Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Velagapudi RK, Paueksakon P. Light chain deposition disease. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/kidneylightchaindepositiondisease.html. Accessed April 2nd, 2025.
Definition / general
- Monoclonal gammopathy of renal significance (MGRS) is defined by the 2019 consensus statement as any B cell or plasma cell clonal lymphoproliferation with both of the following characteristics (Nat Rev Nephrol 2019;15:45)
- One or more kidney lesions that are related to the produced monoclonal immunoglobulin
- Underlying B cell or plasma cell clone does not cause tumor complications or meet any current hematological criteria for specific therapy
- Light chain deposition disease is generically included under plasma cell dyscrasias or dysproteinemias affecting the kidney
- May occur with malignant plasma cell or lymphoplasmacytic neoplasms or may be in the spectrum of MGRS
- Part of a broad spectrum of diseases known as monoclonal immunoglobulin deposition diseases (MIDD), which include heavy chain deposition disease (HCDD), light chain deposition disease (LCDD) and light and heavy chain deposition disease (LHCD)
- MIDD, including LCDD, HCDD, etc. are considered a subtype of MRGS
Essential features
- Systemic disease that primarily involves the kidneys and is characterized by light chain deposits that may be identified in any or all renal compartments (glomeruli, tubules, interstitium and vessels)
- Monotypic staining of glomerular basement membrane (GBM) or tubular basement membrane (TBM) with or without mesangium, for either kappa or lambda light chain, a single heavy chain or single light and heavy chain by immunofluorescence microscopy
- Powdery deposits along same locations by electron microscopy
- May coexist with other manifestations of the monoclonal light chains, such as amyloidosis, light chain proximal tubulopathy or light chain cast nephropathy
Terminology
- Included under a broad spectrum of monoclonal gammopathies or monoclonal immunoglobulin deposition diseases (MIDD)
ICD coding
Epidemiology
- Incidence of LCDD in autopsy series of patients with myeloma has been reported to be 3 - 5% (Arch Pathol Lab Med 1990;114:986, Arch Pathol Lab Med 2004;128:875)
- Light chain deposits can frequently be seen in other organs (in up to 75% of cases), most notably liver, heart and lungs (J Am Soc Nephrol 2001;12:1482)
- MIDD predominantly affects patients older than 50 years; the largest cohort of patients studied with renal MIDD, including 64 cases (51 LCDD, 7 HCDD and 6 LHCD), revealed a mean age at diagnosis of 56 years with 36% of the patients being less than 50 years of age (Clin J Am Soc Nephrol 2012;7:231)
Sites
- Kidney, glomeruli and tubulointerstitium
Pathophysiology
- Commonly composed of kappa chain deposits (80%), specifically the VκIV subgroup, which has a longer complementarity determining region 1 loop, which is part of an antigen binding site
- These immunoglobulins display peculiar physicochemical properties of the variable domain, including unusual hydrophobic residues that are abnormally glycosylated and positive charge
- This promotes aggregation and increases precipitation of the light chains as amorphous deposits along the negatively charged glomerular and tubular basement membranes (N Engl J Med 2021;384:1931)
- Light chains that precipitate can also stimulate synthesis of extracellular matrix proteins by mesangial cells, contributing to the advanced lesion of nodular sclerosis
