Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Yasin I, Schwetye K. Gliosarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorgliosarcoma.html. Accessed March 30th, 2025.
Definition / general
- Rare, classic variant of glioblastoma (GBM), WHO grade 4
- Biphasic glial and mesenchymal differentiation
Essential features
- De novo: primary gliosarcoma (GS); secondary GS develops in previously resected or irradiated glioblastoma
- Biphasic glial and mesenchymal differentiation
- Clinical and genetic similarities to glioblastoma; likewise, treatment and management is similar to that of glioblastoma
- Overall, an aggressive tumor with slightly worse prognosis than glioblastoma (Neuro Oncol 2009;11:183)
Terminology
- Gliosarcoma (GS)
- Classic variant of glioblastoma (GBM)
ICD coding
- ICD-10: C71.9 - malignant neoplasm of brain, unspecified
Epidemiology
- Comprises ~2% of all glioblastoma cases (Neuro Oncol 2009;11:183)
- Typically affects adults in the fourth to seventh decades of life (Neuro Oncol 2009;11:183)
- Slight male predominance, similar to glioblastoma (Neuro Oncol 2009;11:183)
- Pediatric incidence is rare but up to ~13% of all gliosarcoma; age range of 3 - 19 years (BMC Pediatr 2021;21:101)
Sites
- Majority are supratentorial, with tendency towards peripheral localization and dural attachment (Eur Radiol 2019;29:429)
- Temporal > parietal > frontal > occipital lobes; rarely involve occipital region or posterior fossa (Neuro Oncol 2009;11:183)
- Can involve and invade into overlying meninges, bone and extracranial soft tissue (Case Rep Radiol 2016;2016:1762195)
- Mesenchymal / sarcomatous component is associated with propensity to invade and metastasize
- Gliosarcoma has greater propensity for extracranial metastasis than other CNS tumors, including glioblastoma (J Neurooncol 2007;83:39)
- Most common sites of extracranial metastases include lung, liver and lymph node but are also reported in adrenal gland, spleen, kidney, oral mucosa, skin, bone marrow and bone
- Metastatic tumor deposits contain sarcomatous with or without glial components
Pathophysiology
- Similar genetic alterations (p53 mutations, p16 deletions and PTEN mutations) both in glial and sarcomatous components support monoclonal origin (Am J Pathol 2000;156:425)
Etiology
- Prior history of radiation for CNS tumor is a known risk factor
Clinical features
- Presents similarly to glioblastoma
- Symptoms and signs depend on tumor location, increased intracranial pressure
- Common symptoms include headache, nausea, vomiting, seizures, dysphasia, dizziness, cognitive dysfunction, altered mental status and personality changes
- Common signs include focal neurological deficits, visual field defects and papilledema
- Reference: Cancer 2010;116:1358
Diagnosis
- Based on clinical history, imaging, histology, immunohistochemical and molecular features
Radiology description
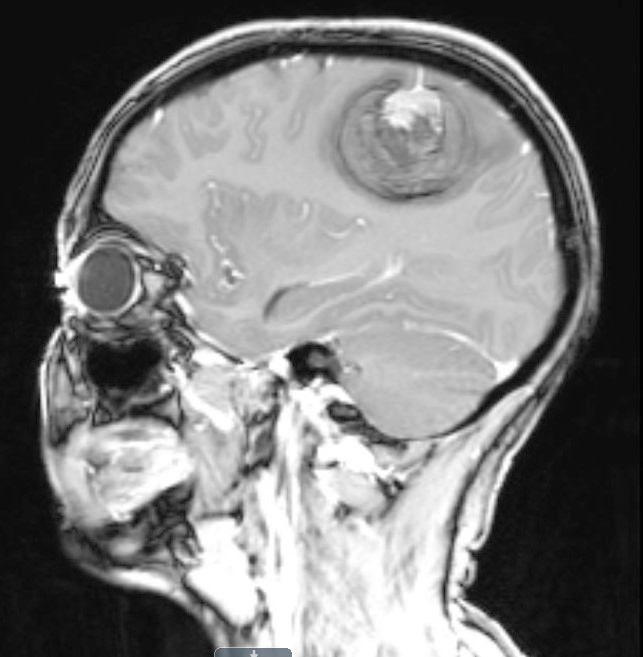
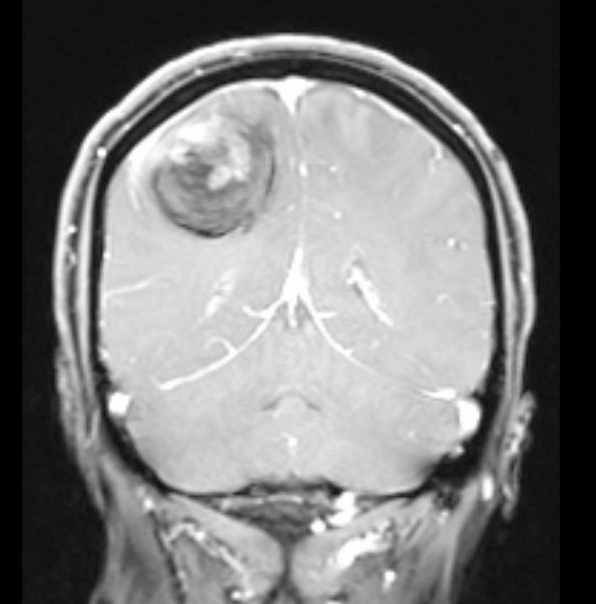
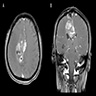
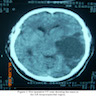
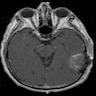
- Usually infiltrative, supratentorial, peripheral, cortical based with dural base / invasion or skull involvement (Eur Radiol 2019;29:429)
- Rarely infratentorial or intraventricular
- CT imaging:
- Large necrotic areas and heterogenous contrast enhancement, similar to glioblastoma (AJNR Am J Neuroradiol 1985;6:527, Neurosurgery 1990;26:261)
- Hyperdense with well defined margins homogenous enhancement, mimicking meningioma (AJNR Am J Neuroradiol 1985;6:527, Neurosurgery 1990;26:261)
- MRI:
- Well demarcated mass with a heterogeneous appearance (Eur Radiol 2019;29:429)
- Heterogeneously isointense to hypointense on the T1 weighted images (Eur Radiol 2019;29:429)
- Mixed hypointense / homointense / hyperintense on the T2 weighted images (Eur Radiol 2019;29:429)
- Unevenly thickened walls with strong rim and a ring-like or paliform enhancement patterns in 78.26% (36/46) of cases (Eur Radiol 2019;29:429)
- Hemorrhage at different stages; salt and pepper sign on T2 or T1 weighted images in 67.39% (31/46) of cases (Eur Radiol 2019;29:429)
Radiology images
Contributed by Nicolas Kostelecky, M.D.
