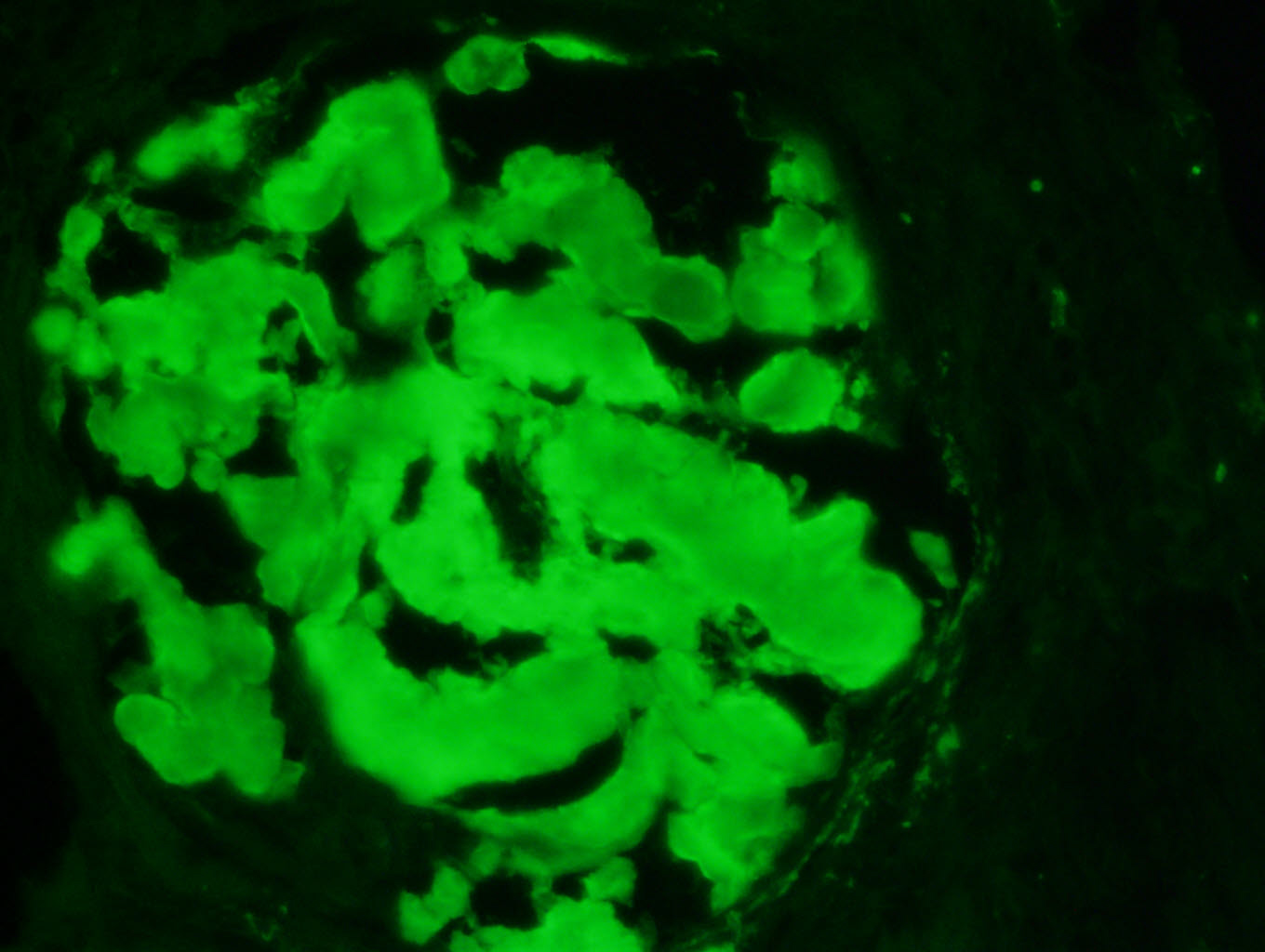
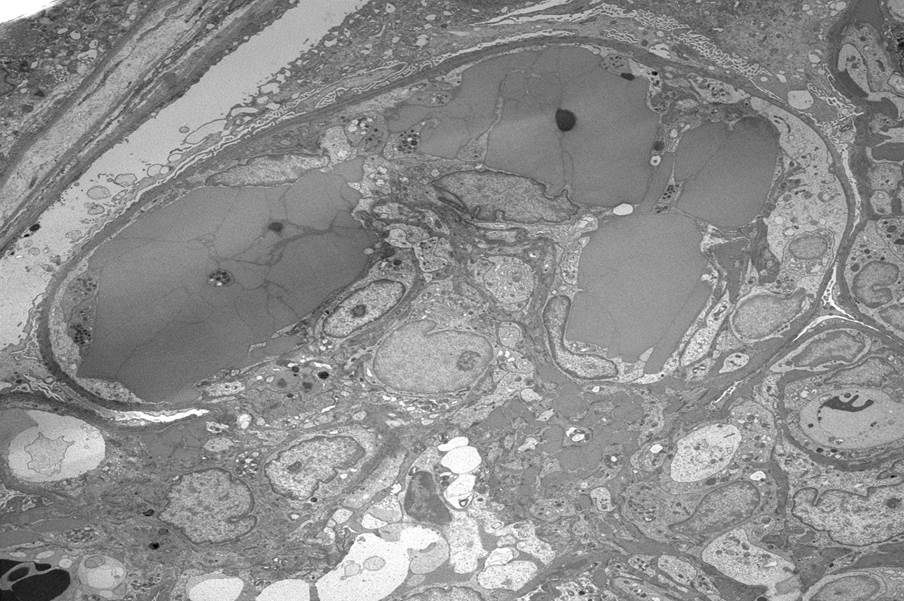
Immunofluorescence images
Contributed by Rajib K. Gupta, M.D. and Ashley Flowers, M.D.