- Interactions of glomerulopathic light chains with peripheral glomerular capillary walls result in alterations of the glomerular permeability, leading to proteinuria
Etiology
- Clonal proliferation of B cells / plasma cells that lead to deposition of abnormal light chains in various regions in the kidney
Clinical features
- Clinical presentation includes proteinuria (more than 90% and usually nephrotic range), kidney insufficiency / acute kidney injury (about 30%), hematuria, hypertension and select cases with tubular dysfunction (Adv Anat Pathol 2004;11:49, Arch Pathol Lab Med 1989;113:781, N Engl J Med 1976;294:71)
- Overall, the most common clinical presentation is proteinuria (usually nephrotic range but can also be nonnephrotic), hypertension and renal insufficiency (Adv Anat Pathol 2004;11:49, Arch Pathol Lab Med 1989;113:781, N Engl J Med 1976;294:71)
- Clinical evidence of dysproteinemia is present in a majority of the patients with plasma cell dyscarasias (myeloma); additionally, classic features of multiple myeloma have been noted in 59% of patients (Arch Pathol Lab Med 2009;133:249)
- Minority of patients with LCDD have no detectable monoclonal proteins in urine or serum (Clin J Am Soc Nephrol 2012;7:231)
- LCDD may coexist with light chain cast nephropathy in up to 21% of the affected patients and may also be associated, though much less commonly, with amyloid light chain amyloidosis (J Am Soc Nephrol 2001;12:1482, Ann Intern Med 1990;112:455)
- In cases without malignant hematopoietic neoplasm, considered to be within the spectrum of MGRS
Diagnosis
- Acute or chronic kidney injury, with proteinuria (may or may not be nephrotic range)
Laboratory
- Monoclonal proteins (M spike) in serum or urine protein electrophoresis or immunofixation
- Serum or urine free light chain assays
Prognostic factors
- 5 year survival of about 67% in the largest series
- Associated myeloma cast nephropathy is a poor prognostic factor
- Diabetes is a risk factor for progression
- Recurs in 75% of transplant patients
Case reports
- 54 year old woman with new onset of nephrotic syndrome and preserved kidney function (Acta Haematol 2024 Jan 16 [Epub ahead of print])
- 54 year old woman status post-living related kidney transplantation (from sister) followed by a diagnosis of proteinuria 6 months posttransplant (Nephron 2023;147:96)
- 72 year old woman with nephrotic syndrome (Cureus 2022;14:e26357)
- 83 year old woman with a history of a monoclonal gammopathy of undetermined significance (MGUS) with shortness of breath and anasarca (Diseases 2023;11:24)
Treatment
- Targeting the underlying plasma cell clone using steroids, cyclophosphamide, melphalan / autologous stem cell transplant, bortezomib
- Supportive therapy
Microscopic (histologic) description
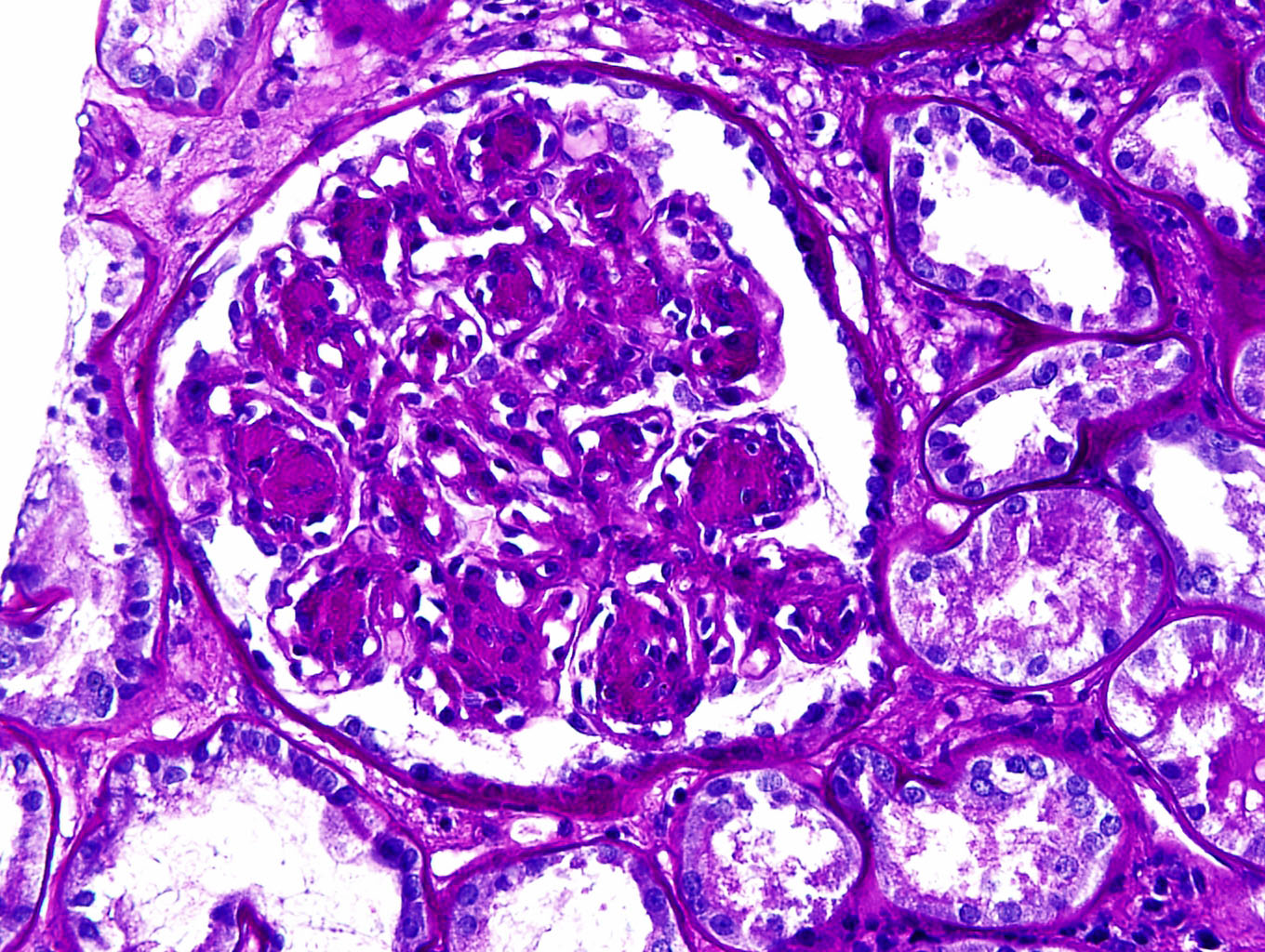
- Variable morphologic patterns seen, including nodular glomerulopathy, mesangioproliferative, membranoproliferative or crescentic patterns
- Most characteristic finding is nodular glomerulopathy / glomerulosclerosis that mimics diabetic nephropathy
- Mesangial nodules are argyrophilic (Jones silver positive) with or without lamellations and composed of matrix proteins admixed with monotypic light chains
- Some cases show associated mesangial hypercellularity
- Glomerular capillary basement membranes appear thickened because of deposits
- Light chain deposits are Congo red negative
