Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Abdelzaher E. Subependymoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorsubependymoma.html. Accessed April 3rd, 2025.
Definition / general
- Glioma characterized by the clustering of uniform to mildly pleomorphic tumor cell nuclei in an abundant fibrillary matrix prone to microcystic change
- CNS WHO grade 1
Essential features
- Slow growing tumors that tend to occur within the ventricles of middle aged and elderly adults (Brain Pathol 2008;18:469)
- Histologically characterized by clustered nuclei in an abundant fibrillary background
- Benign biological behavior with excellent prognosis (J Neurosurg 2021;136:736)
Terminology
- Not recommended by WHO: subependymal glomerate astrocytoma (J Neuropathol Exp Neurol 1954;13:30)
ICD coding
Epidemiology
- Rare; < 1% of intracranial neoplasms and ~8% of ependymal tumors (Arch Pathol Lab Med 1999;123:873, J Neurosurg 2021;136:736)
- Age
- Peak incidence in middle aged and elderly adults (mean: 46.7 plus or minus 18.1 years) (J Neurooncol 2007;85:297, Neurol India 2012;60:379, Arch Pathol Lab Med 1999;123:306, J Neurosurg 2021;136:736)
- Rare in children (World Neurosurg 2017;101:599)
- M > F (70.2%) (J Neurosurg 2021;136:736, J Neurooncol 2007;85:297)
Sites
- Typically intraventricular, rarely intraparenchymal (1.1%) (J Neurosurg 2021;136:736, Childs Nerv Syst 2021;37:1759)
- Most common
- Lateral ventricles (44.5%): more likely to originate from the walls or horns (51.7%) rather than the septum pellucidum (41.4%) or trigone (10.3%) (J Neurosurg 2021;136:736)
- Fourth ventricle (43.1%): more likely to arise from the floor of the fourth ventricle (53.6%) rather than the roof (10.6%) (J Neurosurg 2021;136:736)
- Less common: third ventricle (3.7%), spinal cord (eccentric intramedullary masses preferentially at cervicothoracic segments) (J Neurooncol 2007;85:297, Arch Pathol Lab Med 1999;123:306, Neurosurgery 1996;38:251, J Neurosurg 2021;136:736, Indian J Neurosurg 2019;8:64)
- Rare: cerebral, cerebellar, brain stem and cerebellopontine angle (Childs Nerv Syst 2021;37:1759, AJNR Am J Neuroradiol 2008;29:190, Surg Neurol Int 2021;12:154, Korean J Radiol 2014;15:151, Brain Pathol 2008;18:469)
Pathophysiology
