Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Grading | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: DeWitt J. Diffuse midline glioma, H3 K27 altered. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumordmgh3k27m.html. Accessed March 31st, 2025.
Definition / general
- WHO 2021 definition: diffuse midline glioma, H3 K27-altered, is an infiltrative midline glioma with loss of H3 p.K28me3 (K27me3) and usually, either an H3 c.83A>T p.K28M (K27M) substitution in one of the histone H3 isoforms (H3.1, H3.2 or H3.3), aberrant overexpression of EZHIP or an EGFR mutation (CNS WHO grade 4)
Essential features
- Predominant in children but also occurs in adults
- Arises in the midline; the brain stem, thalamus and spinal cord are the most common locations
- High grade features, such as mitotic activity, microvascular proliferation and necrosis, may be seen but are not necessary for the diagnosis
- Diffusely infiltrative of both adjacent and distant brain structures
- Poor prognosis with 2 year survival rate of < 10%
Terminology
- Brain stem and pontine lesions previously termed brain stem glioma and diffuse intrinsic pontine glioma (DIPG)
ICD coding
- ICD-10: C71.9 - malignant neoplasm of brain, unspecified
Epidemiology
- Official incidence data are not available; this is because, historically, infiltrative gliomas arising in the midline have not been distinguished from other infiltrative gliomas in large registries
- M = F
- Median age at diagnosis is 5 - 11 years, with tumors that arise in the pons occurring at a younger age (~7 years) than those that arise in the thalamus (~11 years) (Neuro Oncol 2014;16:iv1)
- Those harboring EGFR amplifications, containing mutations in isoform histone H3.3 or resulting from EZHIP overexpression, typically occur at 7 to 8 years of age, while those with K27M mutations in isoform histone H3.1 or H3.2 tend to occur in younger patients (~5 years) (Cancer Cell 2017;32:520)
Sites
- Most commonly located in the spinal cord, pons and thalamus
