Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Intraoperative frozen / smear cytology images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: De Los Santos Y, Cai C. Ependymoma overview. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorependymoma.html. Accessed April 3rd, 2025.
Definition / general
- Circumscribed neuroepithelial tumor with histological and molecular evidence of ependymal differentiation that may arise in the supratentorial region, posterior fossa or spinal cord
Essential features
- 2021 CNS WHO classification has reclassified ependymal tumors according to their location and methylation profiles
- Some traditional histological subtypes, including the papillary, clear cell, tanycytic and anaplastic subtypes, have been discontinued
- Supratentorial ependymal tumors include supratentorial ependymoma ZFTA fusion positive (ST-ZFTA), supratentorial ependymoma, YAP1 fusion positive (ST-YAP1) or supratentorial subependymoma (ST-SE)
- Posterior fossa ependymal tumors include posterior fossa ependymoma group A (PFA), group B (PFB) and posterior fossa subependymoma (PF-SE)
- Spinal ependymal tumors include spinal ependymoma (SP-EP), spinal ependymoma, MYCN amplified (SP-MYCN), spinal subependymoma (SP-SE) and myxopapillary ependymoma (SP-MP)
- Common pathology features across all ependymoma subtypes include perivascular pseudorosettes, ependymal rosettes, positive GFAP and S100 cytoplasmic positivity, dot-like EMA perinuclear reactivity and negative Olig2 nuclear reactivity
ICD coding
- ICD-10
- C71.0 - malignant neoplasm of cerebrum, except lobes and ventricles
- C71.1 - malignant neoplasm of frontal lobe
- C71.2 - malignant neoplasm of temporal lobe
