Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Pathophysiology | Clinical features & test indications of conditions where serum free light chain testing is useful | Laboratory | Common commercial platforms and methodologies | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Keren DF. Serum free light chain test. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/chemistryfreelightchains.html. Accessed April 1st, 2025.
Definition / general
- Serum free light chain testing is required for detecting and monitoring patients with monoclonal gammopathies (Arch Pathol Lab Med 2022;146:575)
Essential features
- Measures the concentration of serum free kappa and lambda immunoglobulin light chains
- Recommended to be used in connection with serum protein electrophoresis (SPEP)
- Abnormal ratio of serum free kappa/serum free lambda (rFLC) supports diagnosis of a monoclonal gammopathy
- Elevated rFLC may also be found in patients with chronic kidney disease or chronic inflammation
- Used to calculate risk of progression
Terminology
- Bence Jones protein
- Monoclonal protein (M protein)
- Paraprotein
ICD coding
Pathophysiology
- Plasma cells produce immunoglobulin light chains in excess of immunoglobulin heavy chains
- Most free kappa light chains circulate as monomers (~22.5 kDa) while most free lambda light chains circulate as dimers (~44 kDa)
- Due to their small size, their half life ranges from 2 to 6 hours (Br J Haematol 2004;126:348)
- When the monoclonal free light chains (MFLC) pass into the renal tubules, they may bind to uromodulin (also known as Tamm-Horsfall protein) leading to cast formation that obstructs the distal tubules and can cause pyelonephritis (J Clin Invest 1997;99:732)
- MFLC may form the beta pleated sheets of AL (light chain type) amyloid that deposit in and damage a wide variety of organs: cardiac, renal, neural, hepatic and gastrointestinal
- MFLC that do not form beta pleated sheets also may deposit in glomeruli or cardiac tissues as monoclonal immunoglobulin (or light chain deposition) disease (Clin J Am Soc Nephrol 2012;7:231)
Clinical features & test indications of conditions where serum free light chain testing is useful
- Non-IgM monoclonal gammopathy of undetermined significance (non-IgM MGUS): no clinical features
- Serum monoclonal protein (non-IgM type) < 3 g/dL
- Bone marrow clonal plasma cells < 10%
- In low risk patients (IgG isotype, M protein < 1.5 g/dL, normal ratio of free kappa/free lambda), bone marrow biopsy can be deferred
- No CRAB (increased calcium, renal disease [elevated serum creatinine ≥ 2 mg/dL], anemia or bone lesions) symptoms of end organ damage due to the monoclonal process (Lancet Oncol 2014;15:e538)
- Abnormal free light chain ratio (< 0.26 or > 1.65) is associated with higher risk for progression when using the Freelite assay (manufactured by The Binding Site, Thermo Fisher Scientific) on a BNII nephelometer (manufactured by Siemens) (N Engl J Med 2018;378:241)
- Caution: this range has not been validated for this purpose on contemporary instruments or other methods to measure FLC (see Laboratory below)
- Smoldering multiple myeloma (SMM): no clinical features
- IgG or IgA monoclonal protein present, with measurement of the M protein (≥ 3 g/dL) or
- Bone marrow clonal plasma cells 10 - 60%
- No CRAB signs of end organ damage, anemia or bone lesions due to the monoclonal process
