Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Bhaijee F, Akhtar I. Gastrointestinal stromal tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/esophagusGIST.html. Accessed March 30th, 2025.
Definition / general
- Most common mesenchymal tumor of the gastrointestinal tract (Transl Gastroenterol Hepatol 2018;3:6, World J Gastroenterol 2015;21:5630)
- Comprises 0.1 - 3.0% of all gastrointestinal tumors
Essential features
- Rare in esophagus < 1% (0.7%); most common site is stomach (Mod Pathol 2022;35:554)
- Most are due to mutations in proto-oncogene KIT (exon 11)
- 3 histologic types: spindle, epithelioid and mixed
- Diagnosis confirmed immunohistochemically using KIT and DOG1 antibodies
- Prognosis: depends on tumor size, mitotic rate and site of origin
- Treatment: surgical excision or imatinib
Terminology
- Gastrointestinal stromal tumor (GIST)
- Size range is 0.1 - 12.0 cm (median: 2.5 cm), majority are ≤ 5 cm (Mod Pathol 2022;35:554)
- Microscopic GIST: sporadic interstitial cell of Cajal hyperplasia, seedling GIST, minimal GIST
- Range in size from 4 - 10 mm in diameter and immunohistochemically positive for KIT (Am J Pathol 2002;160:1567)
- Historic terms:
- Gastrointestinal smooth muscle tumor
- Gastrointestinal autonomic nerve tumor
- Leiomyoblastoma
- Smooth muscle tumor of uncertain malignant potential
- Gastrointestinal pacemaker cell tumor
ICD coding
- ICD-10: C49.A1 - GIST, esophagus
Epidemiology
- Very rare in esophagus: < 1% of all GIST arise in esophagus (0.7%); they most frequently arise in stomach (60.3%), followed by small intestine (33.2%), rectum (3.1%) and colon (2.9%) (Mod Pathol 2022;35:554)
- Annual incidence 0.1 - 0.3 per million
- More common in men (M:F = 1.7:1)
- Average age: < 60 years (median: 67)
