Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Intraoperative frozen / smear cytology images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Child DD, Yoda R. Central neurocytoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnstumorcentralneurocytoma.html. Accessed April 2nd, 2025.
Definition / general
- Rare, well differentiated, intraventricular neoplasm with neuroepithelial differentiation, typically arising near the foramen of Monro
Essential features
- Rare tumor, comprising 0.1 - 0.5% of all primary CNS neoplasms (J Neurooncol 2016;126:193)
- Intraventricular localization, usually involving the lateral or third ventricle(s) (Brain Pathol 1993;3:297)
- Clinical symptoms generally result from increased intracranial pressure due to obstructive hydrocephalus (Int J Radiat Oncol Biol Phys 2007;67:1145, J Clin Neurosci 2013;20:679)
- Microscopically appears as sheets of uniform, small - medium, round cells with fine chromatin stippling (salt and pepper) and occasional perinuclear clearing, interspersed with patches of fibrillary matrix (Brain Pathol 1993;3:297)
- CNS WHO grade 2 (Brain Tumor Res Treat 2016;4:49)
Terminology
- Central neurocytoma
ICD coding
- ICD-O:
- ICD-10:
- ICD-11:
Epidemiology
- ~0.1 - 0.5% of all primary brain tumors (J Neurooncol 2016;126:193)
- Overall incidence per 100,000 person years: 0.022, with individual rates varying by reported race (J Neurooncol 2019;143:123)
- Asian / Pacific Islander: 0.038
- Non-Hispanic White: 0.035
- Black: 0.026
- Hispanic White: 0.020
- Mean age at presentation: 20 - 34 years (J Neurooncol 2019;143:123)
- F:M = 1.02:1 (Brain Pathol 1993;3:297)
Sites
- Intraventricular mass, classically arising in the supratentorial ventricular system (Brain Pathol 1993;3:297)
- Anterior lateral ventricle (~50%)
- Lateral and third ventricle (~15%)
- Both lateral ventricles (~13%)
