Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Redding-Ochoa J, Morris M. Ischemic stroke / infarct. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnsstroke.html. Accessed March 31st, 2025.
Definition / general
- Episode of acute neurological dysfunction caused by focal cerebral, spinal cord or retinal infarction (Stroke 2013;44:2064)
- CNS infarction is defined as cell death due to ischemic injury in the CNS based on imaging, neuropathology or clinical assessment
Essential features
- Brain infarcts are common and the leading cause of neurologic disability worldwide
- Commonly associated with hypertension, cardioembolic events and atherosclerosis
- Infarct morphology depends on the interval between stroke onset and death
- Acute changes → blurring of gray-white matter junction (gross), edema and red neurons (micro)
- Subacute → cracking artifact (gross), dense macrophage infiltration and neovascularization (micro)
- Chronic → cavitated lesions (gross) with macrophages and surrounding gliosis (micro)
Terminology
- Brain / spinal cord infarct
ICD coding
Epidemiology
- Leading cause of neurologic disability worldwide (Lancet Neurol 2019;18:459)
- 11.5 - 13.6 million strokes per year worldwide, with the majority being ischemic stroke (Lancet Glob Health 2013;1:e259, Lancet Neurol 2019;18:459)
- Estimated 795,000 ischemic strokes per year in the United States (Circulation 2022;145:e153)
- 2.8 - 5.5 million deaths worldwide per year (Lancet Glob Health 2013;1:e259, Lancet Neurol 2019;18:459)
- Nonmodifiable risk factors include
- Increasing age (Lancet 2014;383:245)
- Male sex, with 133 male cases versus 99 female cases per 100,000 person years (Circ Res 2017;120:439)
- Rare genetic causes of stroke, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) or its autosomal recessive counterpart, CARASIL
- Modifiable risk factors account for 91.5% of population attributed risk and include (Lancet 2010;376:112)
- Hypertension, the strongest risk factor (odds ratio [OR] 3.14, 99% confidence interval [CI] 2.67 - 3.71)
- Sedentary lifestyle, diet, obesity, smoking, psychosocial stress, depression, diabetes, high alcohol consumption, high ApoB:ApoA1 ratio, cardiac disease
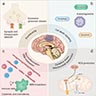
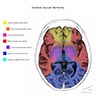
Sites
- Ischemic stroke can affect any site in the central nervous system
- Middle cerebral artery (MCA) is the most affected vessel in the brain by thromboembolism (e.g., internal carotid atherosclerotic plaque fragmentation, atrial fibrillation) (StatPearls: Neuroanatomy, Middle Cerebral Artery [Accessed 7 July 2023])
- Large vessel ischemic strokes occur within the vascular territory supplied by the large artery affected
- Large vessel strokes are commonly secondary to atherosclerosis, either with a local thrombus or embolic event (Nat Rev Dis Primers 2019;5:70)
- Internal carotid artery is the most frequently affected in western countries
- Intracranial atherosclerosis accounts for 30 - 50% of ischemic strokes in Asian populations
- Aortic arch, neck and intracranial vessels are common embolic sources
