Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Burns DK. Abscess. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cnsabscess.html. Accessed March 27th, 2025.
Definition / general
- Localized, space occupying lesion within the brain composed of purulent (neutrophil rich) exudate and other inflammatory cells reacting to the presence of bacteria within the CNS parenchyma
- Can also be caused by nonbacterial pathogens, including fungi, protozoa and helminths
Essential features
- Localized, pyogenic infections of brain parenchyma that occur via direct spread of contiguous infections, hematogenous spread or CNS trauma
- Streptococcus spp. are the most common pathogens, although a wide range of organisms can cause disease; culture negative cases are not uncommon
- Pathogenesis involves binding of microorganism to Toll-like receptors (TLRs), with subsequent release of proinflammatory molecules, blood brain barrier disruption, activation of astrocytes and resident microglia and influx of inflammatory cells
- Radiographic imaging, particularly CT and MRI, is critical to accurate diagnosis; classical appearance is that of ring enhancing lesions with edema and mass effect
- Biopsy with culture of the abscess provides the most specific diagnostic information; no other specific laboratory tests
Terminology
- Synonyms include cerebral abscess and intracranial abscess
- Localized collections of purulent exudate in adjacent extra-axial regions (i.e., subdural or epidural spaces) are more accurately referred to as empyemas
ICD coding
- ICD-10: G06.0 - intracranial abscess and granuloma
Epidemiology
- Prevalence 0.4 - 0.9 per 100,000 population in developed countries
- Male predominance (2 - 3:1)
- Most common between third and fifth decades of life
- Risk factors include bacteremia, intravenous drug abuse, bacterial endocarditis, congenital cardiac defects with right to left shunts, hereditary hemorrhagic telangiectasia with pulmonary arteriovenous malformation, bronchiectasis, intra-abdominal infections, lung abscesses, paranasal sinus and ear infections, dental infections and immunosuppression (Neurohospitalist 2014;4:196)
- Source of infection never identified in some cases (cryptogenic abscesses) (Clin Microbiol Infect 2017;23:614)
Sites
- Can occur anywhere but most common in frontal and temporal lobes; cases associated with sinusitis most commonly involve frontal lobes and cases associated with otitis typically involve temporal lobes and much less commonly cerebellum (Neurology 2014;82:806, Neurohospitalist 2014;4:196)
- Hematogenous abscesses favor middle cerebral artery territory and are more likely to be multiple (Int J Surg 2011;9:136)
- Hematogenous abscesses typically located at gray-white junctions or in deeper cerebral white matter
Pathophysiology
- Microorganisms reach brain parenchyma through 1 of 3 routes: hematogenous dissemination, extension from contiguous sites of infection and implantation via CNS trauma (including neurosurgical procedures)
- Initial reaction to organisms involves binding of Toll-like receptors (especially TLR2 and TL4) to components of bacterial cell wall
- TLRs interact MyD88, a cytoplasmic protein that plays a central role in the activation of the innate immune response
- Activation of MyD88 dependent signaling pathways results in release of a host of proinflammatory cytokines and chemokines (including IL1 and TNF), blood brain barrier disruption, upregulation of ICAM1 and other adhesion molecules
- Proinflammatory molecular pathways activate astrocytes and resident microglia and trigger an influx of neutrophils, followed by recruitment macrophages and T lymphocytes
- References: J Neuroinflammation 2004;1:16, J Immunol 2007;178:4528, J Neuropathol Exp Neurol 2005;64:27, Am J Pathol 2008;172:132
Etiology
- Common infectious agents include Streptococcus spp. (particularly S. milleri), Staphylococcus aureus, Enterobacteriaceae and Bacteroides and other anaerobic spp.