- Extraglomerular changes include ribbon-like thickening of tubular basement membranes and thickened vascular walls; sometimes interstitial deposits in the form of PAS positive aggregates can be seen
- Can have associated light chain cast nephropathy
- Can coexist with other paraprotein related diseases including amyloidosis and light chain cast nephropathy (Kidney Int 2022;101:152)
Microscopic (histologic) images
Immunofluorescence description
- Monotypic light chain deposits for either kappa or lambda can be seen in a linear pattern along the glomerular capillary basement membranes, mesangium, tubular basement membranes, vessels and rarely interstitium
- Alternatively, monotypic heavy chain staining can be seen with or without monotypic light chain staining (heavy chain deposition disease or heavy and light chain deposition disease)
- If IgG heavy chain, restriction to single IgG heavy chain subclass
- Pattern and intensity depend on the amount of light chain deposits
- In most cases of LCDD, the pathogenic light chain is kappa isotype, with a kappa to lambda ratio of 9:1
- Immunoglobulin heavy chains and complements are usually negative (in light chain deposition disease)
- Immunofluorescence studies on paraffin embedded tissue after pronase digestion can be performed in suspicious cases with negative immunofluorescence on frozen tissue
- References: J Am Soc Nephrol 2018;29:1810, Curr Opin Nephrol Hypertens 2016;25:127
Immunofluorescence images
Positive stains
- Kappa or lambda IHC stains can be done to demonstrate monoclonal staining in biopsies without any tissue submitted for immunofluorescence studies
- Paraffin immunofluorescence can be performed to demonstrate monoclonal staining or masked monoclonal staining, even on biopsies with tissue submitted for immunofluorescence
Negative stains
Electron microscopy description
- Granular to powdery electron dense deposits are seen in all renal compartments
- In glomerular capillary walls, the powdery deposits form a thin band along the lamina rara interna and larger deposits may pool along subendothelial region
- Deposits are present along the outer aspect of tubular basement membranes
- References: J Am Soc Nephrol 2018;29:1810, Curr Opin Nephrol Hypertens 2016;25:127
Electron microscopy images
Videos
Washington University in St. Louis Nephrology Web Series - multiple myeloma and the kidney
Sample pathology report
- Kidney, percutaneous needle biopsy:
- Light chain deposition disease (see comment)
- Comment: The biopsy shows 22 glomeruli by light microscopy, 7 of which are globally sclerosed, with ~60% interstitial fibrosis. Glomeruli show segmental to global nodular expansion of mesangium with segmental hypercellularity. Tubular basement membranes appear prominent with mild interstitial lymphocytic infiltrate in areas of scarring with rare tubulitis. Immunofluorescence performed on frozen tissue shows diffuse, ribbon-like staining of glomerular basement membranes and tubular basement membranes for kappa (2 - 3+) without any lambda and focal, segmental, chunky mesangial staining for kappa without lambda staining. Immunofluorescence performed on paraffin embedded tissue by pronase digestion shows a similar pattern of staining for kappa without immunoglobulin or lambda staining. There are powdery deposits along the inner aspect of the glomerular basement membranes and the outer aspect of the tubular basement membranes as well as amorphous deposits in the mesangium by electron microscopy. Taken together, the findings are diagnostic of kappa restricted light chain deposition disease.
