Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesCite this page: Gober-Wilcox J, Crookston K. Factor XIII deficiency. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/coagulationfactorXIIIdef.html. Accessed January 10th, 2025.
Definition / general
- Factor XIII deficiency is a congenital disorder that is inherited as an autosomal recessive trait and is associated with a variable bleeding tendency
- Acquired factor XIII deficiency is associated with liver failure, inflammatory bowel disease, leukemia, disseminated intravascular coagulation, Henoch-Schonlein purpura, systemic lupus erythematosus and exposure to certain drugs (phenytoin, isoniazid, valproate)
Terminology
- Factor XIII is also known as fibrin stabilizing factor
Epidemiology
- Estimated at 1 in 2 to 5 million births
- More frequent in regions where consanguineous marriages are more common
Sites
- Skin and mucosa, joints, soft tissue, gastrointestinal tract, genitourinary tract, CNS (see clinical features below)
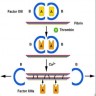
Pathophysiology
- Factor XIII is a transglutaminase that circulates as a zymogen comprised of 2 catalytic A subunits and 2 carrier B subunits
- The A subunit is synthesized in platelets, monocytes and macrophages while the B subunit is synthesized in the liver; the A and B dimers then assemble in the plasma to form a heterotetramer
- Factor XIII is activated by thrombin and is responsible for catalyzing the final step in the coagulation cascade by cross-linking fibrin (in the presence of calcium)
- Deficiency is due to a defect in either the A gene (type 2) or B gene (type 1)
Etiology
- Inherited as an autosomal recessive trait
- Most cases are due to mutations in A subunit gene on chromosome 6
- More than 70 mutations have been identified, most of which are missense and nonsense mutations
- Only 5 mutations in FXIII B deficient patients have been identified; gene is on chromosome 1
Clinical features
- Variable bleeding tendency, from mild to severe depending on factor levels
- Umbilical cord stump bleeding, intracranial hemorrhage, soft tissue hematoma, bleeding after circumcision, gastrointestinal bleeding, gingival bleeding, epistaxis, hematuria, surgical site bleeding, menorrhagia, joint bleeding, delayed healing, spontaneous abortion, recurrent miscarriage
- Plasma half life is 9 - 12 days
- Factor XIII levels above 3 - 5% are usually sufficient to prevent spontaneous bleeding
- Severe bleeding typically occurs in individuals with < 1% circulating levels
- Compound heterozygotes are usually asymptomatic
Laboratory
- Normal PT, PTT, thrombin time, fibrinogen
- Screening test for factor XIII deficiency uses the clot solubility test in which patient plasma is incubated with thrombin and calcium; deficiency will cause the clot to dissolve in the presence of urea or acid
- A standard mixing test using patient plasma and normal pooled plasma is usually performed to rule out the presence of an inhibitor
- Confirmatory testing uses a quantitative factor XIII activity assay
Prognostic factors
- Although there is a life-long risk of bleeding, prognosis is excellent due to good response to treatment; subsequent risk of development of inhibitors is low
Case reports
- Spontaneous splenic rupture in a patient with factor XIII deficiency (Pediatr Blood Cancer 2008;50:113)
Treatment
- Factor XIII concentrate, FFP or cryoprecipitate for replacement therapy or for treatment of acute bleeding episodes:
- Factor XIII concentrate: 10 - 20 U/kg at 4 - 6 week intervals
- FFP: 10 mL/kg every 4 - 6 weeks
- Cryoprecipitate: 1 bag per 10 - 20 kg every 3 - 4 weeks
- To prevent miscarriage, maintain factor XIII levels > 10% in early gestation and > 30% at time of delivery to prevent significant bleeds
Differential diagnosis
- Acquired factor XIII deficiency
Additional references