- Polymicrobial infections common in cases associated with dental infections, paranasal sinus and otic infections and trauma
- References: Clin Microbiol Infect 2017;23:614, Neurohospitalist 2014;4:196, Curr Opin Infect Dis 2017;30:129
Clinical features
- Classical triad includes headache (49 - 93%), fever (14 - 88%) and focal neurological deficits (29 - 71%) but all elements of triad are only seen in a minority of patients; other manifestations include altered mental status, nausea and vomiting, progressive behavioral changes and seizures (Clin Microbiol Infect 2017;23:614, Neurology 2014;82:806)
Diagnosis
- Diagnosis is based on a combination of a thorough history and physical examination coupled with appropriate brain imaging studies, particularly MRI (Curr Opin Infect Dis 2017;30:129)
- Biopsy may be helpful in distinguishing abscesses from other infectious and noninfectious processes (e.g., neoplasms)
- Delayed diagnosis associated with worse prognosis
Laboratory
- Preoperative laboratory tests are of limited value in the diagnosis of brain abscess; may see leukocytosis and elevated C reactive protein and erythrocyte sedimentation rate but these may be absent; blood cultures positive in a minority of patients (Clin Microbiol Infect 2017;23:614, Neurohospitalist 2014;4:196)
- Cerebrospinal fluid (CSF) findings usually nonspecific and risks of lumbar puncture need to be carefully considered in patients with space occupying CNS lesions and mass effect (S Afr Med J 2000;90:609, Neurology 2014;82:806)
- Intraoperative cultures should always be obtained in cases of suspected brain abscess
- Material obtained from the suspected abscess should be submitted directly from the operating room to the microbiology laboratory for both aerobic and anaerobic bacterial cultures
