Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology / etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Al-Husseinawi E, Lakis NS. Pituitary hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorpituitaryhyperplasia.html. Accessed March 31st, 2025.
Definition / general
- Nonneoplastic absolute increase in the number of 1 or more adenohypophyseal cell subtypes seen radiologically as enlargement of the pituitary gland (Pituitary 1999;1:169)
- Relatively common due to physiological and pathological conditions (Pituitary 1999;1:169)
- Remains unrecognized and occurs in heterogenous setting (Endocrinol Diabetes Metab Case Rep 2015;2015:150017)
Essential features
- Nodular or diffuse hyperplasia of polymorphic acini with intact reticulin, without effacement of gland architecture
- MRI essentially shows symmetrical and diffuse enlargement, similar intensity to gray matter (Pituitary 1999;1:169)
ICD coding
- ICD-10: E23.7 - disorder of the pituitary gland, unspecified
Epidemiology
- Very rare, accounting for < 1% of sellar surgical specimens
- Autopsy prevalence 26% (Pituitary 1999;1:169)
- Higher incidence in primary hypothyroidism (Clin Case Rep 2020;9:629)
- Prolactin (PRL) cell hyperplasia in pregnancy (Endocrinol Diabetes Metab Case Rep 2015;2015:150017)
- Age: youth and menopause (AJNR Am J Neuroradiol 1997;18:551)
Sites
- Adenohypophysis
Pathophysiology / etiology
- Physiologic response
- Most common: prolactin cell hyperplasia in pregnancy and lactation, peaks immediately postpartum
- Puberty: pituitary height peaks in the 20 - 29 year age group (F > M)
- Elderly: nonfunctional hyperplasia due to basophil cells
- Pars intermedia derived proopiomelanocortin (POMC) cells invasion into the neurohypophysis, women in the 50 - 59 year age group (Hormones (Athens) 2003;2:149)
- Pathologic hyperplasia due to end organ insufficiency
- Longstanding primary hypothyroidism causes TSH cell hyperplasia, young females
- Gonadotroph hyperplasia and sellar expansion in primary hypogonadism due to Klinefelter or Turner syndrome
- Polycystic ovary syndrome: hyperprolactinemia and lactotroph hyperplasia
- ACTH cell hyperplasia due to hypocortisolism in Addison disease
- Rare: pituitary transcription factor gene, PROP1 mutations
- Pathologic hyperplasia associated with ectopic excess of releasing hormones
- GH releasing hormone (GHRH) or corticotropin releasing hormone (CRH) ectopic release causing somatotroph or corticotroph hyperplasia secreted by pancreatic islet cell tumor, pheochromocytoma, bronchial and thymic carcinoid tumors
- ACTH cell hyperplasia due to corticotropin releasing hormone secretion from hypothalamic hamartoma or neuroendocrine tumors
- Iatrogenic: treatment with antipsychotics and excess estrogen in transgender women are associated with increased secretion of prolactin (Int Clin Psychopharmacol 2019;34:89, Int J Mol Sci 2020;21:2024, BMJ Case Rep 2009;2009:bcr02.2009.1589)
- Syndromic: mammosomatotroph hyperplasia in McCune-Albright syndrome, Carney complex, MEN1 related GHRH associated, X linked acrogigantism syndrome (XLAG)
- Idiopathic
- Hereditary (J Clin Endocrinol Metab 2011;96:E2078)
Clinical features
- Mass effect
- Visual disturbance, bitemporal hemianopia, diplopia
- Headaches
- Hormone related hyperplasia
- GH cell: gigantism or acromegaly
- Prolactin cell: hyperprolactinemia
- ACTH cell: Cushing disease
- TSH cell:
- Longstanding primary hypothyroidism results in TSH hyperplasia
- Hyperprolactinemia
- Elevated levels of TRH that stimulates both pituitary TSH and PRL cells due to lack of negative feedback of thyroxin (T4) on the hypothalamus (Pituitary 1999;1:169, Endocrinol Diabetes Metab Case Rep 2015;2015:150056)
- LH / FSH: result of early onset hypogonadism
Diagnosis
- History and physical
- Increase or decrease corresponding serum hormone levels
- Imaging
- Biopsy / excision
- Rarely removed surgically
- Most likely identified at autopsy
- References: StatPears: Pituitary Hyperplasia In Primary Hypothyroidism [Accessed 8 April 2021], Case Rep Endocrinol 2019;2019:2012546
Laboratory
- Hormonal levels as indicated
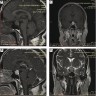
Radiology description
- Symmetric 2 - 3x enlargement of pituitary gland on CT / MRI
- Homogeneously enhancing gland with convex superior margin
- No sellar destruction
- Intensity similar to gray matter
- Homogenous gadolinium uptake
- Reference: StatPearls: Pituitary Gland Imaging [Accessed 13 May 2021]
Prognostic factors
- Excellent with medical treatment
- Rarely associated with adenoma (Pituitary 1999;1:169)
Case reports
- 10 year old girl with growth retardation and obesity (Medicine (Baltimore) 2018;97:e12703)
- 12 year old girl with headaches and fatigue (Clin Case Rep 2020;9:629)
- 19 year old woman with abdominal pain and diarrhea and 35 year old woman with secondary amenorrhea and headaches (Endocrinol Diabetes Metab Case Rep 2015;2015:150017)
- 35 year old man with bitemporal hemianopsia (Clin Pract 2015;5:733)
Treatment
- Usually correct underlying endocrinologic disturbance
- Surgery rarely indicated
- Visual field defects
- Progression in size to establish diagnosis by pathology
- GH and ACTH hyperplasia
- References: StatPears: Pituitary Hyperplasia In Primary Hypothyroidism [Accessed 8 April 2021], AJNR Am J Neuroradiol 1997;18:551