- C71.3 - malignant neoplasm of parietal lobe
- C71.4 - malignant neoplasm of occipital lobe
- C71.5 - malignant neoplasm of cerebral ventricle
- C71.7 - malignant neoplasm of brain stem
- C71.9 - malignant neoplasm of brain, unspecified
- C72.9 - malignant neoplasm of central nervous system, unspecified
- D33.2 - benign neoplasm of brain, unspecified
Epidemiology
- Ependymal tumors comprise 1.9% of all primary CNS tumors and 6.9% of primary glial neoplasms, with incidence rates of ~0.4/100,000 in the U.S. (Neuro Oncol 2015;17:iv1)
- ST-ZFTA, ST-YAP1 and PFA predominantly occur in children, while SP-MYCN predominantly occurs in middle aged adults; other subtypes (ST-SE, PF-SE, PFB, SP-EP, SP-MP, SP-SE) can occur in all ages (Acta Neuropathol 2024;147:24)
- M:F = 1.38:1 (Acta Neuropathol 2024;147:24)
Sites
- Typically associated with ventricular lining in the spinal cord or cerebrum but can occur intraparenchymally / paraventricularly, particularly in supratentorial tumors
- Spinal ependymomas commonly in cervical and thoracic spine
- Spinal myxopapillary ependymoma predominantly in conus medullaris and filum terminale
- Posterior fossa ependymomas around fourth ventricle
- References: Childs Nerv Syst 2003;19:270, Crit Rev Oncol Hematol 2007;63:81
Pathophysiology
- Single cell RNA sequencing across all major molecular ependymoma groups revealed hierarchical cellular populations, including undifferentiated neural stem cells, radial glia cells and more differentiated cells towards ependymal, astrocytic and neuronal lineages (Cancer Cell 2020;38:44)
- Proportion of undifferentiated or less differentiated cells correlates with poor prognosis and increased recurrence
