Table of Contents
Definition / general | Essential features | ICD coding | Etiology | Case reports | Gross examination | Gross images | Microscopic (histologic) images | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Ghandili M, Giffen MA. Sudden cardiac death. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/heartsuddendeath.html. Accessed March 31st, 2025.
Definition / general
- Unexpected death of an individual not attributable to extracardiac causes within 1 hour of symptom onset or within 24 hours of last being known in good health (if death is unwitnessed) (Intern Med J 2019;49:826)
- Some definitions extend the time range with symptom onset up to 24 hours without diagnosed cardiac conditions within that time frame
Essential features
- Sudden cardiac death is the unexpected death of an individual from apparent cardiac causes without symptoms or within 1 hour of symptom onset
- Careful gross and microscopic examination is essential to rule out identifiable causes of sudden cardiac death
- A negative autopsy without observable gross or microscopic findings should warrant additional testing, including genetic testing, especially if there is a history of sudden cardiac death in the family
ICD coding
- ICD-10:
- I46.1 - sudden cardiac death, so described
- Other cardiovascular disorders:
- I10-16 - hypertensive diseases
- I20-25 - ischemic heart diseases
- I33 - acute and subacute endocarditis
- I34 - nonrheumatic mitral valve disorders
- I35 - nonrheumatic aortic valve disorders
- I36 - nonrheumatic tricuspid valve disorders
- I37 - nonrheumatic pulmonary valve disorders
- I38 - endocarditis, valve not specified
- I40 - acute myocarditis
- I42 - cardiomyopathy
- I44 - atrioventricular and left bundle branch block
- I45 - other conduction disorders
- I5A - nonischemic myocardial injury (nontraumatic)
Etiology
- Most common causes of sudden cardiac death vary by age:
- Under the age of 35: most common etiologies include congenital anomalies, heritable disorders and idiopathic causes (Circulation 2016;133:1006)
- Over the age of 35: most common etiology is atherosclerotic cardiovascular disease and its sequela (Circ Res 2015;116:1887)
- Atherosclerotic and hypertensive cardiovascular disorders:
- Coronary artery atherosclerosis (most common cause over age 35):
- Approximately 75 - 90% of all sudden deaths due to natural disease among those 35 - 64 years of age
- Associated with hypertension and diabetes mellitus
- Accelerated atherosclerosis is associated with stimulant drug use (especially cocaine), particularly in males less than 35 years of age and premenopausal women (Atherosclerosis 2017;262:154)
- Accelerated atherosclerosis in younger persons may be associated with genetic disorders related to lipid and triglyceride metabolism (Cardiol Clin 2015;33:267)
- May have significant impact on primary family members
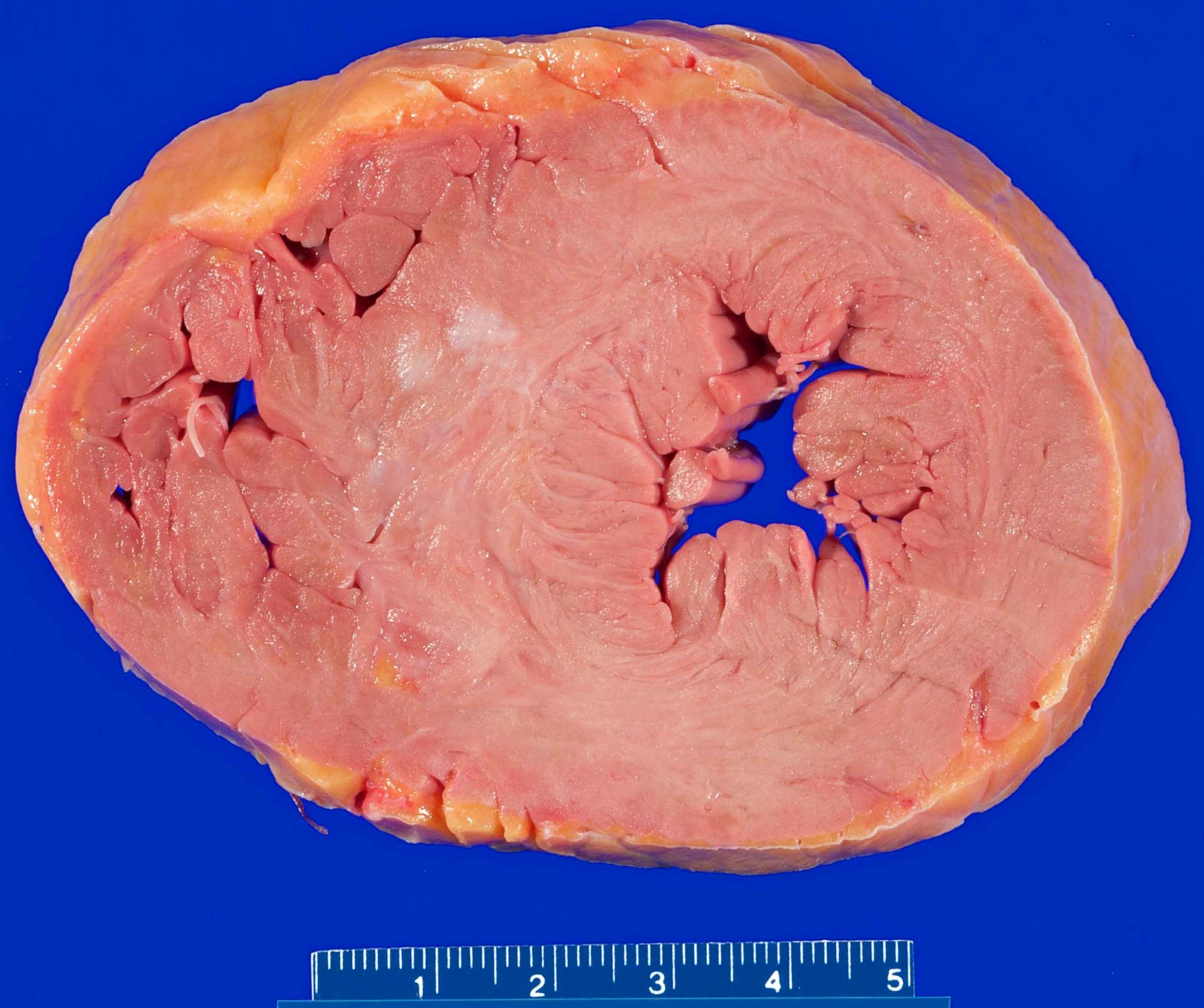
- Gross findings:
- Myocardium may demonstrate either ventricular dilation or hypertrophy in chronic disease
- Coronary luminal stenosis may be concentric or eccentric (it is unclear if either type has an increased risk of adverse events); a variety of grading systems are used and are up to practitioner preferences
- Some pathologists use numeric values to indicate degree of stenosis (i.e., 10%, 50%, etc.)
- Some pathologists use a qualitative scale (i.e., mild, moderate, severe, occlusive, etc.)