- Plaques are located predominantly at branching points, bifurcations and vessel curvatures (e.g., tip of the basilar artery, close to the bifurcation of the common carotid artery) (AJR Am J Roentgenol 2000;174:1657)
- Arterial dissection often occurs in extracranial carotids and vertebral arteries (Nat Rev Dis Primers 2019;5:70)
- Strokes from small vessel disease classically involve perforating arteries that supply deep subcortical (e.g., basal ganglia, thalamus) and brainstem structures (Nat Rev Dis Primers 2019;5:70)
- Infarcts due to more global decreases in blood flow or blood pressure (hypotension, hypoperfusion) affect regions at the border between arterial territories (watershed infarcts)
- Atherosclerosis and embolic events can contribute to watershed type infarcts (Stroke 2006;37:841)
- Full term or mature infant brains show injury predominantly in the neocortex (i.e., laminar necrosis), including the watershed regions, basal ganglia and thalamus, subcortical white matter and brainstem (e.g., midbrain tectum) (Ann Neurol 1987;21:202, Pediatr Clin North Am 1993;40:1061)
- In preterm or immature infant brains with hypoxic ischemic injury, the most affected regions are
- Periventricular (e.g., periventricular leukomalacia) (Semin Pediatr Neurol 2009;16:167)
- Basis pontis and subiculum, either independently or in association (i.e., pontosubicular necrosis) (Neuropediatrics 1993;24:204)
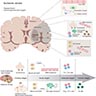
Pathophysiology
- Liquefactive necrosis is the major type of necrosis in the brain
- Occurs via release of enzymes that digest the cells' components, although the exact mechanism in the brain is not elucidated yet (StatPearls: Cell Liquefactive Necrosis [Accessed 7 July 2023])
- Ischemic stroke results from insufficient blood flow to an area of the central nervous system, resulting in cell death
- Blood flow can be decreased due to occlusion of a vessel, most commonly from thromboembolic events (Nat Rev Dis Primers 2019;5:70)
- Atherosclerosis: include in situ arterial obstruction of blood flow or production of embolic fragments derived from unstable atherosclerotic plaques
- Emboli travel to distal vascular territories, obstruct the blood supply and cause ischemia; necrosis ensues when the collateral blood supply is insufficient to meet the tissue's metabolic demands (e.g., end arteries)
- Atherosclerotic occlusion in the parent vessel of a perforator artery can be a cause of lacunar infarct (Stroke 2010;41:2822)
- Cardiac disease: atrial fibrillation, flutter, patent foramen ovale, infective endocarditis, hypokinetic heart segment with associated thromboembolism
- Atrial fibrillation and flutter cause blood to stagnate in the atria, inducing clots and thromboembolism with downstream obstruction of CNS vessels
- Atherosclerosis: include in situ arterial obstruction of blood flow or production of embolic fragments derived from unstable atherosclerotic plaques
- Mild to moderate ischemia or short duration may result in selective neuronal necrosis
- Severe or long duration of the ischemic insult results in necrosis, irrespective of cell type
- Impaired cerebral flow autoregulation in preterm infants is closely associated with periventricular leukomalacia and intraventricular hemorrhage, the most common forms of preterm encephalopathies (Pediatrics 2000;106:625)