- 25% incidental, asymptomatic tumors at diagnosis; may be incidental finding in patients undergoing resection of upper GI neoplasms (Am J Cancer Res 2014;5:333, Can J Surg 2012;55:366, Dtsch Med Wochenschr 2005;130:2380)
- GISTs comprise < 25% of mesenchymal esophageal tumors, leiomyomas being the most common (Am J Surg Pathol 2000;24:211)
Sites
- Most common location is lower esophagus, followed by middle esophagus; rare in upper esophagus (Am J Cancer Res 2014;5:333, Medicine (Baltimore) 2016;95:e2446)
Pathophysiology
- GISTs arise from interstitial cells of Cajal (ICC)
- Driver event involves gain of function mutations in KIT and PDGFRA most frequently
- Most of these tumors respond to the tyrosine kinase inhibitor, imatinib
- Less frequently, a BRAF V600E mutant is involved as the driving force and these cases are less responsive to imatinib
- Etv1, a major transcriptional regulator of ICC - intramuscular and ICC - myenteric, induces BRAF expression in a mouse model
- Smooth muscle cells have recently been proposed as an alternative cell of origin for GIST, and these tumors are BRAF V600E driven and resistant to imatinib (J Pathol 2020;252:441)
Etiology
- Mostly sporadic
- 5% of GISTs arise in patients with:
- Neurofibromatosis type I syndrome (NF1, multiple small intestinal tumors)
- Carney triad (gastric epithelioid GISTs in young females) (Arch Pathol Lab Med 2006;130:1466)
- Familial GIST syndrome: rare, autosomal dominant genetic disorder, characterized by germline KIT or PDGFRA mutations, early onset of multiple GIST tumors, skin pigmentation abnormalities (Cancer 2015;121:2960, Arch Pathol Lab Med 2006;130:1466)
Clinical features
- Dysphagia, most common (51%), weight loss (20%), bleeding (10%) and chest pain (Diagn Interv Radiol 2010;16:217)
- Dysphagia and odynophagia (30%), gastroesophageal reflux (8%) and epigastric pain (4%) (Mod Pathol 2022;35:554)
- Esophageal mass, stricture, perforation