- Aberrant radial glia-like cells are potential cells of origin for supratentorial ependymoma, with ZFTA::RELA fusions and neural stem cell-like cells the origin of posterior fossa ependymoma (Cancer Cell 2020;38:44)
- Spinal ependymoma likely arises from mature adult ependymal cells due to their highly similar transcriptomic profiles through single cell RNA sequencing (Acta Neuropathol 2024;147:22)
Etiology
- Unknown
Clinical features
- Spinal cord ependymomas present with pain, weakness, sensory loss or radiculopathy, depending on level affected
- Intracerebral ependymomas present with obstructive hydrocephalic symptoms as well as features of mass effect, neurologic deficits or seizures, depending on location
- Reference: Pediatr Neurosurg 2019;54:98
Diagnosis
- Diagnosis is made by integrating histologic features, tumor location and molecular findings (Neuro Oncol 2021;23:1231)
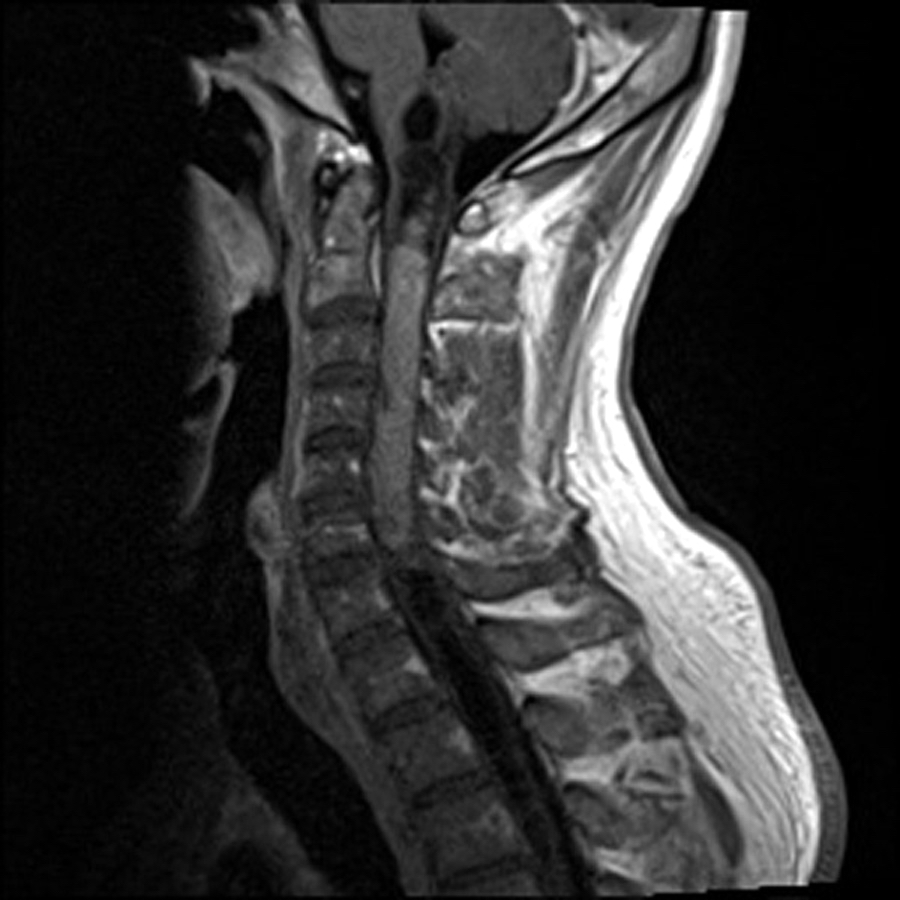
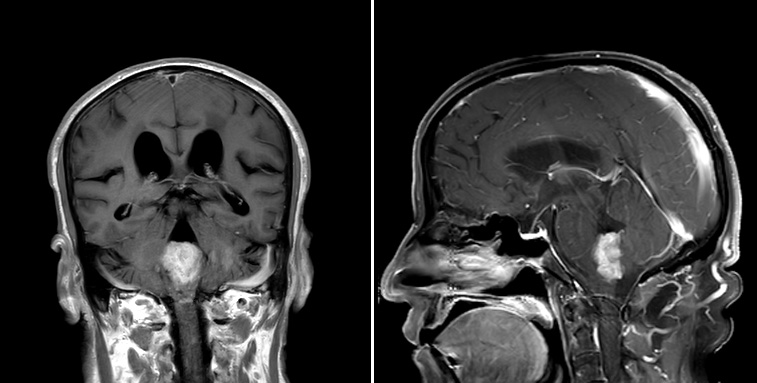
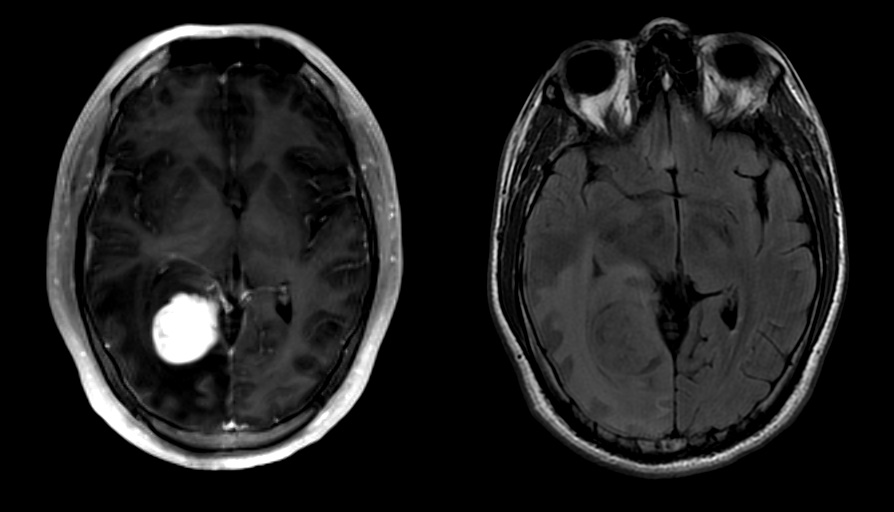
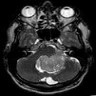
Radiology description
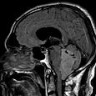
- Commonly discrete, well circumscribed mass with avid uniform contrast enhancement
- Hyperdense on computed tomography and T2 hyperintense on magnetic resonance imaging
- Reference: Childs Nerv Syst 2009;25:1203
Radiology images
Prognostic factors
- Spinal ependymoma (SPEP), generally indolent
- Recent studies suggest the presence of an NF2 mutation may confer a higher risk of recurrence
- Spinal ependymoma, MYCN amplified (SPMYCN) has poor prognosis
- Ependymomas with ZFTA fusions
- 6% of ependymomas occur in posterior fossa and those occur exclusively in children and have worse prognosis
