Table of Contents
Definition / general | Essential features | Terminology | Diagrams / tables | Organization | Project definition | Functional / needs assessment | Software selection | Hardware acquisition and installation | Software installation | System configuration and customization | Integration with lab instruments | Data migration | Training | Testing | Security | Go live and support | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Batra H, Parwani A. LIS installation & implementation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/informaticslisinstallation.html. Accessed March 31st, 2025.
Definition / general
- A laboratory information system (LIS) is a type of healthcare software that records, manages and stores patient data from all stages of testing for clinical laboratories
- Collection of software, operating systems and hardware designed to serve the operational processes of a laboratory
- A LIS processes, stores and manages data from all stages of medical processes and tests, including hematology, chemistry, immunology and microbiology
Essential features
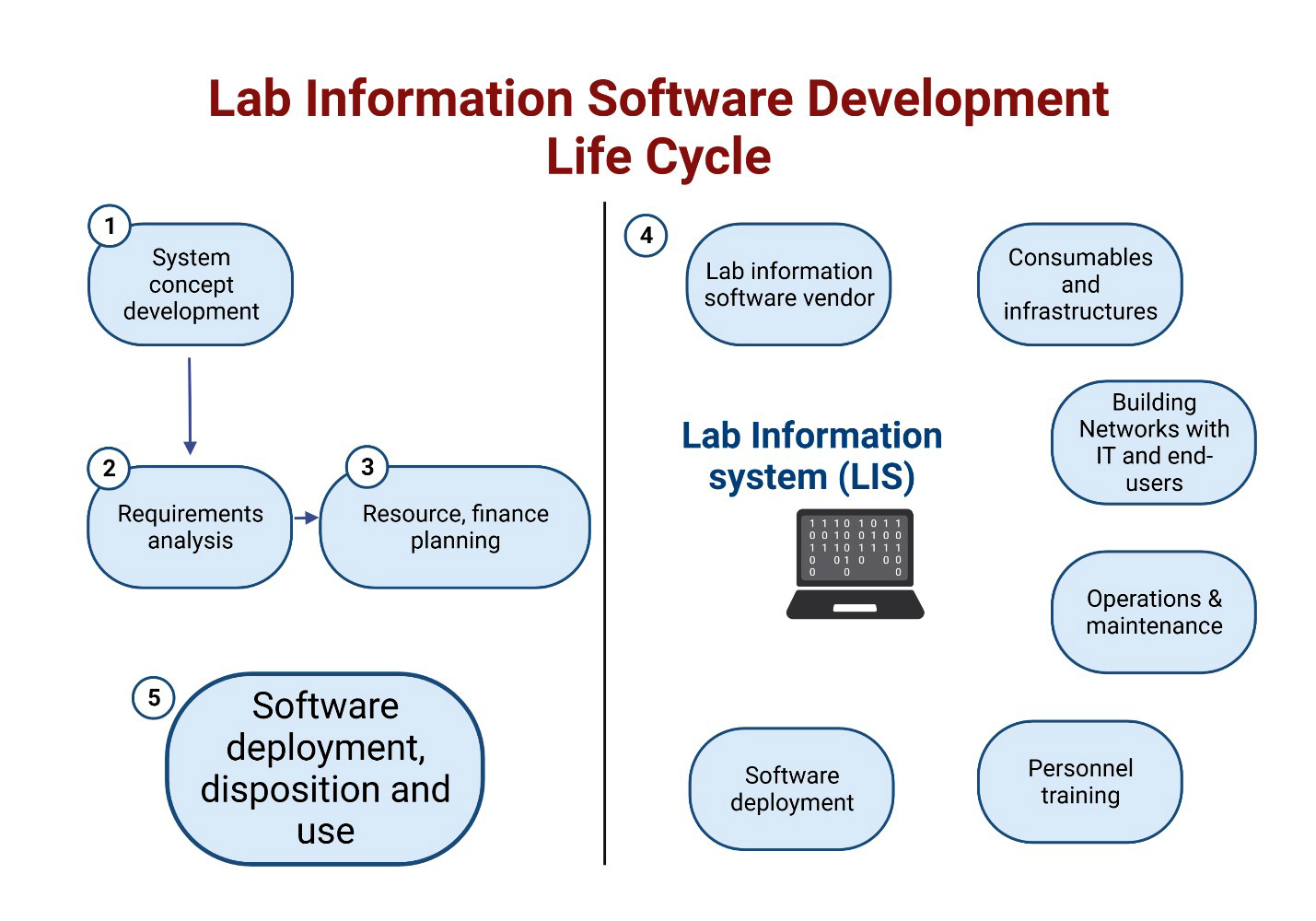
- Process of choosing and implementing a LIS involves 8 steps or phases
- Project definition
- Functional requirements
- Functional design
- Implementation plan
- System integration
- System evaluation (end user feedback)
- Final documentation
- Go live
Terminology
- Laboratory information management system (LIMS)
Diagrams / tables
Organization
- Define governance structure
- Project sponsor
- Initiates the project
- Provides strategic direction
- High level goals
- Funding and project approval
- Is almost always the final decision making authority
- Technical working group
- Overlooks the process
- Promotes communication among stakeholders
- Makes strategic planning and important policy decisions
- Laboratory leadership and policy makers, key staff of laboratory, information technologists, partners
- Project management teams
- Implement the LIS while coordinating budgets, tasks, human and financial resources and managing for risks
- Maintain a communication bridge between involved personnel
- Should have key people who are versed in information technology and possess laboratory technical expertise
- Lab technical advisor / supervisor
- This step is usually a part of enterprise resource planning (ERP)
- References: Computational Collective Intelligence 2015;9330:377, Am J Clin Pathol 2017;147:261
Project definition
- What is to be achieved and by whom, when and why
- Foundation for the project; typically 1 page long
- Plan integration with other systems (such as ERP, financial, etc.), instrument integration for automatic data acquisition and longterm maintenance and growth (future data migration)
- ERP systems support laboratories by (PLoS One 2021;16:e0260798)
- Reducing costs and improving productivity
- Standardizing business; integrating and improving business processes
- Increasing flexibility
- Integrating acquisitions
- Key features of ERP include
- Global financial capabilities
- Advanced planning and scheduling
- Product configurators
- Supply chain management
- Customer relationship management
- Business intelligence
- Component (object oriented) architecture
- Vendors of ERP: SAP, PeopleSoft, J.D. Edwards, Oracle, among others
- Gathering of several groups or teams to understand their needs and agree on the required features for a successful project outcome
Functional / needs assessment
- Crucial step for defining the specific requirements for your LIS
- Serves as the proposal to send to potential bidders, minus the budget information and any proprietary information
- Each lab is unique
- Examples of functional requirements and design
- Technical specifications, such as
- Data storage capacity
- Networking capabilities
- Processing speed
- Security features
- Functional requirements
- Workflow management (types of tests, types of samples, etc.)
- Reporting capabilities
- User management (needs to be defined based on lab)
- Typical list to include would be
- Sample login
- Sample tracking / barcode support / quoting / billing
- Scheduling
- Biometric identification
- Chain of custody
- Instrument integration
- Result entry / audit trail
- Quality assurance (QA) / quality control (QC) / specification checking
- Result reporting
- Web integration / links to enterprise software
- Archiving and data warehousing
- Backup of the data with well tested storing and scheduled maintenance
- Technical specifications, such as
- Reference: J Eval Clin Pract 2019;25:788
Software selection
