Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Ding CKC, Wen KW. Serrated lesions - general. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/colonserratedlesions.html. Accessed March 31st, 2025.
Definition / general
- Lesions and polyps that are characterized by a serrated (sawtooth or stellate) architecture of the epithelium
- Include hyperplastic polyps (HPs), sessile serrated lesions (SSLs), traditional serrated adenomas (TSAs) and serrated adenoma, unclassified
Essential features
- Colorectal epithelial lesion with serrated architecture and arises from serrated pathway (BRAF or KRAS mutation)
- Include hyperplastic polyps, sessile serrated lesions, traditional serrated adenomas and serrated adenoma, unclassified
- Presence of dysplasia should be reported but grading of dysplasia is not recommended
- Size or location alone is not diagnostic for either hyperplastic polyps or sessile serrated lesions
Terminology
- Names that usually refer to sessile serrated lesions but are not recommended by WHO 5th edition (2019):
- Sessile serrated adenoma or sessile serrated polyp
ICD coding
- ICD-O: 8213/0 - serrated adenoma
- ICD-11:
- DB35.0 - hyperplastic polyp of large intestine
- 2E92.4 & XH2F06 - benign neoplasm of the large intestine & sessile serrated adenoma
- 2E92.4 & XH63V9 - benign neoplasm of the large intestine & sessile serrated polyp
- 2E92.4 & XH9PD9 - benign neoplasm of the large intestine & traditional sessile serrated adenoma
Epidemiology
- Hyperplastic polyps: ~30% of all colorectal polyps
- Sessile serrated lesions: ~10% of all colorectal polyps
- Traditional serrated adenomas: ~1% of all colorectal polyps
- References: Virology 2014;449:181, Am J Gastroenterol 2010;105:2656, Histopathology 2010;56:581, Gastroenterology 2006;131:1400, Gut 2018;67:456
Sites
- Hyperplastic polyps:
- Include goblet cell rich hyperplastic polyps (GCHPs) and microvesicular hyperplastic polyps (MVHPs)