Positive stains
- Kappa & lambda light chains
- Positivity by routine (frozen) or pronase IF of glomeruli or tubules
- IHC staining of interstitial histiocytes in CSH
- IHC staining of tubular basement membranes in MIDD (not very sensitive)
- In situ hybridization (ISH) staining of interstitial clonal plasma or B cells when present
- Weak PAS positive (AL amyloid) or strong PAS positive (FGN)
- May be silver positive (AL amyloid, FGN)
- Congo red positive staining of glomeruli, tubules, vessel walls and interstitium in AL or AH amyloid
- Thioflavin T positive staining (similar to Congo red stain) observed under fluorescent microscope
- DNAJB9 staining in FGN
- References: Clin J Am Soc Nephrol 2018;13:128, J Am Soc Nephrol 2016;27:1555, Arch Pathol Lab Med 2010;134:512, Ann Diagn Pathol 2019;43:151403
Negative stains
- Congo red and thioflavin T stains negative in all nonamyloid fibrillary MGRS cases (ITGN and most cases of FGN)
- Nonfuchsinophilic (trichrome negative) - both AL amyloid and FGN
- May be silver negative (AL amyloid, FGN)
- References: Clin J Am Soc Nephrol 2018;13:128, Arch Pathol Lab Med 2010;134:512
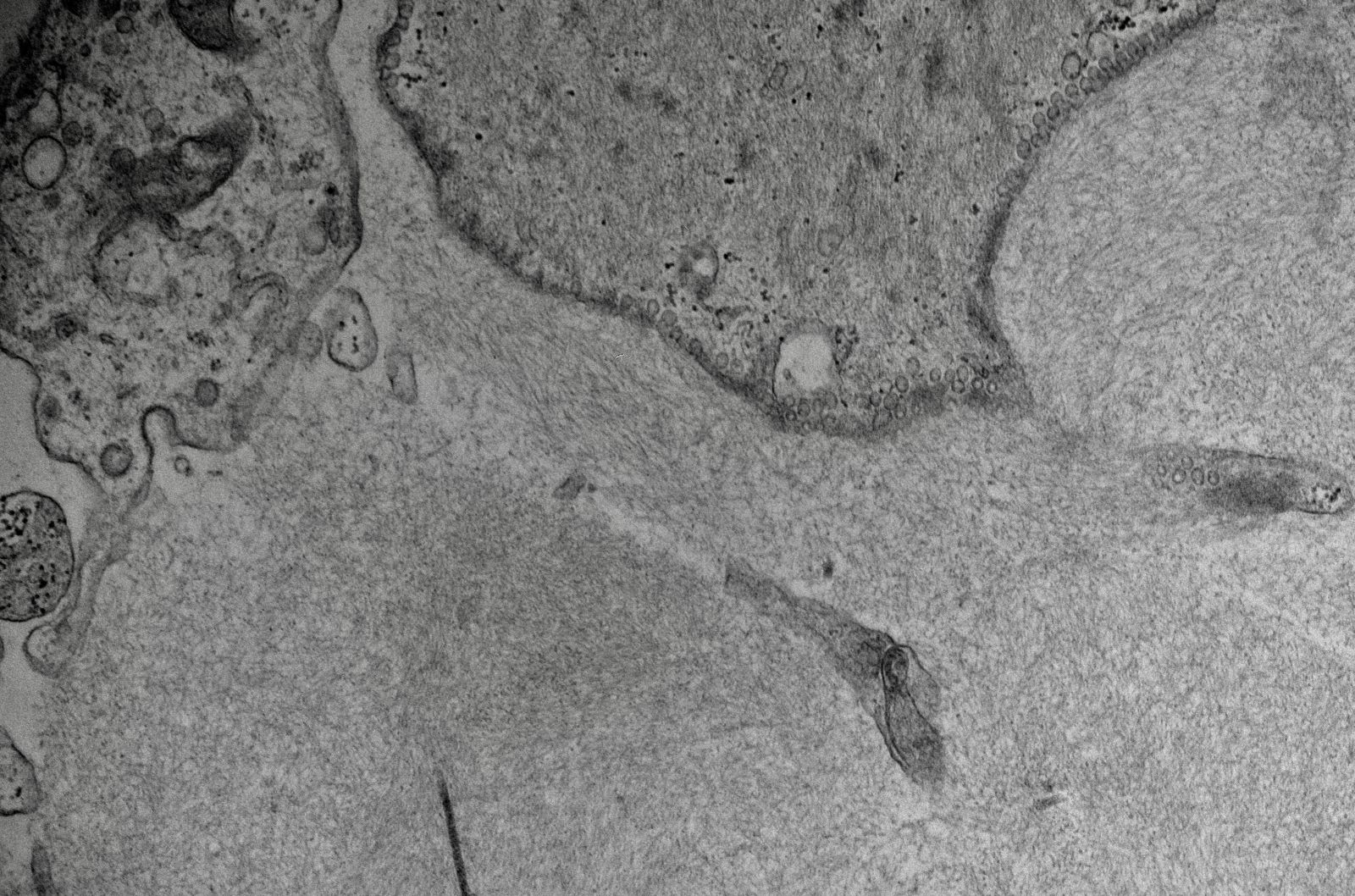
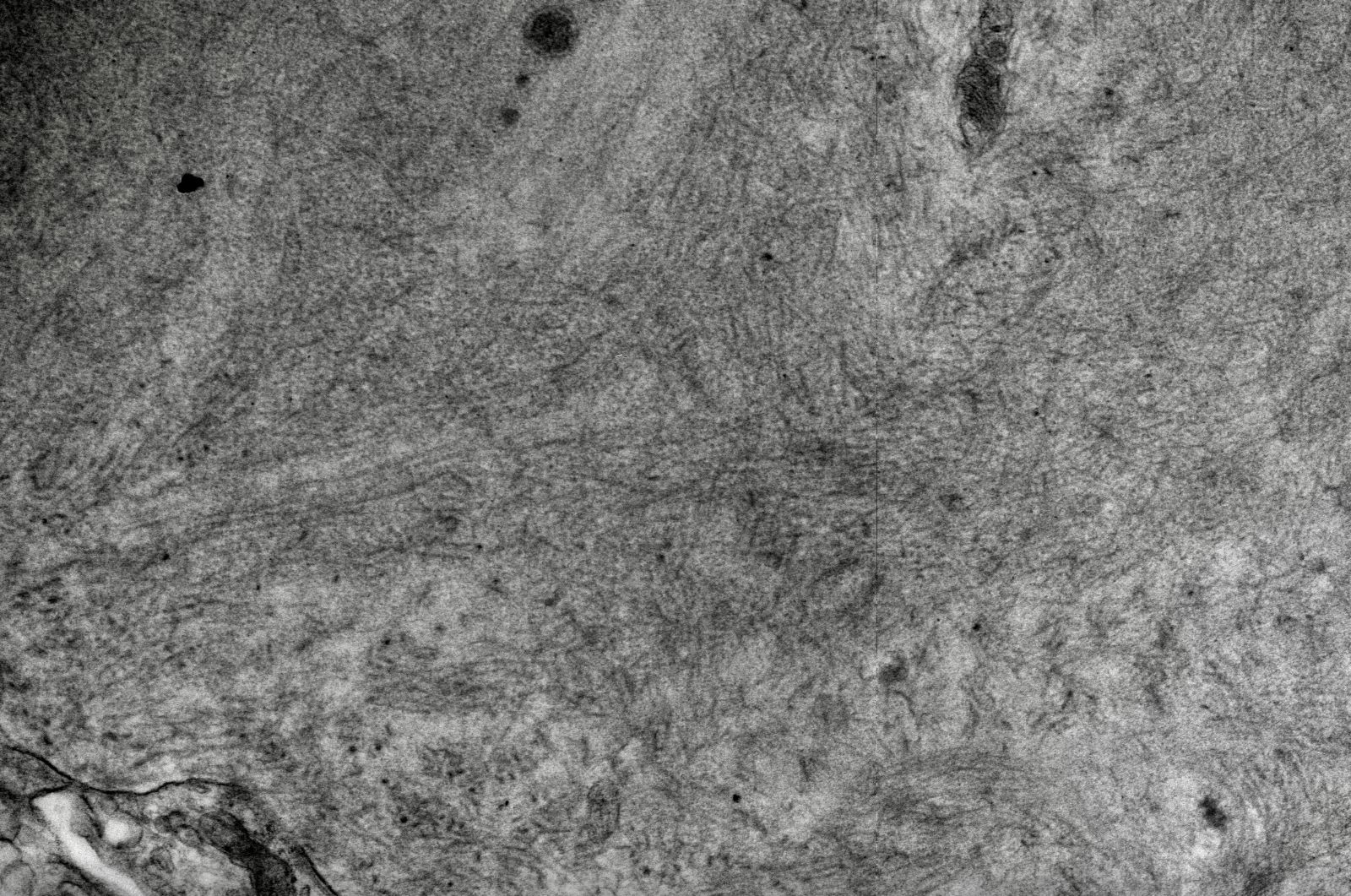
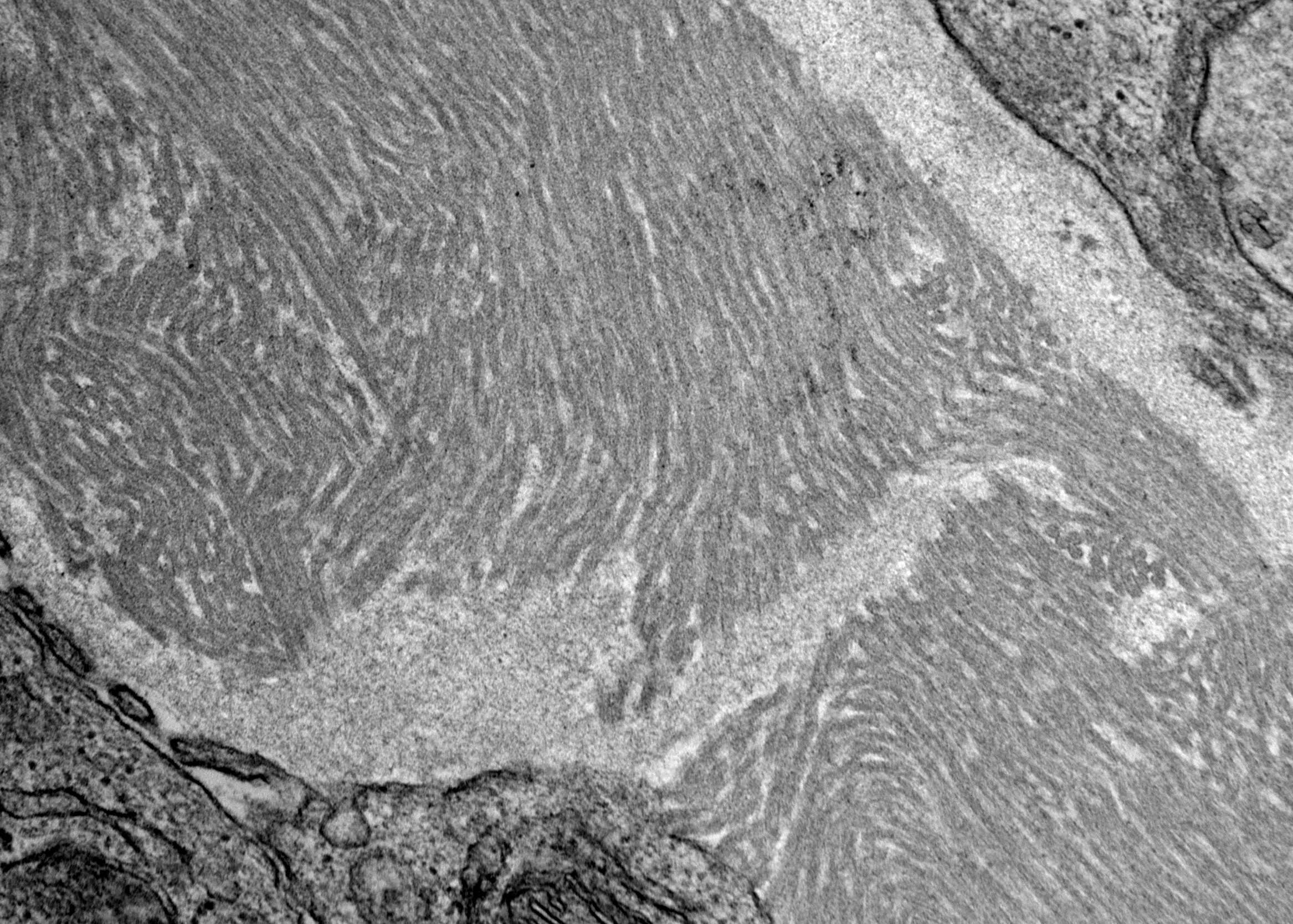
Electron microscopy description