- Cultures for mycobacteria should be obtained if risk factors for tuberculosis are present, along with cultures for fungi in immunocompromised hosts (Clin Microbiol Infect 2017;23:614)
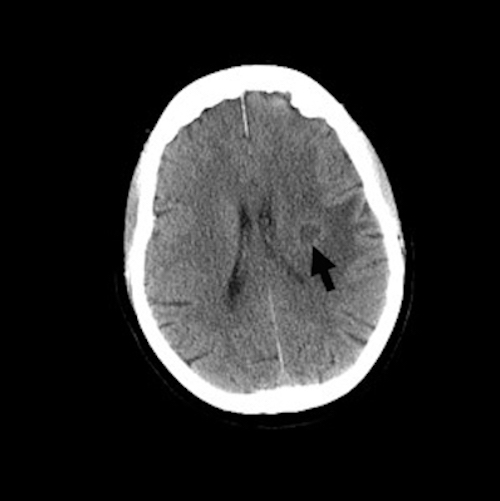
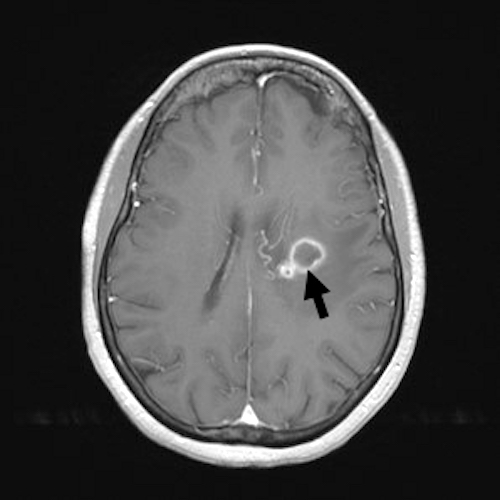
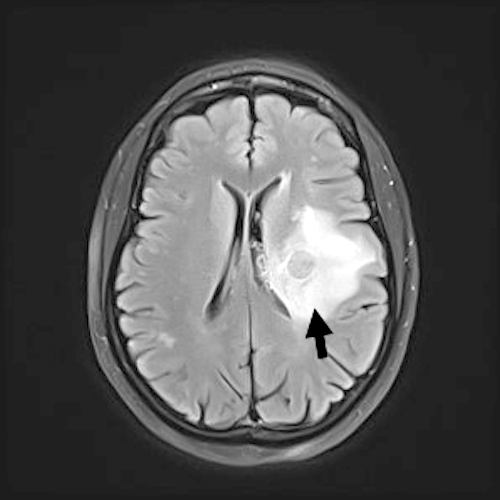
Radiology description
- Brain imaging critical in the diagnosis of suspected brain abscess
- Early lesions may appear as only an area of edema, with or without contrast enhancement
- More fully developed abscesses are mass lesion(s) with a central necrotic core (hypodense on CT, hypointense on T1 weighted MRI, hyperintense on T2 weighted MRI and diffusion weighted MRI), surrounded by contrast enhancing smooth rim and edema in adjacent brain; fluid attenuated inversion recovery (FLAIR) sequences highlight edema surrounding abscess(es)
- MRI more sensitive than CT in visualizing lesions
- Additional imaging techniques (e.g., 1H nuclear MR spectroscopy) may be helpful in distinguishing bacterial abscesses from neoplastic lesions
- References: J Popul Ther Clin Pharmacol 2020;27:e11, Radiographics 2015;35:1555
Prognostic factors
- Mortality: ~10% in developed countries
- Old age, delayed diagnosis and treatment, ventricular rupture, rapid progression of symptoms and coma associated with poorer prognosis
- Chronic sequelae include seizures, permanent focal neurological deficits and, in younger children, cognitive impairment
- Reference: BMC Infect Dis 2012;12:332
Case reports
- 30 year old previously healthy man and 45 year old previously healthy woman who developed brain abscesses of unknown origin (Front Neurosci 2019;12:1054)
