Table of Contents
Definition / general | Essential features | CPT coding | Sites | Cytologic sampling | Diagrams / tables | Laboratory | Cytology description | Cytology images | Molecular / cytogenetics description | Videos | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Upadhyay P, Neupane S, Baskota SU. WHO reporting system for lung cytopathology. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cytopathologylungWHO.html. Accessed March 28th, 2025.
Definition / general
- In 2022, the International Academy of Cytology (IAC) and the International Agency for Research on Cancer / World Health Organization (WHO) published the WHO Reporting System for Lung Cytopathology to standardize cytopathology reporting globally (Acta Cytol 2023;67:80, J Am Soc Cytopathol 2023;12:251)
- WHO system highlights the significance of cell preparation techniques and has established best practice guidelines for using immunocytochemistry (ICC), in situ hybridization and molecular techniques
- 5 categories for reporting lung cytopathology: insufficient / inadequate / nondiagnostic, benign, atypical, suspicious for malignancy, malignancy
Essential features
- WHO system for reporting lung cytopathology includes 5 categories: insufficient / inadequate / nondiagnostic, benign, atypical, suspicious for malignancy, malignancy
- Types of samples included in this classification system include sputum, bronchial wash (BW), bronchoalveolar lavage (BAL), bronchial brush (BB), fine needle aspiration biopsy (FNAB) (transthoracic, percutaneous, transesophageal and endobronchial ultrasound guided) and biopsies (including mediastinoscopy)
- A definitive category should be used with an attempt for a definitive diagnosis; if no definitive diagnosis can be made, a differential diagnosis should be listed