- Embryological origin of these tumors remains uncertain; possible precursors include subependymal glia, astrocytes of the subependymal plate, ependymal cells and a mixture of astrocytes and ependymal cells (Brain Pathol 2008;18:469, J Neurosurg 2021;136:736, Arch Pathol Lab Med 1999;123:306)
- Specific mechanisms by which the documented chromosomal or genetic abnormalities contribute to tumorigenesis are currently unknown
Etiology
- Predisposing factors are unknown
- Rare familial cases are reported (Br J Neurosurg 1987;1:317, J Neurosurg 1994;80:1108, J Neurosurg 2014;121:570)
- TRPS1 gene alterations are reported in sporadic and familial cases (J Neurooncol 2017;134:133, Clin Genet 2023;103:717)
- Losses of chromosomes 19 and 6 appear to play a role in many sporadic cases (Neuro Oncol 2018;20:1616)
- Isolated cases have been described in patients with hereditary aniridia, PAX6 mutation and PTPN11 mutation associated Noonan syndrome (Brain Pathol 2010;20:1033, Case Rep Neurol Med 2019;2019:6091059)
- Rarely associated with craniopharyngiomas (Clin Neuropathol 1996;15:63)
Clinical features
- Often clinically silent and discovered only incidentally on neuroimaging for unrelated reasons or at autopsy (16.1 - 32.5%); asymptomatic cases usually do not exceed 1 cm in diameter (Arch Pathol Lab Med 1999;123:873, J Neurosurg 2021;136:736, J Neurooncol 2007;85:297)
- Clinical presentation is often nonspecific and depends on the location and size of the tumor (Neurol India 2012;60:379, J Neurosurg 1978;49:689, J Neurooncol 2007;85:297, Clin Neurol Neurosurg 1997;99:17)
- Symptomatic tumors usually range from 3 - 5 cm in diameter
- Presenting symptoms can be divided into symptoms caused by ventricular obstruction and intracranial hypertension or those attributable to the compression of neural structures
- Most common: headache (55.5%), followed by nausea or vomiting (20.9%) and dizziness or vertigo (18%) (J Neurooncol 2007;85:297)
- Less common: focal neurologic deficits, seizures (in supratentorial intraparenchymal tumors) and occasionally intratumoral / intraventricular hemorrhage (J Neurooncol 2007;85:297, Childs Nerv Syst 2021;37:1759)
- Subependymomas around the foramen of Monro can cause acute hydrocephalus regardless of tumor size (Neurol India 2012;60:379)
Diagnosis
- Neuroimaging: MRI and CT (AJR Am J Roentgenol 1995;165:1245)
- Biopsy
- WHO essential diagnostic criteria
- Circumscribed glioma with clustering of tumor cell nuclei within expansive, focally microcystic fibrillary matrix
