Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style question #2Cite this page: Voudouri M, Zanazzi G. Diffuse low grade glioma, MAPK pathway altered. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumordiffuselowgradegliomaMAPK.html. Accessed April 3rd, 2025.
Definition / general
- Low grade, infiltrative, pediatric glioma with an alteration in a MAP kinase pathway gene such as FGFR1 or BRAF
- Tumor is IDH wild type, histone H3 wild type and does not have a homozygous deletion of CDKN2A
Essential features
- Predominantly in children
- Arises anywhere in the central nervous system, often in the cerebral hemispheres or posterior fossa
- Histopathologically heterogeneous, often resembling low grade astrocytoma or oligodendroglioma
- MAP kinase pathway alteration without alterations in IDH1 / IDH2, histone H3 or homozygous deletion of CDKN2A
Terminology
- Pediatric low grade glioma
- Formerly, pediatric oligodendroglioma or diffuse astrocytoma
ICD coding
Epidemiology
- Predominantly in children
- Precise incidence data not yet available
Sites
- Cerebral hemispheres, diencephalon, brainstem, cerebellum and spinal cord, with possible site predilections depending on histology and genetic alterations (Cancer Cell 2020;37:569)
Pathophysiology
- Missense mutation, intragenic duplication or fusion may constitutively activate a receptor tyrosine kinase, such as FGFR or NTRK, leading to aberrant recruitment of the G protein RAS, which activates the RAF / MEK / ERK cascade (Acta Neuropathol Commun 2020;8:30)
- Missense mutations in RAS or RAF (especially BRAF V600E) can cause gliomagenesis in the absence of an upstream alteration (Acta Neuropathol Commun 2020;8:30)
Etiology
- Precise etiology is unclear
Clinical features
- Symptoms related to increased intracranial pressure, such as headache, nausea and vomiting
- May have site dependent neurological deficits
- Longstanding epilepsy is common
Diagnosis
- Identification of a MAPK pathway alteration (BRAF, FGFR1, FGFR2, NTRK1, NTRK2, NTRK3, MAP2K1, MET alterations) that is confirmed by sequencing in a diffuse low grade glioma (Nat Genet 2013;45:602, Acta Neuropathol 2016;131:833, Cancer Cell 2020;37:569)
- Absent IDH, histone H3 K27 or H3.3 G34R / V mutations
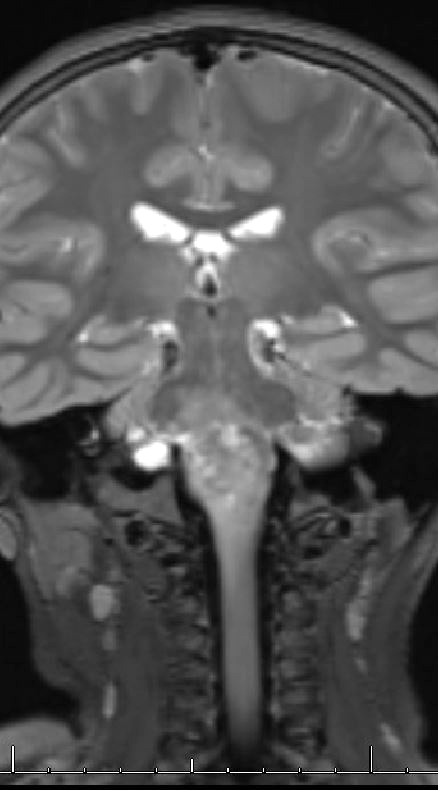