Images hosted on other servers:
Prognostic factors
- Median overall survival with treatment (both primary and secondary gliosarcoma): 17.5 months (J Neurooncol 2015;125:401)
- Median overall survival with treatment (primary gliosarcoma alone): 24.7 months (J Neurooncol 2015;125:401)
- Most important prognostic factors of overall survival: age, extent of resection and adjuvant radiotherapy (Neuro Oncol 2009;11:183)
Case reports
- 8 year old boy with giant parieto-occipital gliosarcoma (Surg Neurol Int 2018;9:111)
- 16 year old boy, 23 year old woman, 35 year old man and 35 year old woman with gliosarcoma (World J Oncol 2017;8:53)
- 37 year old woman with primary gliosarcoma; 2 recurrences and extracranial metastasis, including molecular evidence for clonal evolution (Cold Spring Harb Mol Case Stud 2020;6:a004671)
- 69 year old man with primary gliosarcoma and multiple extracranial metastases (Brain Tumor Res Treat 2020;8:53)
Treatment
- Multimodal therapy, similar to glioblastoma (J Clin Neurosci 2014;21:478, Clin Neurol Neurosurg 2019;182:98)
- Treatment includes maximal surgical resection, followed by radiotherapy and chemotherapy (J Clin Neurosci 2014;21:478, Clin Neurol Neurosurg 2019;182:98)
- Given the higher propensity to metastasize, screening for extracranial metastases is recommended (J Neurooncol 2007;83:39)
Gross description
- Usually well circumscribed, lobular mass in superficial location, weakly attached to dura (Neurosurgery 1990;26:261)
- Glial component is soft with foci of yellowish necrosis or red-brown recent and remote hemorrhage
- Sarcomatous component is firm and well circumscribed, facilitating separation from adjacent brain tissue
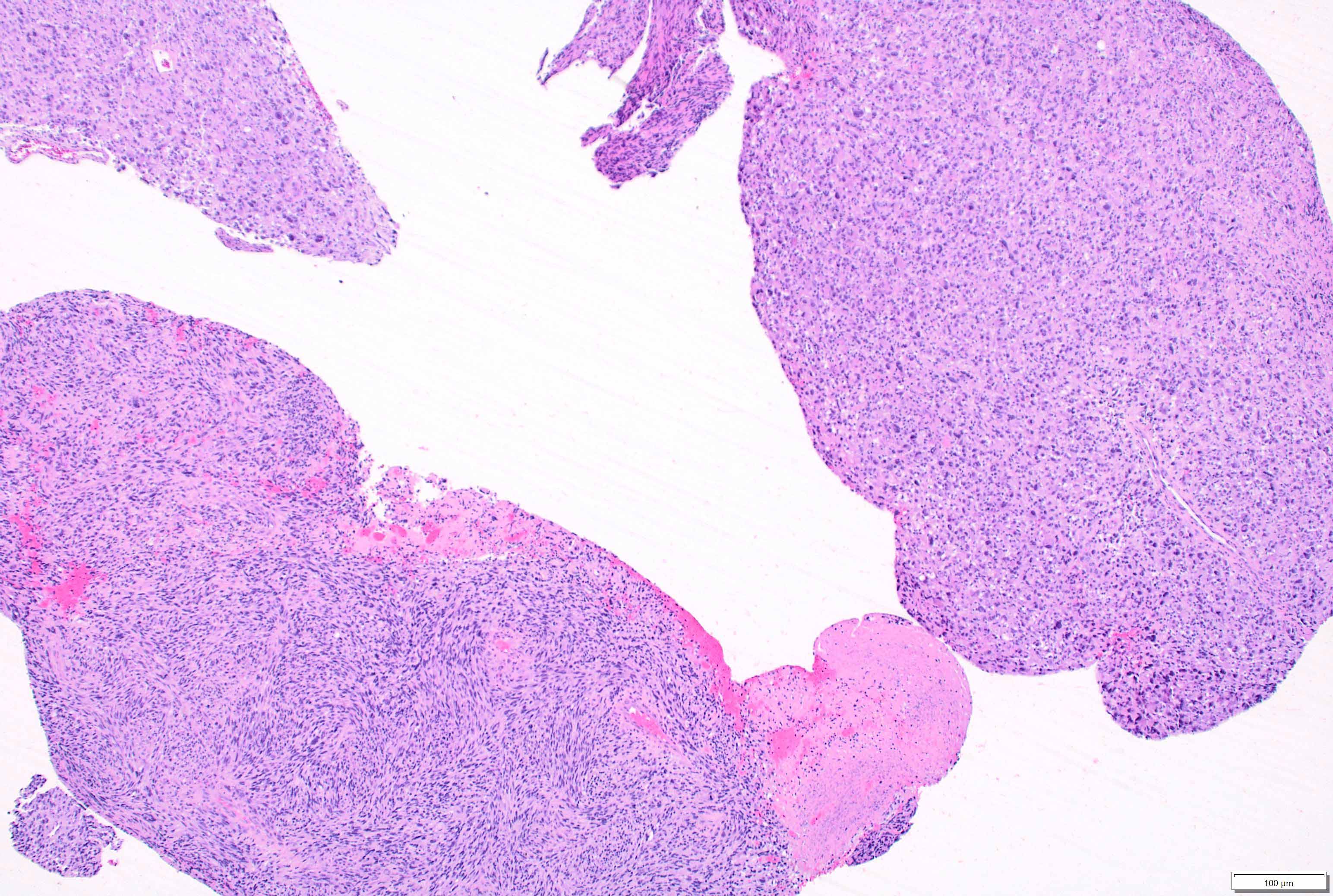
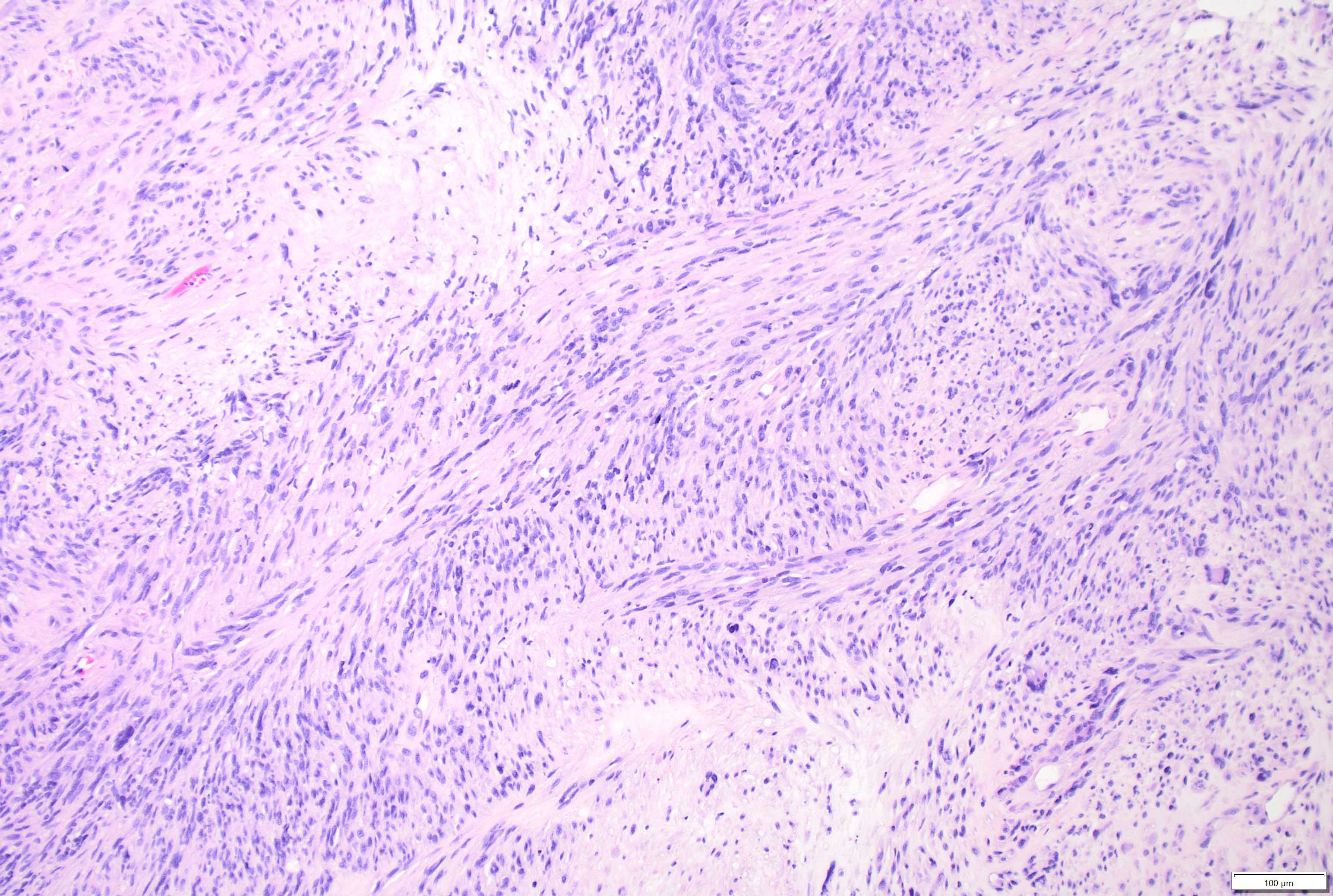
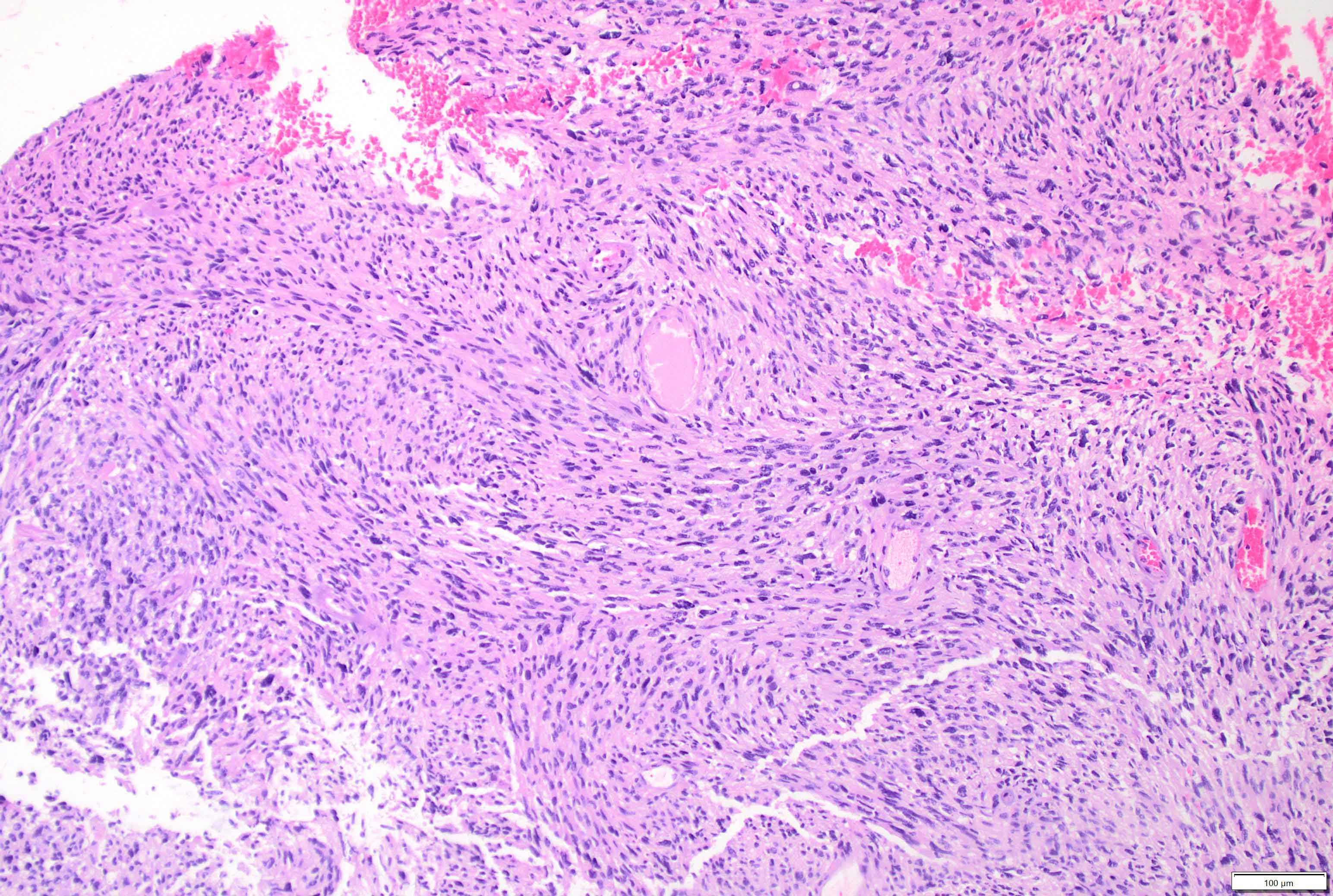
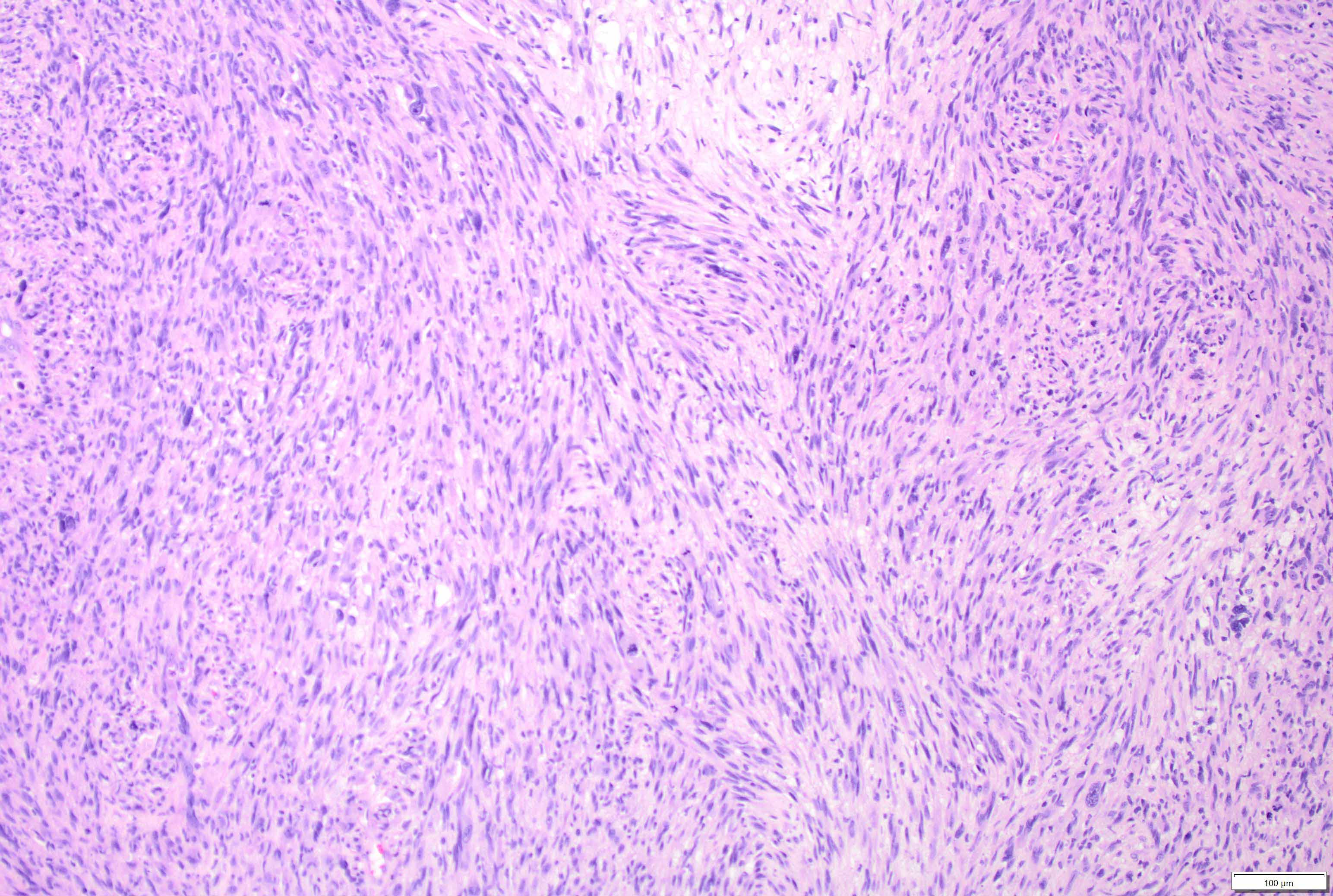
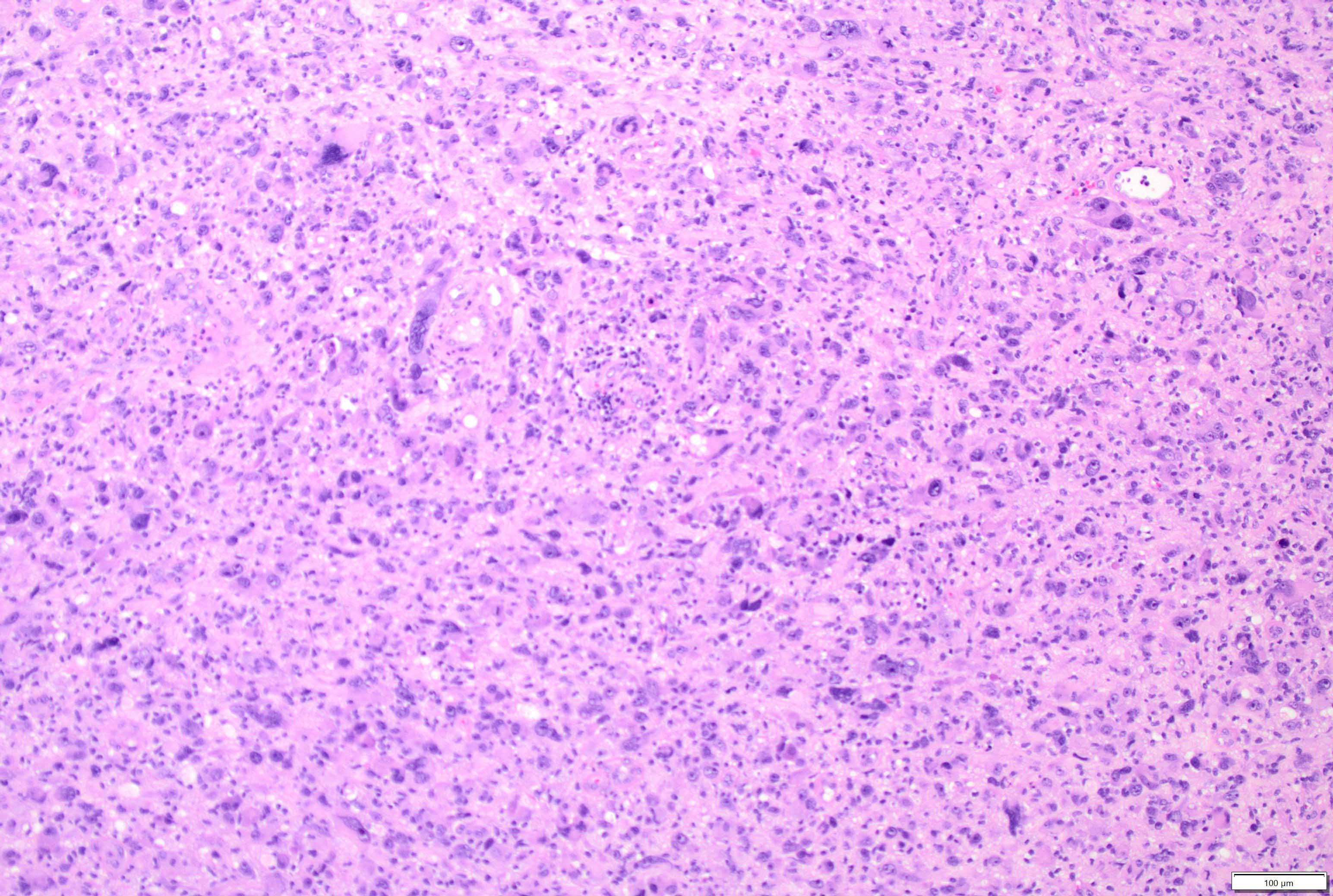
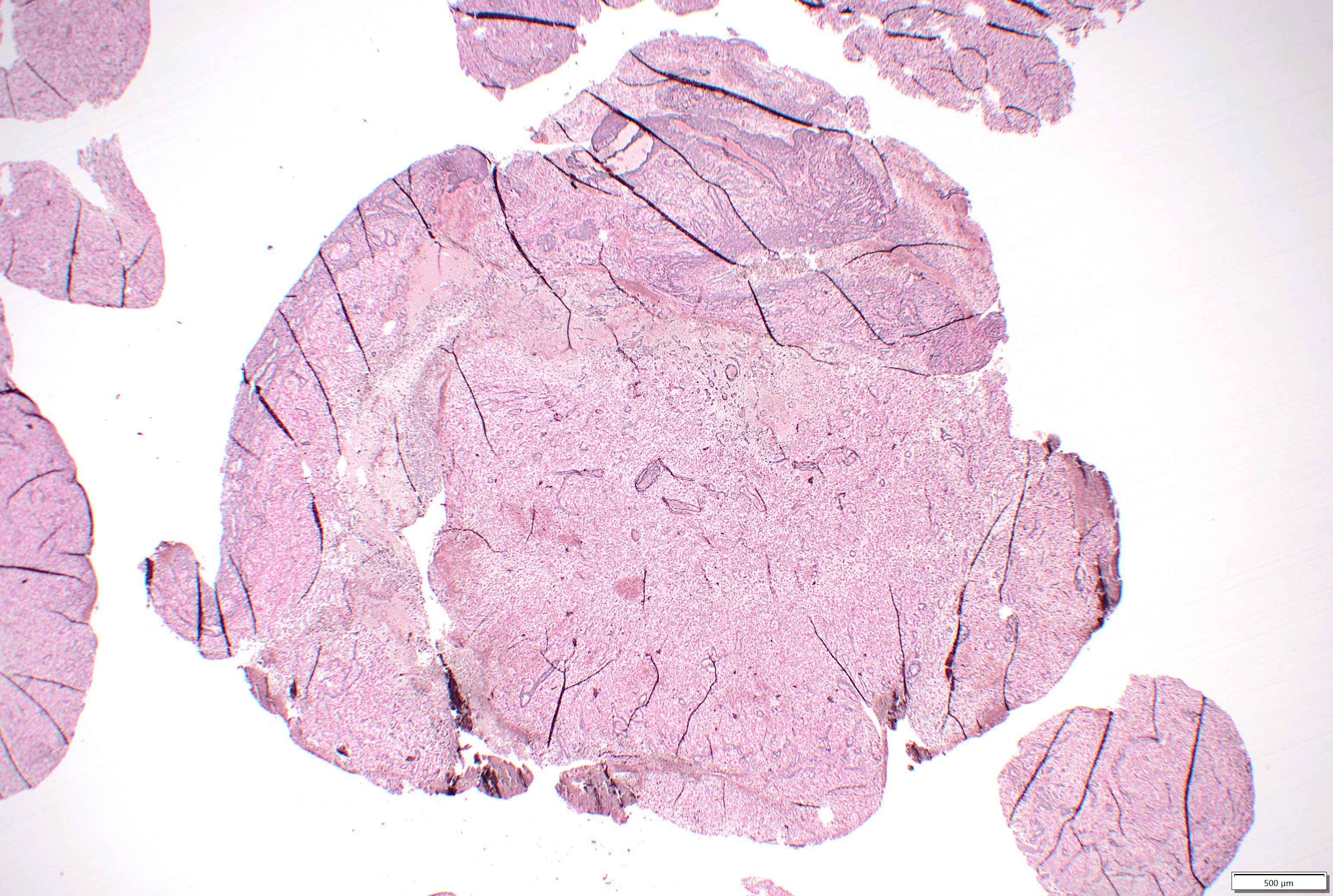
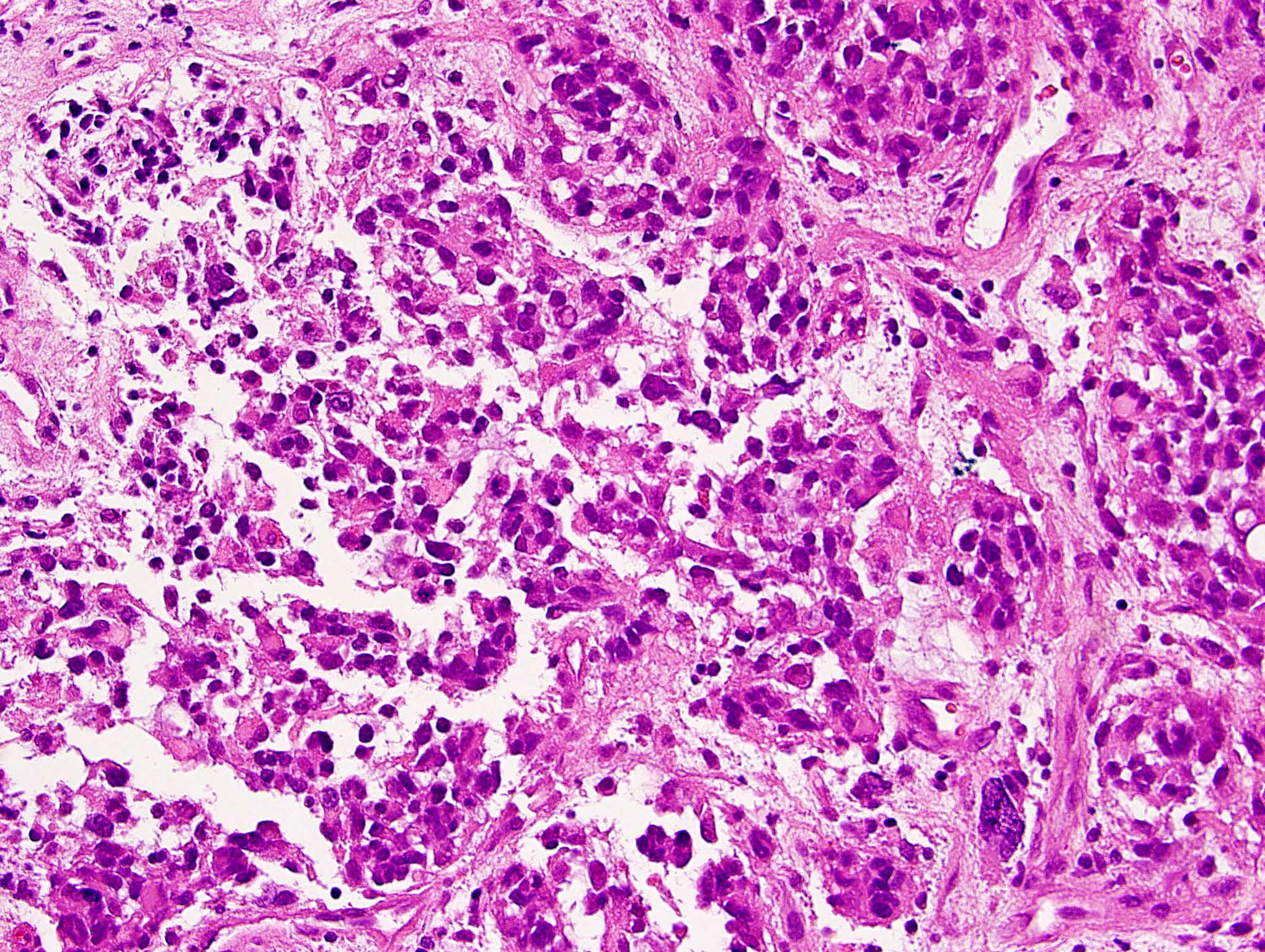
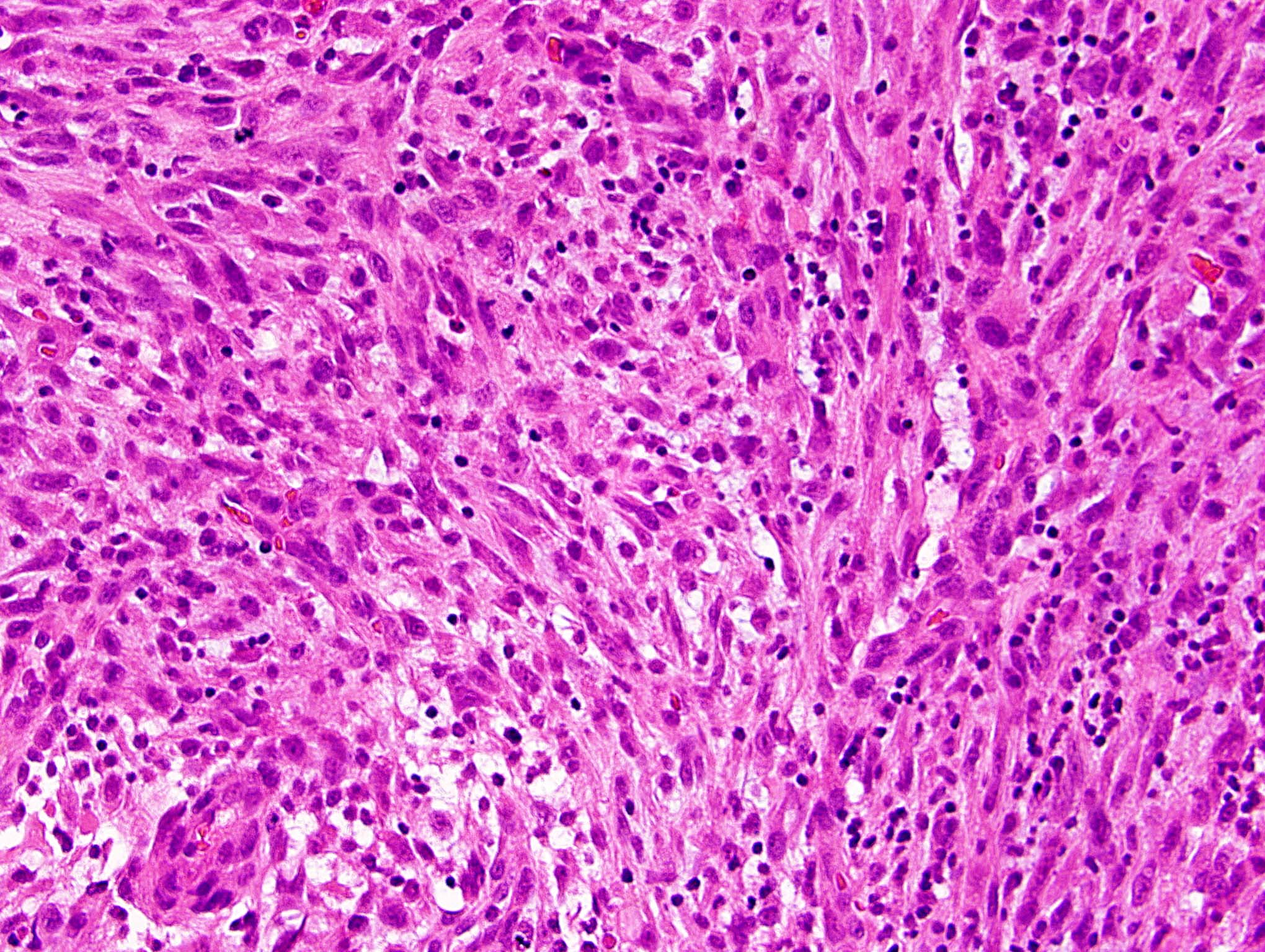
Microscopic (histologic) description
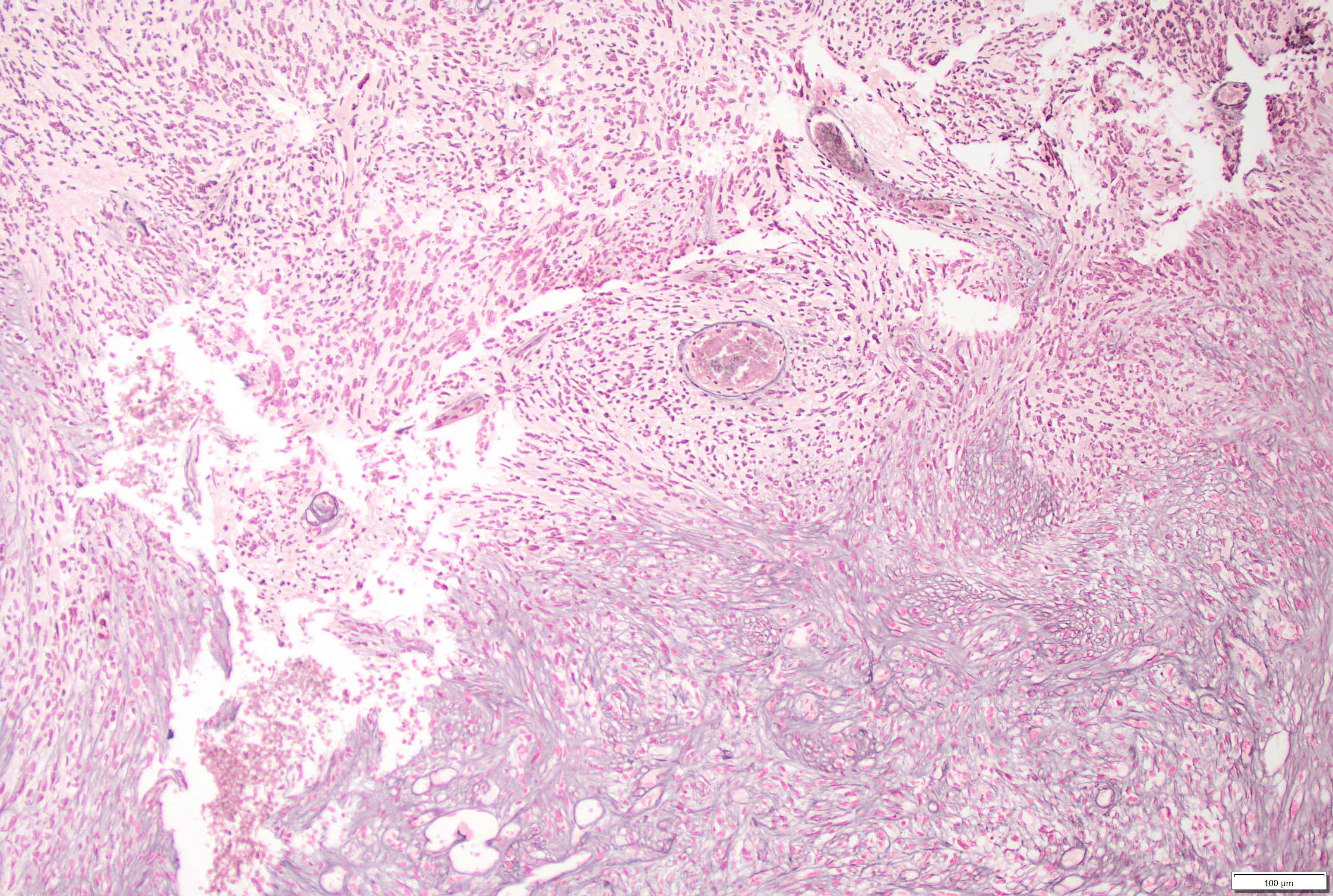
- Mixed tumor of biphasic differentiation with glial and sarcomatous components