- Generally, the greater degree of luminal stenosis, the higher the risk of cardiac event
- Coronary calcifications should be noted as rigidity of the vascular wall may increase the risk of cardiac events even absent atherosclerosis (Int J Cardiol Heart Vasc 2016;14:41)
- Early infarcts may show no changes
- Nitroblue tetrazolium staining may be used to help accentuate early infarcts (Br J Exp Pathol 1978;59:254)
- Acute and subacute infarcts may demonstrate pale discoloration or hemorrhage
- Marked softening of the myocardium may be present in subacute infarcts
- Subacute infarcts (typically 3 - 7 days) may evolve into ventricular rupture with development of hemopericardium and cardiac tamponade
- Remote infarcts will be pale, fibrous and often with thinning of the myocardium
- Compensatory hypertrophy may be present in the viable myocardium
- Microscopic findings:
- Myocyte abnormalities cannot be detected by routine histologic staining for 4 - 6 hours after myocardial infarction
- People dying rapidly of myocardial infarctions may have no myocyte abnormalities (such as by ischemia induced dysrhythmia / arrhythmia)
- Early changes are nonspecific and may include cytoplasmic hypereosinophilia, myofiber irregularities and edema
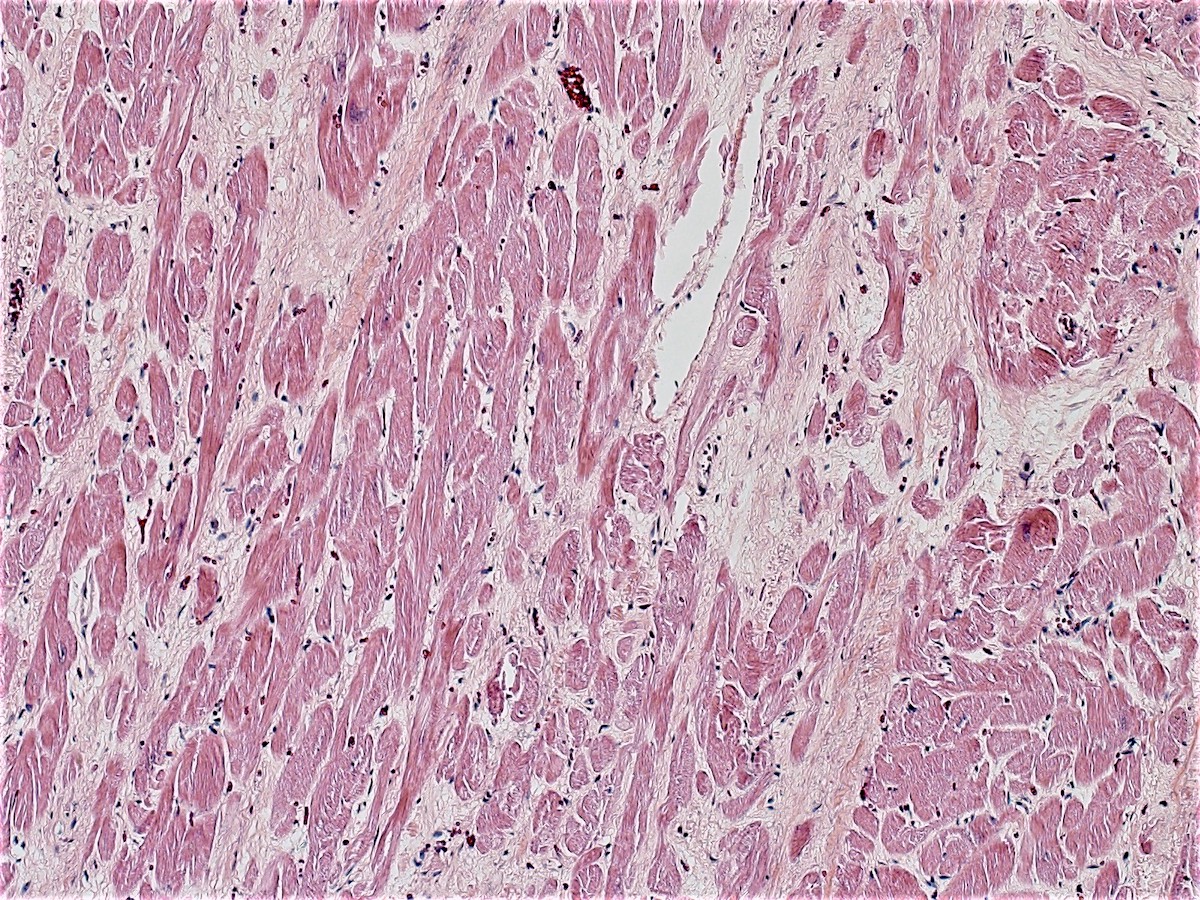
- Contraction band necrosis is evidence of irreversible ischemic change as the sarcomeric proteins begin to break down
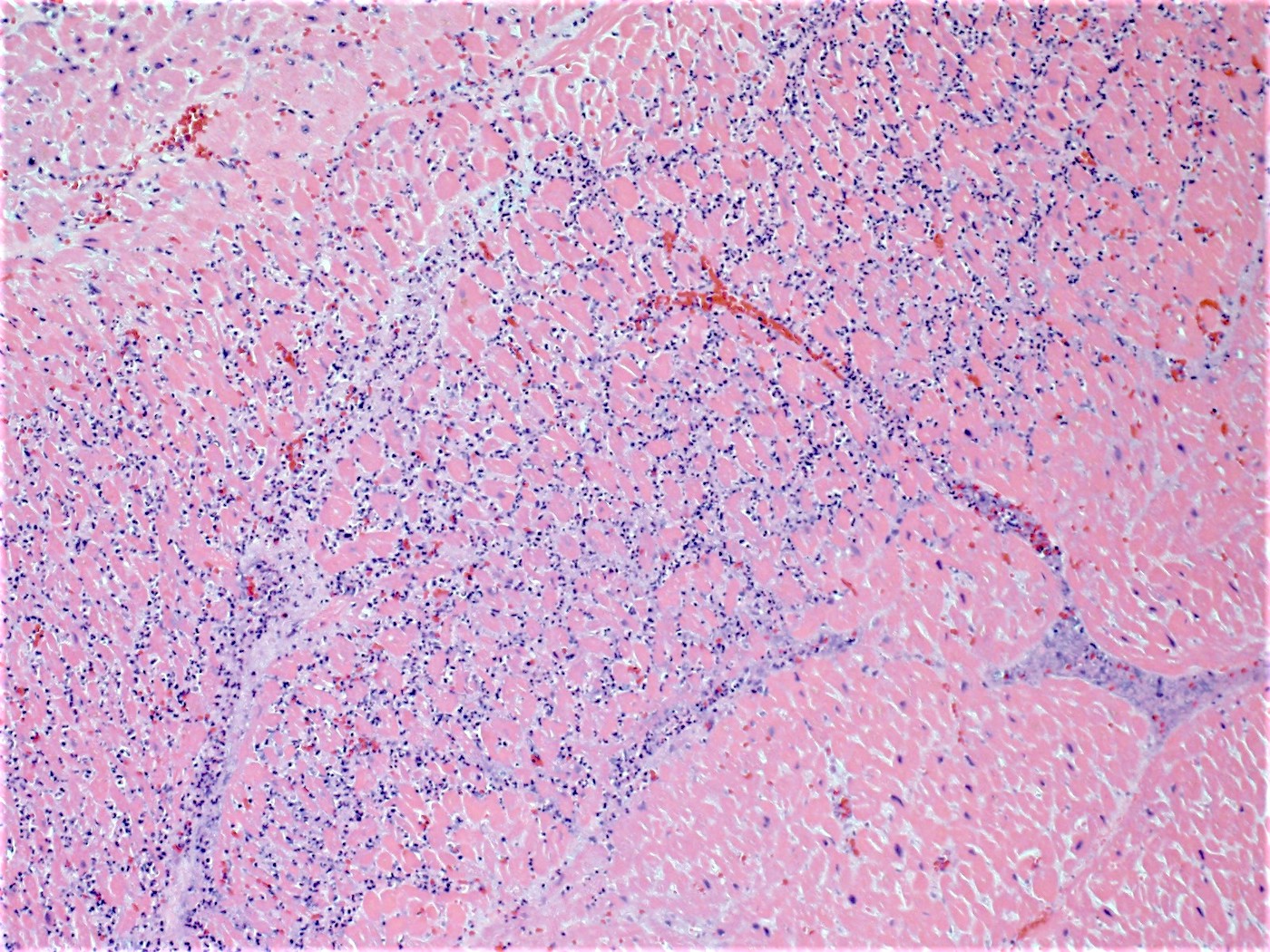
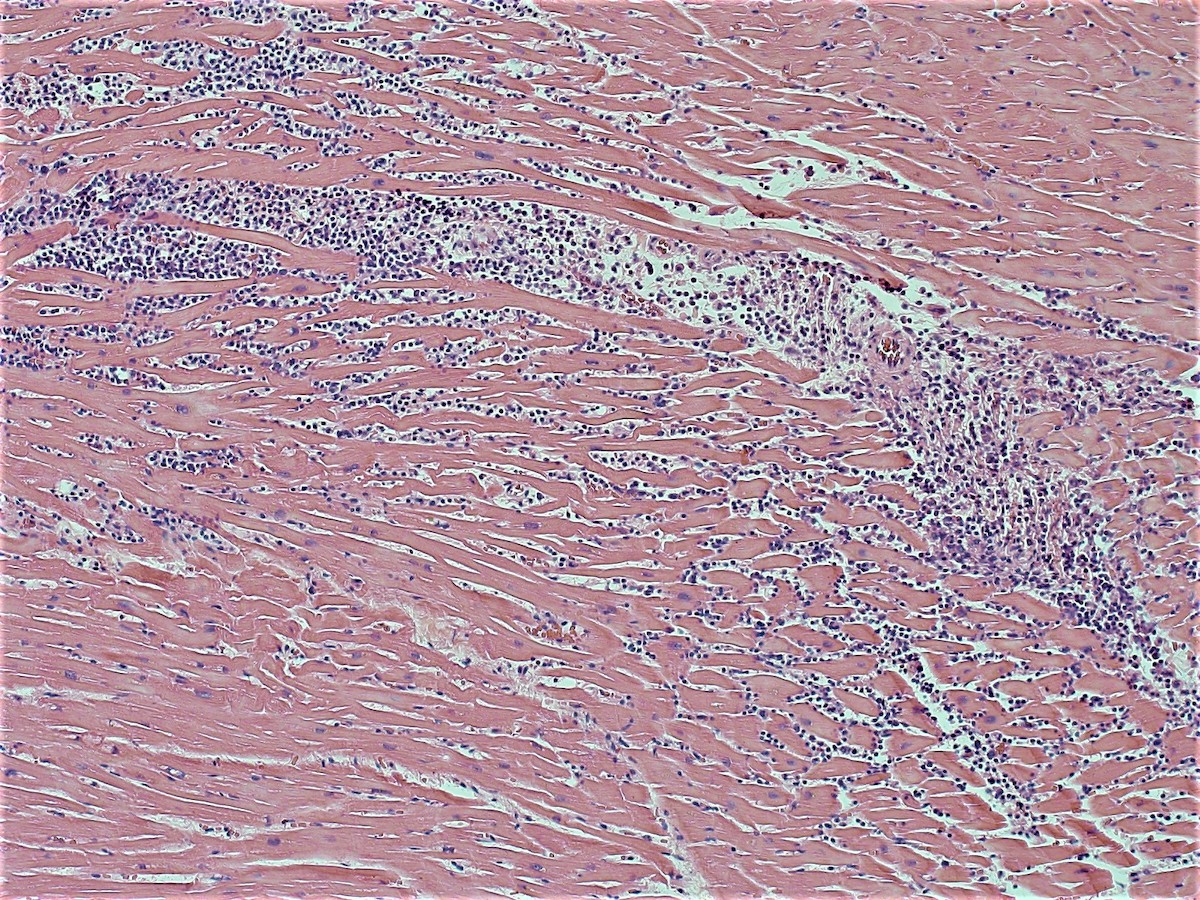
- Coagulative necrosis begins within 24 hours after the ischemic event associated with hemorrhage, neutrophilic infiltrates and continuing myocyte changes (include nuclear pyknosis and dropout)
- Subacute infarcts evolve with macrophage infiltration and necrosis; fibroblasts will also begin infiltrating
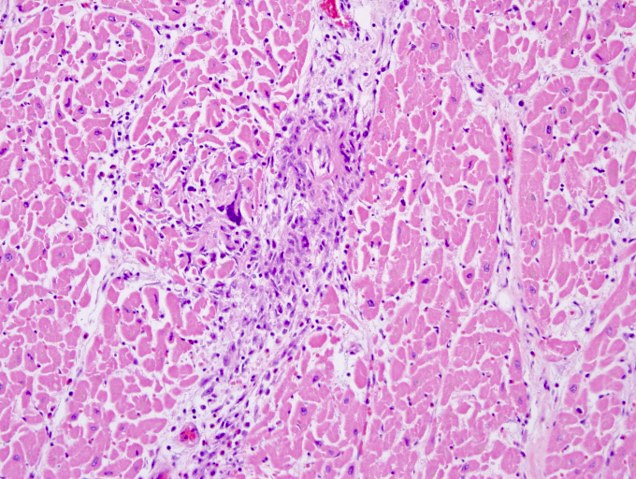
- Remote infarcts / chronic ischemic change associated with cardiac myocyte drop out and resultant interstitial fibrosis and fibroblast proliferation (varying depending on length of time and chronicity of ischemia)
- May also see hemosiderin laden macrophages
- Cross sections of involved coronary arteries should be taken to determine if a clot is postmortem or antemortem
- Ruptured fibrous capsule or hemorrhage within the wall of a vessel indicate acute plaque rupture