- Documented sites of origin include
- Foramen of Monro
- Septum pellucidum
- Corpus callosum
- Hypothalamus
- Large tumors may involve multiple sites
- Rarely reported in fourth ventricle and spinal cord (J Neurosurg 1994;81:288, Acta Neuropathol 2005;109:346, Mol Clin Oncol 2018;8:539)
- Neurocytomas occurring outside the ventricular system are termed extraventricular neurocytomas and are considered distinct entities (Acta Neurochir (Wien) 2014;156:349)
Pathophysiology
- Cell of origin
- Currently unknown, though favored to be a neuroglial progenitor cell due to dual differentiation potential (capable of forming both neurons and glial cells) (J Neurosci Res 1998;51:526, Lab Invest 1991;64:585)
- Frequent occurrence within lateral ventricle suggests origination from residual germinal matrix cells of subependymal plate (Lab Invest 1991;64:585, No Shinkei Geka 1995;23:1083)
- Infratentorial variants may arise from circumventricular organs (Acta Neuropathol 2005;109:346)
Etiology
- Unknown at this time
Clinical features
- Most common presenting symptoms are related to increased intracranial pressure that is due to obstructive hydrocephalus (Int J Radiat Oncol Biol Phys 2007;67:1145, J Clin Neurosci 2013;20:679)
- Headache
- Vomiting
- Visual field changes
- Papilledema
- Presenting symptoms generally present for a short time prior to diagnosis (median: 1.7 - 3 months) (Int J Radiat Oncol Biol Phys 2007;67:1145)
Diagnosis
- Based primarily on histologic and immunophenotypic features (Brain Pathol 1993;3:297)
- Requires intraventricular localization, oligodendroglioma-like cytology and synaptophysin expression
- Correlation with radiologic studies (e.g., MRI, CT) is essential; supportive findings include
- Intraventricular localization (required)
- Enhancing, multicystic mass
- MRI: T1 isointense, T2 heterogeneous, FLAIR hyperintense
- CT: may show calcifications