- Mostly found in distal colon and rectum (left)
- Sessile serrated lesions: mostly found in proximal colon (right), particularly sessile serrated lesions with dysplasia
- Traditional serrated adenomas: mostly found in distal colon (left); exception: flat traditional serrated adenomas (more often in the proximal colon)
Pathophysiology
- About 30% of all colorectal carcinomas arise via serrated neoplasia pathway (Histopathology 2015;66:49, J Gastroenterol 2013;48:287)
- Lesions arise from BRAF serrated pathway:
- BRAF mutation → microvesicular hyperplastic polyps → CpG island methylator phenotype (CIMP) high → sessile serrated lesions
- Sessile serrated lesions → MLH1 methylation / WNT activation → MLH1 deficient sessile serrated lesions with dysplasia → BRAF mutated mismatch repair (MMR) deficient carcinoma
- Sessile serrated lesions → WNT activation + TP53 mutation → MLH1 proficient sessile serrated lesions with dysplasia → BRAF mutated mismatch repair proficient carcinoma
- BRAF mutation + CpG island methylator phenotype → BRAF mutated traditional serrated adenomas → WNT activation / TP53 mutation → BRAF mutated traditional serrated adenomas with high grade dysplasia → p16 silencing → BRAF mutated mismatch repair proficient carcinoma
- BRAF mutation → microvesicular hyperplastic polyps → CpG island methylator phenotype (CIMP) high → sessile serrated lesions
- Lesions arise from KRAS serrated pathway:
- Goblet cell rich hyperplastic polyps → CpG island methylator phenotype low → KRAS mutated traditional serrated adenomas → WNT activation + TP53 mutation → KRAS mutated traditional serrated adenomas with high grade dysplasia → KRAS mutated mismatch repair proficient carcinoma
- Activation of BRAF or KRAS is thought to be mutually exclusive (Mod Pathol 2015;28:414, Gastroenterology 2006;131:1400, Am J Surg Pathol 2014;38:1290, Am J Surg Pathol 2004;28:423)
Etiology
- Similar to colorectal adenocarcinoma