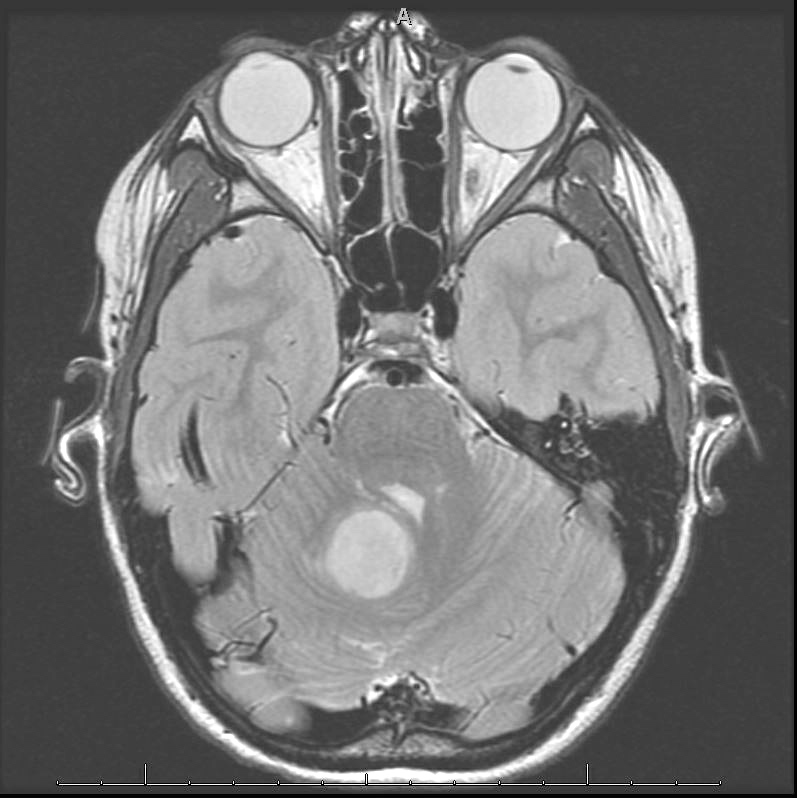
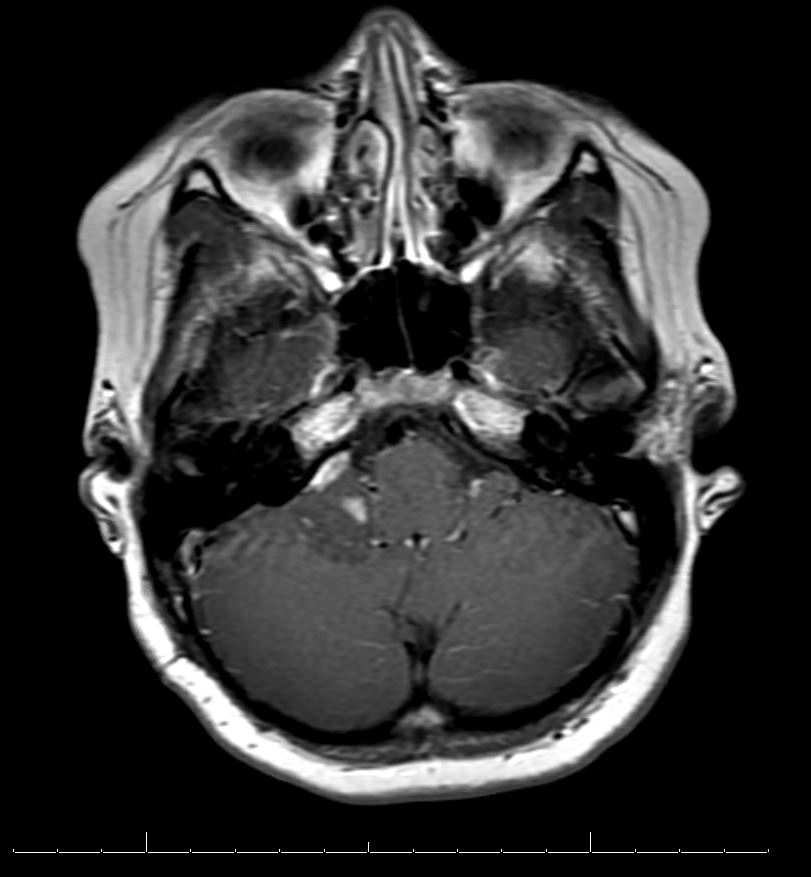
Radiology description
- Diffuse lesion, highlighted by T2 FLAIR on MRI, with possible cystic elements
- May be heterogeneously enhancing on MRI
Radiology images
Prognostic factors
- Currently unclear but rarely undergo malignant transformation
Case reports
- 9 year old boy with brainstem mass growing slowly over 8 years and low grade astrocytoma containing FGFR2-VPS35 fusion (Cold Spring Harb Mol Case Stud 2020;6:a005660)
- 15 year old with seizure and temporal lobe mass containing oligodendroglioma-like cells and BRAF V600E mutation (Brain Pathol 2020;30:515)
- 16 year old boy with developmental delay, germline 7q11.22 deletion and a low grade astrocytoma with BRAF V600E mutation in right temporal lobe (Clin Case Rep 2017;6:274)
Treatment
- Surgical resection, although this may be limited by involvement of critical brain structures
- MEK inhibitors, such as selumetinib and trametinib, show promising effects in initial studies (Neuro Oncol 2017;19:1135, BMC Cancer 2019;19:1250)