- Further research is needed to stratify the risk of malignancy (ROM) of each of the proposed categories; however, this first edition of the WHO system for classification of lung cytopathology has listed risk of malignancy for each category as 40 - 60% for inadequate, 20 - 40% for benign, 50 - 60% for atypical, 54.5 - 90% for suspicious for malignancy and > 90% (approaching 100%) for malignancy
- Every attempt needs to be made to give a definitive diagnosis with the help of clinical, microbial and imaging correlation, immunocytochemistry, special stains and molecular studies whenever applicable
CPT coding
- 88104 - direct smears
- 88108 - concentrated cytology specimen
- 88112 - selective enhanced cytology specimen such as liquid based preparations
- 88173 - fine needle aspiration
- 88305 - cell block
- 88342 - immunohistochemistry first stain
- 88341 - immunohistochemistry additional stains
- 88312 - special stains for microorganisms
- 88313 - special stains, others
Sites
- Upper respiratory tract, lung, pleura, bronchus, bronchioles
Cytologic sampling
- Specimen types
- Sputum, bronchial wash, bronchoalveolar lavage, bronchial brush, fine needle aspiration cytology (transbronchial, percutaneous, endobronchial and transesophageal ultrasound / computed tomography [CT] guided)
- Techniques for cytologic sampling of upper respiratory tract and lung lesions
- Fine needle aspiration biopsy: main procedure for sampling for mass seen in imaging
- Mainly 2 approaches: transcutaneous or transbronchial / transtracheal
- Sputum
- Bronchial washing
- Bronchial brushing
- Fine needle aspiration biopsy: main procedure for sampling for mass seen in imaging
- References: Int J Clin Exp Pathol 2010;3:367, Onco Targets Ther 2013;6:1553, Arch Pathol Lab Med 2018;142:253, Respiration 1992;59:44
Diagrams / tables
Table 1: WHO Reporting System for Lung Cytopathology (Acta Cytol 2023;67:80, J Am Soc Cytopathol 2023;12:251)