- Myocyte abnormalities cannot be detected by routine histologic staining for 4 - 6 hours after myocardial infarction
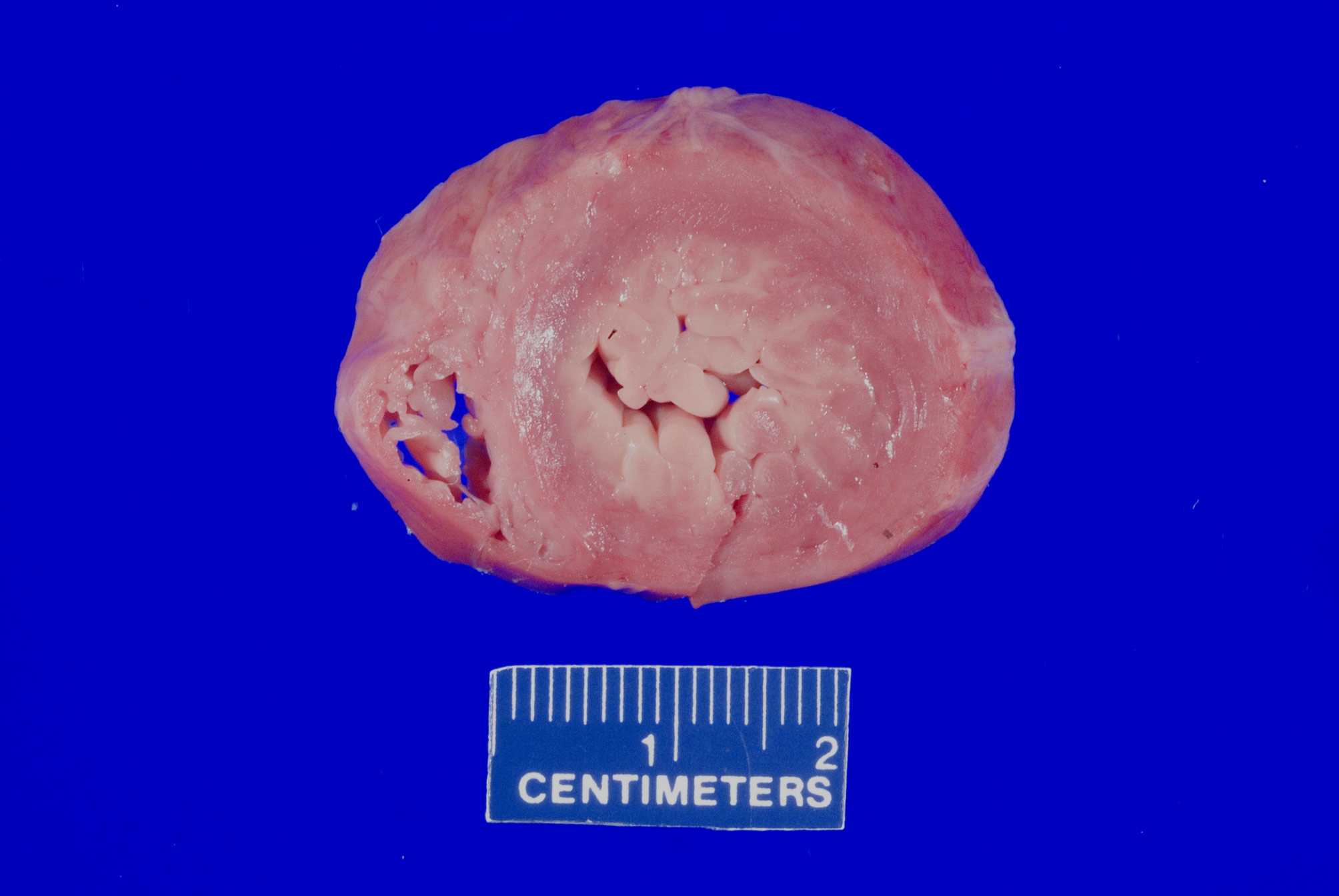
- Hypertensive cardiomyopathy:
- Can present as a chronic disease process (heart failure) or sudden cardiac death (via dysrhythmia / arrhythmia)
- Gross findings:
- Most commonly concentric (less commonly eccentric) thickening of the left ventricular wall without outflow tract obstruction
- End stage hypertensive damage may result in dilation and thinning of the ventricular walls
- Microscopic findings:
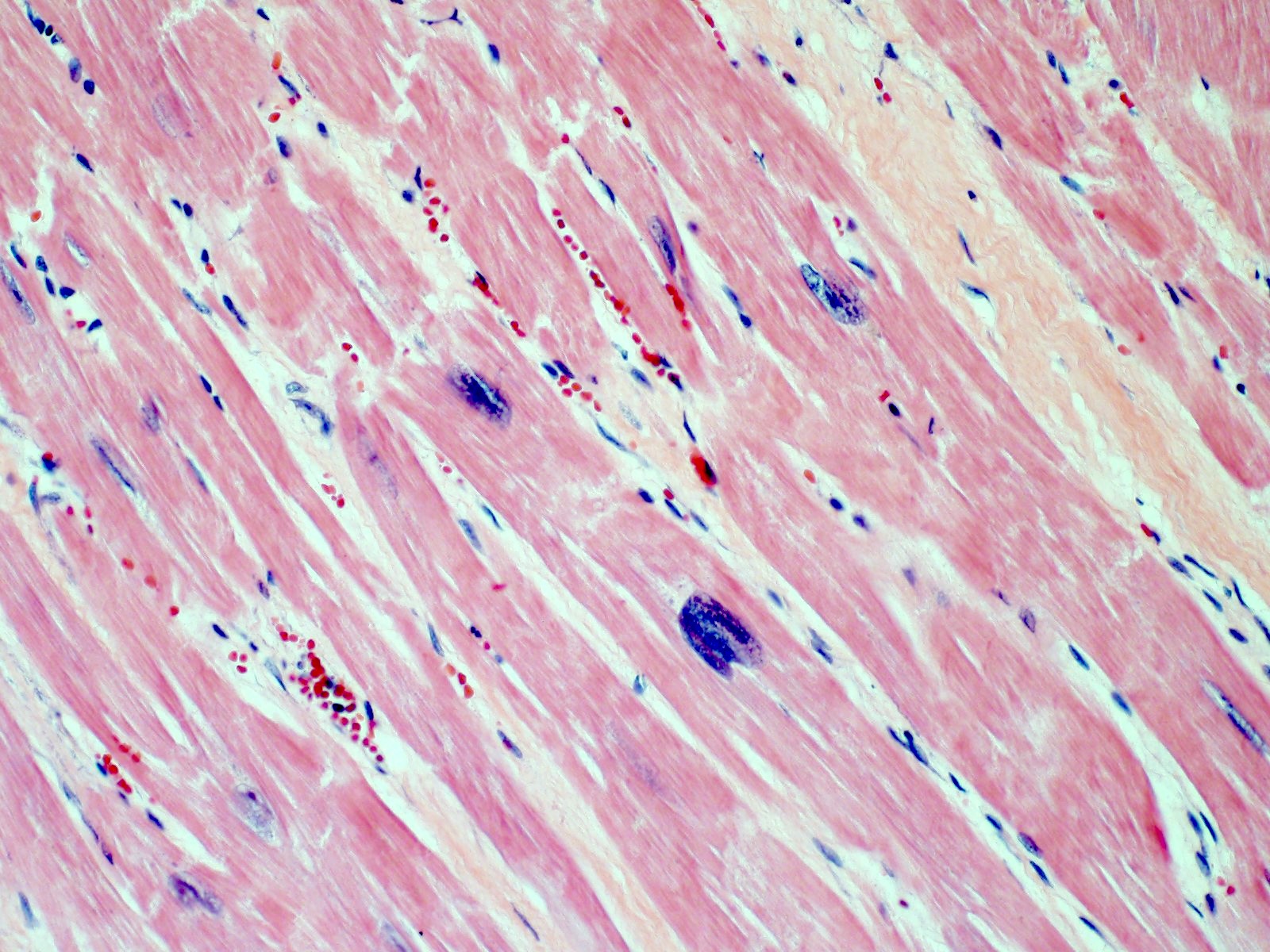
- Enlarged nuclei with squaring of the nuclear edges (aka boxcar nuclei); enlarged myofiber diameter (Am Heart J 2009;158:799)
- Coronary artery atherosclerosis (most common cause over age 35):
- Aortic abnormalities:
- Aortic aneurysm and dissection:
- Risk factors include hypertension, hyperlipidemia, tobacco smoking and male gender
- Unruptured aneurysm can lead to vascular compromise in downstream body areas or compression of adjacent structures
- Ruptured aneurysm can lead to rapid exsanguination
- Congenital aortic stricture (coarctation) and post valvular stenosis:
- Can lead to hypertensive complications upstream of the site of narrowing and hypotensive complications downstream from the site of narrowing
- May be associated with other vascular or congenital abnormalities
- Infective complications of the aorta:
- Syphilis is the most commonly associated agent; rare in the United States due to the availability of treatments
- Aortic aneurysm and dissection:
- Coronary artery abnormalities:
- Anomalous coronary arteries
- Coronary arterial arteritis and aneurysm
- Fibromuscular dysplasia
- Other nonischemic coronary artery disorder
- Myocardial bridging (intramural coronary artery) (J Invasive Cardiol 2015;27:521)
- Controversial cause of sudden cardiac death
- Proposed mechanism is that myocardial contraction causes constriction of the intraluminal segment, resulting in reversible ischemia
- Cardiomyopathies:
- Myocardial dysfunction due to primary myocardial disease or secondary to systemic disease or treatment of systemic disease (such as cardiotoxic chemotherapeutic agents)
- Primary or secondary dilated cardiomyopathy