- Occasionally arises in the cerebellum
Pathophysiology
- No genetic susceptibility is known; however, rare cases of H3 K27-altered tumors have been seen in patients with genetic tumor syndromes such as NF1, Li-Fraumeni syndrome or mismatch repair deficiency
Etiology
- Tumorigenesis is thought to be driven by disruption of polycomb repressive complex 2 (PRC2) mediated differentiation and self renewal (Cancer Cell 2019;35:140, Genes Dev 2014;28:1758)
- Common genetic and epigenetic characteristics of midline gliomas are suggestive of a distinct developmental origin (Nat Rev Cancer 2014;14:651, Nat Rev Cancer 2014;14:92)
- Nestin- / Olig2- expressing neural precursor-like cell has been suggested as the possible cell of origin in one study (Proc Natl Acad Sci USA 2011;108:4453)
- Another found the majority of tumor cells resembled an oligodendrocyte precursor cell (Science 2018;360:331)
Clinical features
- Patients typically present with evidence of cerebrospinal fluid obstruction or brain stem dysfunction, such as cranial nerve abnormalities, ataxia and long tract signs that develop over a short period of time (Front Oncol 2012;2:205)
- Tumors that arise in the thalamus are often associated with motor weakness and gait disturbance (Neuro Oncol 2011;13:680)
Diagnosis
- Typically diagnosed by imaging (MRI) and stereotactic biopsy; resection is often not possible given the involvement of critical brain stem structures
Grading
- Grade 4 of 4
- Presence of H3 K27-alteration in an infiltrative glioma arising in the midline is sufficient for a grade 4 designation, even in the absence of necrosis or microvascular proliferation (Acta Neuropathol 2014;128:573)
Radiology description
- On MRI, typically T1 hypointense and T2 hypertintense
- Contrast enhancement (typically < 25% of tumor volume), hemorrhage or necrosis may be seen (J Neurooncol 2011;105:119)
- Tumors arising in the pons typically present as large expansile masses that occupy > 67% of the pons (Front Oncol 2012;2:205)
- Exophytic component may be present
