Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Shi M, Jevremovic D. T prolymphocytic leukemia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/lymphomanonBTcellpro.html. Accessed December 23rd, 2024.
Definition / general
- T prolymphocytic leukemia (T PLL) is an aggressive, mature T cell leukemia, composed of small to medium sized mature T cells, usually with high white blood cell (WBC) count and widespread organ involvement
- No significant changes in WHO 5 and ICC classifications (Leukemia 2022;36:1720, Blood 2022;140:1229)
Essential features
- Aggressive leukemia of mature T cells
- High white blood cell count (lymphocytosis)
- Usually CD4+
- Usually TCL1 positive by immunophenotyping and TCL1 rearranged by FISH
- Difficult to treat, poor prognosis
Terminology
- T cell chronic lymphocytic leukemia (small cell variant of T cell prolymphocytic leukemia)
ICD coding
Epidemiology
- Rare (2% of mature lymphocytic leukemias)
- Adults and elderly (> 30 years, median age: 65 years)
Sites
- Widespread: peripheral blood, bone marrow, lymph nodes, spleen, liver, skin
Pathophysiology
- Combination of overexpression of TCL1 family of proteins (which stimulate AKT / protein kinase B driven proliferation) and functional deficit of ATM protein (Nat Commun 2018;9:697)
Etiology
- Unknown at this time
- Higher risk in patients with ataxia-telangiectasia (germline ATM mutations)
Clinical features
- High tumor burden with high white blood cell count (median 50 - 60 x 109/L), bone marrow involvement (in 100% of patients) with cytopenias, hepatosplenomegaly, lymphadenopathy, skin and mucosal lesions, other organ involvement / dysfunction (Am J Hematol 2017;92:441)
- Prominent constitutional symptoms
- 20 - 30% present with inactive disease and progress to active disease within 1 - 2 years (criteria for progression: lymphocyte doubling time (LDT) of less than 6 months or lymphocyte count increase by > 50% in 2 months) (Blood 2019;134:1132)
Diagnosis
- Peripheral blood: morphology + flow cytometry with or without fluorescent in situ hybridization (FISH)
- Bone marrow or solid tissue biopsy (lymph node, spleen, liver, skin, other): morphology + phenotyping (immunohistochemistry or flow cytometry) with or without FISH
- Note: FISH not necessary if TCL1 overexpression in neoplastic T cells can be shown by IHC or flow (J Clin Pathol 2018;71:309)
