Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Laboratory diagnosis | Additional testing for primary adrenal insufficiency | Factors that can impact test results | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Lahorewala S, Bertholf RL. Adrenal insufficiency-diagnosis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/chemistryadrenalinsufficiency.html. Accessed April 3rd, 2025.
Definition / general
- Deficiency in the production of glucocorticoids (e.g., cortisol) due to a disorder of the adrenal gland (primary adrenal insufficiency), inadequate pituitary adrenocorticotrophin hormone (ACTH; secondary adrenal insufficiency) or suppression of ACTH due to decreased corticotrophin releasing hormone (CRH; tertiary adrenal insufficiency)
Essential features
- Clinical presentation of adrenal insufficiency is nonspecific; therefore, diagnosis is confirmed via laboratory testing
- Low basal cortisol levels are indicative of insufficiency; abnormal response on the ACTH stimulation test is confirmatory
- Concurrent high or low pituitary ACTH levels can differentiate between primary and secondary / tertiary adrenal insufficiency, respectively
- Additional laboratory work up is needed to confirm the etiology of primary adrenal insufficiency; the most common causes are autoimmune adrenalitis in adults and congenital adrenal hyperplasia in children
Diagrams / tables
Clinical features
- Clinical symptoms are often vague and nonspecific
- Weight loss, anorexia, nausea and vomiting, postural dizziness, headaches, weakness, fatigue, hyponatremia, hypoglycemia, muscle cramps and abdominal pain
- Primary adrenal insufficiency only: skin hyperpigmentation, salt craving, hyperkalemia, postural hypotension and volume depletion