- Supratentorial ependymoma with ZFTA fusion and combined homozygous CDKN2A / CDKN2B losses have worse prognosis
- Supratentorial ependymomas with YAP1 fusions have good prognosis
- Of posterior fossa ependymomas
- PFA is characterized by H3K27 trimethyl loss on IHC and has worse prognosis than PFB
- Gain of 1q and loss of 6q are additional risk factors that predict even worse outcomes in PFA
- Complete resection is a predictor of good prognosis in PFA, PFB, PF-SE and SP-MP but not in ST-ZFTA
- References: Acta Neuropathol 2024;147:24, Brain Pathol 2020;30:863, Acta Neuropathol 2024;147:22
Case reports
- 26 year old woman with neck pain (Cureus 2020;12:e7981)
- 28 year old man with incidental cerebellopontine angle mass (Medicine (Baltimore) 2019;98:e15019)
- 37 year old man with cervicomedullary mass (Asian J Neurosurg 2020;15:190)
- 44 year old woman with extramedullary lumbar spine mass (Yeungnam Univ J Med 2020;37:128)
Treatment
- Gross total resection if possible, with or without adjuvant radiotherapy
- Multiple pathway inhibitors (ALK, EGFR, ErbB2, FAK, MEK, mTOR, TEAD, VEGF) are in clinical trials for NF2 associated tumors (Acta Neuropathol 2024;147:22)
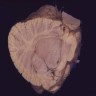
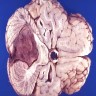
Gross description
- Gray-red tumors with or without cystic degeneration, hemorrhage or necrosis
- Intracranial tumors are well circumscribed
- Ependymomas of fourth ventricle are typically exophytic
Frozen section description
- Cellular tumor with sharp border with brain parenchyma
- Perivascular pseudorosettes, true ependymal rosettes and lumina (J Neurosurg Spine 2018;30:133)
Intraoperative frozen / smear cytology images