- Lack of conspicuous nuclear atypia
- Absent or minimal mitotic activity
- For unresolved lesions: DNA methylation profile aligned with subependymoma
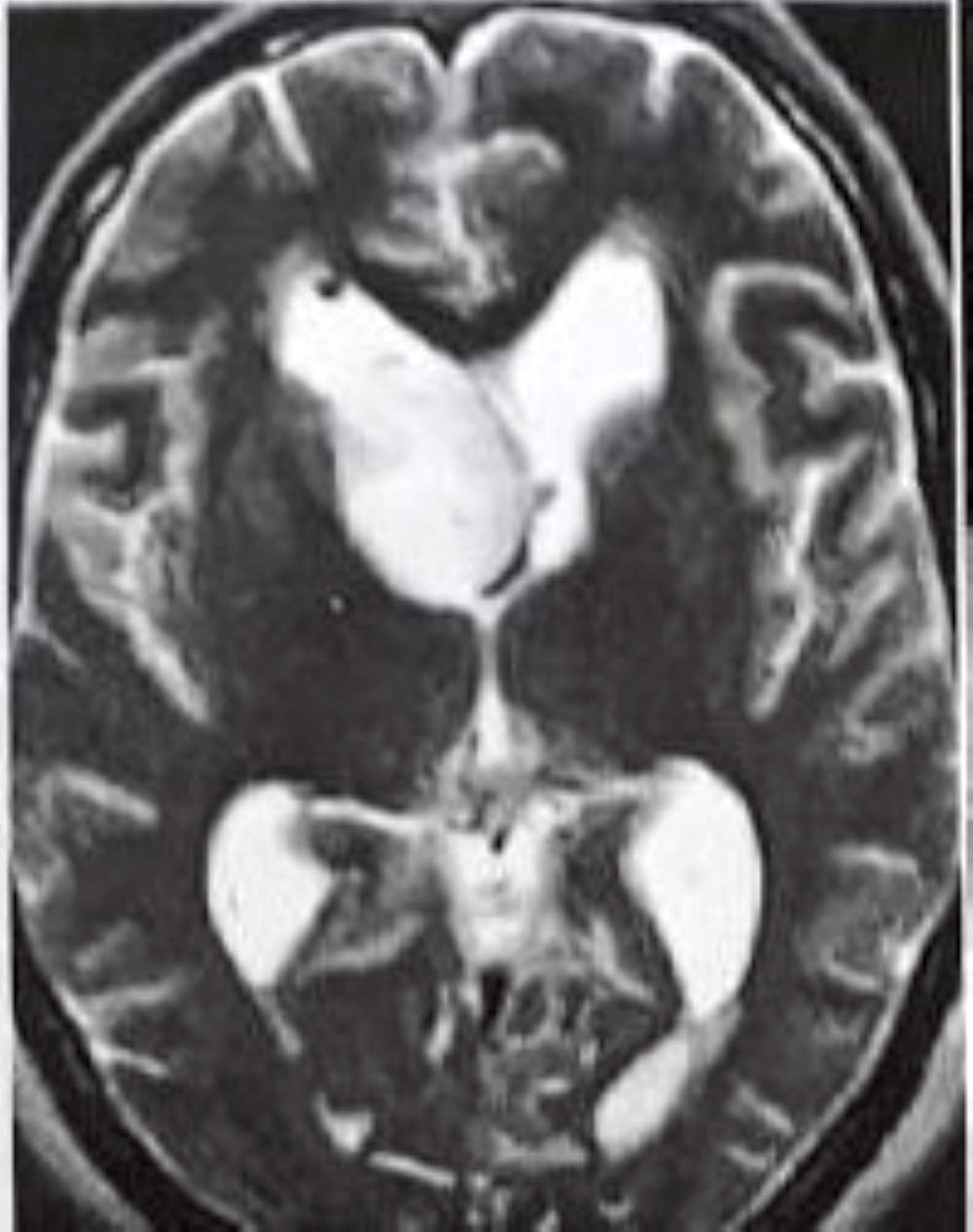
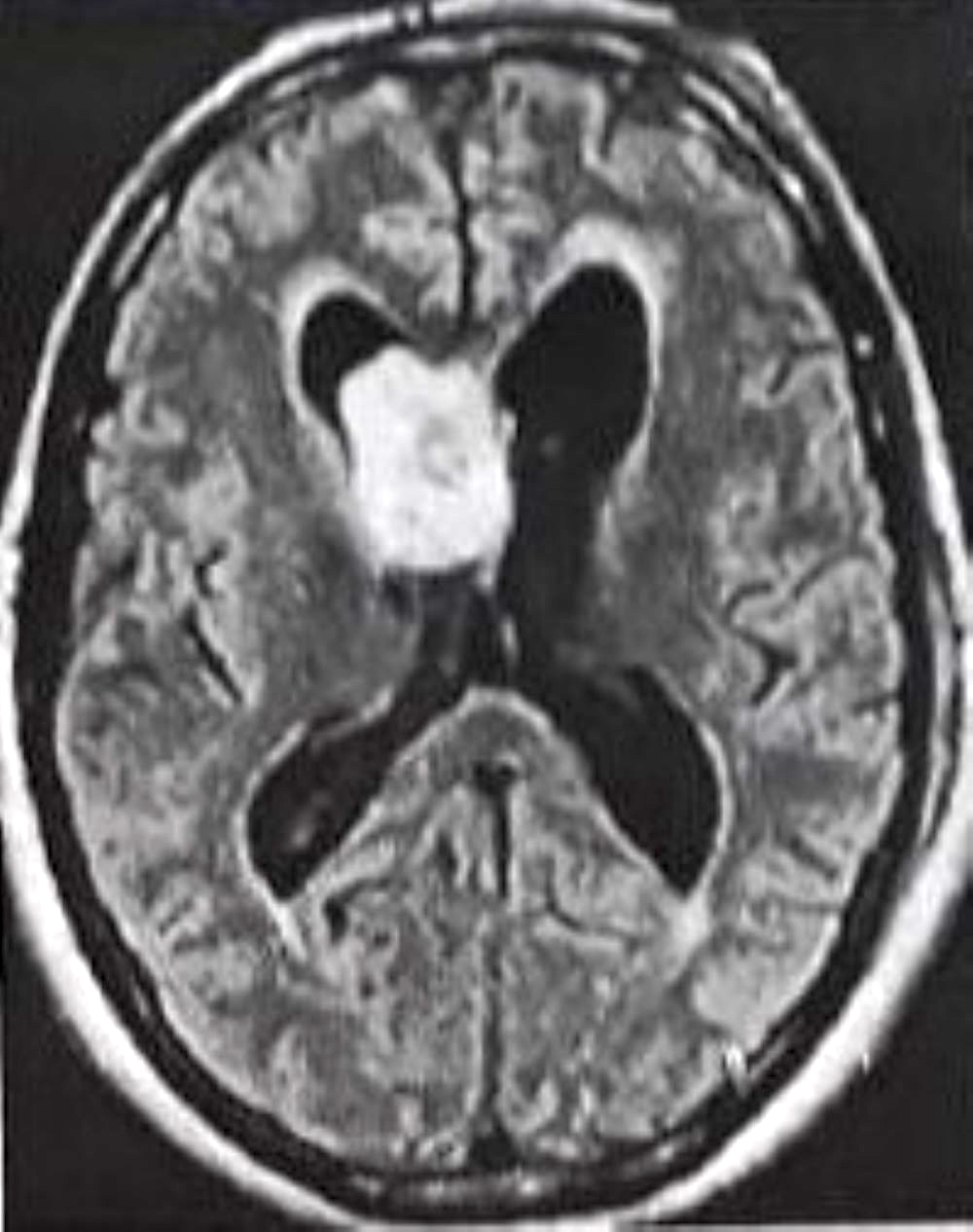
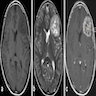
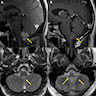
Radiology description
- CT
- Nodular masses with cystic changes (Neurol India 2012;60:379)
- Frequently hypodense (55.6%) rather than isodense (23.6%) or hyperdense masses (20.8%) (J Neurosurg 2021;136:736)
- Absent or minimal enhancement (63.2%); notable contrast enhancement in 36.8% (J Neurosurg 2021;136:736)
- Calcification (23.1%) (J Neurosurg 2021;136:736)
- MRI
- Well circumscribed, sharply demarcated, nodular masses (Neurol India 2012;60:379, J Neurooncol 2007;85:297)
- Hypointense (68%) or isointense (23.8%) on T1 weighted images and hyperintense (> 87%) on T2 weighted images (J Neurosurg 2021;136:736, J Neurooncol 2007;85:297)
- Enhancement is quite variable (J Neurooncol 2007;85:297, Neurol India 2012;60:379)
- Absent or minimal enhancement in the majority
- Notable moderate to marked heterogeneous contrast enhancement (36.7%) (J Neurosurg 2021;136:736)
- Enhancement is more common in posterior fossa subependymomas (AJR Am J Roentgenol 1995;165:1245)
- Hydrocephalus and cystic degeneration are common features (94% and 90.8%, respectively) (J Neurosurg 2021;136:736, J Neurooncol 2007;85:297)
- Peritumoral edema (12.5%) (J Neurooncol 2007;85:297)
- Intraspinal examples appear as eccentric exophytic masses (Indian J Neurosurg 2019;8:64)
Radiology images
Prognostic factors
- Benign biological behavior with excellent prognosis and excellent postoperative functional outcomes with rare postoperative morbidity (J Neurosurg 2021;136:736, Neurol India 2012;60:379)
- Recurrence is rare; usually related to incomplete excision (1.3 - 11.8%) (J Neurosurg 2021;136:736, J Neurooncol 2007;85:297)
- Elevated proliferation index has been correlated with tumor recurrence and rapid growth (Neurol India 2009;57:191, Spine Surg Relat Res 2018;3:91, Pathol Int 2015;65:438)
- Exceptionally rare: subependymal seeding, anaplastic progression or sudden death due to acute obstructive hydrocephalus or subarachnoid hemorrhage (J Neurosurg 2021;136:736)