- Adrenal crisis: life threatening medical emergency mainly seen in primary adrenal insufficiency patients or in secondary / tertiary adrenal insufficiency under conditions of severe physiologic stress
- Features: severe hypotension and shock, often accompanied by loss of consciousness
- Reference: Lancet 2021;397:613
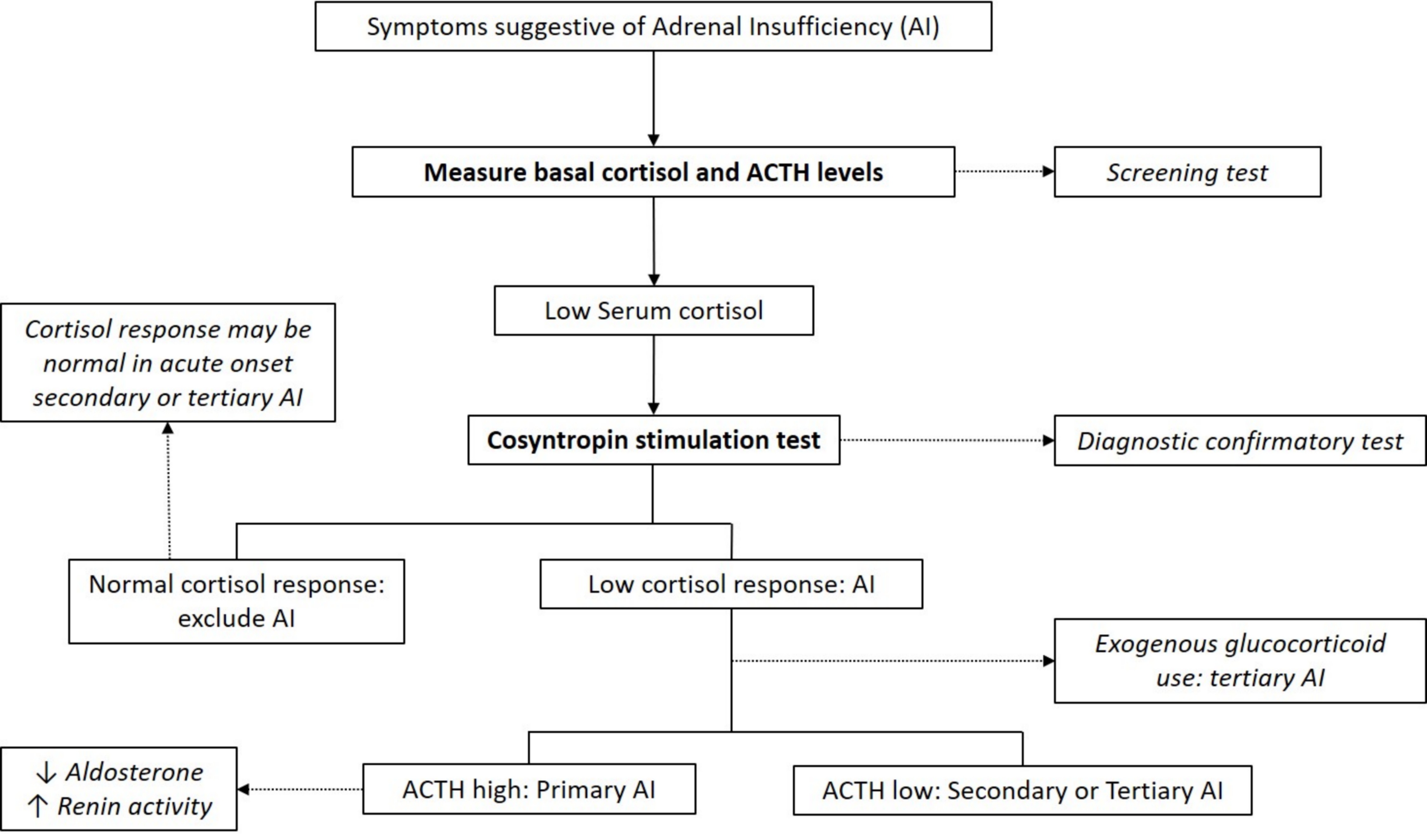
Laboratory diagnosis
- Cortisol typically peaks in the morning
- Early morning (8:00 AM) plasma or serum cortisol < 5 μg/dL (< 140 nmol/L) highly suggestive of adrenal insufficiency (J Clin Endocrinol Metab 2016;101:364)
- Basal cortisol > 14 μg/dl (> 400 nmol/L) on commonly used automated immunoassays excludes adrenal insufficiency (Clin Endocrinol (Oxf) 2017;86:177)
- ACTH levels in conjunction with low morning cortisol:
- High ACTH > 2 fold the upper reference limit → primary adrenal insufficiency (absence of feedback loop to pituitary gland)
- Low ACTH → secondary or tertiary adrenal insufficiency
- Note: serum / plasma cortisol measures total cortisol (i.e., cortisol bound to cortisol binding globulin [CBG] and albumin)
- Free cortisol can be measured in saliva and urine
- Salivary free cortisol can be utilized for screening
- Recommended for the diagnosis of critical illness related corticosteroid insufficiency (CIRCI) in critically ill patients (Crit Care Med 2017;45:2078)
- Urinary free cortisol commonly used for the diagnosis of Cushing syndrome (hypercorticolism); not recommended for adrenal insufficiency testing
- ACTH stimulation test (cosyntropin stimulation test)
- Diagnostic gold standard test for primary adrenal insufficiency
- Cosyntropin: synthetic form of the biologically active region of ACTH
- Standard dose: 250 μg for adults and children ≥ 2 years of age, 125 μg for children < 2 years of age and 15 μg/kg for infants
- Cortisol levels measured at baseline and 30 and 60 minutes after cosyntropin administration