| Diagnostic categories | Average risk of malignancy | Average risk of malignancy according to sampling technique | Recommended management |
| Insufficient / inadequate / nondiagnostic | 40 - 60% | FNAB: 43 - 53% Sputum sample: 0 - 100% Bronchial washing: 38 - 81% Bronchial brushing: 0 - 75% | Repeat sampling
FNAB or transthoracic CT guided FNAB if repetition results in the same diagnosis Correlate clinically and microbiologically with images and discuss at multidisciplinary team (MDT) meeting |
| Benign | 20 - 40% | FNAB: 19 - 64% Sputum sample: 0 - 42% Bronchial washing: 38 - 42% Bronchial brushing: 32 - 38% | Correlate clinically, microbiologically and with images
Benign: follow up in 3 - 6 months No specific diagnosis: repeat sampling (FNAB with or without core needle biopsy [CNB]) and perform limited resection |
| Atypical | 50 - 60% | FNAB: 46 - 55% Sputum sample: 86 - 100% Bronchial washing: 62 - 86% Bronchial brushing: 79 - 100% | Correlate clinically and microbiologically with images and discuss with multidisciplinary team
Repeat sampling:
Benign: follow up in 3 - 6 months No specific diagnosis: repeat FNAB with rapid onsite evaluation (ROSE) with or without core needle biopsy |
| Suspicious for malignancy | 54.5 - 90% | FNAB: 75 - 88% Sputum sample: 100% Bronchial washing: 83 - 100% Bronchial brushing: 75 - 100% | Correlate clinically and microbiologically with images and discuss with multidisciplinary team
Malignant: initiate definitive treatment No specific diagnosis: repeat FNAB with rapid onsite evaluation with or without core needle biopsy |
| Malignancy | 90% | FNAB: 87 - 100% Sputum sample: 100% Bronchial washing: 98 - 100% Bronchial brushing: 94 - 100% | Correlate clinically and microbiologically with images and discuss with multidisciplinary team