- Methylation profiling may aid diagnosis in unresolved cases (J Neurooncol 2022;159:725)
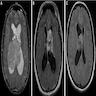
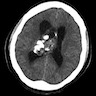
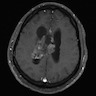
Radiology description
- Noncontrast computed tomography (J Clin Neurosci 2013;20:679, Neurosurg Clin N Am 2015;26:11)
- Highly variable appearance, often with mixed solid and cystic components that appear isodense and hypodense, respectively, to surrounding brain parenchyma
- Calcifications may be seen, typically partial or punctate
- Evidence of hydrocephalus or hemorrhage may be visible
- Magnetic resonance imaging (J Clin Neurosci 2012;19:681, J Clin Neurosci 2013;20:679, Neurosurg Clin N Am 2015;26:11)
- Classically an intraventricular mass with a multicystic, soap bubble appearance characterized by T1 and T2 isointense solid components and T2 hyperintense, fluid filled cysts
- Peripheral cyst walls may form spicules and cause undulation of the adjacent lateral ventricle wall (scalloping)
- Calcifications and flow voids may be seen on T1 sequences
- Heterogenous contrast enhancement
- No surrounding peritumoral edema on T2 / FLAIR
- Proton magnetic resonance spectroscopy (Eur Radiol 2009;19:2049, J Clin Neurosci 2012;19:681, Neurosurg Clin N Am 2015;26:11)
- Characteristic glycine peak at 3.55 ppm
- Prominent choline peak
- Inverted alanine peak
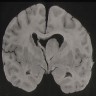
Radiology images
Prognostic factors
- Generally favorable prognosis (J Neurooncol 2016;126:193)
- 5 year overall survival rate: 96%
- 10 year overall survival rate: 82%
- Extent of resection is the only independent prognostic factor (Am J Surg Pathol 2012;36:220)
- Elevated proliferative index is associated with more aggressive behavior, with various thresholds proposed, but no single optimal cutoff established
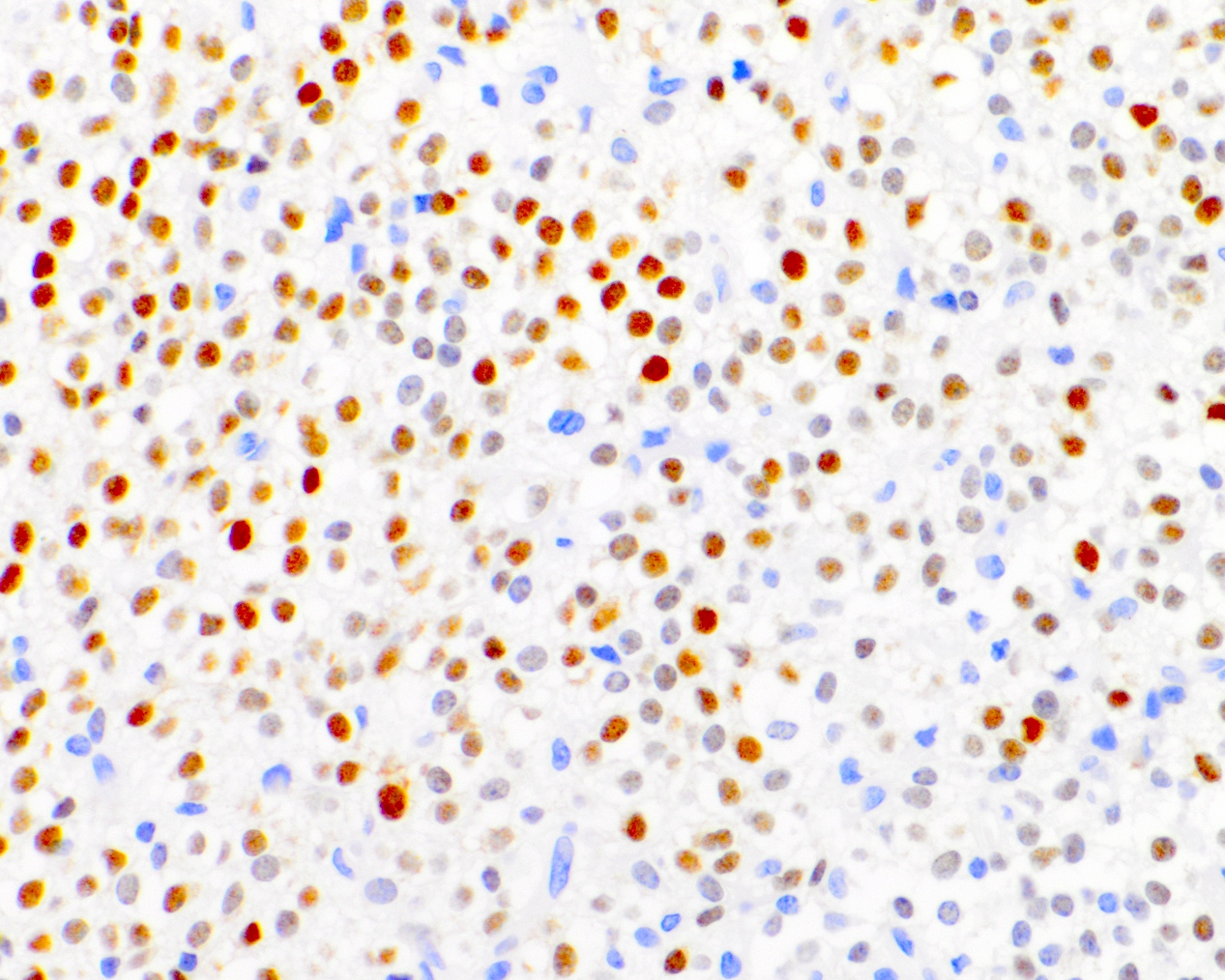