- Rare metastatic spread to lung, liver, bone and peritoneum (Am J Surg Pathol 2000;24:211)
Diagnosis
- May be an incidental finding during imaging or endoscopic examination for other clinical symptoms
- Endoscopy shows as a subepithelial lesion; both leiomyoma and GIST appear similar on CT and EUS (J Thorac Dis 2015;7:E648)
- Biopsy with immunohistochemical stains / molecular required for confirmation
Radiology description
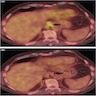
- Barium studies: smooth intraluminal mass or large ulcerative mass extending intraluminally (see radiology image 1)
- Endoscopic ultrasound determines size, shape and intratumoral characteristics of the lesion, its relationship to layers of the bowel wall and enables image guided core needle biopsy or fine needle aspiration (J Gastrointestin Liver Dis 2008;17:131) (see clinical image 1)
- CT / MRI: useful for preoperative and metastatic evaluation
- Well circumscribed, hypoattenuating submucosal mass (Cancer Imaging 2012;12:100) (see radiology image 2)
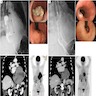
- PET FDG avidity correlates with malignant potential of GIST
- Also used to monitor response to chemotherapy (tyrosine kinase inhibitor therapy) and evaluate postoperative recurrence (see radiology image 3)
Radiology images
Prognostic factors
- Nashville Risk Score is utilized in predicting progression free survival of patients with GIST (Histopathology 2022;80:874, Mod Pathol 2022;35:554)
- Risk stratification based on tumor size, mitotic activity and site of origin per 50 high power fields (HPF or 5 mm²)
- Low risk:
- < 5 cm and < 5 mitoses/50 HPF or 5 mm²
- Intermediate risk:
- < 5 cm and 6 - 10/50 HPF
- 5 - 10 cm and < 5 mitoses/50 HPF or 5 mm²
- High risk:
- > 5 cm and mitoses > 5/5 mm²
- > 10 cm and any mitotic rate
- Any size and > 10/50 HPF or 5 mm²