- Peak cortisol levels < 18 μg/dL (500 nmol/L) at 30 or 60 minutes confirms adrenal insufficiency diagnosis (J Clin Endocrinol Metab 2016;101:364)
- Low dose cosyntropin stimulation test (1 μg) not recommended
- Note:
- Test cannot differentiate between primary and secondary adrenal insufficiency
- Stimulated cortisol levels may appear normal in cases of acute onset secondary or tertiary adrenal insufficiency (e.g., post recent pituitary / brain surgery or trauma)
- Diagnostic gold standard test for primary adrenal insufficiency
- Multiday ACTH stimulation test
- Can differentiate between primary and secondary (or tertiary) adrenal insufficiency
- Rationale:
- Chronic ACTH deficiency in secondary / tertiary adrenal insufficiency leads to adrenocortical atrophy and decreased responsiveness to stimulation
- This results in an absent or low cortisol response 30 - 60 minutes after cosyntropin / Synacthen administration
- Can be overcome by sustained ACTH stimulation, from 8 hours to 5 days
- Dosage: single intramuscular or intravenous dose, 250 μg/day
- Insulin tolerance test (ITT)
- Can differentiate primary from secondary adrenal insufficiency
- Can also identify acute onset central adrenal insufficiency not detected by the ACTH stimulation test
- Rationale: hypoglycemia is a potent stressor for activation of the hypothalamus pituitary adrenocortical axis
- IV insulin (0.1 U/kg body weight) administered after overnight fasting, with samples collected at baseline and variable intervals for up to 120 minutes
- Hypoglycemia confirmed by > 50% reduction in glucose concentration below baseline or at concentration < 45 mg/dL
- Low cortisol (post ITT induced hypoglycemia) confirms adrenal insufficiency
- Concurrent high ACTH → primary adrenal insufficiency; low ACTH → secondary or tertiary adrenal insufficiency