- Increased risk associated with consumption of processed food and red meat, alcohol, excess body fat (Lancet Oncol 2015;16:1599, Ann Oncol 2017;28:1788, N Engl J Med 2016;375:794)
- Decreased risk associated with consumption of dietary fiber and dairy products, increased levels of physical activity (Ann Oncol 2017;28:1788, BMJ 2016;354:i3857)
Diagrams / tables
Clinical features
- Usually asymptomatic; bleeding is rare
- Mostly sessile
- Reference: Am J Gastroenterol 2016;111:516
Diagnosis
- Usually asymptomatic and discovered at surveillance endoscopy (Am J Gastroenterol 2016;111:516)
- Relevant endoscopy modalities:
- Chromoendoscopy
- Narrow band imaging
Prognostic factors
- Low risk features: repeat colonoscopy in 5 years
- No dysplasia
- ≤ 2 polyps
- Size: < 1 cm
- High risk features: repeat colonoscopy in 3 years
- Traditional serrated adenomas
- Sessile serrated lesions with dysplasia
- ≥ 3 polyps
- Size: ≥ 1 cm
- > 10 cumulative adenomatous polyps or SSL
- Reference: NCCN: NCCN Guidelines - Colorectal Cancer Screening [Accessed 12 November 2021]
Case reports
- 12 year old boy with 10 mm polypoid lesion in the lower rectum (World J Gastroenterol 2017;23:4462)
- 45 year old woman with ulcerative colitis and serrated polyposis syndrome (Cureus 2021;13:e14591)
- 66 year old man with positive fecal occult blood test and more than 10 polyps larger than 10 mm (Am J Case Rep 2017;18:304)
Treatment
- Endoscopic polypectomy
- Lesions with low risk pathology features: repeat colonoscopy in 5 years
- Lesions with high risk pathology features: repeat colonoscopy in 3 years
- > 10 cumulative adenomatous polyps or SSL
- Genetic testing for polyposis syndrome
- If genetic testing is negative, repeat colonoscopy within 1 - 3 years
- Incomplete or piecemeal polypectomy for large (> 1 cm) polyp and pathology shows no invasive cancer
- Management depends on if high risk endoscopic features present
- Piecemeal resection of sessile serrated lesions > 2 cm, repeat colonoscopy in 6 months