- Consensus criteria for diagnosis by the T PLL International Study Group (Blood 2019;134:1132)
- All 3 major criteria or the first 2 major and 1 minor criteria are required for diagnosis
- Major criteria:
- 5 x 109/L cells of T PLL phenotype in peripheral blood or bone marrow
- T cell clonality by molecular or flow cytometry methods
- Abnormalities of 14q32 or Xq28 or expression of TCL1A/B or MTCP1
- Minor criteria:
- Abnormalities involving chromosome 11 (11q22.3; ATM)
- Abnormalities in chromosome 8: idic(8)(p11), t(8;8), trisomy 8q
- Abnormalities in chromosome 5, 12, 13, 22 or complex karyotype
- Involvement of specific sites (spleen, effusions)
Laboratory
- Increased peripheral blood lymphocytes, often > 100 x 109/L, increased lactate dehydrogenase (LDH) and beta2 microglobulin (Ann Oncol 2017;28:1554)
- Negative serology for HTLV1
Radiology description
- Prominent hepatosplenomegaly and widespread lymphadenopathy; moderate to high FEV on PET scan
Prognostic factors
- Overall poor prognosis; median survival with active disease is 1 - 2 years
- Worse prognosis: pleural effusion, high LDH (> 1668 IU/l), low hemoglobin (< 9.3 g/dl), complex karyotype (Ann Oncol 2017;28:1554, Am J Hematol 2017;92:441)
Case reports
- 59 year old man with the history of renal transplant (J Med Case Rep 2019;13:223)
- 60 year old woman with a history of chronic myeloid leukemia (CML) (J Clin Pathol 2019;72:511)
- 64 year old man with TCL1 positive hematogones after T cell prolymphocytic leukemia therapy (Hum Pathol 2017;65:175)
- 68 year old man with heart failure (Blood 2017;130:691)
- 75 year old man with unusual phenotype of T cell prolymphocytic leukemia (Blood 2018;132:111)
Treatment
- Only for active disease
- Standard treatment: alemtuzumab (anti-CD52) variable allogeneic bone marrow transplant
- Experimental therapies with BCL2, JAK3 or HDAC inhibitors (Blood 2019;134:1132)
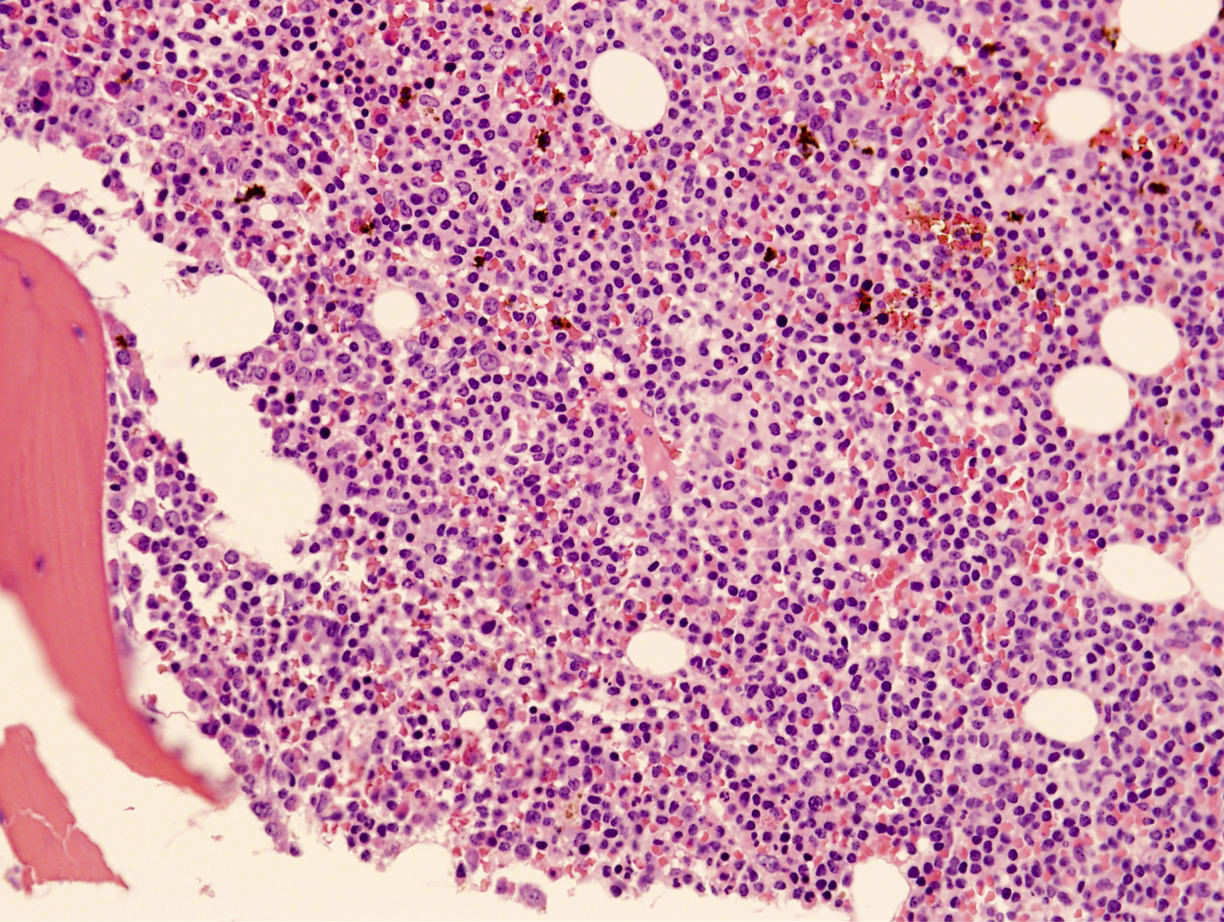
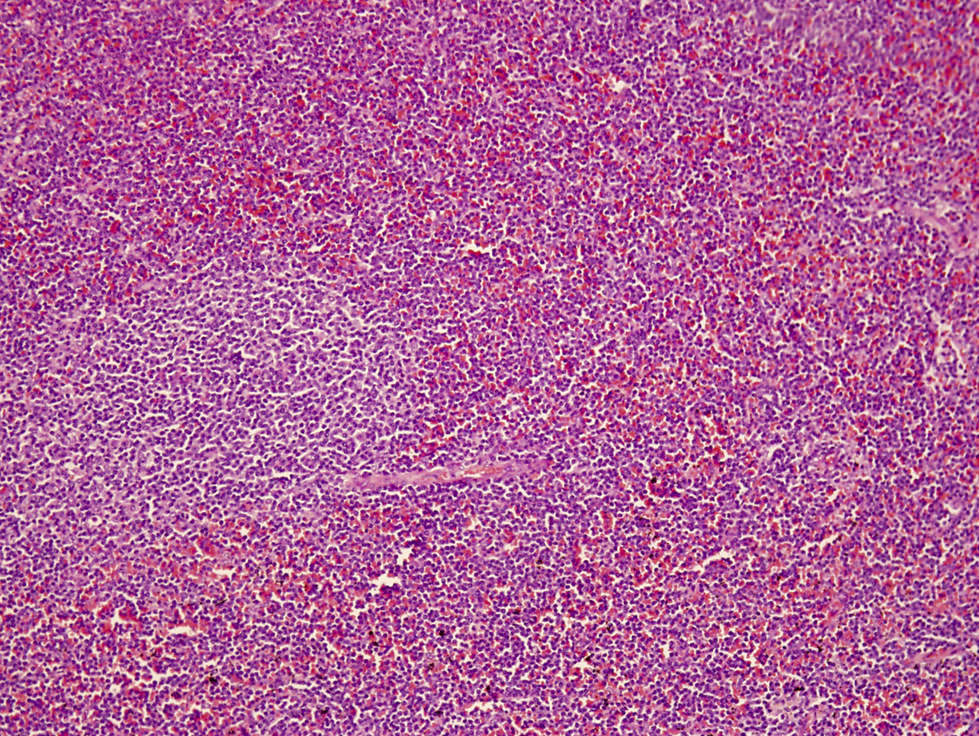
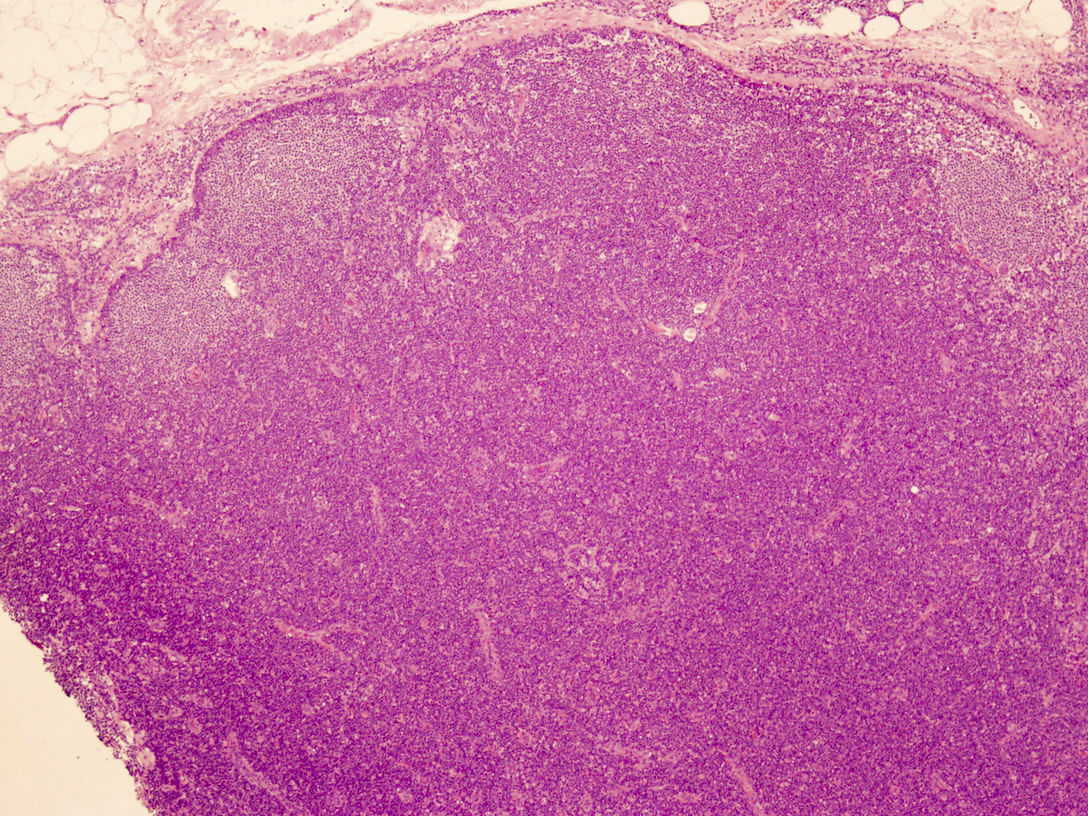
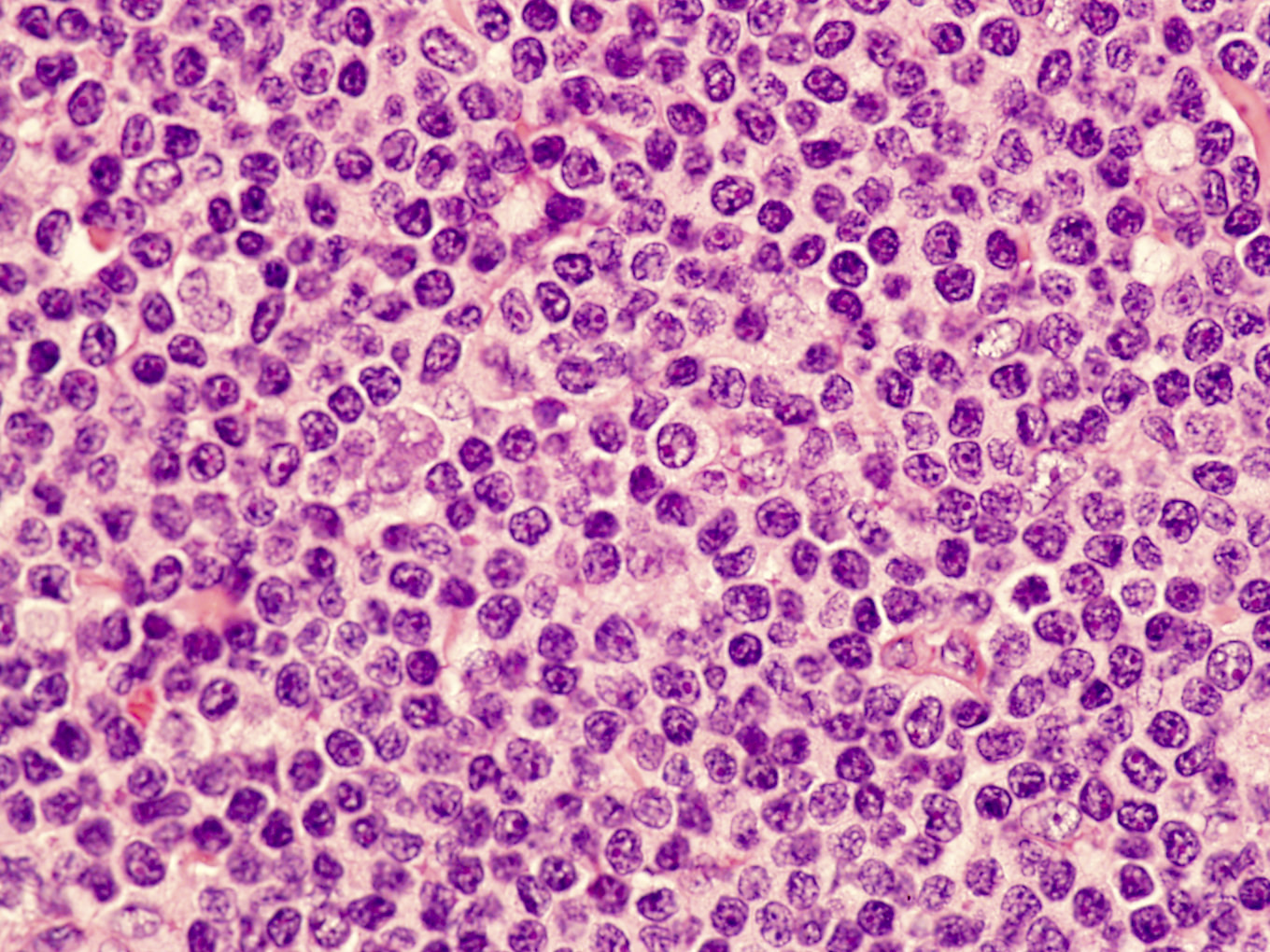
Microscopic (histologic) description
- Perivascular and diffuse tissue infiltrates of uniform small to medium sized lymphocytes
- Red pulp involvement in the spleen
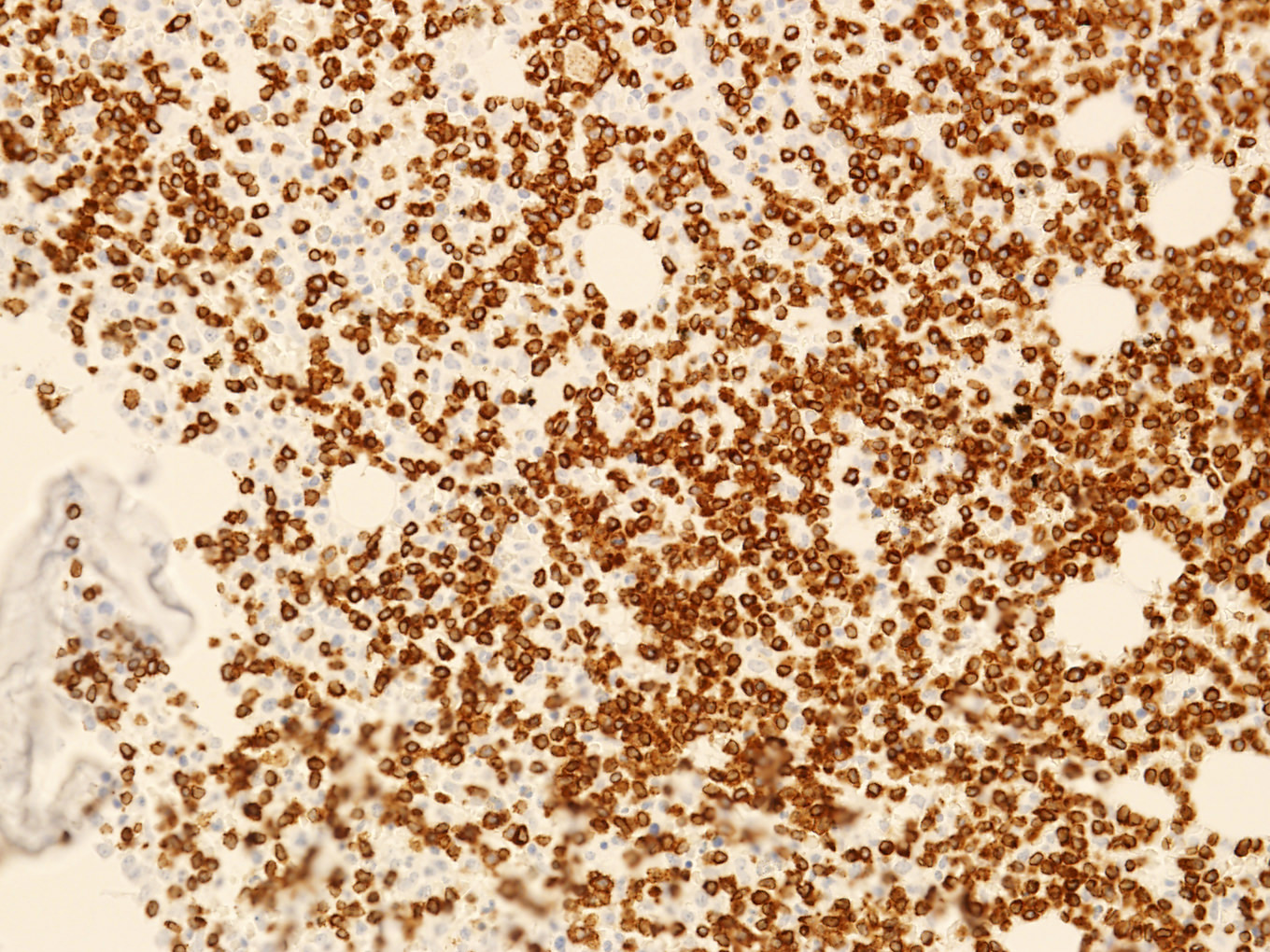
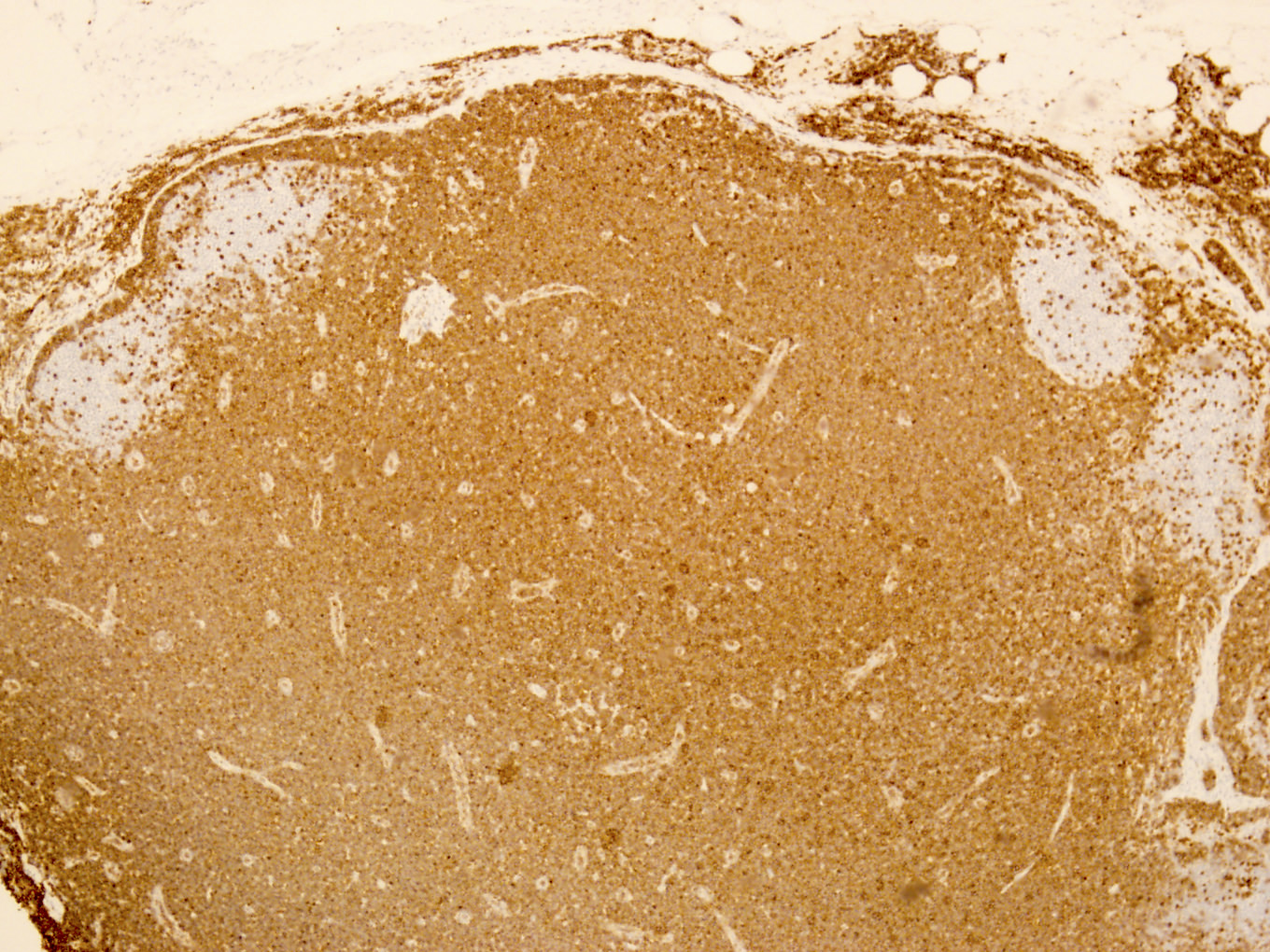