- Less favorable prognosis has been associated with older age and poorly defined tumor borders (J Neurooncol 2007;85:297, J Neurosurg 2015;122:49)
- Assessments of chromosome 19 status and DNA methylation profiling may prove useful in the risk stratification of patients with mixed or morphologically ambiguous lesions (Neuro Oncol 2018;20:1616, Cancer Cell 2015;27:728)
- Prognostic significance of mixed ependymoma subependymoma is controversial (J Neurosurg 1978;49:689, J Neurosurg 2015;122:49)
- Brainstem subependymomas can have H3 K27M mutations but this does not carry the rapidly lethal prognosis of diffuse midline gliomas (Hum Pathol 2019;84:262)
- Cytological pleomorphism, occasional mitoses and necrosis have not proved prognostically significant (Arch Pathol Lab Med 1999;123:306)
- Loss of chromosome 6 and TERT mutations are frequent events in ependymoma component of posterior fossa mixed tumors and confer a less favorable prognosis (Acta Neuropathol 2021;141:959)
Case reports
- 11 year old girl with intraparenchymal subependymoma (Childs Nerv Syst 2021;37:1759)
- 15 year old boy with subependymoma of the cerebellopontine angle and prepontine cistern (AJNR Am J Neuroradiol 2008;29:190)
- 20 year old man with massive subependymoma of the lateral ventricles (Neuroradiology 2005;47:183)
- 25 year old man with mirror image subependymoma (Neurol India 2012;60:684)
- 28 year old man with cerebellar subependymoma (Korean J Radiol 2014;15:151)
- 40 year old man with an intraventricular combined tanycytic ependymoma and subependymoma (Brain Pathol 2013;23:359)
- 57 year old woman with thoracolumbar intramedullary subependymoma with multiple cystic formation (Eur Spine J 2013;22:S317)
Treatment
- Surgery remains the mainstay of management with an ultimate goal of maximal safe tumor resection (World Neurosurg 2017;101:599, J Neurooncol 2007;85:297)
- Role of radiation therapy is controversial (J Neurooncol 2007;85:297, J Neurosurg 2021;136:736, World Neurosurg 2017;101:599, Arch Pathol Lab Med 1999;123:306)
- Targeted therapy is promising: multikinase inhibitors, topoisomerase inhibitor and pSTAT3 / HIF1α inhibitor could counteract disease progression (J Neuroimmunol 2014;277:168, Cancers (Basel) 2021;13:6218)
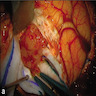
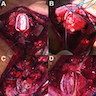
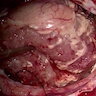
Clinical images