- In general, SSLs without dysplasia are managed like tubular adenomas and SSLs with any grade dysplasia are managed like high risk adenomas but may need even more frequent surveillance
- References: NCCN: NCCN Guidelines - Colorectal Cancer Screening [Accessed 12 November 2021], Gastroenterology 2020;158:1131
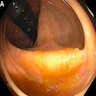
Clinical images
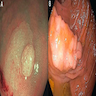
Gross description
- Endoscopic appearance:
- Distal hyperplastic polyps: usually small (< 5 mm), discrete mucosal elevations (Gastrointest Endosc 2003;58:S3)
- Proximal hyperplastic polyps and sessile serrated lesion: pale, poorly defined, sessile to flat lesions covered with a mucus cap and a rim of debris or bubbles imparting a cloud-like surface (Gastrointest Endosc 2011;74:1360, Gastrointest Endosc 2013;77:916, Gastrointest Endosc 2003;58:S3)
- Distal traditional serrated adenomas: usually polypoid, broad based, large protuberant lesions with villiform architecture (raspberry or pinecone appearance) (Am J Surg Pathol 2008;32:21)
- Proximal traditional serrated adenomas: usually flat and laterally spreading (Oncol Lett 2011;2:785)
- Example of gross description in pathology report:
- Specimen is received in formalin and consists of 3 white to red-tan, ovoid, soft tissue fragments, ranging from 0.2 cm up to 0.5 cm
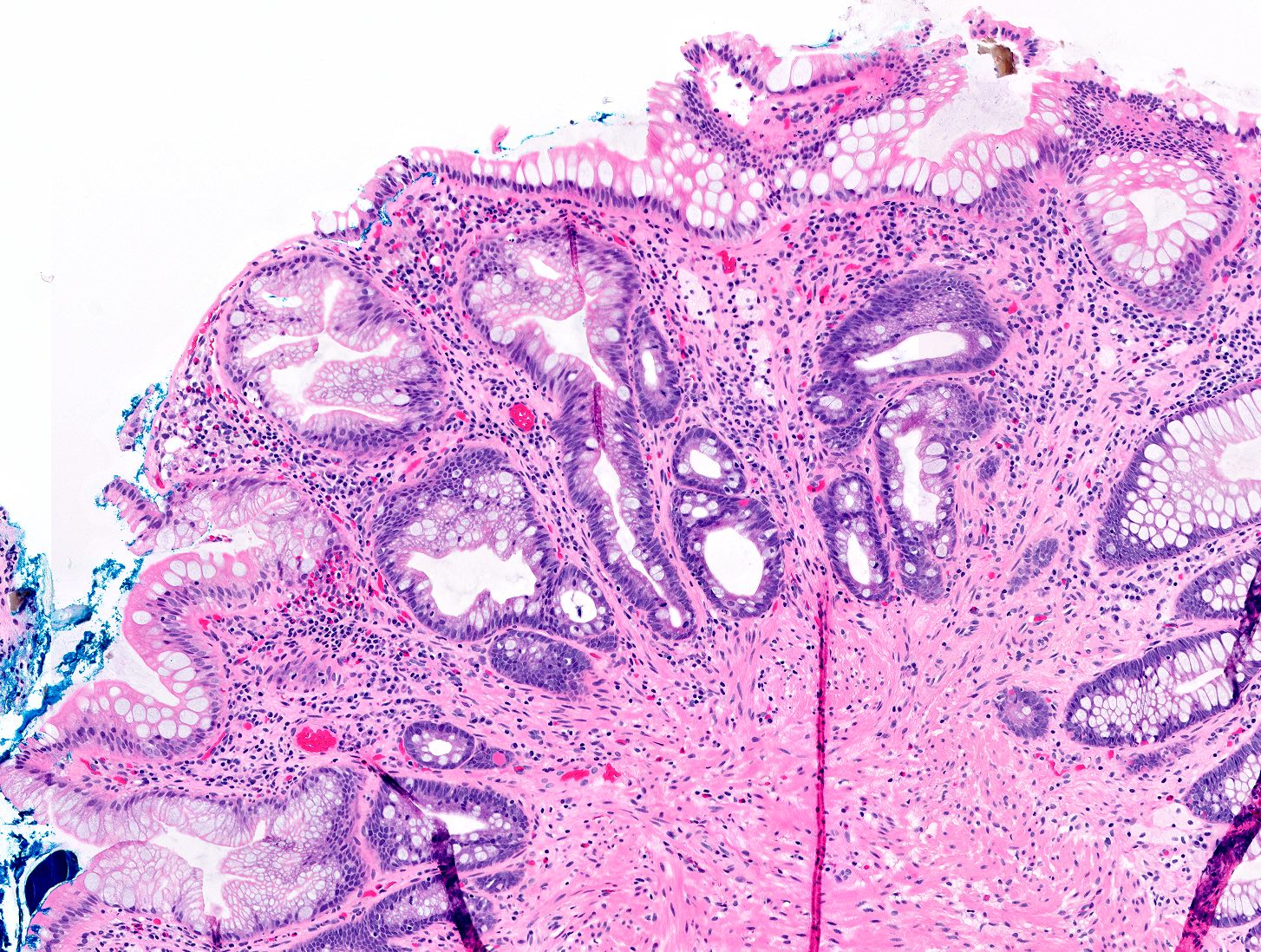
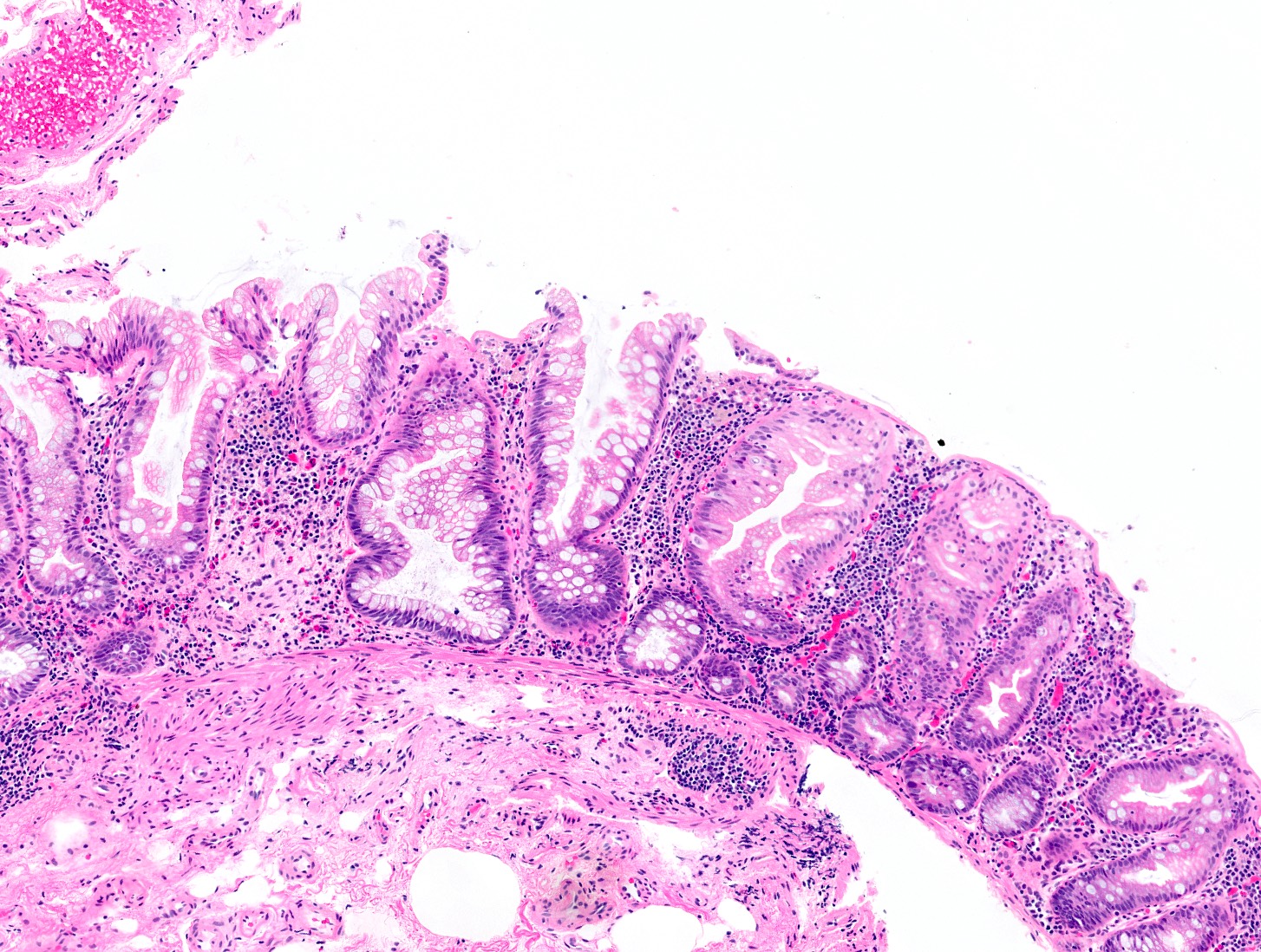
Microscopic (histologic) description
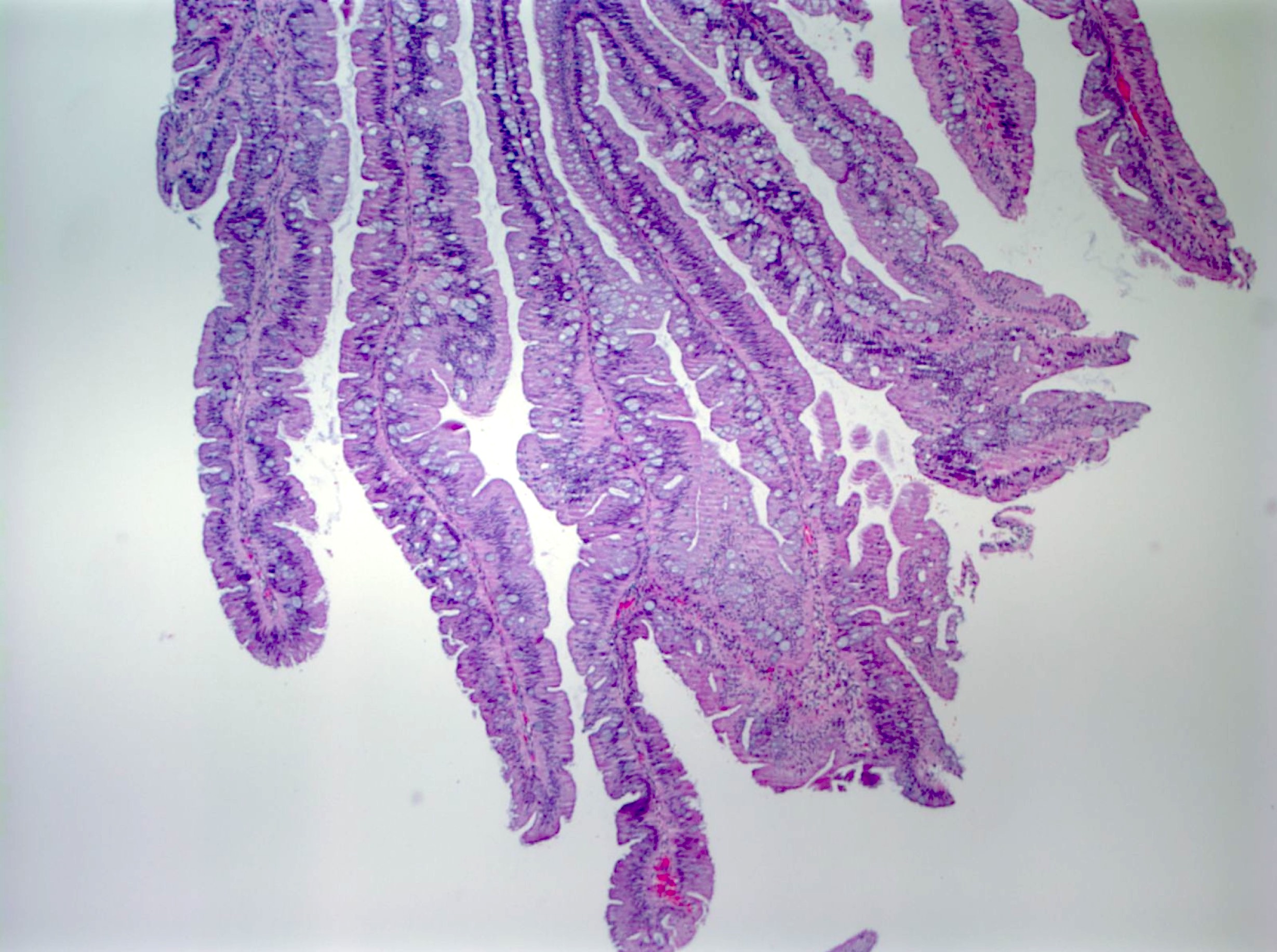
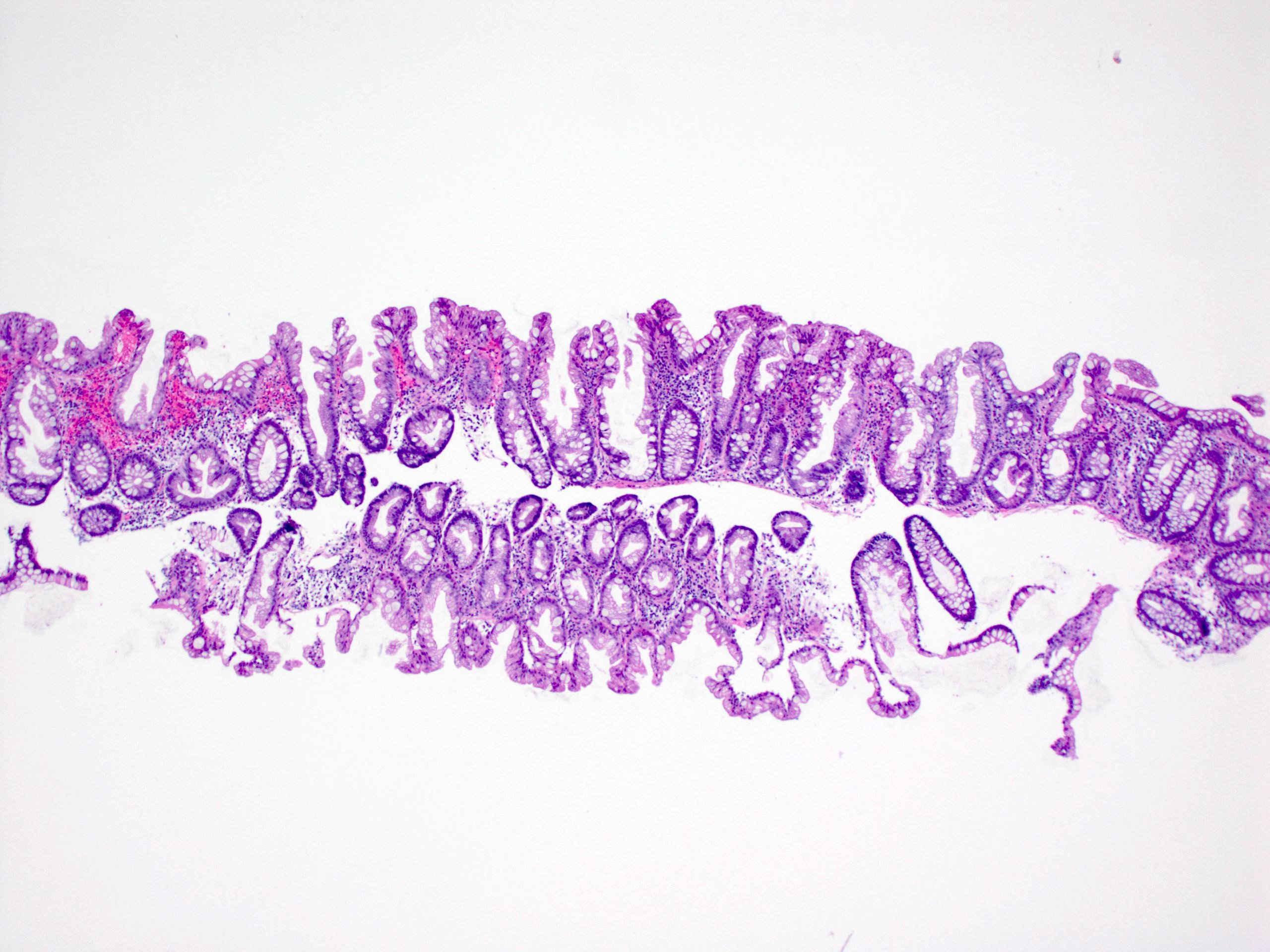
- Hyperplastic polyps:
- Superficial serrated epithelium and funnel shaped, evenly spaced crypts
- Proliferative zones confined to crypt bases
- No basal dilatation, architecture distortion or submucosal misplacement
- No cytologic dysplasia
- Individual crypt branching may occur
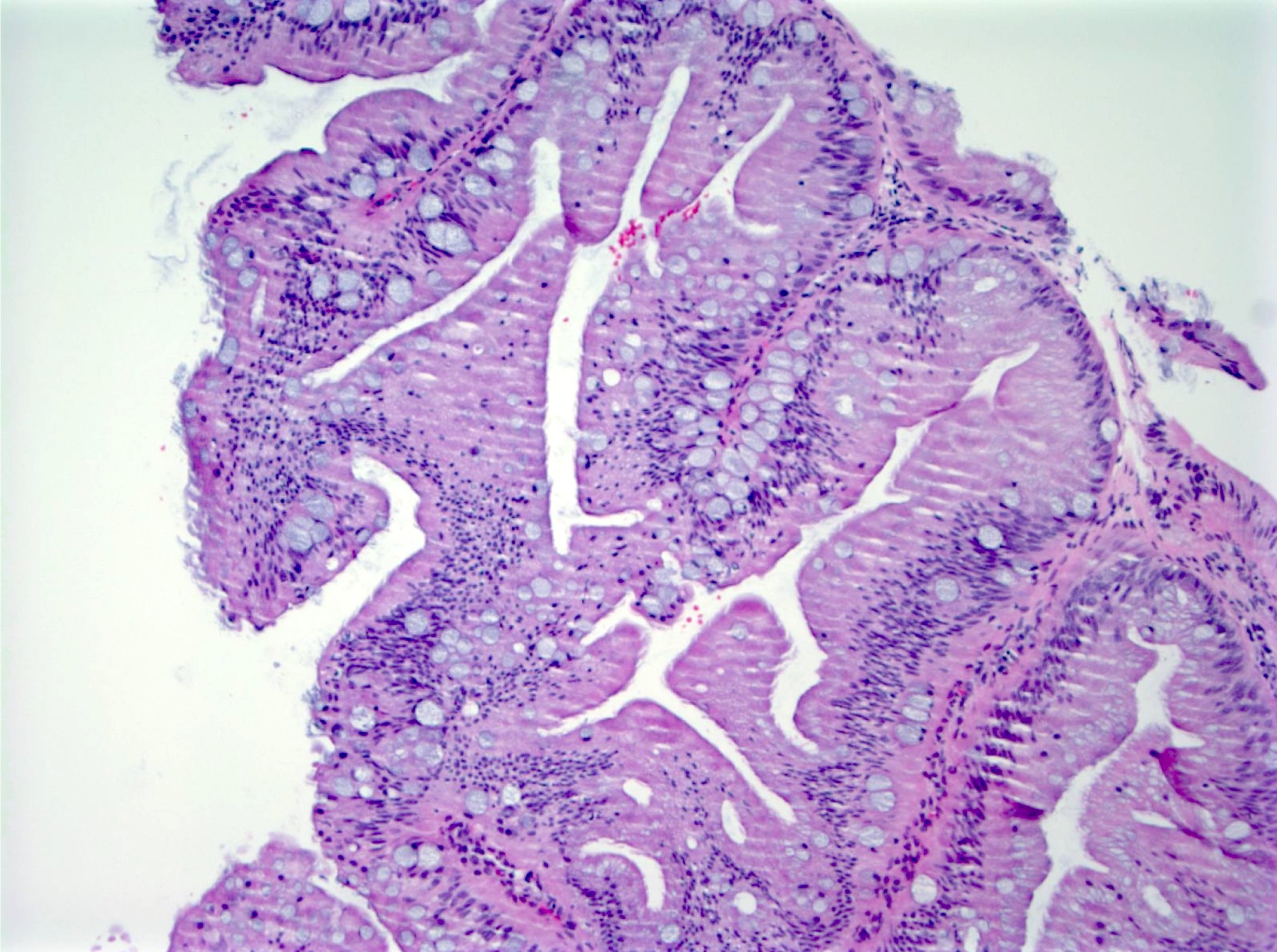
- Microvesicular hyperplastic polyps:
- Microvesicular: abundant cytoplasm with fine apical vacuoles, occasional goblet cells present
- Cross sections of crypts: stellate lumina (star shaped)
- Diagnosis of exclusion: other criteria of sessile serrated lesions are not met
- Goblet cell hyperplastic polyps:
- Subtle superficial epithelial serrations, mimicking reactive epithelium or hyperplasia, thus easily overlooked
- Goblet cell only in epithelium; no microvesicular cell
- Cross sections of crypt: round, not stellate
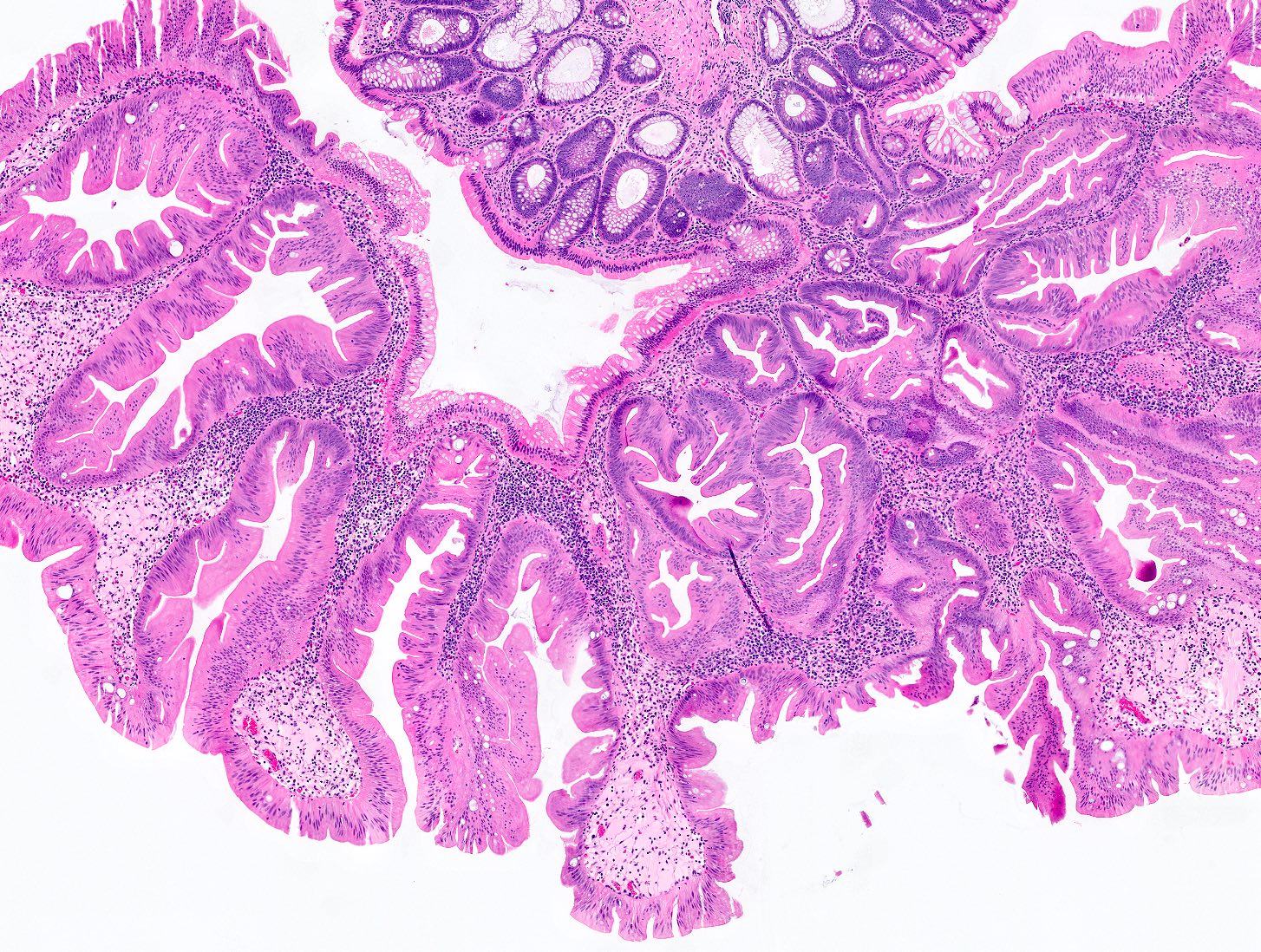
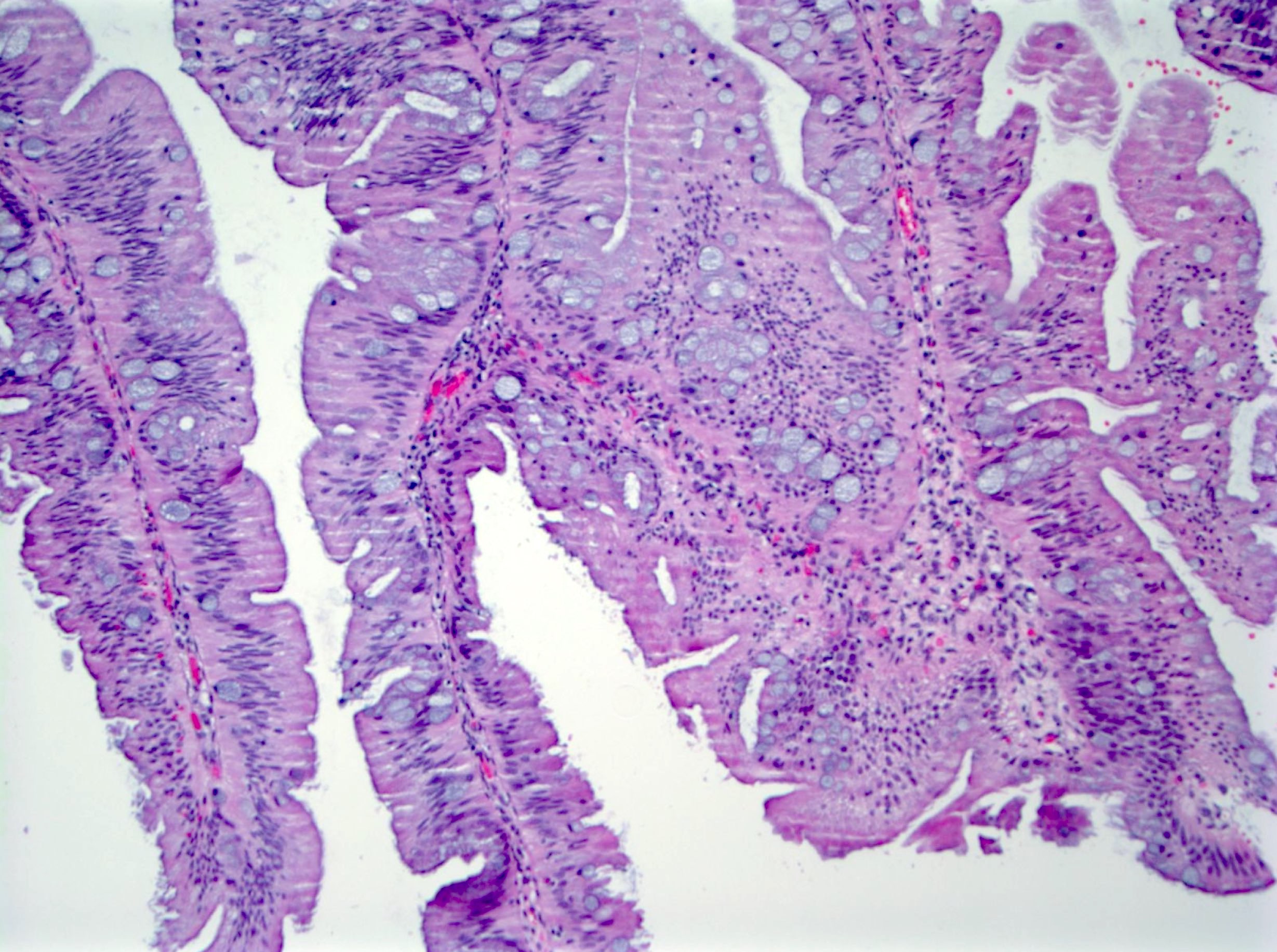
- Sessile serrated lesions:
- Crypts with prominent serrations and overall architecture distortion, defined as unequivocal presence of at least 1 of the following features:
- Horizontal growth along the muscularis mucosae
- Dilation of the crypt base (basal third of the crypt)
- Serrations extending into the crypt base
- Asymmetrical proliferation
- Boot shaped crypts
- Branching of crypts is not sufficient for diagnosis
- Size, location and endoscopic appearance alone should not be used to make the diagnosis of sessile serrated lesions but can be helpful when diagnostic features are ambiguous or in tangential sectioned specimen
- Deeper levels may be helpful for poorly oriented specimen
- Potential pitfall: mucosal herniation by smooth muscle fibers
- Mucosal herniation often associated with lipomatous areas or lymphoid aggregates
- Crypts with prominent serrations and overall architecture distortion, defined as unequivocal presence of at least 1 of the following features:
- Microvesicular hyperplastic polyps and sessile serrated lesions can be associated with stromal proliferation resembling perineural cells (Am J Surg Pathol 2011;35:1373, Ann Diagn Pathol 2018;35:48)
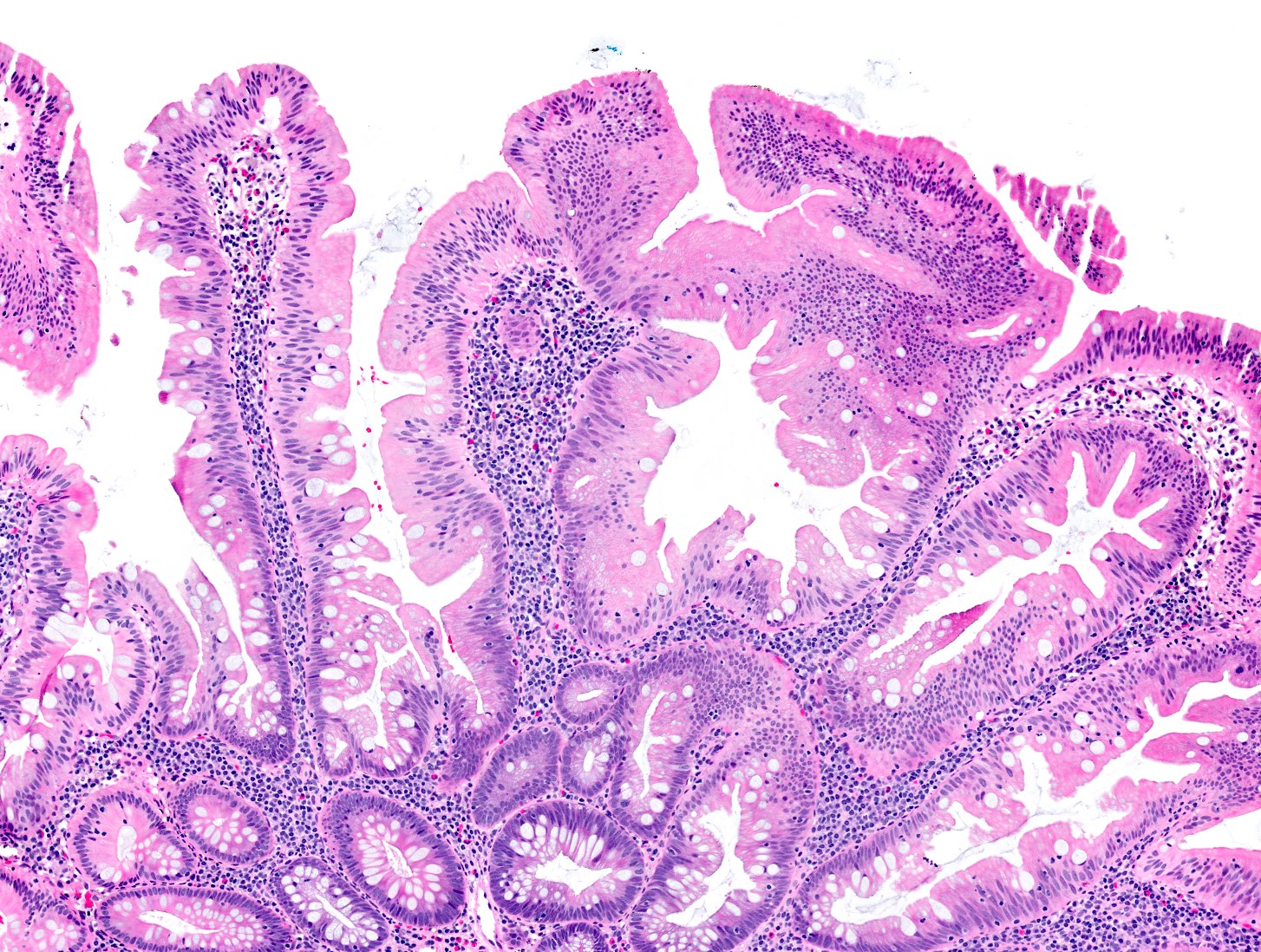
- Sessile serrated lesions with dysplasia:
- 2 - 5% of all sessile serrated lesions
- Dysplastic areas are usually sharply demarcated from the nondysplastic areas (abrupt transition)