- Electron dense amorphous deposits in mesangium and capillary loops, subepithelial or subendothelial (PGNMID, C3G)
- Extremely dense contiguous intramembranous deposits in the capillary glomerular basement membrane (dense deposit variant of C3G)
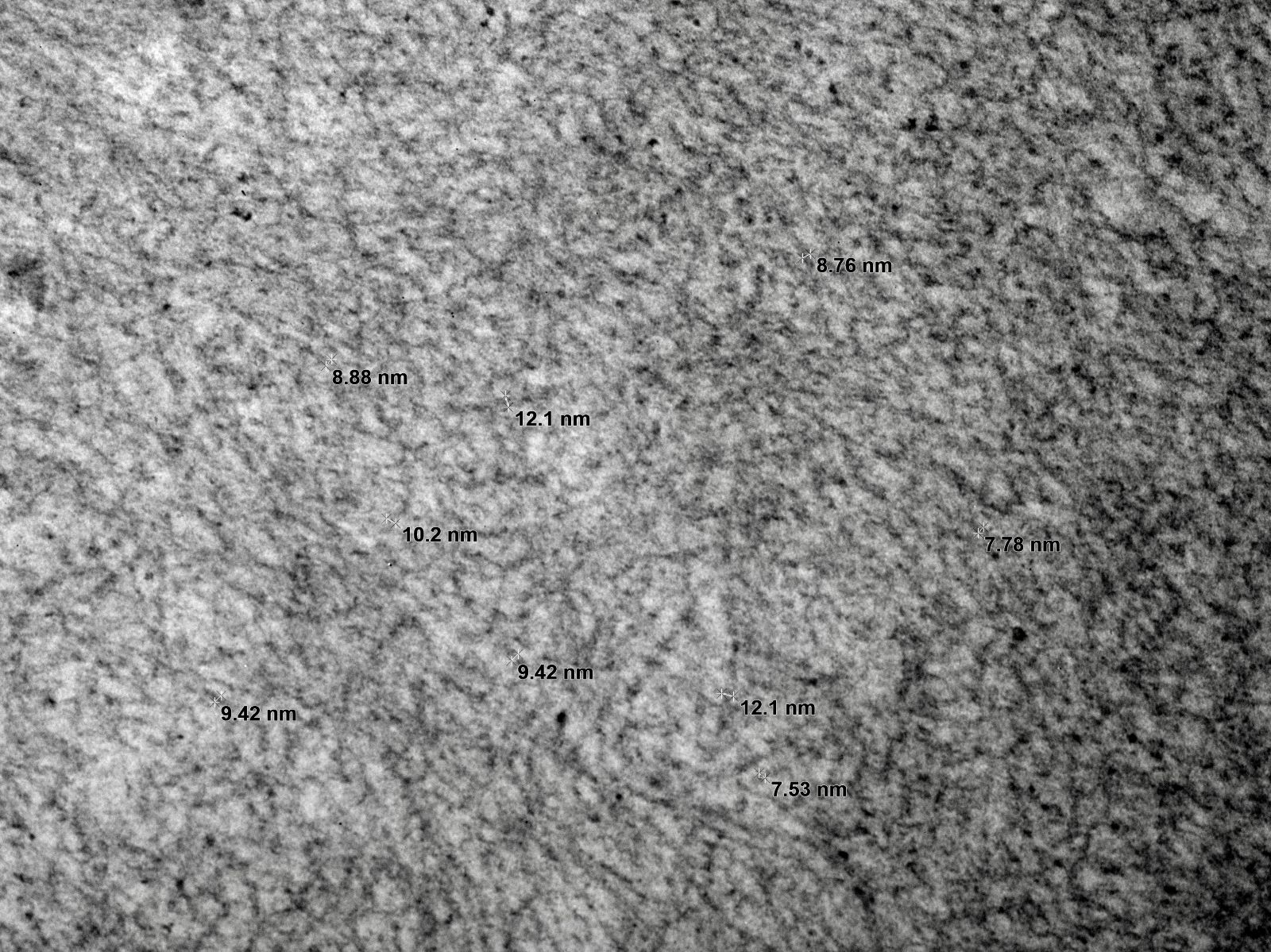
- Randomly arranged fine electron dense fibrils (7 - 12 nm diameter) in mesangium and within capillary loop glomerular basement membranes, usually in subendothelial or intramembranous locations or rarely in subepithelial locations as spikes (AL / AH amyloid)
- Randomly arranged electron dense fibrils (15 - 30 nm) in mesangium and within capillary loop glomerular basement membranes, subendothelial or intramembranous locations (FGN)