- Middle aged adult with a history of chronic sinusitis who developed a brain abscess (BMJ Case Rep 2018;2018:bcr2017223266)
- Case series: brain abscess associated with insertion of a halo pin after C spine injury (Spine (Phila Pa 1976) 2007;32:E271)
Treatment
- Optimum management involves multidisciplinary approach (neurosurgery, infectious disease, neuroradiology, pathology and neurology)
- Empirical broad spectrum antimicrobial therapy covering both Gram positive and Gram negative bacteria is critical due to the wide range of potential pathogens; clinical features (e.g., age of the patient, potential source of infection, immunosuppression) can guide selection of antimicrobials
- Guidelines for neurosurgical intervention vary from center to center; surgical intervention with drainage or excision of abscess is necessary in some patients owing to poor penetration of antimicrobials into abscess cavity and can also be of great value in the identification of the microorganisms causing the abscess
- Other potential indications for surgical intervention include impending herniation, abscesses > 2.5 cm in diameter, location of abscess in paraventricular areas or posterior fossa
- References: Curr Opin Infect Dis 2017;30:129, Neurohospitalist 2014;4:196
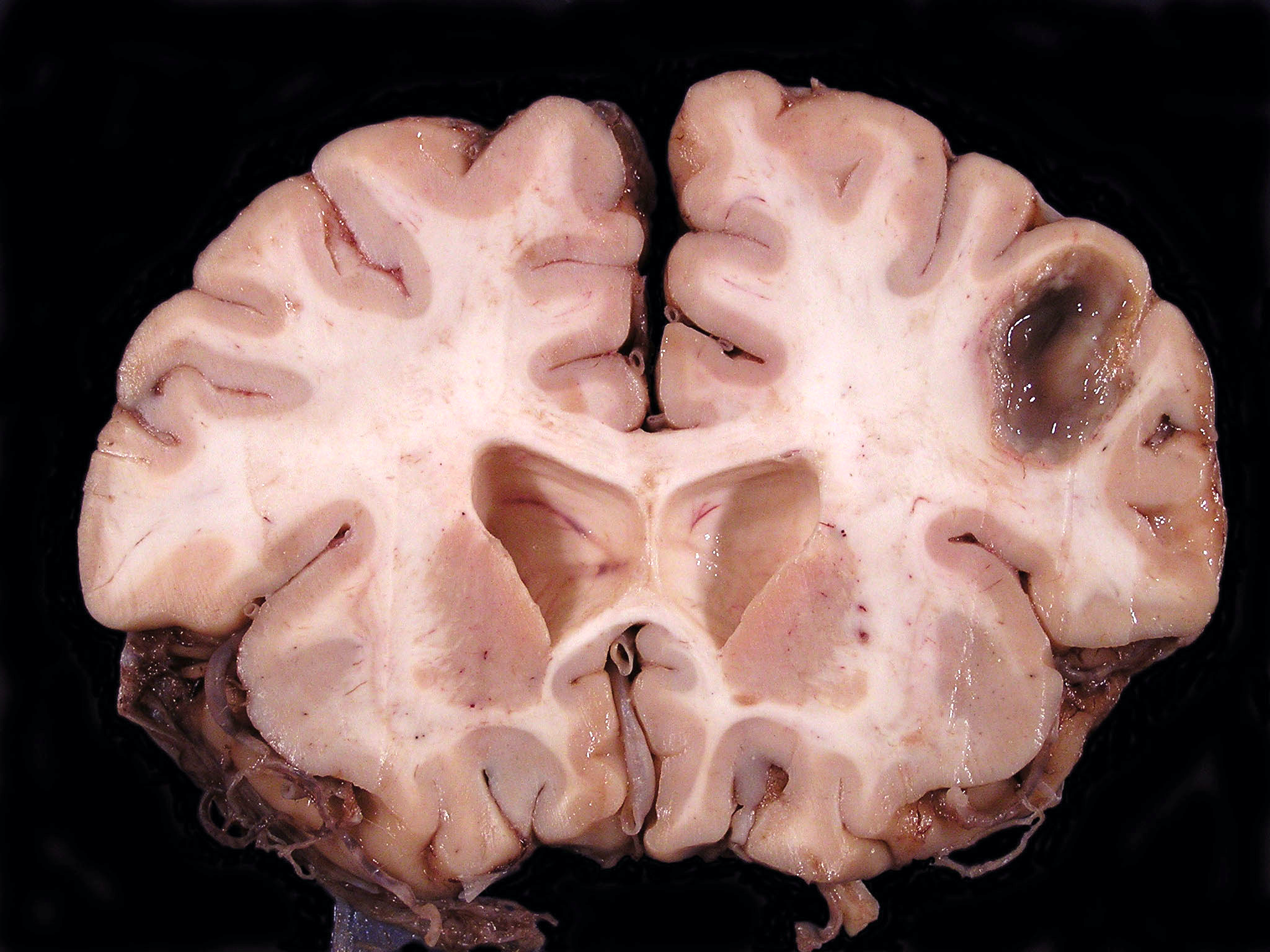
Gross description
- Localized mass, typically well demarcated from adjacent brain, containing a variable quantity of opaque, purulent exudate; older lesions may be cystic, with an irregular shaggy lining surrounded by a fibrous capsule
Frozen section description
- Tissue samples submitted for intraoperative frozen section are usually quite small and the appearance depends on the part of the abscess that was sampled
- Central areas of the abscess present as purulent debris