- In preterm infants, the timing and severity of the ischemic insults along pregnancy are major determinants of the neuropathological picture and clinical outcomes (Clin Perinatol 2014;41:1)
Etiology
- Most frequently associated with atherosclerosis, cardiac pathology and hypertension (Nat Rev Dis Primers 2019;5:70)
- Less common causes of ischemic infarcts include
- Vasculitis
- Coagulation disorders
- Arterial dissection
- Reversible vasoconstriction syndrome
- HIV associated arteriopathy
- Moyamoya disease
- Fat embolism
- Ischemic insults in utero are the most common cause of cerebral injury among preterm / very low body weight infants (Clin Perinatol 2014;41:1)
Diagrams / tables
Clinical features
- Mostly dependent on the location of the ischemic brain tissue
- Common symptoms include
- Contralateral hemiparesis (unilateral weakness of the upper and lower limbs, opposite to the site of the infarct)
- Hemianesthesia (absence of unilateral sensations)
- Aphasia (inability to understand or to express oral language; lesion at the dominant hemisphere)
- Different clinical syndromes are associated with the occlusion of major cerebral arteries (QJM 2013;106:607)
- Middle cerebral artery: presentation depends on whether the ischemic lesion affects the dominant or nondominant hemisphere
- Dominant hemispheric lesions: aphasia, motor and sensory alterations; complete hemiplegia if internal capsule is involved
- Nondominant hemispheric lesions: neglect, anosognosia, motor and sensory deficits, homonymous hemianopia
- Anterior cerebral artery: motor or sensory deficit (leg > face, arm), primitive reflexes (i.e., grasp and sucking, abulia, gait apraxia)
- Posterior cerebral artery: homonymous hemianopia, alexia without agraphia (dominant hemisphere), visual hallucinations, sensory loss, choreoathetosis, spontaneous pain in thalamic lesions
- Vascular dementia: any type of dementia that results from cerebrovascular lesions (i.e., infarcts)
- 2 main clinical syndromes are recognized (Clin Sci (Lond) 2017;131:1059)
- Poststroke vascular cognitive impairment
- Cognitive alterations as an immediate result of a known stroke
- Vascular cognitive impairment without recent stroke
- Cognitive impairment is the result of infarcts that were not detected in the acute setting and tissue injury is documented by imaging or neuropathology
- No alternative causes of dementia explain the observed cognitive decline
- Poststroke vascular cognitive impairment
- 2 main clinical syndromes are recognized (Clin Sci (Lond) 2017;131:1059)
Diagnosis
- Diagnosis of ischemic stroke is made by clinical and imaging findings
- Noncontrast CT
- Primary imaging modality for acute assessment in most centers (Lancet 2018;392:1247)
- Rapid, accessible and highly sensitive diagnosis of intracranial hemorrhage (major contraindication for recanalization therapy)
- Sensitivity is suboptimal (64%) for early ischemic infarct signs in lesions that are < 6 hours from onset (Radiology 2001;219:95)
- CT angiography
- More sensitive for early infarct signs compared to noncontrast CT (Radiology 2007;244:541)