- LIS vendors and products: points to consider while selecting a vendor should include (but are not limited to)
- Different strengths and weaknesses
- Validity of the final, detailed proposal: has anything in the laboratory changed?
- Compliance of proposed system to the specific requirements of the laboratory; check for key features like Health Level Seven (HL7) support for integration with other systems, configurable user roles, customizable workflows, QC management and robust reporting capabilities
- Review of the Gantt chart (a project management tool): realistic timeline and potential shortcomings
- Review of the cost proposal, contract, license agreements and implementation plan
- Final review with the vendor for clear understanding of project goals for seamless integration and progression
- References: Biopreserv Biobank 2017;15:111, Environ Health Prev Med 2015;20:338
Hardware acquisition and installation
- This stage depends heavily on selected software's requirements
- General considerations include
- Number of concurrent users
- Number of data records
- Archive requirements
- External loads on the system from non-LIS applications
- Hardware to be installed for a LIS are usually the following
- Computer hardware
- Central processing unit (CPU): quad core CPU or better (for most present day systems)
- System memory (random access memory [RAM]): 8 GB of RAM and above (for most present day systems)
- Hard drive, tape drives, optical drives and cloud systems
- Recommended standard 232 (RS232) or transmission control protocol / internet protocol (TCP / IP) instrument interfacing
- Although the above are minimum requirements, most present day LIS use the following hardware
- High performance server (e.g., a Dell PowerEdge Server) with a robust processor (e.g., Intel Xeon), sufficient RAM (e.g., 32 GB and above) and storage capacity (e.g., 1 TB solid state drive [SSD] and above)
- Workstations: standard personal computers (PCs) with the latest operating system (OS) (e.g., Windows 10, Windows 11)
- Network equipment: supports fast data transmission (e.g., gigabit ethernet switches) and WiFi for mobile devices if required
- Computer networks: local area networks (LAN) and wide area networks (WAN); consideration for Open Systems Interconnect (OSI) model, which is a uniform standard introduced in 1978 by the International Organization for Standardization (ISO) to facilitate data communication among the disparate equipment from different vendors (Finnell: Clinical Informatics Study Guide - Text and Review, 2nd Edition, 2022)
- Physical layer: most common standard at this level is RS232C
- Data link layer: layer 2 switches, bridges network interface cards (NICs)
- Network layer: hubs and routers
- Transport layer: common transport protocols include TCP / IP and sequence packet exchange / internet packet exchange (SPX / IX)
- Computer hardware
Software installation
- Installation begins with setting up the server OS, then the database management system (DBMS)
- For example, Oracle or SQL Server could be the chosen DBMS for the LIS
- Then, install the LIS server application, followed by the client application on each workstation
- Ensure all components are correctly networked and that security features, such as firewalls and antivirus software, are installed and properly configured
- Sample software installation for a DBMS using Oracle and SQL Server (Acad Radiol 2015;22:527)
- Operating system: Windows Server 2008 or higher
- Database management system: Oracle (11g or higher) or SQL Server (2012 or higher)
- Java Development Kit (JDK) 1.5 or higher
- Oracle SQL Developer or SQL Server Management Studio
- ERP software such as SAP, PeopleSoft, J.D. Edwards or Oracle
- Hardware and software requirements may vary depending on the specific needs of the laboratory and the LIS being used; it is recommended to consult with the vendor or manufacturer for specific requirements before installation
- Computing models
- Single processing: used by 1 user; currently impractical and obsolete
- Shared processing: allows multiple users to use 1 machine, usually a mainframe, simultaneously; multiple terminals are connected to a mainframe computer
- File server computing: Terminal Services is an example of file server computing in LIS; all application execution, data processing and data storage take place on the server (Microsc Microanal 2021 Apr 28 [Epub ahead of print])
- Client / server computing: distributed application structure that partitions tasks or workload between the providers of a resource or service (called servers) and service requesters (called clients); examples of client - server model are email, world wide web, etc. (Comb Chem High Throughput Screen 2011;14:742)