- Microtubules (hollow cylinders) arranged in stacks in mesangium and capillary loops with fibrillary diameter of 10 - 90 nm (immunotactoid GN)
- Microtubules arranged in mesangium, capillary loop glomerular basement membranes (subendothelial) or as occlusive deposits within capillary lumina (CryoGN)
- Powdery amorphous punctuate deposits along glomerular and tubular basement membranes (MIDD / LCDD)
- Crystals (electron dense or electron lucent) within tubular epithelial cells (crystalline variant of LCPT)
- Abnormal shaped lysosomes or mitochondria within tubular epithelial cells (noncrystalline variant of LCPT)
- Electron dense crystals with lattice substructure occluding glomerular capillary loops and small interstitial vessels (CIN)
- Subendothelial electron lucent widening without any deposits and loose mesangial matrix (TMA)
- Reference: Nat Rev Nephrol 2019;15:45, Arch Pathol Lab Med 2010;134:512, Kidney Int 2004;65:85, J Am Soc Nephrol 2016;27:1555, Kidney Int 2018;94:178, J Am Soc Nephrol 2015;26:525
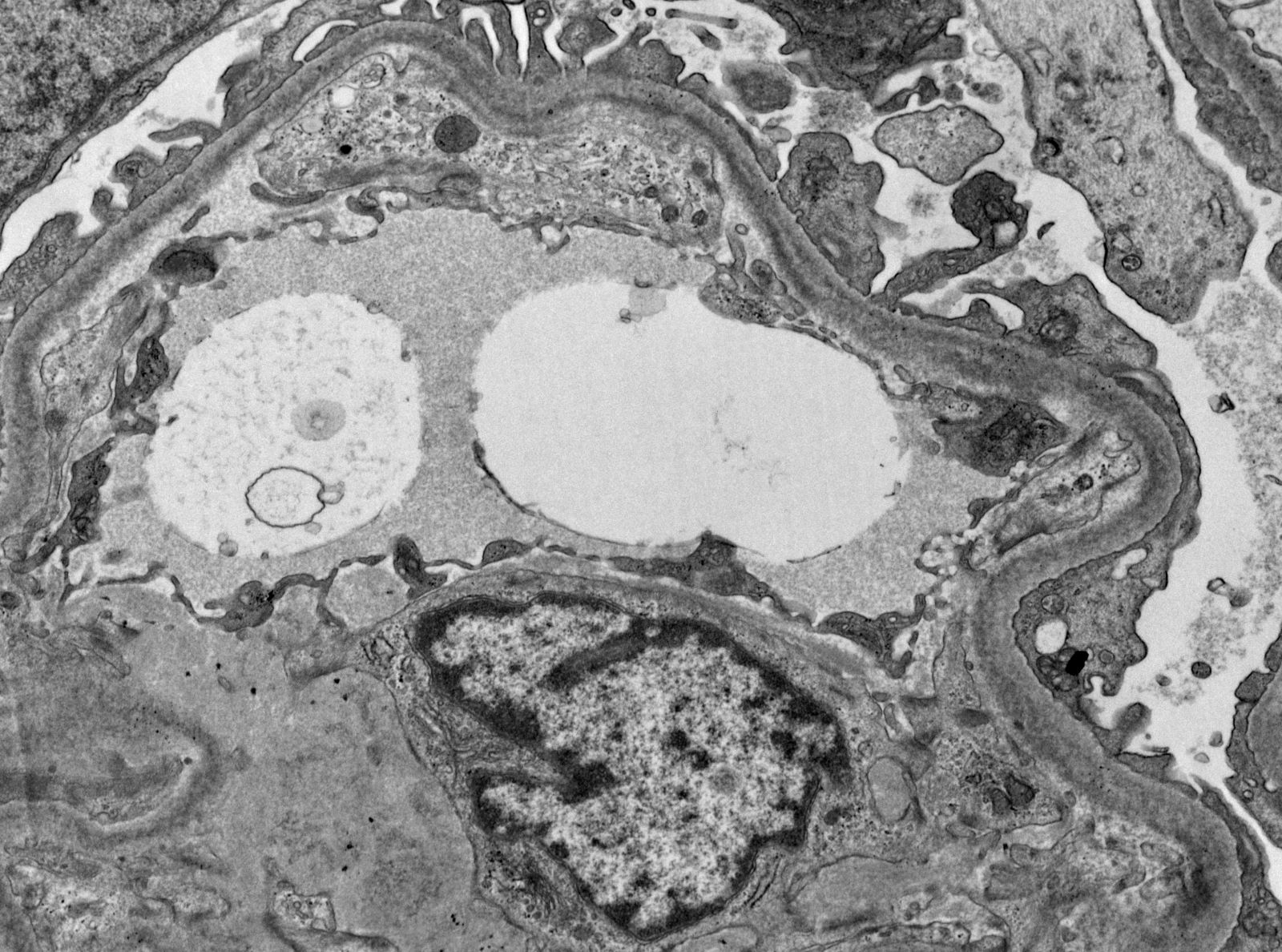
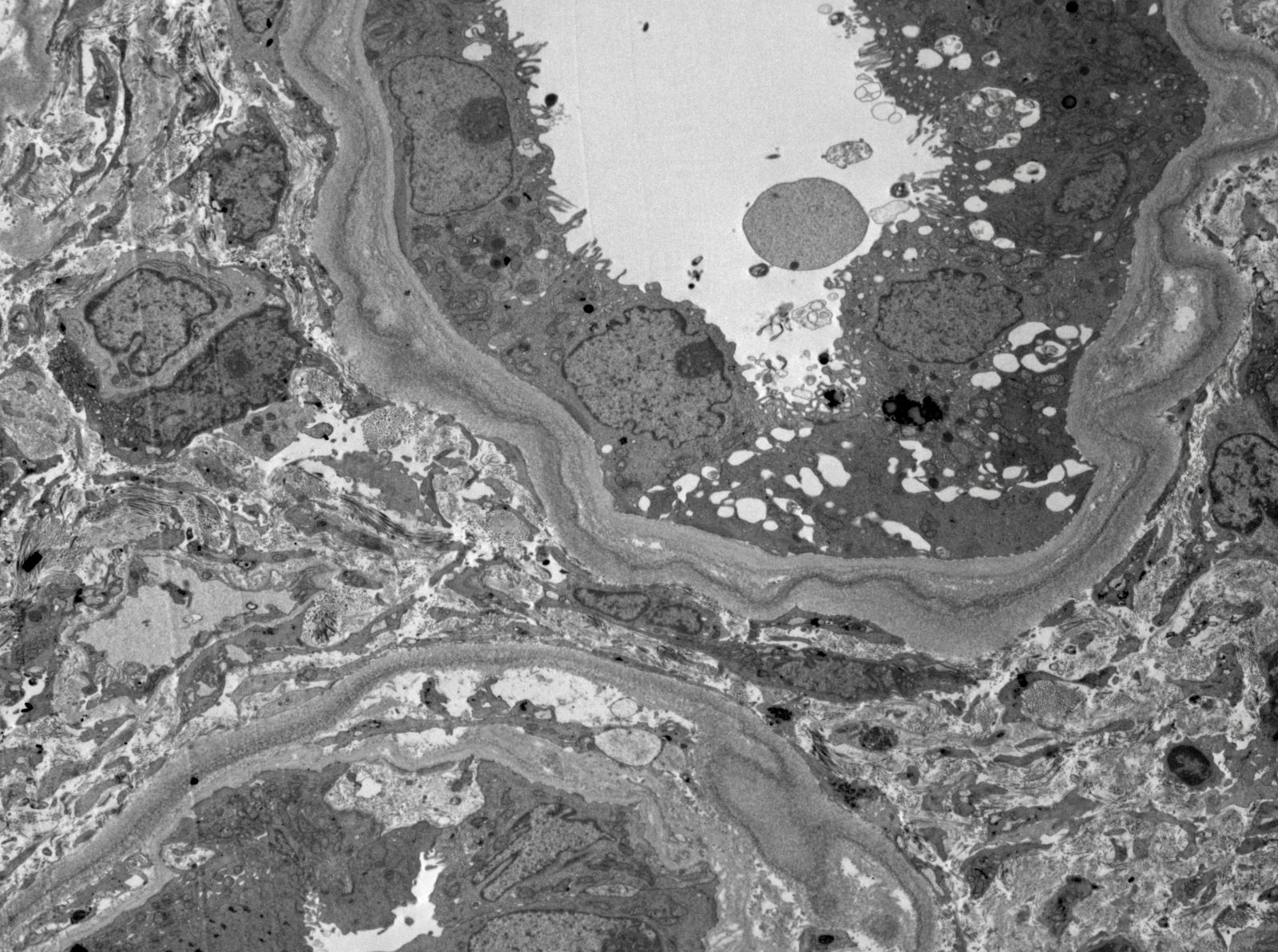
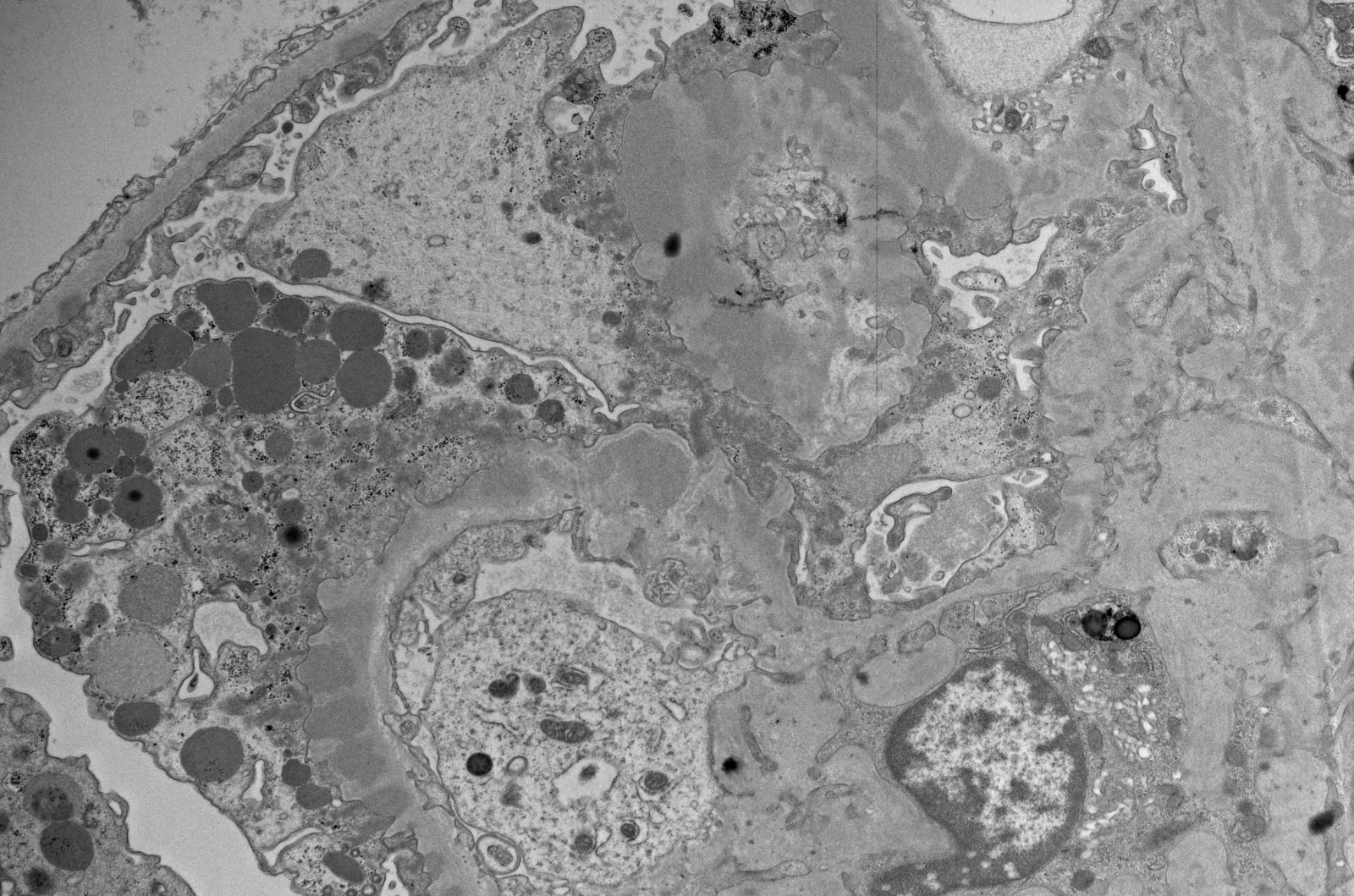
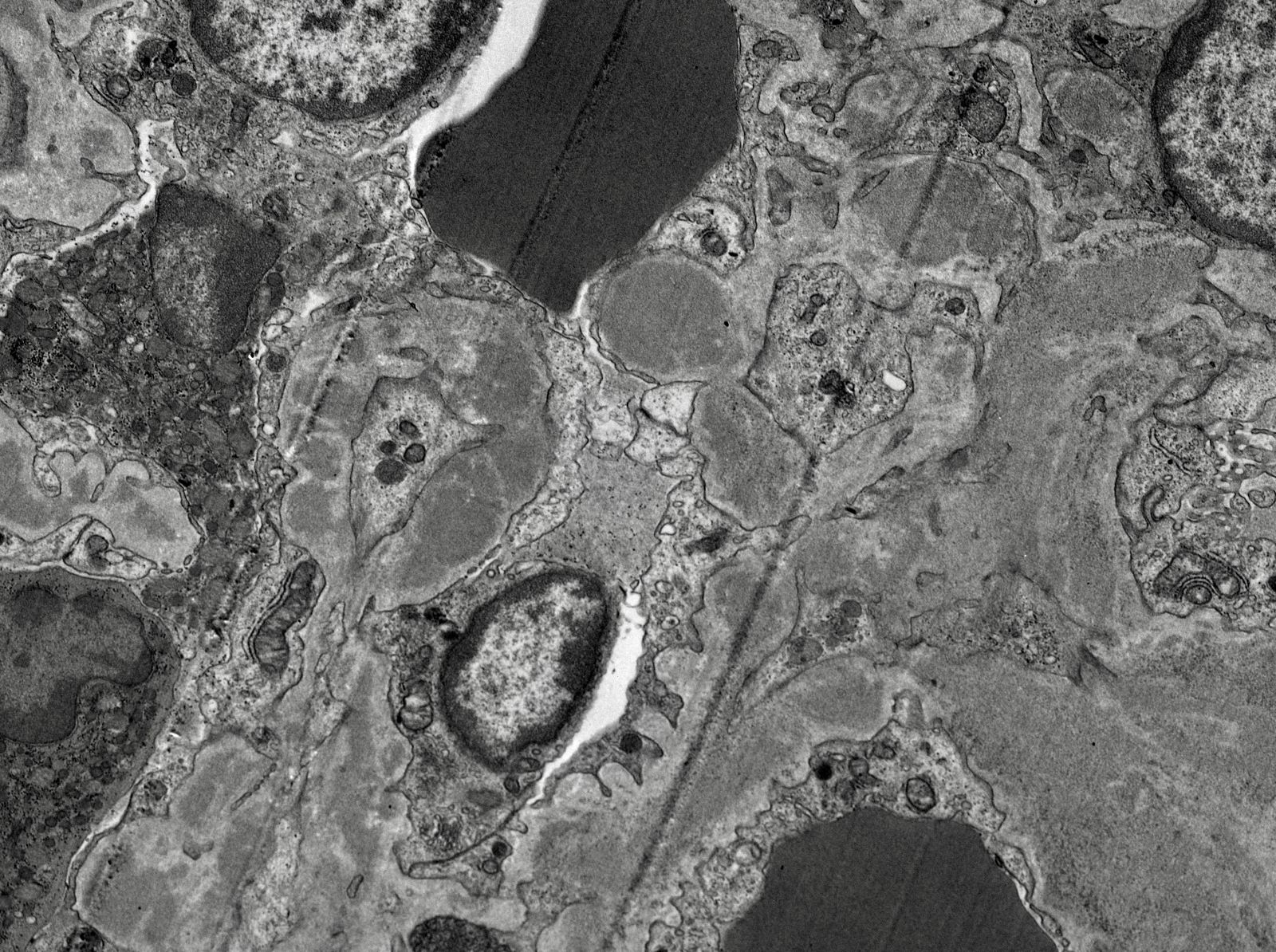
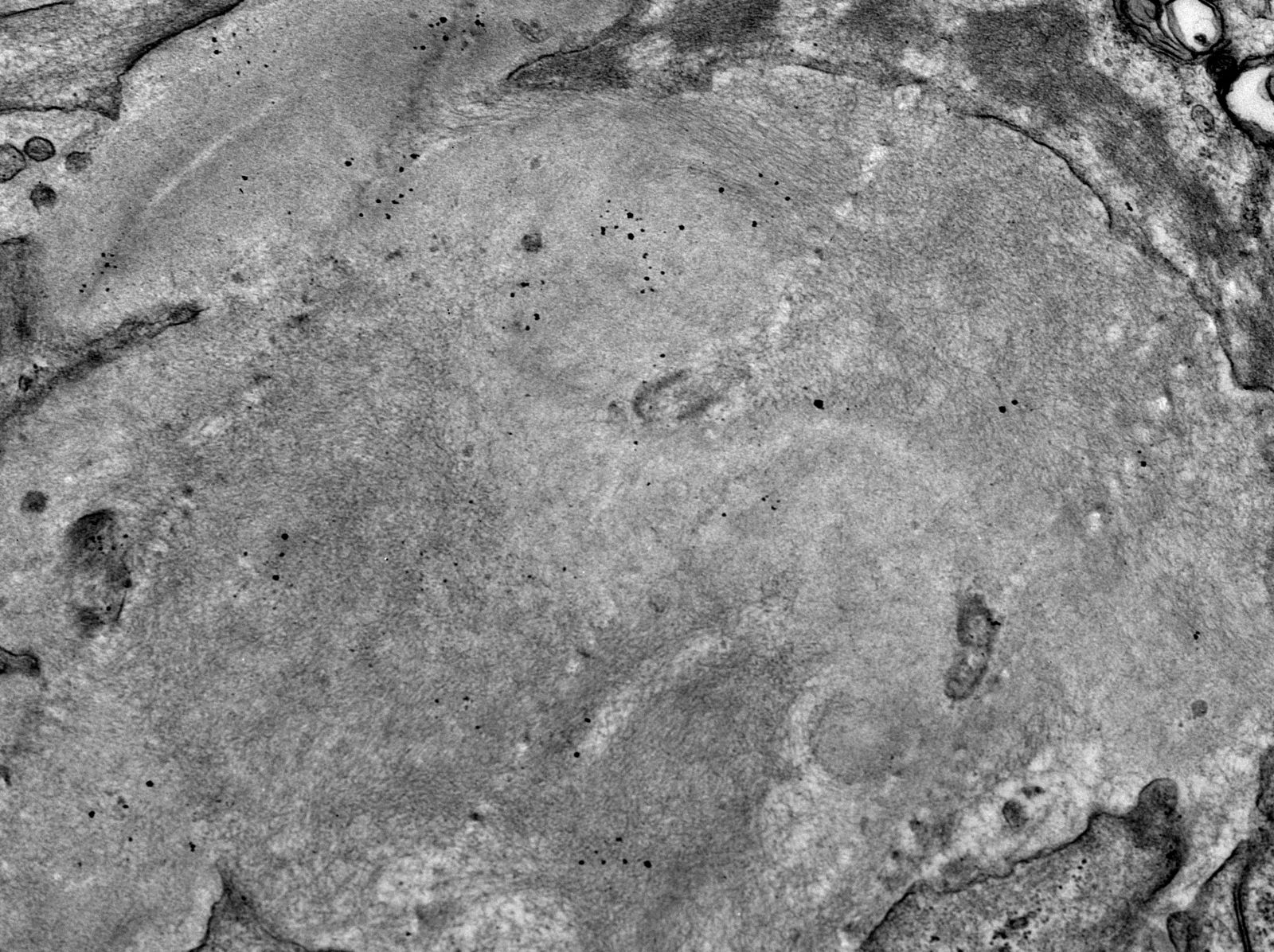
Electron microscopy images
Contributed by Rajib K. Gupta, M.D., Ashley Flowers, M.D., Judy King, M.D., Ph.D. and Xin Gu, M.D.
Sample pathology report
- Kidney, needle biopsy:
- Primary diagnosis (AL amyloid / PGNMID / LCPT, etc.), associated with kappa or lambda light chain restriction (as the case may be) (see comment)
- Comment: Light microscopy, immunofluorescence (frozen or pronase) and electron microscopy support the above findings. Congo red stain is positive (AL or AH amyloid diagnosis) or negative (nonamyloid MGRS diagnosis). The findings are consistent with the patient's documented history of X (if patient has a known diagnosis of hematolymphoid neoplasm) or clinical correlation with appropriate hematologic studies is recommended (if there is no known hematolymphoid diagnosis).