- Proposed Ki67 thresholds: ~2 - 4% (J Neurooncol 2018;140:669, Neurology 2004;62:987, J Neuropathol Exp Neurol 1997;56:551, J Neurooncol 2016;126:193)
- e.g., Ki67 ≤ 4%: 90% 2 year progression free survival; Ki67 > 4%: 48% 2 year progression free survival (J Neurooncol 2016;126:193)
- Mitotic threshold of 3/10 HPF has been proposed (Am J Surg Pathol 2012;36:220, Int J Radiat Oncol Biol Phys 2007;67:1145)
- Proposed Ki67 thresholds: ~2 - 4% (J Neurooncol 2018;140:669, Neurology 2004;62:987, J Neuropathol Exp Neurol 1997;56:551, J Neurooncol 2016;126:193)
Case reports
- 8 year old girl with atypical central neurocytoma arising in the posterior fossa (BMJ Case Rep 2019;12:e231626)
- 13 year old boy with periventricular atypical central neurocytoma harboring unique WSR1::ATF1 fusion and MUTYH mutation (BMJ Case Rep 2019;12:bcr-2018-226455)
- 17 year old boy with right lateral ventricular central neurocytoma and intraventricular hemorrhage (Case Rep Surg 2022;2022:9731987)
- 48 year old man with atypical central neurocytoma and craniospinal drop metastases as well as concomitant pituitary macroadenoma (Asian J Neurosurg 2020;15:140)
- 79 year old woman with third ventricular central neurocytoma treated with gamma knife radiation (Medicine (Baltimore) 2018;97:e13657)
Treatment
- Surgical resection is standard of care (J Clin Neurosci 2013;20:1193)
- When complete resection is not possible, adjuvant radiotherapy improves survival (World Neurosurg 2020;137:e176)
- Adjuvant radiotherapy not recommended with complete resection of atypical central neurocytomas (Front Neurol 2020;11:834)
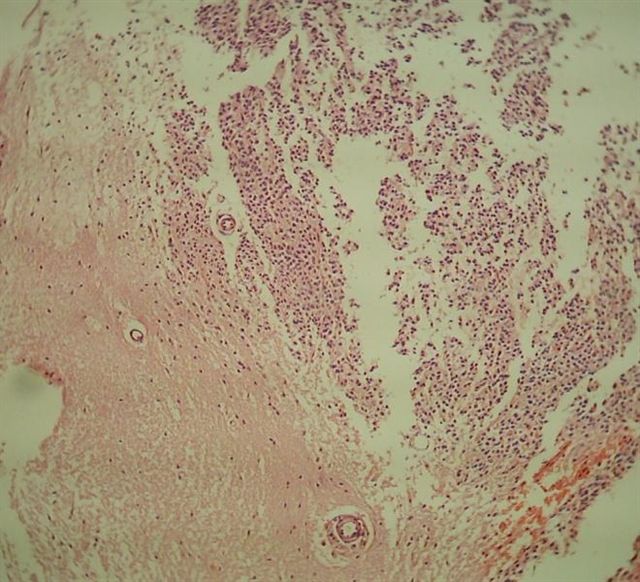
Gross description
- Gray, friable tissue (Brain Pathol 1993;3:297)
- May have macrocalcifications and hemorrhage (Brain Pathol 1993;3:297)
Frozen section description
- Sheets of isomorphous, round cells in a fibrillary background
