Table of Contents
Definition / general | Epidemiology | Terminology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesCite this page: Crookston K, Rosenbaum LS, Gober-Wilcox J. Factor VII deficiency. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/coagulationfactorVIIdef.html. Accessed March 31st, 2025.
Definition / general
- Factor VII deficiency is a rare congenital bleeding disorder that is inherited as an autosomal recessive trait, and is characterized by variable bleeding symptoms, ranging from asymptomatic to life threatening hemorrhage
Epidemiology
- Factor VII deficiency is the most common of the rare congenital coagulation disorders
- Incidence is 1 in 500,000
Terminology
- Also known as proconvertin deficiency
- The deficiency is classified as:
- Type I: decreased synthesis or increased clearance (quantitative defect)
- Type II: dysfunctional molecule (qualitative defect)
- Type I: decreased synthesis or increased clearance (quantitative defect)
Sites
- Bleeding into skin and mucosa, joint and muscle, genitourinary tract, gastrointestinal tract, CNS (see clinical features below)
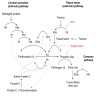
Pathophysiology
- Factor VII is a vitamin K-dependent coagulation factor that is synthesized in the liver (Wikipedia)
- Circulating factor VII forms a complex with exposed tissue factor (from injured vascular endothelium), and is activated by proteases to initiate the extrinsic coagulation cascade to form a fibrin clot
- Biologic half-life is 3.5 hours
Images hosted on other servers:
Etiology
- Autosomal recessive inheritance
- More than 130 mutations have been described, predominantly missense and splice-site mutations and less commonly small deletion and nonsense mutations
- Other environmental and genetic factors influence phenotype since patients with identical factor VII gene mutations have been shown to have discordant bleeding severity
Clinical features
- Patients with a homozygous or compound heterozygous genotype develop bleeding symptoms while heterozygotes are typically asymptomatic
- There is little correlation of factor levels and bleeding symptoms
- Patients exhibit easy bruising, epistaxis, soft tissue hematoma, menorrhagia, menometrorrhagia, postpartum bleeding, postoperative bleeding, hemarthrosis, retroperitoneal bleeding, gastrointestinal bleeding, intracranial hemorrhage
- Paradoxically, thrombotic episodes have been reported (e.g. deep venous thrombosis) in 3 - 4%, but in most cases, thrombotic risk factors were identified (Haemophilia 2008;14:564)
- Few cases have been reported of inhibitor development after replacement therapy
Laboratory
- Prolonged PT
- Normal PTT (although may be prolonged)
- Obtain specific factor VII activity assay to confirm
Prognostic factors
- Patients with severe deficiency typically have life-threatening bleeds (e.g. intracranial, gastrointestinal) within the first 6 months of life
Case reports
- Multiple cerebral aneurysms in congenital factor VII deficiency (AJNR Am J Neuroradiol 2004;25:784)
Treatment
- For mild hemorrhage, recommended to maintain factor levels of 5% - 10% of normal to stop bleeding; for surgical procedures, recommended to maintain levels of 15% - 20% of normal
- Recombinant factor VIIa is the treatment of choice; single dose for mild to moderate bleeding, or every 4 - 6 hours for severe bleeding episodes
- Due to the short half-life of factor VII (3.5 hr), it is difficult to give fresh frozen plasma (FFP) every 4 - 6 hours to maintain levels without producing volume overload
- Plasma derived factor VII concentrates and prothrombin complex concentrates are associated with post-treatment thrombosis
Differential diagnosis
- Acquired factor VII deficiency: due to warfarin, vitamin K deficiency, liver disease
- Acquired factor VII inhibitors
- Familial combined factor deficiencies: e.g. factor VI/factor VIII or factor II, VII, IX and X
Additional references