Table of Contents
Definition / general | Essential features | Terminology | Historical laboratory perspective | Middleware in the laboratory | Enterprise perspective | Trends in middleware | Specialized middleware | Applications | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: McClintock D, Williams C. Middleware. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/informaticsmiddleware.html. Accessed April 3rd, 2025.
Definition / general
- Software that connects various health information systems (HISs) or instruments / devices (either within the laboratory or throughout the enterprise), sometimes referred to as software glue
- In addition to handling interfacing, it oftentimes fills functional gaps of primary health information systems
- May also refer to secondary specialized information systems (e.g., point of care testing, core lab automation, HLA)
Essential features
- Middleware is software that interfaces 2 or more information systems together, often providing additional functionality that would be otherwise lacking
- Laboratory middleware can reduce both the complexity and number of connections required when interfacing laboratory instrumentation, software and health information systems (e.g., laboratory information systems [LIS] and electronic health records [EHR])
- Contemporary middleware solutions can ingest multiple data feeds to provide value added analysis and insights for laboratory management dashboards
Terminology
- Interface: method to transfer data; typically refers to 1 or more of the following
- Machine to machine communication
- Machine to software system communication
- Software system to software system communication
- LIS: may be standalone or a module integrated within an EHR; LISs can be composed of 1 or more modules and may be laboratory discipline specific
- Health level seven (HL7): messaging standard for the exchange of healthcare information; most common version used today is HL7 v2.x all types of interfaces; however, more are now moving towards the recent HL7 FHIR standard
- Admit / discharge / transfer (ADT): ubiquitous HL7 message type providing demographic information and updates on patients (also known as ADT interface or ADT feed)
- ASTM: a standards organization
- When used in the laboratory medicine context, ASTM generally refers to one of the earliest bidirectional interface types to connect instruments to the LIS
- While many information technology (IT) vendors currently prefer HL7, it is common for instruments to support both types of interfaces for backward compatibility
- Driver: software program that enables a specific hardware device or other software to work with another application or system
- Typically used to describe how computer peripherals (e.g., printers, keyboards) communicate with the base operating system
- For laboratories, describes how instruments and other devices connect with primary systems
Historical laboratory perspective
- As LISs were developed and deployed, initial approaches favored direct instrument connections for data transfer