- Minimal pleomorphism
- Necrosis or mitotic figures are typically absent
- Reference: Acta Cytol 2004;48:194
Intraoperative frozen / smear cytology images
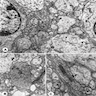
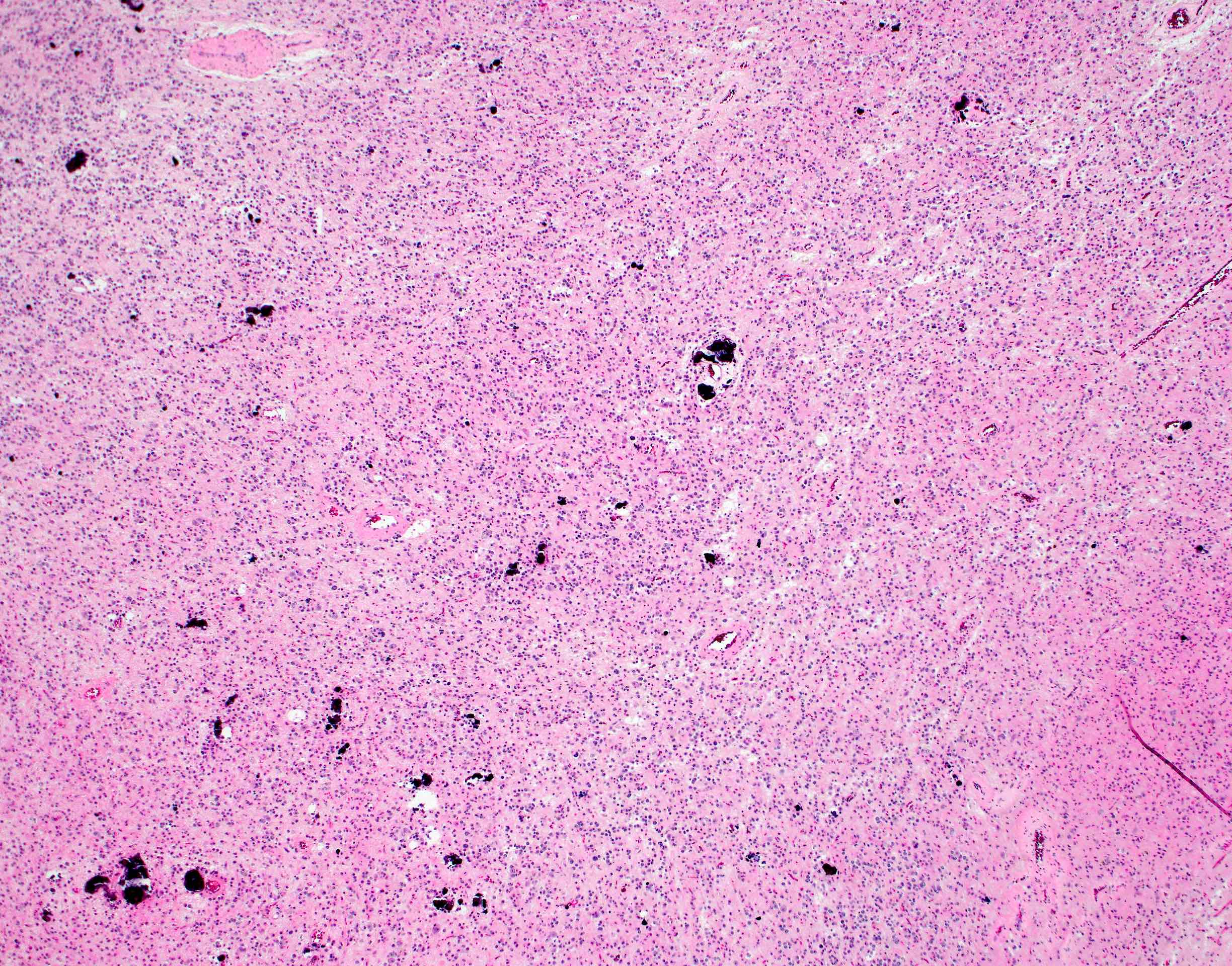
Microscopic (histologic) description
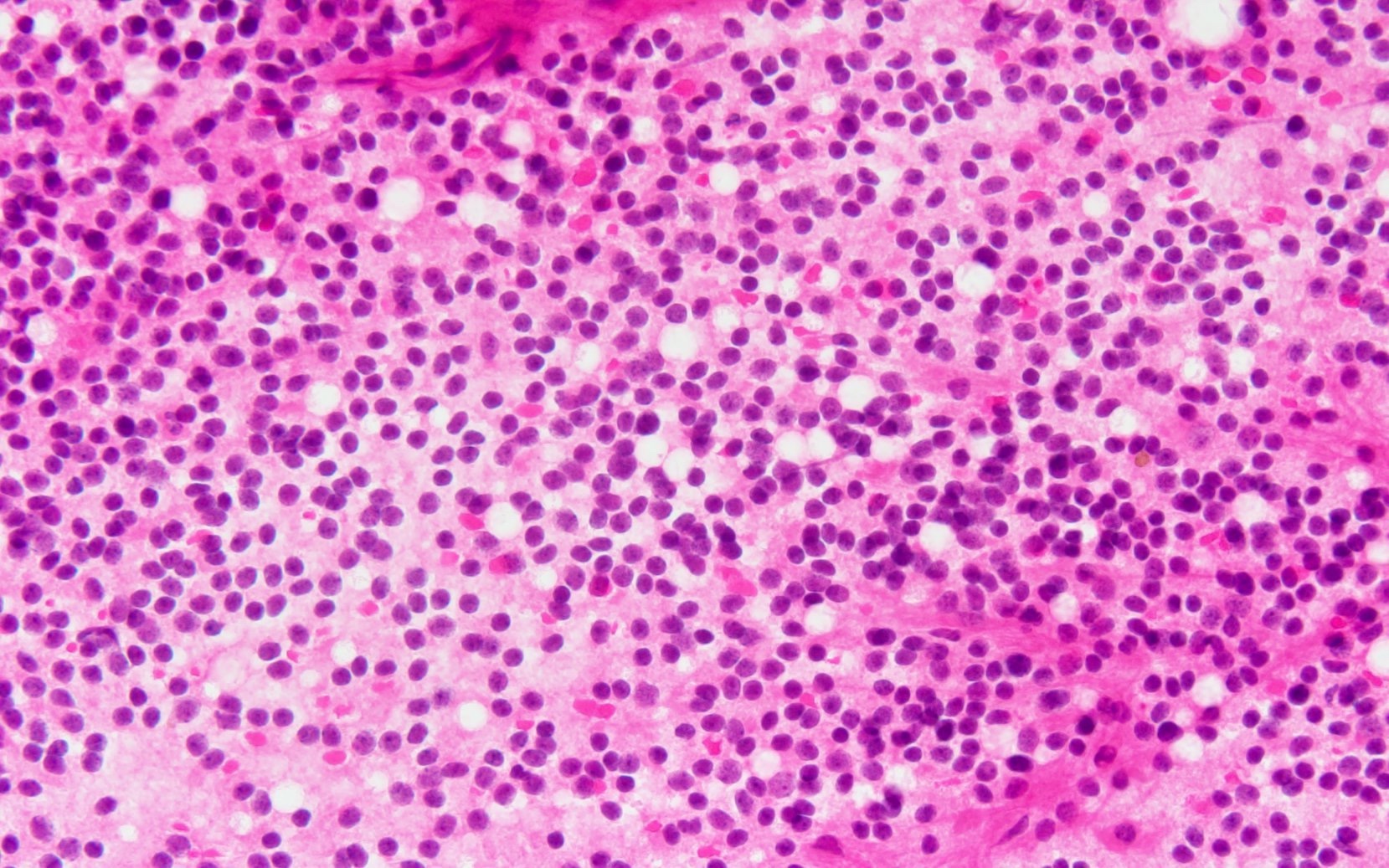
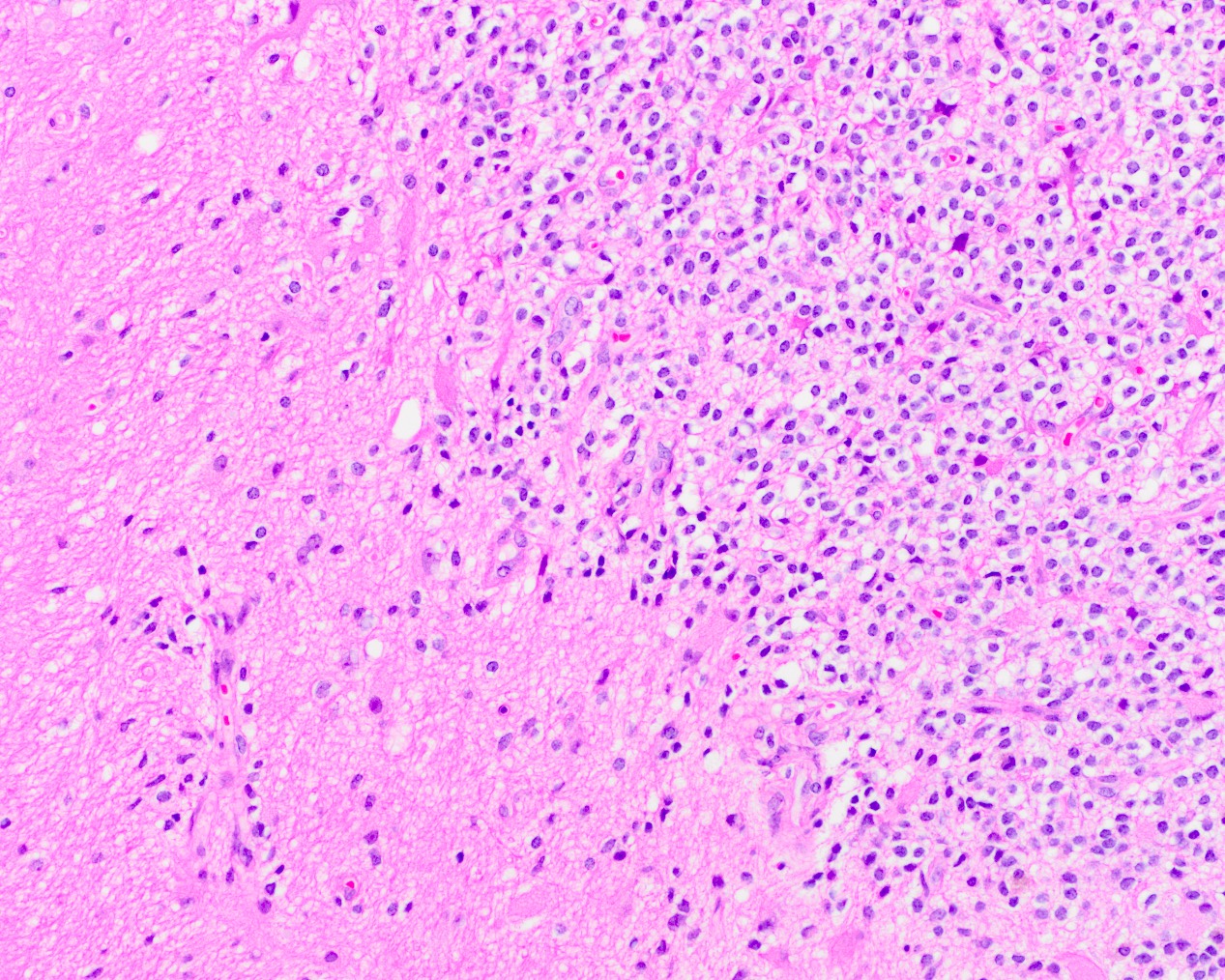
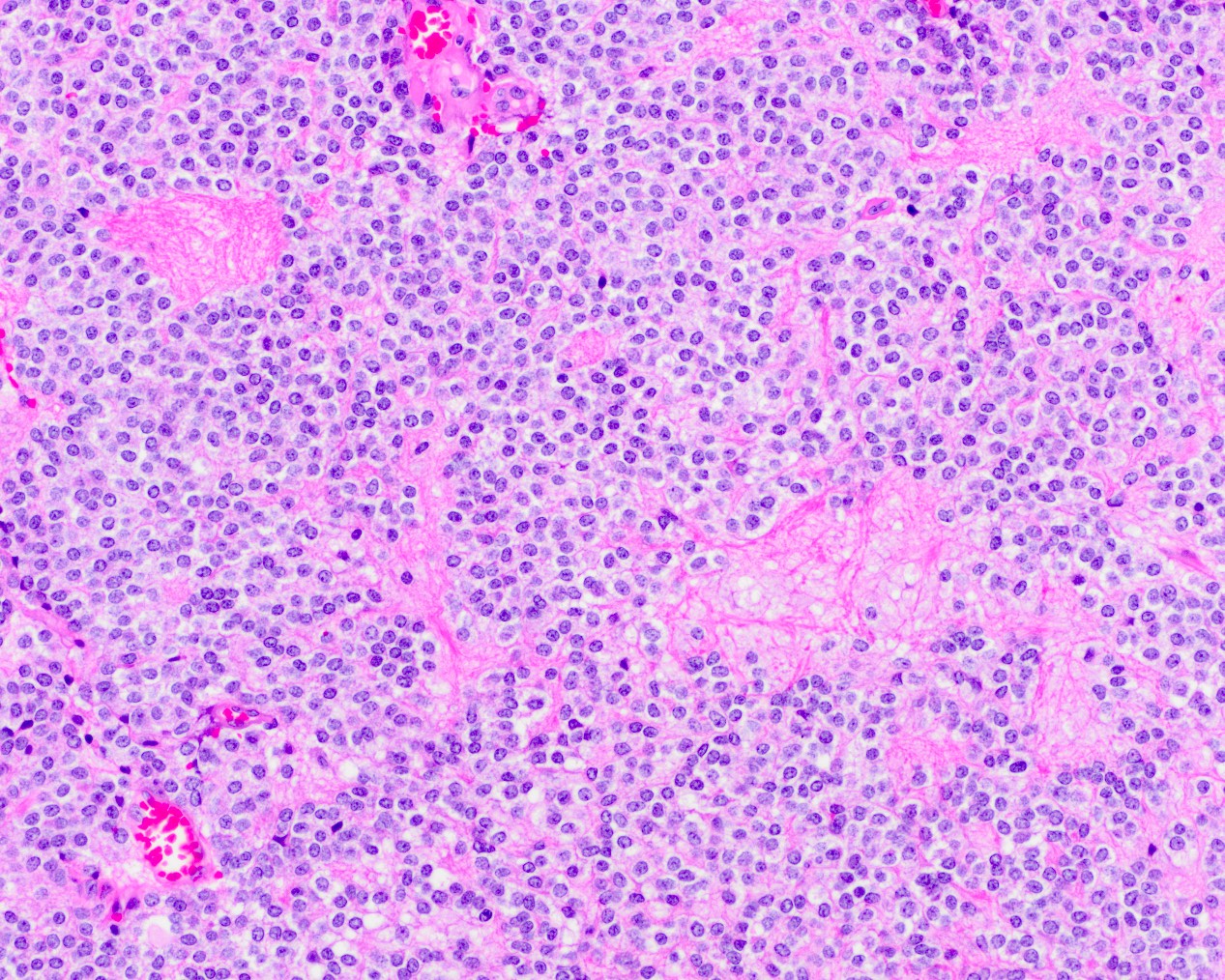
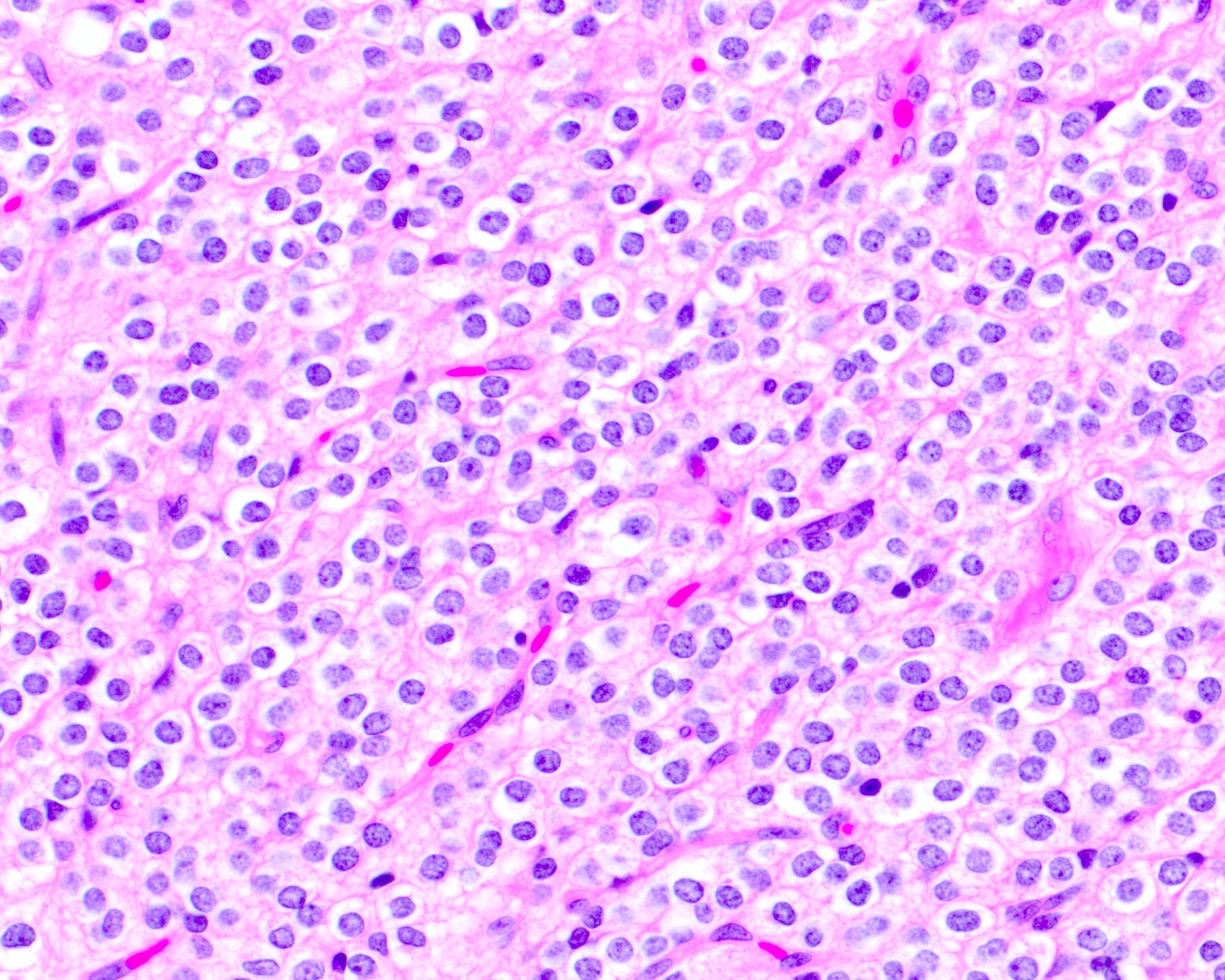
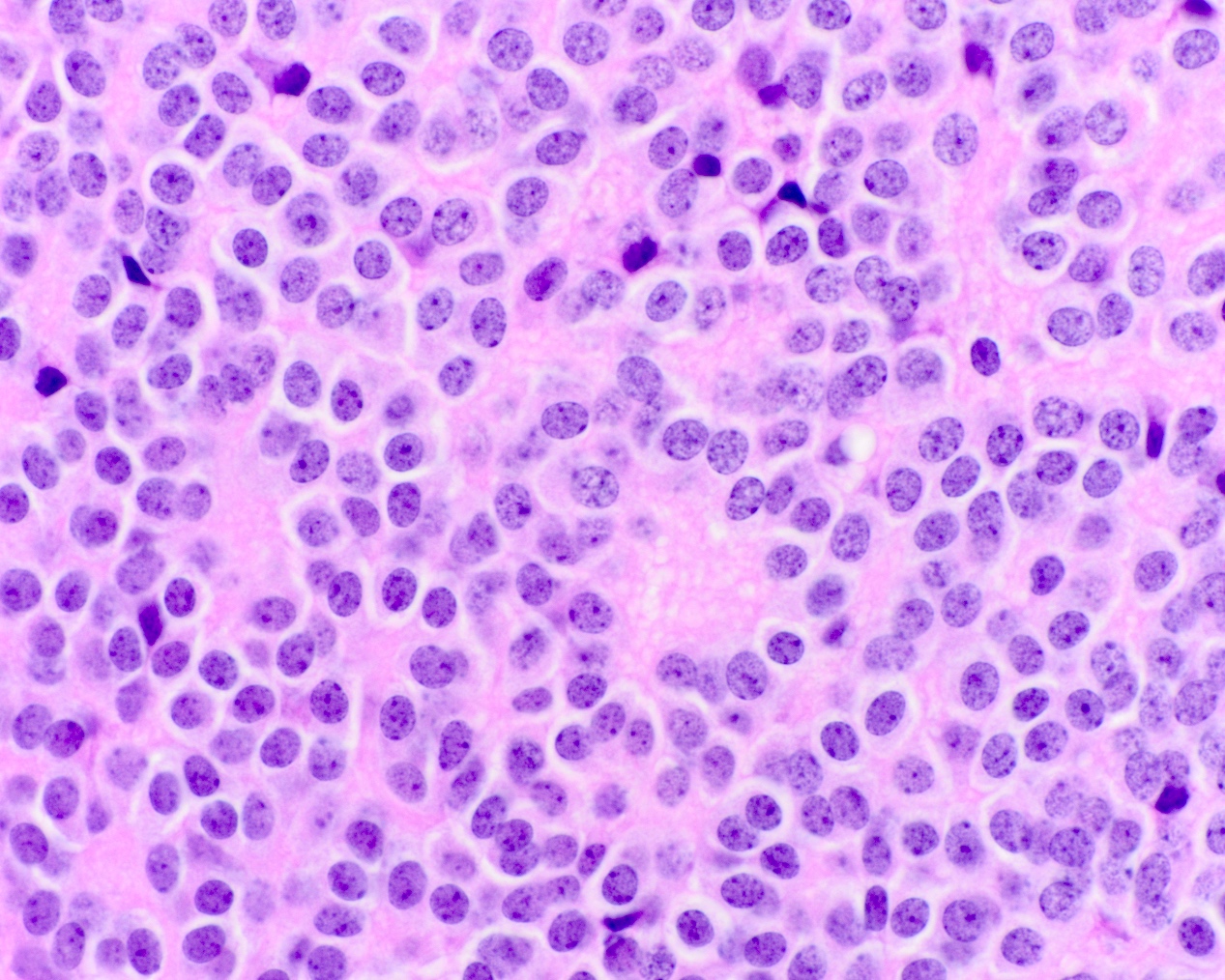
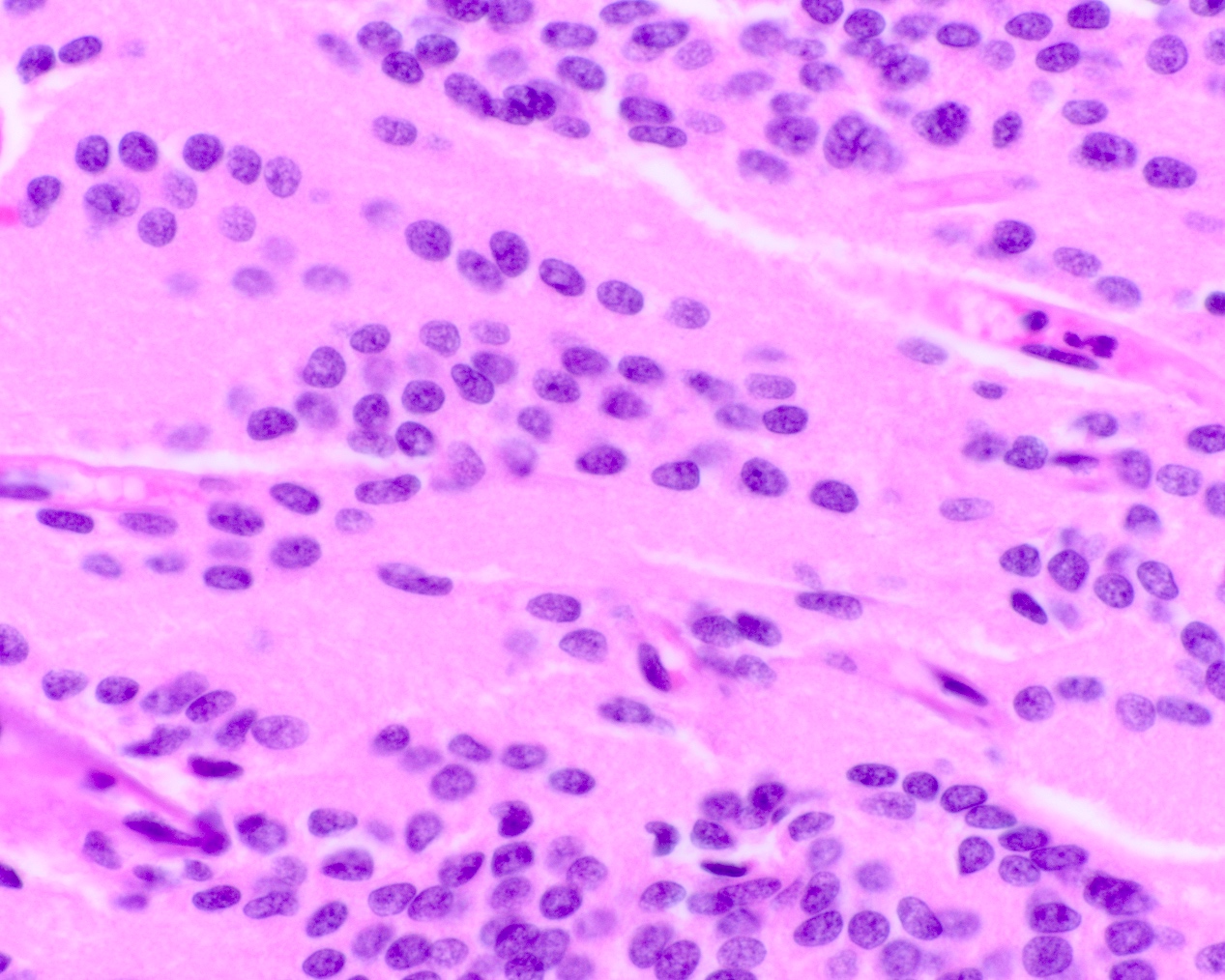
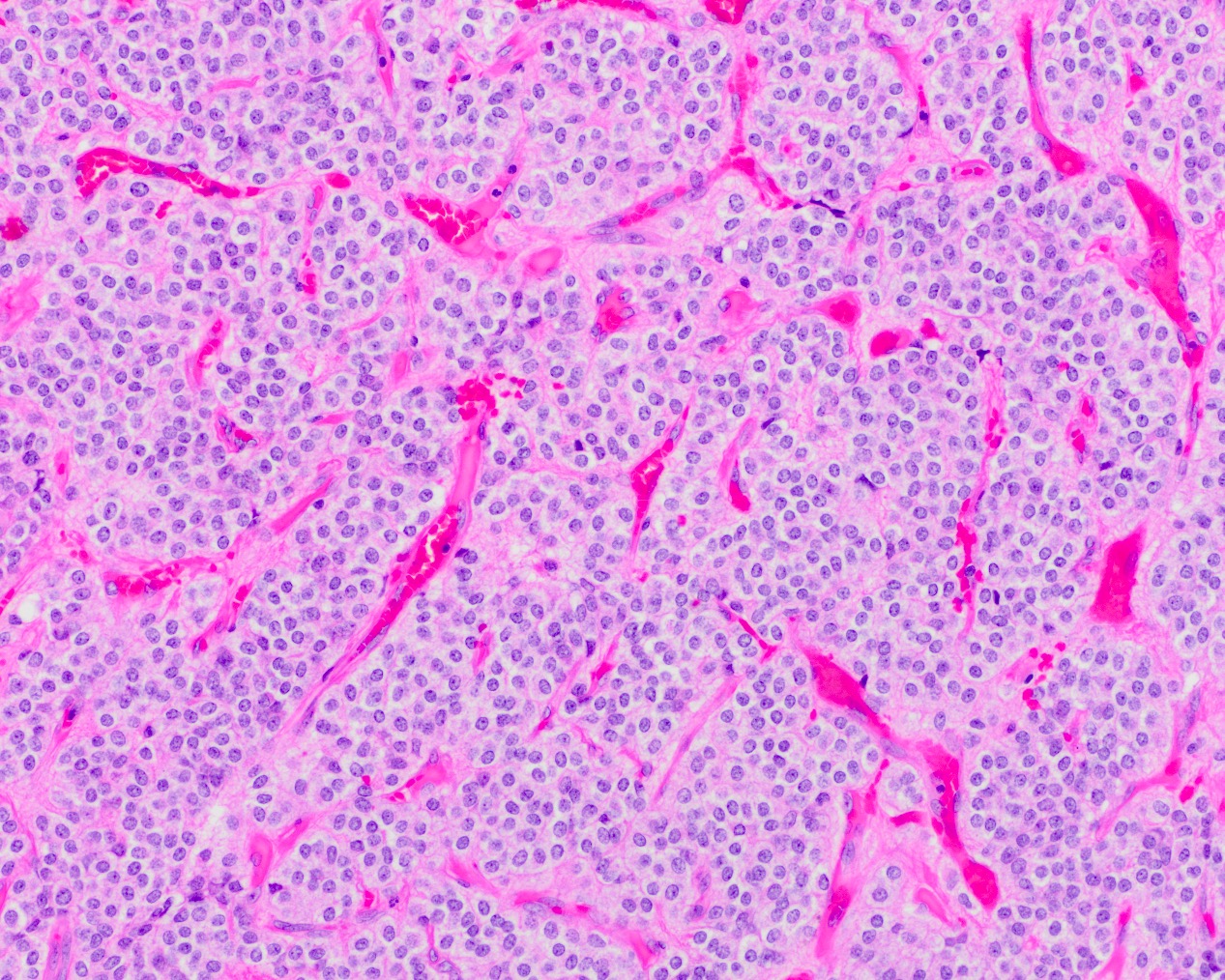
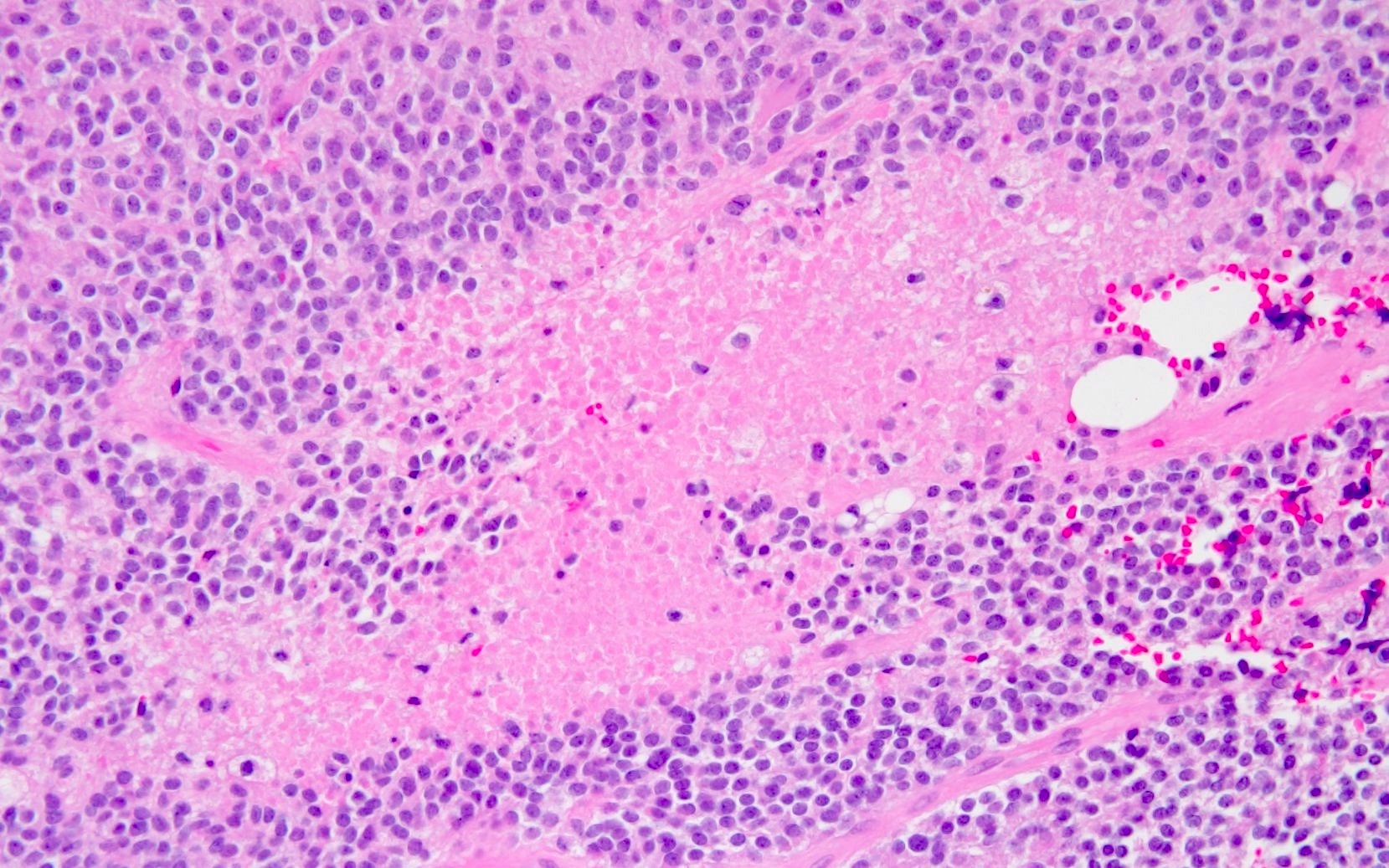
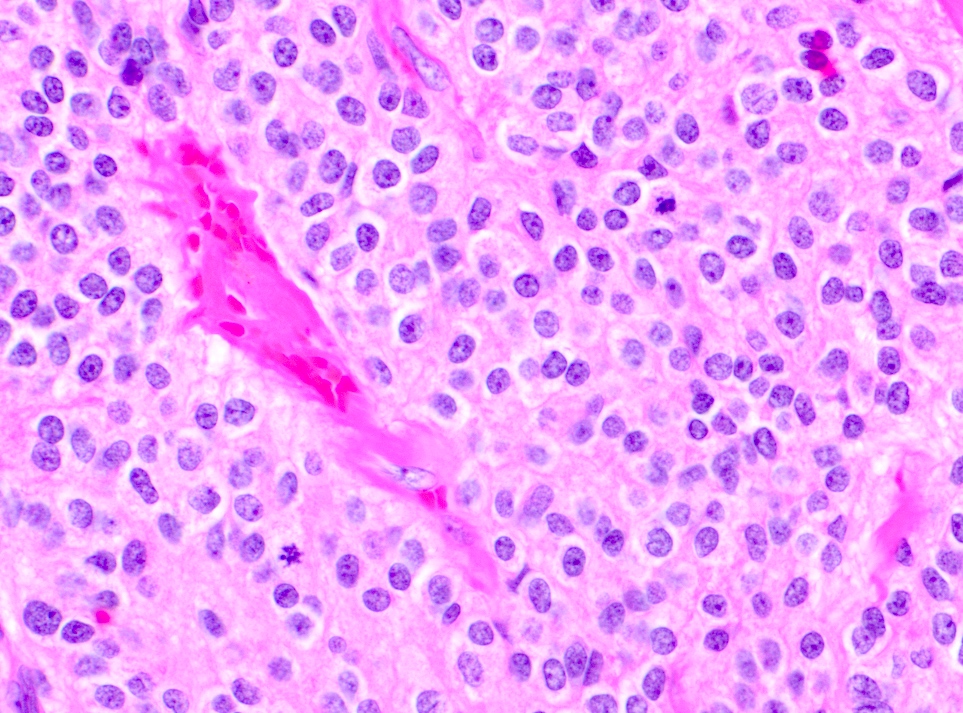
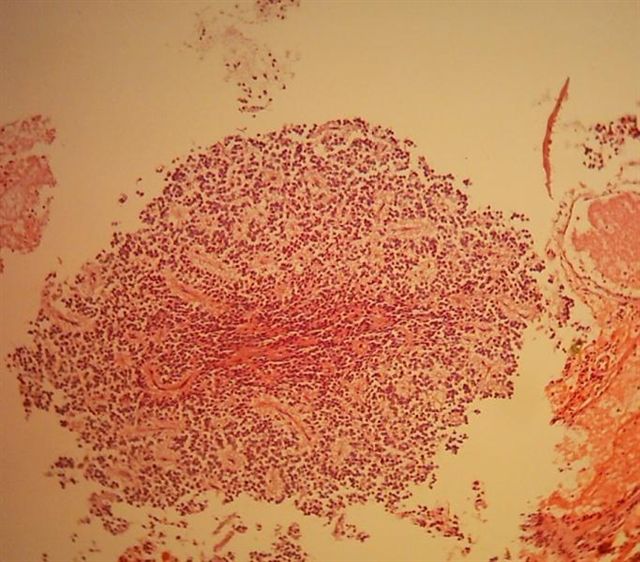
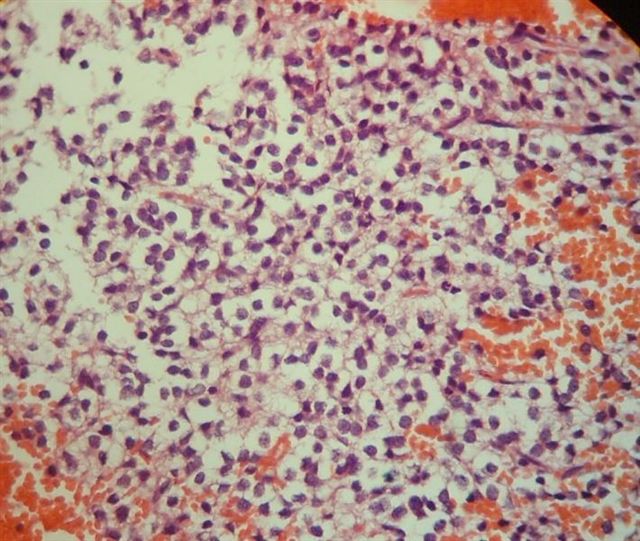
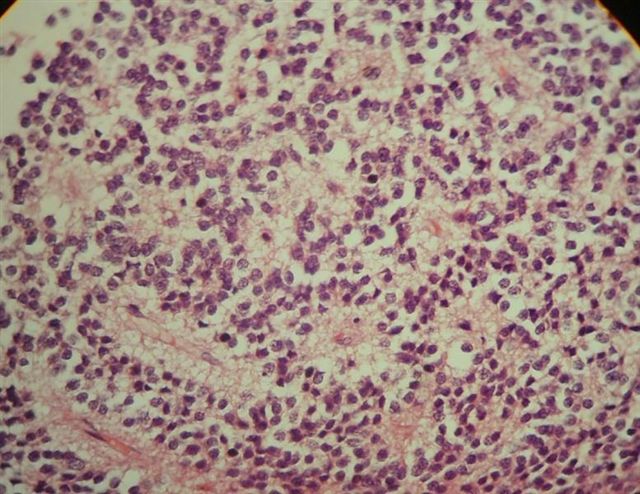
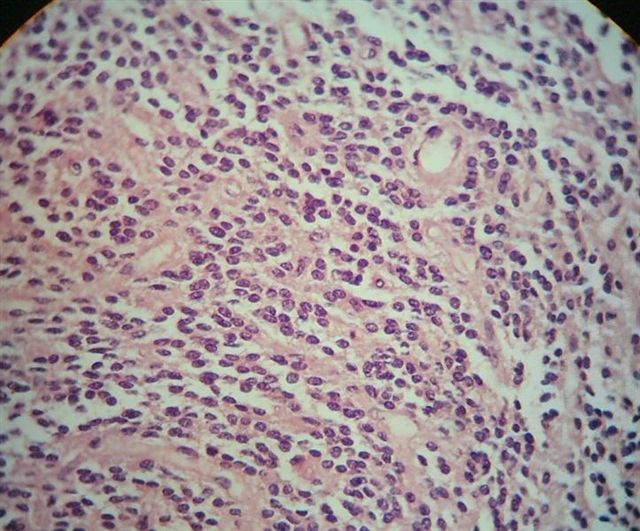
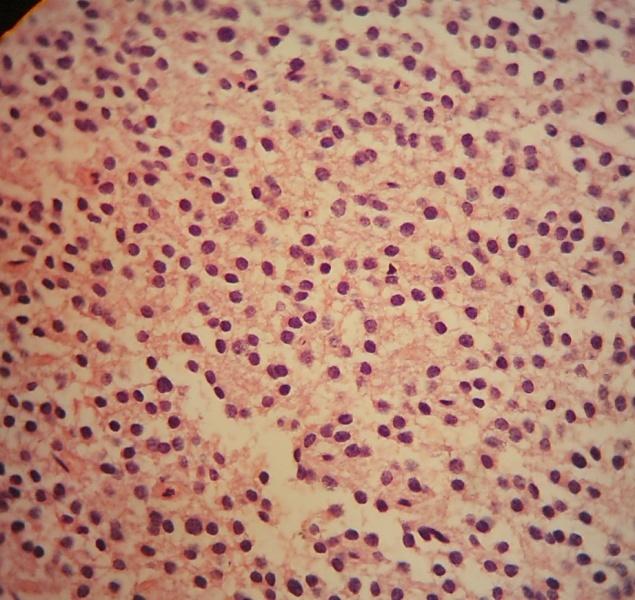
- Neuroepithelial neoplasm composed of uniform, small - medium cells growing in sheets with indistinct cytoplasm (Brain Pathol 1993;3:297)
- Nuclei are round with regular contours, finely stippled (salt and pepper) chromatin and micronucleoli
- Perinuclear clearing may be prominent (similar to oligodendrogliomas)
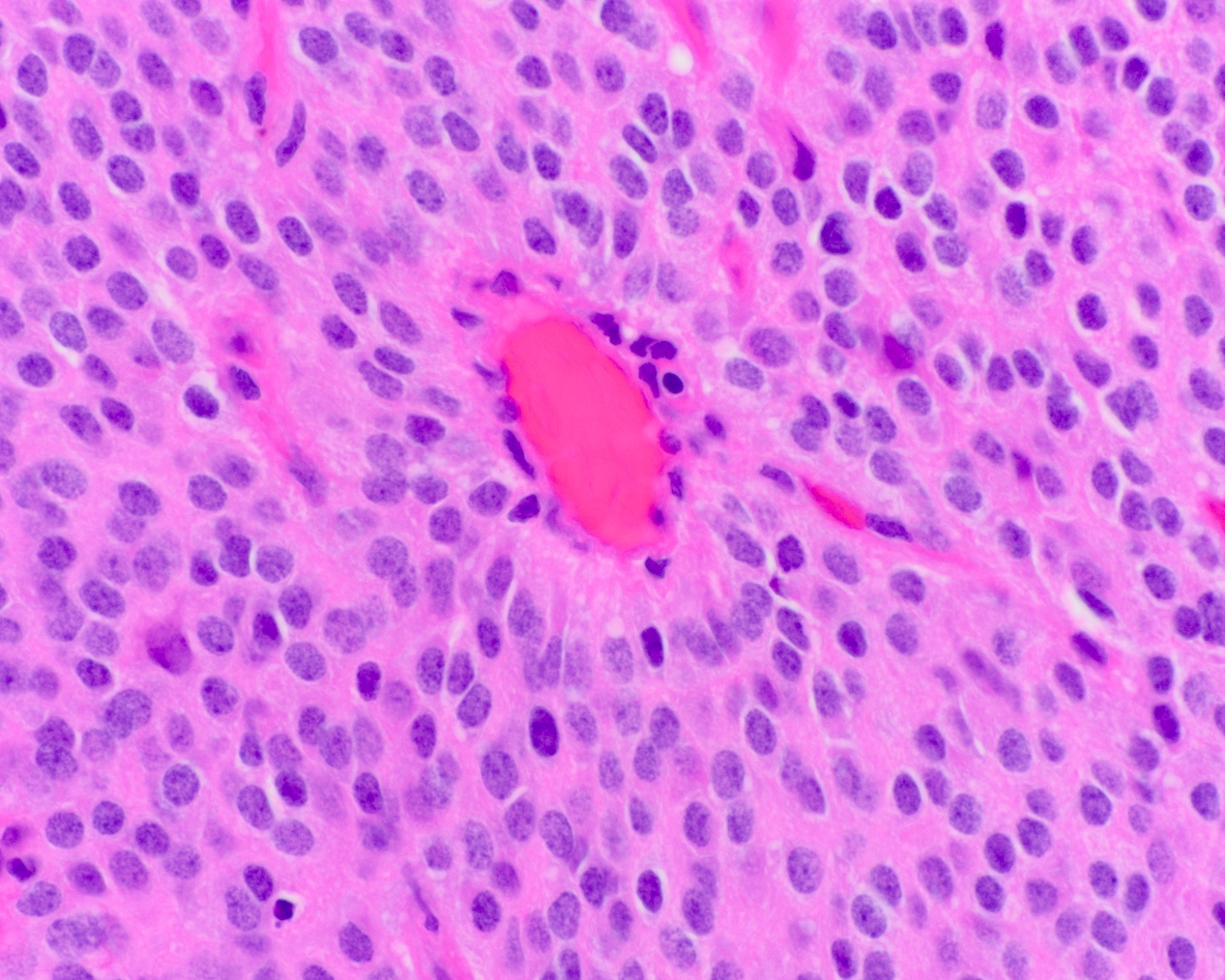
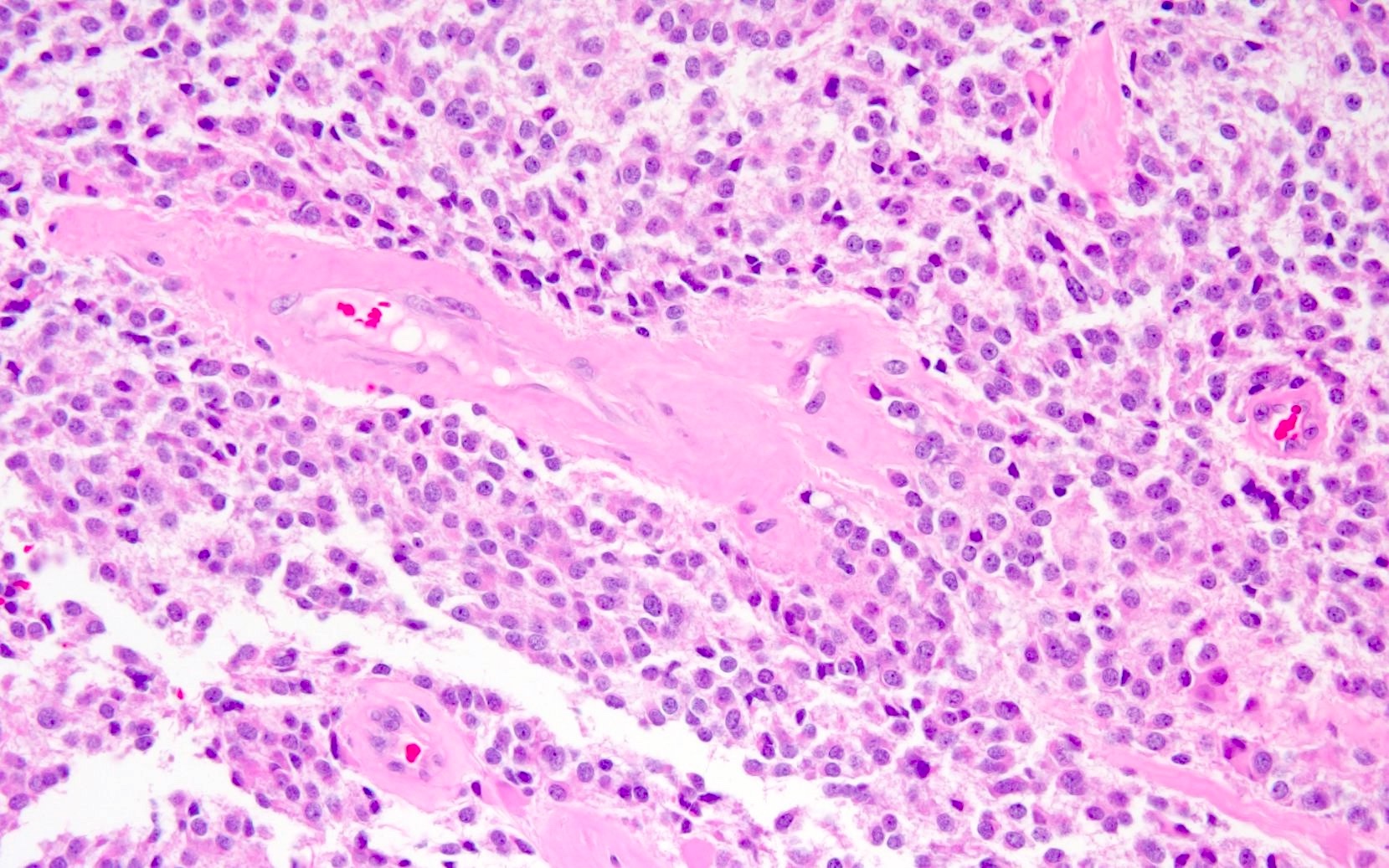
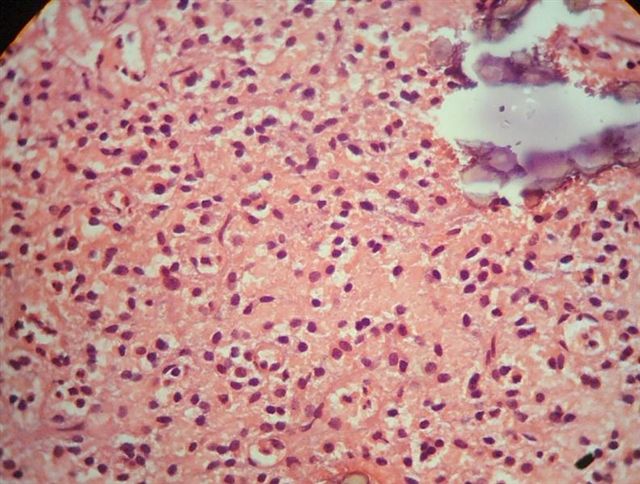
- Arborizing capillaries
- Large hyalinized blood vessels
- Other morphologic features may include
- Honeycomb-like architecture
- Patches of fibrillar, neuropil-like matrix mimicking pineocytomatous rosettes
- Perivascular pseudorosettes
- Homer-Wright rosettes
- Ganglioid cells
- Calcification is usually distributed throughout the tumor and may be prominent
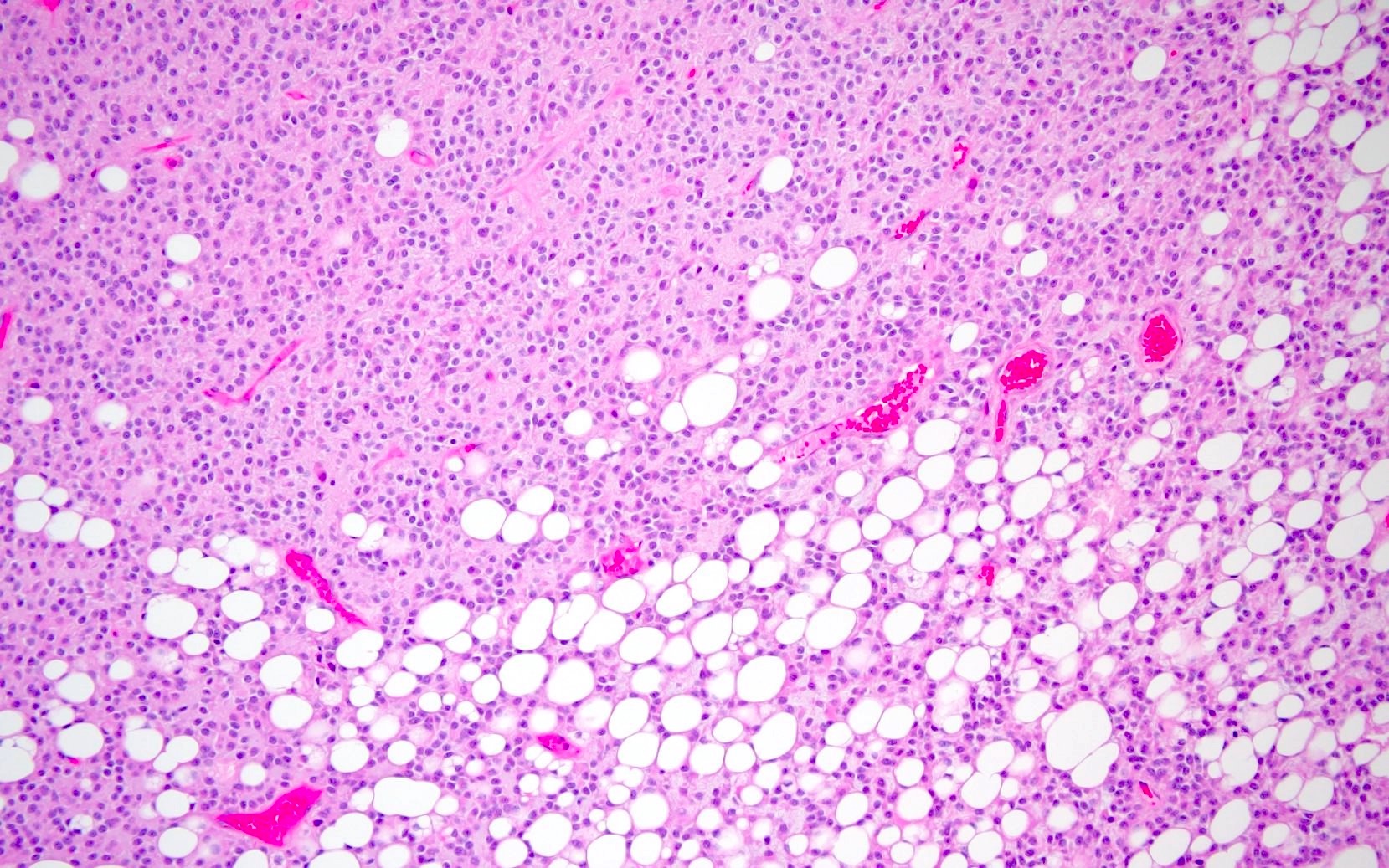
- Lipomatous differentiation occurs rarely (World Neurosurg 2018;120:214)
- Hemorrhage is sometimes present; hemosiderin laden macrophages may be seen (Neurosurg Rev 2001;24:48)