Gross description
- At autopsy or in the rare event the entire gland is removed, it is diffusely enlarged and tan-white with no discernible nodules
- Lack of well defined lesion
- Specimen may be fragmented
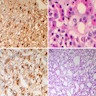
Microscopic (histologic) description
- Diffuse or nodular hyperplasia
- Unevenly enlarged acini
- Usually single cell type
- Relative cellular monomorphism within affected acinus
- Noncompressive; indistinct demarcation
- Difficult to diagnose in fragmented specimen
- Numerical increase in pituitary cells without alteration in architecture
- Rare mitotic activity
- GH cell hyperplasia: chromophobe to pale eosinophilic polygonal cells
- PRL cell hyperplasia: chromophobe with rare microcalcifications
- ACTH cell hyperplasia: amphophilic with large vacuoles (lysosomes) and Crooke cell change
- TSH cell hyperplasia: chromophobe, occasional spindle cells and multiple large PAS+ lysosomes
- LH / FSH hyperplasia: hypervacuolization (castration cells) (Endocrinol Diabetes Metab Case Rep 2015;2015:150017)
Cytology description
- Hypercellular smear with heterogeneous cell populations
Positive stains
- Reticulin is essential for the diagnosis, highlights retention of acinar architecture and may show some expanded acini
- Synaptophysin
- Cell type routine cytoplasmic stains prolactin, GH, TSH, LH, FSH, ACTH
- PAS highlights lysosomes in TSH hyperplasia
- Ki67 labeling may be mildly increased (normal gland is completely negative)
Negative stains
- GFAP
- Neurofilament (2F11)
Electron microscopy description
- Same as normal pituitary:
- GH producing somatotrophs:
- Rough endoplasmic reticulum, well formed Golgi complexes and numerous large, dense secretory granules 100 - 250 nm
- PRL producing lactotrophs:
- Elaborate rough endoplasmic reticulum arranged in parallel arrays, occasionally forming concentric structures known as nebenkern formations
- Prominent Golgi complexes and extrusion of secretory granules at the lateral cell borders known as misplaced exocytosis up to 700 nm
- GH and PRL producing mammosomatotroph:
- Pleomorphic heterogenous granules 150 - 1,000 nm and misplaced exocytosis
- TSH producing thyrotrophs:
- Short dilated rough endoplasmic reticulum and small secretory granules that align along the plasma membrane
- ACTH producing corticotrophs:
- Secretory granules are pleomorphic in shape and electron density with indentations and evaginations of granule membranes, resulting in heart and teardrop shapes
- Also small bundles of intermediate (keratin) filaments throughout the cytoplasm
- GH producing somatotrophs:
- Reference: Ann Clin Lab Sci 1979;9:275, Microsc Res Tech 1992;20:107
Molecular / cytogenetics description
- No specific molecular / cytogenetics available
- Reported germline mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene in familiar cases
- Some syndromic association: Carney complex, McCune-Albright syndrome, X linked acrogigantism (XLAG) and less likely, MEN1 related GHRH associated
- Reference: J Clin Endocrinol Metab 2011;96:E2078
Sample pathology report
- Pituitary, transsphenoidal resection:
- Consistent with pituitary hyperplasia (see comment)
- Comment: Histological sections of the specimen show pituitary tissue composed of unevenly enlarged acini comprised of sheets of monomorphic cells with small round nuclei and moderate amounts of granular eosinophilic cytoplasm. Immunostains were performed that show the lesional tissue cells are strongly positive for synaptophysin, chromogranin, EMA. Ki67 stain labels a rare cell. Reticulin stain highlights retention of acinar architecture and expansion of acini. Prolactin hormone is positive. Other hormonal stains (ACTH, LH, FSH, GH) are negative. This immune profile is consistent with benign pituitary hyperplasia, prolactin hormone producing. Clinical and radiological correlation are recommended.
Differential diagnosis
- Normal adenohypophysis:
- Normal size pituitary gland with heterogeneous cell populations within normal acini
- ACTH cells normally aggregate at the lateral wings of anterior pituitary
- Pituitary adenoma:
- Enlarged mass, well defined from normal pituitary gland
- Homogeneous cell populations with splayed and disrupted reticulin network
- Compresses adjacent normal acini
- Hyperplasia more likely to show strong cytoplasmic immunoreactivity to respective hormones throughout the fragments when compared with adenoma
Additional references
Board review style question #1
Physiologic pituitary hyperplasia is mostly seen in
- Corticotrophin releasing hormone ectopic release
- Longstanding primary hypothyroidism
- Polycystic ovary syndrome
- Prolactin cell hyperplasia in pregnancy and lactation
Board review style answer #1
D. Prolactin cell hyperplasia in pregnancy and lactation
Comment Here
Reference: Pituitary hyperplasia
Comment Here
Reference: Pituitary hyperplasia
Board review style question #2
Which of the following is a characteristic microscopic feature of pituitary hyperplasia?
- Compresses adjacent normal acini
- Homogeneous cell populations with splayed and disrupted reticulin network
- Microcalcifications are never identified
- Mild acinar expansion which is noncompressive
Board review style answer #2