- Each deployment of either a new LIS or instrument generally required custom development, minimally on the part of the vendor but typically also by the laboratory
- This model was not efficient, especially as new LISs, instruments and ancillary systems were developed
- Difficult for vendors to maintain / support for numerous drivers or interfaces
- Point to point interfaces require n x (n - 1) interfaces (e.g., interfacing 7 systems together requires 7 x (7 - 1) = 42 interfaces to be built, in the worst case)
- Point to point interfaces require n x (n - 1) interfaces (e.g., interfacing 7 systems together requires 7 x (7 - 1) = 42 interfaces to be built, in the worst case)
- Middleware theoretically reduced the number of drivers developed, easing implementation and maintenance
- With middleware that has the primary purpose of managing interfaces, the number of total interfaces required can be reduced significantly
- Interface engines require n x 2 interfaces to be built (e.g., interfacing 7 systems now only requires 7 x 2 = 14 interfaces)
- Reference: Sinard: Practical Pathology Informatics, 1st Edition, 2005
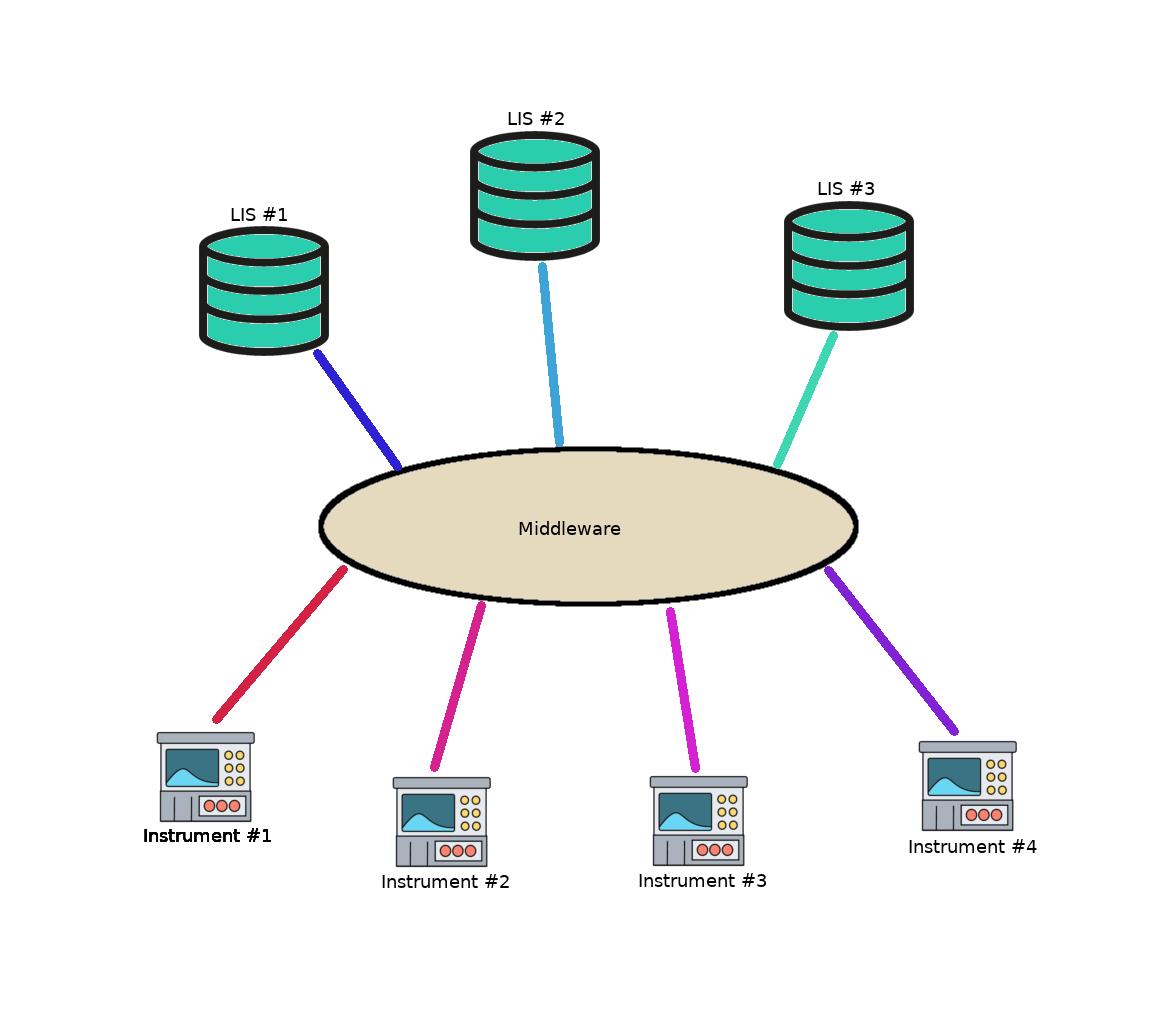
Middleware in the laboratory
- Software layer present between LIS and instruments
- Only requires a single driver per instrument type and 1 driver per LIS / other middleware
- Adding a new instrument or the more challenging task of connecting a new LIS to a laboratory’s instrumentation can be accomplished by installing the appropriate driver
- Rules for sanity checks, delta checks, quality control (QC), etc. can be performed at this layer, preventing questionable results from reaching the LIS and potentially the patient chart
- Results from various platforms can be normalized into the same format (e.g., adjusting semiquantitative results to a laboratory standard dictionary)
- Provides an opportunity to add functionality, such as moving averages, that may not be available within the LIS
- Reference: Pantanowitz: Pathology Informatics - Theory & Practice, 1st Edition, 2012
Enterprise perspective
- Modern healthcare enterprises rely on numerous, interconnected health information systems besides the electronic health record
- As the number of systems increases, the number of direct connections to keep all systems connected and in sync potentially increases exponentially, as shown previously
- Middleware can reduce the overall complexity, simplifying new additions as well as reducing the overall support effort
- This type of middleware, targeting multidisciplinary systems across the enterprise, is often referred to as an integration engine or interface engine
- Some data feeds, such as ADT, may be commonly needed by most of the ancillary systems
- Other niche feeds may only be used for 2 systems to exchange information
- Data feeds can be customized within the middleware for each application
- Data feeds may be forked and used for other purposes, such as external reporting, regulatory compliance, etc.