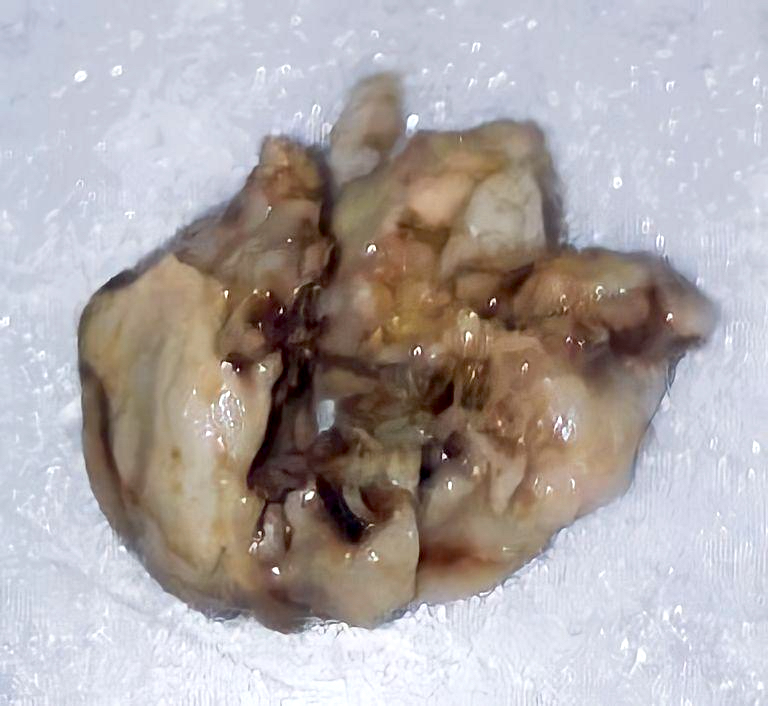
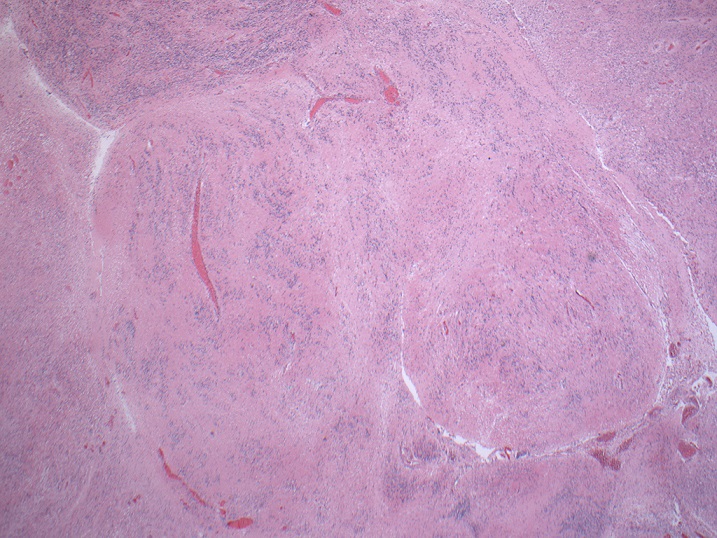
Gross description
- Generally circumscribed, firm, smooth contoured, lobulated, grayish white masses (J Neurooncol 2007;85:297, Case Rep Neurol 2011;3:227, J Neurosurg 1978;49:689)
- Tumors range in size from 1.5 to 60.0 mm (J Neurosurg 2021;136:736)
- Cystic changes
- Calcification with gritty sensation especially in posterior fossa tumors (J Neurooncol 2007;85:297)
- Focal hemorrhage
Frozen section description
- Lobular pattern
- Clusters of monomorphic nuclei with absent or mild pleomorphism; nuclei appear hyperchromatic and pointy with no prominent nucleoli or mitotic figures (Indian J Neurosurg 2019;8:64, OUHSC: Case 501-2 [Accessed 29 January 2024])
- Dense, fine, fibrillary background
- Microcystic formations impart a spongy appearance on low magnification; some bluish mucoid material is identified in some microcysts
Frozen section images
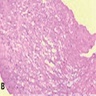
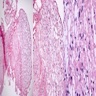
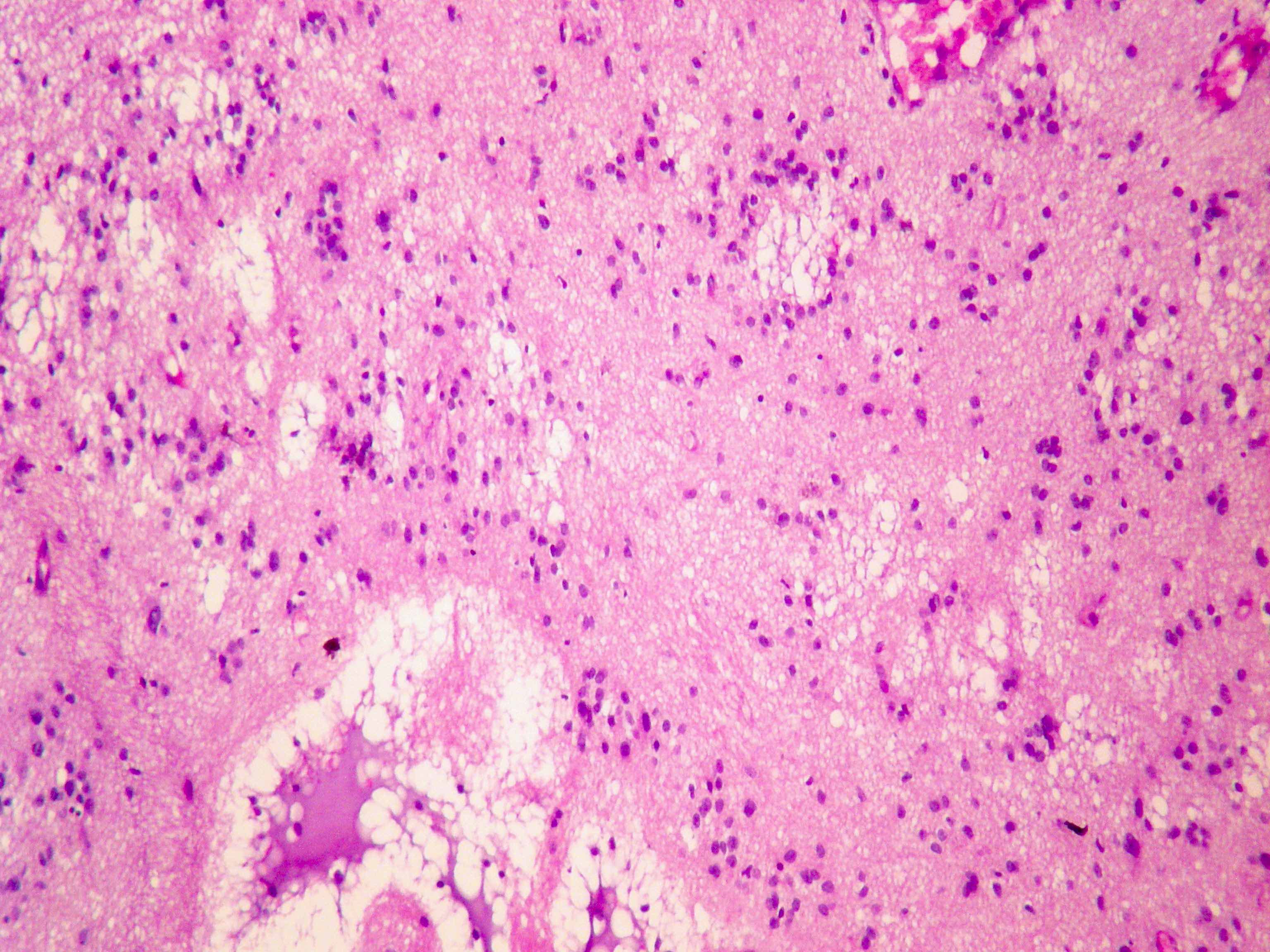
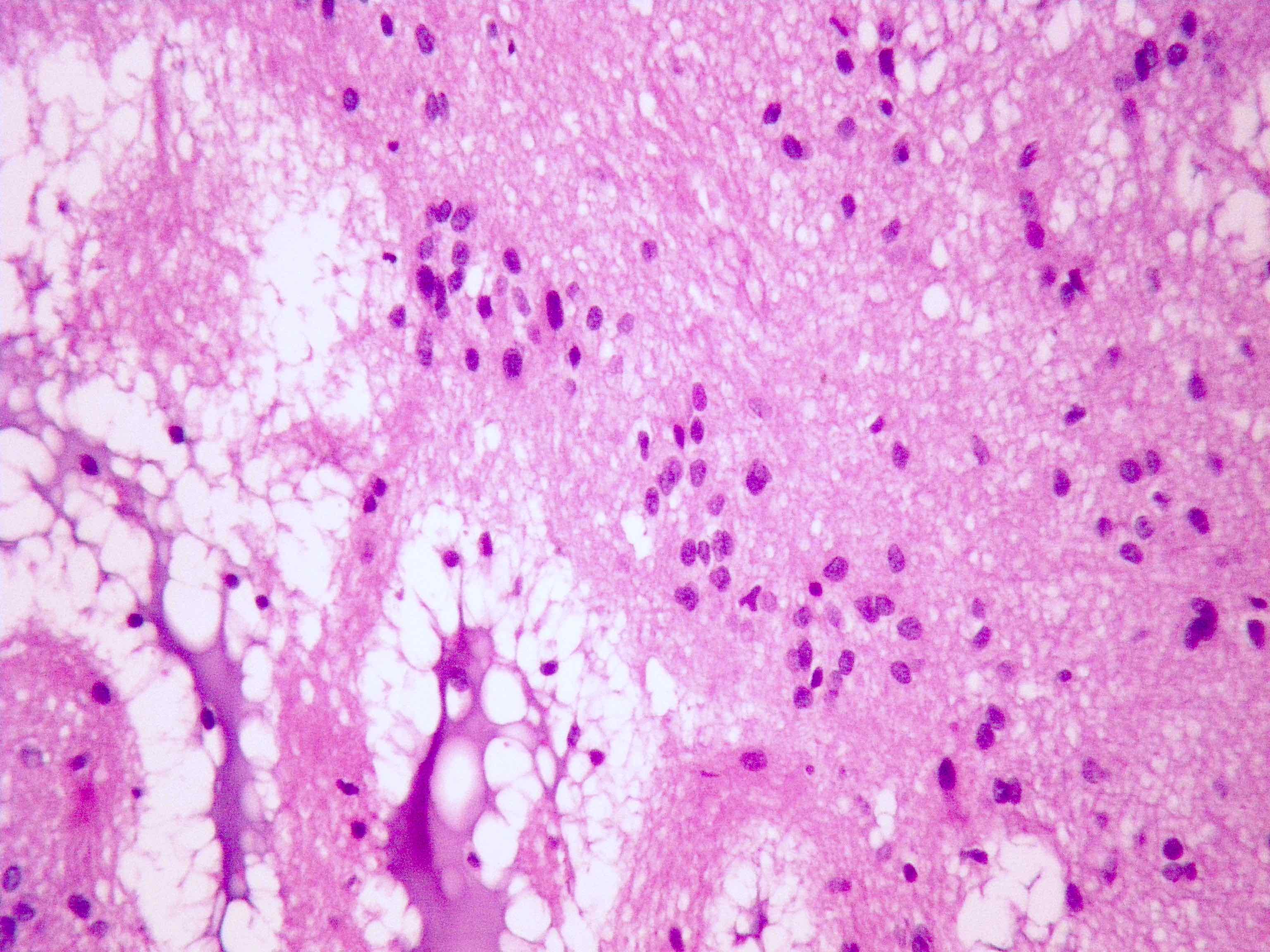
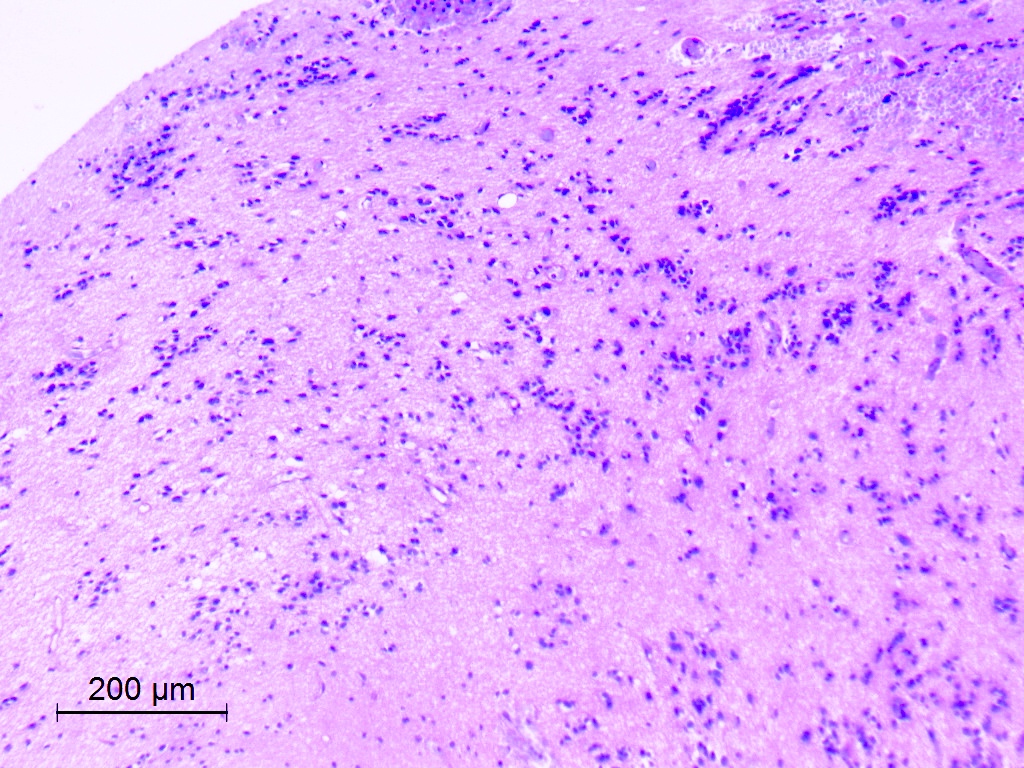
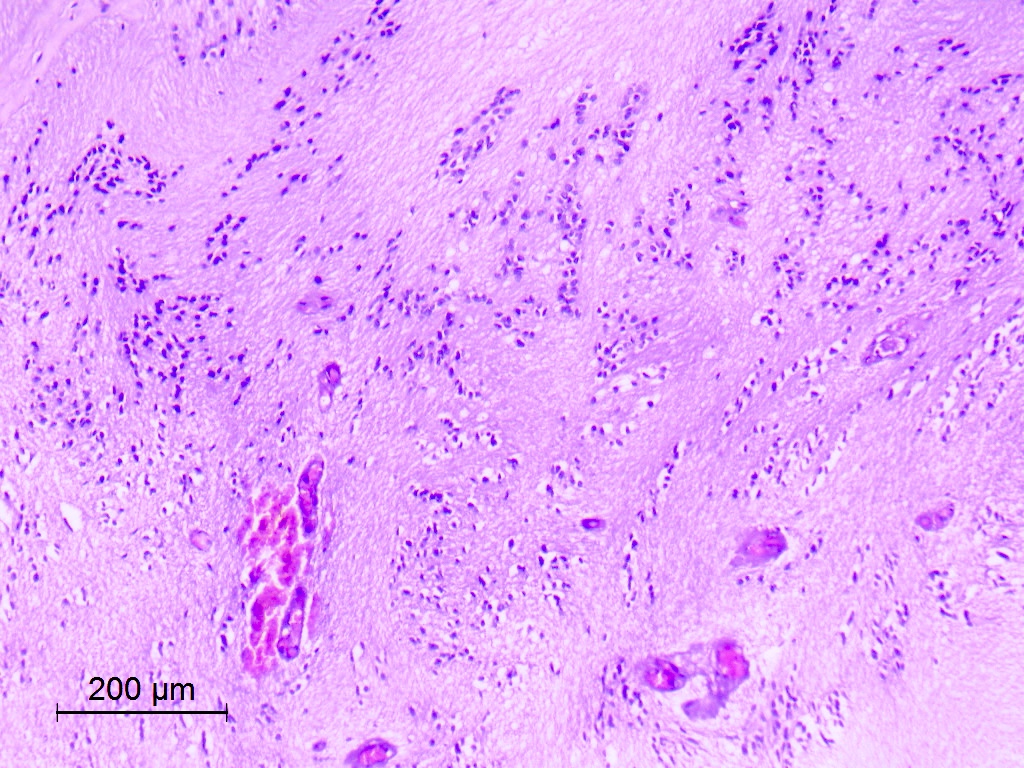
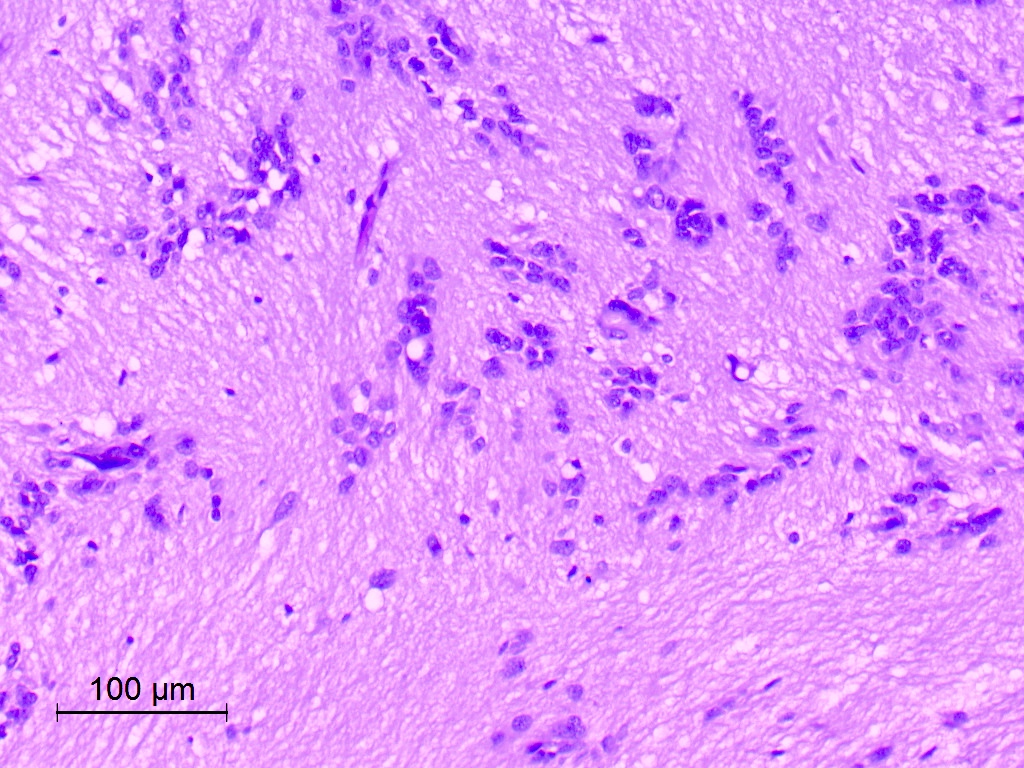
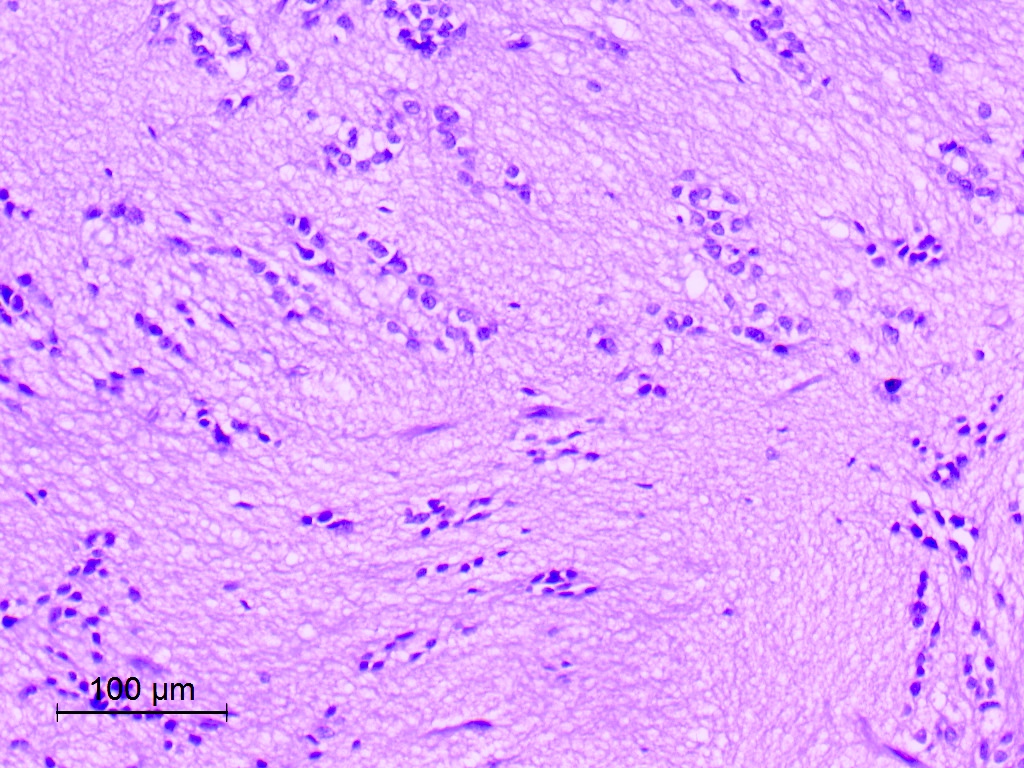
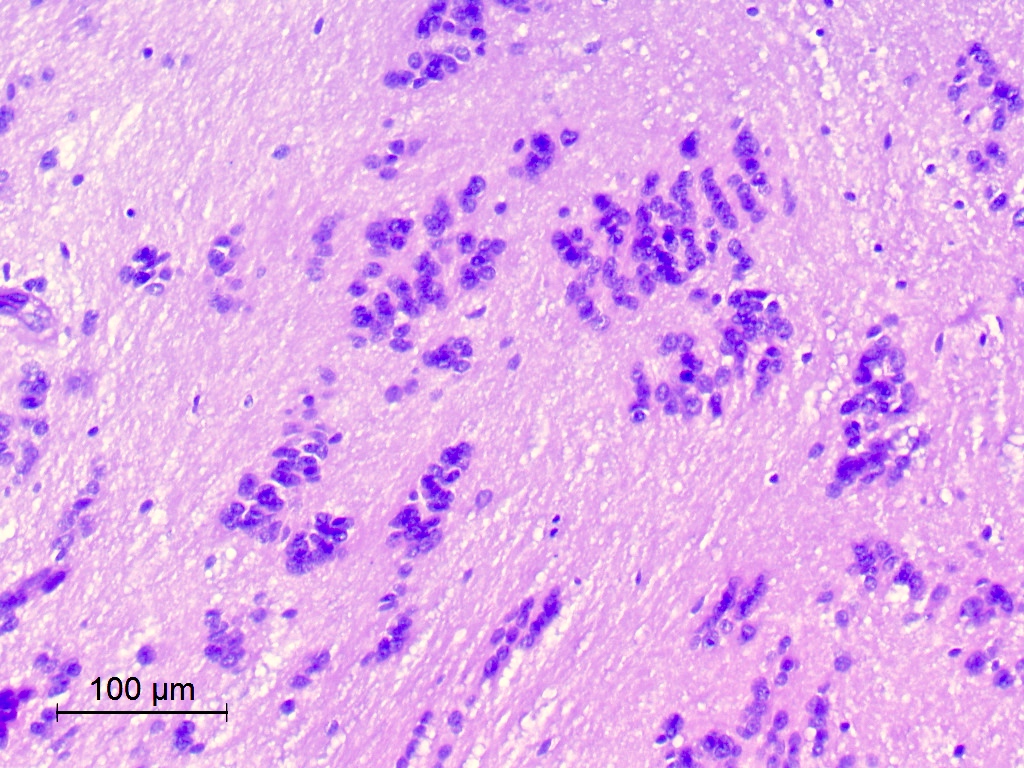
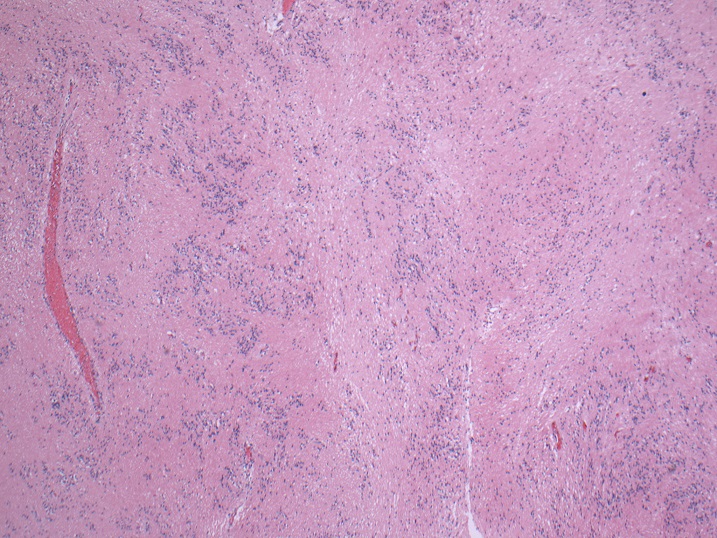
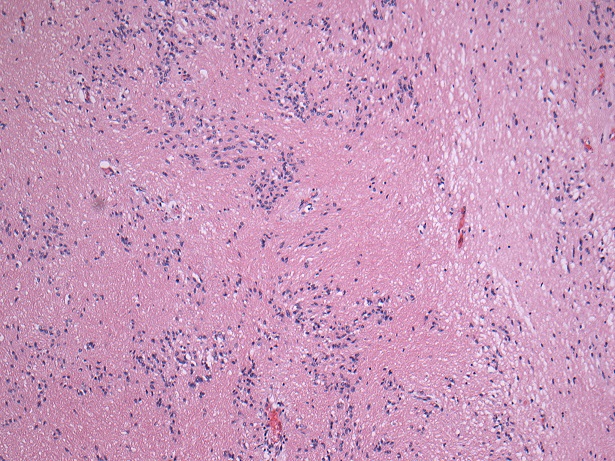
Microscopic (histologic) description
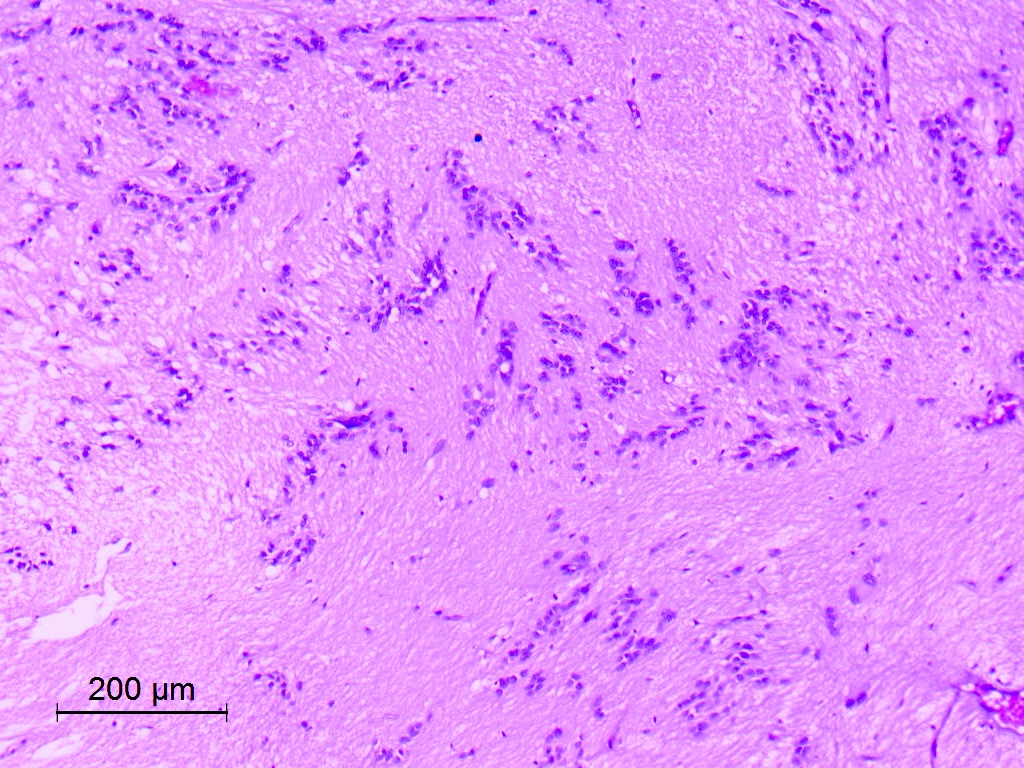
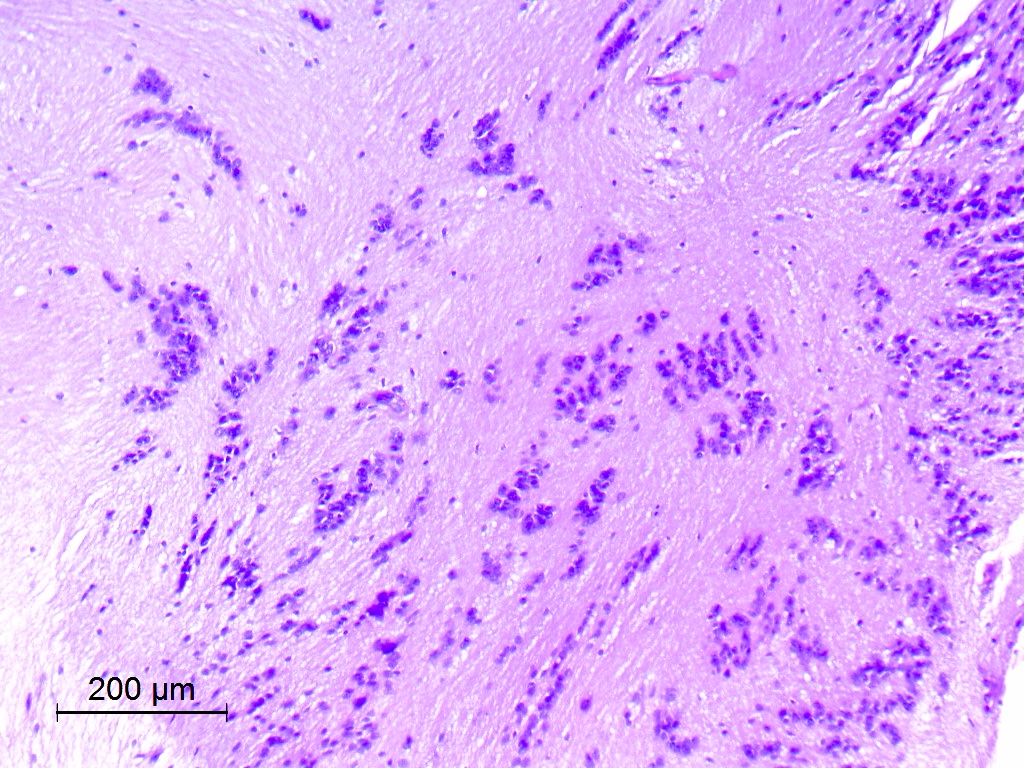
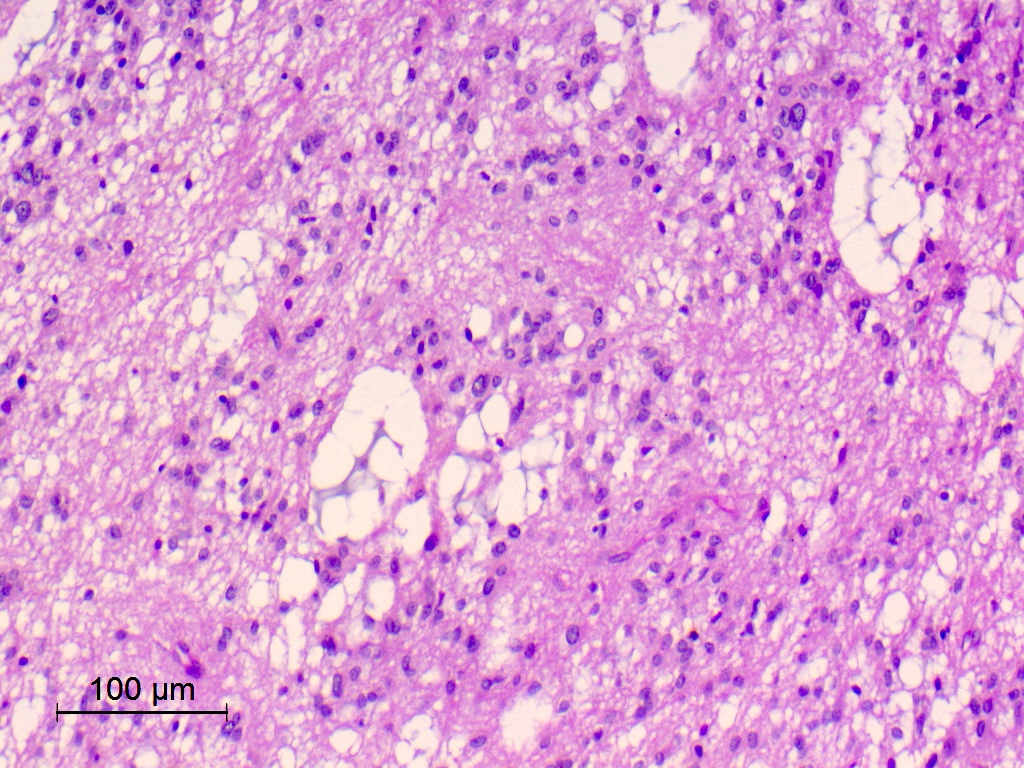
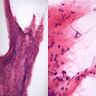
- Nuclear clustering is typical; nuclei are bland, euchromatic, small, round to oval (Neurol India 2012;60:379)
- Abundant densely fibrillar, paucicellular background (Diagn Cytopathol 2018;46:258)
- Common
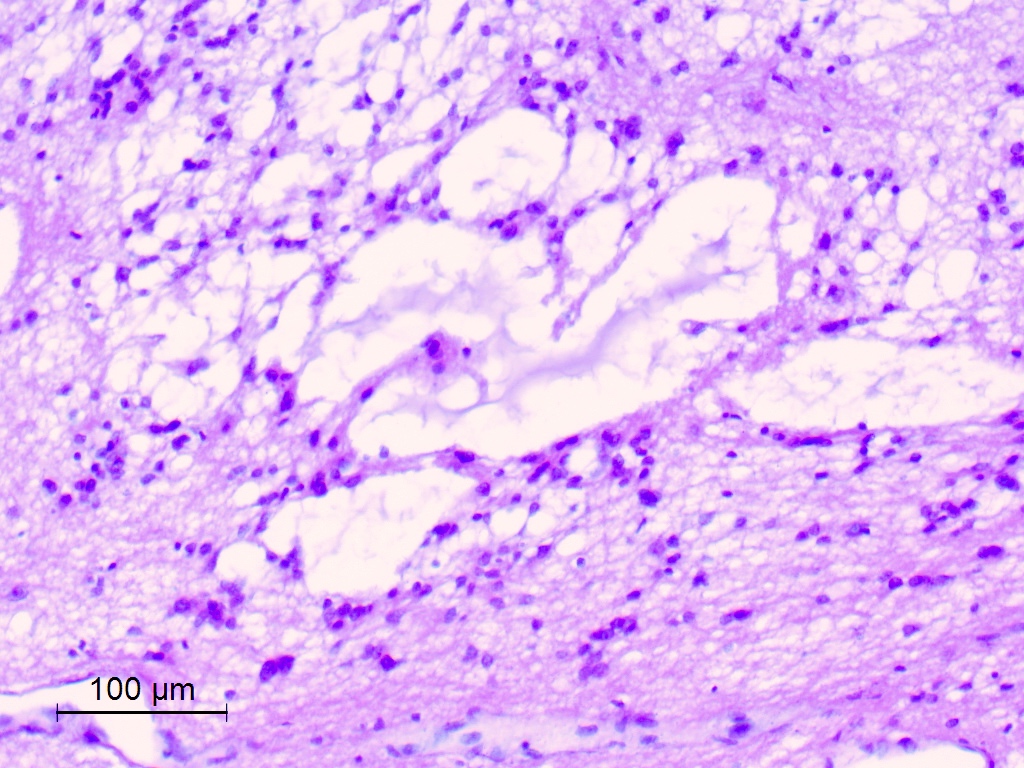
- Microcysts containing mucoid material, especially near foramen of Monro (Diagn Cytopathol 2018;46:258)
- Calcifications, especially in posterior fossa subependymomas (AJR Am J Roentgenol 1995;165:1245)
- Sclerotic and ectatic blood vessels, hemorrhage and hemosiderin deposits (Arch Pathol Lab Med 1999;123:306)
- Occasional
- Focal perivascular pseudorosettes (J Neurooncol 2007;85:297)
- Nuclear pleomorphism (Arch Pathol Lab Med 1999;123:306)
- Proliferative microvascular abnormalities
- Rare
- Low level mitotic activity