- Infiltration into adjacent structures, such as the cerebellar peduncles, midbrain or medulla is frequent
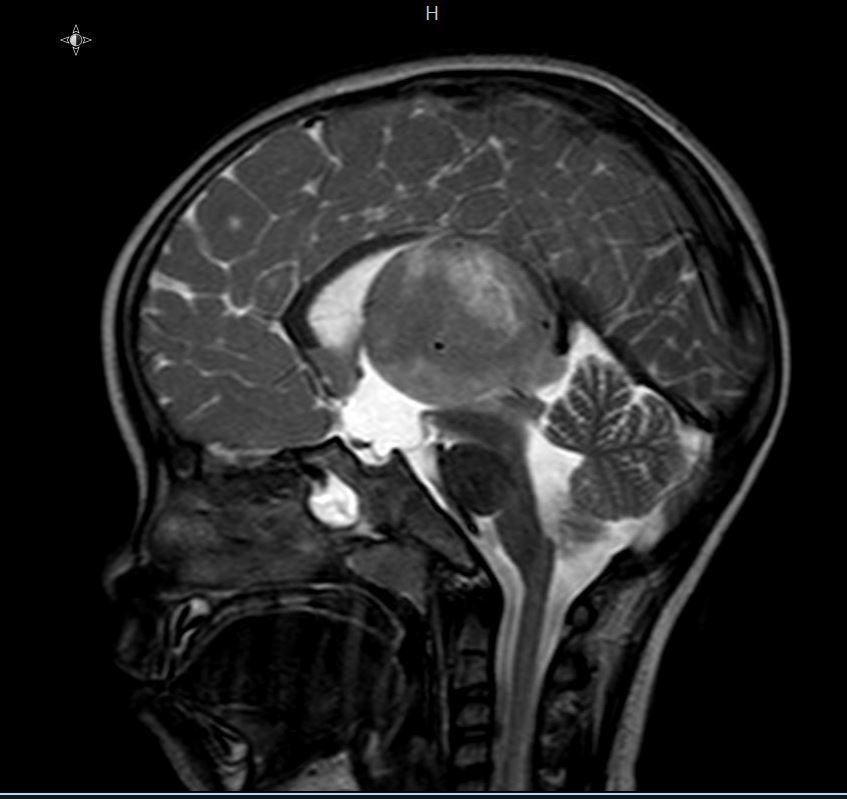
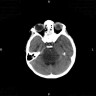
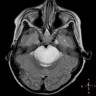
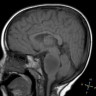
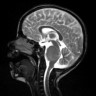
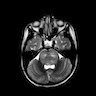
Radiology images
Contributed by Rawia Mubarak Mohamed, M.D. and Najla Saleh Ben Gashir, M.D. (Case #477)
Images hosted on other servers:
Prognostic factors
- Prognosis is almost universally poor
- Presence of an H3 K27M alteration confers a worse prognosis over wild type diffuse midline gliomas (Acta Neuropathol 2014;128:573, Acta Neuropathol 2012;124:439)
Case reports
- 5 year old girl with headache and vomiting for 1 week (Case #477)
- 30 year old woman and 69 year old man with mosaic H3.3 K27M protein expression (Acta Neuropathol 2017;134:961)
- 45 year old man with an H3.1 K27M mutant diffuse cerebellar astrocytoma (Brain Tumor Pathol 2018;35:29)