Malignant: surgical management, systemic treatment or definitive therapy No specific diagnosis: repeat FNAB with rapid onsite evaluation with or without core needle biopsy |
Laboratory
Cytology description
- WHO Reporting System for Lung Cytopathology describes 5 categories with different malignancy risks: insufficient / inadequate / nondiagnostic, benign, atypical, suspicious for malignancy and malignant
- 1 of the 5 categories should be mentioned in the report
- Final diagnosis should be mentioned whenever possible; if the diagnosis is not specific, the differential diagnosis should be reported (Cancer Cytopathol 2023;131:751)
- Categories (see Table 1)
- Insufficient / inadequate / nondiagnostic
- Specimen lacks sufficient material in quantity or quality for a reliable diagnosis
- This includes cases with low cellularity, poor preparation, fixation or staining, obscuring blood, inflammatory cells or other materials
- Reasons for inadequacy should be reported
- Recommendations: insufficient / inadequate to be used where there is insufficient material and nondiagnostic for cases with benign material and mass on imaging
- Risk of malignancy: 40 - 60%, depending on the sampling technique and imaging characteristics of the lung lesion (Acta Cytol 2023;67:80, Diagn Cytopathol 2018;46:725, Radiology 2017;284:228)
- Risk of malignancy according to sampling techniques
- FNAB: 43 - 53%
- Sputum sample: 0 - 100%
- Bronchial washing: 38 - 81%
- Bronchial brushing: 0 - 75%
- Management
- Correlate clinically and microbiologically with images and discuss with a multidisciplinary team
- Repeat noninvasive sampling (sputum, bronchial washing and bronchial brushing)
- Endoscopic guided FNAB and transthoracic computed tomography guided FNAB if repetition results in the same diagnosis
- Benign
- Specimen shows unequivocal cytopathological features that may or may not be diagnostic of specific inflammation or benign neoplasm