- Hypertrophic (and obstructive) cardiomyopathy
- Restrictive cardiomyopathy
- Arrhythmogenic right ventricular cardiomyopathy / dysplasia
- Takotsubo cardiomyopathy
- Inflammatory conditions of the endocardium, myocardium and pericardium:
- Infective endocarditis
- Noninfective endocarditis
- Infective myocarditis
- Noninfective myocarditis
- Noninfective pericarditis
- Noninfectious disorders of the heart valves:
- Primary disorders may be due to congenital abnormalities or due to cardiac flow abnormalities
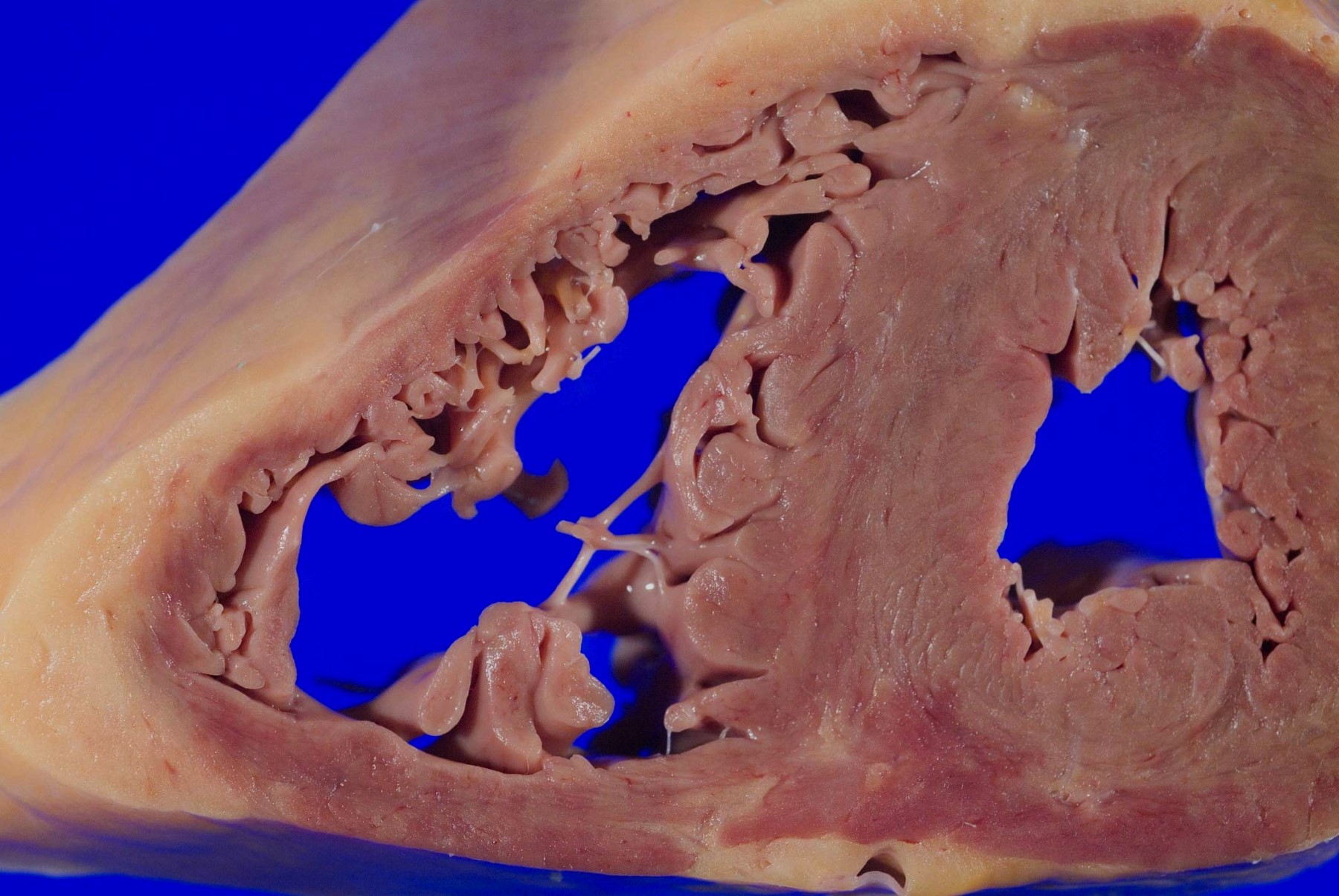
- Mitral valve prolapse (Echocardiography 2019;36:1549)
- Prevalence of 2 - 3% in general population (Circulation 2019;140:952)
- May be associated with Marfan syndrome or other genetic connective tissue disorders
- Gross findings: enlarged, ballooning of the mitral valve leaflets
- Microscopy: myxoid degeneration of the central spongiosa of the valve leaflet (due to buildup of acid mucopolysaccharides)
- Can be seen in cases of sudden death, though controversial if it is causative or coincidental
- Bicuspid aortic valve and other congenital valvular abnormalities (Development 2020;147:dev183020)
- Predisposed to accelerated valvular degeneration due to flow abnormalities around the affected valves
- Degenerative valve disease (including valvular stenosis and regurgitation)
- Physiologic abnormalities of valve function, often associated with valvular sclerosis and changes to the papillary musculature and chordae tendineae
- Degree of functional impairment should be based on antemortem studies
- Morphologic changes do not always accurately depict the degree of physiologic impairment due to compensatory mechanisms during life
- See related topic (Degenerative valve disease)
- Mitral valve prolapse (Echocardiography 2019;36:1549)
- Secondary valvular disorders are most commonly associated with inflammatory and postinfective causes
- Rheumatic heart disease
- Immune complex mediated damage to the cardiac valves after Group A streptococcal infection (most commonly mitral) (Clin Microbiol Rev 2014;27:264)
- Generally considered rare due to the use of antibiotics to treat streptococcal infection
- More commonly seen in elderly patients
- Connective tissue disorders
- Drug induced valvular injury