- Presence of rupture at the time of surgery and male sex independent risk factor
- Low risk:
| Risk stratification of esophageal GISTs using the new Nashville risk score | ||
| Risk category | Tumor size | Mitotic rate/50 HPF or 5 mm² |
| Low | < 5 cm | < 5 |
| Intermediate | < 5 cm | 6 - 10 |
| Intermediate | 5 - 10 cm | < 5 |
| High | > 5 cm | > 5 |
| High | > 10 cm | Any mitotic rate |
| High | Any size | > 10 |
Case reports
- 39 year old man with esophageal perforation (Int J Surg Case Rep 2013;4:636)
- 51 year old woman with bulky tumor in posterior mediastinum (Chirurgia (Bucur) 2015;110:300)
- 53 year old man with pulmonary and bone metastases (Diagn Interv Radiol 2010;16:217)
- 57 year old man with germline KIT mutation (Arch Pathol Lab Med 2007;131:1393)
- 86 year old man with 3 month history of dysphagia presented with a huge gastrointestinal stromal tumor (Surg Case Rep 2022;8:109)
Treatment
- Surgical resection for localized GIST (3 - 5 cm) (Surg Case Rep 2022;8:109)
- Neoadjuvant therapy, small molecule tyrosine kinase inhibitors: imatinib mesylate (STI571, Gleevec), sunitinib maleate may be given preoperatively to reduce the size of tumor
- Imatinib is considered in patients with high mitotic rates or larger tumor sizes to obtain negative microscopic margins (R0 resection) and to reduce the risk of intraoperative complications, including tumor rupture (Histopathology 2017;71:805) (see radiology image 4)
Gross description
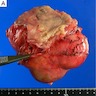
- Lobulated, well circumscribed mass with homogenous fleshy cut surfaces (see gross image 1)
- Exophytic or intramural, centered within the muscularis propria
- Cystic degeneration, hemorrhage, necrosis (more with high grade)
- Overlying esophageal mucosa may be ulcerated (see gross image 2)
- References: Transl Gastroenterol Hepatol 2018;3:6, Int J Surg Case Rep 2013;4:636
Frozen section description