- 51 year old woman with a diffuse midline glioma with primitive neuroectodermal tumor-like appearance and neuropil-like islands (Neuropathology 2018;38:165)
Treatment
- Surgical resection is often highly limited, due to the involvement of critical brain structures (PDQ Cancer Information Summaries: Childhood Astrocytomas Treatment [Accessed 25 February 2019])
- Radiation or chemotherapy is standard
Gross description
- Infiltrative nature causes enlargement and distortion of involved anatomical structures
- Focal discoloration and softening, indicating hemorrhage or necrosis, may be present (Neurol Med Chir (Tokyo) 2003;43:375)
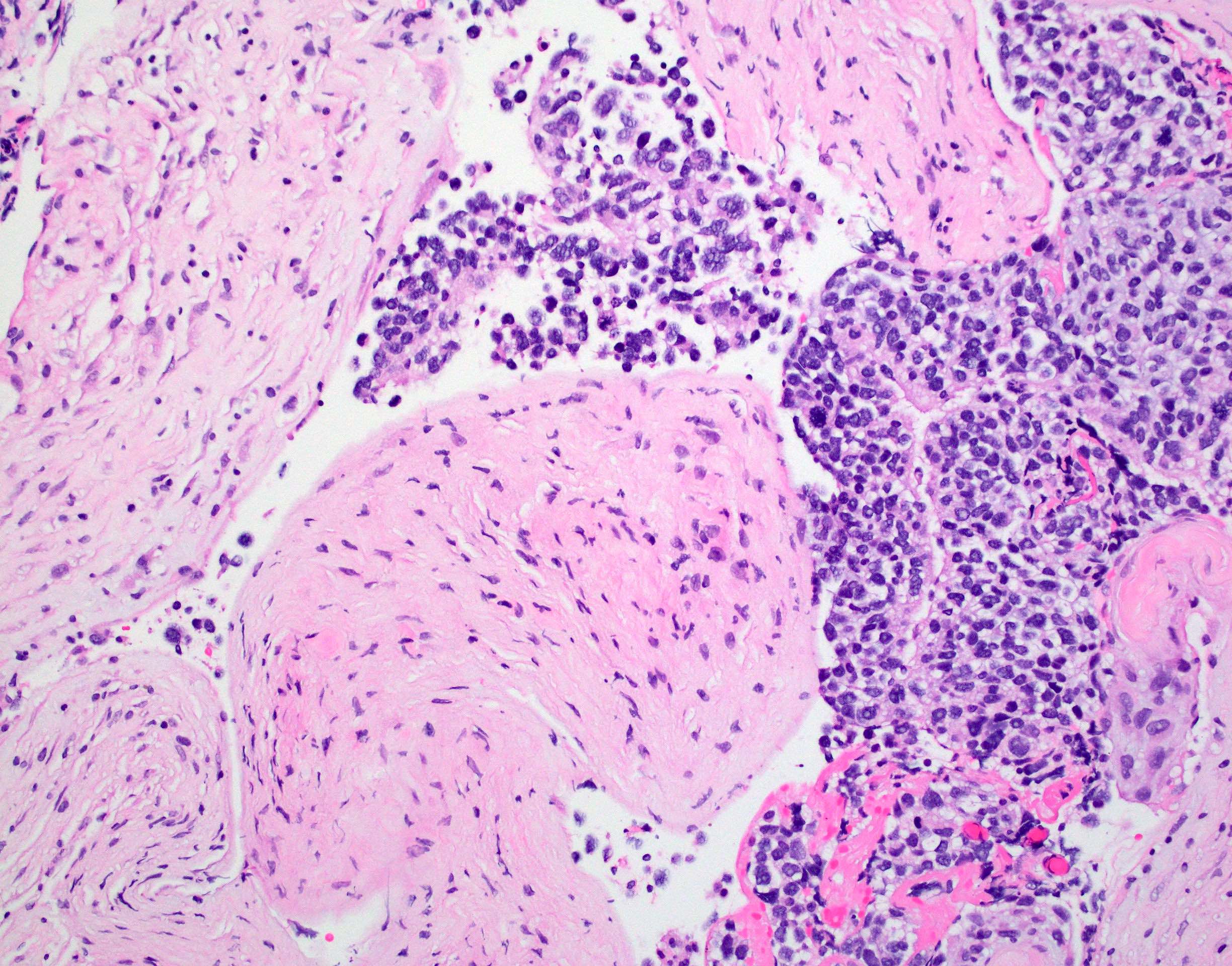
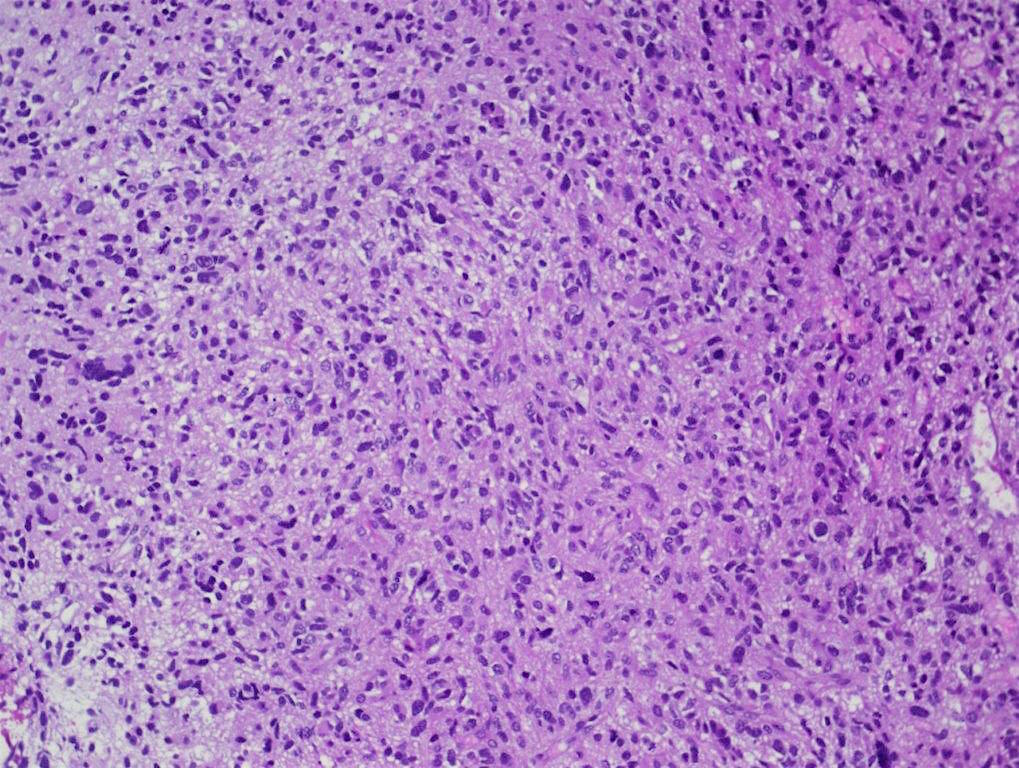
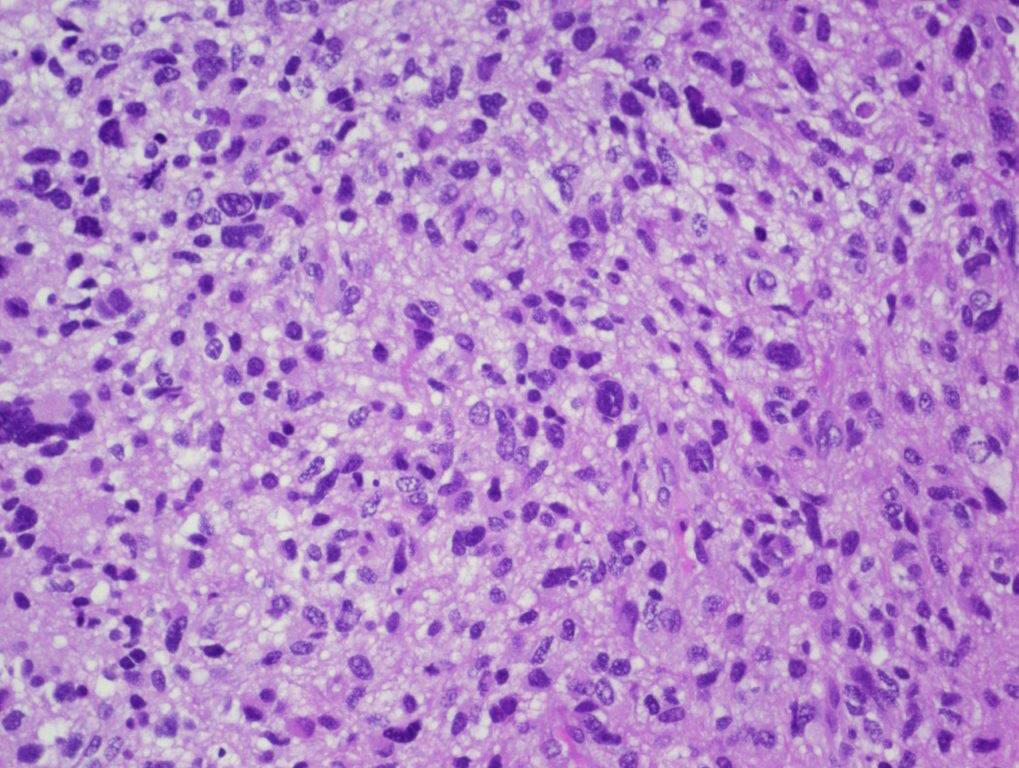
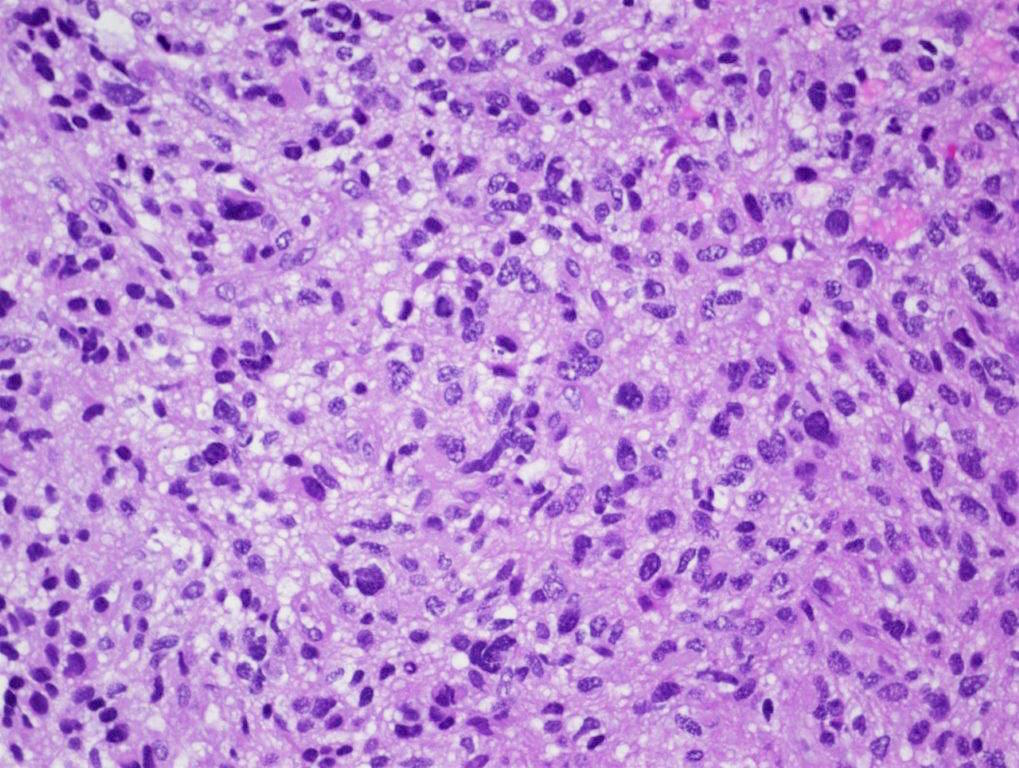
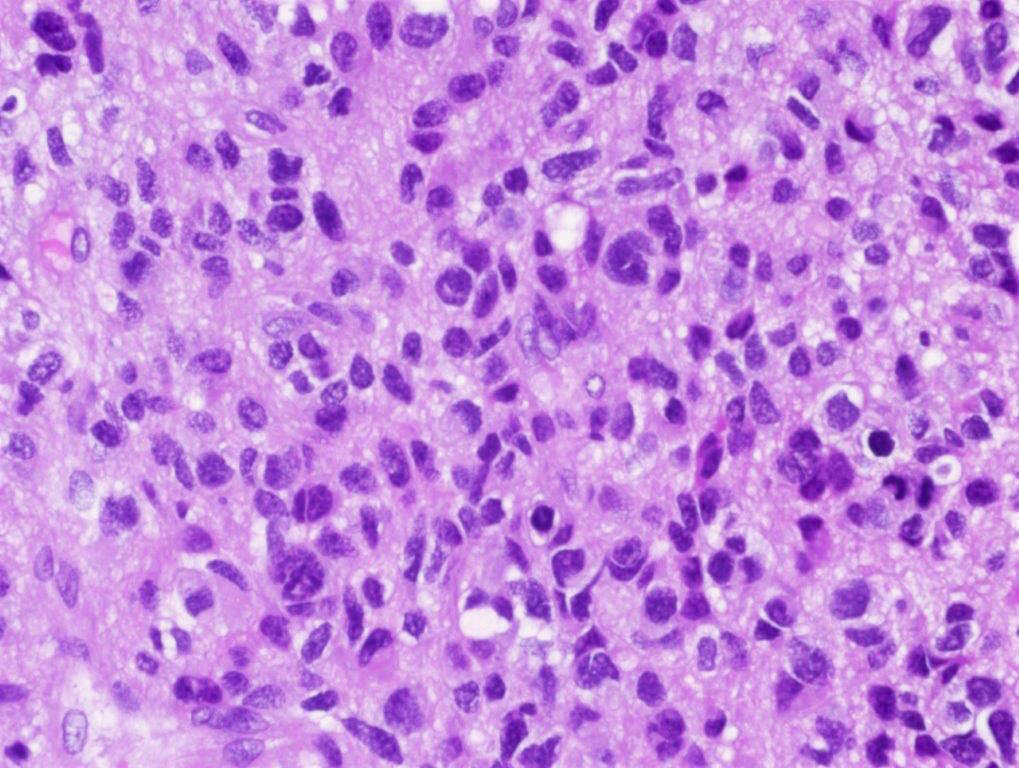
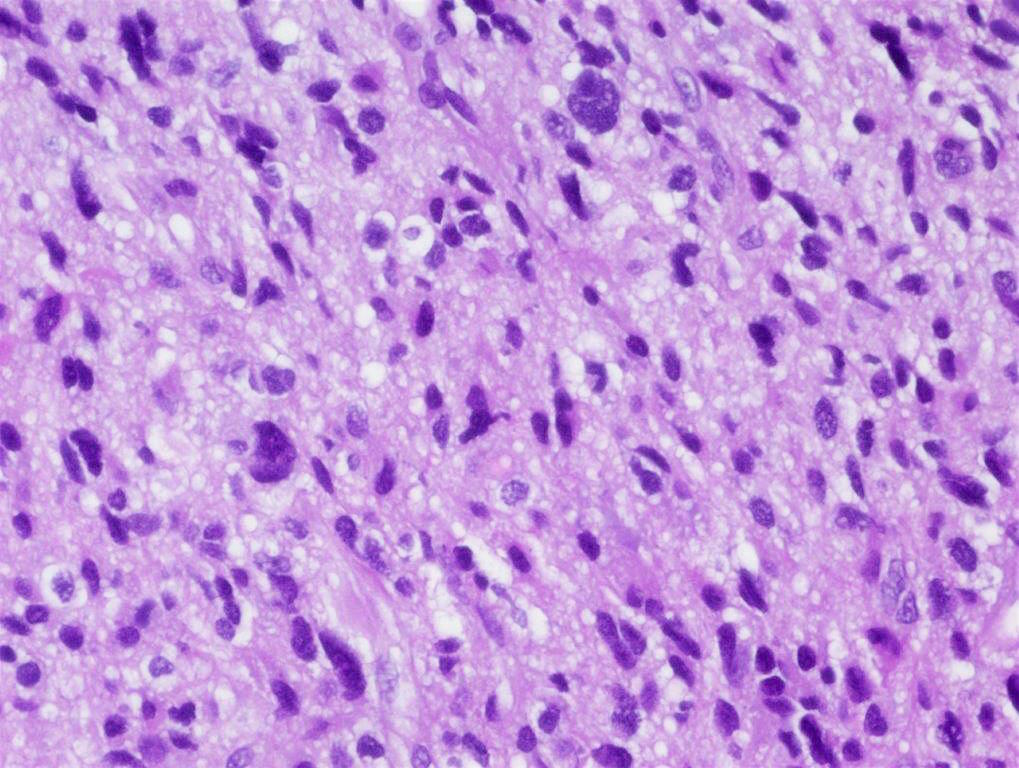
Microscopic (histologic) description
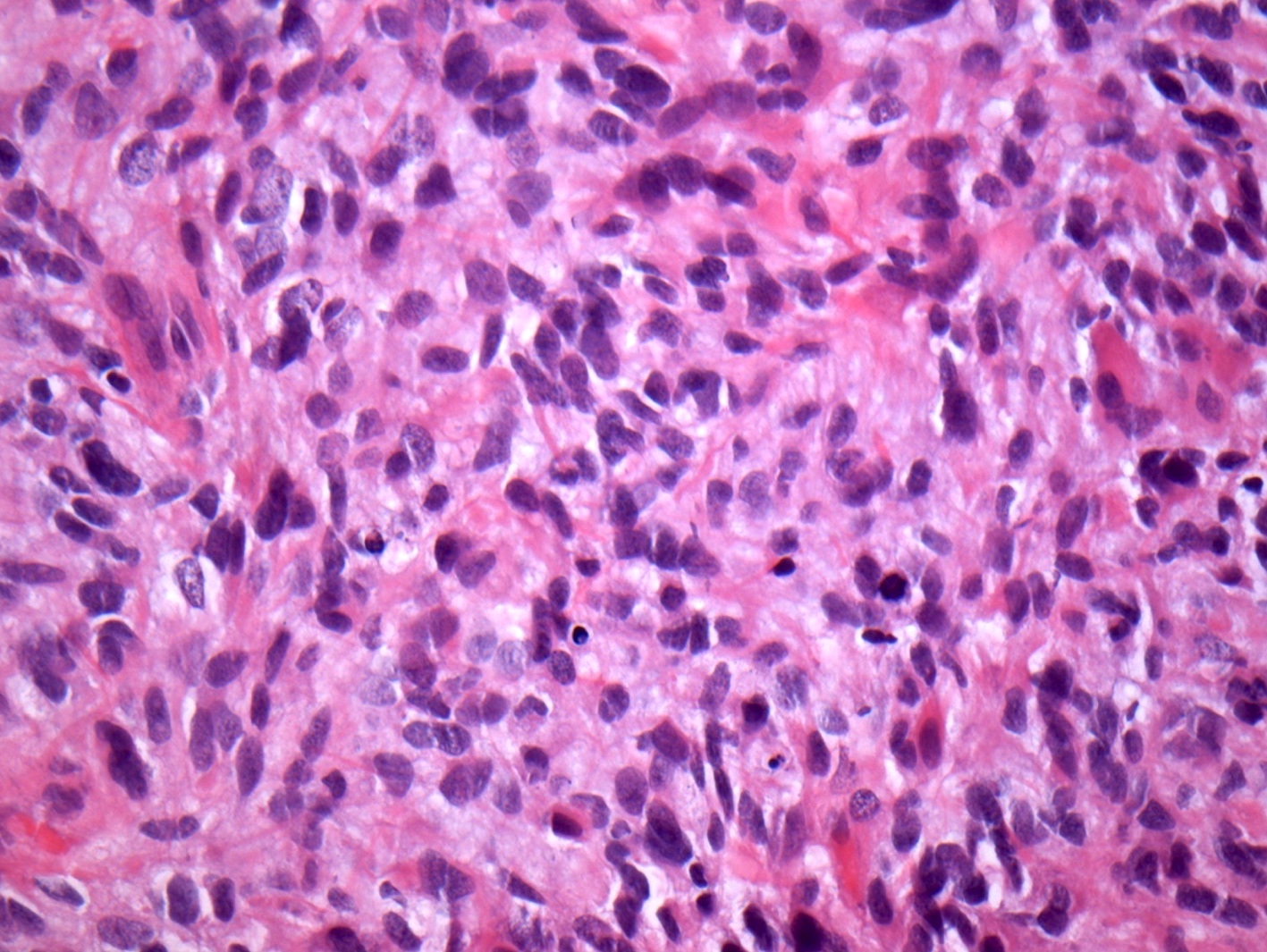
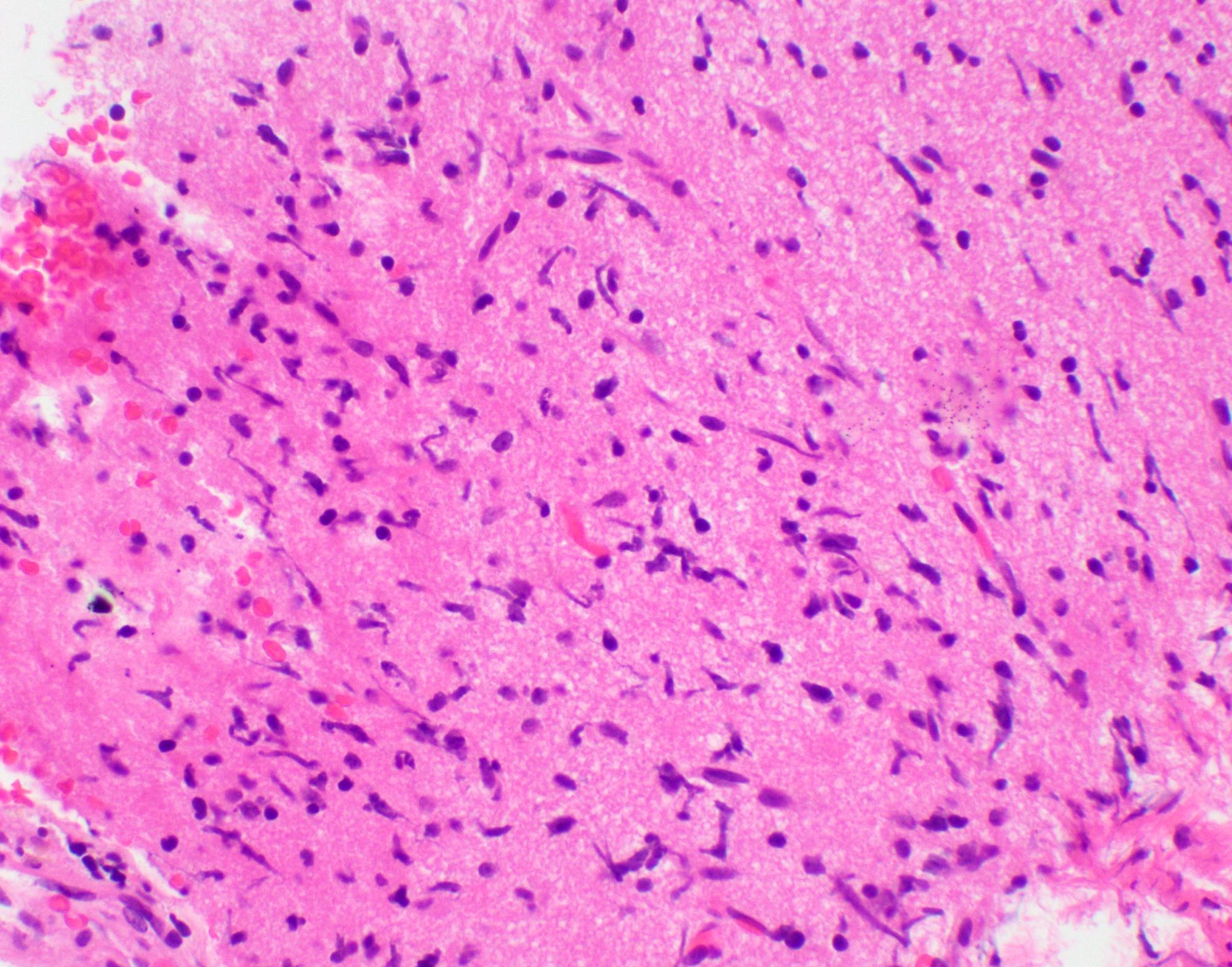