- More peripheral areas may contain only reactive astrocytes
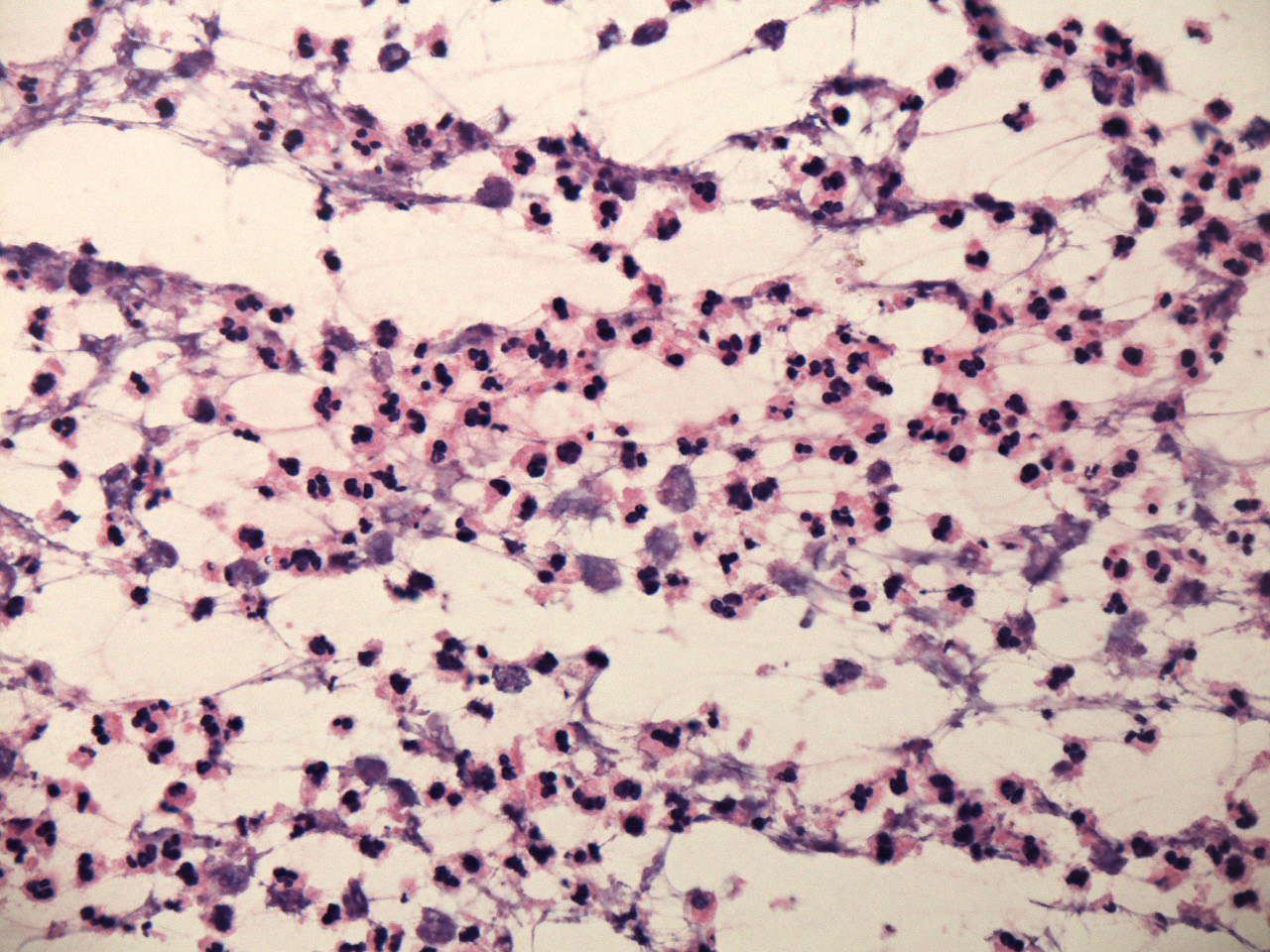
Microscopic (histologic) description
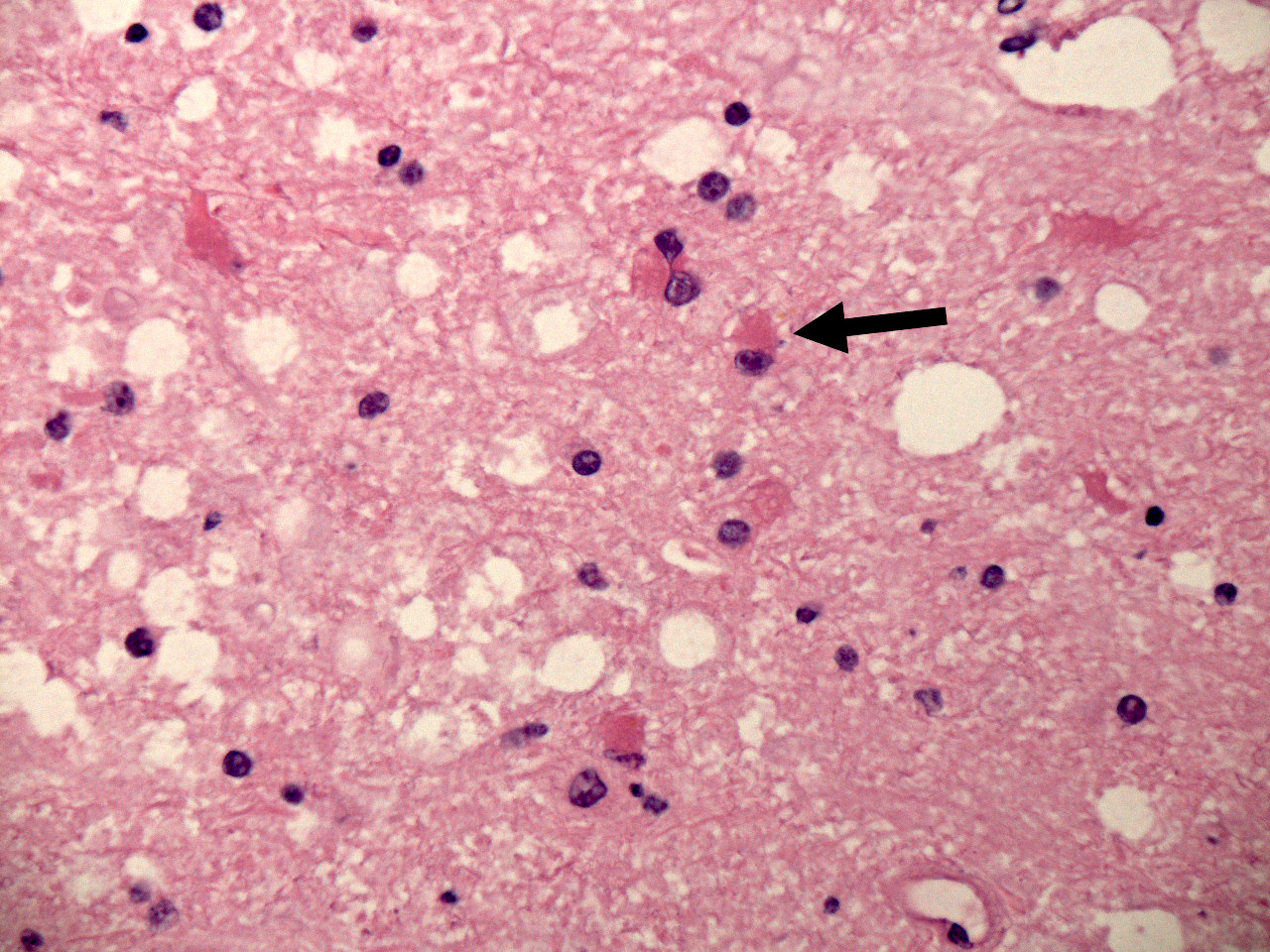
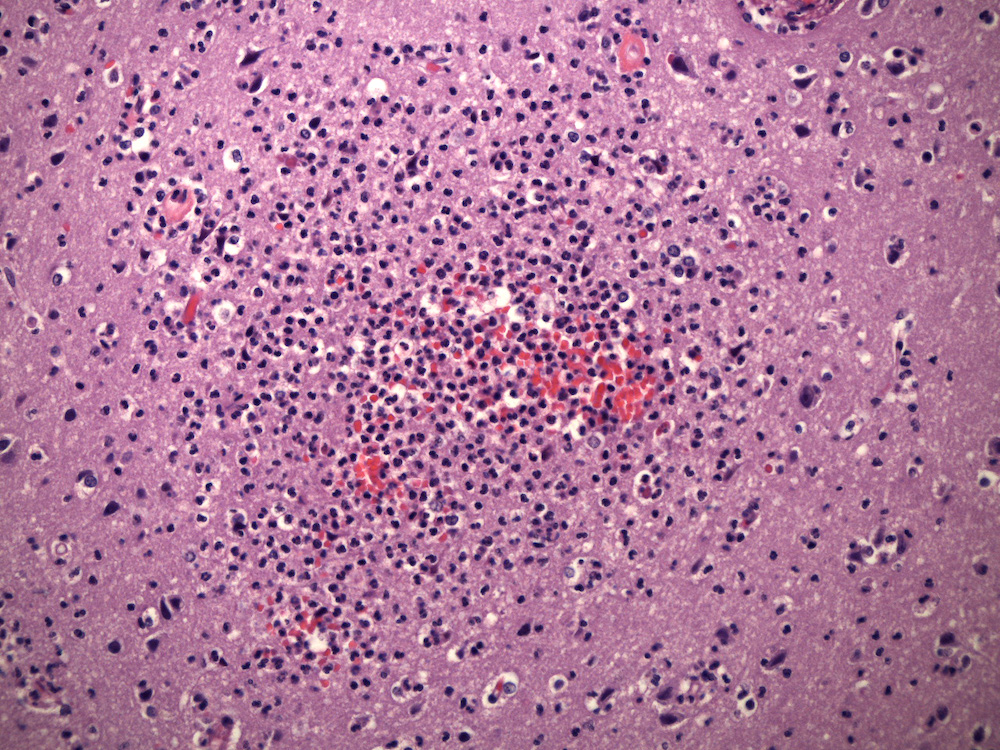
- Early lesions appear microscopically as foci of cerebritis, characterized by edema and neutrophilic infiltration
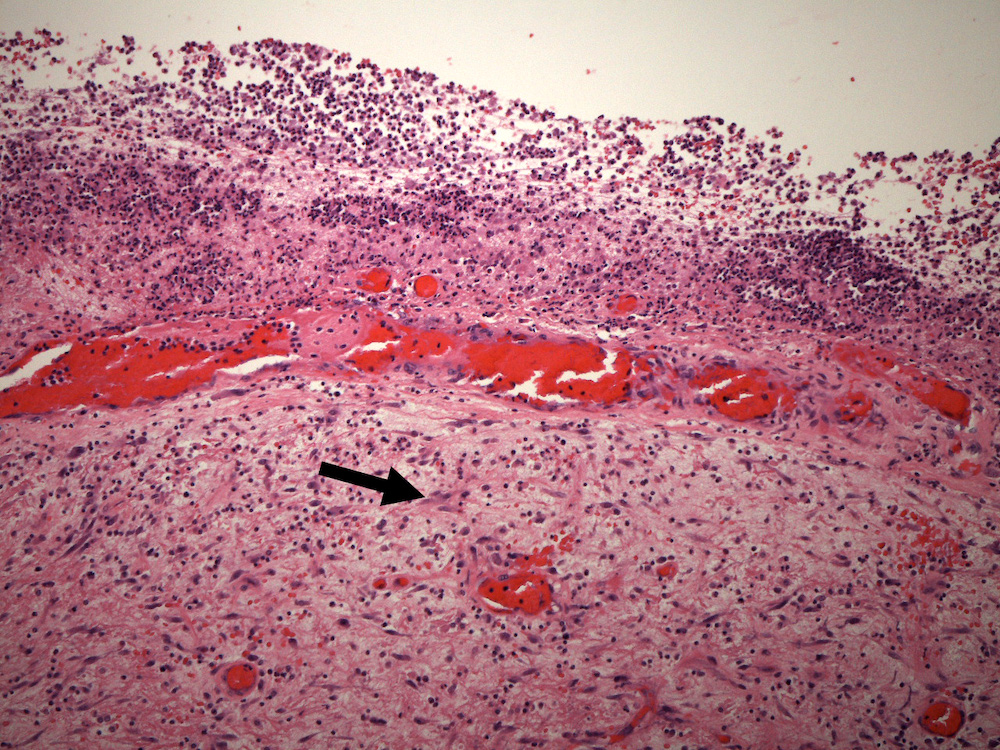
- Later lesions contain a central core of necrotic debris, organisms and neutrophils surrounded by a variably developed rim of granulation tissue, a mixed inflammatory infiltrates and activated macrophages and reactive astrocytes in the adjacent brain; over time, granulation tissue matures into a denser fibrous capsule
- Histological evolution of abscesses has traditionally been divided into 4 stages (Clin Microbiol Infect 2017;23:614, Int J Surg 2011;9:136):
- Stage I: early cerebritis (1 - 4 days)
- Stage II: late cerebritis (4 - 10 days)
- Stage III: early capsule stage (11 - 14 days)
- Stage IV: late capsule stage (> 14 days)
Cytology description
- As in the case of intraoperative frozen sections, the appearance of intraoperative touch or smear preparations is variable, depending on the area of the abscess that was biopsied; tissue from central areas is dominated by neutrophils in varying stages of degeneration, while tissue from more peripheral areas usually contains only nonspecific reactive elements, including reactive astrocytes
Positive stains
Sample pathology report
- Brain, left basal ganglia mass, stereotactic biopsy:
- Acute inflammation consistent with abscess; Gram positive cocci identified on Gram stain
- Brain, autopsy:
- Organizing abscess, left frontoparietal region; no microorganisms identified