- Spindle cell neoplasm; defer to permanent for definite categorization
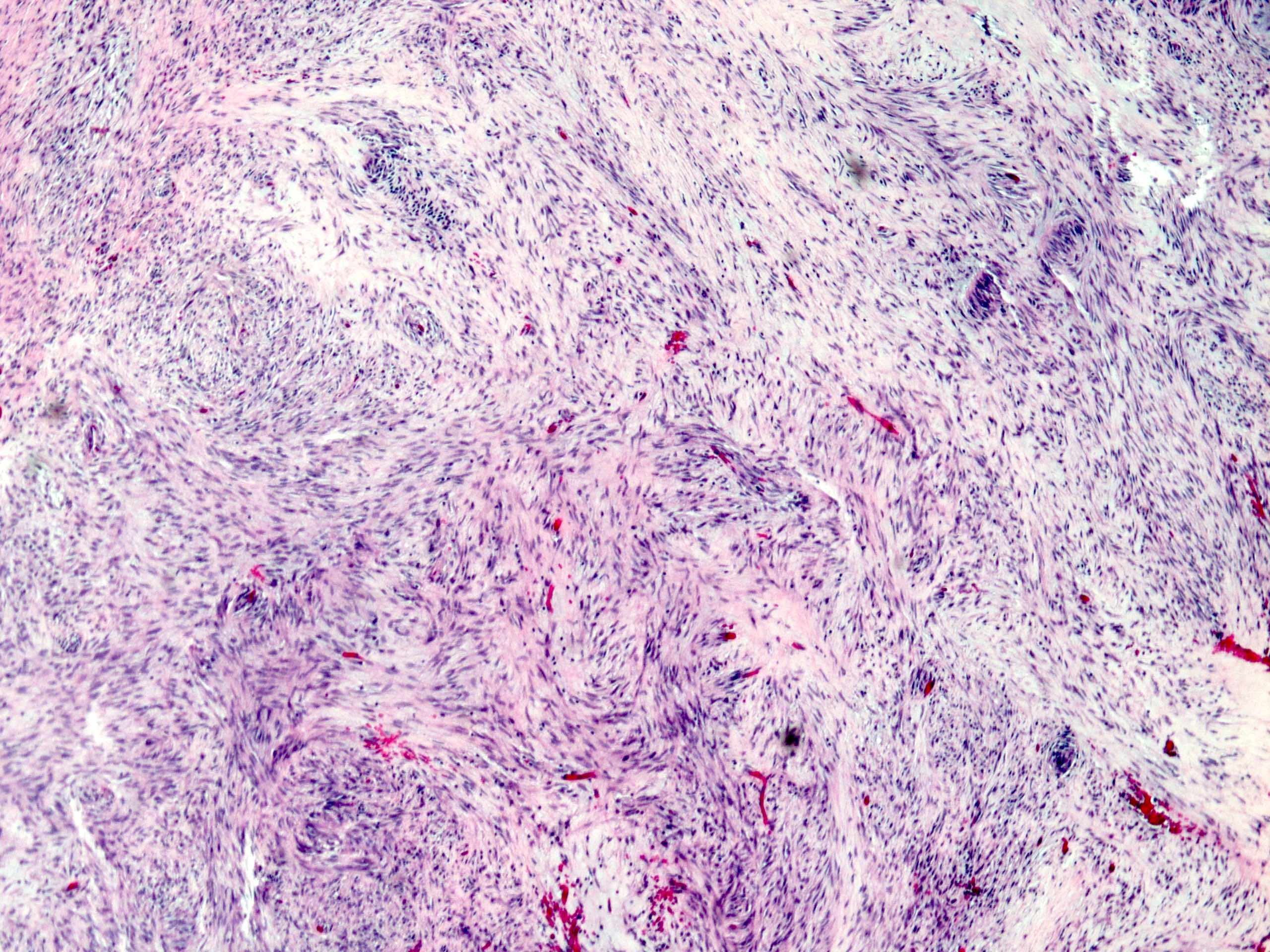
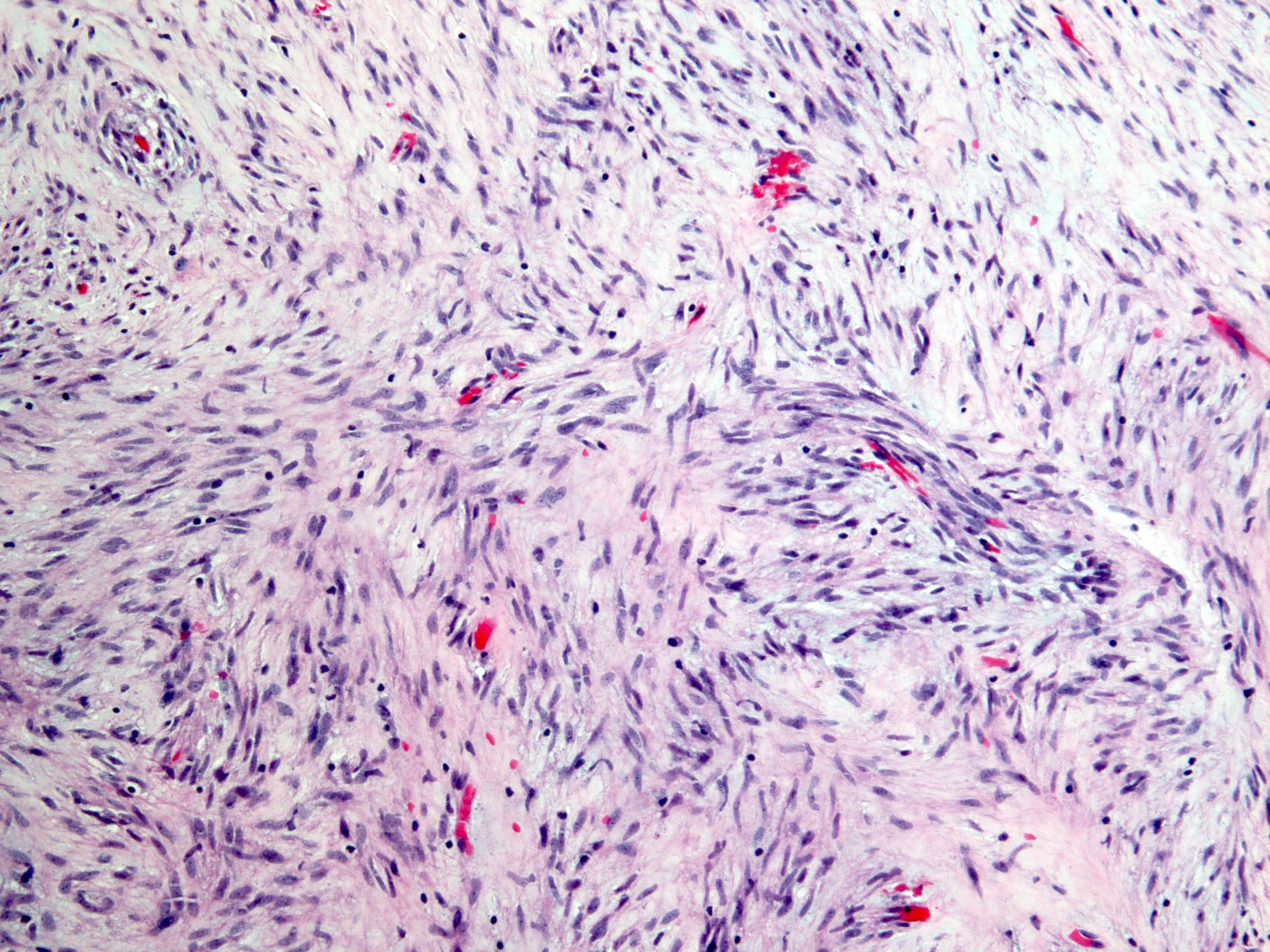
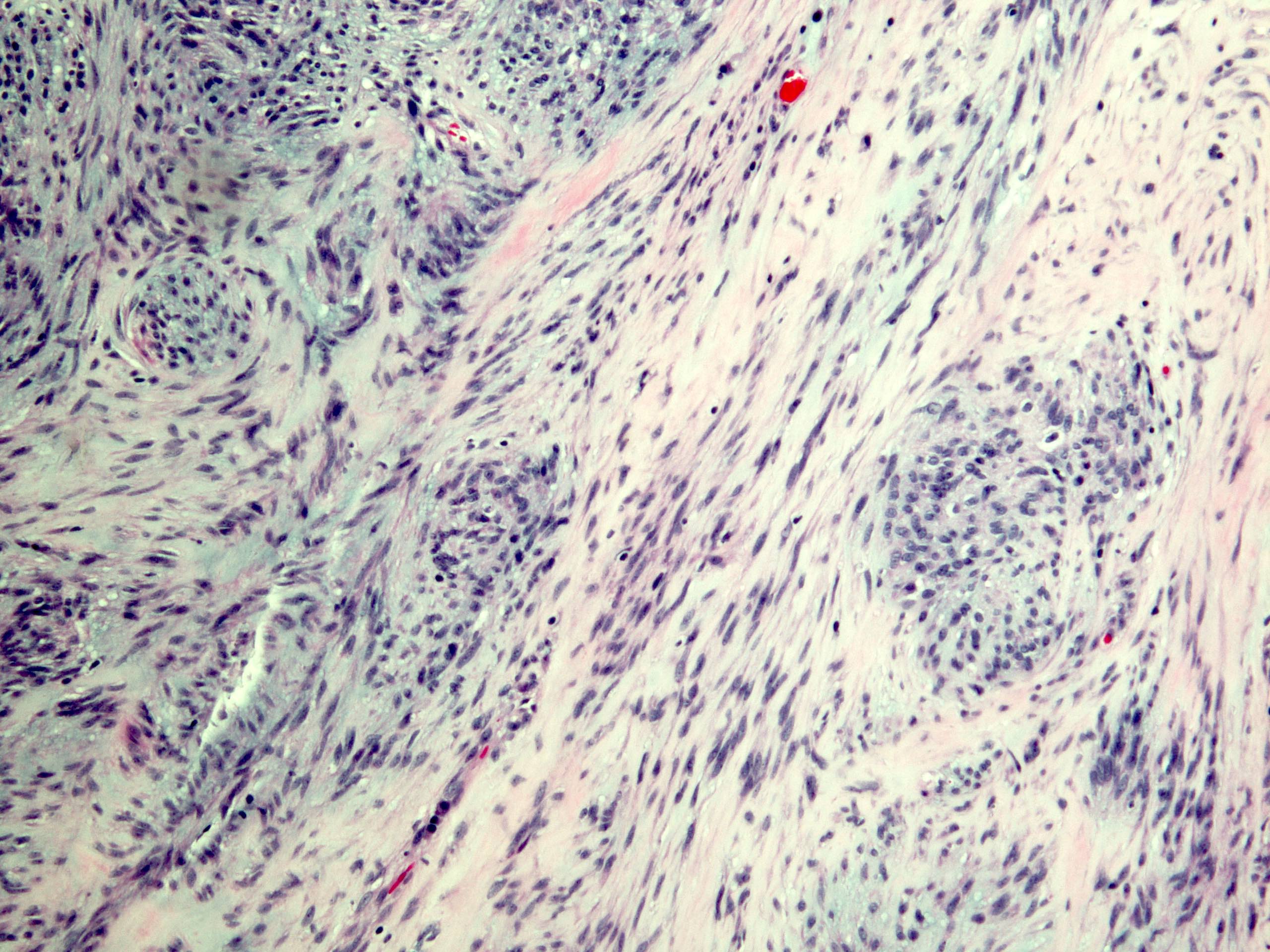
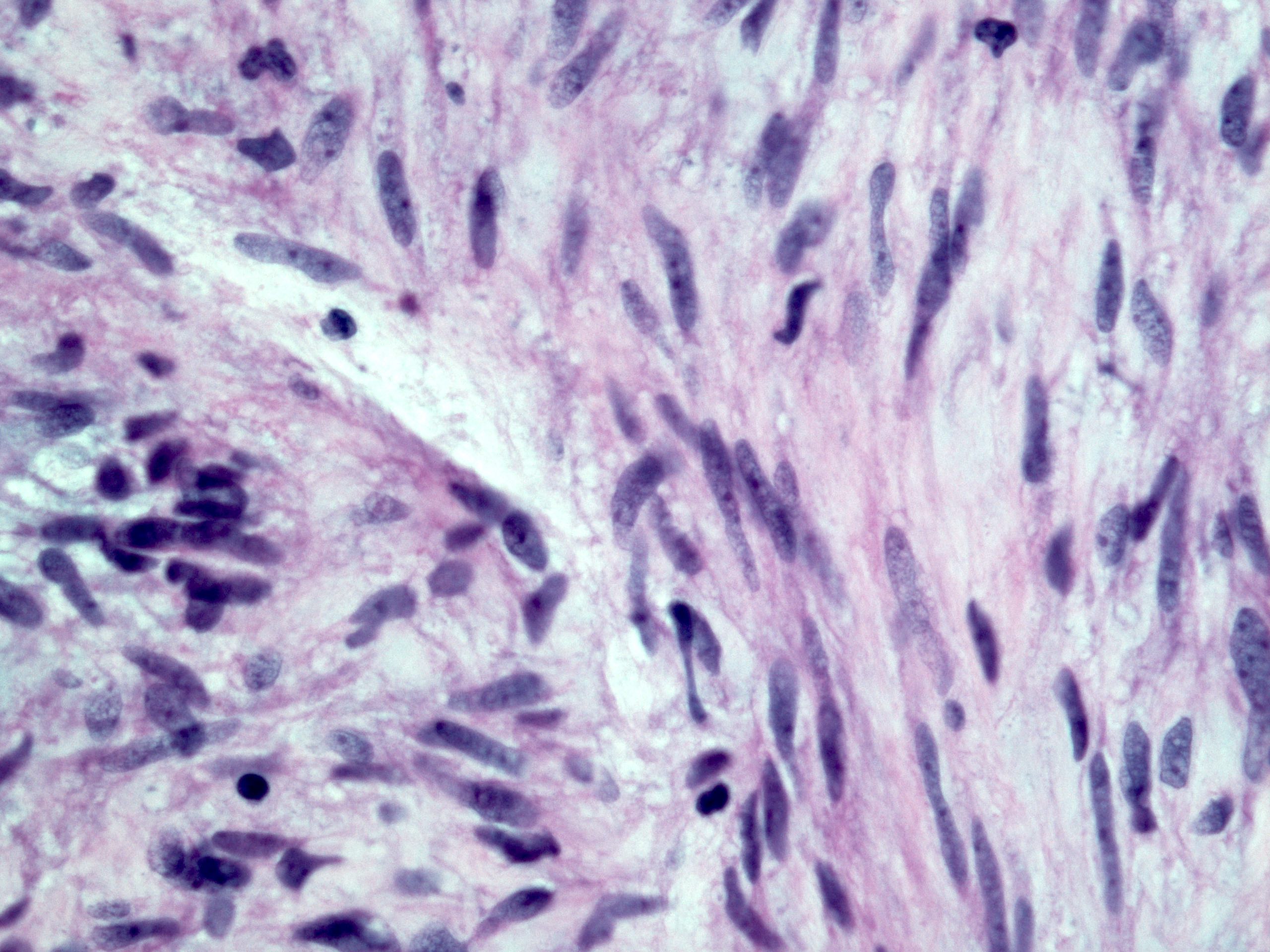
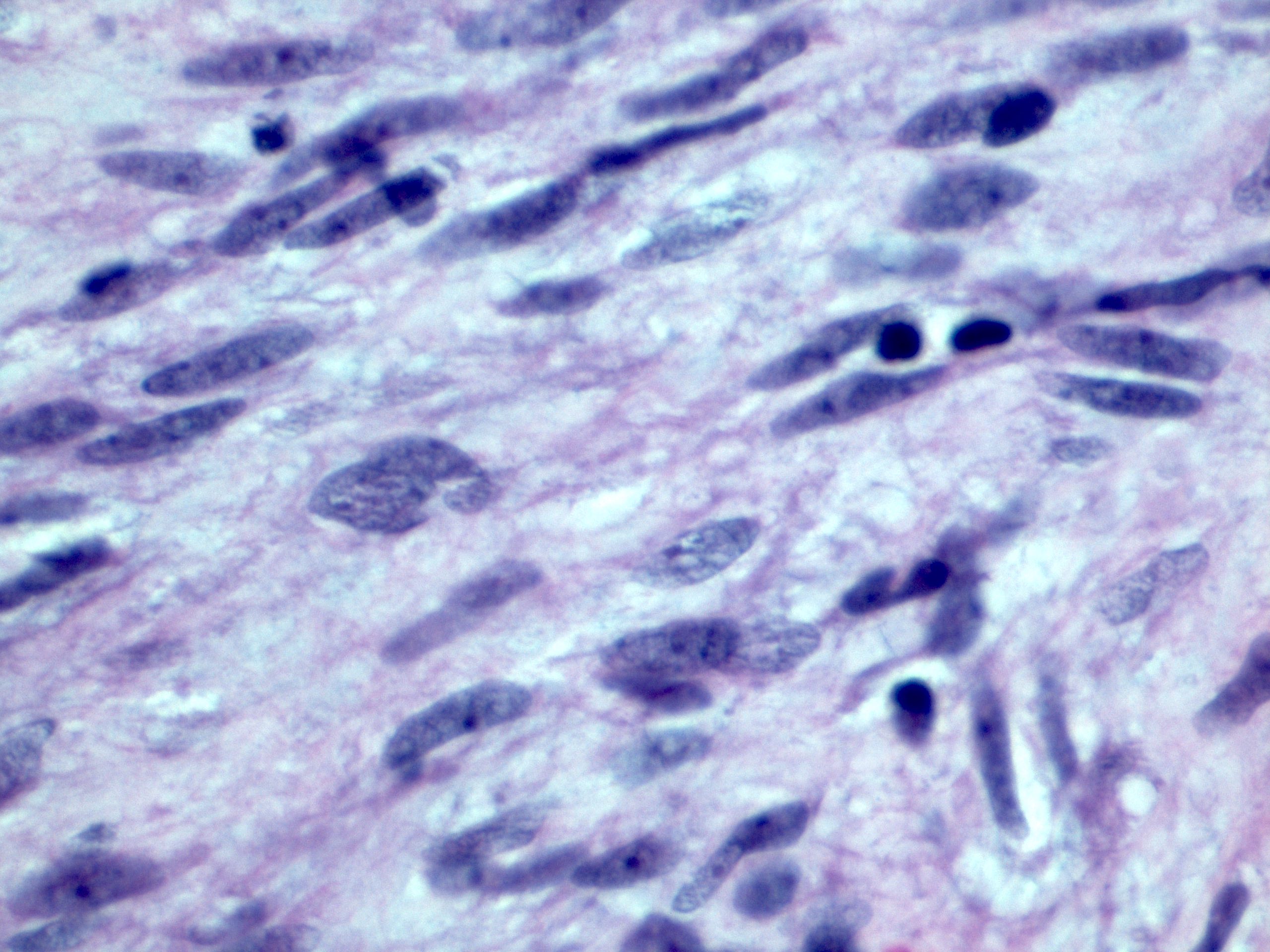
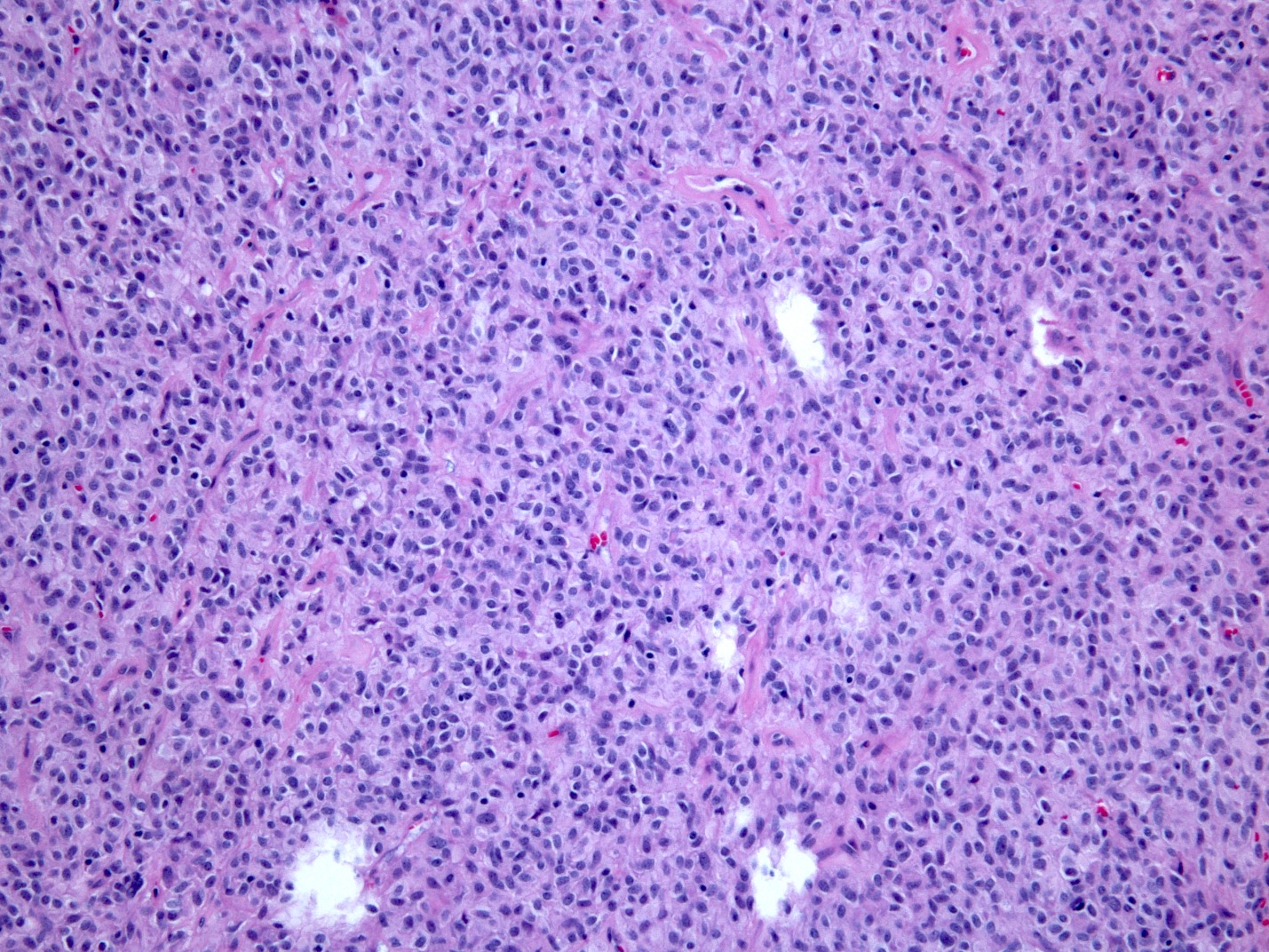
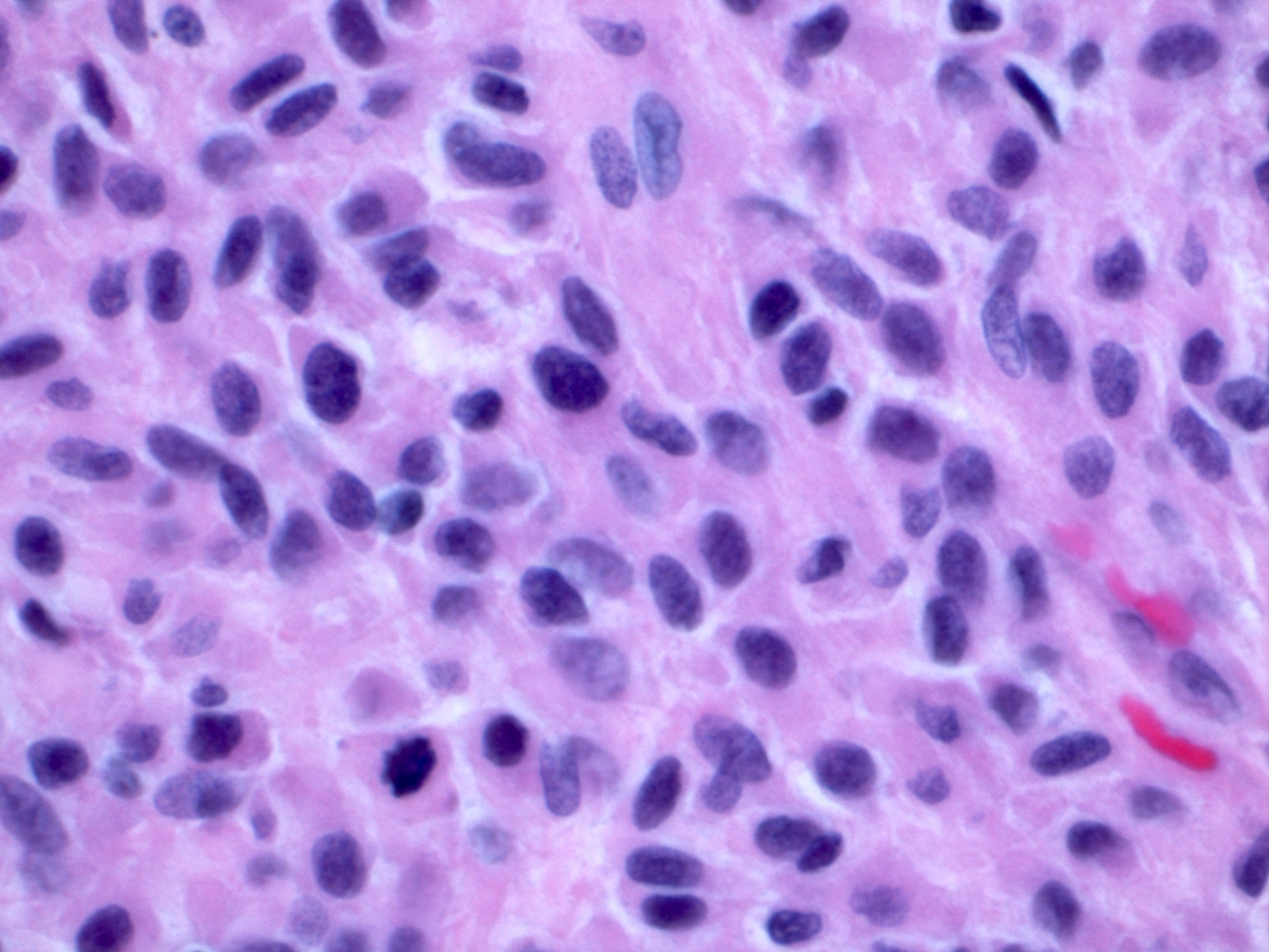
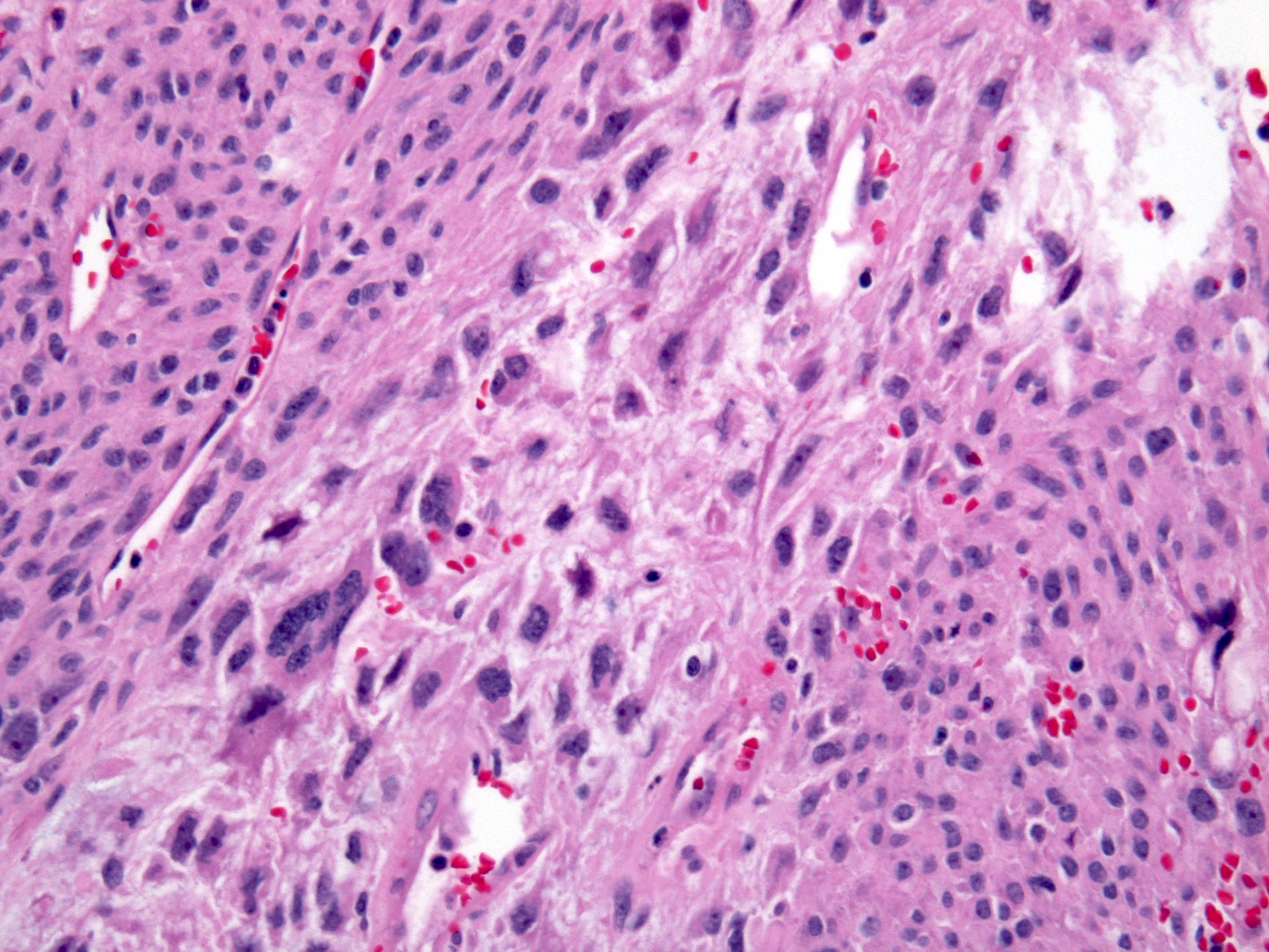
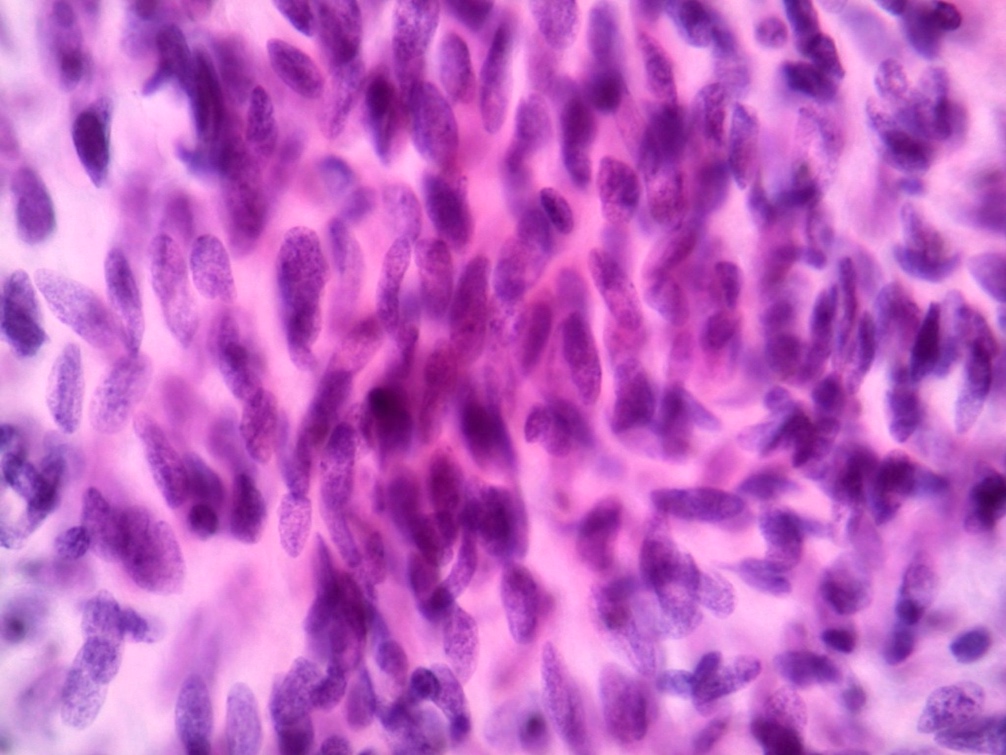
Microscopic (histologic) description
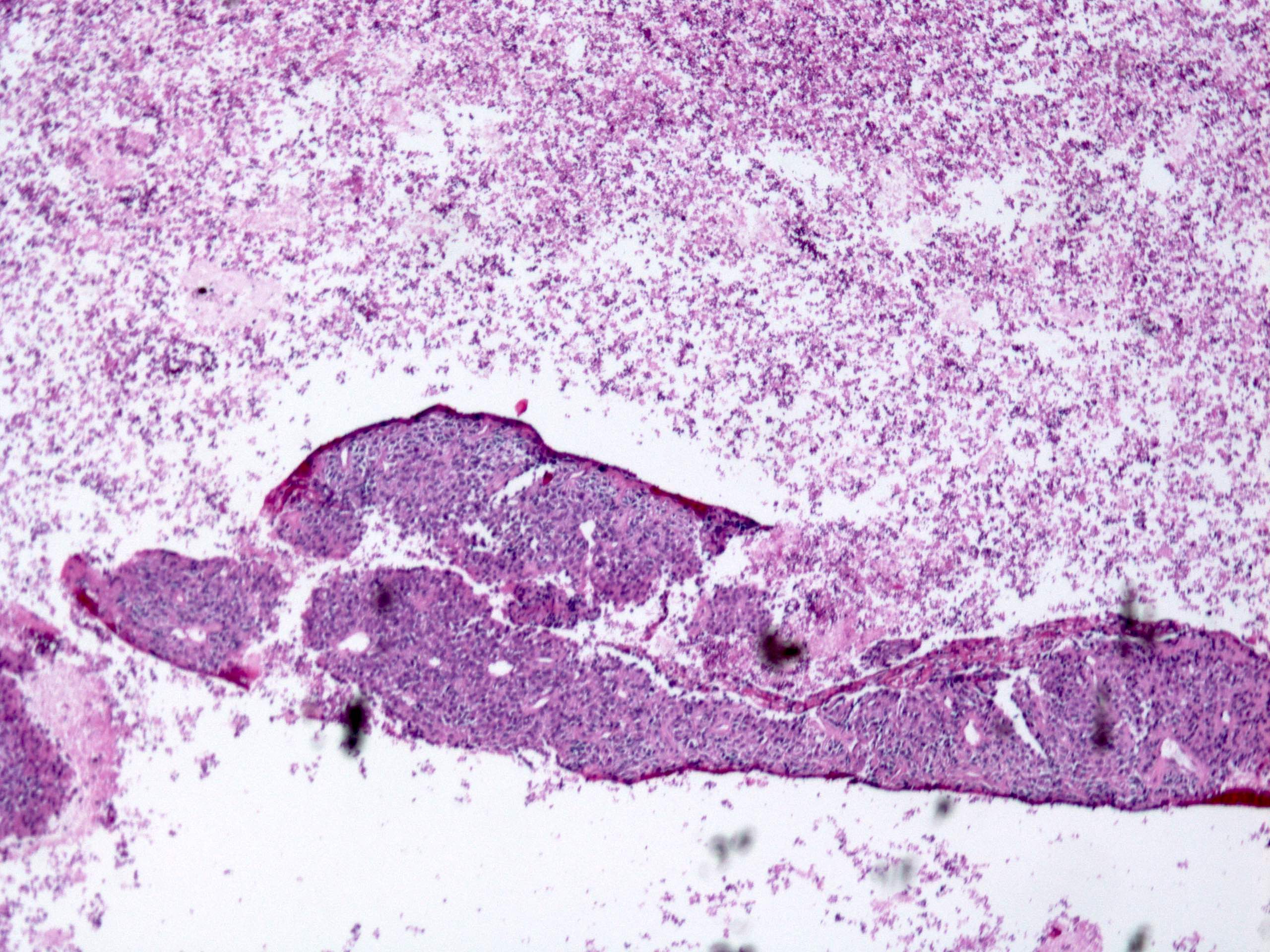
- Histologic heterogeneity: 3 morphologic types - spindle (70%), epithelioid (20%) and mixed (10%)
- Spindle cell type: bland spindle cells with eosinophilic cytoplasm in short fascicles or syncytia, elongated nuclei with inconspicuous nucleoli, indistinct cell borders, paranuclear cytoplasmic vacuoles (more common in gastric), stromal lymphocytes and microcystic stromal degeneration (as in schwannoma), minimal collagen, delicate thin walled vessels, stromal hemorrhage
- Subtypes: sclerosing, palisaded, vacuolated, diffuse hypercellular, can have sarcomatoid features with significant nuclear atypia and mitotic activity
- Epithelioid type: round cells with vesicular chromatin, eosinophilic or clear cytoplasm in nests or sheets, more pleomorphism than spindle type
- Subtypes: sclerosing, discohesive, hypercellular, sarcomatous with significant atypia and mitotic activity
- Mixed type: combination of cells with both spindle and epithelioid type, may have abrupt transition between the 2 or complex comingling
- Spindle cell type: bland spindle cells with eosinophilic cytoplasm in short fascicles or syncytia, elongated nuclei with inconspicuous nucleoli, indistinct cell borders, paranuclear cytoplasmic vacuoles (more common in gastric), stromal lymphocytes and microcystic stromal degeneration (as in schwannoma), minimal collagen, delicate thin walled vessels, stromal hemorrhage