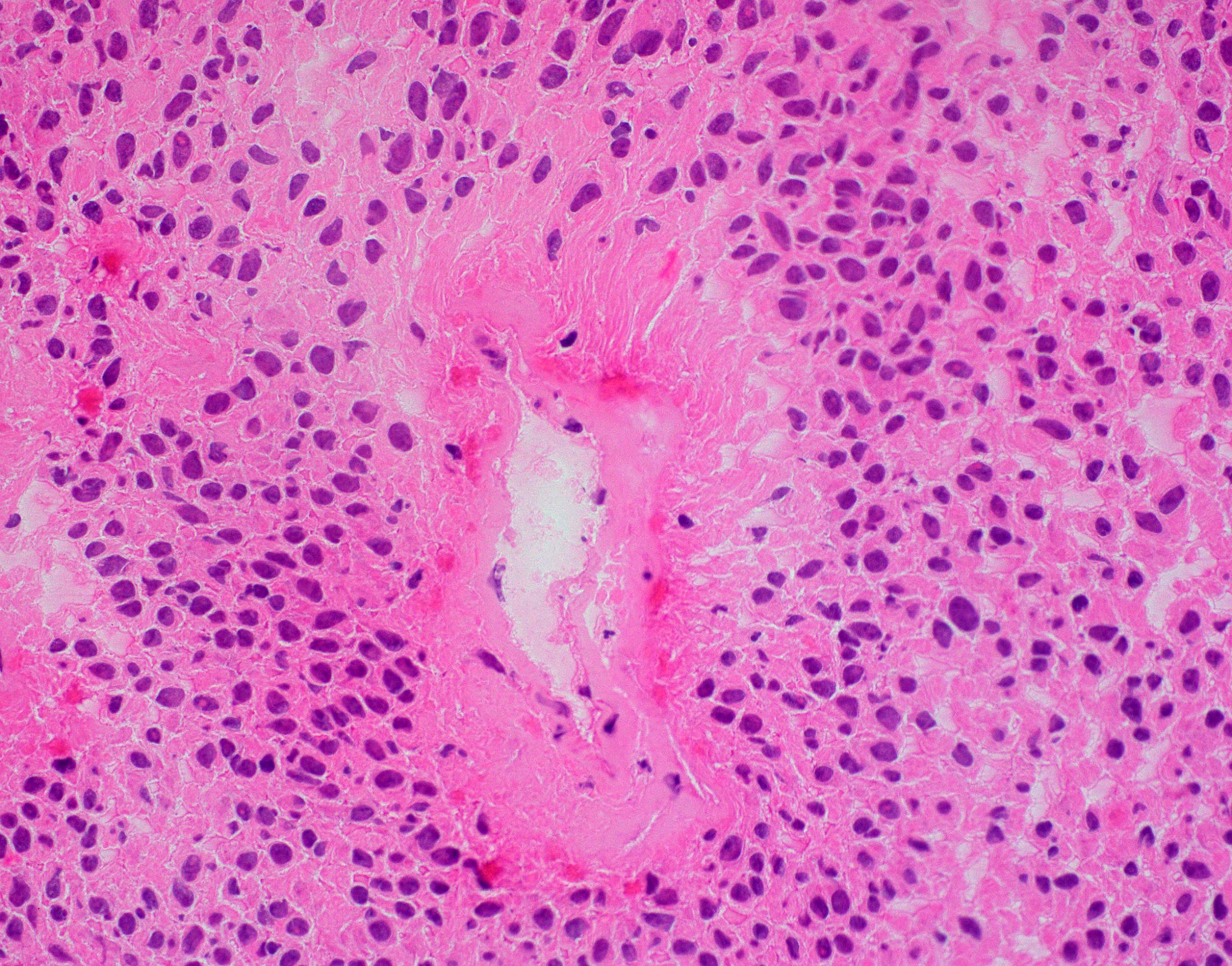
Microscopic (histologic) description
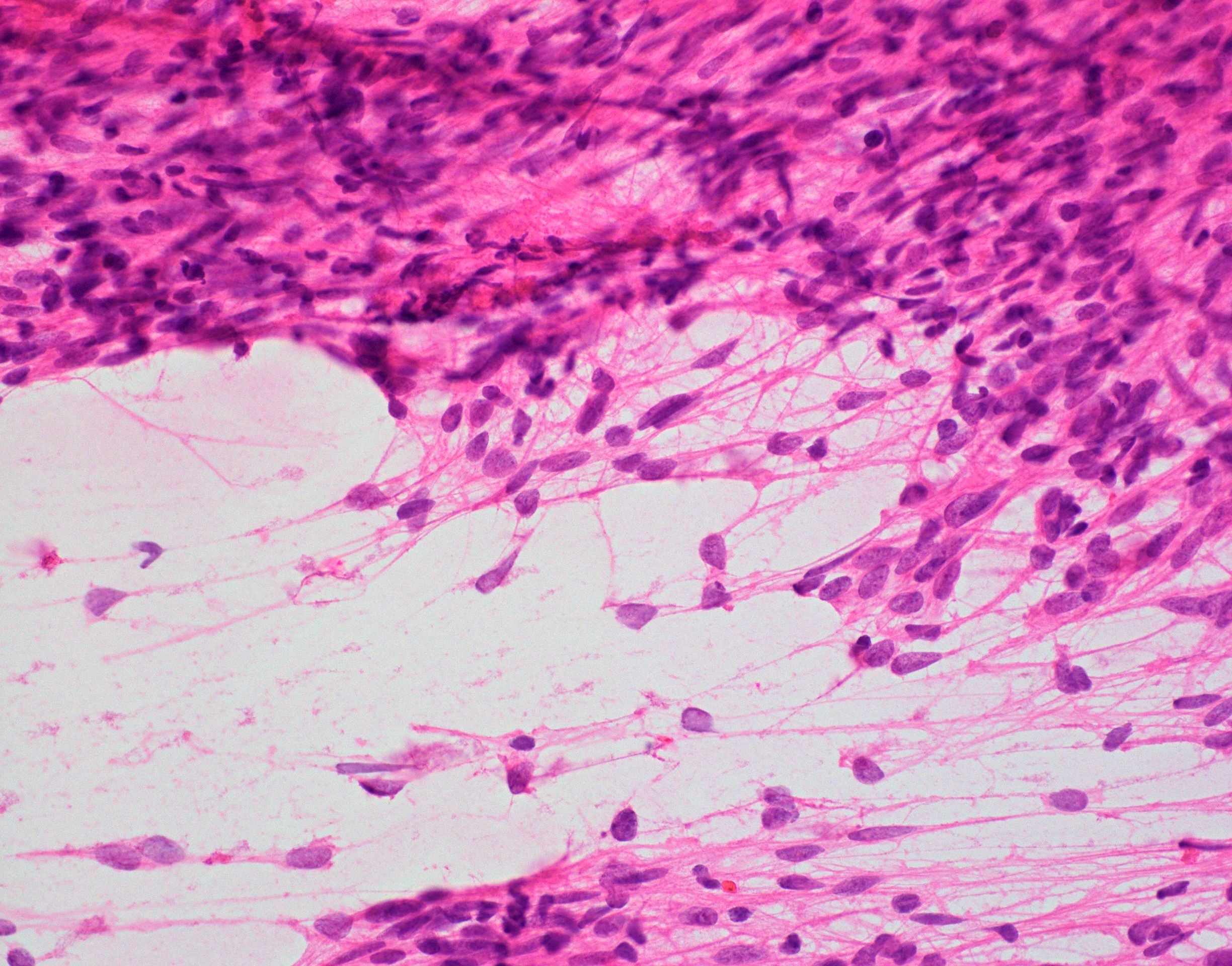
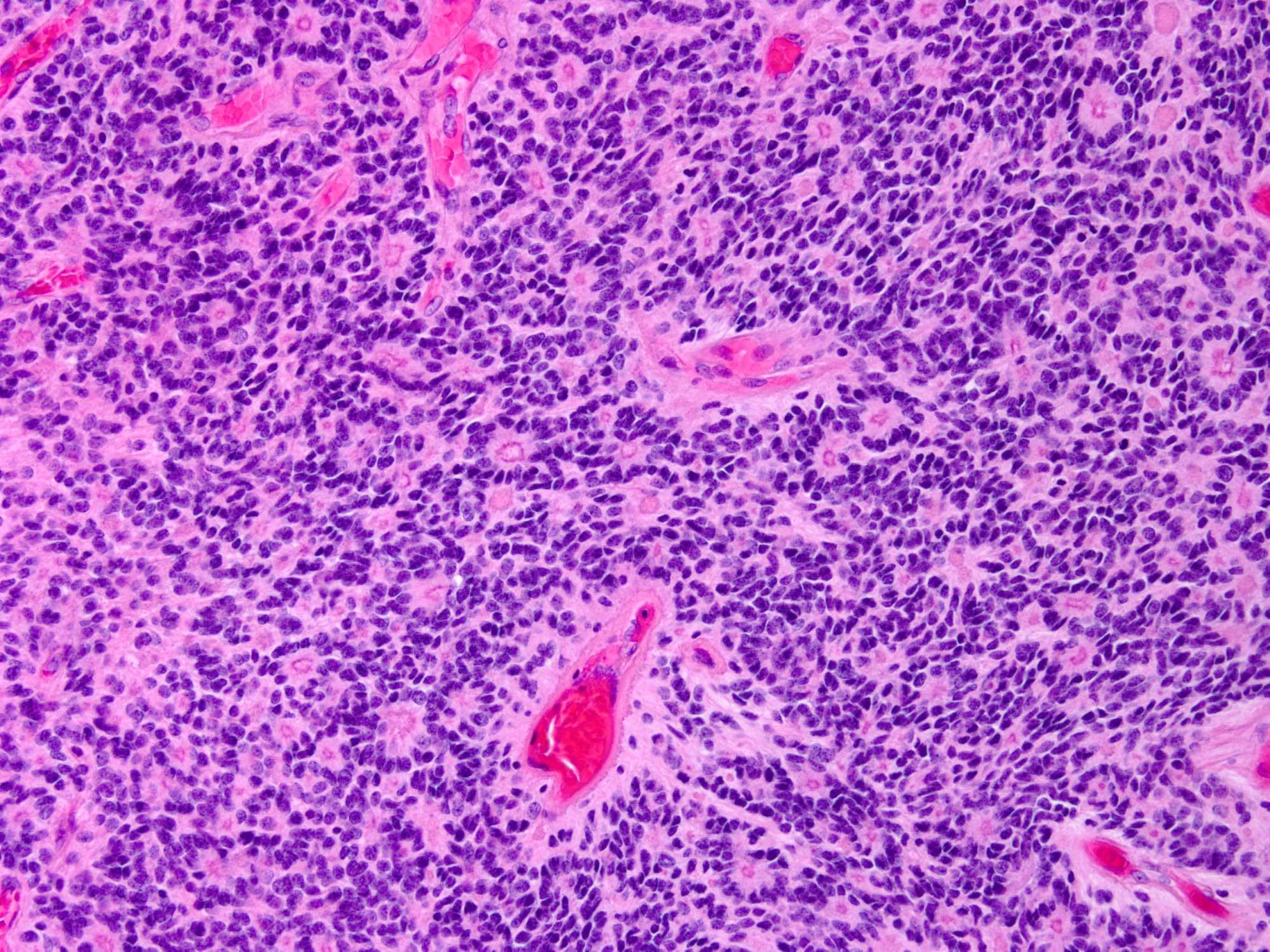
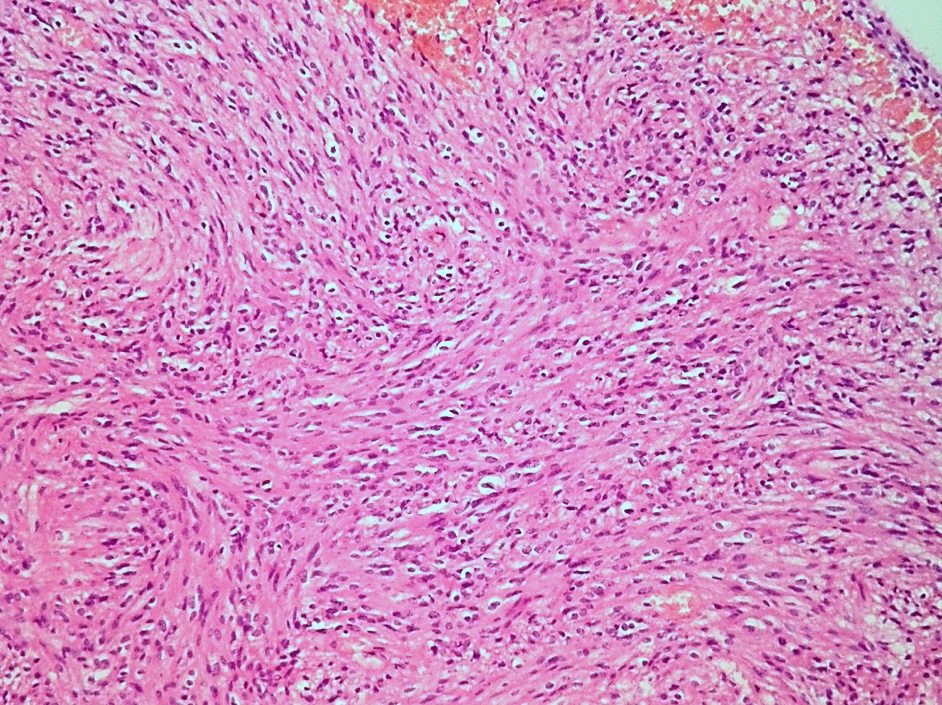
- Cellular tumor with typically sharply circumscribed borders; may be infiltrative
- Monomorphic round to oval cells with speckled chromatin
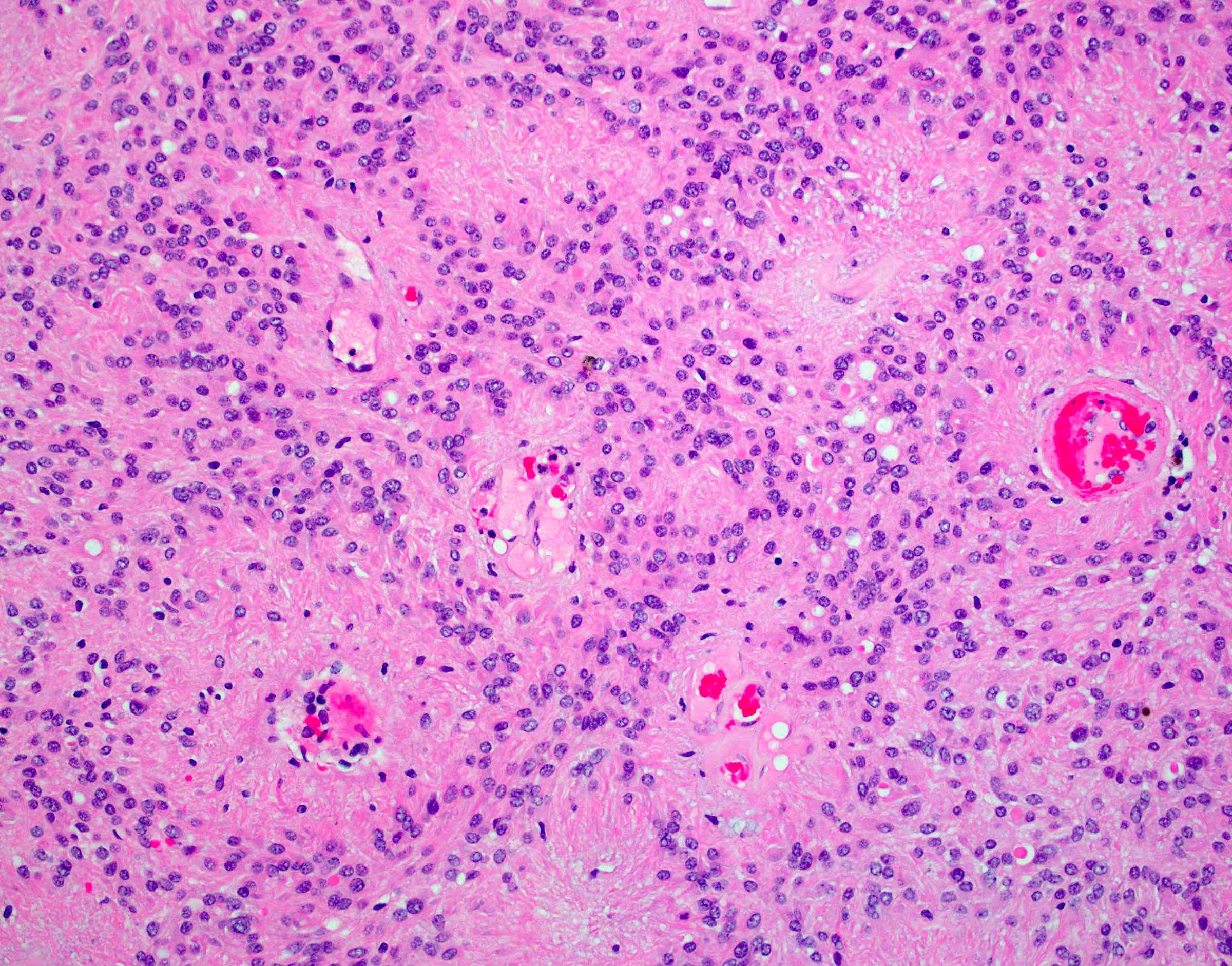
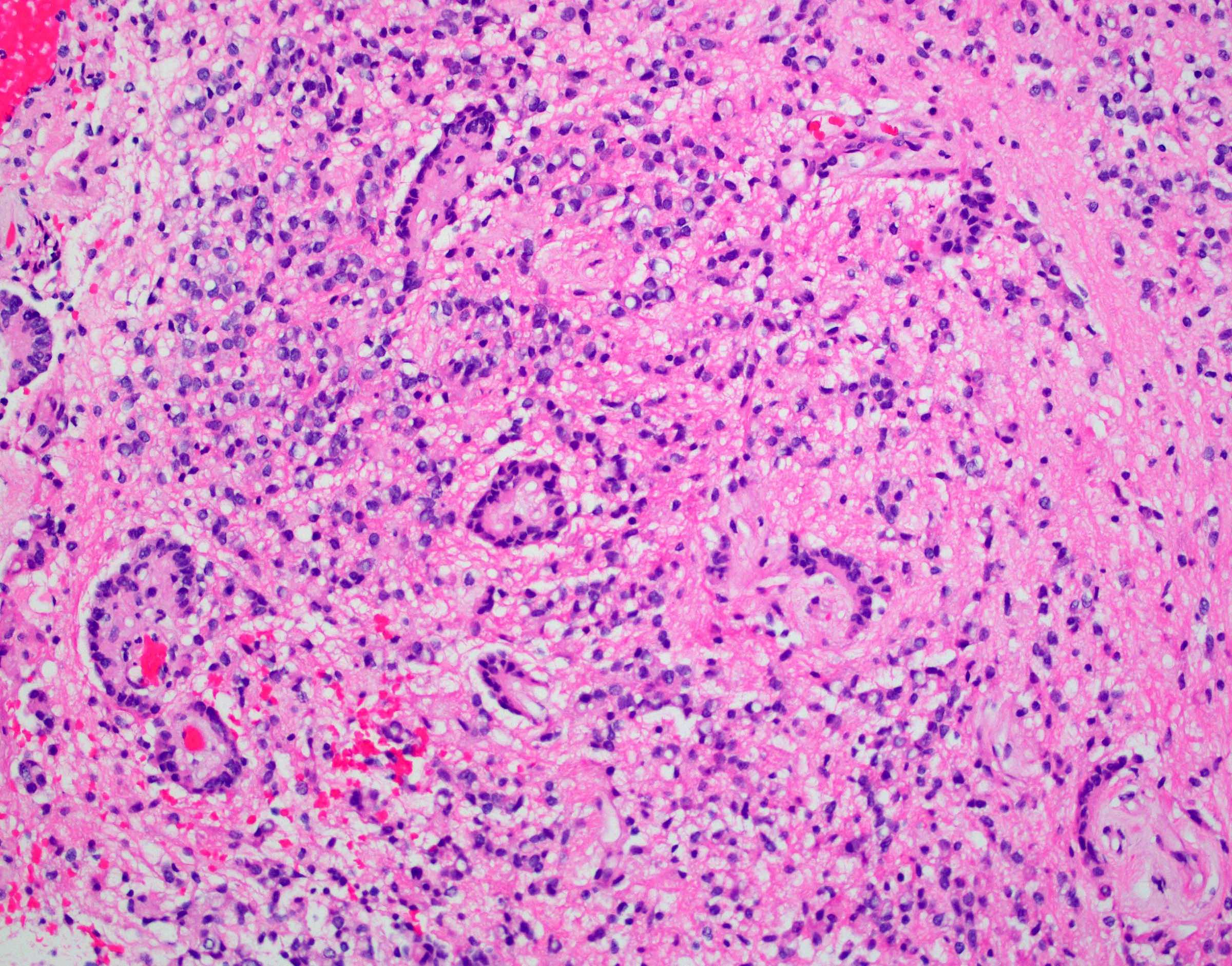
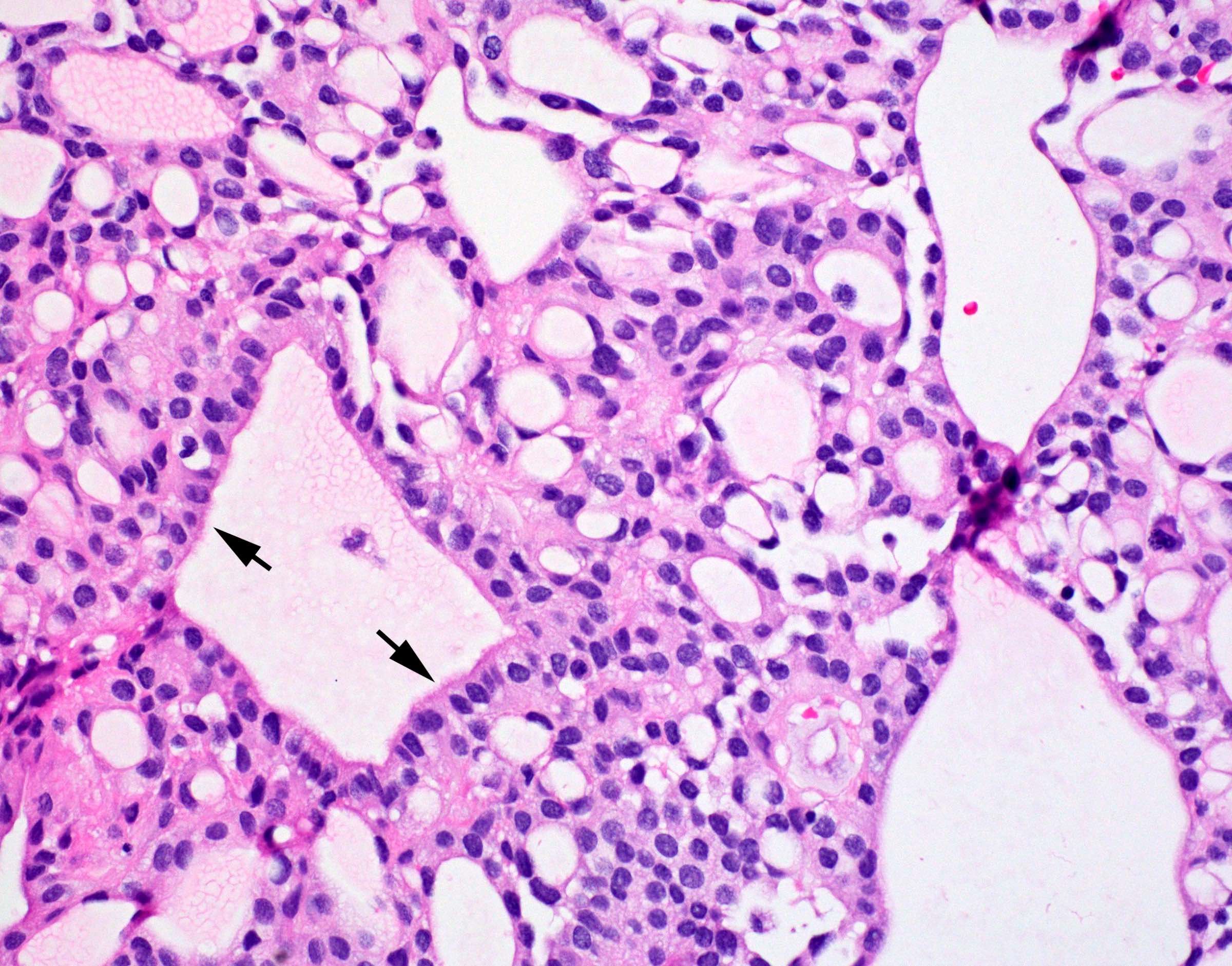
- Perivascular pseudorosettes, true ependymal rosettes, lumina and fibrillar areas
- May see gemistocyte-like cells and hypercellular nodules, particularly in posterior fossa tumors
- Can have nonpalisading necrosis, areas of cystic or myxoid degeneration, calcifications, degenerative atypia, neuronal differentiation and rarely metaplastic elements
- Morphologic subtypes have no clinicopathological significance and include papillary, clear cell and tanycytic
- Utility of histological grading is debated; the 2021 WHO still recommend assigning either WHO grade 2 or grade 3 to an ependymoma, according to its histopathological features as part of the integrated diagnosis
- Myxopapillary ependymoma are now assigned WHO grade 2 in the 2021 WHO CNS tumor classification
- Subependymoma remains a WHO grade 1 (Neuro Oncol 2021;23:1231)
Microscopic (histologic) images
Cytology description
- Spindle shaped cells with oval to elongated nuclei and delicate fibrillary cytoplasm with occasional intracytoplasmic lumina, which can be arranged around blood vessels
- Occasional nuclear grooves and inclusions can be seen (J Pathol Transl Med 2019;53:104)
Positive stains
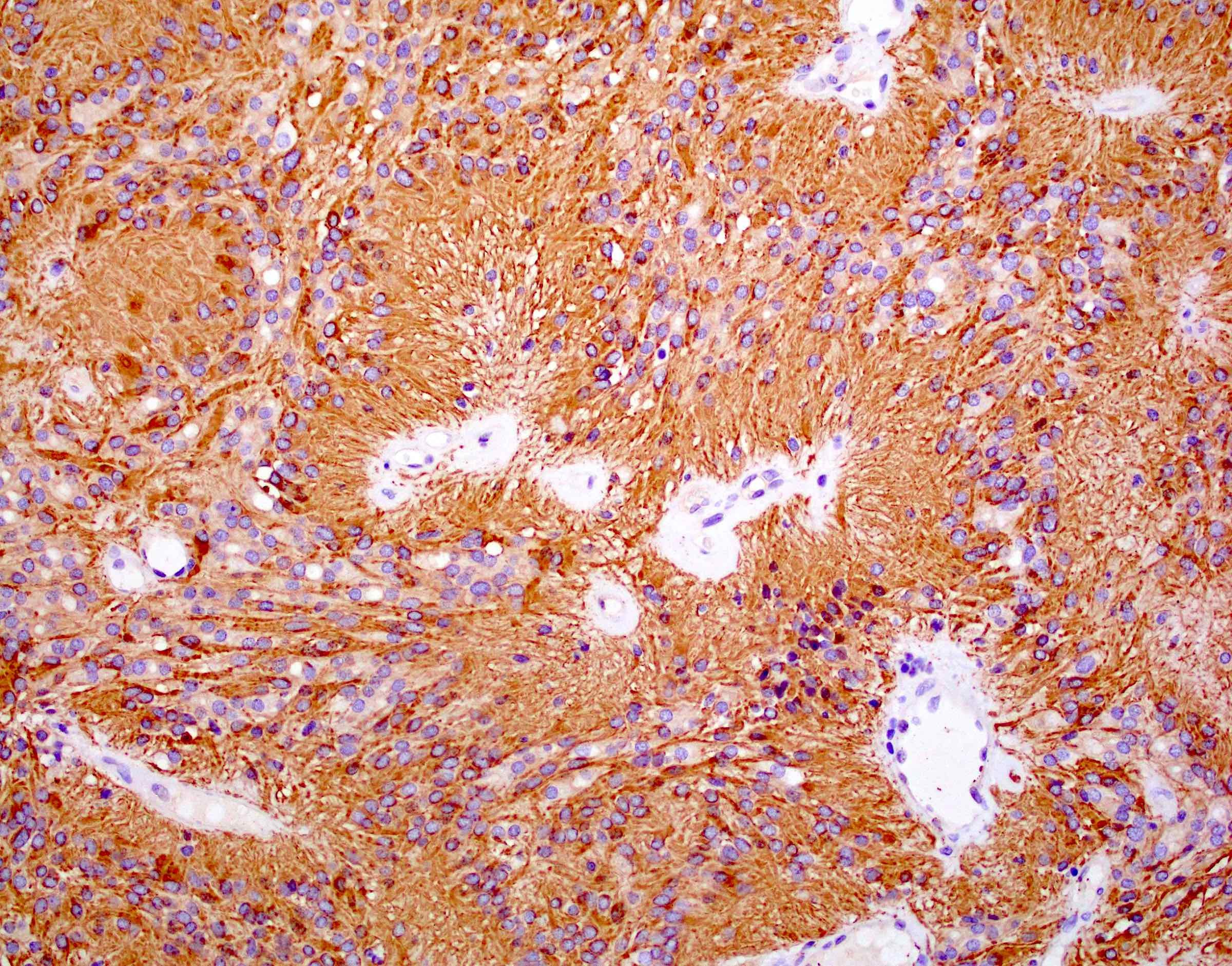