- Used for characterization of collateral vasculature and the occlusive thrombi at extracranial and intracranial arteries (Br J Radiol 2018;91:20170573)
- MRI
- Diffusion weighted MRI: most accurately measures the infarct core (i.e., nonsalvageable tissue) (Radiology 2009;251:627)
- Perfusion MRI
- PET
- Gold standard for viable (i.e., penumbra) identification
- Replaced by diffusion weighted imaging due to long processing time and radioactivity exposure (Front Cardiovasc Med 2022;9:861913)
- Vascular dementia
- Diagnostic criteria for neuropathological validation are lacking
- Features of vascular lesions involved in development of dementia are number, volume, location and origin (Acta Neuropathol 2016;131:659)
- Noncontrast CT
Laboratory
- Coagulation tests (i.e., aPTT, PT, TT) are relevant to rule out coagulation disorders (contraindication for thrombolysis)
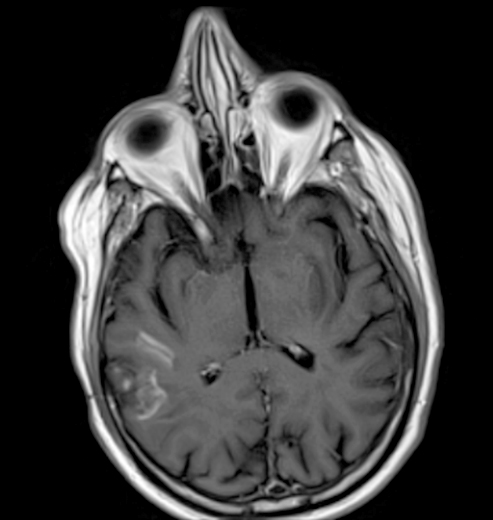
Radiology description
- CT unenhanced
- Early / acute signs: loss of definition between gray and white matter, cortical hypoattenuation and effacement of the sulci (Radiology 2005;235:444)
- Loss of the insular cortex in acute middle cerebral artery (MCA) infarcts (Radiology 1990;176:801)
- Hyperdensity of the MCA (i.e., hyperdense artery sign) is observed in 30 - 40% of patients with acute MCA infarcts (Stroke 1992;23:317)
- Chronic infarcts show cavitation sometimes with mineralization
- MRI
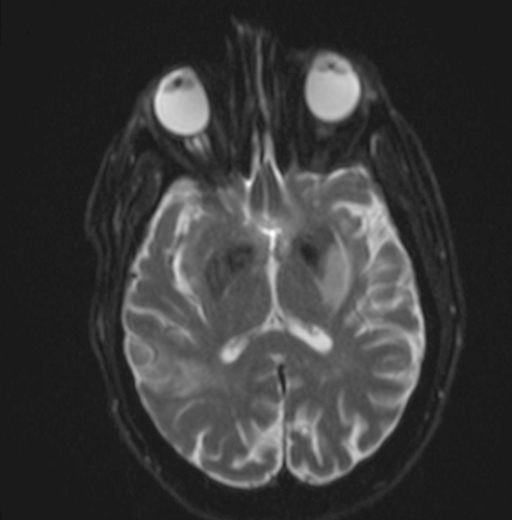
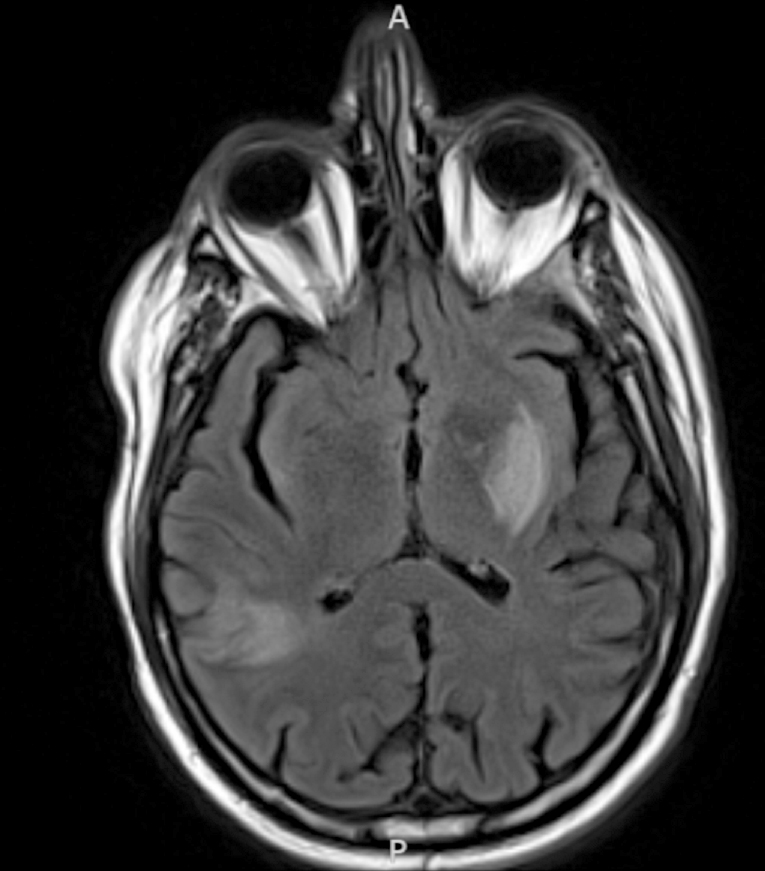
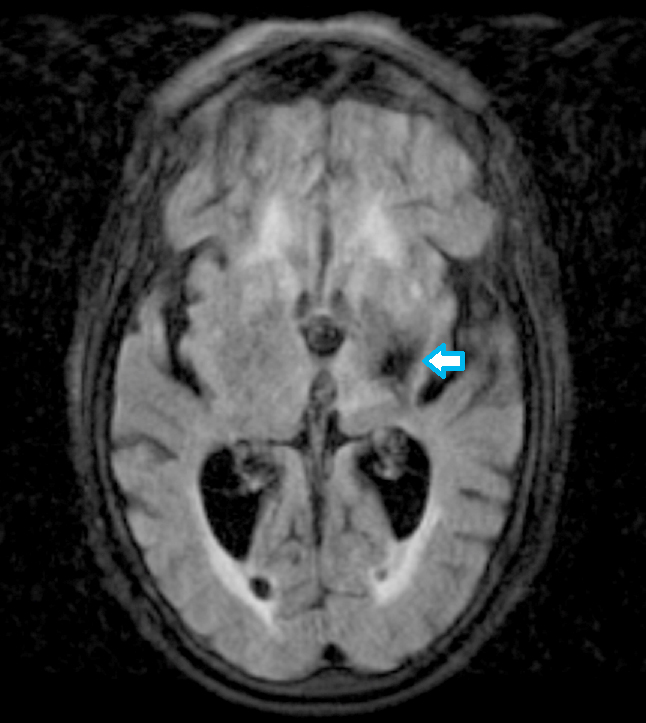
- Diffusion weighted imaging (DWI): hyperintense signal in the acutely infarcted tissue; low signal on apparent diffusion coefficient (ADC) map
- Infarcts are often T2 hyperintense
- Collateral flow on FLAIR: linear or serpentine hyperintensities distal to the site of obstruction (Neurology 2009;72:1134)
- Perfusion weighted imaging (PWI) - DWI mismatch: used to estimate the salvageable tissue (i.e., penumbra) in acute infarct (Neuroimaging Clin N Am 2021;31:177, AJNR Am J Neuroradiol 2015;36:32)
- Ischemic areas on PWI are compared with areas shown by DWI (i.e., the infarct core or nonsalvageable tissue); the mismatch represents the volume of salvageable tissue with reperfusion therapy
- Chronic infarcts show absence of contrast enhancement in strokes older than 4 months (Radiographics 2012;32:1285)
Radiology images
Prognostic factors
- Age at presentation and degree of neurologic impairment are major predictive factors in the acute setting (Neurology 2011;77:965, Neurology 1999;53:126)
- Infarct volume and location assessed by neuroimaging must be considered for prognostic assessment
- Stroke due to acute lesions of the proximal internal carotid, basilar or major intracranial arteries show an increased risk for a poor outcome (Stroke 2011;42:2419, Arch Neurol 2006;63:1287, Stroke 2013;44:1310)
Case reports
- 25 year old woman with postpartum ischemic stroke (Cureus 2020;12:e9975)
- 37 year old woman with Percheron artery embolic infarct (Ann Neurosci 2016;23:124)
- 79 year old man with acute ischemic stroke and COVID-19 infection (Diagn Pathol 2020;15:78)
Treatment