- Ruby on Rails based platform: QTREDS is a Ruby on Rails based platform for Omics Lab that helps researchers to have a complete knowledge of the laboratory activities at each step (BMC Bioinformatics 2014;15:S13)
- Cloud based LIS: organizes all sample information (metadata, workflows, samples, results and instruments) at all times
- SaaS LIS platforms provide flexibility to present sample data clearly and concisely for a variety of stakeholders; most widely used (Comb Chem High Throughput Screen 2011;14:742)
- Electronic lab notebook (ELN): ELNs are used for organizing lab experiments; the 2 systems can track samples through their processes
- ELNs are more specific for individual experiments, whereas a LIS will center around collecting and reporting aggregate sample data (EMBO Rep 2020;21:e49862)
System configuration and customization
- Configure the LIS according to lab's workflow
- Setting up a workflow that includes order entry, sample tracking, result entry and report generation
- User roles might include technologist (can enter results), pathologist (can approve results) and clerk (can enter orders)
- LIS might be configured to print barcodes for samples, to apply certain QC rules or to generate specific report formats
- Customization might include developing a specific data input form or report template
- Reference: Opie: Cannabis Laboratory Fundamentals, 1st Edition, 2021
Integration with lab instruments
- Connect medical instruments with the LIS; vendor solutions are typically classified into
- Instrument vendors who wrote output files from their instruments to function only with their LIS (thus providing a sales advantage)
- Vendors using proprietary software that usually require vendor involvement to interface instrument to the LIS
- Vendors who attempted to provide some standardization with a generic output
- Common output formats: comma separated values (CSV), Microsoft Access or Excel spreadsheets
- Example: integration of an automated 5 part hematology analyzer
- This would involve setting up an interface using HL7 or American Society for Testing and Materials (ASTM) standards, possibly involving a middleware, such as Data Innovations's Instrument Manager
- Goal is to automate the flow of information from the instrument to the LIS so results can be recorded automatically without manual data entry
- Questions to ask the vendor: format of the output file, the ability to interface the instrument to a LIS and if any additional software must be purchased
- Reference: Opie: Cannabis Laboratory Fundamentals, 1st Edition, 2021
Data migration
- Transitioning from an old system; involves writing scripts to export data from the old system and import it into the new one, keeping in mind that the data structures might be different
- E.g., might need to map different types of hematology results from the old to the new system, considering various factors, such as test codes and units
Training
- Every user role needs training on the new system
- Train staff on how to enter orders
- Train technologists on how to enter results
- Train pathologists on how to approve results and generate reports
- Training should also cover data security and privacy regulations, such as the Health Insurance Portability and Accountability Act (HIPAA)
- Reference: Opie: Cannabis Laboratory Fundamentals, 1st Edition, 2021
Testing
- System evaluation
- Checklist to compare before and after the installation of the system to ensure that all needs have been met
- LIS software is adaptable and able to accommodate change
- Integration testing would test the HL7 interfaces with other systems, such as an electronic medical record (EMR) system or the medical instruments in the lab
- Coordinated and continuous update of functional requirements phase and system evaluation documentation
- QC: operational techniques and tests required to maintain and improve the quality of a product or service
- QA: activities that monitor and evaluate the performance of QC procedures employed in the manufacture of products and services
- QC / QA role of LIS: user warnings, graph (control charts and trend analysis) and report generation, full audit trail, automatic calculations, automated sample tracking, online standard operating procedures (SOPs), maintenance of standards, instrument calibration, tracking training records and much more
- System validation
- Validation scripts, which allow end users to run through a series of functions, input a known value and make sure that the output is what was expected, based on the input
