Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Immunofluorescence description | Immunofluorescence images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Oakes F, Glass C. Takotsubo cardiomyopathy. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/hearttakotsubo.html. Accessed April 2nd, 2025.
Definition / general
- Takotsubo cardiomyopathy is a process of acute myocardial injury which occurs in the setting of excessive catecholamines and is sometimes associated with an identifiable physical or emotional stressor
- Characterized by balloon-like deformation of the ventricular myocardium, which produces profound but potentially short lived functional impairment
- Risk of severe complications is greatest in the acute setting but may persist chronically even after recovery
Essential features
- Ventricular wall motion abnormality in a regional ballooning pattern
- Evidence of reversible ischemic damage without widespread cell death
- Leading hypothesis suggests process is catecholamine mediated
- Clinical presentation mimics acute coronary syndrome
- Absence of severe coronary artery disease or thrombus on imaging
- Usually self limiting and reversible but carries risk of severe complications
Terminology
- Broken heart syndrome
- Stress cardiomyopathy
- Apical ballooning syndrome
- Happy heart syndrome
- Ampulla cardiomyopathy
Epidemiology
- 1 - 3 % of all people presenting with acute coronary syndrome, 5 - 6% of all female patients presenting with acute coronary syndrome (Eur Heart J 2018;39:2032)
- F > M (90% female)
- Average age: 66.8 years old
- Commonly have comorbid psychiatric or neurological disorder (N Engl J Med 2015;373:929)
Sites
- Left ventricular myocardium
- Apical (81.7%) > midventricular (14.6%) > basal (2.2%) > focal (1.5%) (N Engl J Med 2015;373:929)
Pathophysiology
- Catecholamine hypothesis:
- Sudden extreme stress (emotional or physical) → increase in circulating catecholamines → multivessel coronary vasospasm → acute cardiac dysfunction (Heart 2017;103:1461)
- Physical stressors capable of inducing catecholamine excess include thyrotoxicosis, pheochromocytoma and acute subarachnoid hemorrhage with sympathetic storm
- Multiple mechanisms of damage to cardiomyocytes:
- Vasoconstriction of coronary microvasculature leading to ischemia
- Catecholamine toxicity of cardiomyocytes
- Interstitial mononuclear inflammatory response (Eur Heart J 2018;39:2032)
- Cardiomyocyte damage → characteristic ballooning of ventricular walls:
- High level of wall motion abnormality
- Large area of ballooning relative to troponin levels suggests stretch and nonapoptotic myocardial injury (Heart 2018;104:96)
- Localization to ventricular myocardium may be related to apical - basal gradient of sympathetic innervation and beta adrenergic receptor density (Eur Heart J 2018;39:2032)
Etiology
- Classically associated with an emotional or physical stressor
- Stressor not always identifiable
- Emotional stressor can be negative (e.g. loss of a loved one) or positive (e.g. very happy events)
- Physical stressor can be related to pre-existing medical conditions (e.g. acute critical illness, respiratory failure) or iatrogenic (e.g. surgery, exercise tests)
- Loss of sympatholytic estrogens thought to play a role in predisposing postmenopausal women to this condition (Heart 2017;103:1461)
Clinical features
- Mimics acute coronary syndrome
- Acute chest pain (60 - 100%) > dyspnea > syncope (Herz 2010;35:240)
- Sinus tachycardia
- Reduced left ventricular ejection fraction (LVEF) with recovery of systolic LV function over time (N Engl J Med 2015;373:929)
- Symptoms rapidly improving within several hours after admission (Eur Heart J 2007;28:2456)
- Associated conditions: any cause of high catecholamine levels
Diagnosis
- Echocardiographic findings: left ventricular wall motion abnormalities (see Radiology description)
- Coronary angiography: absence of coronary artery disease or thrombus
- Presence of incidental coronary artery disease (10 - 15%) does not preclude diagnosis; this can lead to misclassification as coronary artery disease (N Engl J Med 2015;373:929)
- New ECG abnormalities: ST elevation or T wave inversion (Herz 2010;35:240)
- Postmortem gross examination may reveal ventricular ballooning with or without wall rupture (Forensic Sci Med Pathol 2019 Nov 9 [Epub ahead of print])
- Pathologic examination generally does not play a major role in diagnosis
Laboratory
- Elevated myocardial injury markers (creatine kinase, creatine kinase MB, troponin)
- Elevation less than expected for extent of wall motion abnormality (Heart 2017;103:1461)
- Mean troponin elevation similar to that seen in acute coronary syndrome (N Engl J Med 2015;373:929)
- Serum cardiac brain natriuretic peptides (BNP) elevated compared with patients with acute coronary syndrome