- SDH deficient: epithelioid or mixed with spindle cell component, multinodular, minimal nuclear pleomorphism, occasional atypical mitosis (gastric location strictly, not seen in esophagus)
- Dedifferentiated: anaplastic morphology with unusual phenotype such as loss of KIT expression or aberrant expression of markers such as cytokeratin
- Stromal skeinoid fibers (< 20%): hyaline or fibrillary brightly eosinophilic PAS+ structures, representing nodular tangles of collagen fibers; more common in small or large bowel GIST (Case Rep Gastroenterol 2014;8:257)
- Rarely: cytologic atypia, myxoid stroma
- Variable mitotic activity
- Microscopic GIST: < 10 mm, spindle cells with hyalinized stroma and variable calcification (Pathology 2008;40:9, Histopathology 2017;70:211)
- Treated GIST can microscopically have hyalinized areas or areas of necrosis
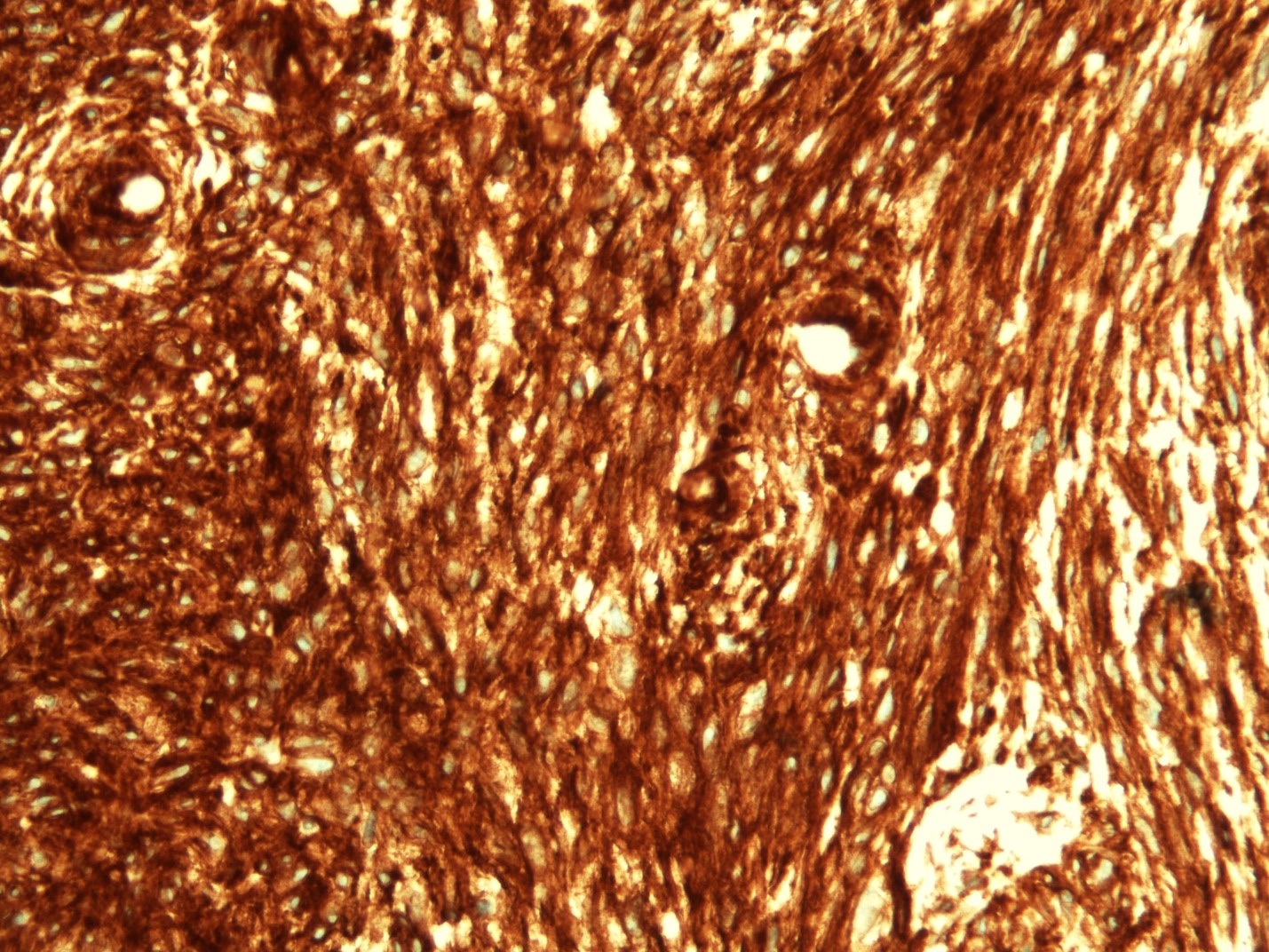
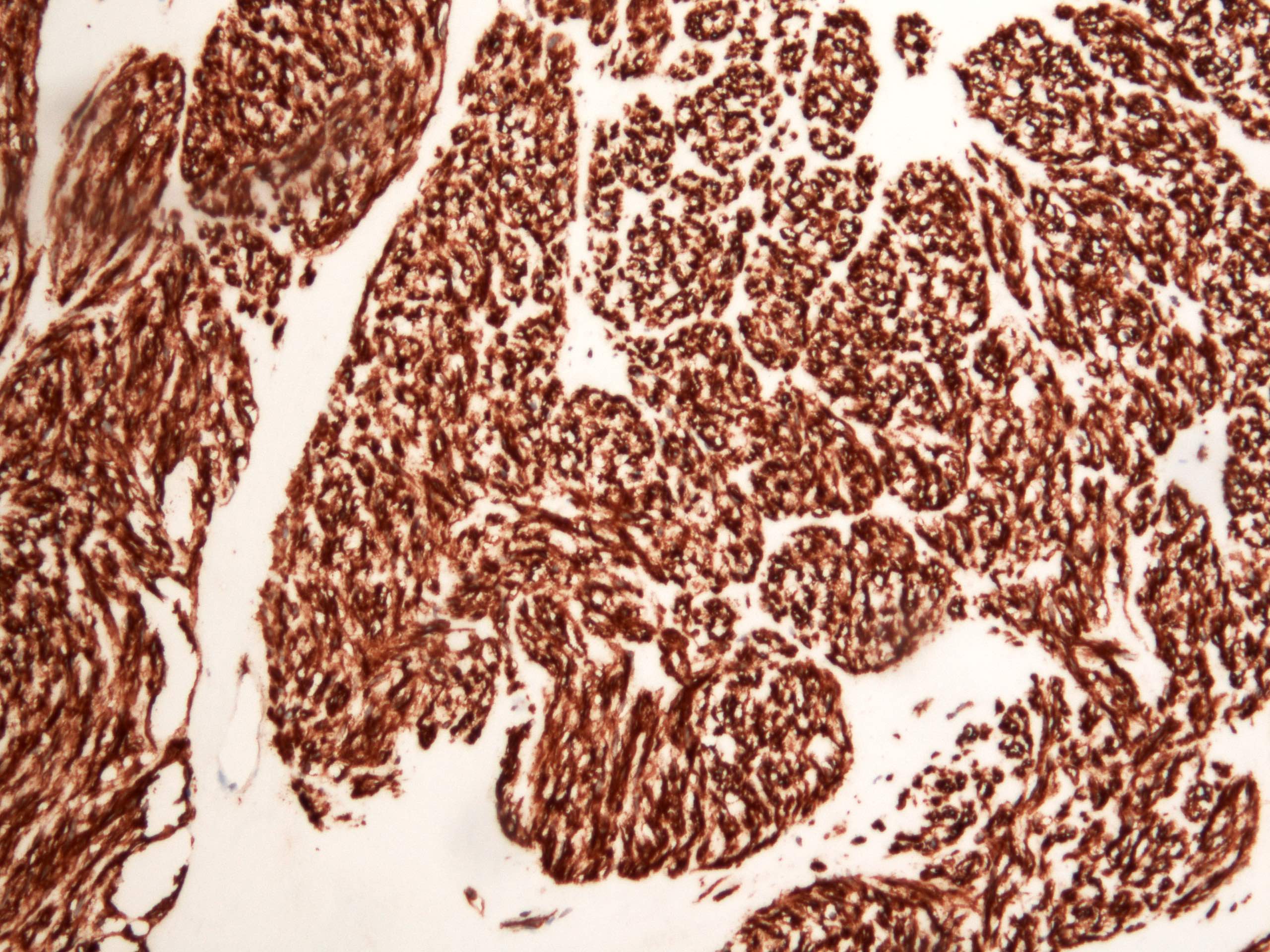
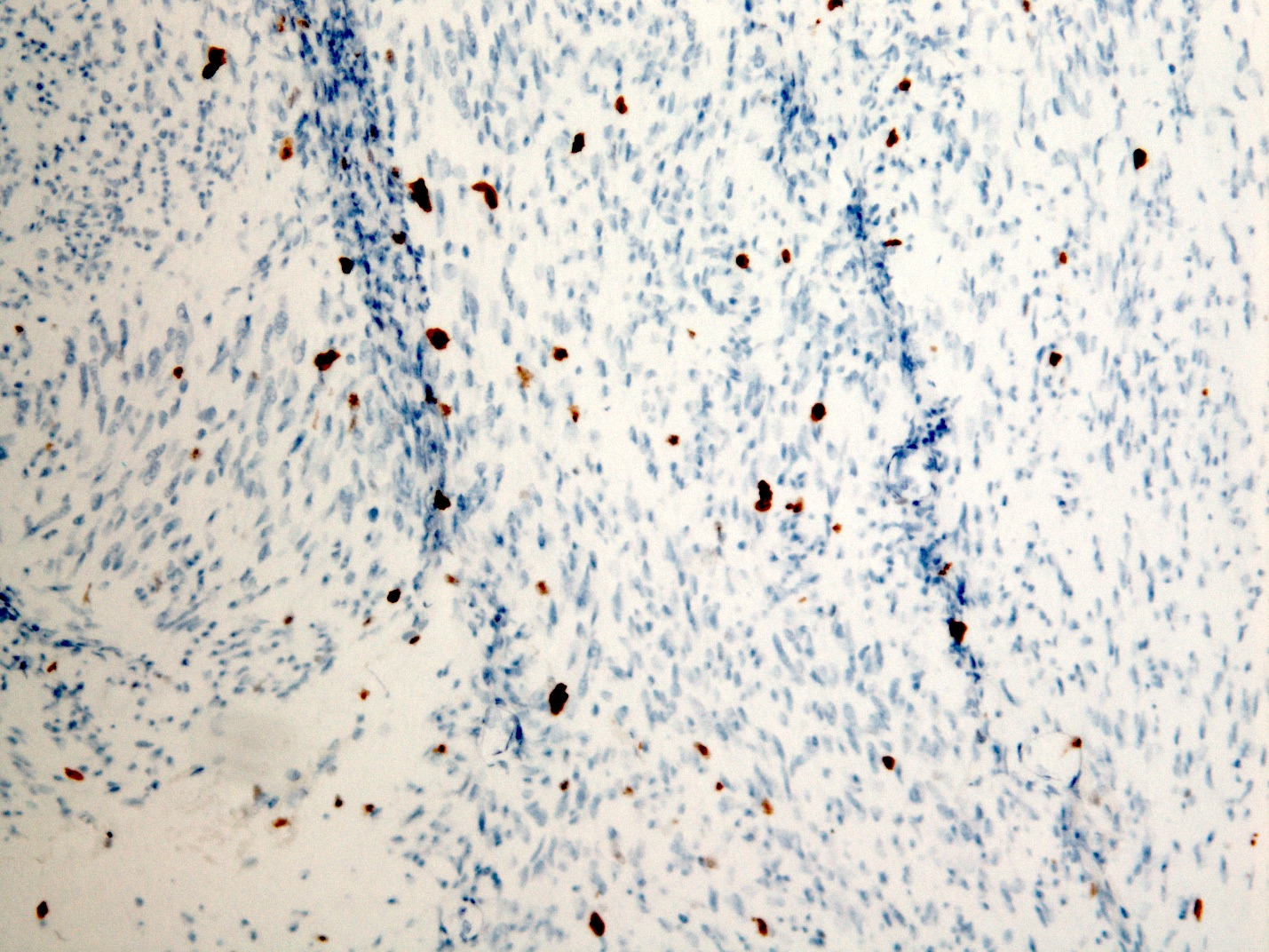
Microscopic (histologic) images
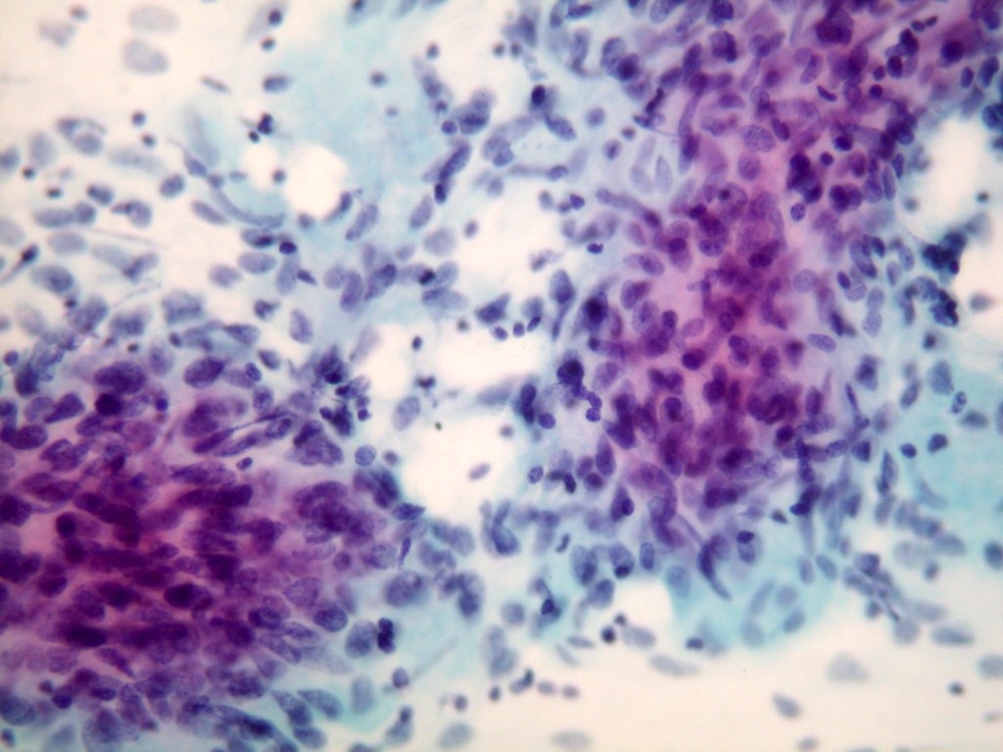
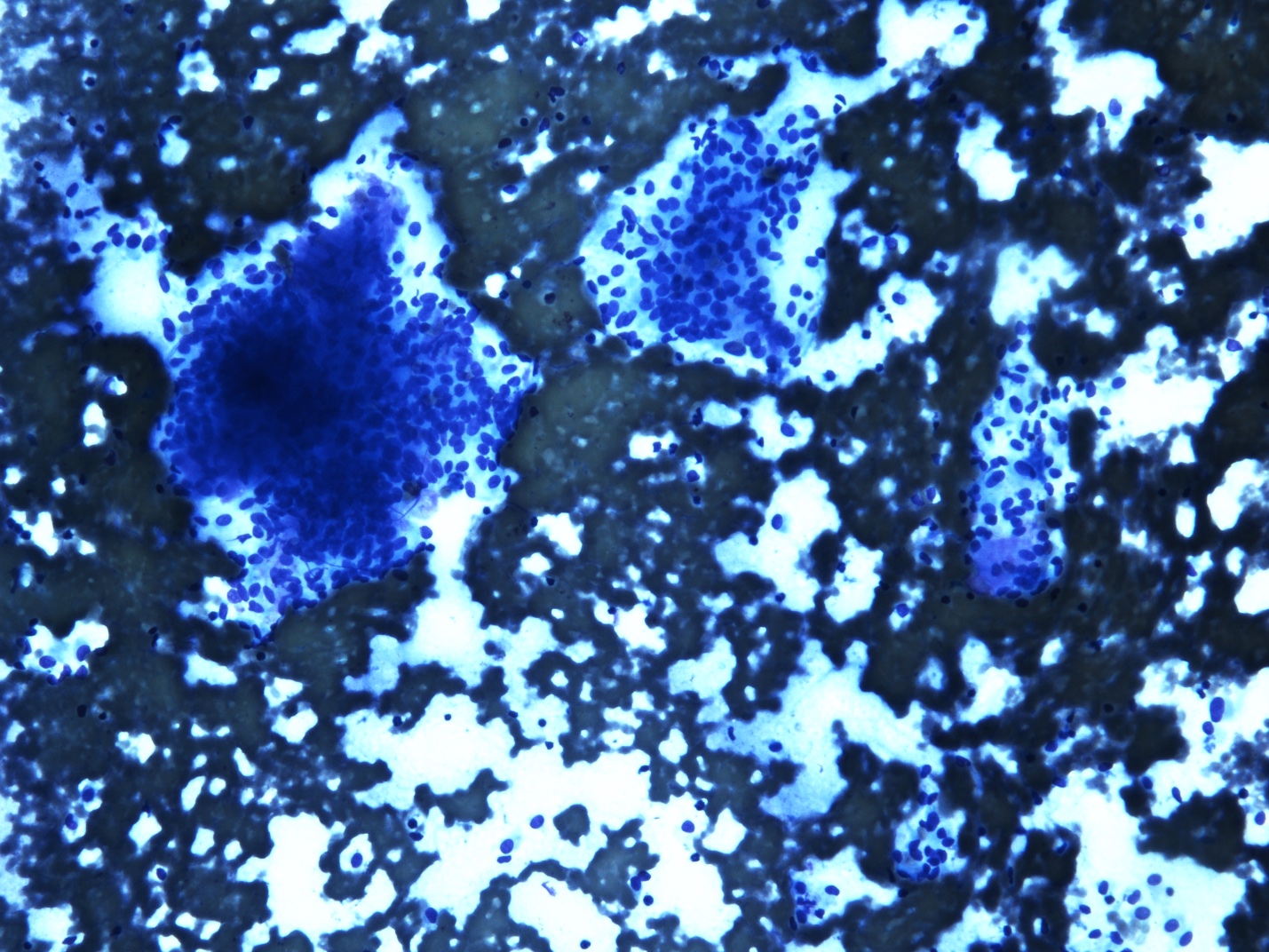
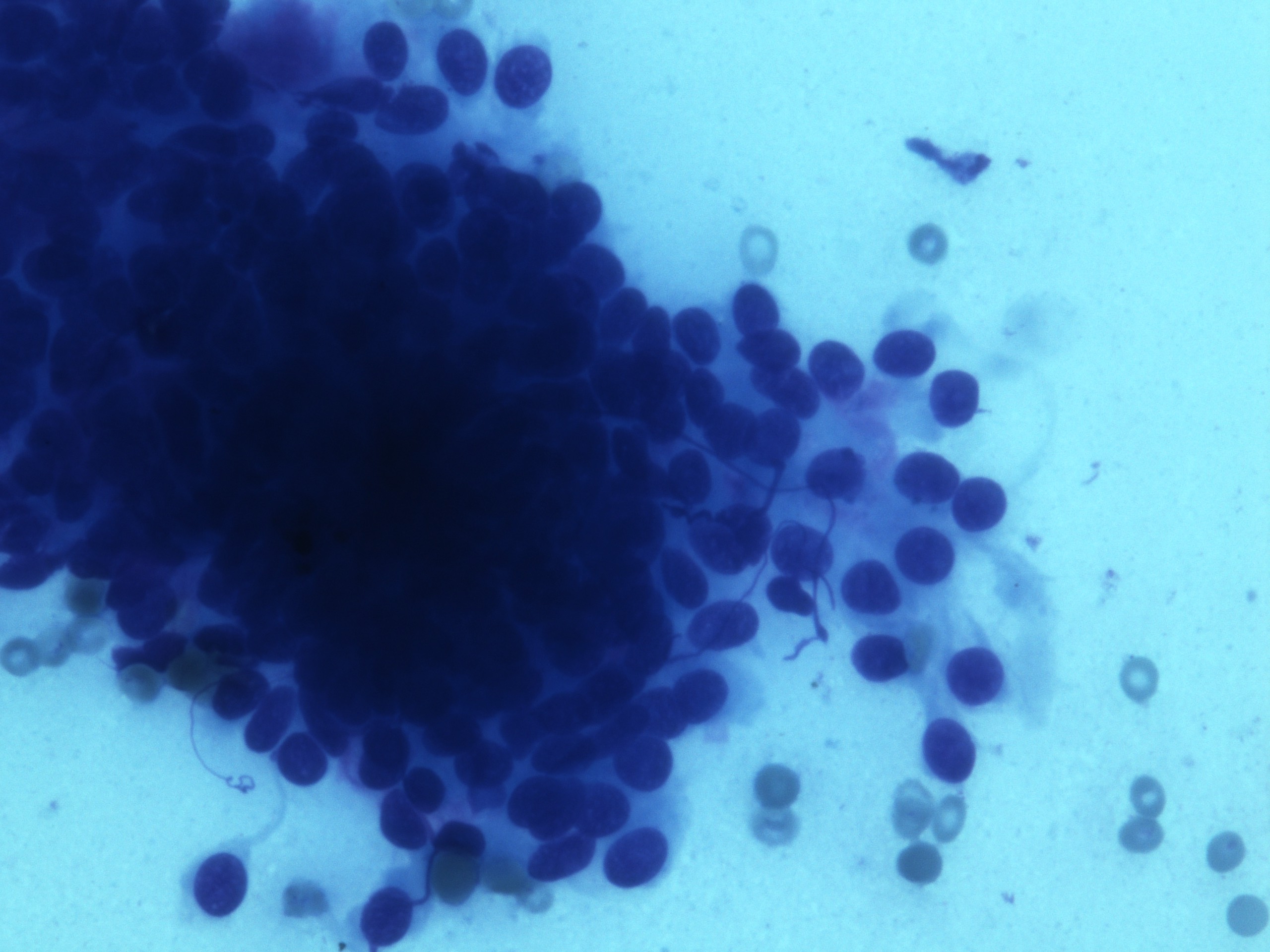
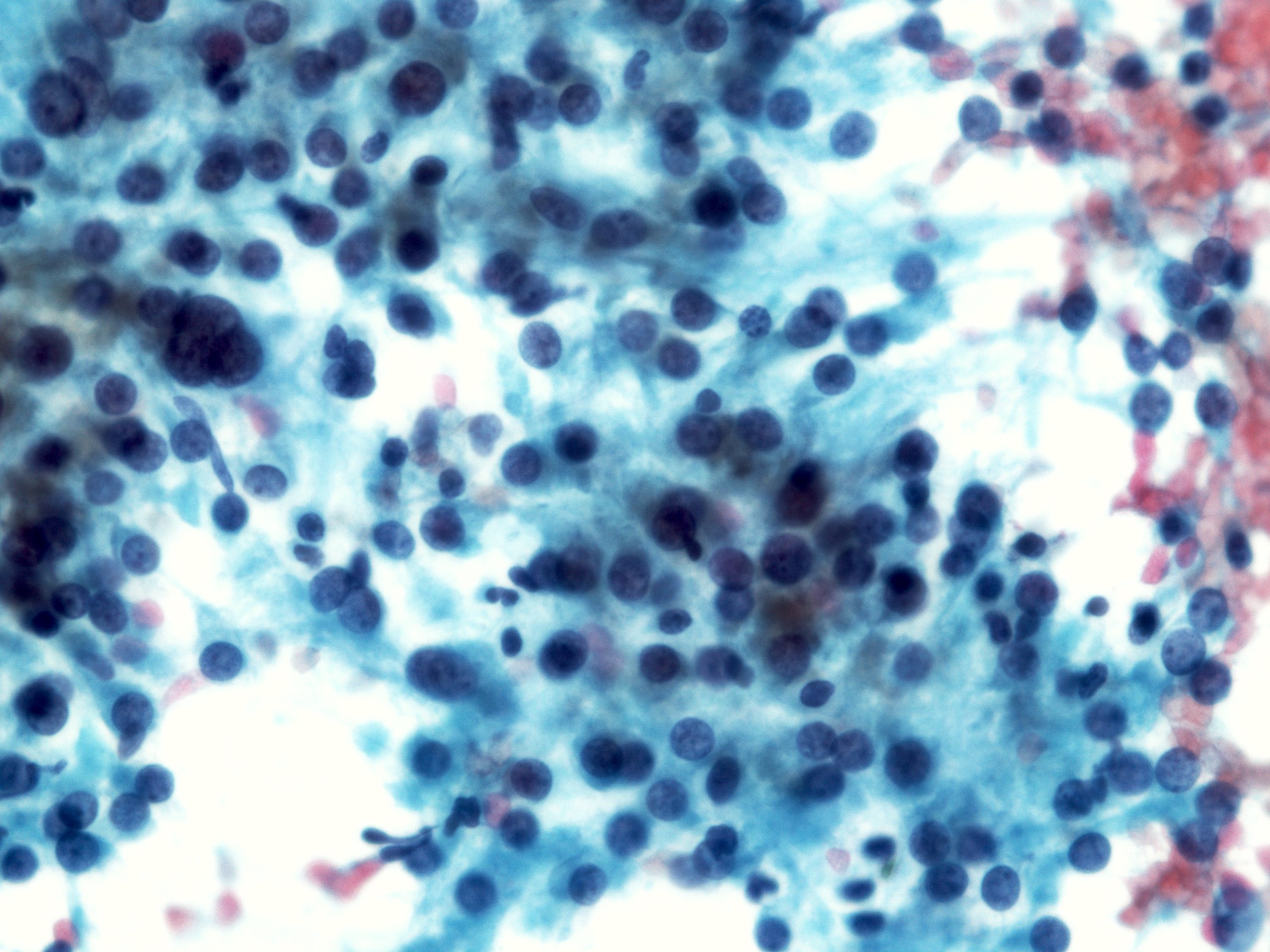
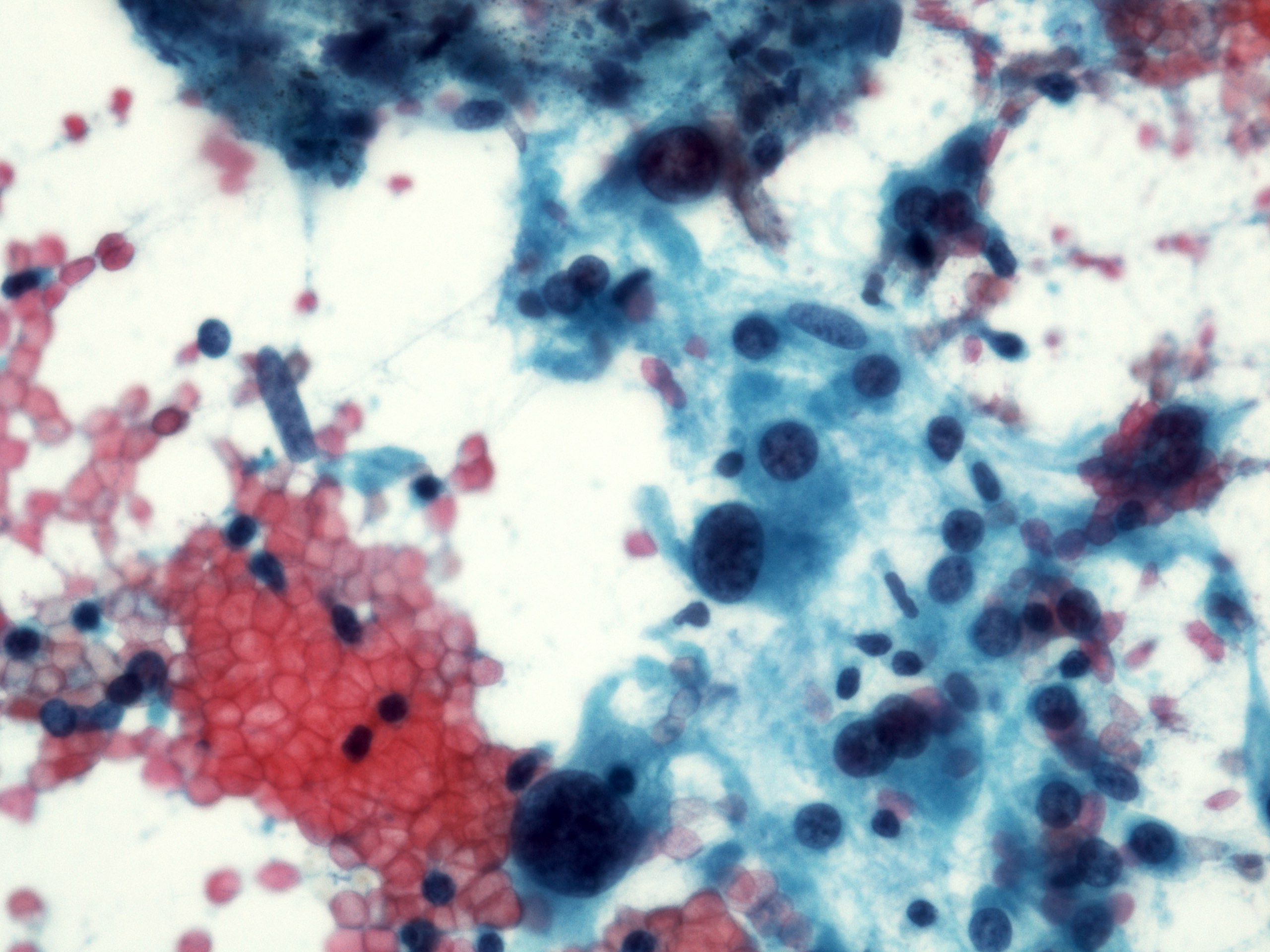
Cytology description
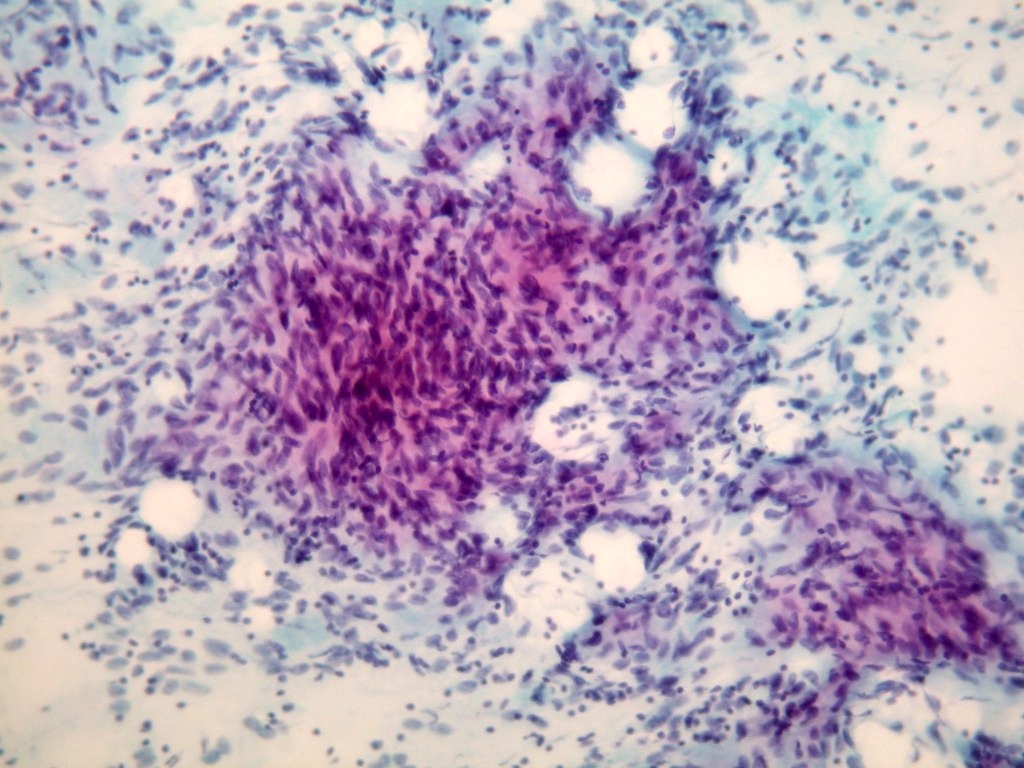
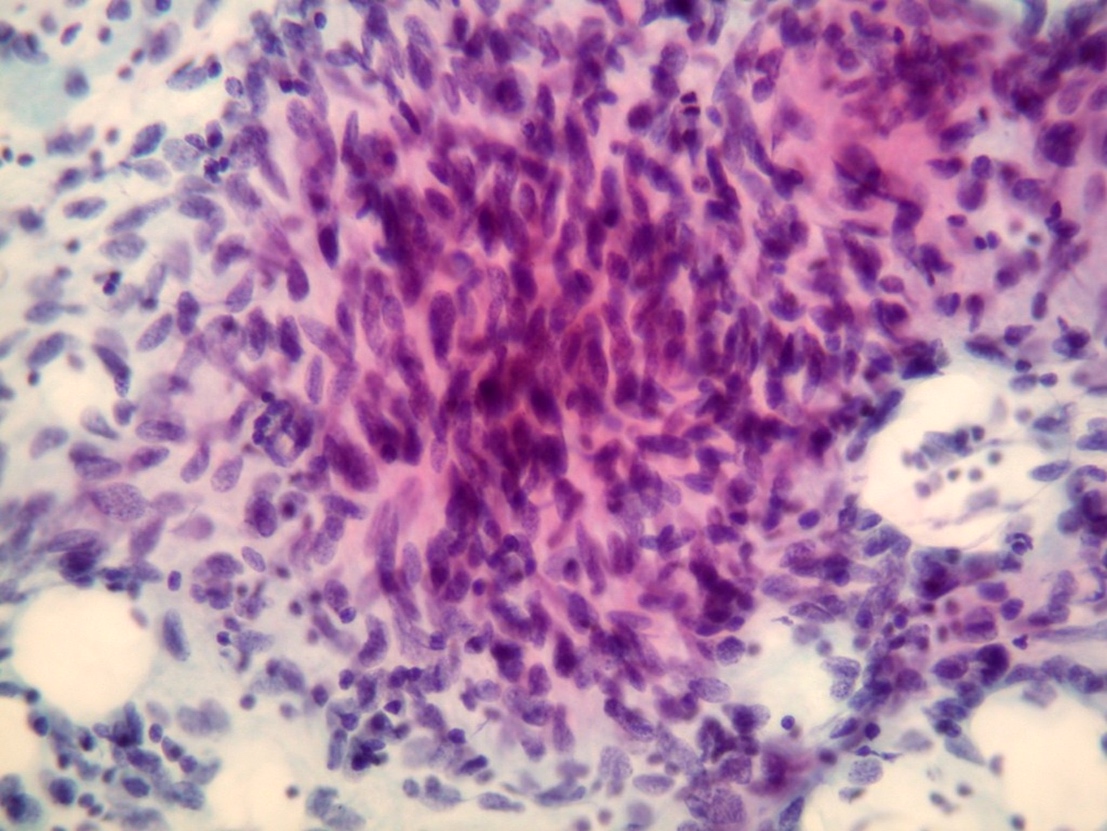
- Endoscopic ultrasound guided fine needle aspiration may be primary diagnostic modality
- Cellular smears, single cells and loosely cohesive fascicles of monomorphic spindle to epithelioid cells
- Irregularly outlined clusters of cells
- Prominent vascular pattern is commonly observed
- Spindle / round / oval nuclei, vesicular chromatin, wispy cytoplasm with long extensions
- Perinuclear or paranuclear vacuoles may be present
- Nuclear palisading can occur