- 2 common forms of treatment for acute ischemic stroke available if within 6 - 8 hours of onset (Neurologia 2014;29:102)
- Systemic thrombolysis
- Thrombectomy
- Neuroimaging guides decision making for thrombolysis in patients with an unknown time of onset
- Selection of patients is based on recognition of a mismatch between DWI and FLAIR at the site of the infarct (N Engl J Med 2018;379:611, Int J Stroke 2016;11:260)
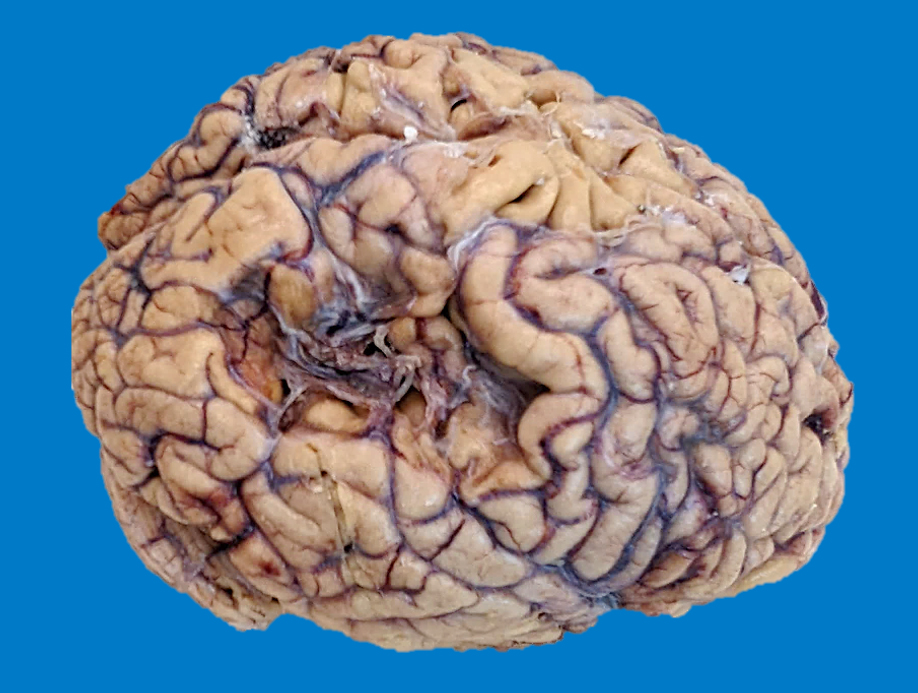
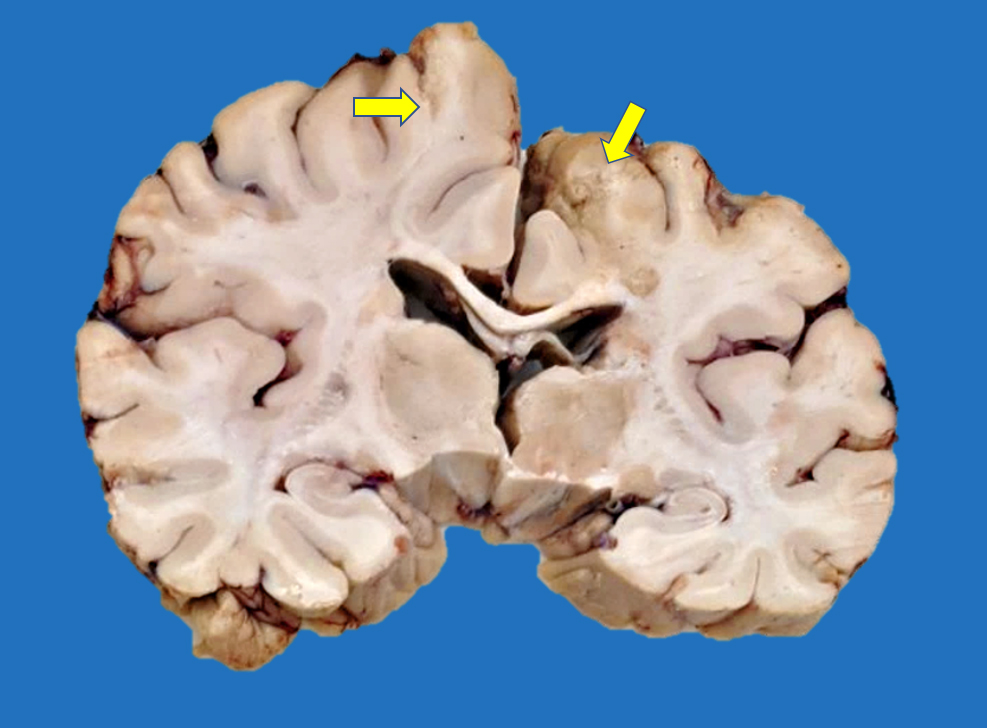
Gross description
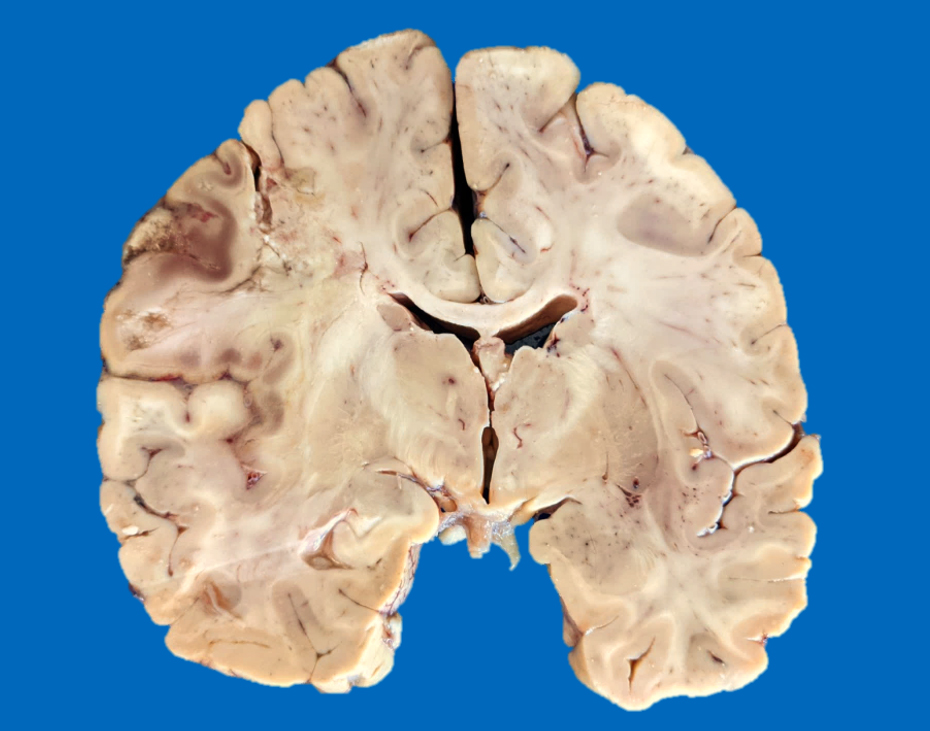
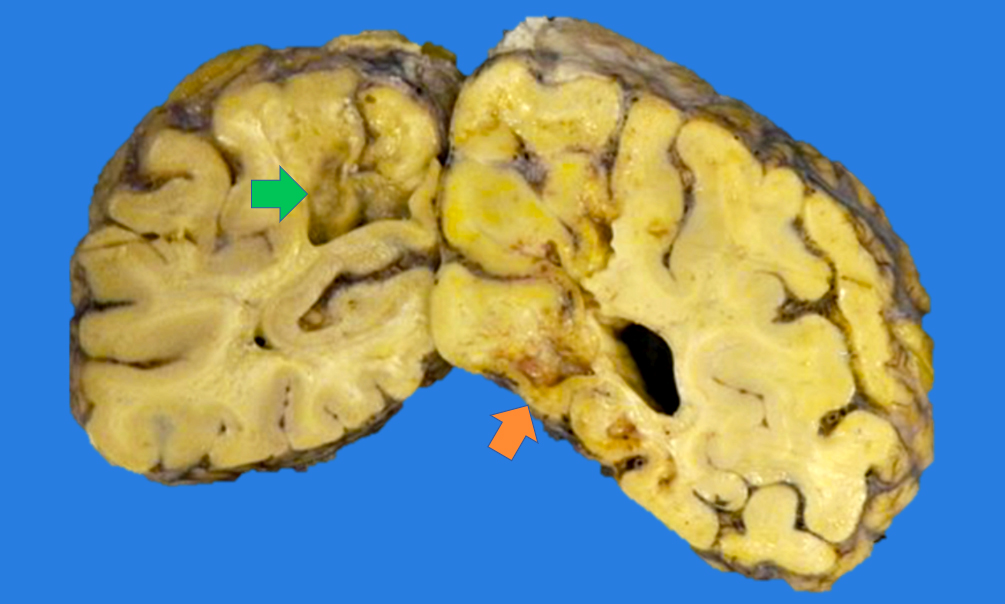
- Time between stroke and death is a major determinant of the gross features
- Completely accurate dating of an infarct based on the gross features is not possible, especially cavitated lesions
- < 8 hours to 48 hours
- Undetectable if the infarct occurred < 8 hours before death
- Congestion of gray matter and edema (Histopathology 2011;58:333)
- Dusky discoloration and blurring of the gray-white matter junction
- Ill demarcated borders
- Reperfusion of an ischemic lesion where vessels are also affected can become hemorrhagic (Stroke 1986;17:586)
- Hemorrhagic discoloration can be limited to a specific vascular territory
- Commonly observed as secondary infarcts in the setting of brain herniations (e.g., obstruction of pericallosal artery in subfalcine herniation, obstruction of posterior cerebral artery in uncal herniation)
- 2 days to a few months (Histopathology 2011;58:333)
- Marked tissue softening
- Cracking artifact: demarcates the necrotic area
- Tissue edema (e.g., midline shift, narrowing of the sulci, convexity flattening)
- Dusky discoloration and blurring of gray-white matter junction
- Cerebellar infarcts show effacement of the folia
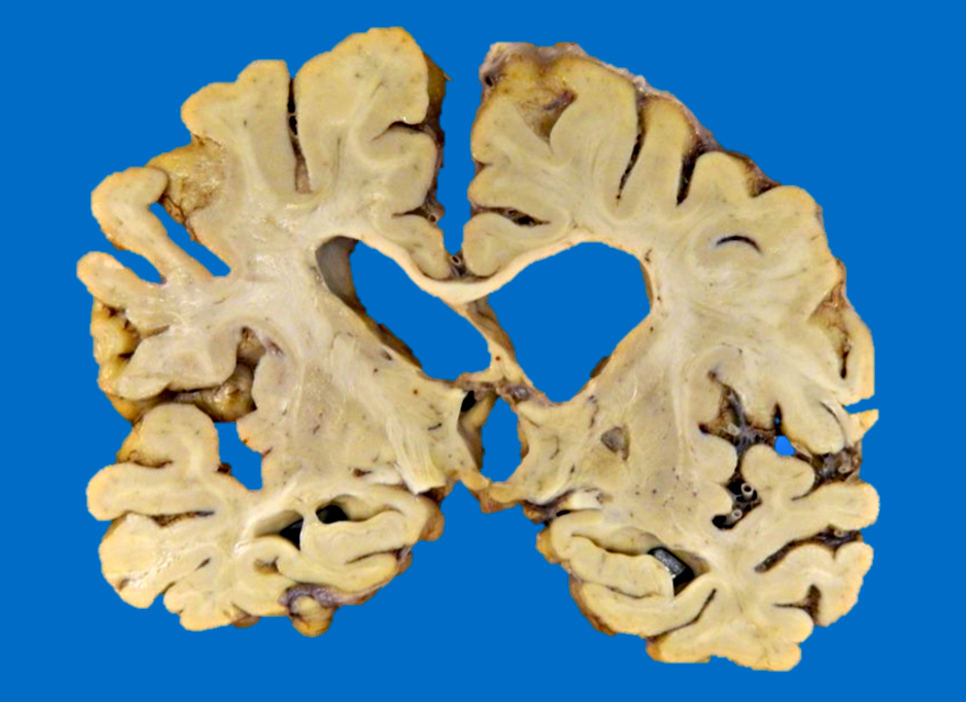
- Chronic (months - years)
- Cavitation (i.e., cystic infarct)
- Thin cortical remnant overlying the cavitation
- In long term survivors of severe global hypoxic ischemic encephalopathy, a markedly thin cortex can be observed due to laminar necrosis
- Lateral ventricle asymmetry can be seen (i.e., ex vacuo ventricular dilation of the affected hemisphere)
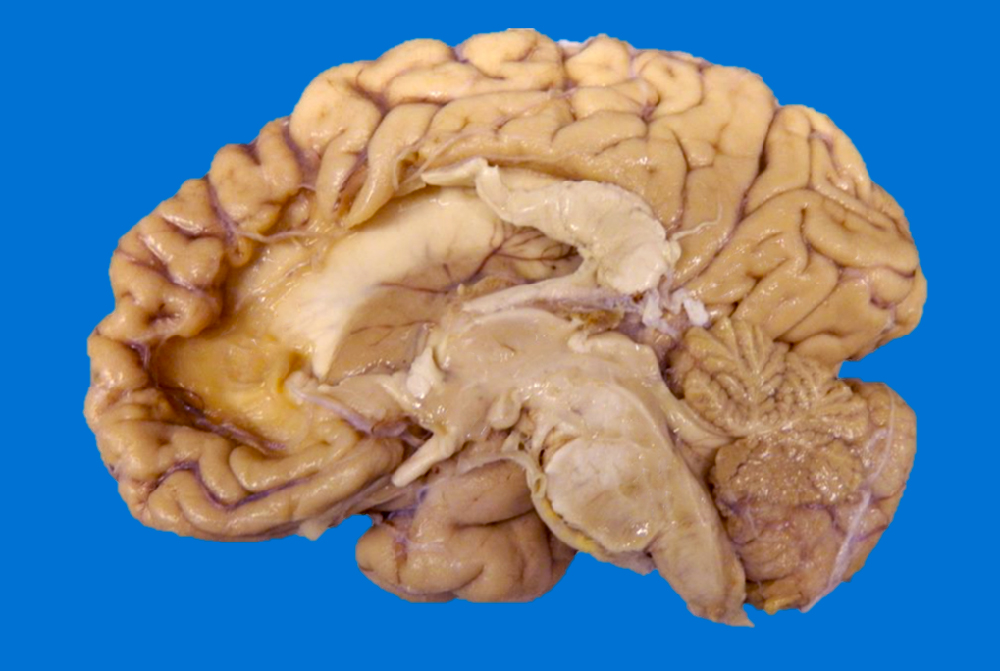
- Anterograde loss white matter volume due to an ischemic insult (e.g., atrophy of the ipsilateral cerebral peduncle and pyramid after an MCA infarct with degeneration of the corticospinal tract), ipsilateral brainstem atrophy after extensive supratentorial stroke (corticospinal tract and frontopontine fiber degeneration) and subsequent contralateral cerebellar atrophy due to transsynaptic degeneration (AJNR Am J Neuroradiol 2008;29:354, Eur Radiol 2006;16:592)
- Postinfarct anterograde white matter loss of volume occurs due to Wallerian degeneration or transsynaptic degeneration (AJR Am J Roentgenol 1998;171:813)
- Lacunar infarct: arbitrarily defined as cystic infarcts < 10 mm in length
- Predominantly found at basal ganglia, internal capsule, pons
- Binswanger disease
- White matter structures, including corpus callosum, corona radiata, internal capsule and anterior commissure shows marked loss of volume, discoloration, softening and granular texture predominating in the periventricular areas (Neurology 1995;45:626, J Neurol Sci 2010;299:9)