Differential diagnosis
- Neoplasms (particularly metastatic carcinoma, high grade glial neoplasms, lymphoma)
- Atypical, pleomorphic neoplastic epithelial, glial or lymphoid cells
- Inflammatory infiltrates may be present but do not usually contain large numbers of neutrophils
- Mycobacterial infections:
- Lesions contain a granulomatous inflammatory infiltrate dominated by epithelioid macrophages and multinucleated giant cells, usually associated with caseous necrosis (neutrophils may be conspicuous in acute lesions)
- Acid fast (Ziehl-Neelsen or Fite) stains may demonstrate acid fast bacilli
- Nonbacterial infections (some parasitic and fungal infections):
- Inflammatory infiltrates may be granulomatous or predominantly lymphocytic, depending upon the pathogen; neutrophilic infiltrates are less common
- Parasitic organisms usually visible in H&E sections, while pathogenic fungi are best demonstrated with silver stains (Grocott-Gomori methenamine silver)
- Infarcts:
- Early lesions contain necrotic red neurons and fragmented, necrotic neuropil accompanied in the initial phases (1 - 2 days) accompanied by predominantly neutrophilic infiltrates; no organisms are demonstrable in gram, acid fast or GMS silver stains
- Macrophages begin to infiltrate lesion beginning at about 48 hours, with older lesions composed of variable numbers of lipid laden macrophages (gitter cells), cystic, liquified brain parenchyma and reactive astrocytes
- Organizing hemorrhage:
- Numerous erythrocytes and hemosiderin laden macrophages
- Astrocytic reaction develops at the periphery of the lesion, which is typically well demarcated from the surrounding neuropil
- Radiation necrosis:
- Prominent areas of coagulation necrosis, usually confined to white matter, accompanied by vascular changes that include vascular telangiectasia, hyaline vascular sclerosis with luminal stenosis and occasional lipid laden macrophages in vessel walls
- Tumefactive demyelination:
- Rare
- Horseshoe pattern of enhancement at periphery of lesion
- Macrophagic infiltrates, striking myelin loss with relative axonal sparing (although some degree of axonal injury / loss is usually seen)
Board review style question #1
A 46 year old man with a history of intravenous drug abuse presented with a 3 day history of fatigue, shortness of breath, fever and headache. Clinical evaluation revealed evidence of severe aortic regurgitation. Blood cultures were obtained in the emergency department (ED) and the patient was started on empirical antimicrobial therapy for presumed bacterial endocarditis. During transport from the ED to the ward, the patient sustained a cardiac arrest. Resuscitation was unsuccessful. A postmortem examination confirmed the presence of infective endocarditis involving the aortic valve, associated with destruction of the valve leaflets. Postmortem examination of the brain revealed the lesions shown above. Which one of the following is the earliest event in the development of the brain lesion?
- Binding of bacteria to Toll-like receptors (TLRs)
- Disruption of the blood brain barrier
- Inactivation of MyD88
- Migration of neutrophils into the lesion
- Release of IL1α
Board review style answer #1
A. Binding of bacteria to Toll-like receptors (TLRs). Experimental models have demonstrated that binding of bacteria to TLRs is the initial step in the development of brain abscesses. TLR binding triggers an innate immune response via activation of MyD88 dependent pathways, which results in the release of cytokines and chemokines, disruption of the blood brain barrier, activation of astrocytes and resident microglia and migration of inflammatory cells to the site of infection.