- Ratio of the involved serum free light chain isotype (iFLC) to the uninvolved free light chain isotype (uFLC) of > 20 (iFLC/uFLC > 20) is 1 of 3 risk factors for progression; the other risk factors are bone marrow plasma cells percentage > 20% and the M protein > 2 g/dL (Blood Cancer J 2018;8:59)
- Multiple myeloma (MM), also known as plasma cell myeloma (PCM)
- Bone marrow clonal plasma cells ≥ 10% or extramedullary plasmacytoma and 1 or more of the following myeloma defining events (solitary plasmacytoma with ≥ 10% clonal bone marrow plasma cells is considered as multiple myeloma)
- CRAB symptom(s) attributed to the plasma cell proliferative process (any 1)
- Increased serum calcium > 1 mg/dL (> 0.25 mmol/L) higher than the upper limit of normal or > 11 mg/dL (> 2.75 mmol/L)
- Renal insufficiency: serum creatinine > 2 mg/dL (> 177 μmol/L) or creatinine clearance < 50 mL/min
- Anemia: hemoglobin value of < 10 g/dL or a value of > 2 g/dL below the lower limit of normal
- Bone lesions: 1 or more osteolytic lesions on skeletal radiography, computed tomography (CT or positron emission tomography CT [PET CT])
- Bone marrow clonal plasma cells ≥ 60%
- Involved / uninvolved serum free light chain ratio ≥ 100 (involved FLC level must be ≥ 100 mg/L)
- Caution: this level was established using the Freelite method on a BNII nephelometer
- This level has not been verified at this time on other methods for free light chain measurement or on the Freelite method on other instruments (see Laboratory below)
- > 1 focal lesion on magnetic resonance imaging (MRI) studies (at least 5 mm in size)
- CRAB symptom(s) attributed to the plasma cell proliferative process (any 1)
- Bone marrow clonal plasma cells ≥ 10% or extramedullary plasmacytoma and 1 or more of the following myeloma defining events (solitary plasmacytoma with ≥ 10% clonal bone marrow plasma cells is considered as multiple myeloma)
- IgM monoclonal gammopathy of undetermined significance (IgM MGUS): no clinical features
- Serum IgM monoclonal protein < 3 g/dL
- Bone marrow clonal plasma cells < 10%
- No evidence of anemia, constitutional symptoms, hyperviscosity, lymphadenopathy or hepatosplenomegaly attributed to the underlying lymphoproliferative disorder
- Abnormal free light chain ratio (< 0.26 or > 1.65) is associated with higher risk for progression when using the Freelite system on a BNII nephelometer (Am J Hematol 2023;98:348)
- Caution: this range has not been validated for this purpose on contemporary instruments or other methods to measure FLC (see Laboratory below)
- Waldenström macroglobulinemia
- IgM M monoclonal protein (any size) and
- ≥ 10% Bone marrow infiltration by small lymphoplasmacytic cells with specific phenotype excluding other lymphoproliferative disorders
- Anemia, constitutional symptoms, hyperviscosity, lymphadenopathy or hepatosplenomegaly due to the lymphoproliferative disorder
- Serum FLC has been recommended to follow response to therapy and progression of disease (Leuk Lymphoma 2008;49:864)
- Light chain monoclonal gammopathy of undetermined significance (light chain MGUS): no clinical features
- No M protein on SPEP or immunofixation
- No underlying lymphoproliferative disease
- Abnormal FLC ratio, determined with Freelite assay on Optilite Analyzer (manufactured by The Binding Site, Thermo Fisher Scientific)
- eGFR ≥ 60 mL/min/1.73 m²
- Age < 70 years: FLC ratio < 0.44 or > 2.16
- Age ≥ 70 years: FLC ratio < 0.46 or > 2.59
- Elevated iFLC
- Age < 70 years: kappa > 39.0 or lambda > 36.7 mg/L
- Age ≥ 70 years: kappa > 55.8 or lambda > 48.0 mg/L
- eGFR < 60 mL/min/1.73 m²