- Heterogenous morphology (multiple dysplastic patterns) within single lesion
- Architectural changes including:
- Villous architecture
- Crypt elongation
- Crowding of crypts with complex branching
- Cribriforming
- Excessive or reduced luminal serration
- Cytological changes including:
- Intestinal dysplasia, mimicking tubular adenoma
- Prominent nucleoli
- Eosinophilic cytoplasm
- Increased mitoses
- 4 dysplasia patterns described in sessile serrated lesions (Mod Pathol 2017;30:1728):
- Dysplasia, not otherwise specified:
- Most common pattern
- Easily identifiable architectural changes and obvious cytologic atypia
- Frequently associated with MLH1 loss
- Minimal deviation:
- Subtle architectural changes (crypt crowding)
- Cells with hypermucinous cytoplasm or gastric type change
- Mild hyperchromasia
- Mitotic figures present at higher part of the crypts
- Requires MLH1 loss for diagnosis
- Serrated dysplasia:
- Dense eosinophilic appearance at low magnification
- Closely packed small glands with reduced serration and cribriforming
- Marked nuclear atypia
- Rarely associated with MLH1 loss (retained MLH1 expression)
- Adenomatous dysplasia: similar appearance to conventional adenomas; rarely associated with MLH1 loss
- Dysplasia, not otherwise specified:
- Multiple patterns are often found in a single lesion
- Loss of MLH1 expression is not specific for sessile serrated lesions with dysplasia but can help when dysplastic features are subtle
- Grading of dysplasia is not recommended because of low reproducibility, high heterogeneity of the lesion and lack of correlation of MLH1 expression loss (Mod Pathol 2017;30:1728, Histopathology 2015;66:49)
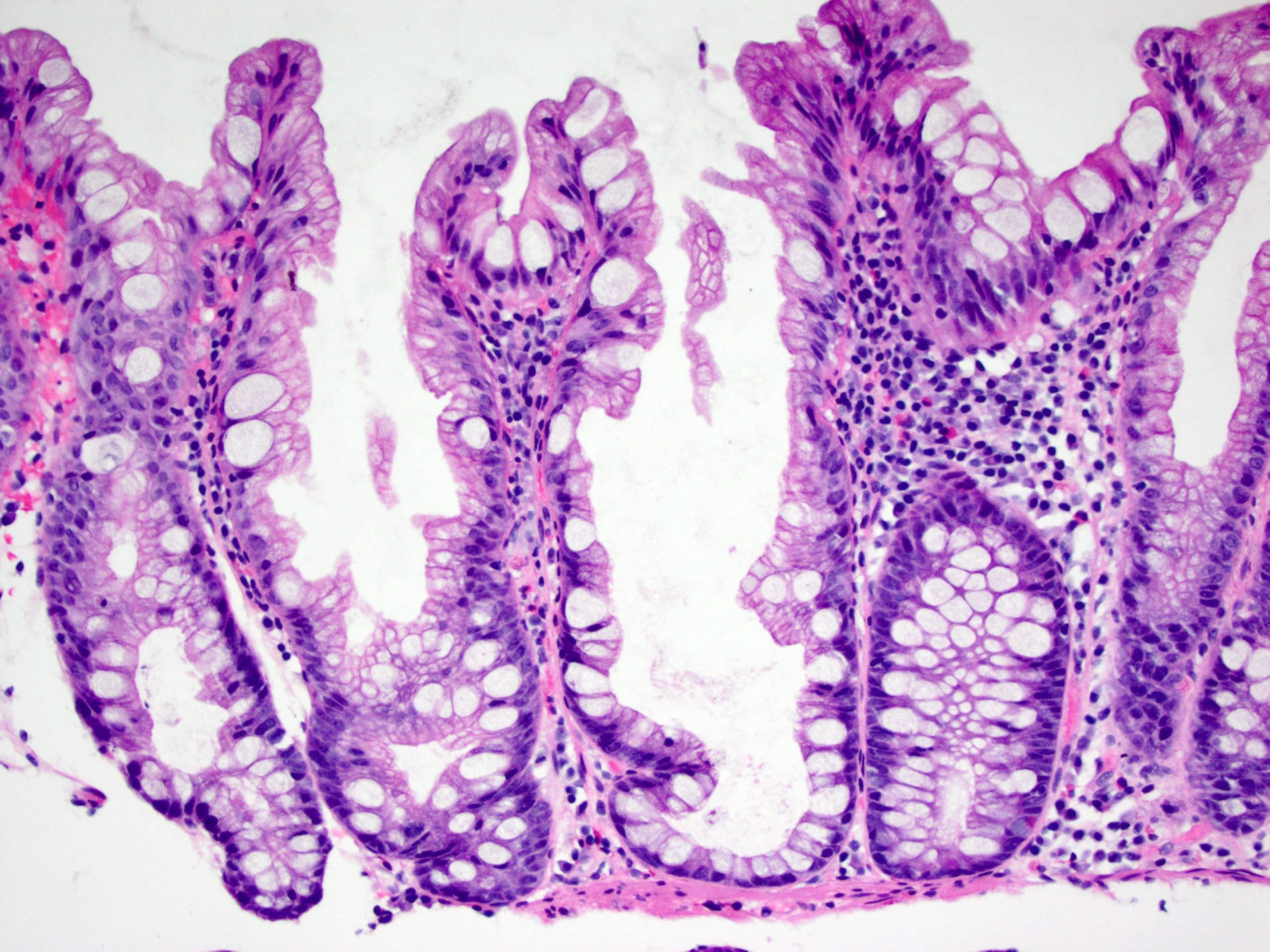
- Traditional serrated adenomas:
- 3 major morphologic features (Am J Surg Pathol 1990;14:524):
- Slit-like serration (mimicking small intestinal mucosa)
- Tall columnar cells with intense eosinophilic cytoplasm and bland elongated (pencillate) nuclei