- Stripped spindled to epithelioid nuclei
- Cohesive fragments with admixed delicate, thin walled vessels
- Features of malignant behavior of tumor predicted by cytologic atypia, cellular dyscohesion, nuclear pleomorphism, prominent nucleoli, increased mitotic activity, prominent necrosis
- Reference: DeMay: The Art & Science of Cytopathology, 2nd Edition, 2012
Cytology images
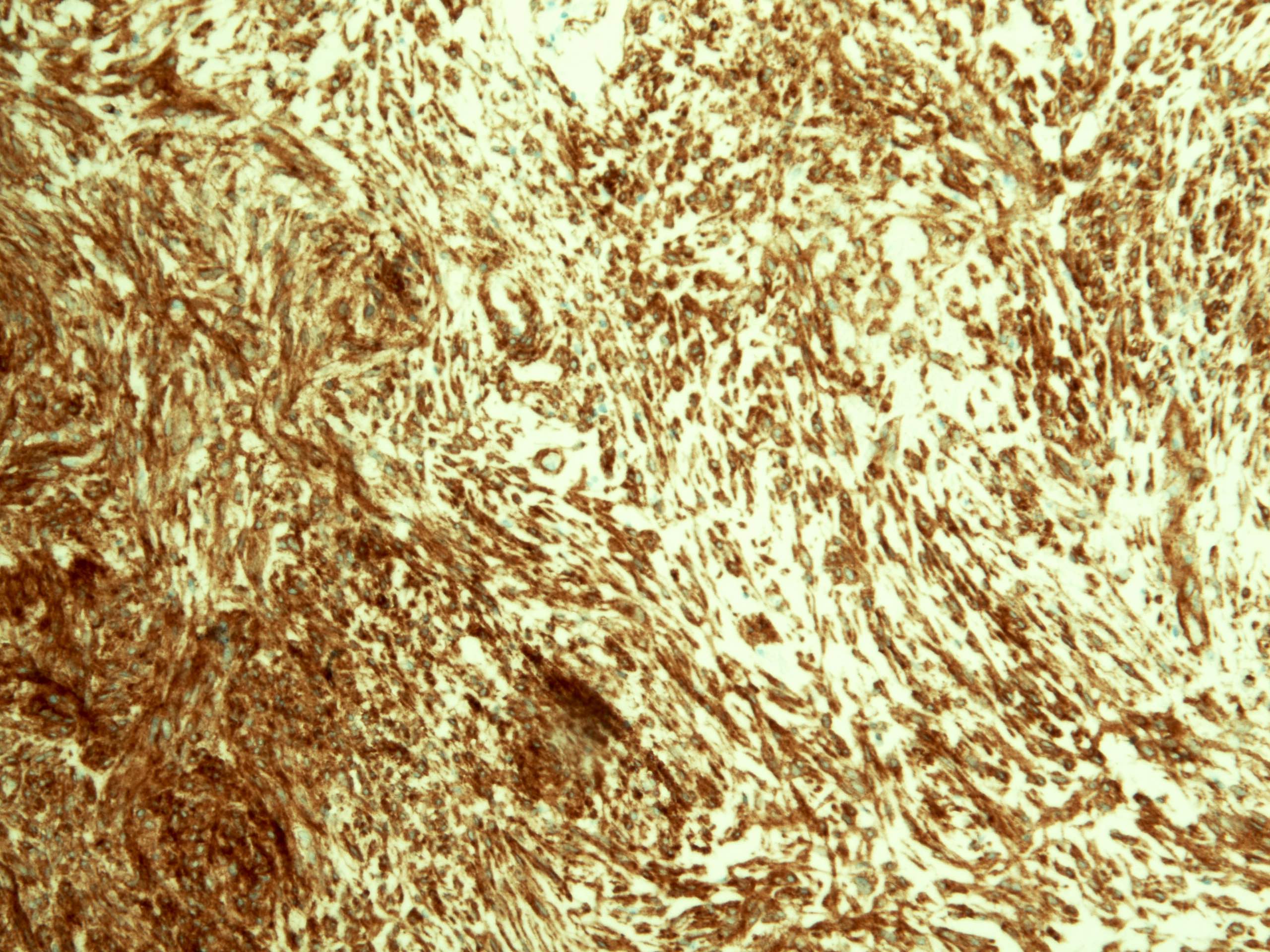
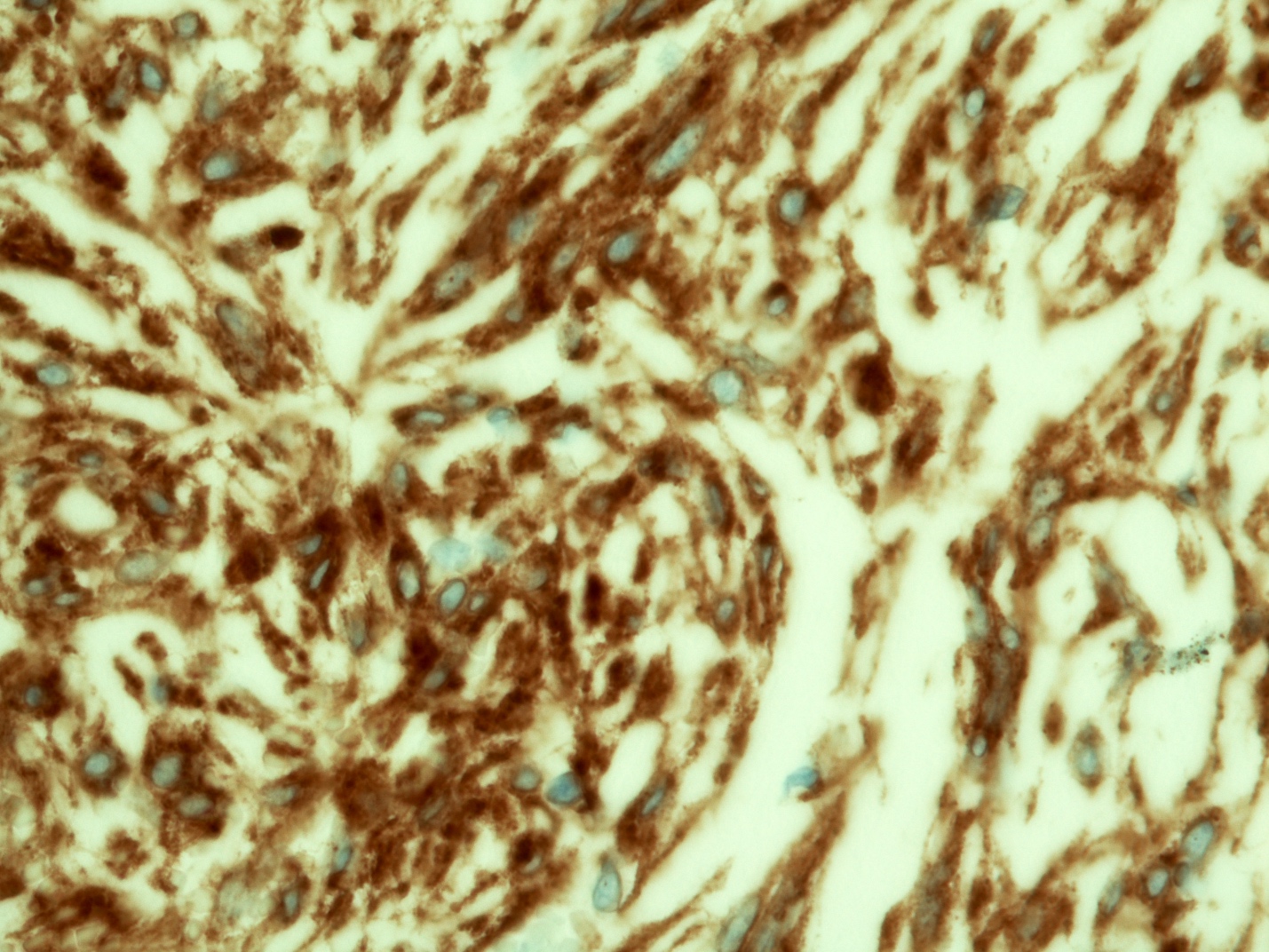
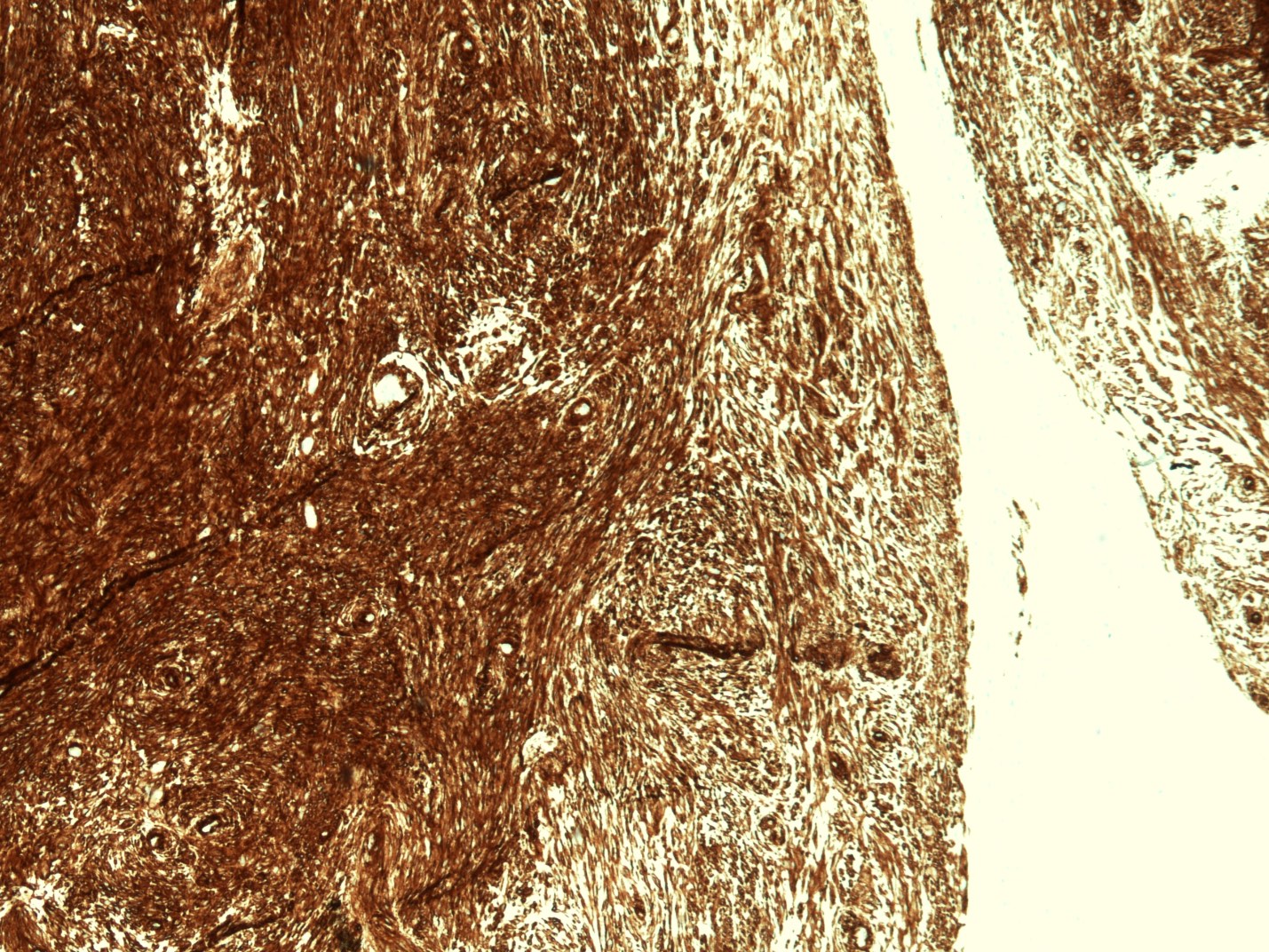
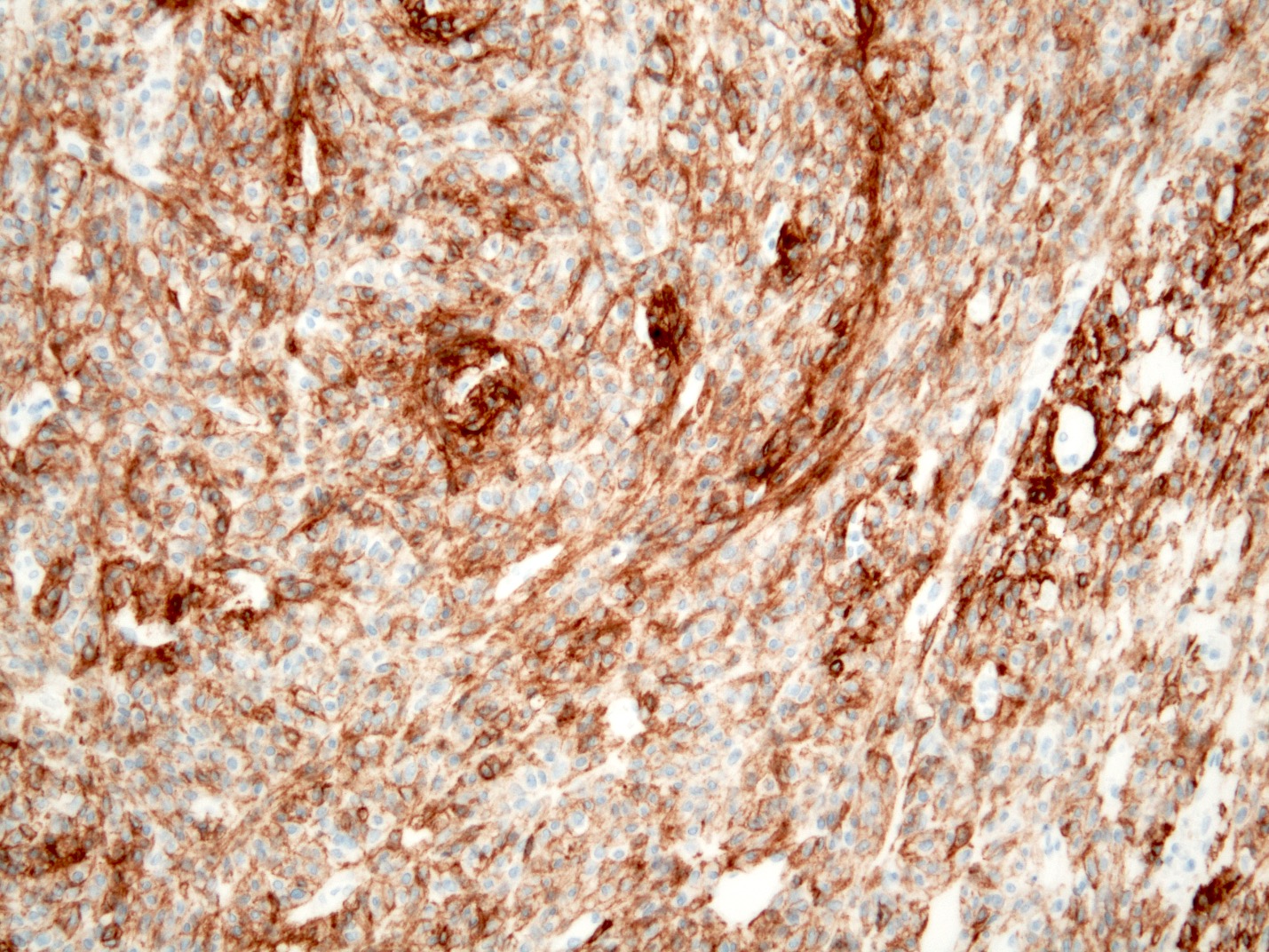
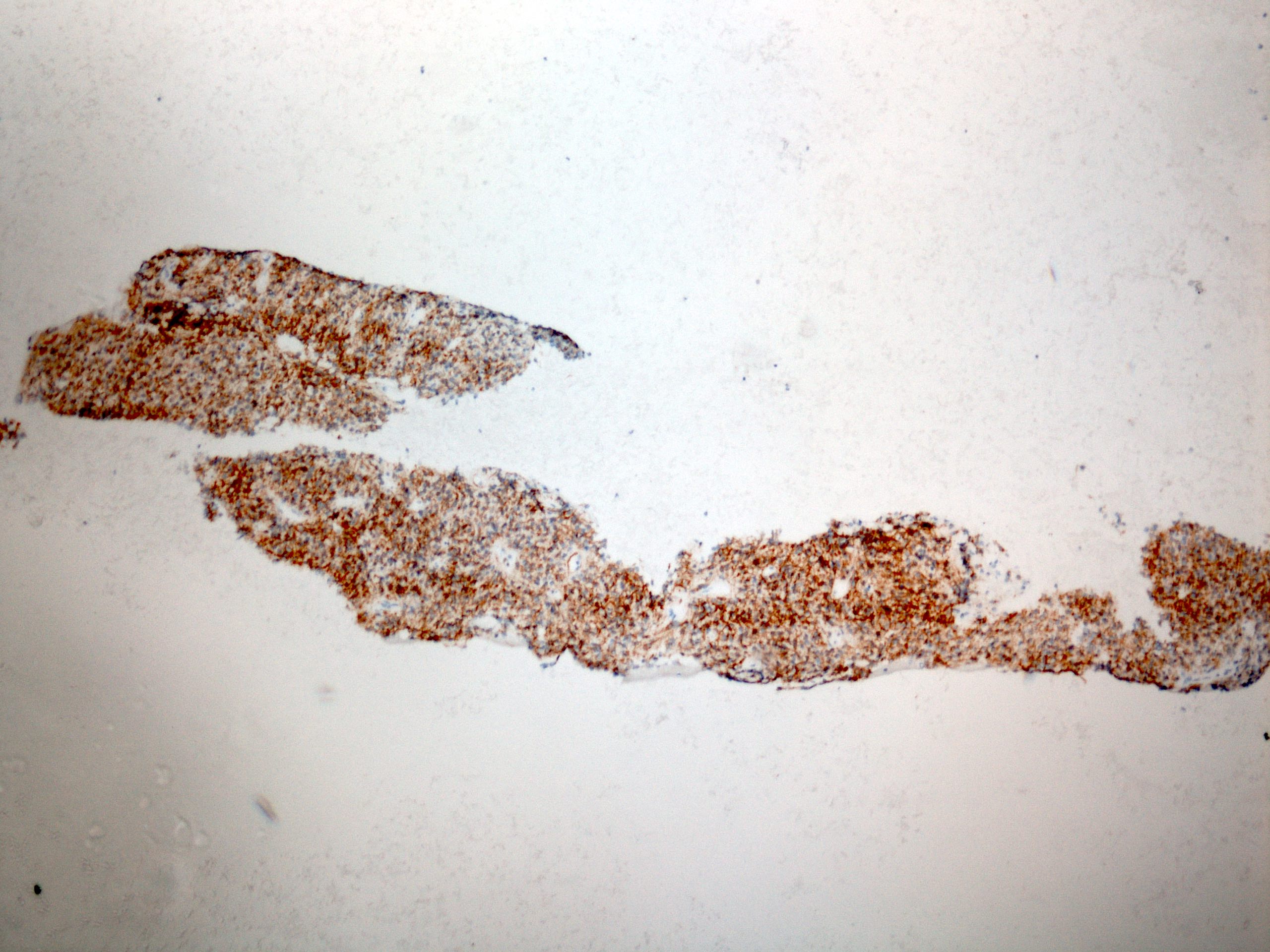
Positive stains
- CD117 / KIT (95%), membranous and cytoplasmic or concentrated in a dot-like perinuclear pattern (globular) diffuse, strong (Am J Surg Pathol 2009;33:1401)
- DOG1 (ANO1) (98%) (Am J Pathol 2004;165:107)
- CD34 (75%), SMA (45%, focal), desmin (5%, focal), S100 (5%, focal) (Histopathology 2017;71:805, Mod Pathol 2011;24:866)
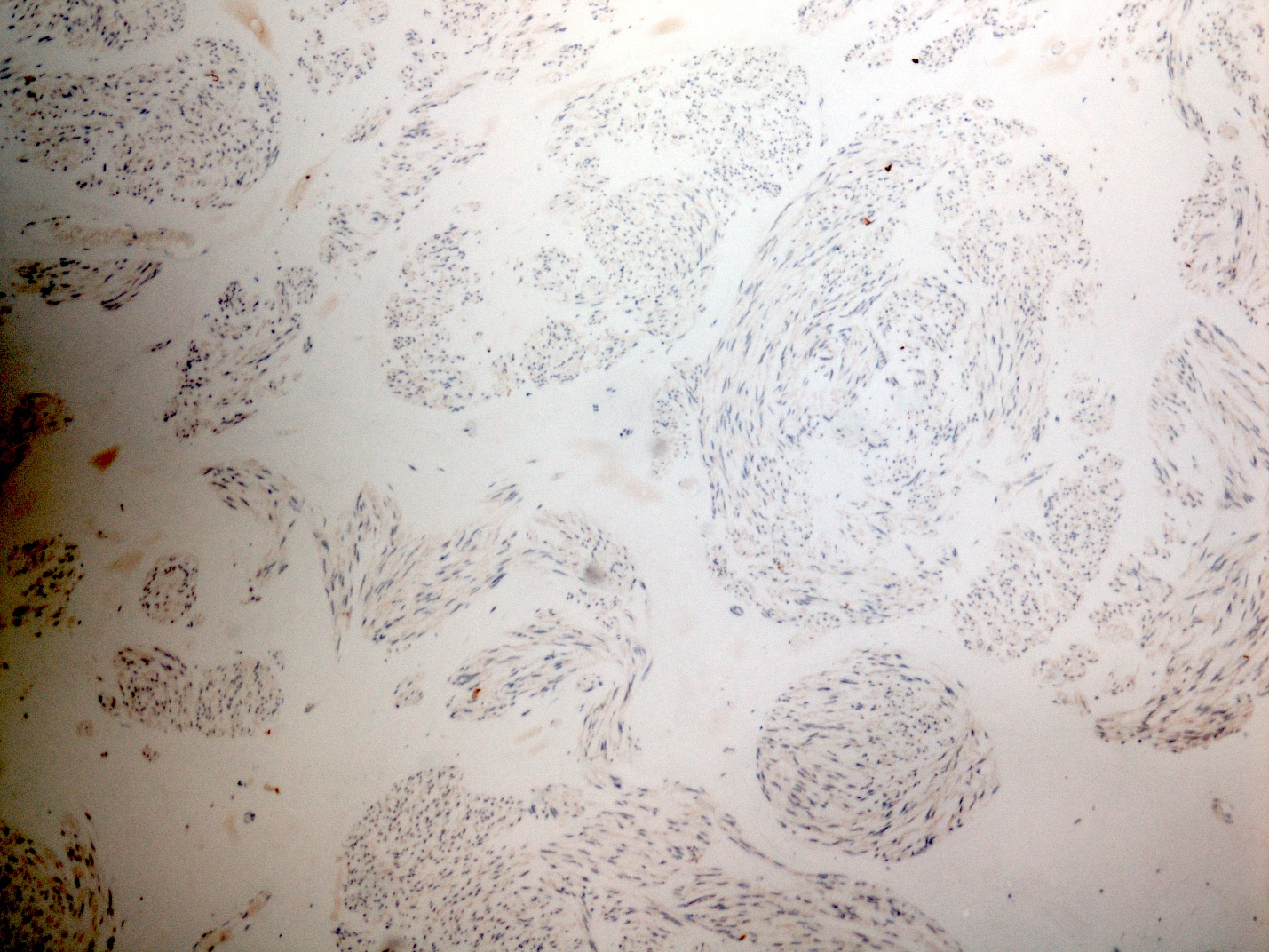
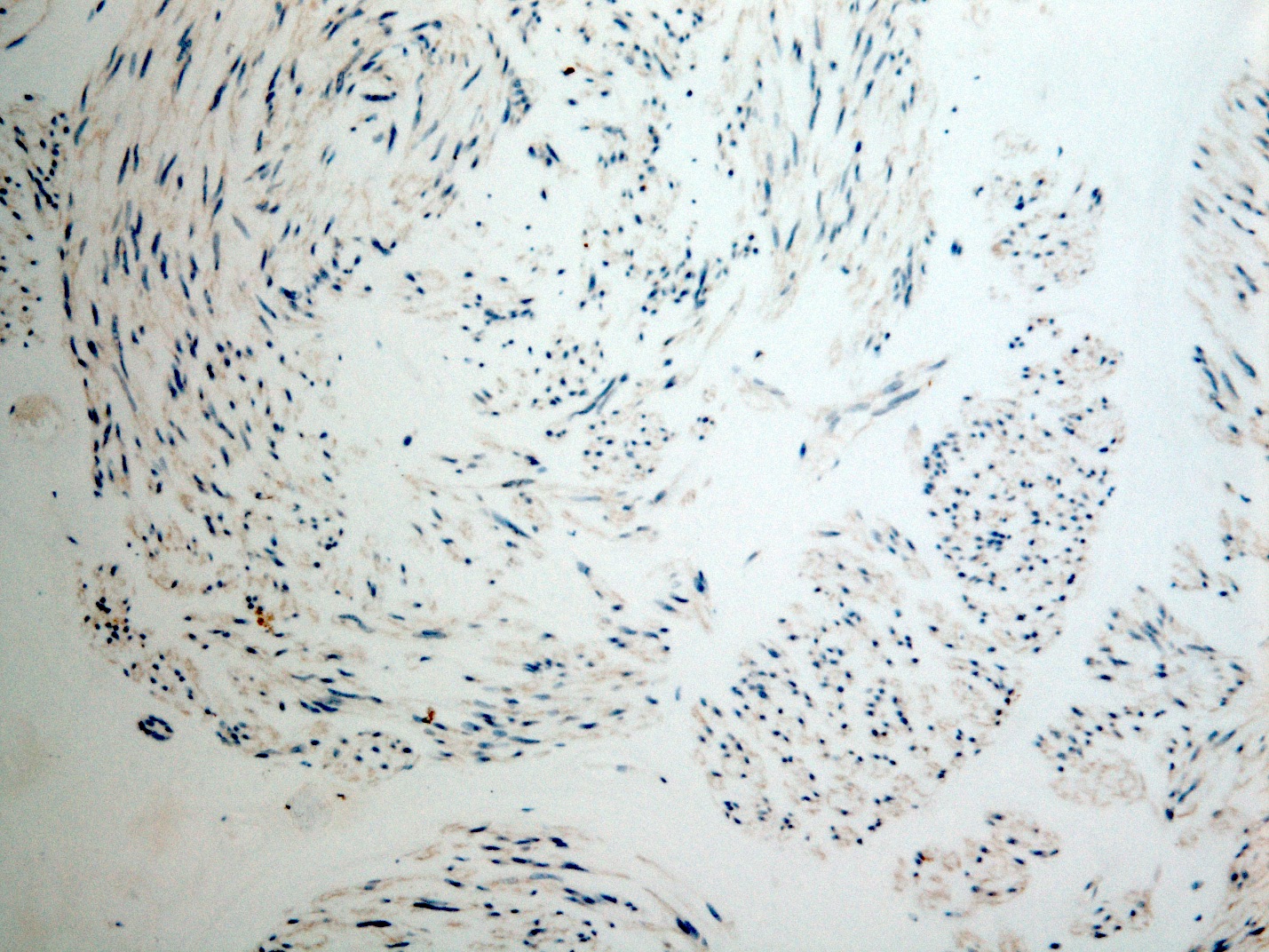
Negative stains
Electron microscopy description
- Relative lack of differentiation (Endocr Relat Cancer 2007;14:853)
- Incomplete smooth muscle or neuroaxonal differentiation
- No bundles of actin filaments (seen in true smooth muscle tumors)
Molecular / cytogenetics description
- Mutually exclusive activating mutations in the proto-oncogene KIT (75%) or platelet derived growth factor alpha (PDGFRA) receptor tyrosine kinase (10%)
- In frame deletions, point mutations, duplications, insertions
- Most common: KIT juxtamembrane domain (exon 11) mutations
- Confers a better response to tyrosine kinase inhibitor therapy
- References: Science 2003;299:708, Cancers (Basel) 2019;11:679, Semin Diagn Pathol 2006;23:91
Sample pathology report
- Esophagus, distal, esophagectomy:
- Gastrointestinal stromal tumor (GIST), mixed spindle and epithelioid type, 4.0 x 3.5 x 2.4 cm (see synoptic report)