- Prosthetic heart valves
- Rheumatic heart disease
- Primary disorders may be due to congenital abnormalities or due to cardiac flow abnormalities
- Congenital heart disease:
- Primary cardiovascular disorders are the most common congenital disorders, affecting ~0.08% of live births (Circ Res 2017;120:908)
- Complications of congenital heart defects vary widely from asymptomatic to severe and life threatening
- Asymptomatic, undiagnosed cardiac defects can progress to life threatening disease if not treated
- All disorders arise from abnormal embryogenesis and development
- See related topic (Heart - embryology)
- Disorders can include atrial septal defects, ventricular septal defects, outflow tract abnormalities and abnormalities of the great vessels
- See related topics (Tetralogy of Fallot and VACTERL association)
- Neoplastic lesions:
- Primary and secondary cardiac neoplasms are exceedingly rare entities (Curr Treat Options Oncol 2019;20:66)
- Death can occur due to a variety of mechanisms including cardiac flow obstruction, heart failure and conduction abnormalities resulting in arrhythmia
- See related topics under benign and malignant tumor headings (Heart)
- Primary and secondary cardiac neoplasms are exceedingly rare entities (Curr Treat Options Oncol 2019;20:66)
- Disorders of the conduction system:
- Conduction system is comprised of specialized fibers allowing for the rapid passage of electrical signals through the heart in a synchronized manner
- Primary or secondary abnormalities of these specialized fibers may result in asynchronous transmission and the development of dysrhythmias and arrhythmia (Circulation 2011;123:904)
- Nearly all disorders of conduction will have no gross anatomic findings at the time of autopsy unless due to prior injury or secondary to other disease process
- Functional (primary) disturbances include channelopathies (such as Long QT syndrome), pre-excitation syndromes, sick sinus syndrome, vagal inhibition and spontaneous idiopathic dysrhythmias associated with excitation (either physical or emotional excitation)
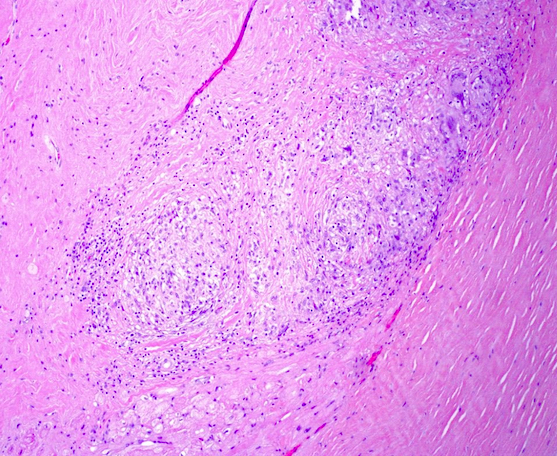
- Structural disturbance (primary or secondary) include Lev’s disease, collagen vascular disorders with cardiac involvement and infiltrative involvement of systemic disease (hemochromatosis, amyloidosis, etc.)