- Designated as atypical central neurocytoma when anaplastic features are seen (J Neurosurg 1992;76:32, Brain Pathol 1993;3:297)
- Brisk mitotic activity
- Microvascular proliferation
- Necrosis
Microscopic (histologic) images
Cytology description
- Cerebral spinal fluid cytology may be positive in the presence of disseminated tumor (J Neurosurg 1992;76:32)
- Crowded, cellular spheres composed of uniform small - medium cells
- Scant, cyanophilic cytoplasm on Papanicolau stain
- Neurocytic rosettes may be seen
- Squash preparations / direct smears (Acta Cytol 2004;48:194, Acta Cytol 2010;54:209)
- Monotonous, round cells with ill defined cytoplasm and without aggregation or clustering
- Nuclei tend to have finely granular chromatin and micronucleoli
- Hemosiderin laden macrophages or reactive astrocytes may be present
Positive stains
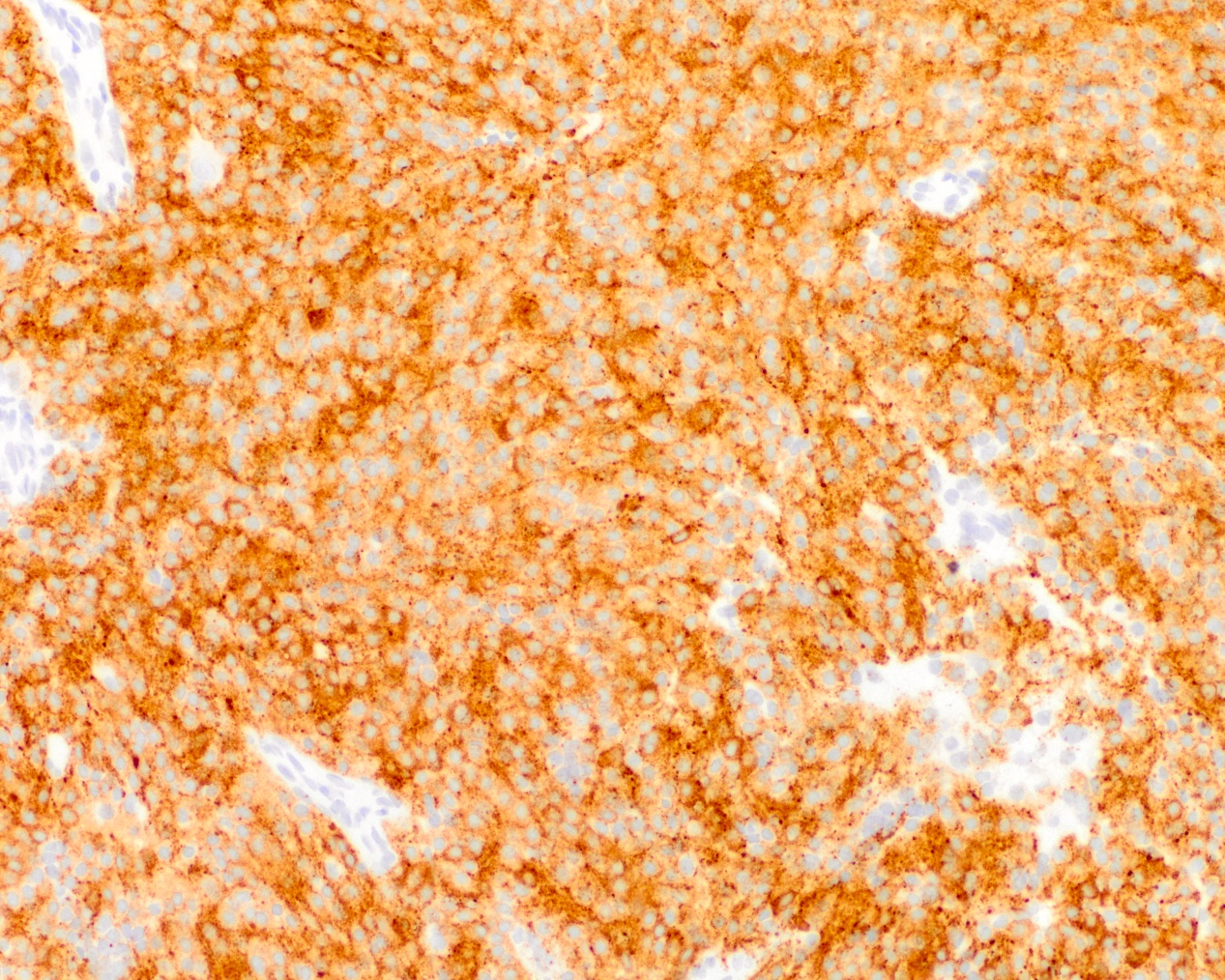
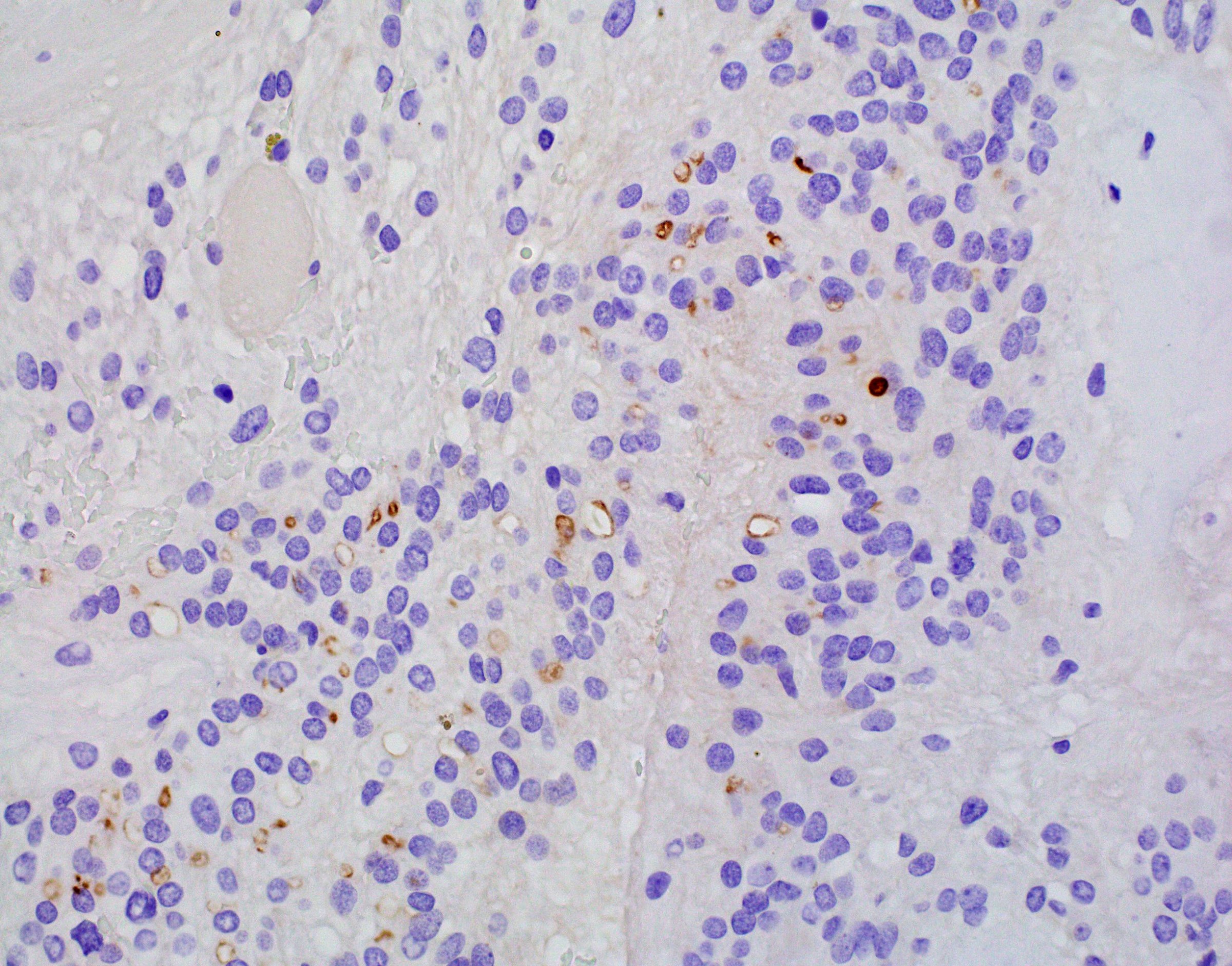
- Synaptophysin (Brain Pathol 1993;3:297)
- NeuN (Pathol Res Pract 2003;199:463)
- MAP2 (Brain Pathol 1993;3:297)
- Neuron specific enolase (Brain Pathol 1993;3:297)
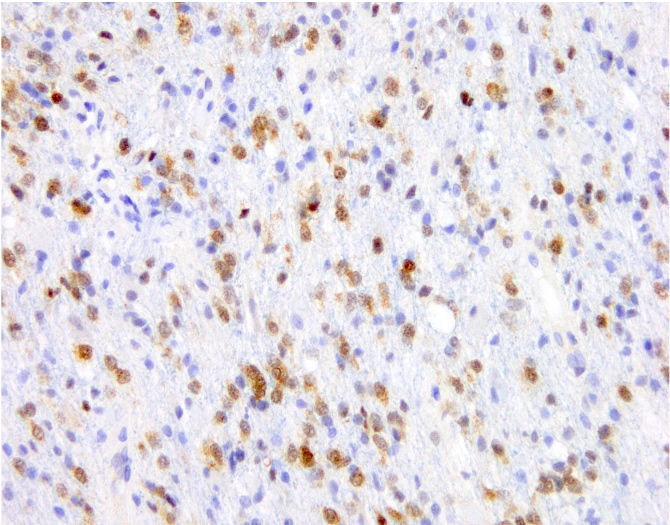
- Ki67: cutoff for atypical central neurocytoma has not been established but suggested values range from ~2 - 4% (Neurology 2004;62:987, J Mol Biol 1989;210:709, J Neurooncol 2018;140:669, J Neuropathol Exp Neurol 1997;56:551)
- TTF1
- Clone 8G7G3/1 (7 - 21%) (World Neurosurg 2022;159:e62, Mod Pathol 2017;30:318)
- Clone SPT24 (47%) (Mod Pathol 2017;30:318)
Negative stains
- Chromogranin A (Brain Pathol 1993;3:297)
- Neurofilament (Am J Surg Pathol 2012;36:220)
- Olig2 (J Neurooncol 2017;135:57)
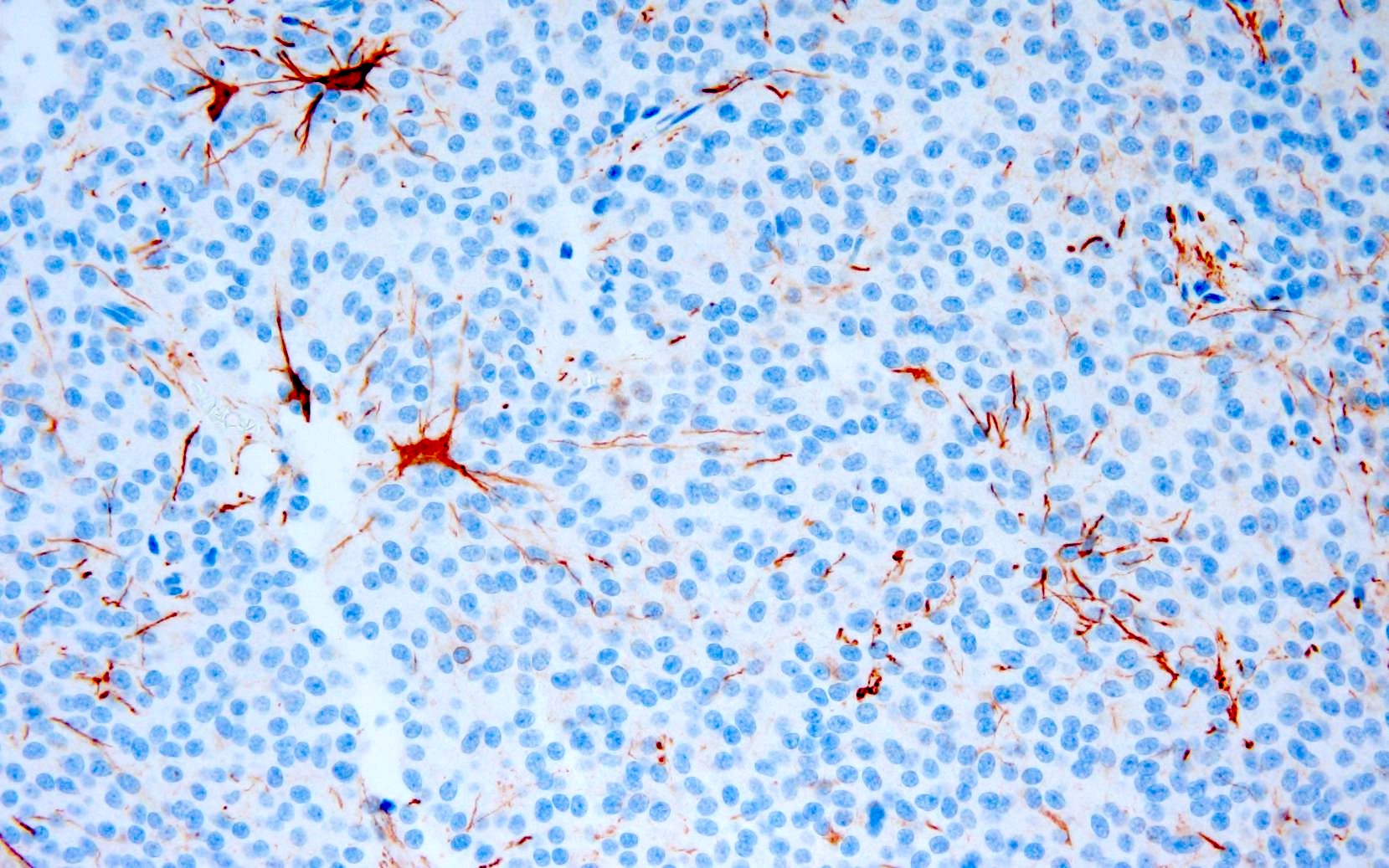
- GFAP: generally stains only entrapped astrocytes, though may be positive in rare cases (J Neuropathol Exp Neurol 1997;56:551, Acta Neuropathol 1996;91:573)
Electron microscopy description