- Margins are negative
- AJCC pathologic stage: pTxNx
- Reference: CAP: Protocol for the Examination of Specimens from Patients with Gastrointestinal Stromal Tumor (GIST) [Accessed 12 October 2022]
Differential diagnosis
- Leiomyoma / leiomyosarcoma:
- Spindle cells with cigar shaped nuclei (blunt ended), brightly eosinophilic cytoplasm, arranged in fascicles
- Desmin+, h-caldesmon+; diffusely SMA+, CD117-
- Schwannoma:
- Desmoid fibromatosis:
- Beta catenin+, CD117 variable, CD34-
- Inflammatory fibroid polyp:
- Inflammatory myofibroblastic tumor:
- ALK rearrangements
- CD117-
- Prominent inflammatory infiltrates
- Melanoma:
- MelanA+, tyrosinase+, MITF+, SOX10+
- Malignant peripheral nerve sheath tumor:
- Carcinoma:
- Solitary fibrous tumor:
Additional references
Board review style question #1
Board review style answer #1
A. 3 cm mass and mitosis < 3/5 mm². Size more than 5 cm, mitosis greater than 5 per 5 mm² and site are associated with disease progression in esophageal GIST. Tumor size > 5 cm and mitotic rate > 5/5 mm² are independent risk factors for progression. Esophageal and colonic GIST are more aggressive than gastric GIST. Of the choices above, A is low risk, D is high risk and others have moderate risk of progression (Mod Pathol 2022;35:554).
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor
Board review style question #2
Succinate dehydrogenase deficient GIST is seen exclusively in which part of the gastrointestinal system?
- Colon
- Esophagus
- Ileum
- Stomach
Board review style answer #2
D. Stomach. Succinate dehydrogenase deficient GIST has a female preponderance and seen almost exclusively in stomach (predilection for distal stomach and antrum) (Am J Surg Pathol 2010;34:636).
Comment Here
Reference: Gastrointestinal stromal tumor
Comment Here
Reference: Gastrointestinal stromal tumor