- Microscopy may reveal no abnormalities, nonspecific fibrosis or discrete lesions
- If conduction studies are indicated at the time of autopsy, specialized dissection methods should be undertaken and planned for prior to examination (Anat Sci Educ 2009;2:78)
- Careful and extensive sampling may be indicated
- In cases of negative autopsy, testing for genetic mutations which can lead to cardiac dysrhythmias may be indicated (Trends Cardiovasc Med 2017;27:207)
- Testing availability and appropriate samples vary by laboratory; confirm with your preferred testing provider prior to examination
- Medical records will be useful in determining if prior events occurred, what their triggers may be and what work up was conducted (especially for individuals with vagal or excitation type events)
- Conduction system is comprised of specialized fibers allowing for the rapid passage of electrical signals through the heart in a synchronized manner
Case reports
- 21 year old man with sudden cardiac death due to left ventricular noncompaction, arrhythmogenic right ventricular cardiomyopathy and acute myocardial infarction (Egypt Heart J 2017;69:157)
- 24 year old man with sudden cardiac death due to acute myocardial infarction in the setting of anabolic steroid abuse (Forensic Sci Res 2019;4:267)
- 35 year old man with sudden cardiac death due to tuberculosis (Breathe (Sheff) 2015;11:67)
- 37 year old man with sudden cardiac death due to stellate ganglionitis (Auton Neurosci 2021;234:102837)
- 58 year old man with sudden cardiac death due to cardiac involvement by lymphoma (J Formos Med Assoc 2018;117:939)
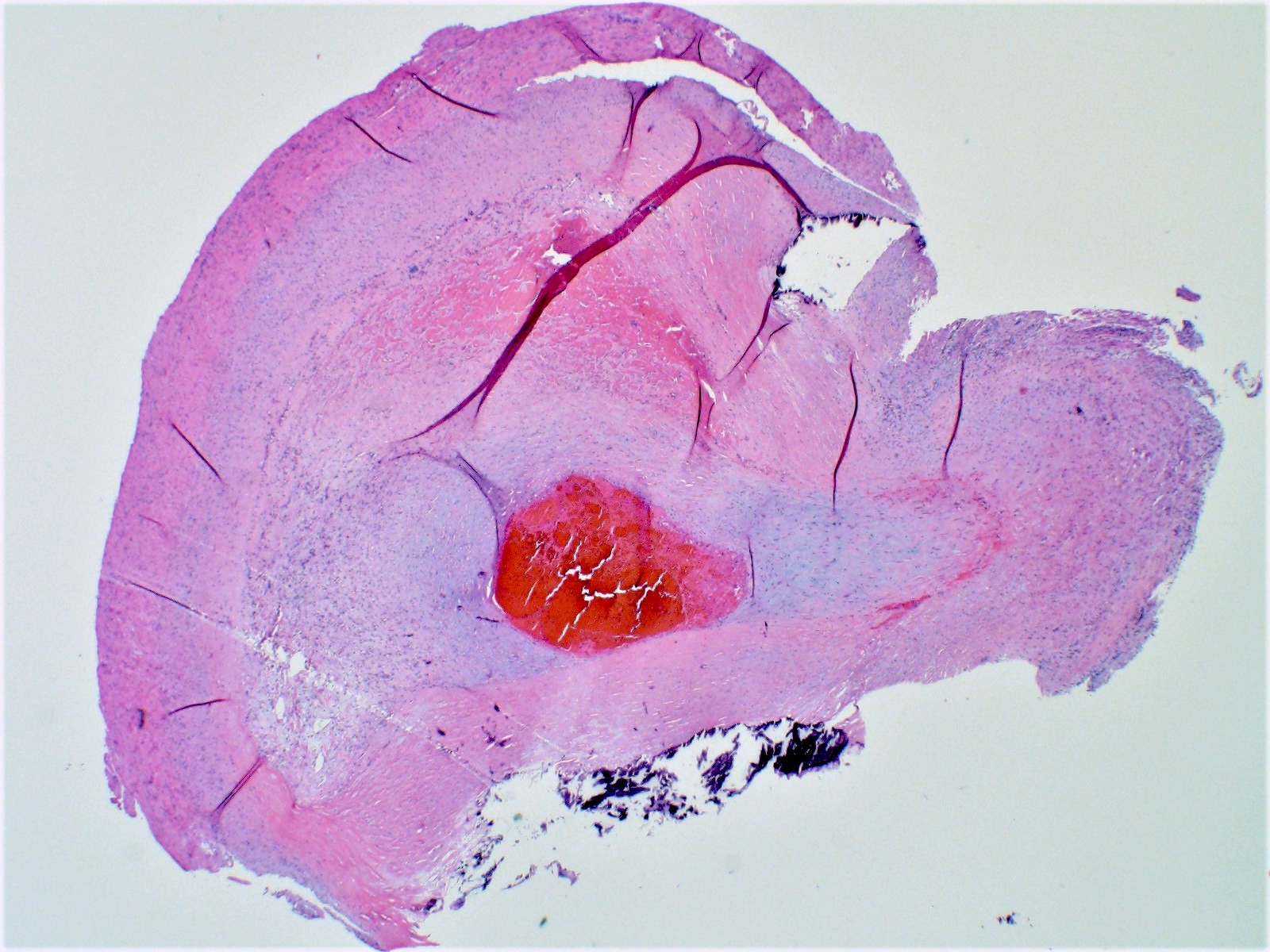
Gross examination
- Cardiac examination should include weight with excess valvular tissue removed
- Note any external features, including general size and shape of the heart, areas of discoloration or defects
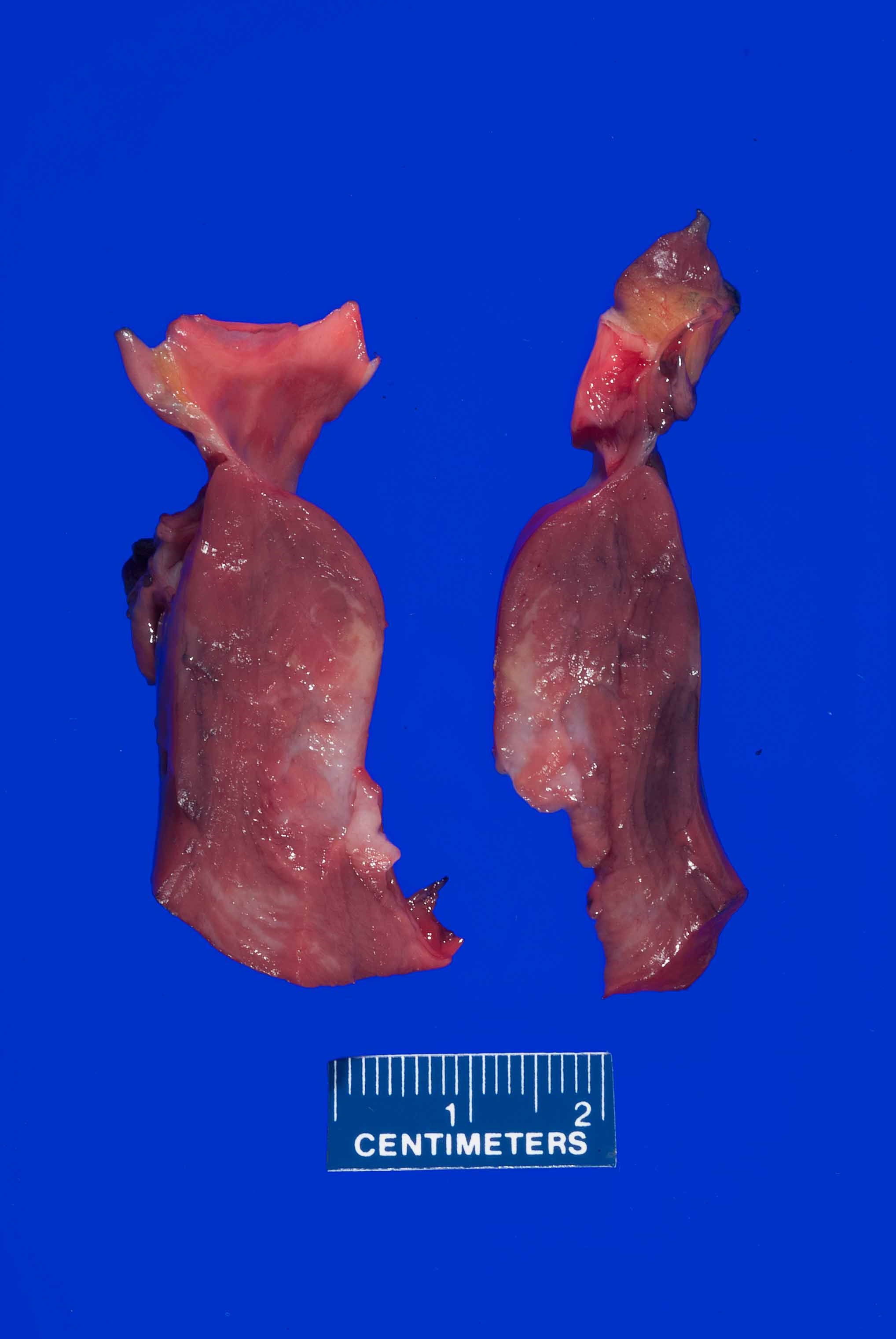
- Coronary arteries and their major branches should be identified and sectioned at 3 mm intervals along their course (Virchows Arch 2017;471:691)
- Note any stenosis, calcification, perivascular hemorrhage, thrombus or dissection
- Note areas of myocardial bridging including length of the involved segment