Comment Here
Reference: Abscess
Comment Here
Reference: Abscess
Board review style question #2
Which one of the following provides the most specific information in the evaluation of a patient with a suspected brain abscess?
- Cerebrospinal fluid culture
- Complete blood count
- Erythrocyte sedimentation rate
- Magnetic resonance imaging
- Peripheral blood culture
Board review style answer #2
D. Magnetic resonance imaging. MRI plays a critical role in the evaluation of a patient with a suspected brain abscess. Although biopsy may be necessary to distinguish brain abscesses from other processes causing contrast enhancing, space occupying brain lesions (e.g., neoplasms), of the choices listed, radiographic imaging, particularly MRI, provides the most specific information about the presence or absence of a brain abscess. Abnormal peripheral blood counts and elevated erythrocyte sedimentation rates can be seen in patients with brain abscesses but are nonspecific and such tests may be normal in patients with brain abscesses. A positive blood culture can be helpful in identifying the responsible pathogen in cases of brain abscess but is also nonspecific by itself. Blood cultures, moreover, may be negative. Lumbar puncture and cerebrospinal fluid (CSF) studies are seldom useful in the evaluation of suspected brain abscesses and are usually contraindicated due to the danger of herniation in patients with expanding intracranial masses of any type.
Comment Here
Reference: Abscess
Comment Here
Reference: Abscess