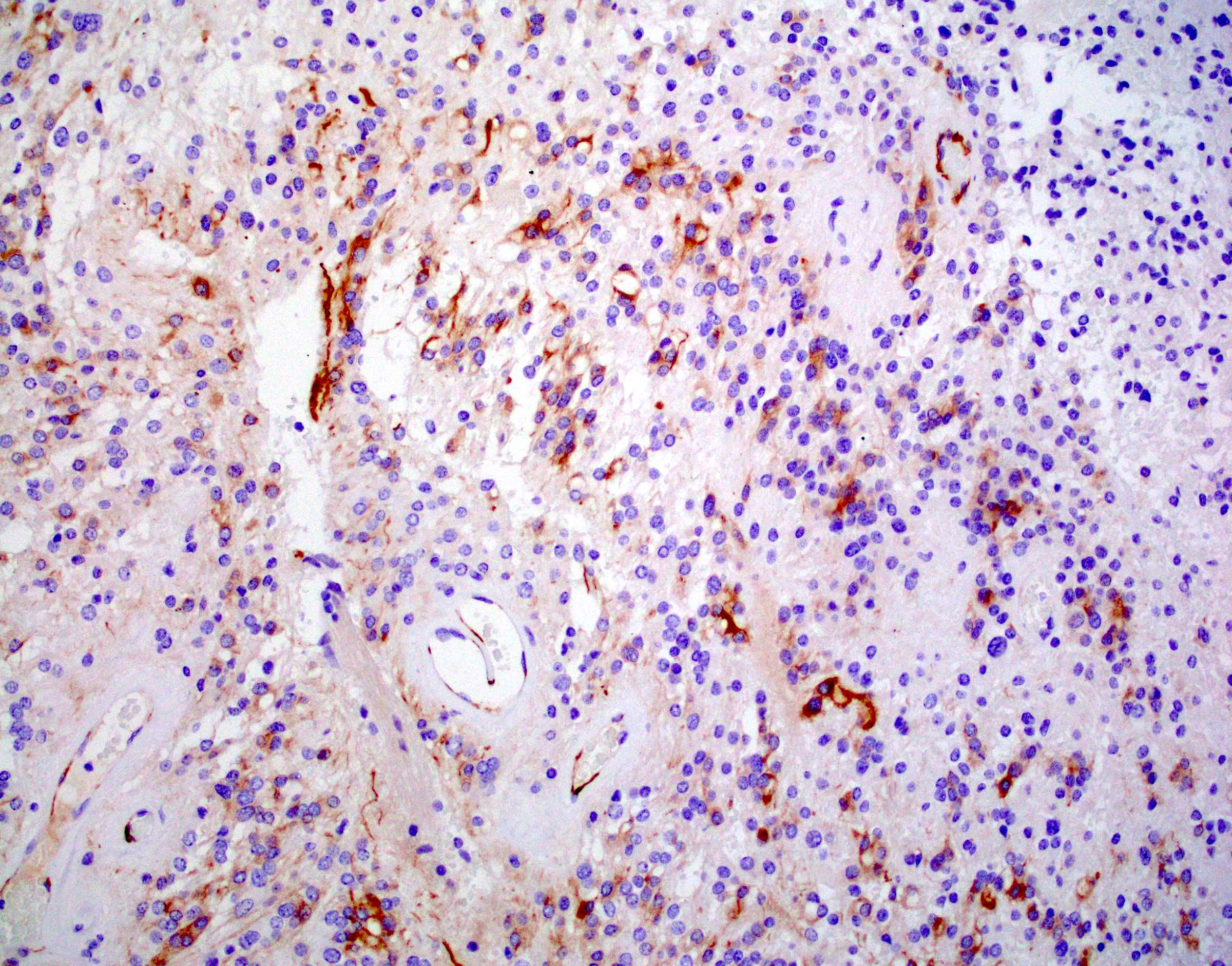
- Positive for S100, GFAP, vimentin
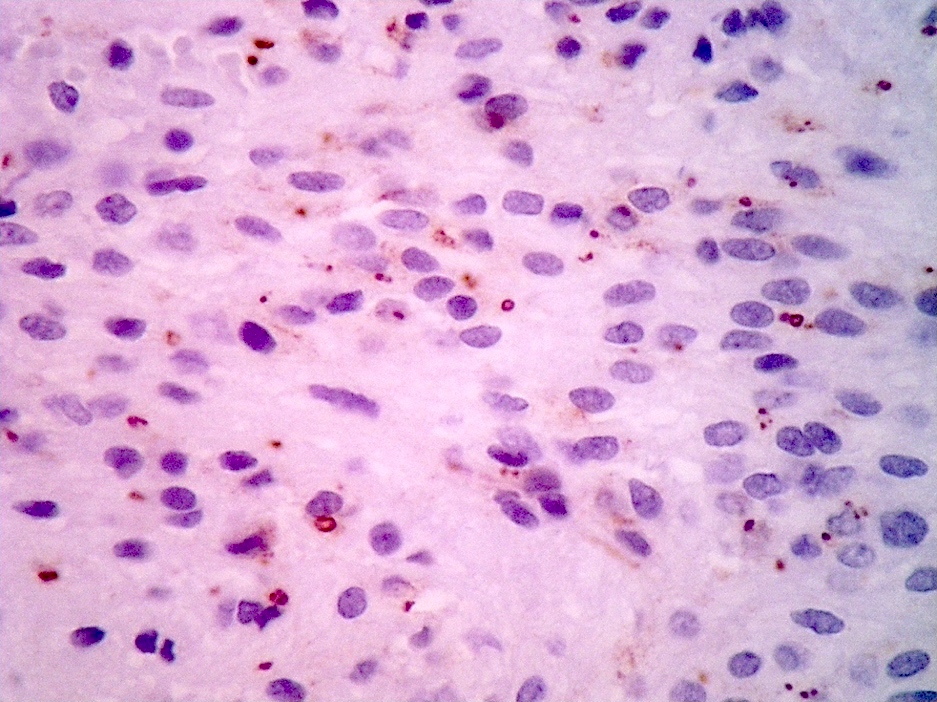
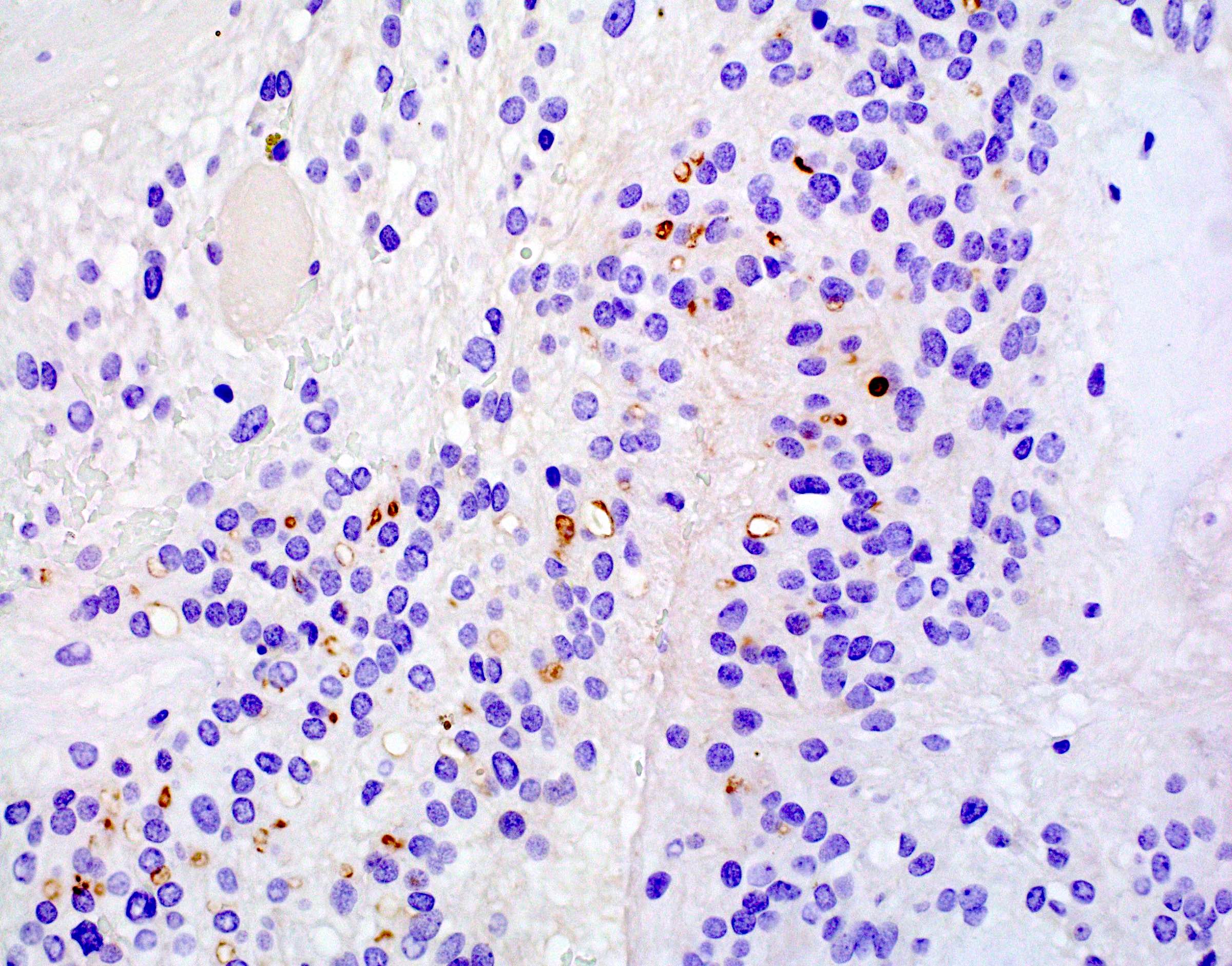
- Perinuclear dot-like pattern of EMA and D2-40 staining
- CD56 staining in lumina and tumor cells
- Variable membranous or dot-like staining for CD99
- Can have focal staining for keratin (CAM 5.2) and synaptophysin
- L1CAM can be positive in some supratentorial ependymomas but mostly is seen in RELA fusion tumors (Am J Surg Pathol 2019;43:56)
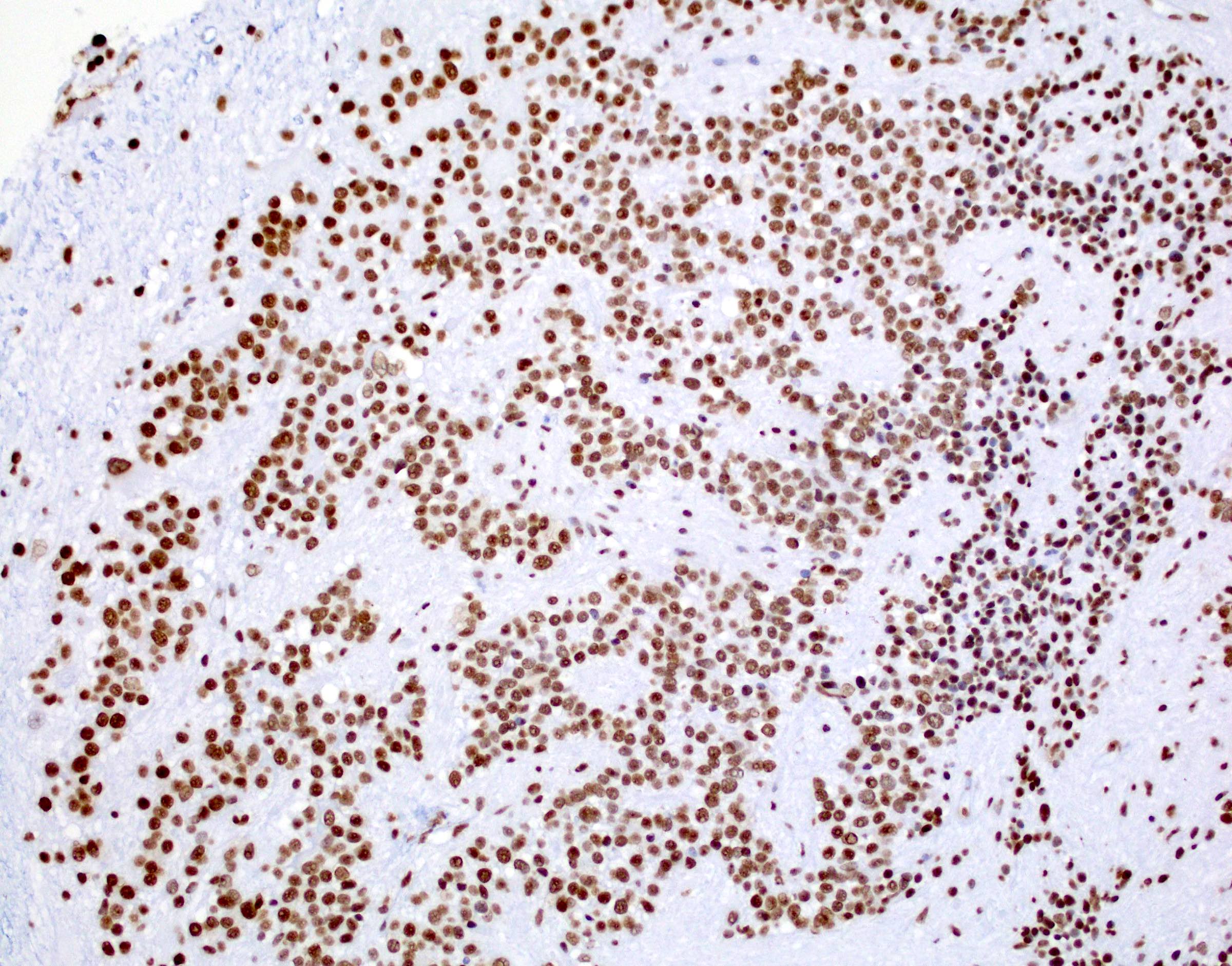
- In posterior fossa ependymomas, decreased expression of H3K27me3 is seen in posterior fossa group A, which has a worse prognosis (Acta Neuropathol 2017;134:705)
Negative stains
- Negative for neuronal markers (NeuN, chromogranin), Olig2 and IDH1
- Negative for reticulin
Electron microscopy description
- Features of ependymal cells
- Cilia with a 9+2 microtubular pattern
- Blepharoblasts and microvilli of the lumina
- Zipper-like junctional complexes of lateral aspect
- Lack of basement membrane
- Reference: CNS Oncol 2014;3:49
Molecular / cytogenetics description
- Molecular groups based on fusion and methylation profiling (Cancer Cell 2015;27:728)
- Supratentorial ependymoma, ZFTA fusion positive (formerly C11orf95::RELA fusion tumors), comprises 60% of supratentoral ependymomas and portends a poor prognosis
- Supratentorial ependymoma, YAP1 fusion positive, has a better prognosis
- Posterior fossa ependymoma, group A
- Posterior fossa ependymoma, group B
- Spinal ependymomas with NF2 mutations
- Spinal ependymoma, MYCN amplified
- Molecular characterization of histopathological ependymoma variants (Acta Neuropathol 2020;139:305)