- 4 circulating miRNAs identified as possible biomarkers (Heart 2017;103:1461)
Radiology description
- Coronary angiography: normal and unobstructed coronary arteries
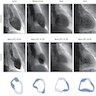
- Left ventriculography: 4 different wall motion patterns identified
- Apical, midventricular, basal and focal (see Radiology images) (Heart 2017;103:1461)
- Echocardiography: wall motion abnormality of left ventricular midsegments with or without apical involvement; severely reduced LV function
- Cardiac MRI: wall motion abnormality (Herz 2010;35:240)
Radiology images
Prognostic factors
- Complete functional recovery expected within 12 ± 3 days but chronic complications are possible (Eur Heart J 2007;28:2456)
- Complications = major cardiac / cerebrovascular events
- Rate is 9.9% per patient year (N Engl J Med 2015;373:929)
- Frequent: acute heart failure, LV outflow tract obstruction, cardiogenic shock, mitral regurgitation
- Moderate: Afib, LV thrombus, cardiac arrest, AV block
- Rate is 9.9% per patient year (N Engl J Med 2015;373:929)
- Rare: tachyarrhythmia, bradyarrhythmia, torsades de pointes, death, Vtach or Vfib, acute ventricular septal defect (VSD) (Eur Heart J 2018;39:2047)
- Unfavorable prognostic factors (re: in hospital complications):
- Older age
- Male
- Physical stressor
- Lower LVEF at admission
- Higher neutrophil / lymphocyte ratio at admission
- Higher WBCs / mean platelet volume ratio at admission (Clinical Cardiology 2020;43:1294)
- Higher troponins
- Acute neuro or psychiatric disease (N Engl J Med 2015;373:929)
- Presence of atrial fibrillation (JAMA Cardiol 2016;1:335)
- Mortality = 5.6% per patient year, similar to ST elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI) (Int J Cardiol 2015;185:282)
Case reports
- 27 year old man with iatrogenic epinephrine overdose induced cardiomyopathy (Proc (Bayl Univ Med Cent) 2019;32:567)
- 57 year old woman develops cardiomyopathy in the setting of COVID-19 infection (BMJ Case Rep 2020;13:e236811)
- 66 year old woman with cardiomyopathy associated heart failure as the presenting symptom of a pheochromocytoma (Tex Heart Inst J 2019;46:124)
- 73 year old woman with mild traumatic brain injury presenting in cardiogenic shock (Neurocrit Care 2017;26:284)
- 80 year old woman with exertional chest pain and vomiting in the absence of an identifiable stressor (Cureus 2019;11:e5749)
- 85 year old woman with acute chest pain in the setting of severe anxiety (QJM 2020;113:488)
Treatment
- Supportive care during acute phase and treatment of any complications, such as heart failure or arrhythmia
- ACE inhibitors and angiotensin II receptor blockers (ARBs) associated with improved survival at 1 year (N Engl J Med 2015;373:929)
- No evidence for improved survival benefit with beta blockers
Gross description
- Apical biventricular ballooning
- Death associated with Takotsubo cardiomyopathy is often due to complications, such as cardiac wall rupture, which are notable on gross examination (Forensic Sci Med Pathol 2019 Nov 9 [Epub ahead of print])
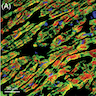
Microscopic (histologic) description
- Acute phase (day of admission)
- Hypertrophied myocytes (> 20 μm)
- Intracytoplasmic glycogen + PAS staining
- Recovery phase (12 ± 3 days after admission)
- Reduced PAS staining (Eur Heart J 2007;28:2456)
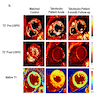
Immunofluorescence description
- Intracellular proteins show diminished density and disrupted intracellular organization during the acute phase of illness
- Alpha actinin
- N terminal dystrophin
- C terminal dystrophin
- Connexin 43
- Titin (T12, Tz1) (Eur Heart J 2007;28:2456)
- Cardiomyocytes demonstrate more normal cross striated and homogenous staining after recovery of contractile function (Eur Heart J 2007;28:2456)
Immunofluorescence images
Positive stains
- Cardiomyocyte staining is well described but not commonly used in diagnostic practice
- Alpha actinin:
- Acute: located in border areas of myocytes
- Recovered: regular distribution
- Actin:
- Acute: reduced in amount, displaced to border of myocytes
- Recovered: regular distribution
- Dystrophin (amino terminal):
- Acute: reduced
- Recovered: regular distribution
- Dystrophin (carboxy terminal):
- Acute: regular distribution
- Recovered: regular distribution
- Connexin 43:
- Acute: myocardial organization disturbed, profound loss of gap junctions
- Recovered: reorganization of cell - cell contact
- Titin:
- Acute: missing from some myocytes, abnormal punctate pattern
- Recovered: most myocytes showed recovery of regular cross striated pattern
- Fibronectin:
- Acute: widened interstitial space with large amounts of fibronectin between cells
- Recovered: reduced from acute but still increased compared with controls
- Collagen 1:
- Acute: increased in amount
- Recovered: reduced from acute but still increased compared with controls