- Not utilized in routine diagnostics
- Cells typically appear more uniform than on H&E preparations (Brain Pathol 1993;3:297)
- Nuclei (Brain Pathol 1993;3:297, Pathol Res Pract 1995;191:100, Acta Neuropathol 1997;94:425)
- Regular and round
- Finely dispersed chromatin
- Small, well defined nucleoli
- Cytoplasm (Brain Pathol 1993;3:297, Pathol Res Pract 1995;191:100, Acta Neuropathol 1997;94:425)
- Prominent golgi apparatus
- Abundant mitochondria
- Dumbbell shaped lysosomal inclusions
- Parallel microtubule arrays
- Membrane bound, dense core neurosecretory granules
- Matrix (Brain Pathol 1993;3:297, Pathol Res Pract 1995;191:100, Acta Neuropathol 1997;94:425)
- Numerous cytoplasmic projections separating adjacent cell bodies
- Occasional synapses
Electron microscopy images
Molecular / cytogenetics description
- No known recurrent mutations or chromosomal imbalances (Acta Neuropathol 2018;136:181)
- Microarray analysis (Acta Neuropathol 2007;113:303)
- One study reports frequent copy number aberrations including frequent MYCN gain
- No strong sensitivity or specificity for any region
- Transcriptomic analysis showed overexpression of genes related to (Neuropathology 2013;33:149)
- Wnt / beta catenin signaling pathway
- Sonic hedgehog signaling pathway
- Calcium function
- Maintenance of neural progenitors
- Methylation profile is unique to central neurocytoma but cannot distinguish between classic and atypical variants (Acta Neuropathol 2018;136:181, J Neurooncol 2022;159:725)
- Negative for 1p / 19q codeletion (J Neurosurg 2002;97:1350)
Sample pathology report
- Brain, intraventricular mass, resection:
- Central neurocytoma, CNS WHO grade 2
Differential diagnosis
- Oligodendroglioma:
- Infiltrative growth
- IHC (J Neurooncol 2017;135:57)
- Strong and diffuse Olig2 positivity
- May be positive for IDH1 R132H