- Includes suppurative or granulomatous inflammation and pulmonary hamartomas
- Report should mention if there is no correlation between cytopathological and imaging findings
- Risk of malignancy: 20 - 40% (Acta Cytol 2023;67:80, Acta Cytol 2020;64:452, Virchows Arch 2021;478:45)
- Risk of malignancy according to sampling techniques
- FNAB: 19 - 64%
- Sputum sample: 0 - 42%
- Bronchial washing: 38 - 42%
- Bronchial brushing: 32 - 38%
- Management
- Correlate clinically and microbiologically with images
- Routine follow up in 3 - 6 months if a diagnosis is benign
- Repeat sampling
- Repeat core needle biopsy or limited resection if the diagnosis is not confirmed
- Atypical
- Specimen shows the features mostly seen in benign lesions (with the possibility of a malignant lesion as well) but it lacks sufficient features in quality or quantity to diagnose either benign or malignant lesions
- Includes reactive changes (metaplasia and hyperplasia), infections (viral), acute respiratory distress syndrome and posttherapy changes
- Risk of malignancy: 50 - 60% (Diagn Cytopathol 2016;44:399, Diagn Cytopathol 2018;46:725, Acta Cytol 2022;66:124)
- Risk of malignancy according to sampling techniques
- FNAB: 46 - 55%
- Sputum sample: 86 - 100%
- Bronchial washing: 62 - 86%
- Bronchial brushing: 79 - 100%
- Management
- Correlate clinically and microbiologically with images and discuss with a multidisciplinary team
- Routine follow up in 3 - 6 months if a diagnosis is benign
- Repeat FNAB with rapid onsite evaluation with or without core needle biopsy if the diagnosis is not confirmed
- Suspicious for malignancy
- Specimen shows cytopathologic features suggestive of malignancy but lacks sufficient features in quality or quantity to make an unequivocal diagnosis of malignancy