- Lacunar or large infarcts are frequently observed
- Cerebellar white matter is also commonly affected
- Associated with vascular dementia
Gross images
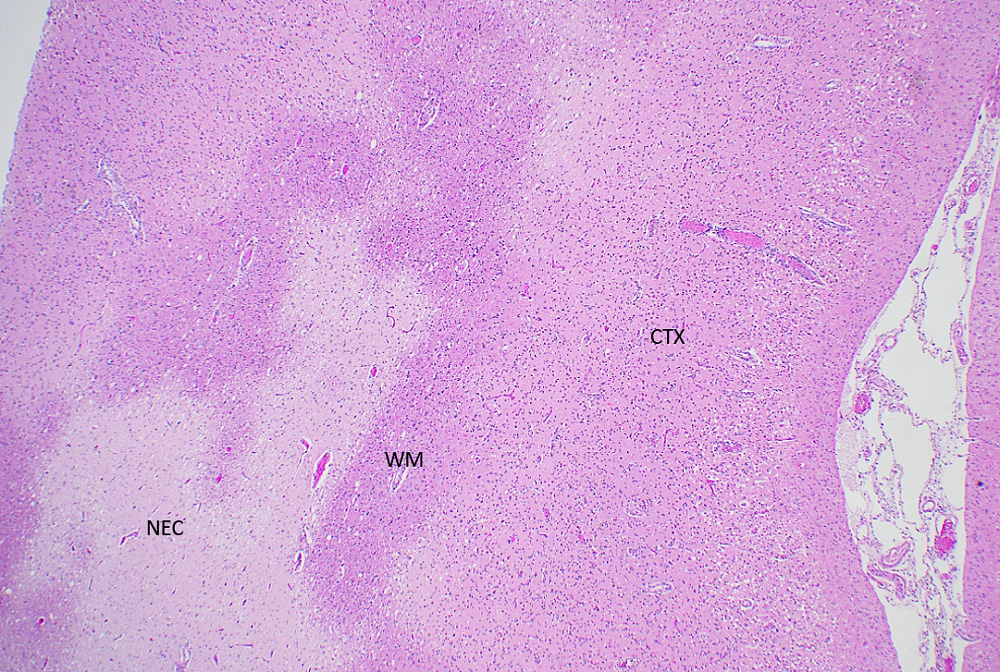
Microscopic (histologic) description
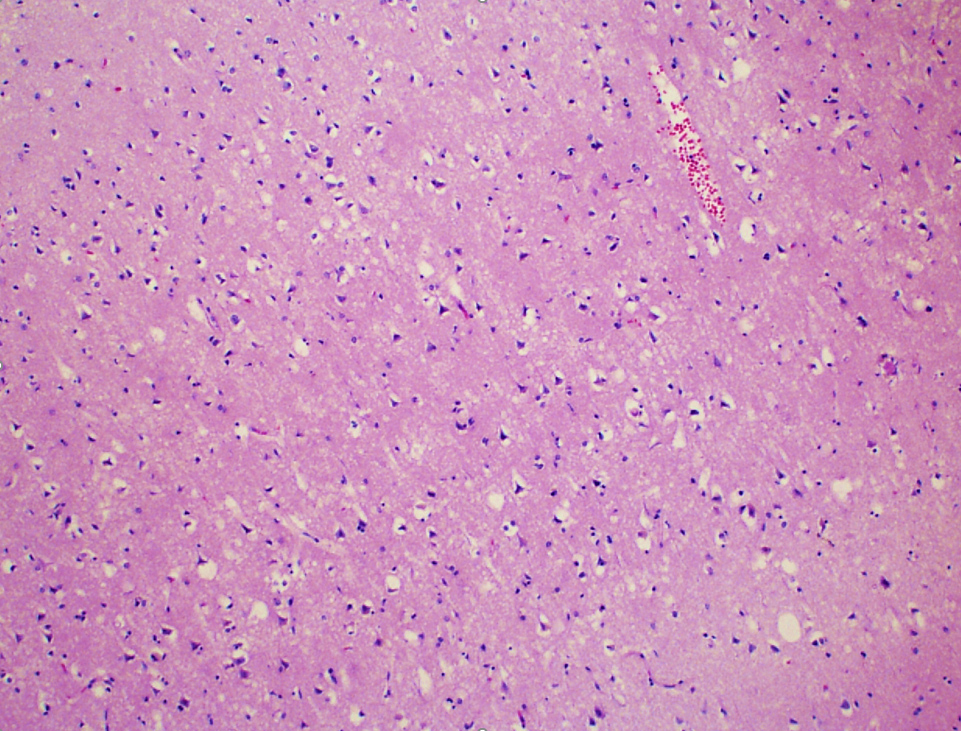
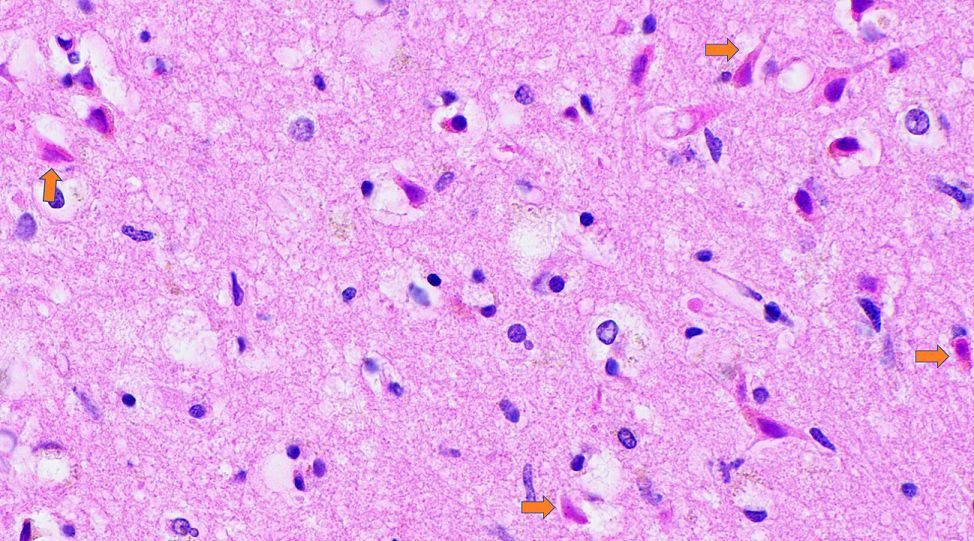
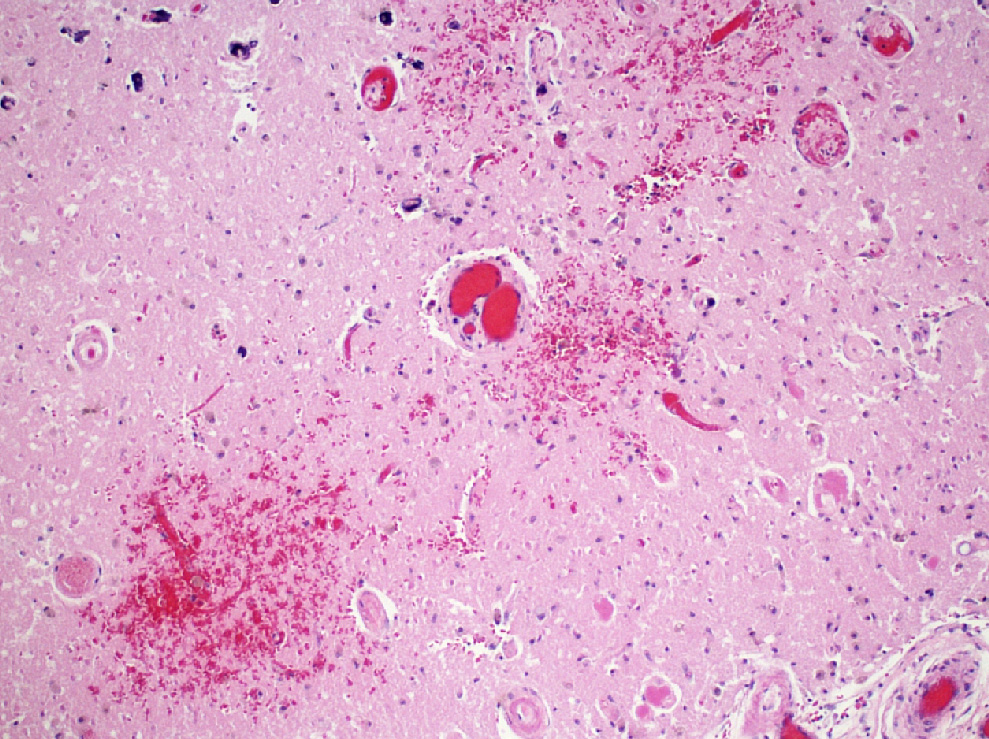
- Acute infarct (1 - 4 days) (Histopathology 2011;58:333)
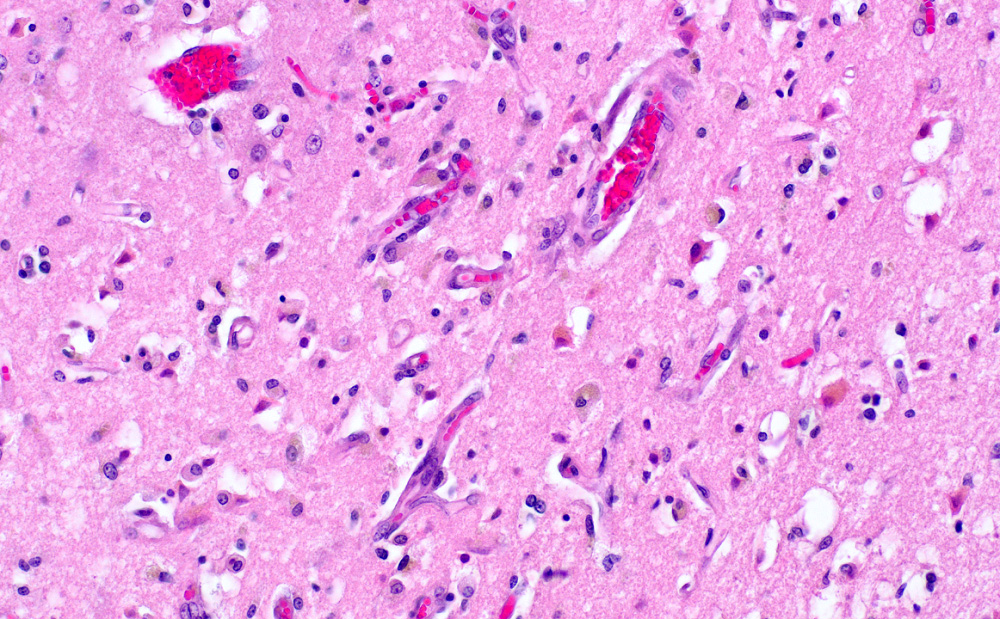
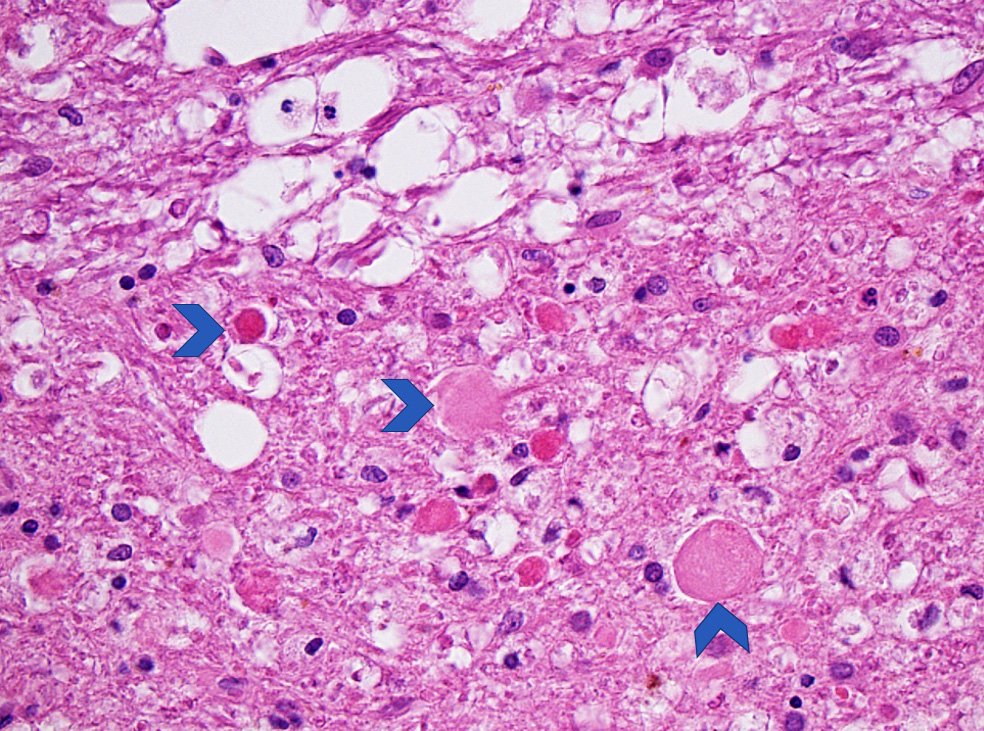
- Neuronal changes: hypereosinophilic perikaryon (red dead neurons), cell body shrinkage, pyknosis (i.e., nuclear hyperchromasia), loss of demarcation of the nuclear features in later phases
- Neurons are more susceptible to ischemia than glia
- Neuropil vacuolation (i.e., tissue edema)
- May have some neutrophilic infiltrate
- Pannecrosis: all cell populations (i.e., neurons, glia, blood vessels) are necrotic; dead cells remain visible as hypereosinophilic structures that preserve the cell and nuclear outlines (pale neurons or "ghosts")
- Laminar necrosis
- Occurs due to variable susceptibility to hypoxia among the cortical histological layers
- In the mature brain, the most vulnerable neurons are located in cortical layers III, V, VI, Purkinje cells in the cerebellum and pyramidal cells of CA1 field in the hippocampus proper
- Geographic necrosis: liquefied necrotic tissue is well demarcated from the adjacent viable tissue
- Neuronal changes: hypereosinophilic perikaryon (red dead neurons), cell body shrinkage, pyknosis (i.e., nuclear hyperchromasia), loss of demarcation of the nuclear features in later phases
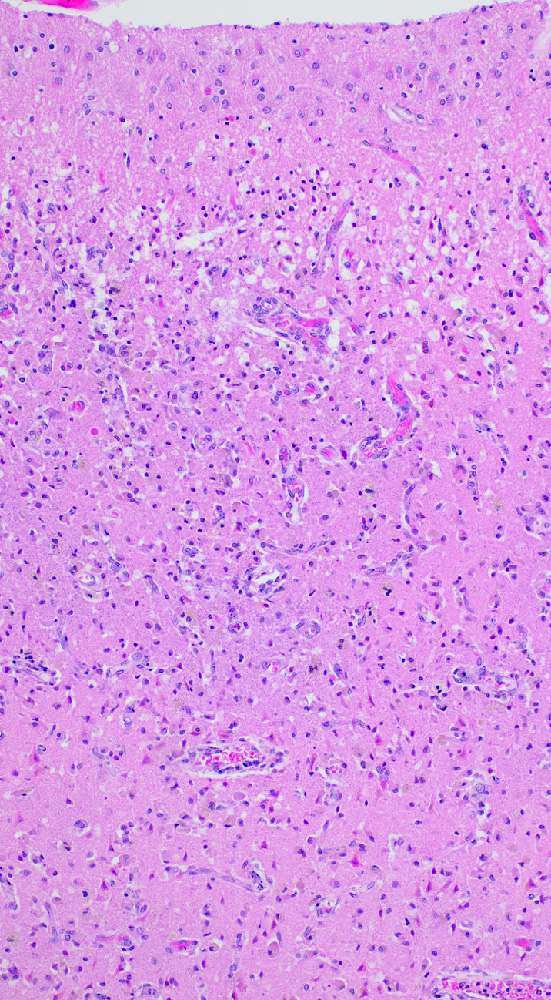
- Subacute (5 - 14 days)
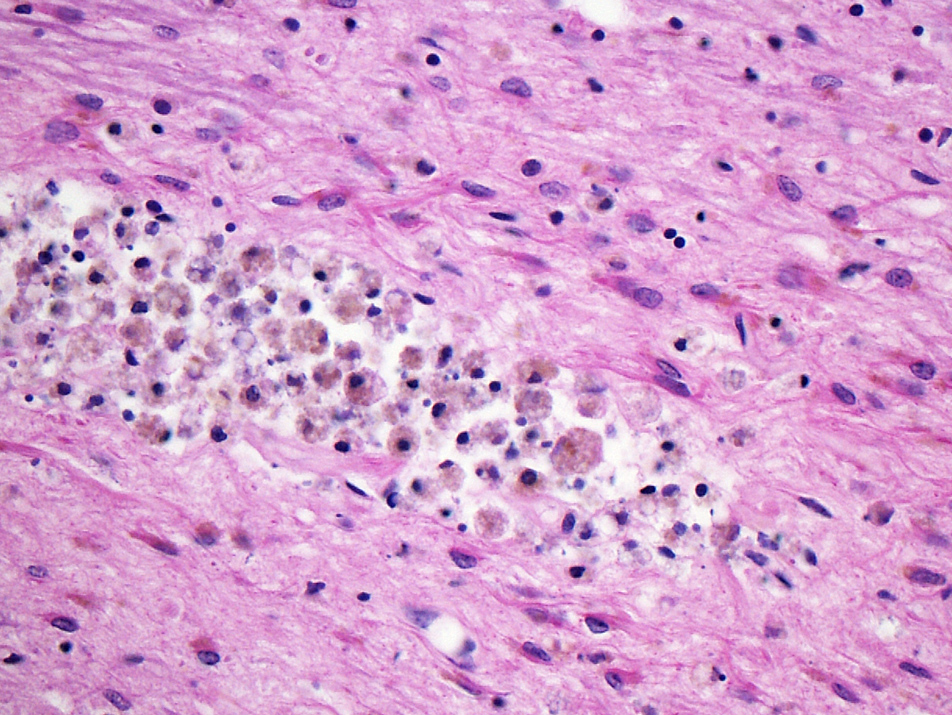
- Dense macrophage infiltration and scattered siderophages
- Variable neutrophilic infiltration
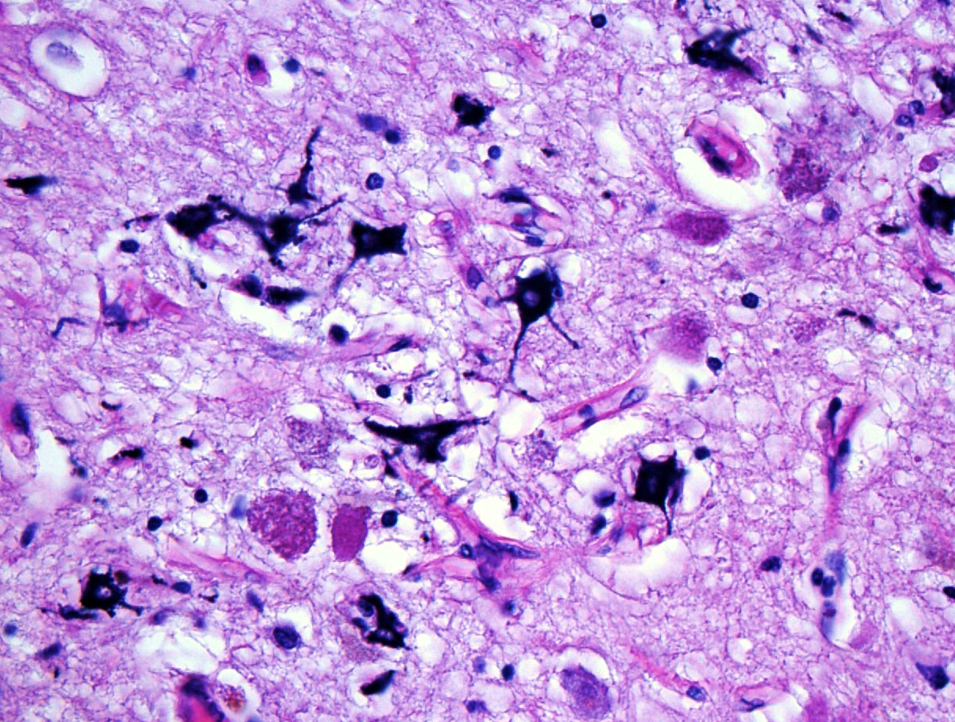
- Peripheral reactive astrocytosis and microglial activation (i.e., rod shaped microglia)
- Hypereosinophilic neurons are still present in gray matter
- Neovascularization of necrotic tissue and reactive endothelial cells
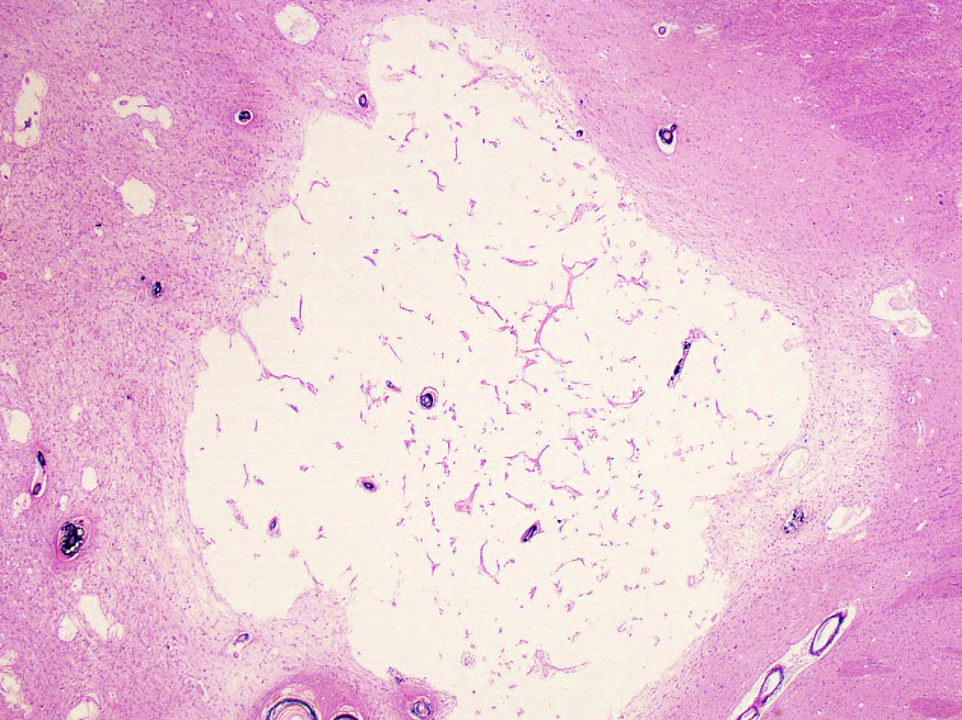
- Chronic (15 days - years)
- Cavitated lesion with vessels and macrophages surrounded by a glial scar
- Reactive astrocytes in the edge of the cavitation
- Can be piloid gliosis with Rosenthal fibers, particularly in brainstem infarcts
- Scattered hemosiderin laden macrophages typically present
- Axonal balloons can occur in all phases of ischemic injury (i.e., dilation of axons indicate injury with subsequent defective axonal transport)
- Neuronal ferrugination occurs occasionally in subacute to chronic infarcts, characterized by perykaryal mineralization (i.e., prominent basophilia)
- Vascular dementia
- Diagnosis is based on the absence of a primary neurodegenerative disease known to cause dementia (e.g., Alzheimer disease, Lewy body dementia)
- Multivariable model based likelihood showed that the presence of 1 or 2 microinfarcts on routine neuropathological sections indicates a burden of hundreds of microinfarcts in that brain (Neurology 2013;80:1365)
- Binswanger disease