- Lacks diffuse neuronal differentiation (e.g., NeuN, synaptophysin)
- Molecular
- IDH1 / 2 mutation
- Chromosome 1p / 19q codeletion
- Infiltrative growth
- Ependymoma:
- Ganglioglioma:
- Mixed neuronal (ganglion cells) and glial neoplastic components
- IHC
- Positive for GFAP and CD56, frequently BRAF V600E positive (J Neurosci Rural Pract 2021;12:807, Pediatr Neurosurg 2019;54:36)
- Pineocytoma:
- Pineal region location
- IHC: positive for NFP (strong and diffuse)
- Distinct DNA methylation profile
- Choroid plexus papilloma
- Well developed papillary architecture
- IHC: positive for Kir7.1
- Subependymoma:
- Meningioma:
Additional references
Board review style question #1
Board review style answer #1
D. Synaptophysin. Synaptophysin expression is the most reliable diagnostic marker for central neurocytoma, which generally shows low to absent immunoreactivity for chromogranin, GFAP and Olig2.
Comment Here
Reference: Central neurocytoma
Comment Here
Reference: Central neurocytoma
Board review style question #2
Which of the following histological features is necessary for the designation of atypical central neurocytoma?
- Hypercellularity
- Lipomatous differentiation
- Macronucleoli
- Microvascular proliferation
Board review style answer #2
D. Microvascular proliferation: Brisk mitotic activity, necrosis and microvascular proliferation are the 3 histological features necessary for the classification of an atypical central neurocytoma. Answers A and C are incorrect because hypercellularity and macronucleoli are 2 of several criteria used for the diagnosis of an atypical meningioma but are not used to evaluate for atypical central neurocytoma. Answer B is incorrect because lipomatous differentiation may be seen in rare cases of central neurocytoma but does not confer an atypical designation.
Comment Here
Reference: Central neurocytoma
Comment Here
Reference: Central neurocytoma