- This category offers risk stratification and maintains a high positive predictive value for malignant diagnosis (Diagn Cytopathol 2016;44:399, Acta Cytol 2020;64:452, Diagn Cytopathol 2022;50:164)
- Report should mention suspected malignancies, which include non-small cell carcinoma, neuroendocrine tumors, small and large cell neuroendocrine carcinomas, lymphoma, sarcoma and metastatic carcinomas
- There are no defined cytopathological criteria but the presence of significant cytopathological atypia, including nuclear enlargement, anisonucleosis, nuclear crowding, varying chromatin, variability in cell size and shape and other features associated with malignancy
- When metastatic or neuroendocrine tumor is suspected, ancillary studies (e.g., immunocytochemistry) help change the category from suspicious for malignancy to malignant
- 5% of cases reported in this category (Diagn Cytopathol 2018;46:725)
- Risk of malignancy: 54.5 - 90% (Diagn Cytopathol 2016;44:399, Diagn Cytopathol 2018;46:725, Acta Cytol 2022;66:124)
- Risk of malignancy according to sampling techniques
- FNAB: 75 - 88%
- Sputum sample: 100%
- Bronchial washing: 83 - 100%
- Bronchial brushing: 75 - 100%
- Management
- Correlate clinically and microbiologically with images and consult with a more experienced cytopathologist
- If malignancy is established, initiate definitive treatment
- If there is no correlation that the lesion is malignant, repeat FNAB with rapid onsite evaluation with or without core needle biopsy
- Malignant
- Specimen shows unequivocal cytopathologic features of malignancy with no discrepant features
- Type of malignancy or differential diagnosis should be mentioned
- Subclassifications should be mentioned based on important diagnostic features and immunocytochemistry