- Proportions of glial and sarcomatous components vary; may show mosaic pattern
- Glial component typically shows typical features of glioblastoma, including pleomorphic astrocytic cells with nuclear atypia, mitosis, pseudopalisading necrosis and microvascular proliferation
- Sarcomatous component shows densely packed, spindle shaped cells with nuclear atypia; can show a variety of morphologies: fibrosarcoma, osteosarcoma, chondrosarcoma, angiosarcoma, rhabdosarcoma or undifferentiated pleomorphic sarcoma
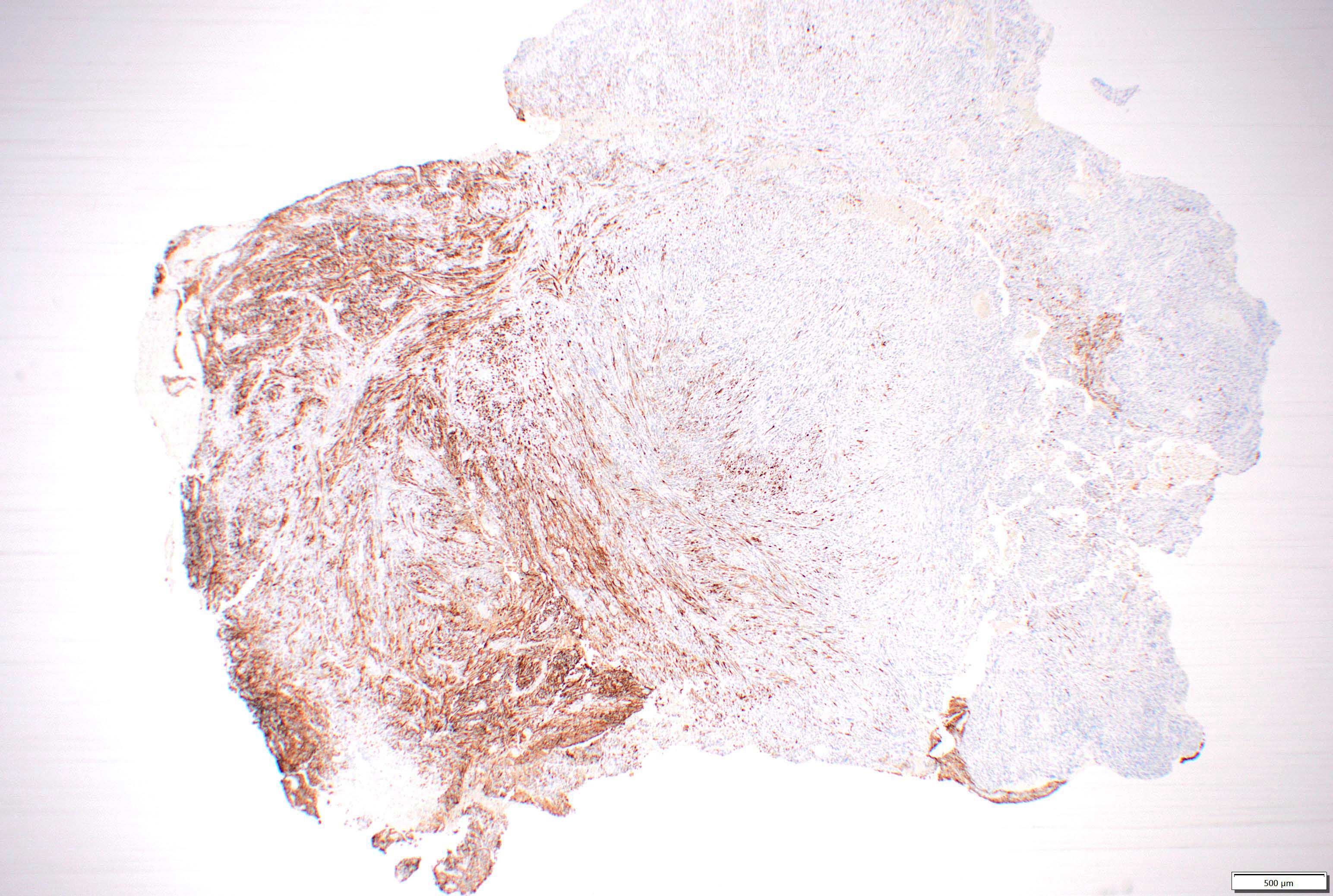
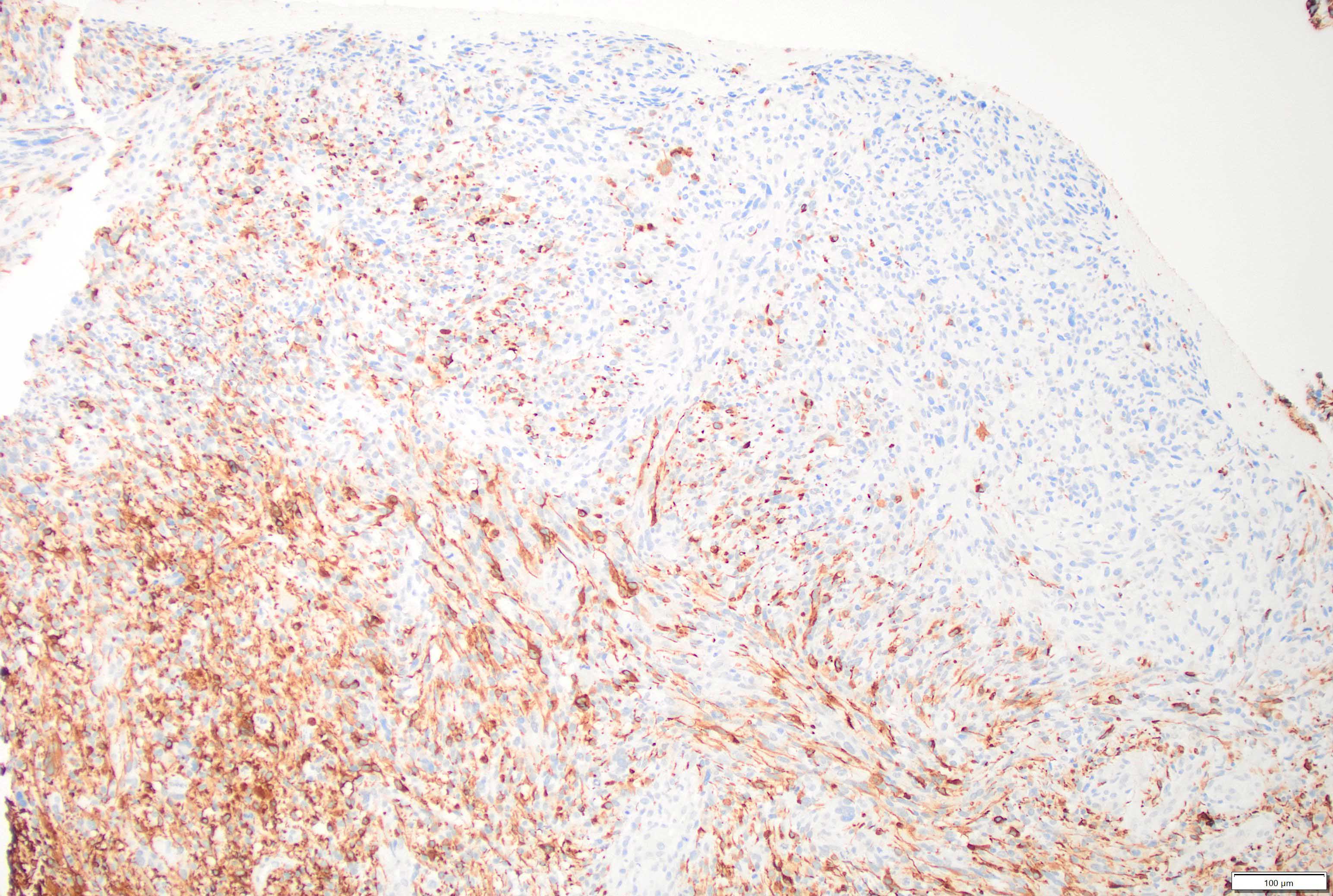
Microscopic (histologic) images
Cytology description
- Smears demonstrate biphasic neoplastic cell populations with mesenchymal and glial components
- Highly cellular, rich arborizing vessels, high mitotic rate and necrosis
- Glial component has pleomorphic round / oval nuclei
- References: Diagn Cytopathol 2009;37:906, Acta Cytol 2016;60:490, Diagn Cytopathol 2004;30:77
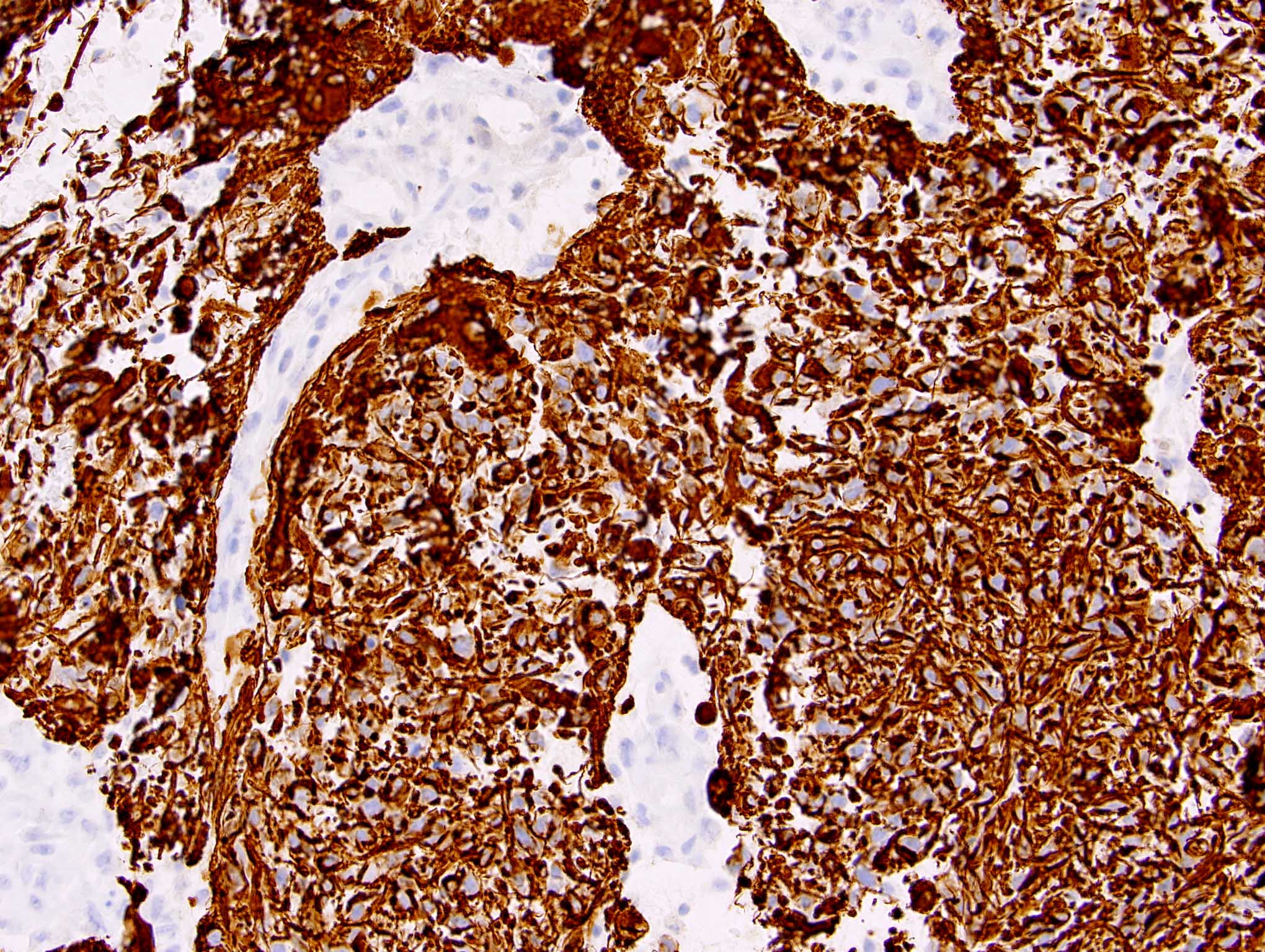
Positive stains
- GFAP in glial region but mostly negative in sarcomatous region