- Validation is a very tedious process and differs from testing; testing evaluates if the software meets the design specifications
- References: Opie: Cannabis Laboratory Fundamentals, 1st Edition, 2021, Qual Assur 2001;9:217
Security
- System security
- Responsibility of the database administrator
- Maintaining the static tables of the LIS
- Usernames and passwords
- Regular backups
- Modifying report templates
- Application security
- Can be done effectively by segregating roles of users as view only, make changes and validation
- Regular password change prompts
- 2 or 3 factor security if the application can be accessed over virtual private network (VPN)
- Network security
- Network administrator typically assigns permissions to individuals or groups to access the network and its modification rights
- Reference: Bioinformatics and Biomedical Engineering 2017;10208:602
Go live and support
- After thorough testing, the LIS can be deployed
- Go live day is selected and users start using the new system
- Support team should be ready to address any issues
- Regular monitoring should be conducted to ensure system performance, security and user satisfaction
- Reference: J Pathol Inform 2017;8:47
Board review style question #1
Which computing model is used in a laboratory information system (LIS) to store and manage data files so that other computers on the same network can access the files?
- Client - server model
- Electronic lab notebook (ELN)
- Ruby on Rails based platform
- Virtual reality integration
Board review style answer #1
A. Client - server model. The client - server model is a distributed application structure that partitions tasks or workload between the providers of a resource or service (called servers) and service requesters (called clients). In a LIS, the client - server model is used to store and manage data files so that other computers on the same network can access the files. Answer C is incorrect as Ruby on Rails is a web development framework and not a specific computing model. It does not dictate the underlying computing model used for data storage and access in a networked environment. Answer D is incorrect as virtual reality integration is focused on creating immersive experiences and environments using virtual reality technology. It is not a computing model for storing and managing data files in a networked environment like a laboratory information system (LIS). Answer B is incorrect as an electronic lab notebook is a system for researchers to store and manage their experimental data. While it involves storing and managing data, it is not a computing model in itself.
Comment Here
Reference: LIS installation & implementation
Comment Here
Reference: LIS installation & implementation
Board review style question #2
What is the role of HL7 (health level seven) in laboratory information systems (LIS)?
- HL7 facilitates the exchange of standardized information between different healthcare systems, enabling interoperability and seamless communication in the laboratory
- HL7 is a programming language used for writing custom scripts to automate laboratory workflows and data analysis processes, ensuring efficiency in LIS operations
- HL7 is primarily used for tracking the movement of laboratory equipment within a healthcare facility, ensuring efficient utilization
- HL7 provides a platform for creating virtual reality simulations within the laboratory, enhancing training for laboratory staff
Board review style answer #2
A. HL7 facilitates the exchange of standardized information between different healthcare systems, enabling interoperability and seamless communication in the laboratory as it is focused on standardizing communication for information exchange in healthcare settings. Answer C is incorrect because the primary focus of HL7 is on the standardization of communication and data exchange, not on equipment tracking. Answer D is incorrect because HL7 is not designed for creating virtual reality simulations. Its purpose is centered around data exchange and interoperability. Answer B is incorrect because HL7 is not a programming language; it is a set of international standards for the exchange, integration, sharing and retrieval of electronic health information. Its primary focus is on standardizing communication formats to enable the seamless exchange of health related data, not on writing custom scripts for laboratory workflows.
Comment Here
Reference: LIS installation & implementation
Comment Here
Reference: LIS installation & implementation