- Tanycytic ependymomas were mostly located in spinal cord; DNA methylation match to spinal or myxopapillary ependymoma
- Clear cell ependymomas were mostly supratentorial; DNA methylation match to RELA fusion positive ependymomas
- Papillary ependymomas match to posterior fossa group B or myxopapillary ependymoma
Videos
Ependymal tumors by Dr. Rodriguez
Sample pathology report
- Brain, fourth ventricular mass, excision:
- Posterior fossa group B ependymoma, WHO grade 2 (see comment)
- Comment: Sections show an ependymal neoplasm with perivascular pseudorosettes and multiple foci of well formed ependymal canals. Solid, papillary and clear cell components are variably present. No necrosis, microvascular proliferation or mitotic figures are identified. Tumor cell nuclei are oval and uniform. Focal areas of hemosiderin laden macrophages are present.
- Immunohistochemically, a subset of the tumor cells express GFAP. EMA is negative. MIB1 proliferation index is < 1%. H3K27me nuclear expression is retained in over 95% of tumor cells.
- The collective findings of floor of fourth ventricle location, adult age and retained H3K27me nuclear expression in tumor cells are most consistent with posterior fossa group B (PFB) ependymoma. Correlation with NGS result is recommended.
Differential diagnosis
- Infiltrating glioma:
- Astroblastoma:
- Astroblastic pseudorosette and IHC profile that resembles ependymoma
- Located almost exclusively in cerebral hemispheres
- Stout broad process that is strongly GFAP+
- MN1 rearrangement detectable by FISH (Cell 2016;164:1060, Brain Tumor Pathol 2019;36:112)
- Medulloblastoma:
- Posterior fossa ependymoma can radiographically and histologically resemble medulloblastoma, with monotonous cells and true rosettes