- Olig2 is positive in the glial region (93% of cases) (Mod Pathol 2013;26:315)
- Reticulin in sarcomatous region and blood vessels in glial region
- Vimentin in sarcomatous region
- Other stains in sarcomatous region depending on differentiation: CD34, desmin, etc.
- p53 may be positive in both glial and sarcomatous region
- References: Neuroradiol J 2013;26:639, Neuropathol Appl Neurobiol 1991;17:177
Negative stains
- Olig2, unlike other glioblastomas
Molecular / cytogenetics description
- Most common genetic aberrations in gliosarcoma similar to those of primary glioblastoma: PTEN and PIK3 mutations, p53 mutation and p16 deletion (Cancers (Basel) 2019;11:284)
- Among 19 commonly altered genes in gliosarcoma: most frequent are TERT promoter (92%), PTEN (66%), TP53 (60%) and NF1 (41%) (Sci Rep 2021;11:18009)
- Altered genes that are potentially targetable by FDA approved therapies: BRAF, EGFR, CDKN2A, NF1 and PTEN (Sci Rep 2021;11:18009)
- Less frequent pathologic EGFR alteration in gliosarcoma as compared to glioblastoma (BMC Neurol 2021;21:231)
- MGMT methylation and IDH1 mutation are rare events in primary gliosarcoma (J Neurooncol 2018;137:303)
- Gliosarcomas overexpress mesenchymal proteins, indicating epithelial mesenchymal transition (EMT) (Brain Pathol 2012;22:670)
Sample pathology report
- Brain, cerebrum, right, mass, excision:
- Gliosarcoma, IDH wildtype, WHO grade 4 (see microscopic description and comment)
- Microscopic examination shows highly pleomorphic, glial and spindled tumor cells with pseudopalisading necrosis and microvascular proliferation. The spindle cell component has herringbone architecture. The more pleomorphic glial elements show high cellularity. Tumor is remarkable for abundant atypical mitosis. There are areas of geographical necrosis.
- IHC: The glial elements are positive for GFAP. Reticulin is markedly increased in the spindle cell areas with concomitant negativity for GFAP. This dual immunostaining pattern with strong reactivity of glial component for GFAP and reticulin positivity in mesenchymal component supports a diagnosis of glioblastoma.