- If coronary artery bypass grafts are present, note the origins, anastomotic site, course and patency
- Serially section the ventricles in a plane parallel to the base of the heart, from apex to base at 1 cm intervals until approximately 2 cm from the atrioventricular valves (Cardiovasc Pathol 2012;21:2)
- Measure the thicknesses of the left ventricular free wall, interventricular septum and right ventricular free wall, excluding the papillary muscles
- Note areas of discoloration, fibrosis or areas of thinning
- Note the distribution and pattern of epicardial fat, especially in the right ventricle
- Note the thickness and areas of discoloration of the epicardium
- Cardiac valves should be inspected for abnormalities prior to sectioning; note areas of obstruction, stenosis or other abnormalities
- Open the heart by following the direction of blood flow
- Posterior incision of the right atrium to right ventricle through the tricuspid valve
- Anterior incision of the right ventricle to the pulmonary artery through the pulmonic valve
- Posterior incision of the left atrium to the left ventricle through the mitral valve
- Anterior incision of the left ventricle to the aorta through the aortic valve
- Measure the circumference of the cardiac valves
- Note any areas of thickening, vegetations, chordae tendineae and papillary muscles
- If prosthetic valves are present note the type of valve, if it is appropriately seated / attached and any abnormalities of the valve or artificial surfaces
- Section the remaining myocardium to include the conduction system
- Microscopic sections should be taken of areas of interest
- The conduction system, including the SA and AV nodes, should be sampled in otherwise negative autopsies
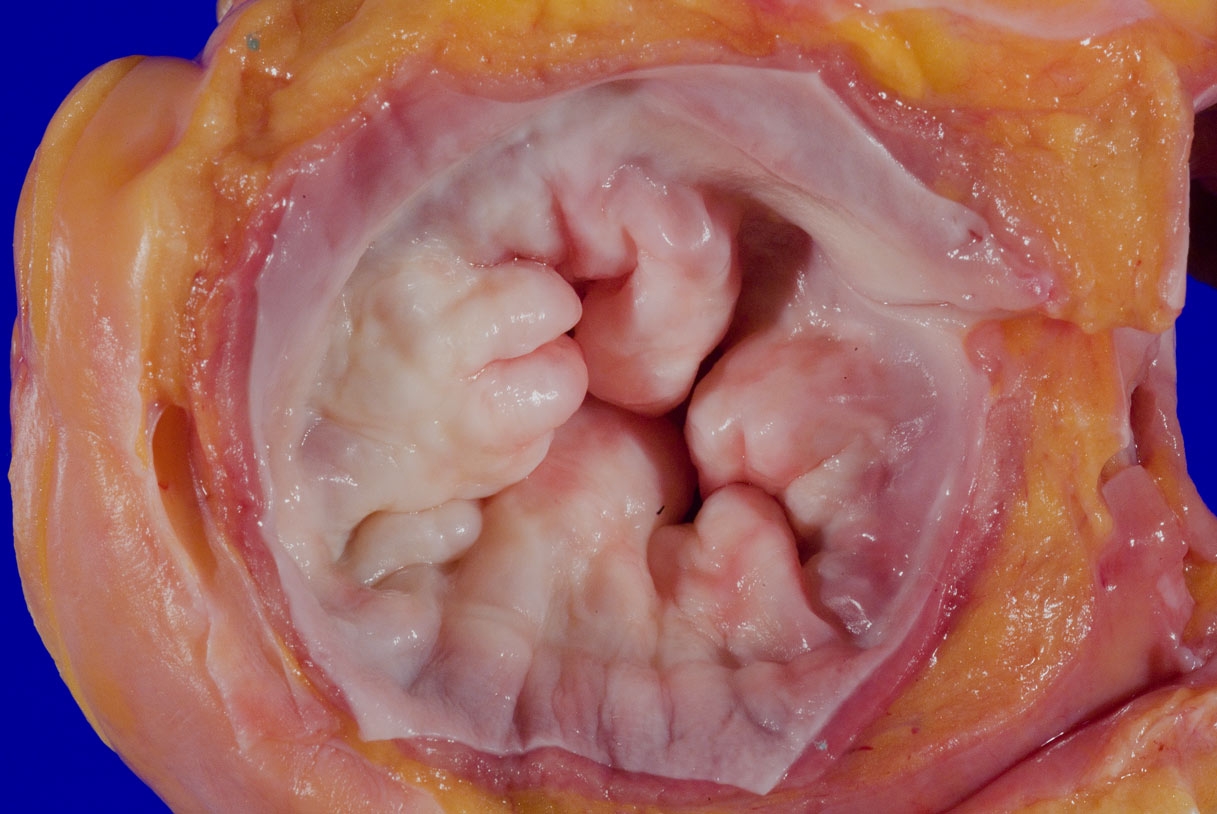
Gross images
Microscopic (histologic) images
Additional references
- Connolly: Autopsy Pathology: A Manual and Atlas, 3rd Edition, 2015, DiMaio: Forensic Pathology, 2nd Edition, 2001, Dolinak: Forensic Pathology - Principles and Practice, 1st Edition, 2005, Miller: Diagnostic Pathology - Cardiovascular, 2nd Edition, 2018, Spitz: Spitz and Fisher's Medicolegal Investigation of Death - Guidelines for the Application of Pathology to Crime Investigation, 4th Edition, 2005
Board review style question #1