- Ectopic crypt formations
- Epithelial buds not connected to the muscularis mucosae
- Can have mild atypia; often with cell crowding and frequent mitoses
- Always noted in polypoid traditional serrated adenomas; less common in flat traditional serrated adenomas
- Diagnosis: ≥ 2 major morphologic features present in ≥ 50% of the polyp
- Other common histological features: tubulovillous architecture, intraepithelial lymphocytes (Am J Surg Pathol 2008;32:21)
- Often adjacent / associated with a precursor polyp (hyperplastic polyps or sessile serrated lesions)
- Variants:
- Goblet cell rich / mucinous traditional serrated adenomas: traditional serrated adenomas with a lot of goblet cells
- Filiform traditional serrated adenomas: traditional serrated adenomas with bulbous and edematous tips of villi
- 3 major morphologic features (Am J Surg Pathol 1990;14:524):
- Traditional serrated adenomas with overt dysplasia:
- Intestinal type dysplasia: resembling dysplasia seen in conventional adenoma
- Serrated type dysplasia: resembling serrated type dysplasia seen in sessile serrated lesions
- No specific surveillance guidelines; however, traditional serrated adenomas with high grade dysplasia considered worrisome (often with molecular abnormalities), which should be reported and necessitate closer surveillance
- Serrated adenoma, unclassified:
- Serrated polyp with dysplasia and morphologically cannot fit into traditional serrated adenomas, sessile serrated lesions or conventional adenoma criteria
- Serrated tubulovillous adenoma
- Molecular features intermediate between traditional serrated adenomas and tubulovillous adenoma (TVA) (Histopathology 2018;73:444, Histopathology 2016;68:578)
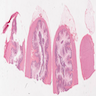
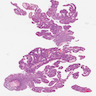
Microscopic (histologic) images
Contributed by Chien-Kuang Cornelia Ding, M.D., Ph.D., Kwun Wah Wen, M.D., Ph.D., Raul S. Gonzalez, M.D. and Enoch Kuo, M.D.