- eGFR 45 - 49: FLC ratio < 0.46 or > 2.62
- eGFR 30 - 44: FLC ratio < 0.48 or > 3.38
- eGFR < 30: FLC ratio < 0.54 or > 3.30
- Elevated FLC
- eGFR 45 - 59: kappa > 83.6 or lambda > 65.1 mg/L
- eGFR 30 - 44: kappa > 103.3 or lambda > 73.2 mg/L
- eGFR < 30: kappa > 265.1 or lambda > 150.9 mg/L
- Further, this range has not been verified at this time on other methods or on the Freelite method on other instruments (Blood 2023;142:535, J Appl Lab Med 2023;8:742, Clin Chem Lab Med 2023;61:e229)
- eGFR ≥ 60 mL/min/1.73 m²
- Increased level of iFLC
- No immunoglobulin heavy chain by immunofixation, immunosubtraction or MASS-FIX
- Bone marrow clonal plasma cells < 10%
- No CRAB signs of end organ damage, anemia or bone lesions due to the monoclonal process
- Urine monoclonal protein < 500 mg/24 h
- Monoclonal gammopathy of renal significance (MGRS): evidence of renal disease due to damage from monoclonal immunoglobulin free light chains or heavy chains
- Demonstration of monoclonal light chain deposition in the kidney usually accompanied by circulating monoclonal FLC (Neth J Med 2019;77:243)
- AL amyloidosis
- Clinical symptoms of broad organ involvement: cardiac, renal, hepatic or gastrointestinal involvement
- Monoclonal FLC indicated by abnormal serum free light chain ratio and increase of involved isotype, monoclonal free light chain band by urine or serum immunofixation or biopsy demonstration of amyloid by Congo red technique or ultrastructure analysis and demonstration of monoclonal free light chain (Blood 2020;136:2620)
- Reference: Mayo Clin Proc 2016;91:101
Laboratory
- Diagnostic range of 0.26 - 1.65 established by using the entire range of individuals' serum as controls on the Freelite assay performed on the Dade-Behring BNII nephelometer in 2002 has not been confirmed as correct with most contemporary methods and instruments
- < 3% of laboratories performing serum FLC assays currently use the BNII instrument with the Freelite assays (CAP Survey 2023)
- Recent studies report that the range differs depending on which method and which instrument the laboratory uses (Clin Biochem 2018;58:100)
- Current studies suggest using a more statistically reproducible 99% range for the specific method and instrument used (Blood Cancer J 2022;12:133, Clin Biochem 2023;118:110604)
- iStopMM study, which uses the Freelite method on the Optilite, reports the 99% range as (Blood 2023;142:535)
Kappa FLC (mg/L) Lambda FLC (mg/L) FLC ratio Age < 70 yr 6.3 - 39.0 5.9 - 36.7 0.44 - 2.16 Age ≥ 70 yr 7.0 - 55.8 6.4 - 48.0 0.46 - 2.59
- Several commercial methods are available for measuring serum free light chains
Common commercial platforms and methodologies
| Company | Methodology | Instrument(s)* |
| The Binding Site (Thermo Fisher Scientific) | Polyclonal anti-light chain reagents Immunonephelometric | Binding Site Optilite Binding Site SPAplus Siemens Nephelometer Roche Cobas c500 |
| Siemens | Monoclonal anti-light chain reagents Immunonephelometric | Siemens Nephelometer |
| Diazyme Reagent | Polyclonal anti-light chain reagents Immunoturbidimetric | Roche Cobas c500 |
| Sebia | Polyclonal anti-light chain reagents Enzyme linked immunosorbent assay (ELISA) | AP22 ELITE analyzer |
Additional references
Board review style question #1
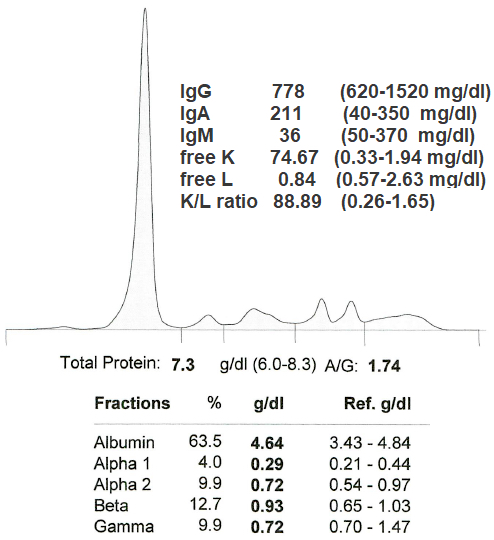
This serum is from a 58 year old woman with back pain and hemoglobin of 7.4 g/dL. Her bone marrow clonal plasma cells were > 60%. Which of the following is the most likely diagnosis?