A 75 year old woman is consented for a hospital autopsy. Her past medical history is significant for hypertension, hyperlipidemia and congestive heart failure. At autopsy, the findings shown above are identified in the mitral valve. Remote infection with which organism is most likely to have precipitated these findings?
- Coxsackie virus A and B
- Enterococci
- Group A streptococci
- Staphylococcus aureus
- Trypanosoma cruzi
Board review style answer #1
C. Group A streptococci. The findings are consistent with chronic rheumatic heart disease due to a remote, untreated Group A streptococcus infection such as streptococcal pharyngitis or scarlet fever. Coxsackie A and B virus are common causative organisms identified in lymphocytic myocarditis but are not known to cause significant valvular disease. Enterococci can cause infective endocarditis but are less common that streptococcal or staphylococcal infections. Staphylococcus aureus is more common in individuals with intravenous drug use, chronic underlying conditions or prosthetic heart valves. Trypanosoma cruzi is a cause of parasitic myocarditis with intracellular forms and pseudocyst formation.
Comment Here
Reference: Sudden cardiac death
Comment Here
Reference: Sudden cardiac death
Board review style question #2
A 55 year old man presents to the emergency department unresponsive after a sudden collapse. His past medical history was significant for gastroesophageal reflux disease (GERD), hypertension and hyperlipidemia. Bedside echocardiogram in the ED revealed a large pericardial effusion determined to be a large hemopericardium. At autopsy he was found to have a ruptured left ventricular free wall with severe atherosclerotic stenosis of the involved left circumflex coronary artery. What is the most common time frame for ventricular wall rupture after a myocardial infarction?
- 1 day
- 3 - 7 days
- 1 weeks
- 3 - 7 weeks
- 1 year
Board review style answer #2
B. 3 - 7 days. The timing of ventricular walls rupture after myocardial infarction varies greatly from 1 day up to 3 weeks after infarction. The most common timeframe for this to occur is within 3 - 7 days after the ischemic event.
Comment Here
Reference: Sudden cardiac death
Comment Here
Reference: Sudden cardiac death