- Molecular: PCR based analysis of IDH1 and IDH2 genes (exon 4) shows wildtype phenotype. Analysis for MGMT gene promoter hypermethylation shows no hypermethylation.
Differential diagnosis
- Glioblastoma with meningeal invasion:
- Lacks alternating areas of glioma and sarcoma
- Oligosarcoma (Acta Neuropathol 2022;143:263):
- Recurrence after oligodendroglioma or de novo
- Belongs to IDH mutant and 1p19q codeleted category in 2021 WHO
- Smooth muscle differentiation by proteomic profiling
- Embedded in a dense network of reticulin fibers
- Frequent p53 accumulation, positivity for SMA and CALD1, loss of Olig2, gain of H3K27me3, CDKN2A / CDKN2B deletions, loss of NF1 and gain of YAP1
- Frequent mutations in IDH, TERT promoter, FUBP1, CIC, NF1 and TP53
- Primary sarcomas of the CNS:
- Lack glial differentiation
- Metastatic sarcomas:
- Clinical history of a primary sarcoma elsewhere
- Lack glial differentiation
Additional references
Board review style question #1
A 58 year old man with a medical history that is significant for resection and treatment (including radiotherapy) of a glioblastoma (IDH wildtype) 2 years prior, presents with a headache. MRI shows a heterogeneously enhancing mass associated with the dura near his prior resection site. The tumor is resected and shows the above on H&E and immunostains. Which of the following is most likely true of this tumor?
- It is a metastatic sarcoma
- It is a recurrent glial malignancy, this time with sarcomatous differentiation: secondary gliosarcoma
- Most likely hypermethylated in the MGMT gene promoter region
- WHO grade is 3, since we do not see necrosis
Board review style answer #1
B. It is a recurrent glial malignancy, this time with sarcomatous differentiation: secondary gliosarcoma
Comment Here
Reference: Gliosarcoma
Comment Here
Reference: Gliosarcoma
Board review style question #2
Gliosarcoma shows which of the following similarities to primary glioblastoma?
- Frequent EGFR alteration and MGMT hypermethylation
- Frequent IDH mutations and loss of ATRX expression
- Frequent infratentorial and spinal cord localization
- Slightly increased incidence in males
Board review style answer #2