- Necrosis (J Neurooncol 2007;85:297)
- Exceptionally rare
- Melanotic pigmentation (Am J Surg Pathol 1990;14:729)
- Sarcomatous change (Neurosurgery 1991;28:761, Am J Surg Pathol 2008;32:699)
- Mixed histologic pattern (subependymoma together with another glial tumor)
- Mixed ependymoma subependymoma, the most common (13.7%) (J Neurosurg 2021;136:736)
- Subependymoma with elements of fibrillary astroglial or (rarely) gemistocytic morphology (J Neurooncol 2007;85:297)
Microscopic (histologic) images
Contributed by Eman Abdelzaher, M.D., Ph.D., David Taylor, M.D. and Nazila Azordegan, M.D.
Cytology description
- Smears poorly; remains as cohesive tissue fragments
- Clusters of bland round or oval nuclei with absent or mild pleomorphism (Diagn Cytopathol 2018;46:258, Indian J Neurosurg 2019;8:64)
- Fine fibrillary matrix of delicate cell processes (Indian J Neurosurg 2019;8:64)
- Microcystic changes (Acta Cytol 2001;45:636)
- Focal mucoid material (Diagn Cytopathol 2018;46:258)
Cytology images
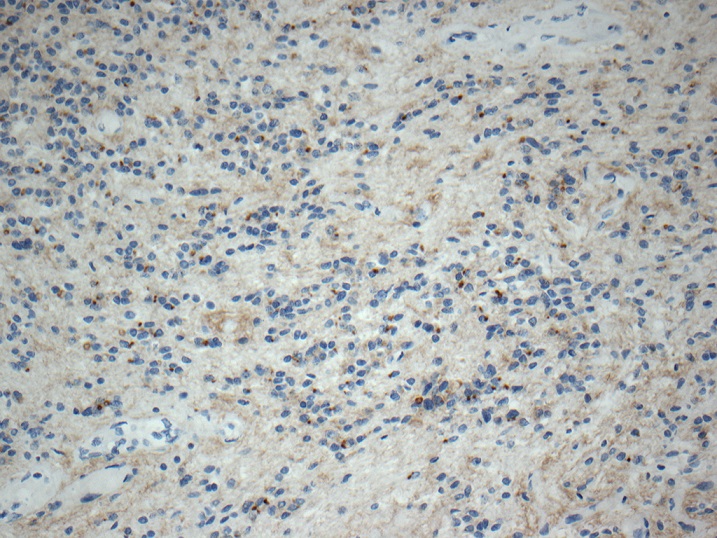
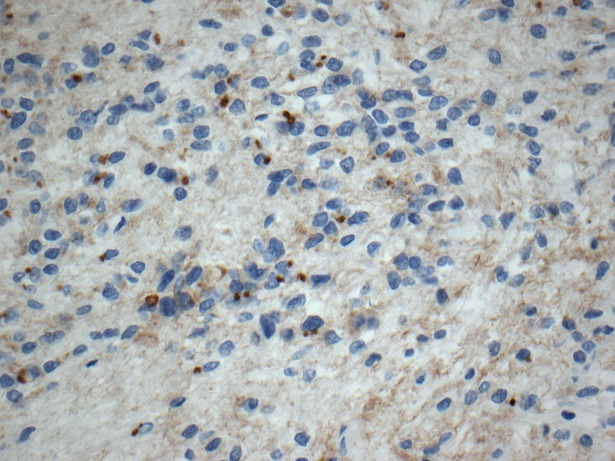
Positive stains
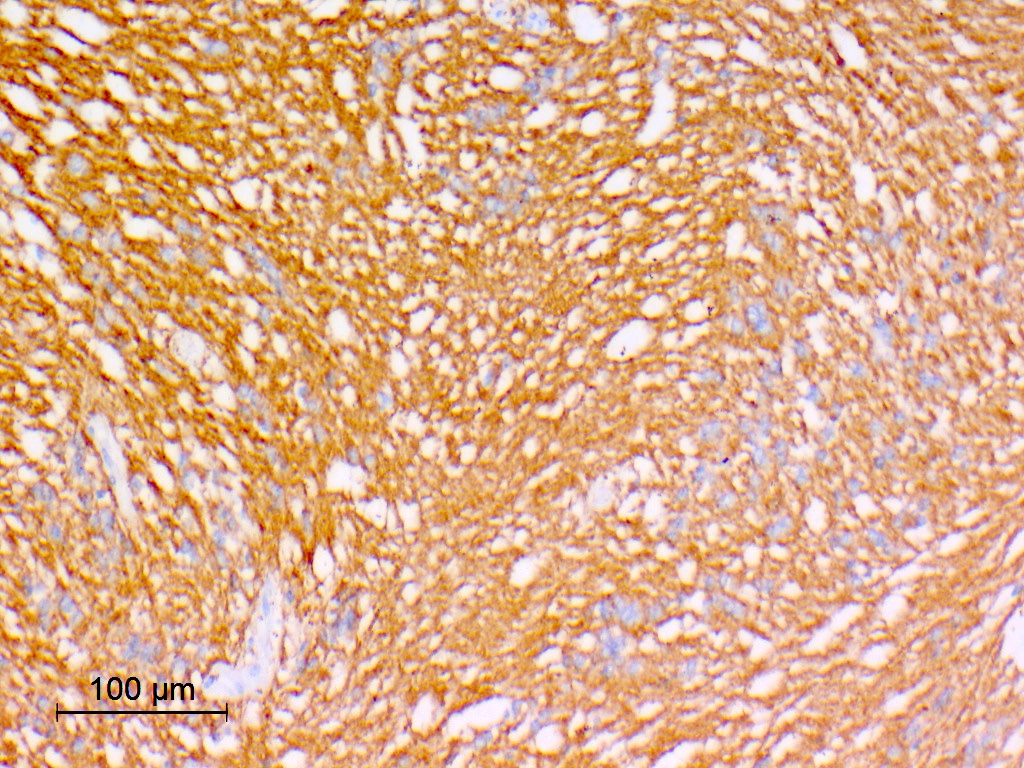
- GFAP: diffuse (100%) (J Neurooncol 2007;85:297, Cancers (Basel) 2021;13:6218, Hum Pathol 2019;84:262)
- S100 (Diagn Cytopathol 2018;46:258)
- ATRX retained (Surg Neurol Int 2021;12:154, Hum Pathol 2019;84:262)
- H3K27me3: retained (except for rare brainstem lesions) (Hum Pathol 2019;84:262)
- MDM2, HIF1α, topoisomerase IIβ, pSTAT3 and nucleolin (J Neuroimmunol 2014;277:168)
- Aquaporin 1 and aquaporin 4 with different distribution in infratentorial and supratentorial subependymomas (PLoS One 2015;10:e0131367)
- Ki67 typically low but can be variable (J Neurooncol 2007;85:297, Arch Pathol Lab Med 1999;123:306, Diagn Cytopathol 2018;46:258, Neurol India 2009;57:191, Spine Surg Relat Res 2018;3:91, Pathol Int 2015;65:438)
Negative stains
- EMA: may show focal, dot-like positivity (Diagn Cytopathol 2018;46:258, Cancers (Basel) 2021;13:6218, Hum Pathol 2019;84:262)
- Synaptophysin (Diagn Cytopathol 2018;46:258, Pathol Int 2003;53:169)
- Olig2 (Hum Pathol 2019;84:262)
- p53 (Hum Pathol 2019;84:262)
- SOX10: may show limited expression (J Neuropathol Exp Neurol 2016;75:295)
- IDH1 p.R132H (Surg Neurol Int 2021;12:154, Hum Pathol 2019;84:262)
- BRAF p.V600E