Differential diagnosis
- Diabetic (nodular) glomerulosclerosis:
- Strong PAS and silver positive mesangial nodules seen on light microscopy, although has an overall nodular glomerulosclerosis pattern
- Mesangial nodules are Congo red negative (positive in AL amyloid) and DNAJB9 negative (positive in FGN, monoclonal type)
- Often these mesangial nodules show peripheral microaneurysms which are not seen in any of the MGRS entities
- Immunofluorescence does not show any light chain restriction for either kappa or lambda
- More than 90% of the time, electron microscopy shows only diffuse nodular expansion of mesangial matrix without any fibrillary substructure
- Rarely, fibrillary substructure may be seen in mesangium (diabetic fibrillosis) but the fibrillary substructure is never seen within capillary loop glomerular basement membranes (intramembranous or subendothelial)
- Membranoproliferative glomerulonephritis (MPGN) due to infection:
- Patient has preceding history of recent infection
- No serologic evidence of monoclonal gammopathy
- No light chain restriction seen on immunofluorescence
- Cryoglobulinemic GN in hepatitis C:
- Patient usually has history of hepatitis C
- No serologic evidence of monoclonal gammopathy
- On serology, cryoglobulin type is usually type 2 or type 3 but typically never type 1
- No light chain restriction seen on immunofluorescence
- Hyaline in blood vessel wall in hypertensive nephrosclerosis:
- Hyaline in blood vessel wall is Congo red negative on light microscopy
- Vessel wall does not show any light chain restriction on immunofluorescence
- On electron microscopy, the vessel wall does not show AL amyloid type fibrillary substructure
- Fibronectin glomerulopathy:
- No serologic evidence of monoclonal gammopathy
- Mutation in fibronectin FN1 gene may be present
- No light chain restriction seen on immunofluorescence
- Mesangial lobules or nodules intensely fuchsinophilic (trichrome positive) and silver negative
- Electron microscopy shows amorphous or granular deposits in mesangium and subendothelial space but no fibrillary deposits akin to AL amyloid, FGN or ITGN
Additional references
Board review style question #1
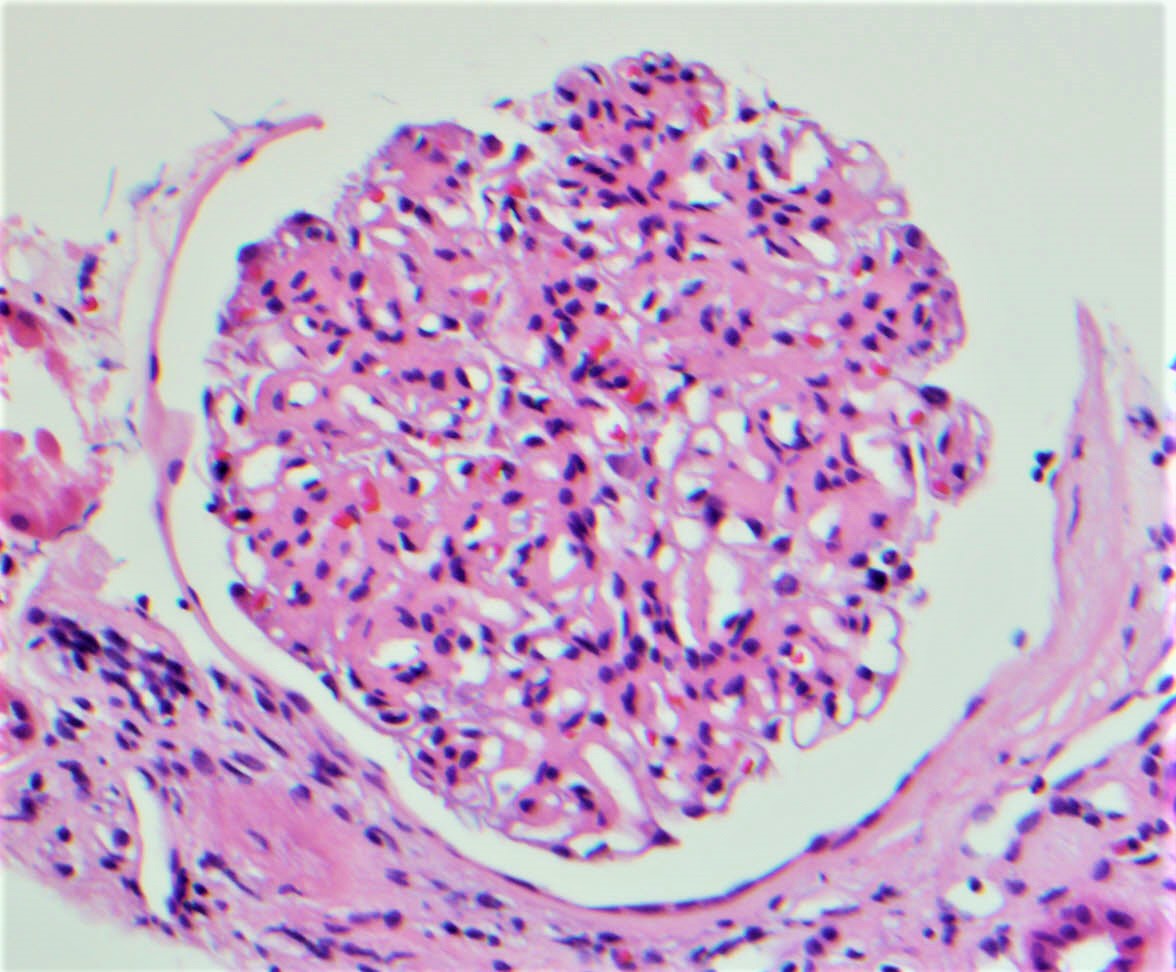
A 70 year old man with known hypertension presents with slow rising creatinine, nephrotic range proteinuria and microscopic proteinuria. Serology is negative for ANA, ANCA, viral hepatitis markers and anti-GBM antibody. Serum protein electrophoresis shows an abnormal M spike. A kidney biopsy is done. Light microscopy shows pale nodular expansion of the mesangium of the glomeruli which is weak PAS positive and silver and trichrome negative and Congo red stain positive, while immunofluorescence shows positive staining of glomeruli and interstitial vessel walls for lambda light chain and negative staining for all other immunoreactants, including for kappa light chain. An electron microscopy image of the glomerulus is given above. What is the likely diagnosis here?
- Amyloid light chain (AL) amyloidosis, lambda restricted
- Light chain deposition disease, lambda restricted
- Light chain proximal tubulopathy, lambda restricted
- Type 1 cryoglobulinemic glomerulonephritis, lambda restricted
Board review style answer #1
A.Amyloid light chain (AL) amyloidosis, lambda restricted
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease
Board review style question #2
A 72 year old woman with known history of gout presents with rapidly progressive renal failure, subnephrotic proteinuria and features of renal tubular acidosis. Serology is largely negative except for a positive urine protein electrophoresis showing an M spike and testing for free light chain level in blood shows elevated kappa free light chain. A kidney biopsy is done. Light microscopy shows weak PAS positive and silver negative crystalline material in the proximal tubule cell cytoplasms and electron lucent crystals packing the same cells on electron microscopy. What will be the characteristic immunofluorescence pattern in this case?
- Prominent granular staining of glomerulus for IgG and C3 on routine immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for C1q on routine immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for kappa on pronase immunofluorescence
- Prominent granular staining of proximal tubular cytoplasms for lambda on routine (frozen) immunofluorescence
Board review style answer #2
C. Prominent granular staining of proximal tubular cytoplasms for kappa on pronase immunofluorescence
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease
Comment Here
Reference: Monoclonal gammopathy of renal significance (MGRS) / paraprotein related kidney disease