- For BRAF V600E mutant tumors, dabrafenib has been used (Clin Cancer Res 2019;25:7303)
Gross description
- Soft, tan-gray tissue
- Hemorrhage may occur (J Neurosurg 2020;134:733)
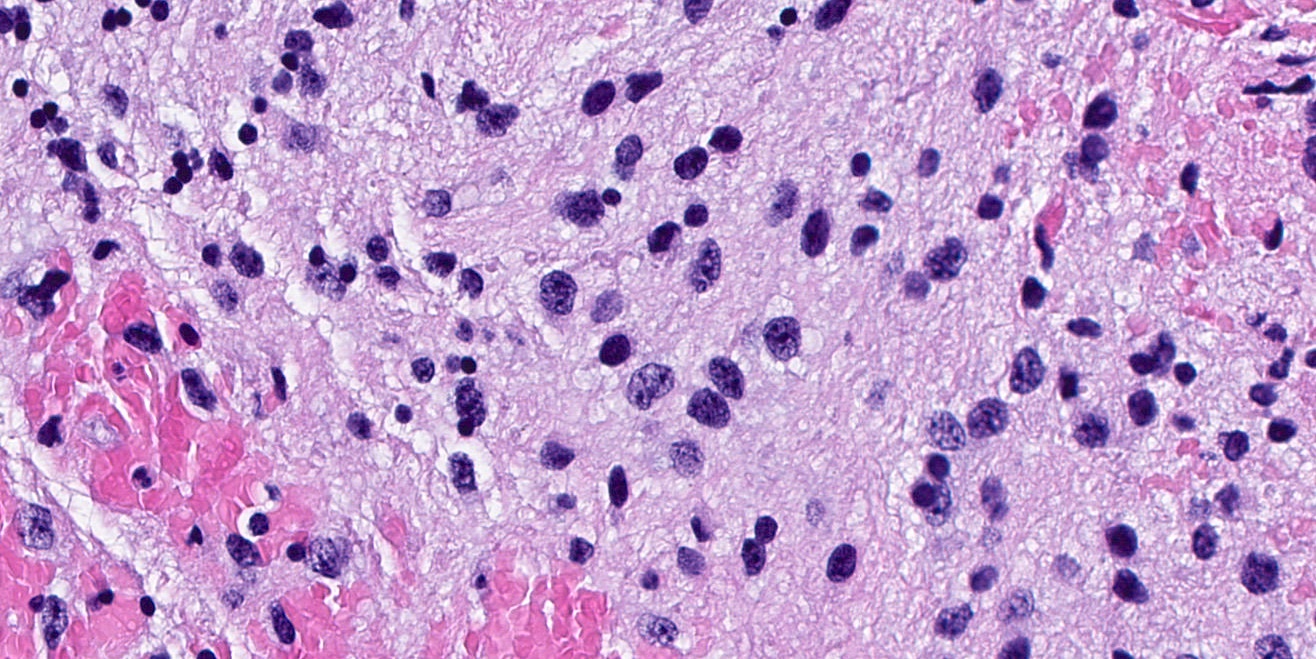
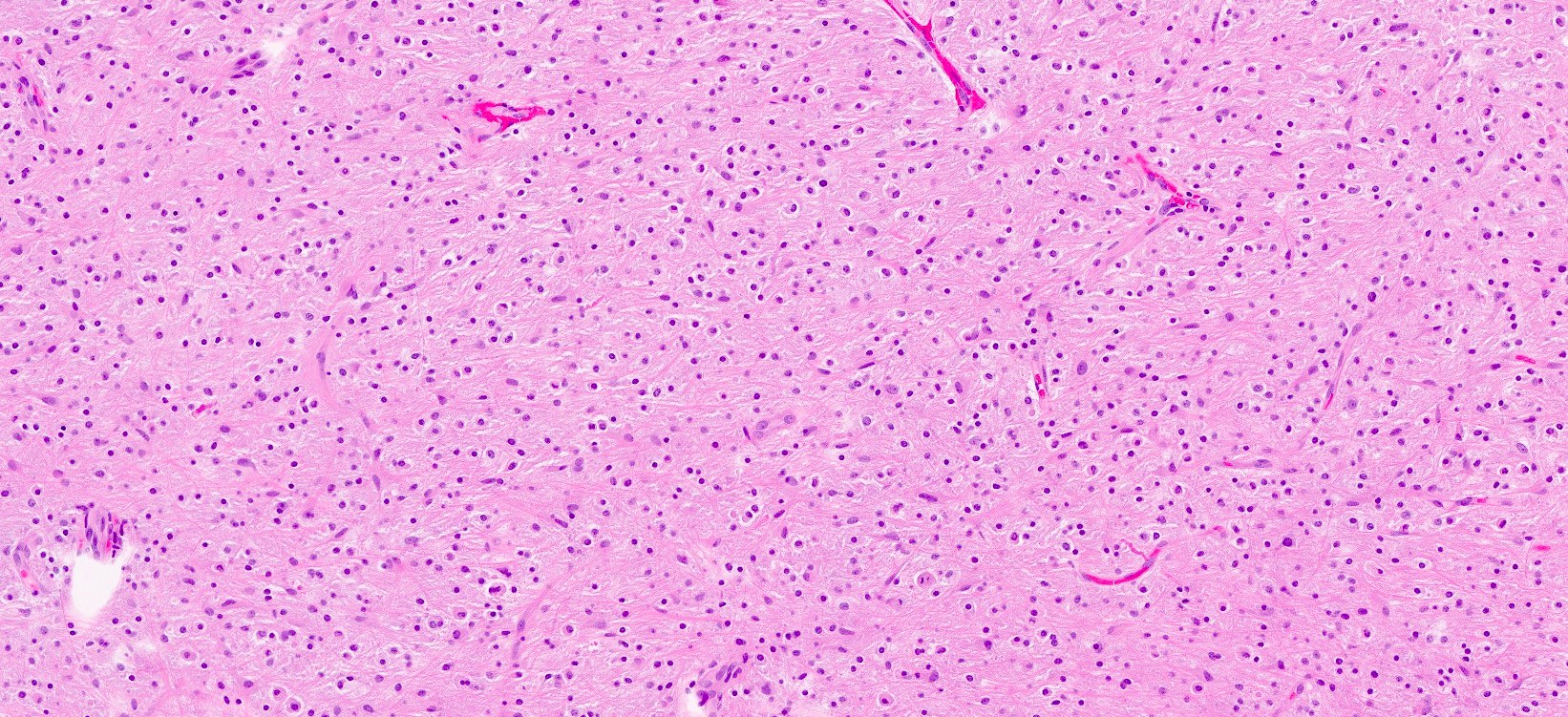
Microscopic (histologic) description
- General features: infiltrative, glial tumor cells without a neuronal component
- BRAF V600 altered tumors tend to have densely fibrillary areas and microcalcifications (Acta Neuropathol 2015;130:575)
- FGFR1 altered tumors tend to have oligodendrocyte-like cells, rare or no mitoses, no necrosis and no microvascular proliferation (Acta Neuropathol 2016;131:833)
- In a large study excluding NF1 patients, 5.9% of pediatric low grade gliomas exhibited astrocytic differentiation and 3.0% exhibited oligodendroglial differentiation (Cancer Cell 2020;37:569)
Microscopic (histologic) images
Positive stains
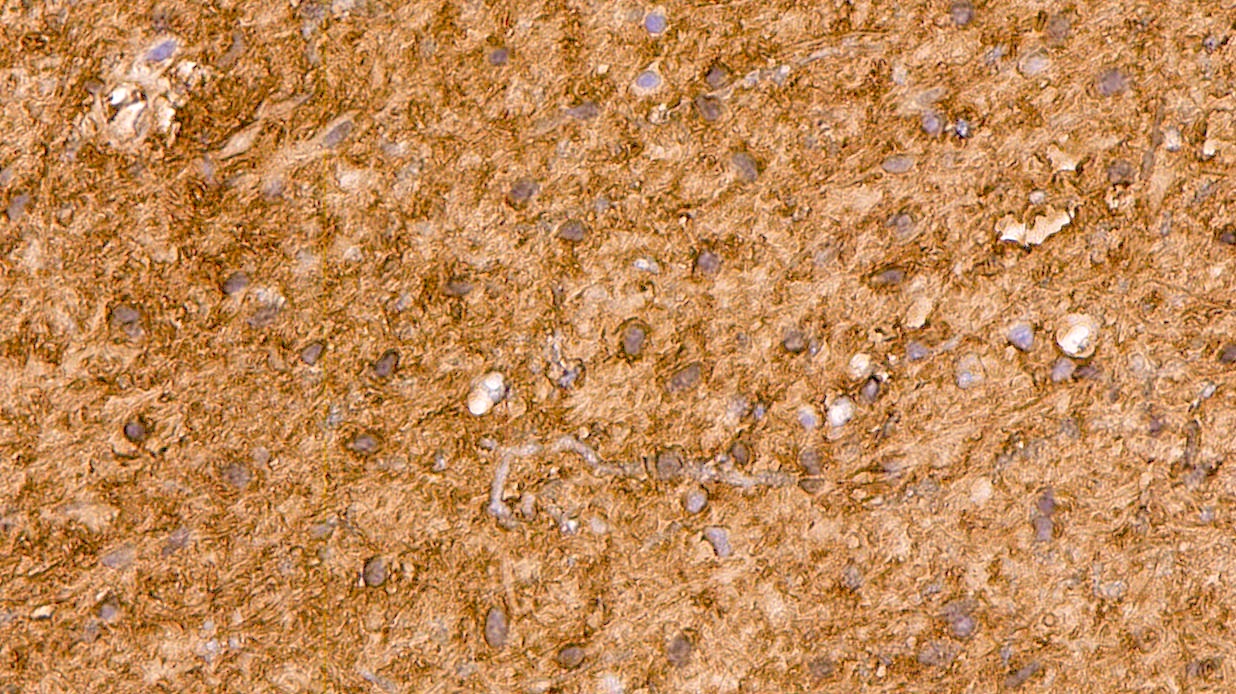
- GFAP (Nat Genet 2013;45:602)
- Olig2 (Am J Surg Pathol 2014;38:1058)
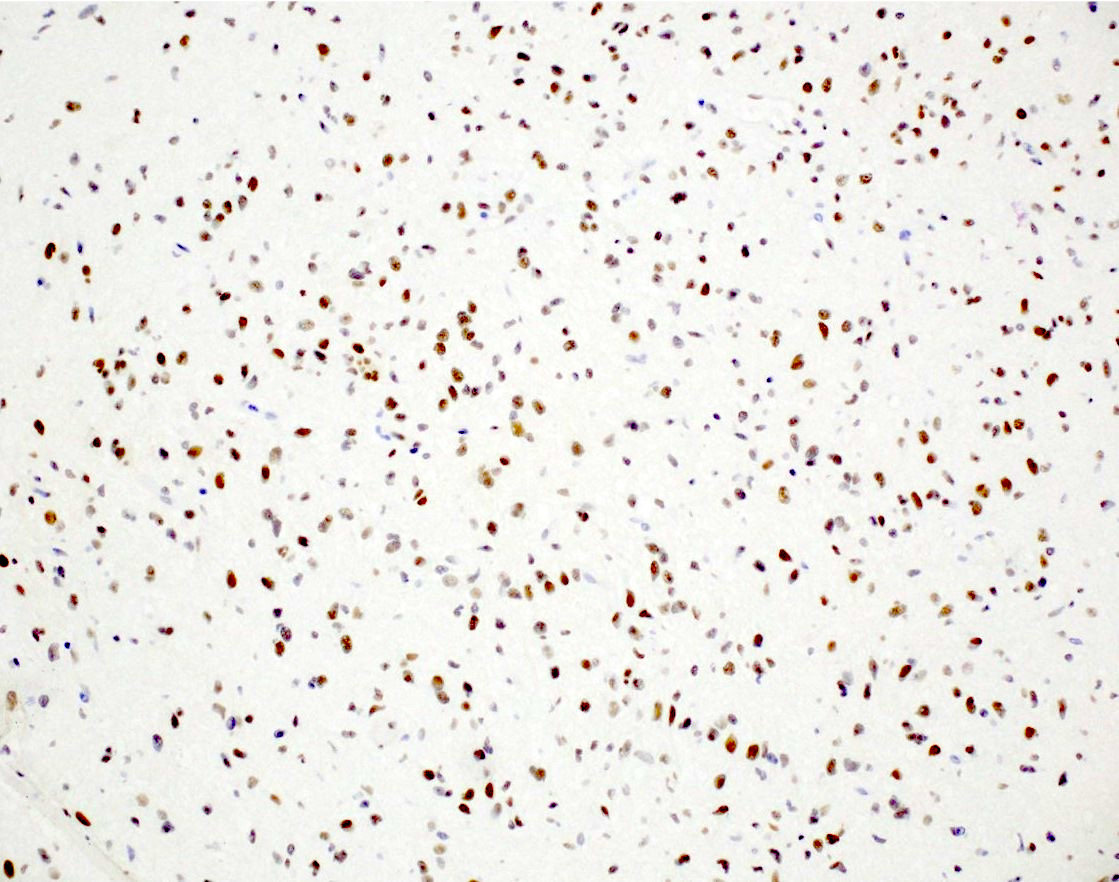
- BRAF V600E (if mutated)
Negative stains
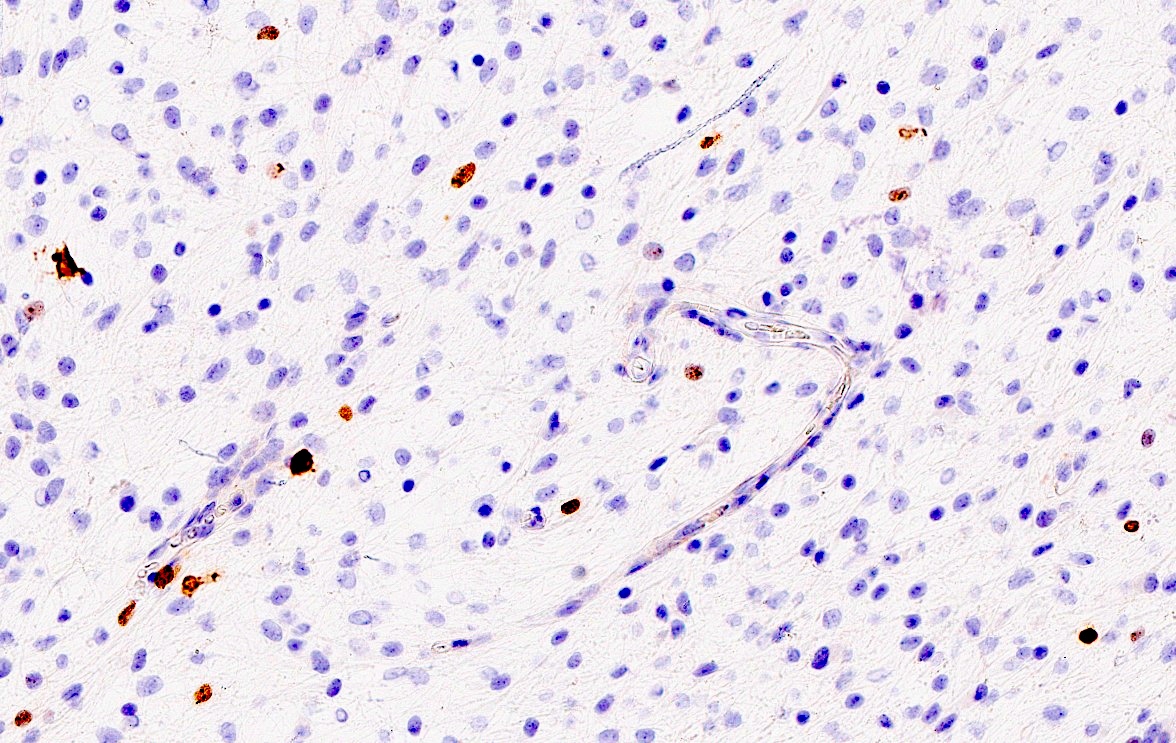
- IDH1 (R132H)
- H3F3A K27M