- H3K27M: negative (except for rare brainstem lesions) (Hum Pathol 2019;84:262)
- Neurofilament protein: no entrapped axons
Electron microscopy description
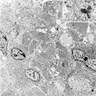
- Demonstrate both astrocytic and ependymal characteristics
- Former is evidenced by intermediate filaments within processes and the latter by microlumens, cilia, microvilli and junctional complexes (J Neurosci Rural Pract 2012;3:366, Pathol Int 2003;53:169)
- Microcystic change (Diagn Cytopathol 2018;46:258)
Molecular / cytogenetics description
- Distinct DNA methylation profiles of subependymomas at different anatomical locations (supratentorial, posterior fossa and spinal) are documented
- Some histologically defined grade 2 and 3 classic ependymomas may also cluster with molecular subgroups of typical subependymomas (Cancer Cell 2015;27:728, Neuro Oncol 2018;20:1616)
- Chromosomal copy number variations are infrequent in subependymomas at different anatomical locations
- Recurrent copy number abnormalities are loss of chromosome 19 (most frequently within posterior fossa subependymomas [79%] and less frequent within supratentorial [50%] and spinal [40%] subependymomas) and partial chromosome 6 loss in spinal and posterior fossa subependymomas (Neuro Oncol 2018;20:1616, Cancer Cell 2015;27:728, Brain Pathol 2008;18:469)
- TRPS1 and PTPN1 gene mutations have been documented (J Neurosurg 2021;136:736)
- Posterior fossa subependymomas express KIT at high levels (Cancer Cell 2015;27:728)
- Brainstem subependymomas can have H3 K27M mutations but this does not carry the rapidly lethal prognosis of diffuse midline gliomas (Hum Pathol 2019;84:262)
- Do not appear to be associated with NF2 mutations that are found in other ependymal neoplasms (J Neurooncol 2007;85:297)
Molecular / cytogenetics images
Videos
Subependymoma
Sample pathology report
- Lateral ventricular mass lesion, gross total resection:
- Subependymoma, CNS WHO grade 1
- Molecular genetics: DNA methylation profile aligned with subependymoma
Differential diagnosis
- Ependymoma:
- Higher cellularity
- No nuclear clustering
- True rosettes present in some cases (AJNR Am J Neuroradiol 2006;27:488)
- Absent or few microcysts
- Some cases show mixed ependymoma subependymoma features
- Subependymal giant cell astrocytoma:
- Large ganglion-like astrocytes
- Strongly associated with tuberous sclerosis