- CD68 (macrophages):
- Acute: multiple extracellular clusters
- Recovered: multiple extracellular clusters
- CD3 (T lymphocytes):
- Acute: increased number
- Recovered: reduced to normal
- Ubiquitin:
- Acute: several ubiquitin positive cells detected but no large accumulations of ubiquitin protein complexes
- Recovered: same as above (Eur Heart J 2007;28:2456)
Negative stains
- Complement 9
- TUNEL (Eur Heart J 2007;28:2456)
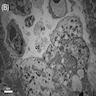
Electron microscopy description
- Acute phase (day of admission)
- Heterogenous vacuoles (varying contents: cellular debris, myelin bodies)
- Enlarged myocytes
- Cytoskeletal and contractile proteins dissolved
- Contractile material mostly seen around edges of myocytes
- Sporadic contraction bands
- Abnormalities in size and shape of clustered mitochondria
- Rounded / oval nuclei
- Interstitial space = widened, containing fibrotic material
- Recovery phase (12 ± 3 days after admission)
- Normal size myocytes
- Fewer, smaller vacuoles
- Regular composition of myocardium
- Intracellular structures nearly normal (Eur Heart J 2007;28:2456)
Molecular / cytogenetics description
- Adrenergic receptor polymorphisms are potentially relevant (Eur Heart J 2018;39:2032)
Videos
Illustrated walkthrough of the essential features of Takotsubo cardiomyopathy
Ventricular angiography video showing the 4 main subtypes of Takotsubo cardiomyopathy
Sample pathology report
- Heart, left ventricle, endomyocardial biopsy:
- Takotsubo cardiomyopathy (see comment)
- Comment: Microscopic examination reveals hypertrophied myocytes with vacuolar degeneration as well as rare contraction bands. PAS staining shows intracellular accumulation of glycogen. Moderate cellular infiltration observed in the interstitium. No necrosis identified. Microscopic appearance consistent with clinical diagnosis of Takotsubo cardiomyopathy in the acute phase, with symptom onset 2 days prior to biopsy.
Differential diagnosis
- Myocardial infarction:
- Positive history of coronary artery disease
- Coronary angiography: significant vessel occlusion
- BNP moderately elevated
- Acute myopericarditis:
- Preceding flu-like symptoms
- Leukocytosis
- Positive viral serology
- Angina:
- Positive history of coronary artery disease
- Improves with nitroglycerin and is relieved by rest
- Anxiety / panic attack:
- ECG normal (with or without sinus tachycardia)
- Cardiac biomarkers normal
Board review style question #1
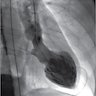
A 68 year old woman experiences acute onset chest pain and dyspnea upon receiving news that she is being evicted from her home. Cardiac imaging (shown above) suggests Takotsubo cardiomyopathy. Which of the following is most likely to be seen on biopsy of the affected myocardium?
- Abundant inflammatory cell infiltrate
- Atherosclerotic plaque in microvessels
- Coagulative necrosis of the cardiac apex
- Myocyte hypertrophy
Board review style answer #1
D. Myocyte hypertrophy. Takotsubo cardiomyopathy is characterized by ischemic damage to the ventricular myocardium, which leads to myocyte hypertrophy, resolving in a matter of days to weeks. In the absence of complications, necrosis is not usually seen. Atherosclerotic plaques are not usually seen in Takotsubo cardiomyopathy and if present are regarded as incidental findings. Some inflammatory cells could be seen but would not be the characteristic finding.
The picture (taken through ventriculography) shows ballooning at the apex of the heart, which is the most common pattern seen in Takotsubo cardiomyopathy.
Comment Here
Reference: Takotsubo cardiomyopathy
The picture (taken through ventriculography) shows ballooning at the apex of the heart, which is the most common pattern seen in Takotsubo cardiomyopathy.
Comment Here
Reference: Takotsubo cardiomyopathy
Board review style question #2
A 72 year old woman presents with acute onset chest pain and dyspnea. Cardiac enzymes are elevated and ECG shows a new ST elevation. Cardiac imaging reveals an enlarged, balloon-like left ventricle which fails to contract during systole. However, coronary angiography reveals no evidence of coronary artery disease or thrombus. The patient improves clinically with minimal supportive care over the next 2 weeks and is discharged to her home. What is the most likely diagnosis?
- Dressler syndrome
- Hypertrophic cardiomyopathy
- Takotsubo cardiomyopathy
- Viral myocarditis
Board review style answer #2
C. Takotsubo cardiomyopathy classically mimics acute coronary syndrome's presentation. Unobstructed coronary arteries, however, point towards Takotsubo cardiomyopathy. The ballooning appearance on echocardiography or ventriculography, as well as her relatively rapid recovery, are characteristic of Takotsubo cardiomyopathy.
Comment Here
Reference: Takotsubo cardiomyopathy
Comment Here
Reference: Takotsubo cardiomyopathy