- Note: high risk test due to the sequelae of severe hypoglycemia
- Metyrapone test
- Metyrapone: blocks the conversion of 11-deoxycortisol to cortisol, causing decreased cortisol while increasing ACTH and 11-deoxycortisol
- No increase in ACTH and 11-deoxycortisol confirms adrenal insufficiency
- Dosage: 30 mg/kg body weight
- Administered at midnight and levels of cortisol, ACTH and 11-deoxycortisol measured next morning at 8:00 AM
- Note: can precipitate an adrenal crisis due to aggravated hypocortisolemia
- CRH stimulation test
- Can differentiate between secondary and tertiary adrenal insufficiency
- Not recommended in routine practice
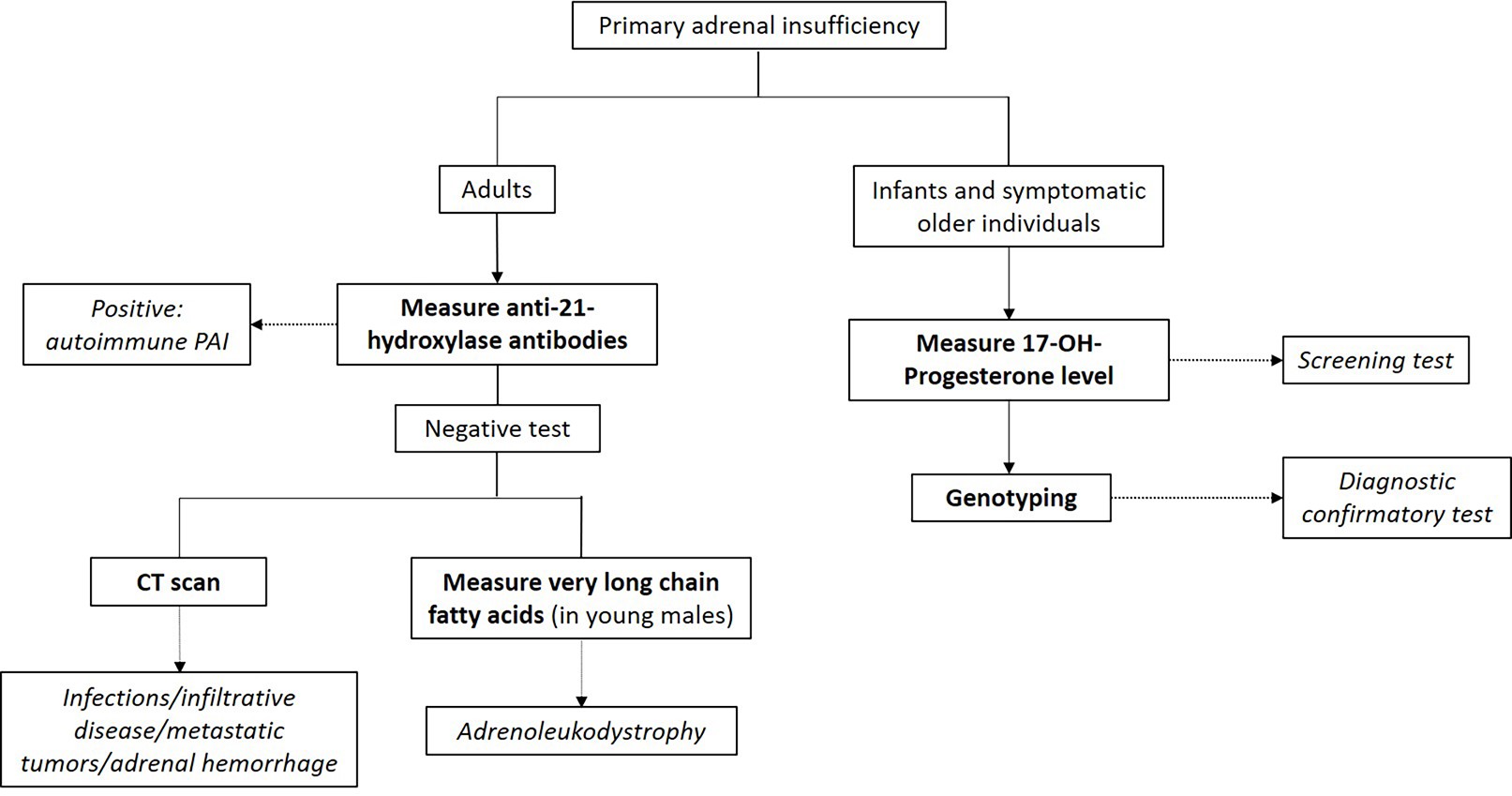
Additional testing for primary adrenal insufficiency
- Mineralocorticoid (body salt balance) function evaluation
- Tests for serum electrolytes, aldosterone levels and plasma renin concentration / activity
- Hyponatremia, hyperkalemia, decreased aldosterone and increased renin concentration (or activity) indicates primary adrenal insufficiency diagnosis
- Autoantibodies against 21-hydroxylase
- Autoimmune primary adrenal insufficiency: most common cause of primary adrenal insufficiency in the western world
- Congenital adrenal hyperplasia (CAH) testing
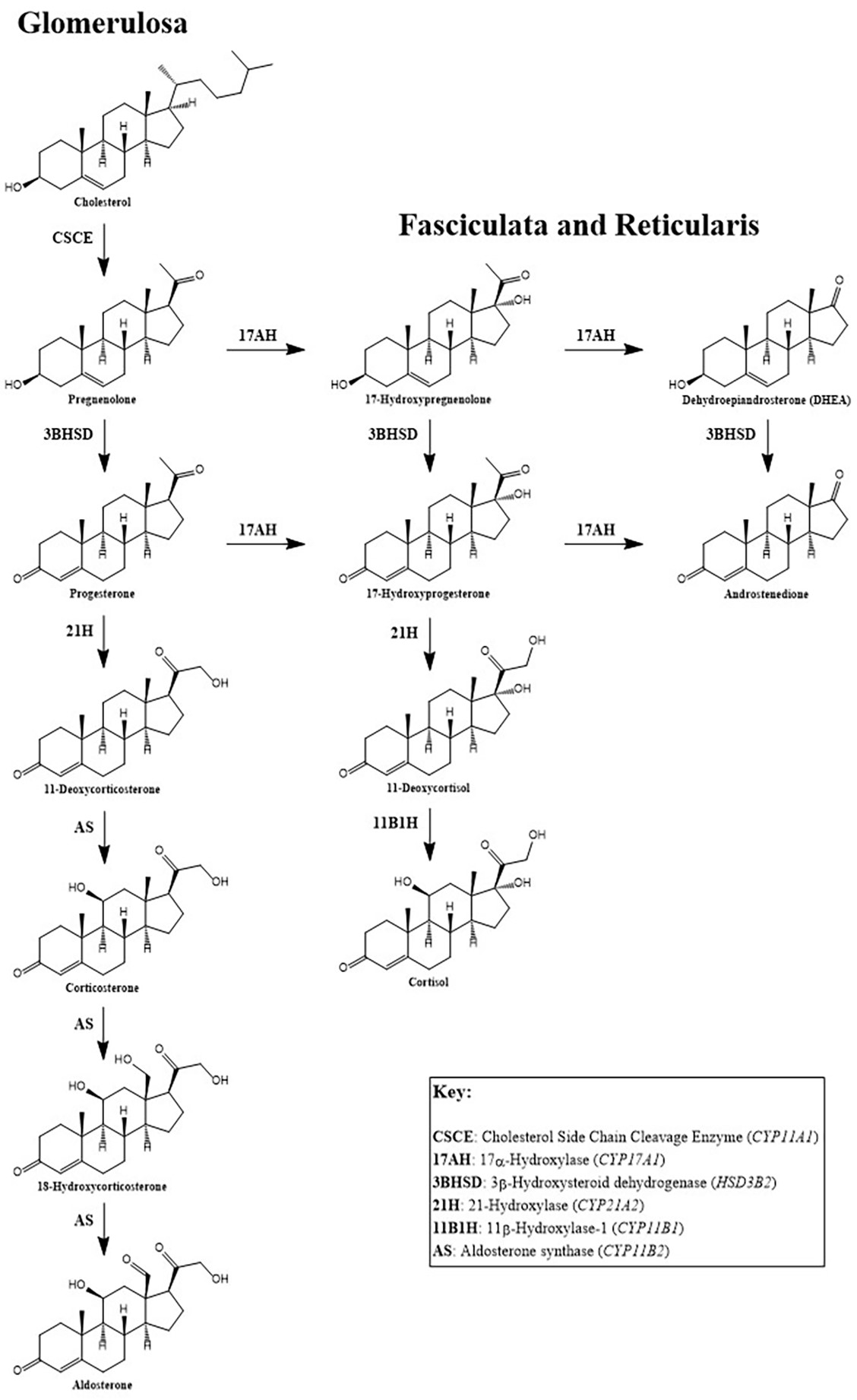
- CAH: autosomal recessive disorder of defective steroidogenesis
- Most cases are due to 21-hydroxylase deficiency (95%), with 11-hydroxylase deficiency accounting for the majority of the remaining cases
- Screening: elevated 17-hydroxyprogesterone
- Second tier screening: 17-hydroxyprogesterone measured by liquid chromatography-mass spectrometry (LC-MS); if not available, cosyntropin stimulation test should be performed (J Clin Endocrinol Metab 2018;103:4043)
- Basal or cosyntropin stimulated 17-hydroxyprogesterone > 1,000 ng/dL is diagnostic