- Includes non-small cell carcinoma, small cell neuroendocrine carcinoma, low grade neuroendocrine tumors, neuroendocrine carcinomas of small or large cell types, salivary gland type carcinomas, mesenchymal tumors and secondary malignancies
- Ancillary techniques (e.g., immunocytochemistry)
- TTF1 and p40 to differentiate adenocarcinoma and squamous cell carcinoma (Diagn Cytopathol 2017;45:598, Acta Cytol 2018;62:318, J Thorac Oncol 2022;17:793, J Thorac Oncol 2011;6:489, Curr Oncol 2012;19:e16)
- Napsin A / p40 dual stains preserve tissue in limited samples (Cancer Cytopathol 2016;124:472)
- INSM1, chromogranin and synaptophysin to diagnose low grade neuroendocrine tumors (previously known as carcinoid and atypical carcinoid) and neuroendocrine carcinomas of predominantly small or large cell types
- Risk of malignancy: > 90% (approaching 100%) (Diagn Cytopathol 2016;44:399, Diagn Cytopathol 2018;46:725, Acta Cytol 2022;66:124, Diagn Cytopathol 2020;48:701)
- Risk of malignancy according to sampling techniques
- FNAB: 87 - 100%
- Sputum sample: 100%
- Bronchial washing: 98 - 100%
- Bronchial brushing: 94 - 100%
- Management
- Correlate clinically and microbiologically with images and discuss with a multidisciplinary team
- If malignancy is confirmed, start surgical management, systemic treatment or definitive therapy
- If there is no correlation that the lesion is malignant, repeat FNAB with rapid onsite evaluation with or without core needle biopsy
- Insufficient / inadequate / nondiagnostic
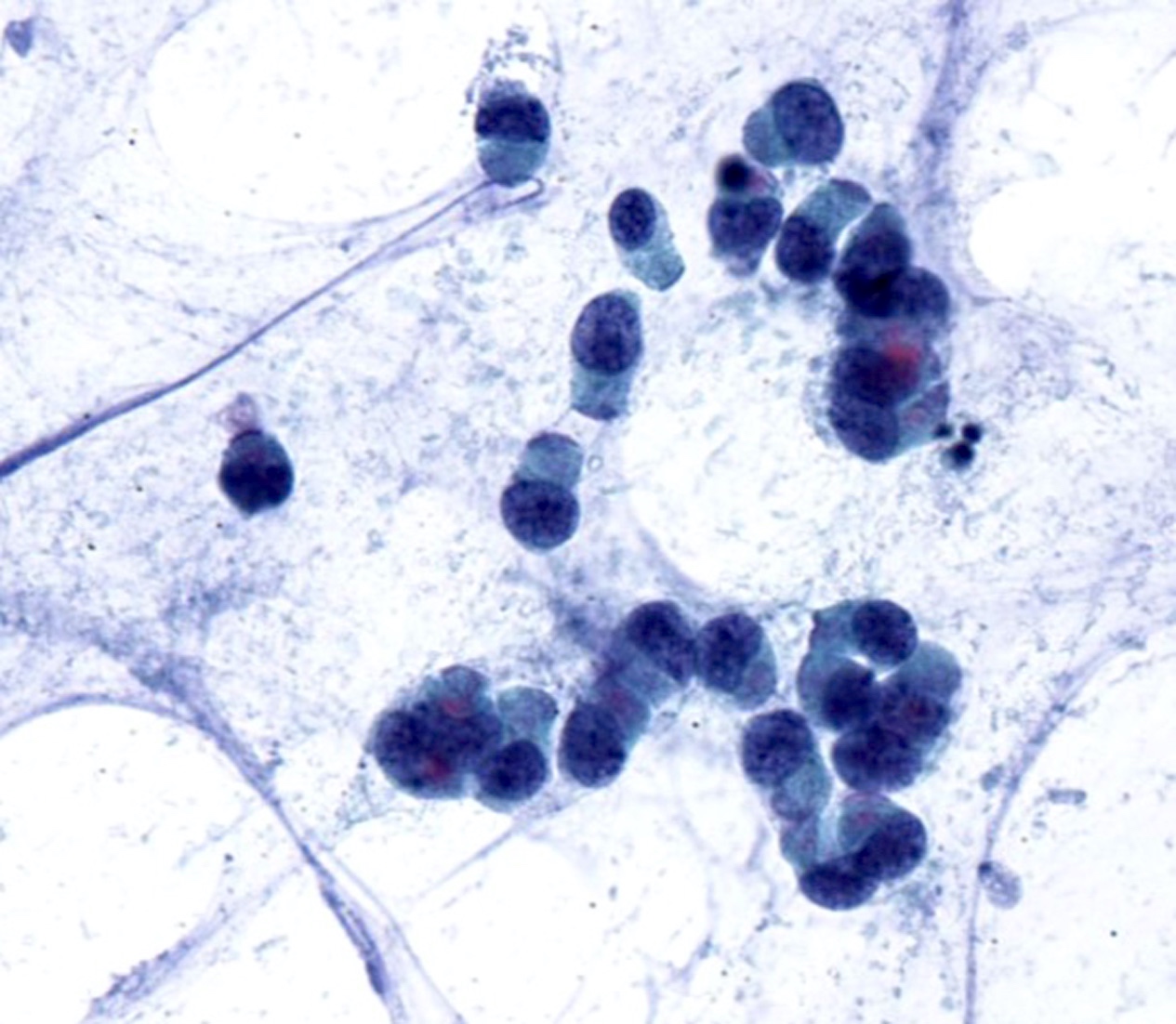
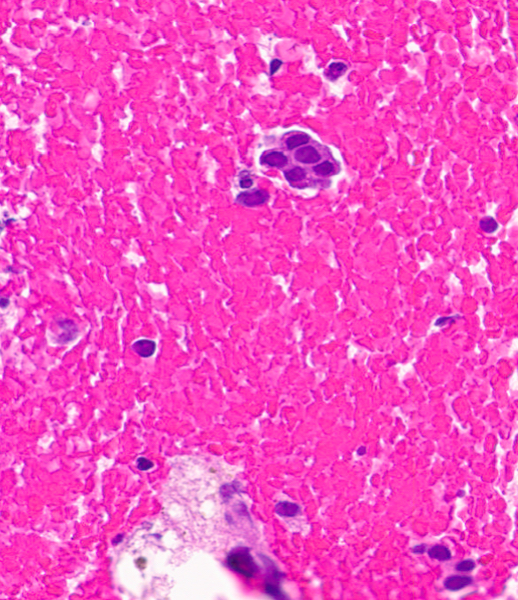
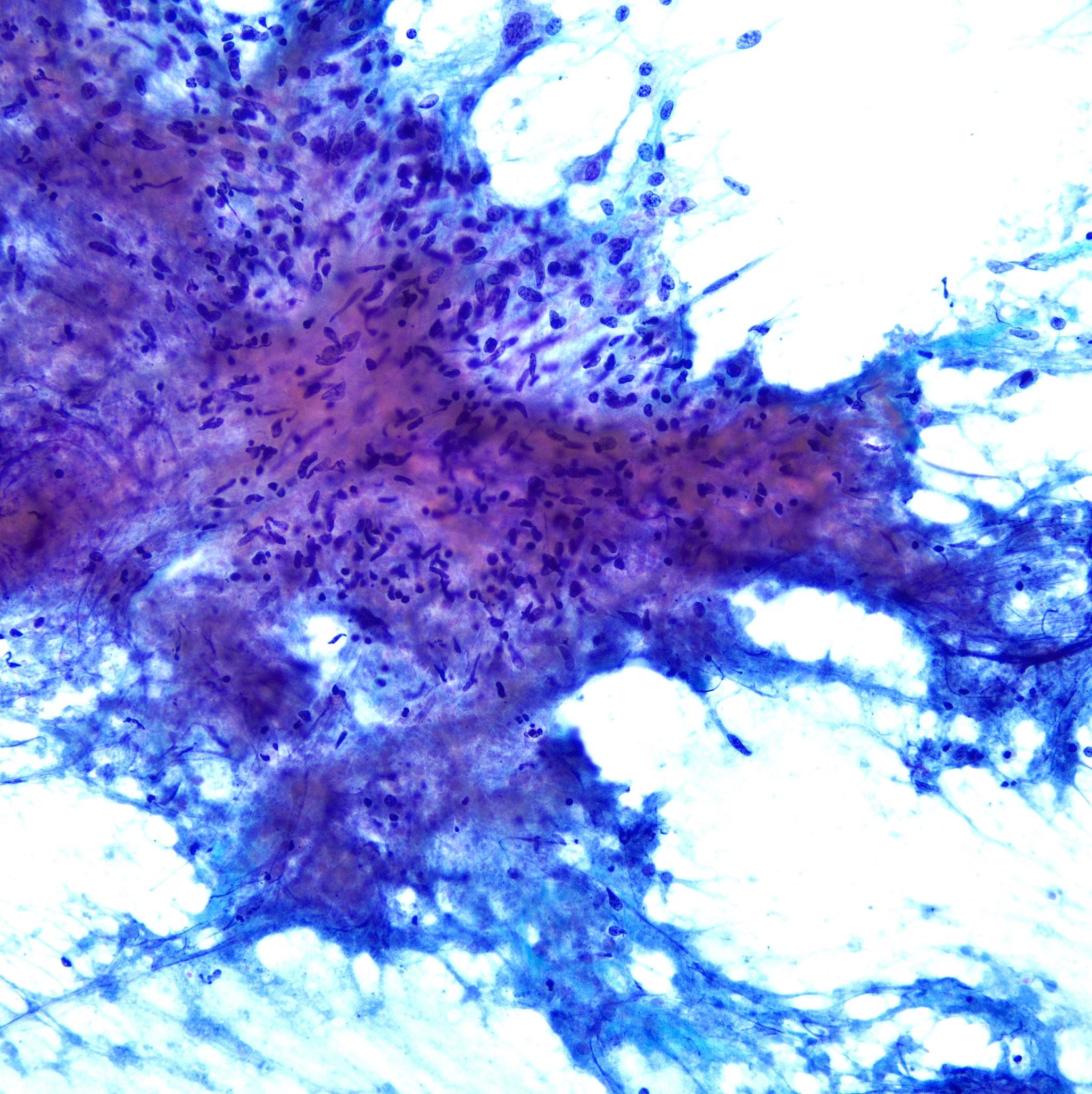
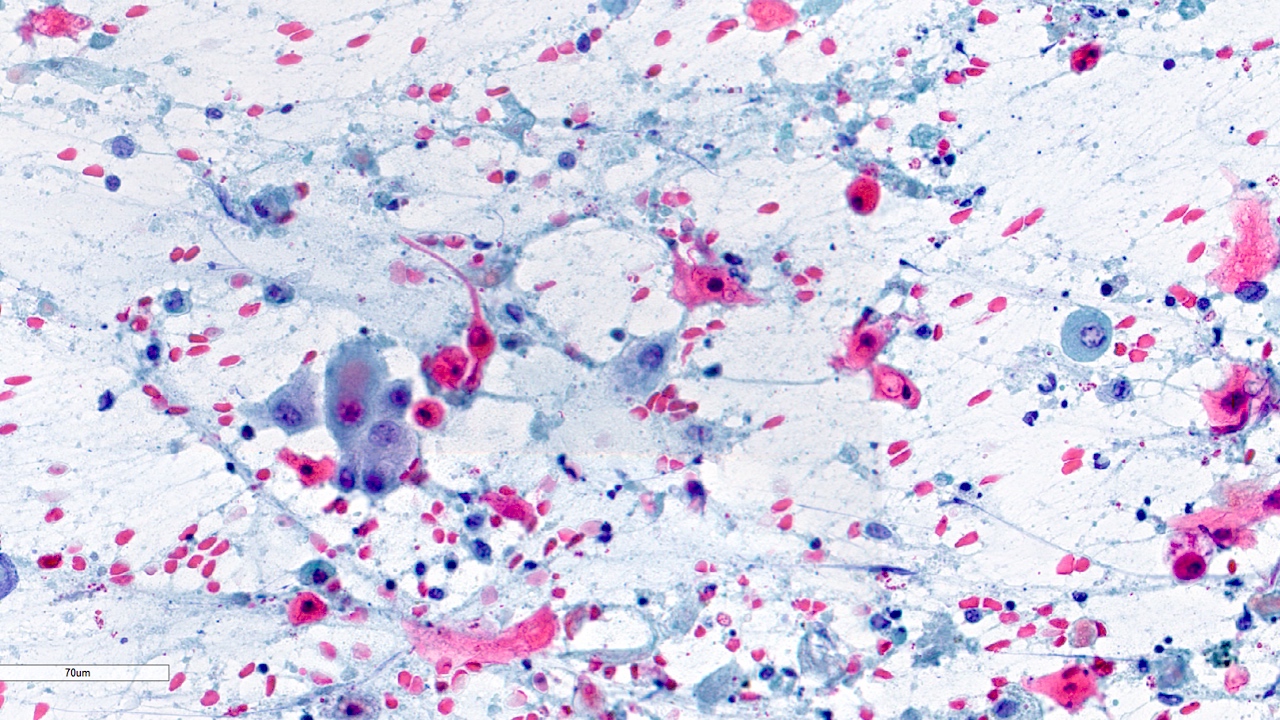
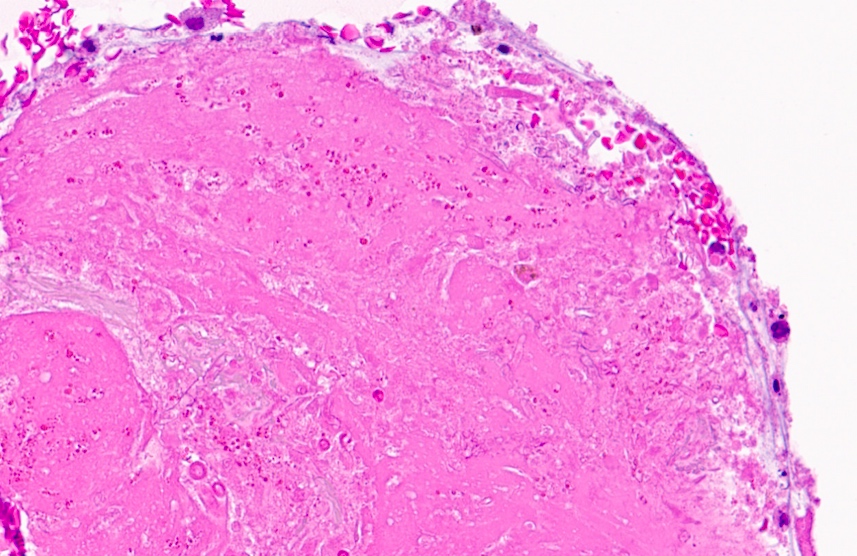
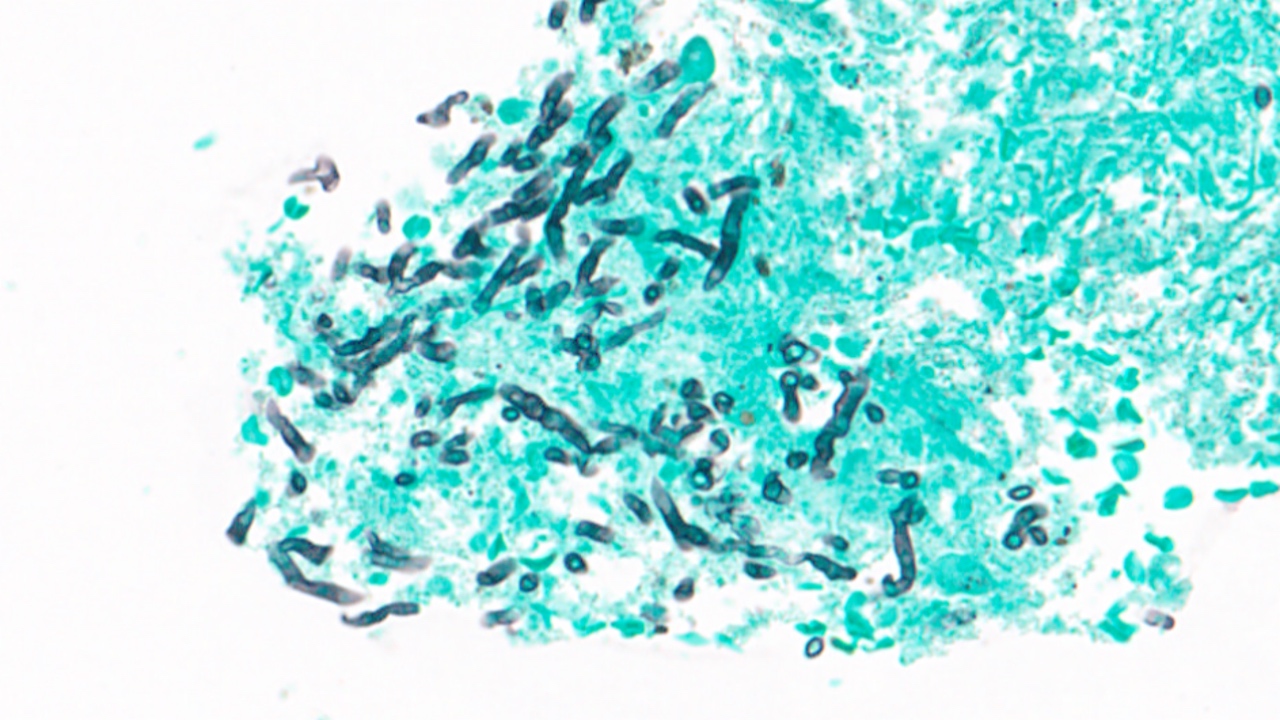
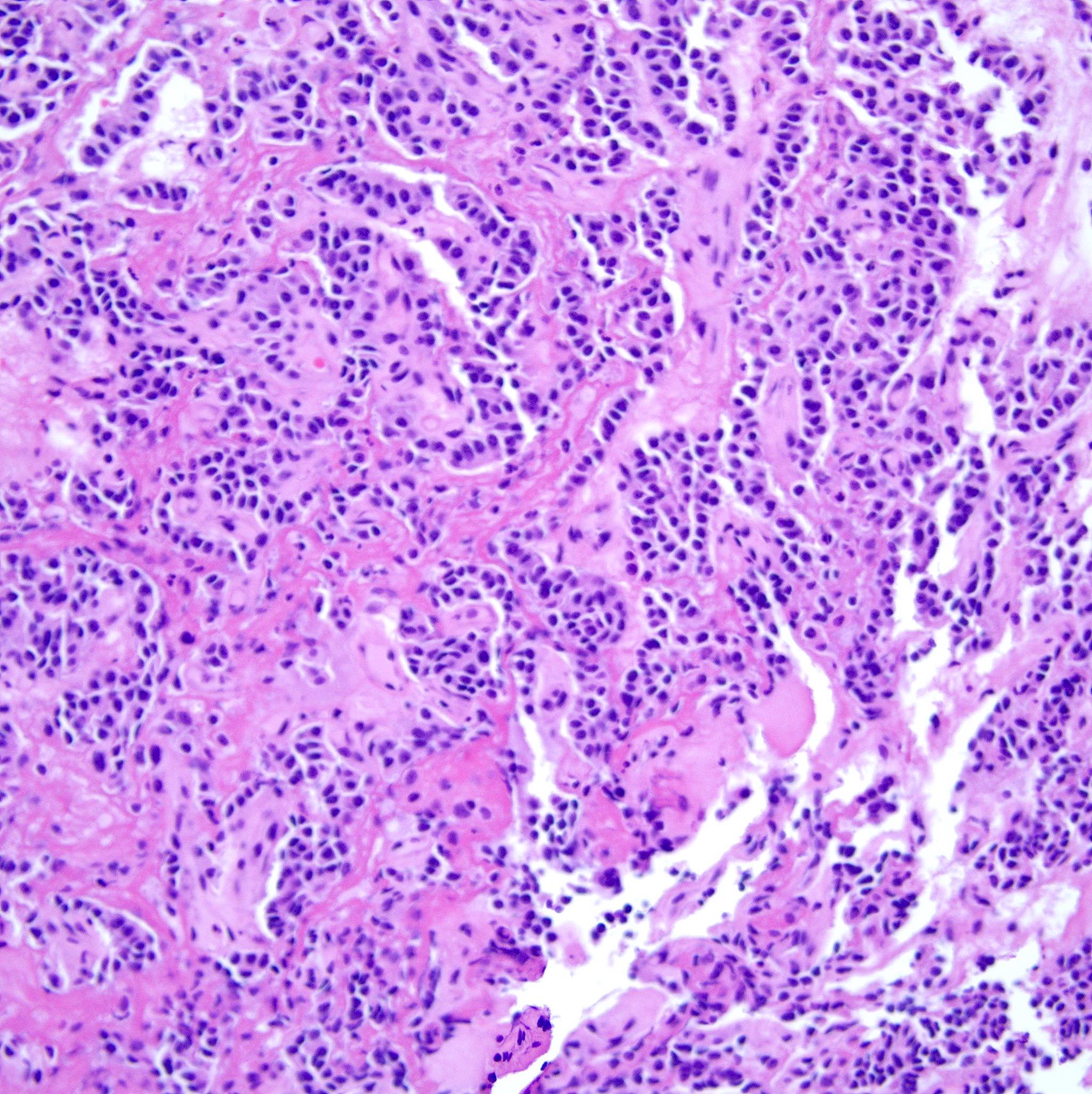
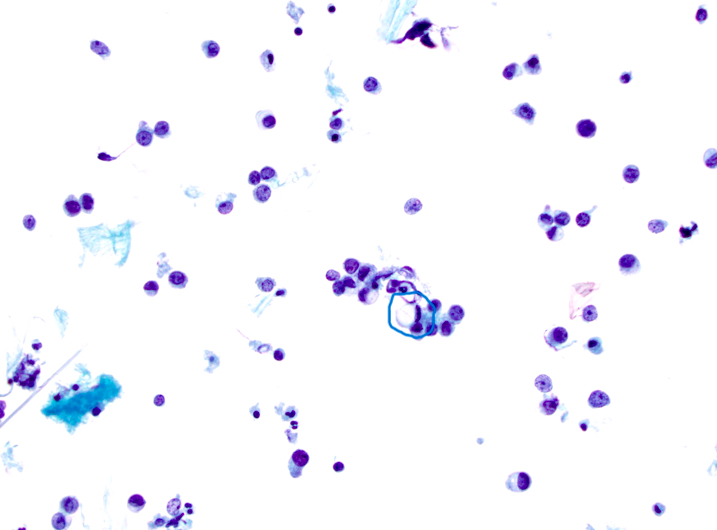
Cytology images
Contributed by Swikrity Upadhyay Baskota, M.B.B.S., M.D. and Samikshya Neupane, M.B.B.S., M.D.