Virtual slides
Negative stains
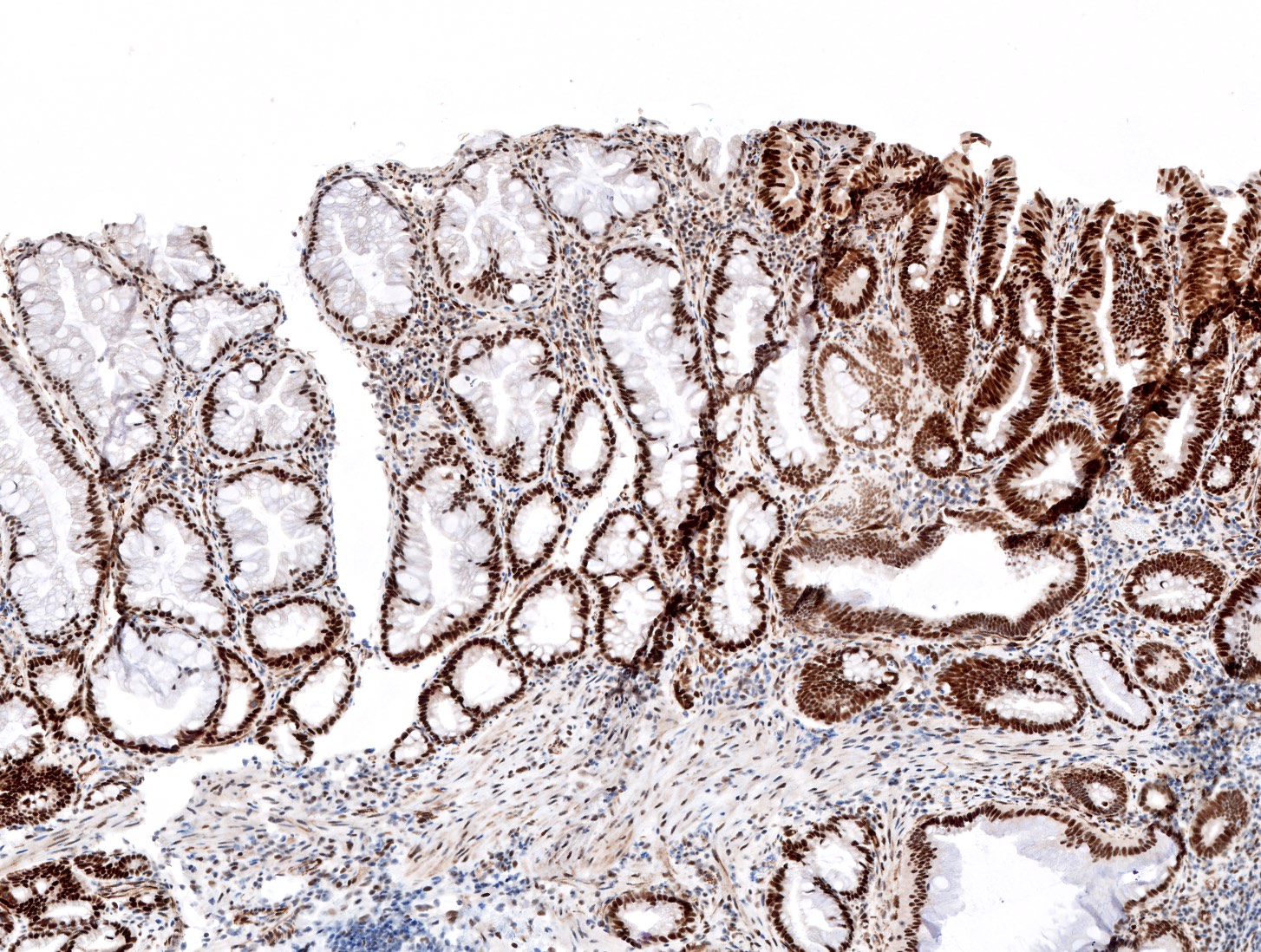
- MLH1 stain (Mod Pathol 2017;30:1728)
- Although not specific, MLH1 expression loss can support the diagnosis of sessile serrated lesions with dysplasia
- Recommended in:
- Equivocal cytological atypia with inflammation, mucosal prolapse or cross section of crypt bases
- Fragmented specimen with separate fragment of epithelial dysplasia from sessile serrated lesions
- Sessile serrated lesions with morphology features support minimal deviation dysplasia (MLH1 loss is required for diagnosis)
Molecular / cytogenetics description
- Molecular / cytogenetic testing is not required for establishing the diagnosis; however, the following tests could be helpful based on clinical settings:
- DNA sequencing to determine:
- BRAF status
- KRAS status
- Activation mutation of genes in WNT pathway
- TP53 status
- CpG methylation analysis
- To determine if the tumor has CpG island methylator phenotype
- Associated with MLH1 methylation and microsatellite instability
- Microsatellite instability (MSI) test / DNA mismatch repair
- MSI high (deficient mismatch repair)
- MSI low (cannot be determined)
- MSI stable (intact mismatch repair)
- DNA sequencing to determine:
Videos
The problematic colorectal polyp by Dr. Rish K. Pai, on the serrated pathway
Sample pathology report
- Transverse colon, polyp, endoscopic mucosal resection:
- Sessile serrated lesion with dysplasia (see comment)
- Comment: Immunohistochemical stain for MLH1 was performed and is intact. This does not exclude the diagnosis of dysplasia within sessile serrated lesion.
- Sigmoid / rectum colon, polyps, biopsy:
- Fragments of serrated polyp (see comment)
- Comment: Sections show fragments of serrated polyp. Given the orientation of the specimen, the base of the serrated polyp is not well visualized to distinguish a hyperplastic polyp from sessile serrated lesion; however, the lack of lumina dilation and some narrow crypts favor a hyperplastic polyp. Level sections were performed to confirm the diagnosis.
Differential diagnosis
- Sessile serrated lesion versus Microvesicular hyperplastic polyps:
- Current recommendation: a polyp with single classical sessile serrated polyp type crypt is sufficient for a diagnosis of sessile serrated lesions over hyperplastic polyps (Am J Surg Pathol 2014;38:158, Am J Gastroenterol 2012;107:1315)
- Deeper levels are often helpful
- Size, location and endoscopic appearance are not part of the criteria for pathological diagnosis of hyperplastic polyps versus sessile serrated lesions
- However, hyperplastic polyps are rarely > 10 mm
- Presumption that hyperplastic polyps rarely exist in the proximal (right) colon is controversial
- Tubular adenoma / tubulovillous adenoma
- Reactive colonic mucosa
Additional references
Board review style question #1
Board review style answer #1
A. Architecture distortion of crypt base is the most reliable morphologic feature to diagnose colorectal sessile serrated lesions. Branching of crypts alone is not diagnostic for sessile serrated lesions. Cytological atypia does not always present in the sessile serrated lesions. Location is not part of the pathology diagnostic criteria of sessile serrated lesions. Serrations of surface epithelium can be seen in hyperplastic polyps.
Comment Here
Reference: Serrated lesions
Comment Here
Reference: Serrated lesions
Board review style question #2
When a colorectal polypectomy specimen shows no dysplasia but with equivocal features for either hyperplastic polyps or sessile serrated lesions, what is the right thing to do?
- Choose to call hyperplastic polyps or sessile serrated lesions based solely on clinical information (size and location)
- Consider taking deeper sections to look for definite diagnostic features
- Report as serrated adenoma, unclassified
- Sign out as serrated lesion and mention equivocal between hyperplastic polyps and sessile serrated lesions without deeper level sections
Board review style answer #2
B. Deeper sections of the tissue can often clarify some equivocal features and may demonstrate more definite diagnostic features, such as basally dilated crypts. Note in answer C, the diagnosis of serrated adenoma, unclassified is only reserved for serrated lesions with dysplasia and cannot fit into other diagnosis entities. Size or location alone should not be used to determine the diagnosis of hyperplastic polyps or sessile serrated lesions.
Comment Here
Reference: Serrated lesions
Comment Here
Reference: Serrated lesions
Board review style question #3
In colorectal sessile serrated lesions, which of the following dysplastic patterns requires loss of MLH1 for diagnosis?
- Adenomatous dysplasia
- Dysplasia, NOS
- Minimal deviation
- Serrated dysplasia
Board review style answer #3