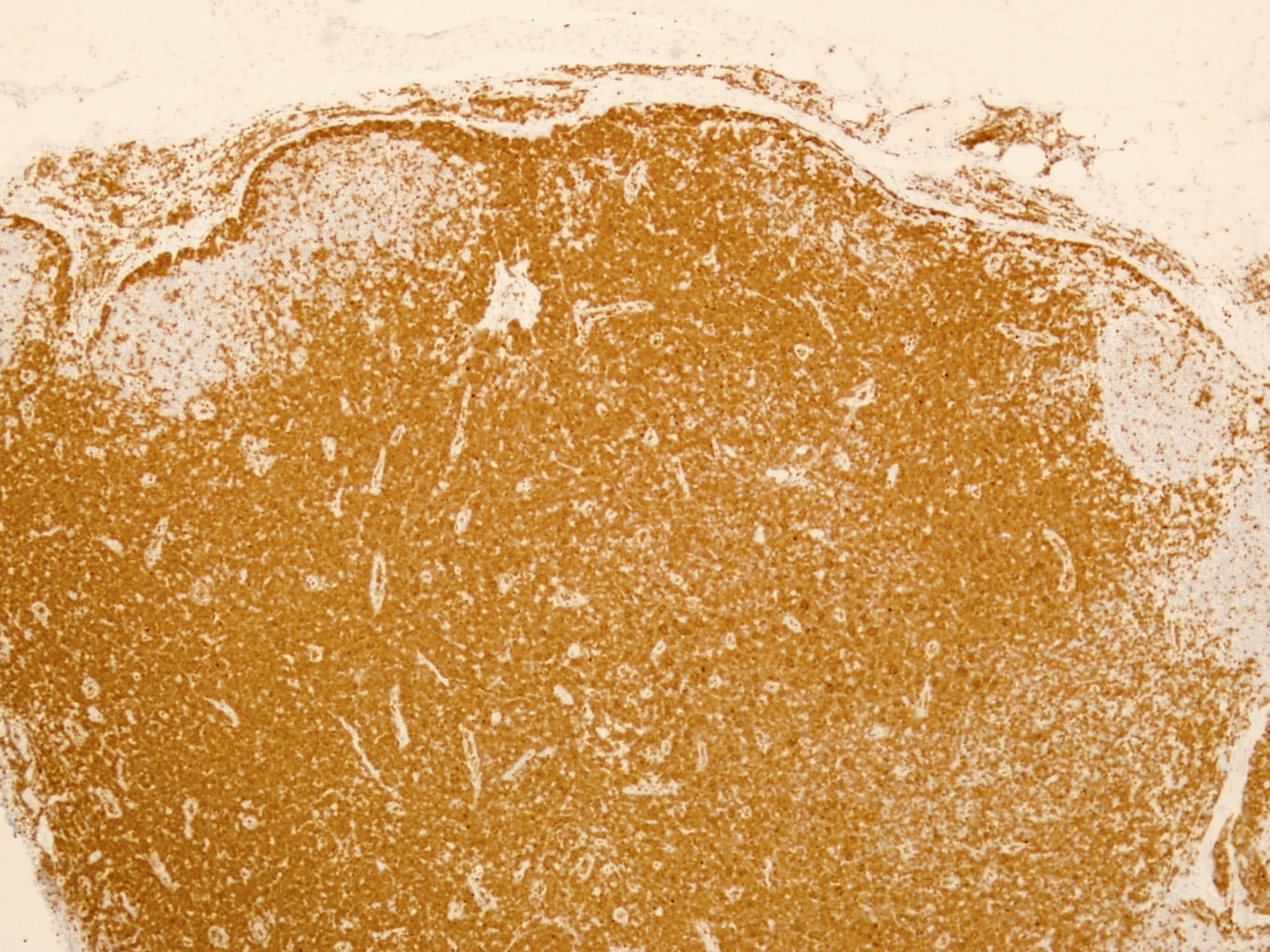
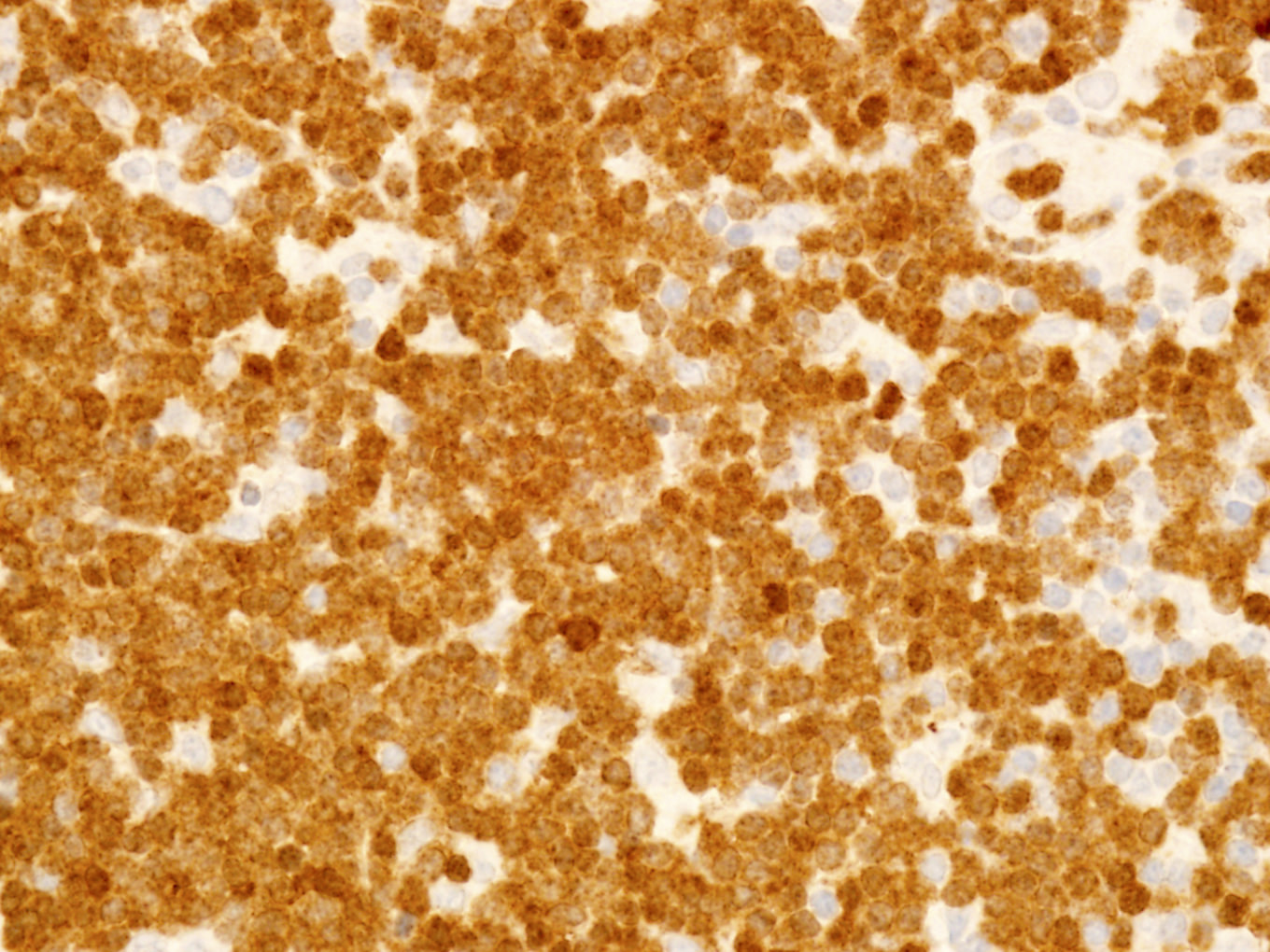
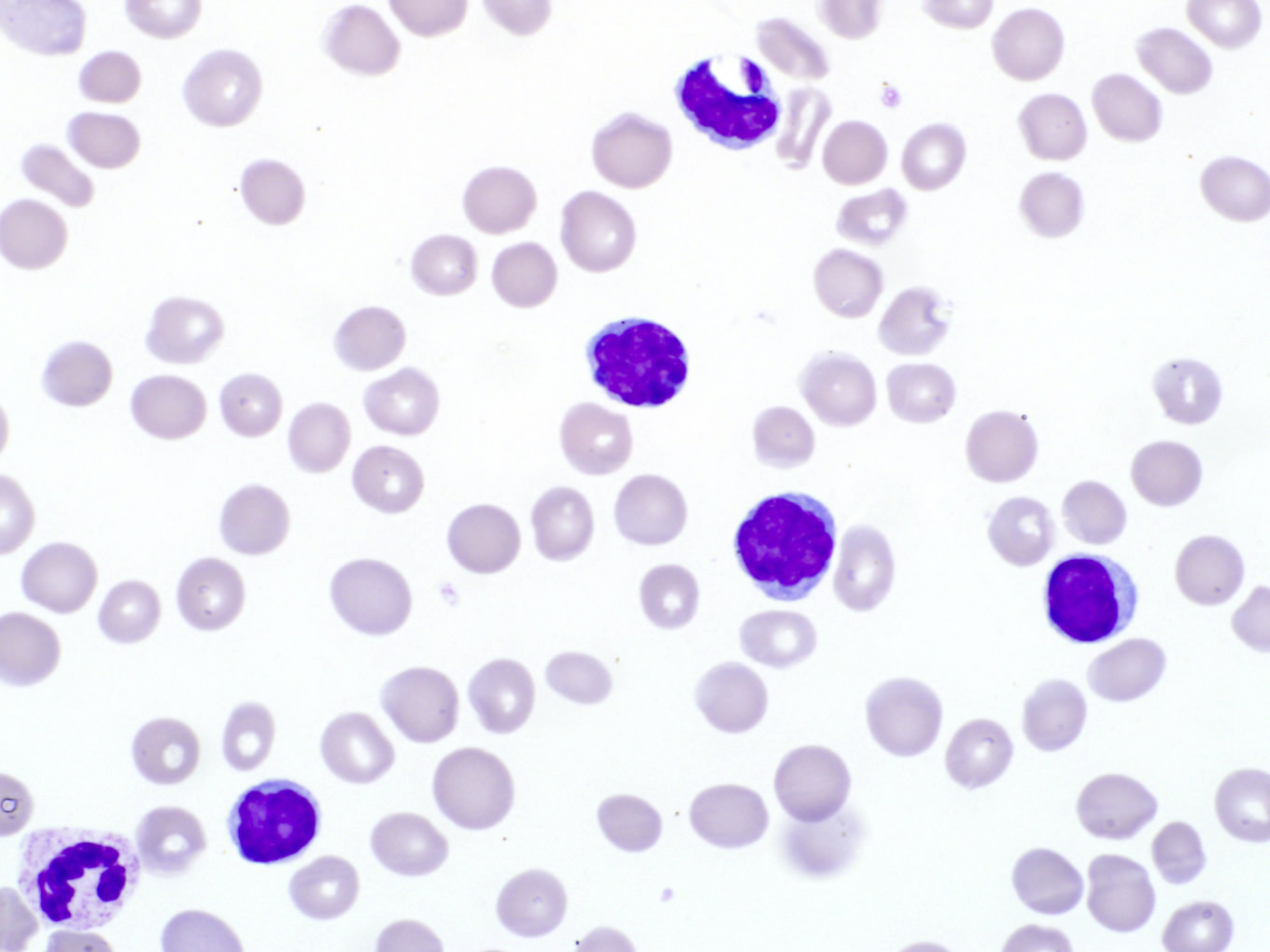
Microscopic (histologic) images
Contributed by Min Shi, M.D., Ph.D. and Dragan Jevremovic, M.D., Ph.D.
Peripheral smear description
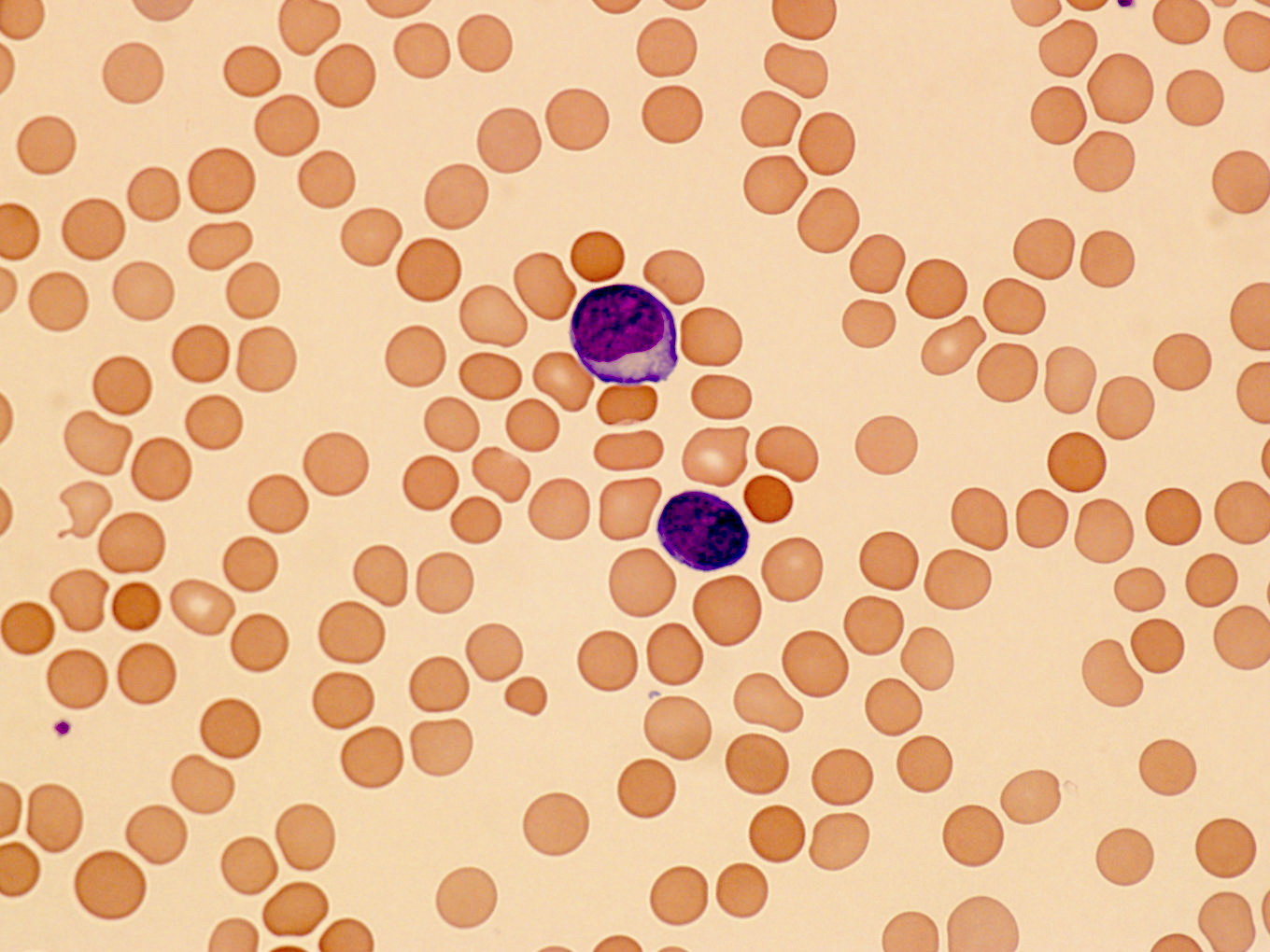
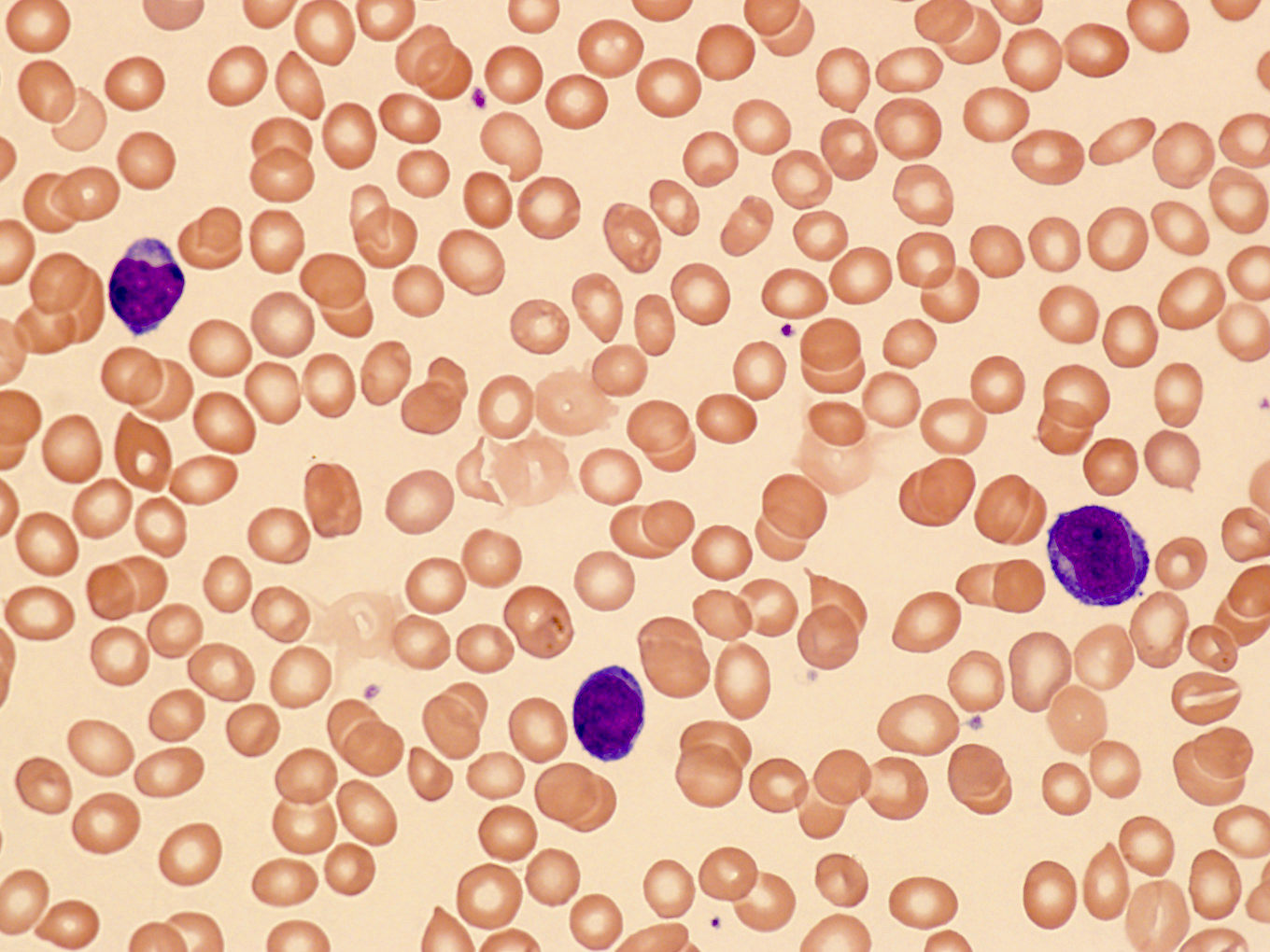
- Lymphocytosis with small to medium sized lymphocytes with cytoplasmic blebs, clumped chromatin and variably prominent central nucleolus
- Small cell variant in 25% of cases: smaller cells without obvious nucleolus
- Cerebriform variant in 5% of cases
- Reference: Blood 2019;134:1132
Peripheral smear images
Positive stains
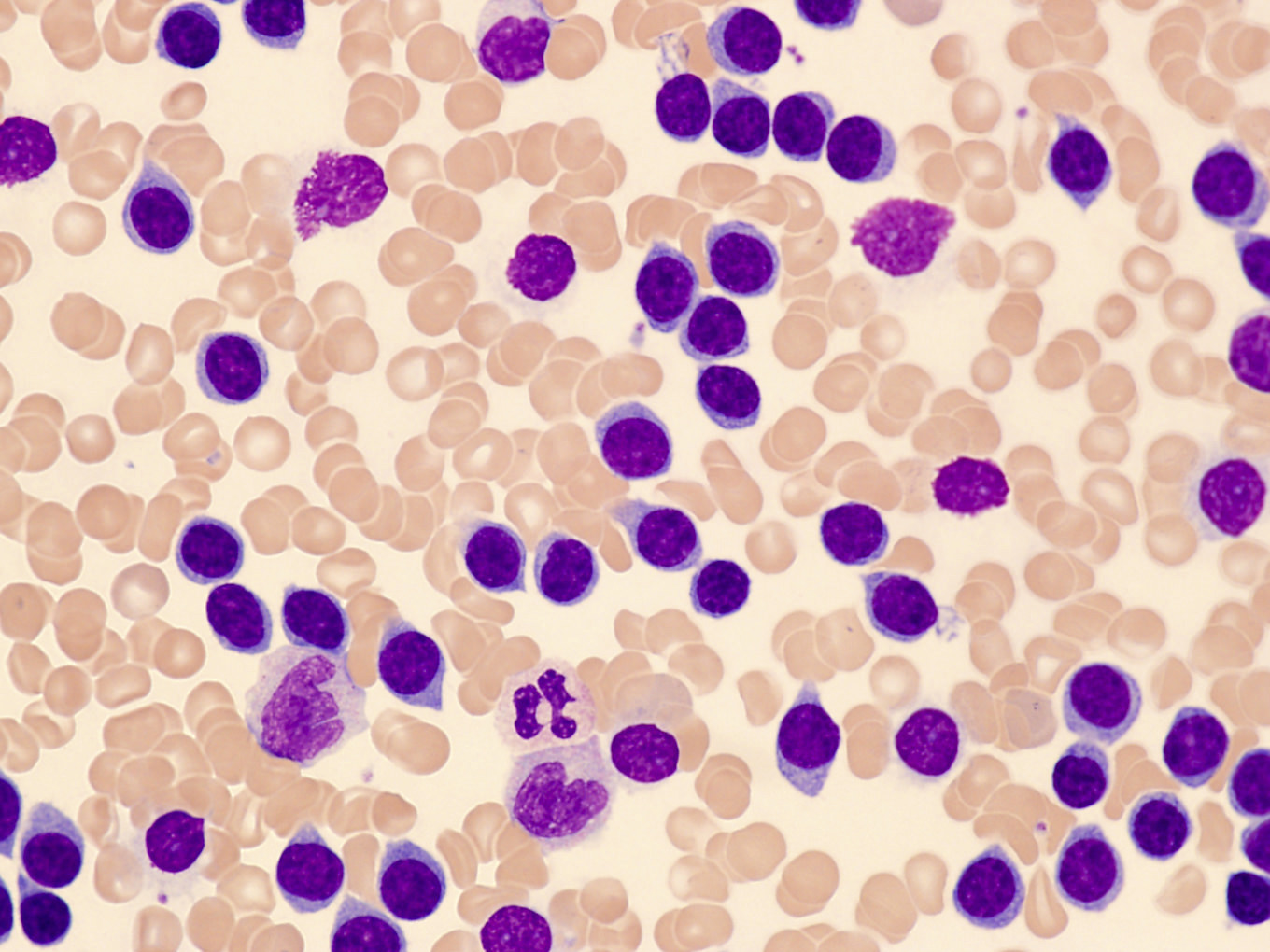
Flow cytometry description
Flow cytometry images
Molecular / cytogenetics description
- Clonal T cell receptor gene rearrangements (TRB@ and TRG@)
- FISH is commonly used for diagnosis; T cell receptor locus rearranged with the TCL1 family of genes:
- TCL1A / TCL1B rearrangement: inv(14)(q11;q32) in 80%, t(14;14)(q11;q32) in 10%
- Rarely MTCP1 rearrangement t(X;14)(q28;q11)
- Rarely negative for TCL1A / TCL1B or MTCP1 rearrangements (Blood 2019;134:1132)