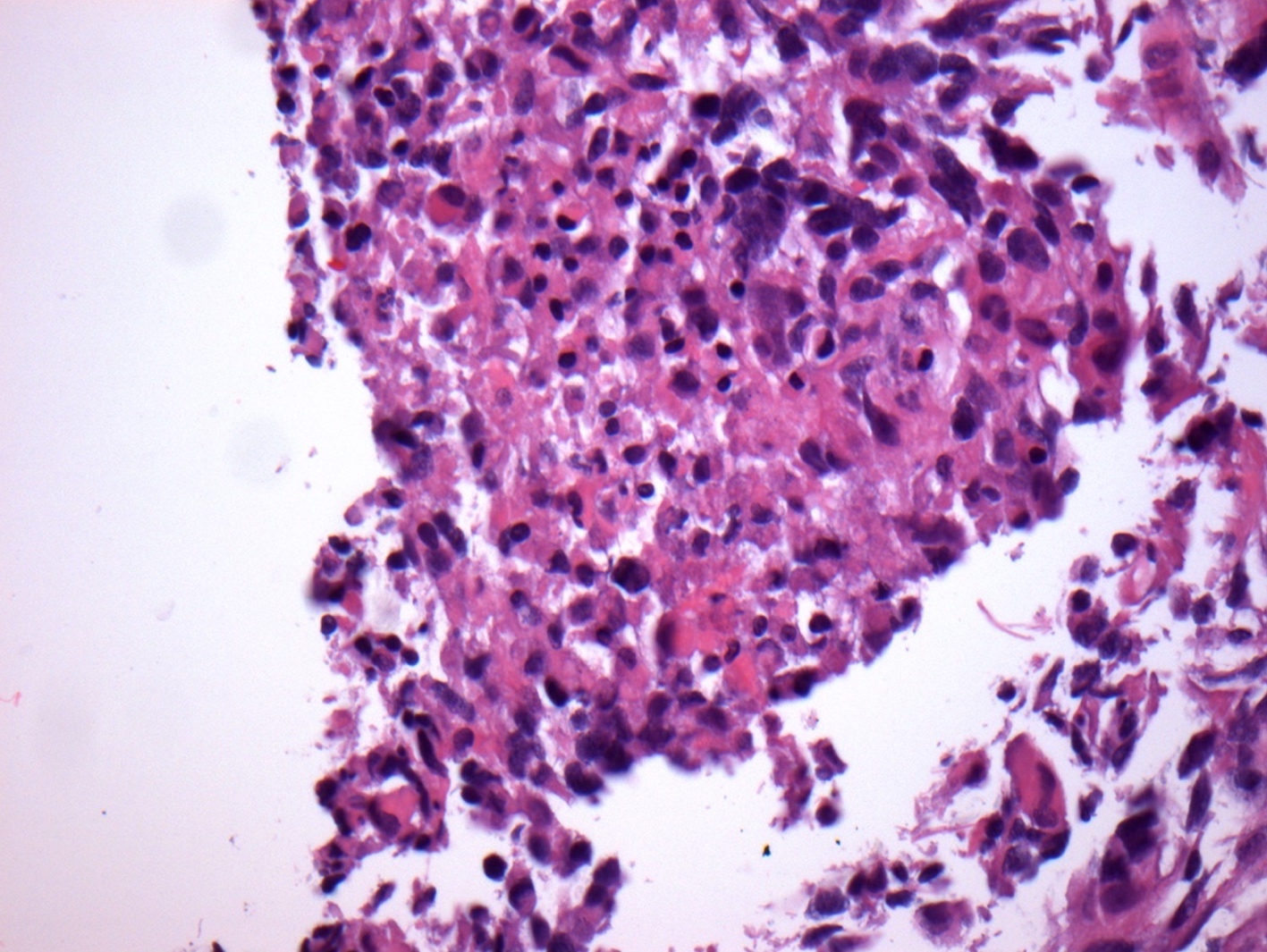
- Tumor cells typically have an astrocytic morphology and may be small and monomorphic to (occasionally) large and pleomorphic (Neurol Med Chir (Tokyo) 2003;43:375)
- Show an infiltrative growth pattern with tumor cells diffusely growing among native neurons and invading into adjacent structures
- Occasionally, an oligodendroglial-like pattern with halos may be seen
- Some cases will not show mitoses, necrosis or microvascular proliferation consistent with a WHO grade 2 histologic appearance; however, in the presence of H3 K27-alteration, WHO grade 4 is warranted given the aggressive nature of these tumors (Acta Neuropathol 2014;128:573)
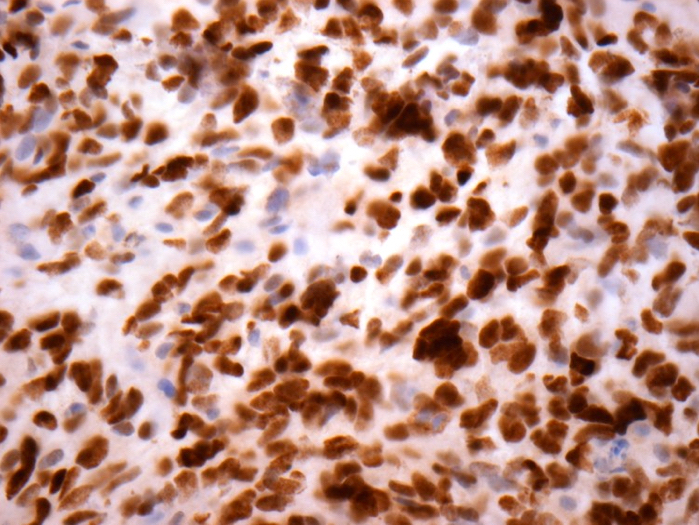
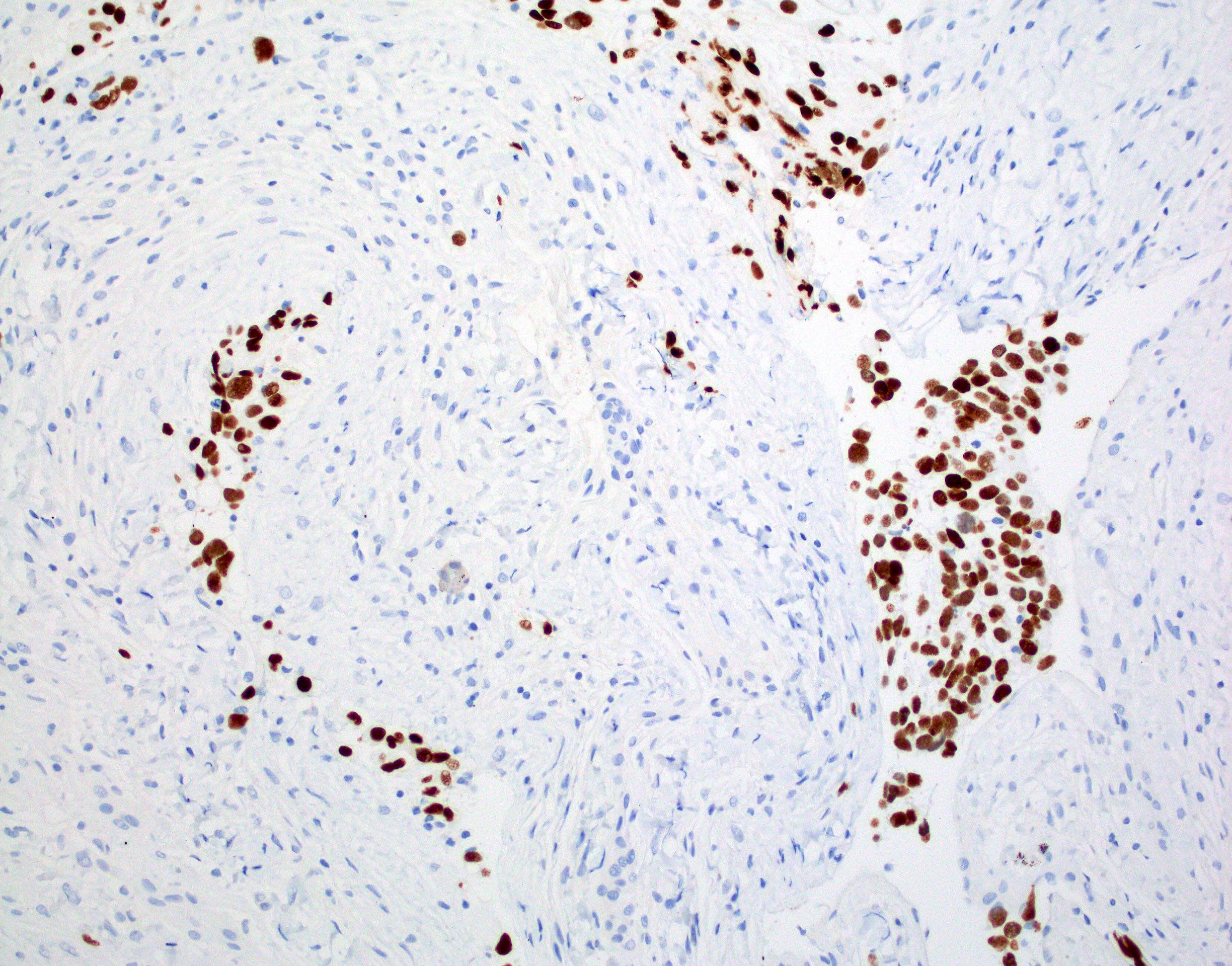
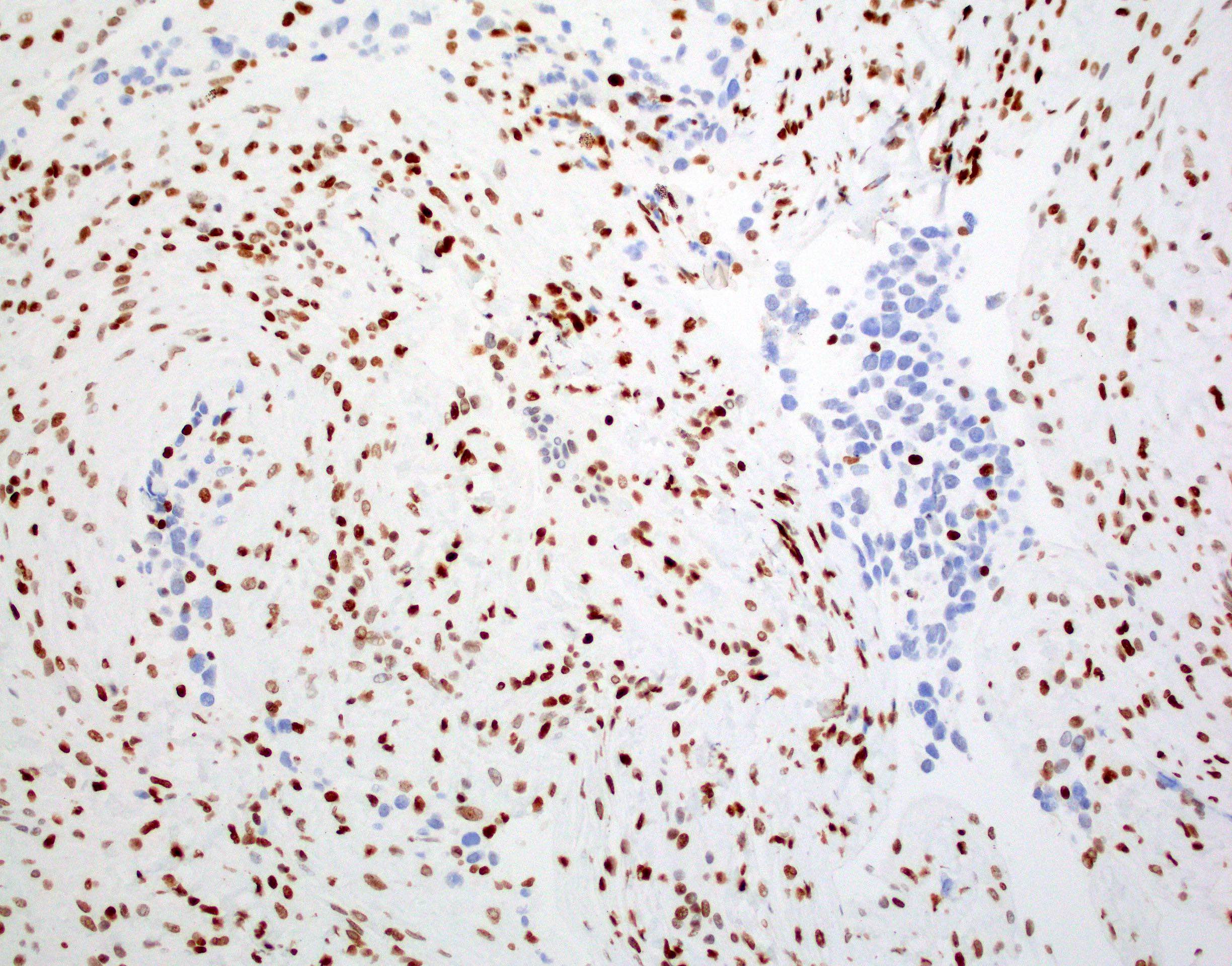
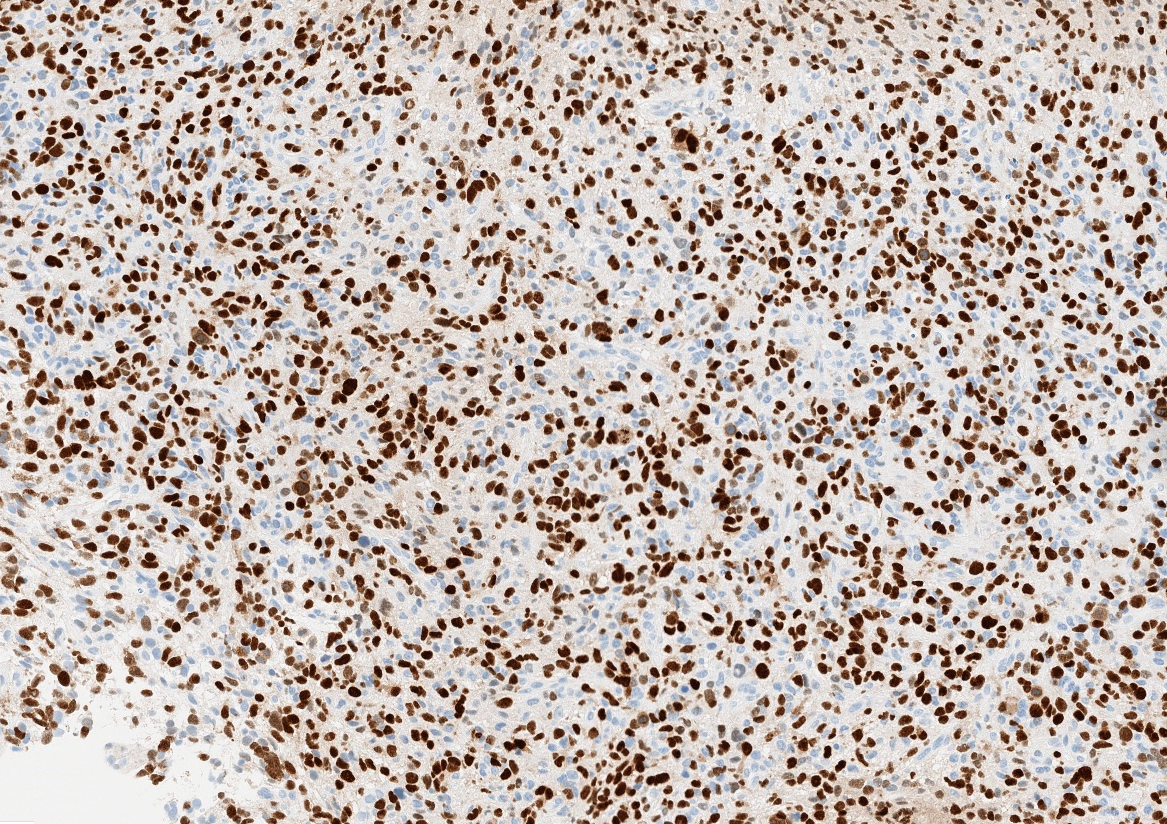
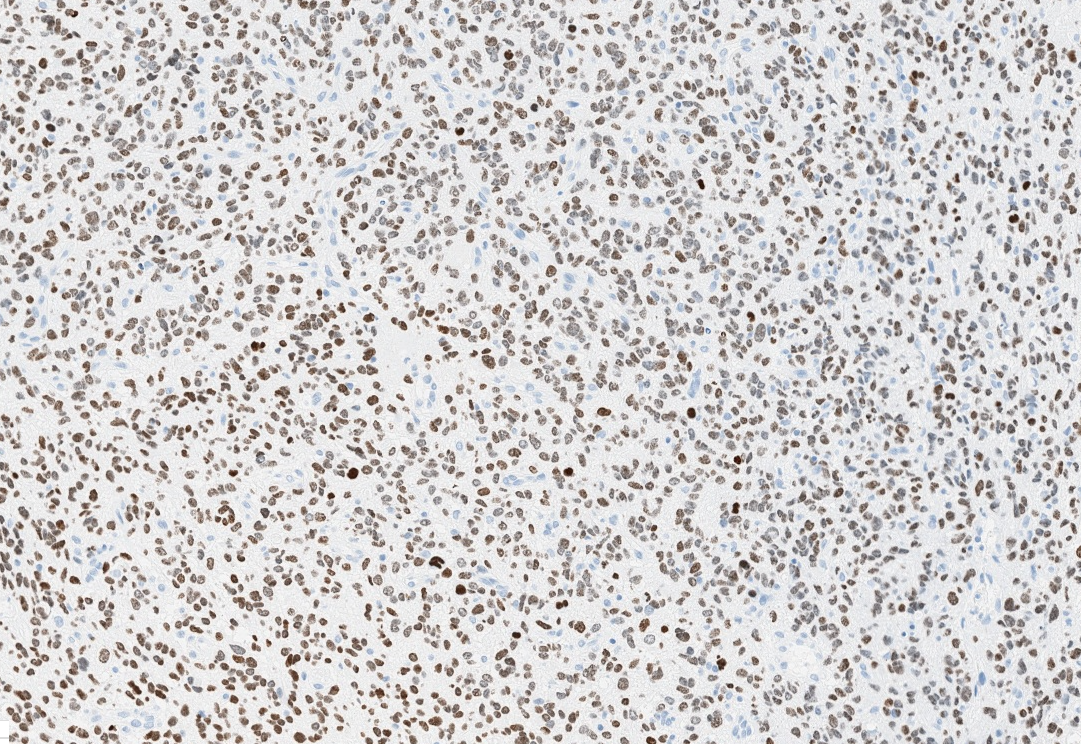
Microscopic (histologic) images
Contributed by John DeWitt, M.D., Ph.D.
Contributed by Chunyu Cai, M.D., Ph.D.