- Usually > 5,000 ng/dL in classic CAH
- 21-deoxycortisol:
- Due to 21-hydroxylase deficiency (in CAH), accumulated 17-hydroxyprogesterone is converted to 21-deoxycortisol by 11-hydroxylase
- Can be used for CAH screening
- More specific than 17-hydroxyprogesterone as a marker for 21-hydroxylase deficiency CAH (J Pediatr 2021 Mar;230:161)
- Virtually absent in normal patients and in CAH cases caused by 11-hydroxylase deficiency
- Confirmatory test: genotyping
Factors that can impact test results
- Levels of CBG: total cortisol can appear to be falsely elevated or decreased due to a corresponding change in CBG levels (J Pediatr Endocrinol Metab 2018;31:107)
- Albumin: cortisol binds to albumin and therefore measured total cortisol can be affected by albumin concentration, albeit to a lesser extent than CBG
- Pregnancy: cortisol and CBG increase in pregnancy and may cause normal appearing morning cortisol levels; higher diagnostic thresholds for the cosyntropin stimulation test are indicated (Curr Opin Endocrinol Diabetes Obes 2017;24:184)
Additional references
Board review style question #1
A patient presents to the clinic with low basal cortisol, high ACTH, hypokalemia, hyperpigmentation and anti 21-hydroxylase antibodies. What is the patient's likely diagnosis?
- Adrenoleukodystrophy
- Autoimmune primary adrenal insufficiency
- Congenital adrenal hyperplasia
- Secondary adrenal insufficiency
Board review style answer #1
B. Autoimmune primary adrenal insufficiency. Low basal cortisol, high ACTH, hypokalemia and hyperpigmentation are all features of primary adrenal insufficiency. Autoantibodies against 21-hydroxylase are detected in 90% of patients with autoimmune adrenalitis.
Comment Here
Reference: Adrenal insufficiency-diagnosis
Comment Here
Reference: Adrenal insufficiency-diagnosis