Trends in middleware
- Middleware once focused on interfacing systems together but vendors are adding additional functionality targeted at end users
- Reporting and analytics
- Vendor provides reports for commonly used metrics; these can be run periodically or on demand and may be accessible in real time depending on the underlying IT infrastructure
- Custom reports for customers to explore their data with in house expertise
- Visualization methods, such as interactive dashboards for monitoring trends and producing graphical representation for distribution, are becoming more common
- Utilization metrics for process improvement, resource management and cost saving initiatives are common analytics functions
- Remote support / monitoring
- Companies are able to remotely monitor, log and pull information for maintenance and billing purposes
- Pre-emptive service can prevent unexpected downtime
- Remote troubleshooting can replace expensive on site service calls in certain situations
Specialized middleware
- Example systems; not an exhaustive list
- Point of care testing (POCT) middleware
- Provides base interfacing capabilities for large numbers of the same device deployed throughout institutions (e.g., 250 glucose meters) that would otherwise traditionally require individual interfaces if connected to the LIS
- Most times includes additional functionality besides providing connectivity for transmitting results to the LIS / electronic health record
- Remote device configuration: devices can be configured and updated remotely via middleware, saving much effort when large numbers of devices are deployed throughout an organization
- Helps meet regulatory requirements specifying who can perform waived and moderate complexity testing, as well as documenting competency
- Locks out users lacking or with expired training
- Can be configured to require quality control measures, such as QC lockout after specific time periods
- More recent functionally may include lab order functionality
- Providers rarely enter an electronic order for specific point of care tests
- Most point of care testing middleware or the LIS can autogenerate orders when a point of care test is performed on a connected instrument (unsolicited orders)
- Some newer point of care testing middleware allows for solicited orders, where the order placed by the provider for a point of care test is linked to the patient result
- Customer relationship management middleware
- Software to aid client service to cultivate relationships and retain referring laboratories
- Facilitates individualized attention - tracks points of contact so customers are greeted by preferred name, have immediate access to individual fee schedules, other miscellaneous features to make customers feel pampered
- Tracks support history so common problems are quickly addressed
- Automation
- Provides baseline interfacing and workflow management capabilities for automation line instrumentation
- Typically the LIS cannot orchestrate the complex workflows required to successfully run a core lab automation line
- Load balancing and distribution based on volume and test availability to maximize throughput are common features
- Communicates with instruments to disable routing when a test or analyzer is offline
- Sample tracking throughout the entire testing process, including storage and disposal of samples as they expire for some systems
- Provides baseline interfacing and workflow management capabilities for automation line instrumentation
- Outreach / reference lab systems
- Acts as both an interface engine with external health information systems and reporting engine for clinical laboratories with external clients
- Manages clients and patients and may include a master patient index
- Other typical functionality includes
- Custom report formatting based on customer preference
- Individualized testing menus, panels and reflexing options
- Client billing
- Transfusion medicine (blood bank) middleware
- Primary purpose is to provide a safe manner for issuing blood products
- Checks ABO compatibility
- Checks markers and historic record for special blood needs (e.g., antibodies, irradiation)
- Checks expiration date / time of products being issued
- Compliant with International Society of Blood Transfusion (ISBT) labeling standards
- Interfaces with blood product administration modules for electronic health records for verification of transfusions
- One of the few health information systems treated as a class 3 medical device by the Food and Drug Administration (FDA); has strict federal regulatory requirements
- Primary purpose is to provide a safe manner for issuing blood products
- Point of care testing (POCT) middleware
- Reference: Surg Pathol Clin 2015;8:175
Applications
- Connects laboratory devices to LIS
- Connects LIS to EHR
- Remote order entry and results review
- Autoverification
- Asset tracking
- Image management
- Reporting and analytics
- Reference: Surg Pathol Clin 2015;8:175
Board review style question #1
What is the most common messaging standard used in healthcare today for interfacing medical hardware and software?
- CPT
- DICOM
- HL7
- LOINC
- SNOMED CT
Board review style answer #1
C. HL7. HL7 and ASTM are the 2 primary protocols used to interface laboratory instruments and other devices throughout the enterprise, to laboratory information systems and electronic health records, with HL7 replacing ASTM as the favored protocol. Answer A is incorrect because CPT is a code set used for billing to document services delivered. Answer D is incorrect because LOINC is a code set used to describe medical laboratory observations. Answer E is incorrect because SNOMED CT is a hierarchical ontology used for a variety of medical documentation purposes.
LOINC, SNOMED CT and CPT codes may be used within an HL7 message to convey meaning in a structured way. Answer B is incorrect because DICOM is primarily used to transfer radiology images; however, there are extensions to support digital pathology images as well.
Comment Here
Reference: Middleware
Comment Here
Reference: Middleware
Board review style question #2
What is the software that provides additional functionality to laboratories but is not included within the primary laboratory information system (LIS) best known as?
- Analytics platform
- Interface engine
- Middleware
- Software modules
Board review style answer #2
C. Middleware. Middleware is the best answer among the options provided, as middleware generally sits between laboratory instruments and the LIS, providing additional communication drivers and perhaps additional functionality that is missing from the LIS. Answer D is incorrect because software modules are generally extensions to existing applications, which would be included as part of the LIS. Answer B is incorrect because interface engines are usually found at the enterprise level and include interfacing the electronic health record, LIS, billing and numerous other systems together. Answer A is incorrect because while analytics platforms could potentially be utilized by laboratories, these are generally found at the enterprise level and would provide additional operational functionality to the laboratory, per se. However, in this context, the analytics platform could be considered a form of specialized middleware.
Comment Here
Reference: Middleware
Comment Here
Reference: Middleware
Board review style question #3
In the context of interface engines, what does the formula (n x 2) reference?
- Cost multiplier for budgeting for interfaces when adding new instrumentation to the laboratory
- Length of time needed for an interface to transmit data
- Number of systems required to create a single point to point interface
- Total number of interfaces required to connect an n number of systems
Board review style answer #3
C. Number of systems required to create a single point to point interface. This references the minimum number of interfaces (unidirectional) required to interface n systems together (i.e., with middleware). In the worst case scenario, n x (n - 1) interfaces are required when using direct connections. Answers A, B and D are incorrect because they are distractors and not relevant to the formula in question.
Comment Here
Reference: Middleware
Comment Here
Reference: Middleware