- Variably immunoreactive for synaptophysin (Neuropathology 2009;29:25)
- Pilocytic astrocytoma:
- Biphasic piloid and spongy areas (Acta Neuropathol 2015;129:775)
- Rosenthal fibers and eosinophilic granular bodies
- Central neurocytoma:
- Cellular
- Uniform oligodendroglioma-like cells
- Neuropil background
- Synaptophysin positive, GFAP negative (Diagn Cytopathol 2018;46:258)
- Adult type diffuse gliomas (oligodendroglioma, IDH mutant and 1p / 19q codeleted or low grade diffuse astrocytomas, IDH mutant):
- Infiltrating growth pattern
- IDH mutations
- 1p / 19q codeletion in oligodendrogliomas (Neuropathol Appl Neurobiol 2022;48:e12790)
Additional references
Board review style question #1
66 year old man presented with a headache. MRI of brain showed a nonenhancing mass lesion projecting into the right lateral ventricle. The mass was surgically excised. The image above shows the characteristic morphology of this lesion. What is the typical microscopic finding seen in this entity?
- High mitotic activity
- Nuclear clustering
- Palisading necrosis
- True ependymal rosettes
Board review style answer #1
B. Nuclear clustering. Nuclear clustering is a typical microscopic finding of subependymomas. Answer A is incorrect because mitoses are absent or rare in typical subependymomas. Answer C is incorrect because palisading necrosis is not a feature of subependymomas. Answer D is incorrect because true ependymal rosettes are not seen in pure subependymomas.
Comment Here
Reference: Subependymoma
Comment Here
Reference: Subependymoma
Board review style question #2
The recently described loss of chromosome 19 in subependymomas is most frequent at which anatomical location?
- Intraparenchymal subependymoma
- Posterior fossa subependymomas
- Spinal subependymomas
- Supratentorial subependymomas
Board review style answer #2
B. Posterior fossa subependymomas. Loss of chromosome 19 is most frequently encountered within posterior fossa subependymomas (79%).
Answers C and D are incorrect because loss of chromosome 19 is less frequent within supratentorial (50%) and spinal (40%) subependymomas. Answer A is incorrect because intraparencymal subependymomas are rare and chromosome 19 loss has not been documented in the studied cases (Int J Surg Pathol 2023;31:69).
Comment Here
Reference: Subependymoma
Comment Here
Reference: Subependymoma