- Complex karyotype in 70 - 80%; abnormalities of chromosomes 6, 8, 12p, 17p
- Mutations in ATM gene on 11q23 in 80 - 90%
- Other mutations / alterations: TP53, JAK / STAT pathway genes IL2RG, JAK1, JAK3, STAT5B (Blood 2014;124:1460)
Molecular / cytogenetics images
Sample pathology report
- Peripheral blood, bone marrow aspirate and biopsy, iliac crest:
- T cell prolymphocytic leukemia, extensively involving peripheral blood and bone marrow, with decreased trilineage hematopoiesis.
- Peripheral blood:
- Complete blood count: hemoglobin 11.2 g/dL; red blood cell 3.55 x 1012/L; mean corpuscular volume 97.2 fL; red blood cell distribution width 15.2%; white blood cell 470.0 x 109/L; PLT 111 x 109/L
- Cell percentage of total cells: neutrophils (4%), lymphocytes (95%), monocytes (1%)
- Peripheral smear: lymphocytosis; small to intermediate lymphocytes with mature chromatin, prominent nucleoli, eccentric nuclei and moderate amounts of basophilic cytoplasm
- Bone marrow aspirate and biopsy:
- Aspirate quality: cellular
- Biopsy quality: adequate
- Myeloid to erythroid (M:E) ratio: normal, 3:1
- Cellularity: hypercellular, 90%