Contributed by Rawia Mubarak Mohamed, M.D. and Najla Saleh Ben Gashir, M.D. (Case #477)
Positive stains
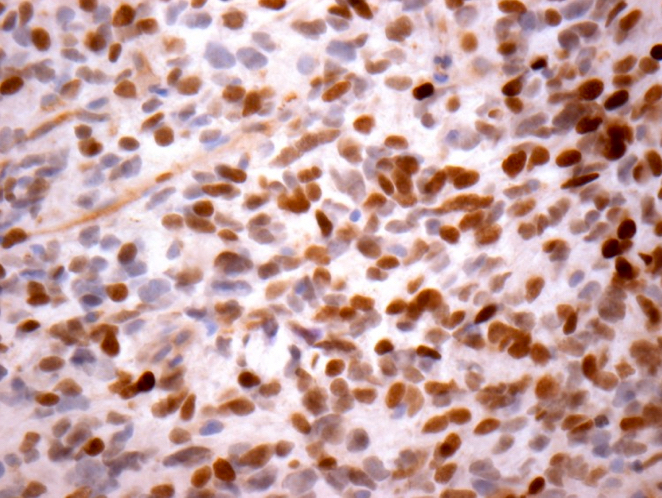
- H3F3A K27M (specific immunostain)
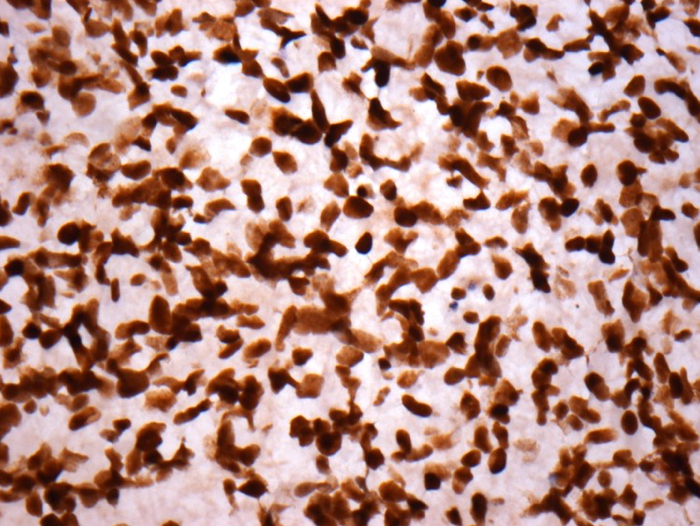
- S100
- ATRX nuclear loss varies by tumor location, very low in pontine gliomas (1/13) but high in thalamus (9/12) or spinal cord gliomas (4/7) (Brain Pathol 2016;26:569)
- GFAP (variably positive)
- Olig2, MAP2
- p53 (strong positivity in ~50% of cases)
- Reference: Brain Pathol 2016;26:569
Negative stains
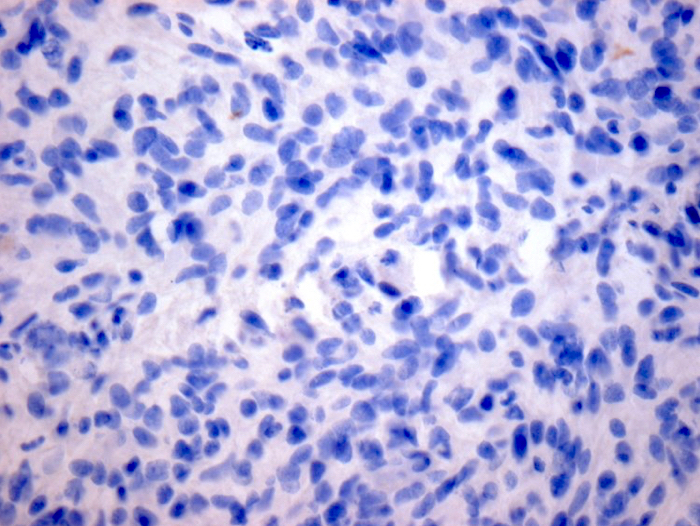
- R132H-IDH1
- Keratins (although cocktails may show cross reactivity with GFAP)
- H3K27me3: H3 K27-altered tumors show loss of H3K27me3 staining, a finding that by itself is not specific, however, may be helpful in identifying diffuse midline gliomas with H3 p.K28I (K27I) mutation or EZHIP overexpression, which are negative for H3K27M IHC but show loss of nuclear H3K27me3 expression
- Reference: Brain Pathol 2016;26:569
Molecular / cytogenetics description
- Various molecular techniques including sequencing, expression arrays and methylation patterns are consistent with 4 distinct subtypes of diffuse midline gliomas that are defined by oncohistone alterations:
- H3.3 c.83A>T p.K28M (K27M) mutant
- H3.1 or 3.2 c.83A>T p.K28M (K27M) mutant
- H3 wild type with aberrant overexpression of EZHIP
- EGFR mutation or amplification
- Additional mutations seen in diffuse midline glioma, H3 K27-altered (Nat Genet 2014;46:451, Nat Genet 2014;46:457, Nat Genet 2014;46:444, J Neurosurg 1999;90:833):
- TP53 (~50%)
- PDGFRA amplification (~30%)
- CDK4 / 6 or CCND1 - 3 amplification (~20%)
- ACVR1 mutation (~20%)
- PPM1D mutation (~15%)
- MYC / PVT1 amplification (~15%)
- ATRX mutation (~15%)
- CDKN2A / B homozygous deletion (< 5%)
Sample pathology report
- Brain, pons, biopsy:
- Integrated diagnosis: diffuse midline glioma, H3 K27-altered, WHO grade 4 of 4 (see comment)
- Histological diagnosis: astrocytoma with proliferative activity
- CNS WHO grade 4 of 4
- Molecular information:
- IDH: negative (R132H immunohistochemistry; consistent with wild type)
- ATRX: nuclear expression retained (immunohistochemistry; consistent with wild type)
- p53: negative (immunohistochemistry; consistent with wild type)
- H3K27M: positive (immunohistochemistry; consistent with mutant)
- Comment: The specimen consists of core biopsy specimens of white matter with moderately atypical infiltrating astrocytic tumor cells. There is no evidence of vascular proliferation or necrosis. Scattered mitoses are seen. Although the histologic grade is that of a grade 3 astrocytoma, the positivity for H3K27M is consistent with a diagnosis of diffuse midline glioma, H3 K27-altered, which are considered grade 4 lesions due to their historically aggressive clinical behavior
- Integrated diagnosis: diffuse midline glioma, H3 K27-altered, WHO grade 4 of 4 (see comment)
Differential diagnosis
- Astrocytoma, IDH mutant, CNS WHO grade 2:
- Grade 2 histology with IDH mutation present
- Astrocytoma, IDH mutant, CNS WHO grade 3:
- Grade 3 histology with IDH mutation present
- Astrocytoma, IDH mutant, CNS WHO grade 4:
- Grade 4 histology with IDH mutation present or lower grade histology with IDH mutation and homozygous deletion of CDKN2A / CDKN2B
- Glioblastoma, IDH wild type:
- Astrocytoma lacking IDH or H3 K27M mutations with either grade 4 histology or any of the following molecular alterations: EGFR amplification, TERT promoter mutation, gain of chromosome 7 / loss of chromosome 10
- Tumors do not show loss of H3 K27 trimethylation
- Posterior fossa (PFA) ependymoma (Childs Nerv Syst 2017;33:1047, Nat Commun 2019;10:2146):
- Will show loss of H3K27Me3 (trimethyl mark) as in diffuse midline glioma, H3 K27-altered and rare cases also contain H3 K27 mutations
- Found in the posterior fossa where diffuse midline glioma, H3 K27-altered may also occur
- Differentiated by ependymal differentiation (round to oval glial tumor cells with perivascular pseudorosettes) as opposed to astrocytic differentiation of diffuse midline glioma, H3 K27-altered
- Molecular studies including sequencing and methylation profiling could be considered in histologically ambiguous cases
Additional references
Board review style question #1
Board review style answer #1
Board review style question #2
A work up of a biopsy of a diffusely infiltrating glial tumor from an 11 year old girl reveals sequencing results that show a methionine substitution for a lysine at position 27 in one of the genes encoding histone H3 (H3 K27M). Where is this tumor most likely arising from?
- Cerebellar hemisphere
- Frontal lobe
- Lateral ventricle
- Temporal lobe
- Thalamus
Board review style answer #2
Board review style question #3
Which statement about diffuse midline glioma, H3 K27-altered is true?
- Despite its name, it is typically not found in the midline
- It may lack high grade histologic features but is still considered grade 4
- The prognosis varies based on the histologic features
- This diagnosis includes midline gliomas that are diffusely infiltrating but have not been tested for the H3 K27M mutation
Board review style answer #3
B. It may lack high grade histologic features but is still considered grade 4. This tumor is considered grade 4 regardless of the histologic features and all of these tumors are considered to have a dismal prognosis. Most of these tumors appear in the midline. Molecular or IHC testing to confirm one of the diagnostic alterations or loss of H3 K27 trimethylation is required for this diagnosis.
Comment Here
Reference: Diffuse midline glioma, H3 K27-altered
Comment Here
Reference: Diffuse midline glioma, H3 K27-altered