- H3F3A G34R / V
- CD34 (although may be present in a few cells) (Acta Neuropathol 2015;130:575)
Molecular / cytogenetics description
- FGFR alterations, such as FGFR1 tyrosine kinase domain duplications (Nat Genet 2013;45:602)
- BRAF alteration, usually V600E mutation (Nat Genet 2013;45:602)
- NTRK fusions (Acta Neuropathol Commun 2020;8:107)
- No CDKN2A / B homozygous deletion, MYB / MYBL1 rearrangement, IDH1 / IDH2 mutation or histone H3 K27 or G34 alterations (Neuro Oncol 2021;23:1231)
- Chromosomal alterations
- Chromosome 1p and 19q intact (Am J Surg Pathol 2014;38:1058)
Sample pathology report
- Brain tumor, right frontal lobe, resection:
- Integrated diagnosis: diffuse low grade glioma, MAPK pathway altered
- Histological diagnosis: diffuse astrocytoma, low grade
- Molecular information:
- IDH1 R132H: negative (immunohistochemistry; consistent with wild type)
- H3 G34R / V: negative (immunohistochemistry; consistent with wild type)
- CDKN2A: intact (fluorescence in situ hybridization)
- BRAF V600E: positive (immunohistochemistry; consistent with mutation)
- Comment: Sections demonstrate a diffusely infiltrating glioma with moderate cellularity. The tumor is composed of fibrillary astrocytic cells. No mitotic activity, microvascular proliferation or necrosis noted. No Rosenthal fibers or eosinophilic granular bodies are present. A CNS WHO grade has yet to be assigned.
Differential diagnosis
- Diffuse astrocytoma, MYB or MYBL1 altered, CNS WHO grade 1:
- MYB or MYBL1 alterations
- Angiocentric glioma, CNS WHO grade 1:
- Angiocentric arrangements of tumor cells, including perivascular pseudorosettes, with EMA positivity
- Polymorphous low grade neuroepithelial tumor of the young, CNS WHO grade 1:
- Histologically variable with an oligodendroglioma-like component and widespread expression of CD34
- Pilocytic astrocytoma, CNS WHO grade 1:
- Well circumscribed, tumor cells with piloid processes, Rosenthal fibers, eosinophilic granular bodies
- Ganglioglioma, CNS WHO grade 1:
- Gangliocytic cells
- Dysembroplastic neuroepithelial tumor, CNS WHO grade 1:
- Glioneuronal elements and glial nodules (if complex DNET)
- Diffuse midline glioma, histone H3 K27 altered, CNS WHO grade 4:
- Presence of histone H3 K27 alteration, typically K27M
- Oligodendroglioma, IDH mutant and 1p / 19q codeleted, CNS WHO grade 2:
- Presence of IDH mutation, chromosome 1p19q codeletion
- Astrocytoma, IDH mutant, CNS WHO grade 2:
- Presence of IDH mutation, ATRX loss
Additional references
Board review style question #1
A nonenhancing tumor was discovered in the right frontal lobe of a 5 year old boy. Resection revealed fibrillary astrocytic tumor cells without mitotic activity, microvascular proliferation or necrosis. Tumor cells showed immunopositivity for Olig2 and GFAP. What molecular alteration would you expect?
- BRAF V600E
- Histone H3 G34R
- Histone H3 K27M
- IDH1 (R132H)
Board review style answer #1
Board review style question #2
An 8 year old girl with lower cranial nerve deficits was found to have a large, infiltrative, minimally enhancing lesion in the left cerebellum, cerebellopontine angle and extending to the thalamus. She underwent a biopsy. Targeted next generation sequencing of the tumor reveals a missense KRAS mutation. What signaling pathway is most likely to be activated in this tumor?
- EGFR
- MAP kinase
- TGF beta
- Wnt
Board review style question #2