- Erythroid precursors: markedly decreased quantity, normal morphology
- Myeloid precursors: markedly decreased quantity, normal morphology, blasts not increased
- Megakaryocytes: markedly decreased quantity, normal morphology and distribution
- Lymphocytes: abnormal (diffuse) infiltrates of small to intermediate sized cells present (90% of the total marrow cellularity)
- Plasma cells: not increased
- Ancillary studies:
- Flow cytometry, bone marrow:
- Blasts: not increased by CD45 / side scatter and CD34
- B cells: no monotypic; normal expression pattern of CD19, CD10, surface kappa and lambda
- T cells / NK cells: distinct T cell population
- Express: CD4, CD2, CD3, CD5, CD7
- Do not express: CD8, CD16, TCR gamma / delta, CD1a, nTdT, cMPO, cCD79a, cCD22
- Estimated size: 95% gated lymphoid events; 87% total analyzed events
- T cell lymphoma FISH, bone marrow: the result is abnormal and indicates 88.5% of nuclei had a rearrangement involving TCL1A; this observation may indicate a clone with inv(14) or t(14;14), which are common rearrangements in T cell prolymphocytic leukemia
- Flow cytometry, bone marrow:
Differential diagnosis
Additional references
Board review style question #1
What is the most common phenotype of T cell prolymphocytic leukemia (T PLL)?
- CD3-, CD19+, CD20+, CD5+, CD23+
- CD3+, CD4+, CD5+, CD7-, CD8-, CD26-
- CD3-, CD4+, CD8+, TdT+
- CD3+, CD4+, CD5+, CD7+, CD8-
- CD3+, CD4-, CD7-, CD8+, CD16+
Board review style answer #1
D. CD3+, CD4+, CD5+, CD7+, CD8-. The most common phenotype of T PLL is that of mature CD4+ T cells expressing pan T cell antigens, including CD7. Answer A is a common B CLL / SLL phenotype. Answer B is a common Sézary syndrome phenotype. Answer C is a common T lymphoblastic leukemia / lymphoma phenotype. Answer E is a common T cell large granulocytic lymphocyte leukemia phenotype.
Comment Here
Reference: T cell prolymphocytic leukemia
Comment Here
Reference: T cell prolymphocytic leukemia
Board review style question #2
Board review style answer #2
D. TCL1 rearrangement on chromosome 14 is the most common genomic alteration, which results in the overexpression of TCL1 protein and drives proliferation of neoplastic cells in T cell prolymphocytic leukemia.
Comment Here
Reference: T cell prolymphocytic leukemia
Comment Here
Reference: T cell prolymphocytic leukemia