- Medulloblastoma is diffusely synaptophysin+
- Subtype specific markers: GAB1, YAP1, filamin A, beta catenin (nuclear)
- Schwannoma:
- Meningioma:
Additional references
Board review style question #1
A biopsy of an enhancing, well circumscribed mass located in the paraventricular white matter of the parietal lobe shows cells with oval nuclei and speckled chromatin forming perivascular pseudorosettes and true rosettes with lumina. Fluorescence in situ hybridization reveals a ZFTA::RELA fusion. What is the best diagnosis?
- Astrocytoma, IDH mutant
- Oligodendroglioma, IDH mutant and 1p / 19q codeleted
- Posterior fossa ependymoma
- Supratentorial ependymoma, YAP1 fusion positive
- Supratentorial ependymoma, ZFTA fusion positive
Board review style answer #1
E. Supratentorial ependymoma, ZFTA fusion positive. The ZFTA::RELA (previously named C11orf95::RELA) fusion defines this tumor entity. Answers A, B, C and D are incorrect because the ZFTA::RELA fusion is exclusively seen in ependymoma and has not been seen in any other CNS tumors.
Comment Here
Reference: Ependymoma overview
Comment Here
Reference: Ependymoma overview
Board review style question #2
Board review style answer #2
B. H3K27me3. The H3K27 trimethylation status distinguishes PFA from PFB. PFA has loss of the H3K27 trimethylation and therefore loss of nuclear staining on H3K27me3 staining, while PFB group ependymoma tumor cells have retained nuclear expression. Answers A, C, D and E are incorrect because they do not distinguish H3K27 trimethylation status.
Comment Here
Reference: Ependymoma overview
Comment Here
Reference: Ependymoma overview