- IgA kappa multiple myeloma
- IgG kappa multiple myeloma
- IgM kappa Waldenström macroglobulinemia
- Kappa light chain multiple myeloma
Board review style answer #1
D. Kappa light chain multiple myeloma is the most likely diagnosis because she meets the IMWG criteria for multiple myeloma; she is anemic with a hemoglobin of 7.4 and her bone marrow clonal plasma cells are > 60%. Kappa light chain myeloma is the most likely isotype because her free K is 35x the upper limit of normal with a free K/L ratio 40x the upper limit of normal. Answers A, B and C are incorrect because it is not likely to be an IgG, IgAK or IgMK process given the absence of an M spike and absence of elevated IgG, IgA and IgM levels.
Comment Here
Reference: Free light chains
Comment Here
Reference: Free light chains
Board review style question #2
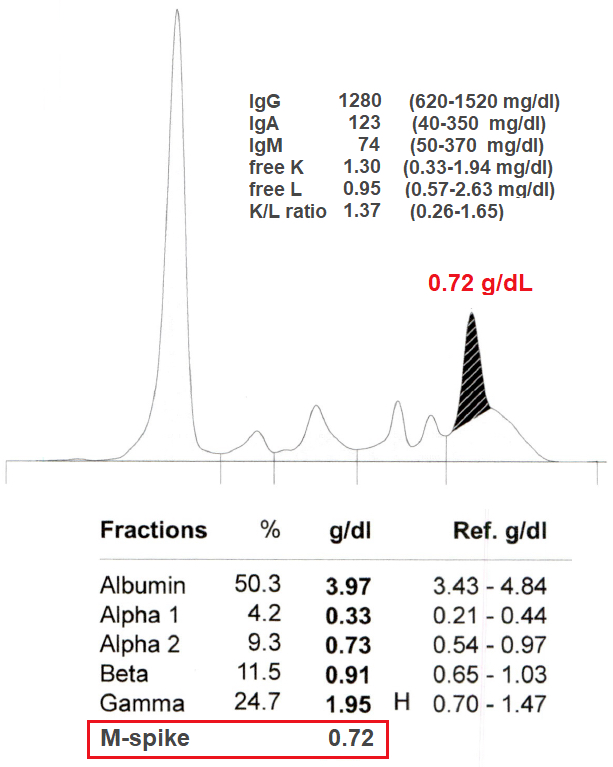
This serum is from a clinically well 76 year old woman with a normal CBC, no evidence of renal disease and normal calcium. The immunofixation identified the M spike as IgGK. Which of the following is the most likely diagnosis?
- Monoclonal gammopathy of undetermined significance (MGUS)
- Multiple myeloma (MM)
- Smoldering multiple myeloma (SMM)
- Waldenström macroglobulinemia (WM)
Board review style answer #2
A. Monoclonal gammopathy of undetermined significance (MGUS) is the most likely diagnosis because she is well, not anemic and her M spike measures only 0.72 g/dL. Answer B is incorrect because the patient is clinically well with no evidence of renal disease and has a normal CBC. Answer C is incorrect because an M spike of ≥ 3g/dL or a bone marrow clonal plasmacytosis of ≥ 10% would be needed to upgrade her condition to SMM. In this case she has a normal free K/L, a normal CBC and no myeloma defining events. Bone marrow biopsy can be deferred in patients categorized as low risk MGUS (IgG isotype, M protein < 1.5 g/dL, normal FLC ratio) and who lack features suspicious for MM. Answer D is incorrect because she is clinically well with a normal CBC and the M protein is not IgM.
Comment Here
Reference: Free light chains
Comment Here
Reference: Free light chains