- Multiple infarcts, typically chronic, with associated white matter rarefaction, myelin loss, loss of oligodendroglia and reactive gliosis
- Subcortical U fibers are usually spared
- Penetrating arteries' walls are markedly thickened and hyalinized with enlarged perivascular spaces
- CAA and CADASIL are uncommonly associated as the underlying cause of Binswanger disease (Neurology 1995;45:626, J Neurol Sci 2010;299:9)
- Reference: J Neuropathol Exp Neurol 1993;52:481
Microscopic (histologic) images
Positive stains
- CD68: highlights macrophages and microglia in subacute and chronic infarcts
- GFAP: positive in reactive astrocytes
- CD61: highlights platelets and thrombi in blood vessels (Histopathology 2011;58:333)
- Neurofilament protein: highlights axonal spheroids in the vicinity of the lesion indicating axonal injury; shows loss of staining in the infarcted areas
Negative stains
- Luxol fast blue: shows loss of myelination associated with the necrotic area; subacute infarcts show loss of axons in the affected area (see demyelination in Differential diagnosis)
- Special stains and immunostains for infectious etiologies are negative
Videos
Brain old infarcts versus cerebral abscess
Brain infarcts tutorial
Sample pathology report
- Brain, autopsy:
- Acute infarct, 10.5 x 6 x 4 cm, left frontal, temporal, parietal lobes (see comment)
- Gross description: External examination of the cerebrum shows a 10.5 x 6 cm area with marked vascular congestion, narrowing of the sulci and softening extends from the left frontal lobe to the superior temporal lobe and parietal lobe. The area of softening spares the superior frontal gyrus. At the base of the brain, severe atherosclerosis is observed in the left middle cerebral artery with > 90% of stenosis.
- On coronal sections, this area is characterized by mild dusky discoloration in the cortical ribbon and in the subjacent white matter with blurring of the gray-white matter junction measuring up to 4 cm in depth. The adjacent left lateral ventricle shows decreased caliber compared with the right lateral ventricle.
- Microscopic description: Low power examination of the frontal, parietal and temporal cortices show marked vacuolation of the neuropil and pericellular retraction artifact. On high power, the neurons are remarkable for cell shrinkage, hypereosinophilic perykarya and loss of demarcation of the nuclear contours. The subjacent white matter is pale with vacuolization and poorly demarcated from the gray matter.
- Comment: The observed distribution of the infarct corresponds to the left MCA vascular territory.
Differential diagnosis
- Putrefaction / "Swiss cheese" artifact:
- Foul smell
- Multiple cavitations not limited to vascular territories and commonly in deep gray matter
- Bacterial colonies without accompanying inflammation
- Cerebral contusions:
- History of trauma
- Coup / countrecoup lesions are primarily located in the orbitofrontal cortices, frontal and temporal poles
- Wedge shaped hemorrhagic lesions with the widest area oriented to the pial surface
- Old contusions: brown, orange (i.e., hemosiderin) discoloration in the rim of the lesion, no superficial cortical remnant
- History of trauma
- Abscess:
- Typically gray-white matter junction
- Well defined borders often with fibrotic rim
- Necrotic areas have abundant acute and chronic inflammation
- Infectious organisms may be present
- Infiltrating glioma:
- Not in the distribution of a vascular territory
- Ill defined lesions which expand and distort brain anatomy
- Hypercellular glial lesion on histology without signs of neuronal ischemic changes
- Mitotic activity and glomeruloid vascular proliferation common in high grade gliomas
- Demyelination (e.g., multiple sclerosis):
- Well demarcated foci of white matter discoloration
- Classically seen around the ventricular system, though Luxol fast blue staining can show demyelination affecting white and gray matter
- Not limited to a specific vascular territory
- Microscopic features depend on the plaque's state of activity
- Inactive: pallor, loss of oligodendrocytes, reactive astrocytes
- Active: venules with mononuclear perivascular cuffing and relative preservation of axons within the lesion
Board review style question #1
Board review style answer #1
B. Hypereosinophilic cytoplasm and nuclear hyperchromasia. The earliest morphological abnormalities observed by H&E are hypereosinophilic cytoplasm and loss of nuclear normal staining features. In the earliest phase of the infarct, the nuclei are hyperchromatic and pyknotic. In later stages, the nuclear chromatin disappears and the nuclei become eosinophilic, giving the impression that it merges with the surrounding eosinophilic cytoplasm. These changes can be observed also with hypoglycemia or excitotoxicity. Answer D is incorrect because mineralization is more associated with subacute to chronic changes in an infarct, rather than acute. Answer A is incorrect because while cell swelling can occur in the setting of acute ischemia, this change is not specific and when it occurs in ischemia it is potentially reversible. Answer C is incorrect because karyorrhexis is not associated with acute neuronal ischemia in adults, though it can be seen in ischemic injury in immature or premature fetal and neonatal acute infarcts.
Comment Here
Reference: Ischemic stroke / infarct
Comment Here
Reference: Ischemic stroke / infarct
Board review style question #2
Which of the following are the most common locations for lacunar infarcts?
- Basal ganglia and pons
- Frontal lobe and hippocampus
- Medulla and cerebellum
- Spinal cord and mamillary bodies
Board review style answer #2
A. Basal ganglia and pons. Lacunar infarcts, defined by < 10 mm in length, are most commonly seen in the diencephalic regions (i.e., basal ganglia, thalamus) and in the brainstem, particularly in the pons and deep white matter (e.g., the internal capsule). These regions share in common that penetrating arteries supply the blood flow in these territories and hypertensive arteriopathy produces damage frequently in penetrating arteries and arterioles. Answers B, C and D are incorrect because although these sites may show lacunar infarcts, they are observed less frequently than lacunar infarcts in the basal ganglia and pons.
Comment Here
Reference: Ischemic stroke / infarct
Comment Here
Reference: Ischemic stroke / infarct