Molecular / cytogenetics description
- Molecular studies are ordered by the cytopathologist or the oncologist once the diagnosis of malignancy has been made
- Commonly ordered molecular markers can be found in the Molecular markers chapter
Videos
The WHO System for Reporting
Lung Cytopathology
by Prof. Dr. Fernando Schmitt
Sample pathology report
- Bronchial brushing:
- Nondiagnostic (see comment)
- Findings: few bronchial epithelial cells with low cellularity
- Comment: Inadequate sample with low cellularity to establish a diagnosis. Resampling is recommended.
- Sputum sample:
- Benign (see comment)
- Findings: presence of alveolar macrophages and inflammatory cells
- Comment: Reactive changes associated with inflammation. No evidence of malignancy.
- Fine needle aspiration biopsy:
- Atypical cells, no definitive diagnosis could be made (see comment)
- Findings: scattered epithelial cells with nuclear enlargement and hyperchromasia; nonspecific findings
- Comment: Resampling is recommended with imaging correlation.
- Bronchoalveolar lavage:
- Suspicious for malignancy (see comment)
- Findings: cells in clusters with enlarged, irregular nuclei, suggestive of malignancy
- Comment: Additional procedures are recommended to confirm the diagnosis.
- Bronchial brushing:
- Malignant, squamous cell carcinoma (see comment)
- Findings: malignant squamous cells with dense cytoplasm and intercellular bridges
- Ancillary testing: p40 positive, TTF1 negative
- Comment: Clinical staging and definitive treatment are recommended.
Notes
- Reporting format examples listed above are essential components of a cytopathology report and are not intended to be mandatory
- Generally, the report should mention the specimen type and diagnostic categories, followed by either a definitive diagnosis or a list of differential diagnoses whenever applicable
- Clear diagnostic summary with a microscopic description along with comments and suggestions for subsequent clinical management should also be made available
- An addendum to report additional ancillary studies can also be added to the report
Board review style question #1
Board review style answer #1
D. A morphologic diagnosis associated with various conditions. Granulomatous inflammation is a cytomorphologic diagnosis observed with or without necrosis and can be associated with a variety of conditions, including not only infections (fungal, parasitic, mycobacterial) but also sarcoidosis, chronic inflammation and malignancies. Answers A, B and C are incorrect because while granulomatous inflammation can be associated with infections, carcinomas and sarcoidosis, granulomatous inflammation can also be seen in a variety of other conditions not limited to these.
Comment Here
Reference: WHO reporting system for lung cytopathology
Comment Here
Reference: WHO reporting system for lung cytopathology
Board review style question #2
Which immunostain(s) will confirm the diagnosis of adenocarcinoma in a lung cytology specimen?
- CD45 immunostaining
- EGFR mutation testing
- p40 and CK5/6 immunostaining
- TTF1 and CK7 immunostaining
Board review style answer #2
D. TTF1 and CK7 immunostaining. The cytomorphologic features of intracytoplasmic mucin, acini or tubule formation along with immunostains positive for nuclear TTF1 and cytokeratin 7 (cytoplasmic) are helpful for diagnosis of adenocarcinoma in cytology specimens.
Answer A is incorrect because CD45 stained cells are used to determine the prognosis of small cell lung cancer (SCLC).
Answer B is incorrect because EGFR mutation testing is molecular testing done to determine the targeted therapies.
Answer C is incorrect because p40 and CK5/6 are positive in squamous cell carcinoma.
Comment Here
Reference: WHO reporting system for lung cytopathology
Comment Here
Reference: WHO reporting system for lung cytopathology