Differential diagnosis
- Diabetic nephropathy:
- Nodular expansion of mesangium and thickened tubular basement membranes
- Absence of monoclonal staining by immunofluorescence and no powdery deposits by electron microscopy
- Proliferative glomerulonephritis with monoclonal IgG deposits:
- Proliferative pattern by light microscopy
- Monoclonal staining by immunofluorescence
- Amorphous deposits along glomerular basement membranes by electron microscopy but without any deposits along tubular basement membranes
- Atypical anti-GBM nephritis:
- Linear glomerular basement membrane staining for heavy and light chains by immunofluorescence but without any deposits by electron microscopy
- ~38% can have monotypic staining by immunofluorescence (Am J Kidney Dis 2024;83:713)
- Amyloidosis:
- Nodular glomerulosclerosis by light microscopy
- But Congo red positive and randomly arranged fibrils by electron microscopy
Additional references
Board review style question #1
A 65 year old man with history of chronic kidney disease now presents with oliguria and edema. Clinically, he is found to have nephrotic syndrome with urinalysis showing 3+ proteinuria without hematuria and urine protein of 4.2 gm/day; laboratory studies show anemia with a hemoglobin of 8.6 g/dL and serum creatinine of 1.7 mg/dL. Serum and urine free light chain assays show elevated kappa levels. Renal biopsy was performed and shows the following findings: casts do not have a fractured appearance or reaction around them and no monoclonal staining of casts by immunofluorescence; Congo red stain is negative; electron microscopy shows powdery deposits along the glomerular basement membranes (GBMs) and tubular basement membranes (TBMs) and no crystals within the tubules. What is the diagnosis?
- Amyloid light chain amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Board review style answer #1
C. Light chain deposition disease. Images show nodular expansion of mesangium and thickened tubular basement membranes by light microscopy, monoclonal staining with kappa along GBMs and TBMs by immunofluorescence and powdery deposits along GBMs by electron microscopy. Answer A is incorrect because a Congo red stain is negative and there are no fibrillary type deposits by electron microscopy. Answer D is incorrect because there is no monoclonal droplet staining within the tubules by immunofluorescence and no intratubular crystals by electron microscopy. Answer B is incorrect because there are no casts typical of light chain cast nephropathy and no clonal staining of casts by immunofluorescence.
Comment Here
Reference: Light chain deposition disease
Comment Here
Reference: Light chain deposition disease
Board review style question #2
A 56 year old woman with a new diagnosis of multiple myeloma presents with edema and fatigue. Laboratory studies show serum creatinine is 2.2 mg/dL. Renal biopsy was performed and shows thickened glomerular and tubular basement membranes. Immunofluorescence staining shows monoclonal staining for kappa only (negative for lambda) along the glomerular basement membranes (GBMs) and tubular basement membranes (TBMs). A Congo red stain is negative and electron microscopy shows powdery deposits along GBMs and TBMs. What is the diagnosis?
- Amyloid light chain amyloidosis
- Light chain cast nephropathy
- Light chain deposition disease
- Light chain proximal tubulopathy
Board review style answer #2
C. Light chain deposition disease. Biopsy findings revealed thickened tubular basement membranes by light microscopy, monoclonal staining with kappa along GBMs and TBMs and powdery deposits along GBMs by electron microscopy. Answer A is incorrect because a Congo red stain is negative and there are no fibrillary type deposits by electron microscopy. Answer B is incorrect because there are no casts typical of light chain cast nephropathy and no clonal staining of casts by immunofluorescence. Answer D is incorrect because there is no monoclonal droplet staining within the tubules by immunofluorescence and no intratubular crystals by electron microscopy.
Comment Here
Reference: Light chain deposition disease
Comment Here
Reference: Light chain deposition disease