Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Monsrud A, Turashvili G. Leiomyosarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/uteruslms.html. Accessed December 24th, 2024.
Definition / general
- Rare, malignant mesenchymal tumor derived from myometrial smooth muscle
- Most common sarcoma of the gynecologic tract
Essential features
- Rare tumor, mostly found in uterine corpus
- 3 subtypes: spindled / conventional (most common), myxoid, epithelioid
- Diagnostic triad: marked cytologic atypia, tumor cell necrosis and increased mitoses (mitotic count depends on subtype)
- Poor prognosis even at low stage (Histopathology 2009;54:355, Curr Probl Cancer 2019;43:283, Asia Pac J Clin Oncol 2020;16:e63)
ICD coding
Epidemiology
- Rare, 3 - 9% of all uterine cancers
- ~70% of uterine sarcomas (Frumovitz: Diagnosis and Treatment of Rare Gynecologic Cancers, 1st Edition, 2022, Lancet Oncol 2009;10:1188)
- Peak incidence is > 50 years old; range of 30 - 70 years (Medicine (Baltimore) 2020;99:e21766)
Sites
- Uterus corpus
- Cervix, rare
Pathophysiology
- Derived from smooth muscle
- Vast array of associated cytogenetic abnormalities but none are consistent or diagnostic
- Most frequently mutated genes: TP53 (~30%), ATRX (~25%) and MED12 (~20%) (Proc Natl Acad Sci U S A 2021;118:e2025182118)
Etiology
- Most patients do not have predisposing risk factors
- Rare associations include:
- Prior pelvic radiation (Arch Gynecol Obstet 2019;300:389, Cancer 1986;58:2003)
- Tamoxifen use for > 5 years (Int J Gynecol Cancer 2008;18:352)
- Very rare cases may arise from a pre-existing leiomyoma (J Minim Invasive Gynecol 2020;27:926, Mod Pathol 2009;22:1303, Int J Gynecol Pathol 2022;41:552)
- Black women have a 2 fold higher risk compared with white women (Br J Cancer 2013;108:727)
- Hereditary retinoblastoma and Li-Fraumeni syndrome are characterized by increased incidence of leiomyosarcoma (Curr Probl Cancer 2019;43:283)
- In one series, increased predisposition reported in Finnish hereditary leiomyomatosis / renal cell carcinoma syndrome kindreds (J Med Genet 2006;43:523)
Clinical features
- Nonspecific symptoms:
- Abnormal uterine bleeding, pelvic or abdominal pain
- Rapidly growing uterine mass in a postmenopausal woman
- Usually an incidental finding, identified in 0.13% of hysterectomies for benign indication and 0.39% of hysterectomies for uterine leiomyomas (Am J Obstet Gynecol 2019;220:179.e1)
Diagnosis
- Myomectomy, hysterectomy
Laboratory
- No laboratory values are diagnostic:
- Some studies suggest leiomyosarcomas have higher levels of lactate dehydrogenase (LDH) versus uterine leiomyoma (BMC Cancer 2020;20:514)
- Variable success as a predictive marker
Radiology description
- No pathognomonic findings
- Difficult to distinguish from benign smooth muscle tumors (Curr Opin Oncol 2021;33:464)
- TVUS:
- Most common initial imaging modality
- Successfully detects uterine leiomyomas
- Does not differentiate between leiomyomas and leiomyosarcomas
- Most common initial imaging modality
- CT: not indicated for assessing uterine masses
- May show irregular central zones of low attenuation, suggesting necrosis and hemorrhage
- MRI, conventional techniques:
- Ill defined borders
- Central nonenhancement
- T1 weighted images with hyperintensity associated with tumoral hemorrhage or necrosis
- T2 weighted images show heterogeneous intermediate to high signal intensities
- Advanced imaging modalities show potential diagnostic improvement:
- Machine learning
- Radiomics
- Texture analysis (Jpn J Radiol 2022;40:385)
Radiology images
Prognostic factors
- Most important prognostic factor is stage
- Additional prognostic measures include age, tumor size, mitotic rate and lymphovascular invasion
- High recurrence rate (50 - 70%) regardless of stage at initial diagnosis
- Poor prognosis even if the tumor is confined to the uterus
- More favorable prognosis if tumor is < 5 cm and confined to the uterus
- 5 year overall survival (OS) rate for all stages is poor, ranging from 15 to 25%
- OS rates are more favorable at low stages (1 - 2), ranging from 40 to 70%
- Morcellation is associated with significantly increased risk of recurrence (Gynecol Oncol 2021;160:99)
- Risk stratification model, including mitoses > 25 per 2.4 mm2 (10 high power fields), atypical mitoses, coagulative necrosis, lymphovascular invasion and serosal abutment, is significantly associated with disease free and disease specific survival in stage I tumors (Mod Pathol 2022;35:794):
- 3 risk groups include low risk (0- 2 points), intermediate risk (3 - 5 points) and high risk (6 - 13 points)
- Serosal abutment and lymphovascular invasion can be omitted for myomectomy or morcellated specimens
Case reports
- 46 year old hemodynamically unstable woman with fever (Cureus 2020;12:e11586)
- 51 year old woman with metastatic leiomyosarcoma to the breast (Case Rep Pathol 2020;2020:8037646)
- 53 year old woman with diffuse abdominal pain and abnormal vaginal bleeding (Clin Pathol 2022;15:2632010X221105224)
- 59 year old woman with microsatellite instability high uterine leiomyosarcoma (Gynecol Oncol Rep 2021;35:100701)
- 68 year old woman with uterine myxoid leiomyosarcoma (Rom J Morphol Embryol 2021;62:883)
Treatment
- Surgical resection (hysterectomy) is standard treatment for patients with localized leiomyosarcoma:
- Adjuvant chemotherapy for early stage disease is controversial
- Bilateral salpingo-oophorectomy is reasonable in peri and postmenopausal patients
- Lymphadenectomy is only indicated if there is evidence of concerning lymph nodes (J Adv Pract Oncol 2022;13:70, StatPearls: Leiomyosarcoma [Accessed 26 October 2022])
- Radiotherapy is indicated for palliative care purposes in advanced or metastatic disease (Cancer Med 2022;11:2906):
- No evidence for increased overall survival
- Adjuvant chemotherapy is indicated for metastatic / recurrent tumors
- Immunotherapy is a potential option for MSI high uterine leiomyosarcoma (Gynecol Oncol Rep 2021;35:100701)
- Hormonal therapy may be an option in hormone receptor positive tumors
Clinical images
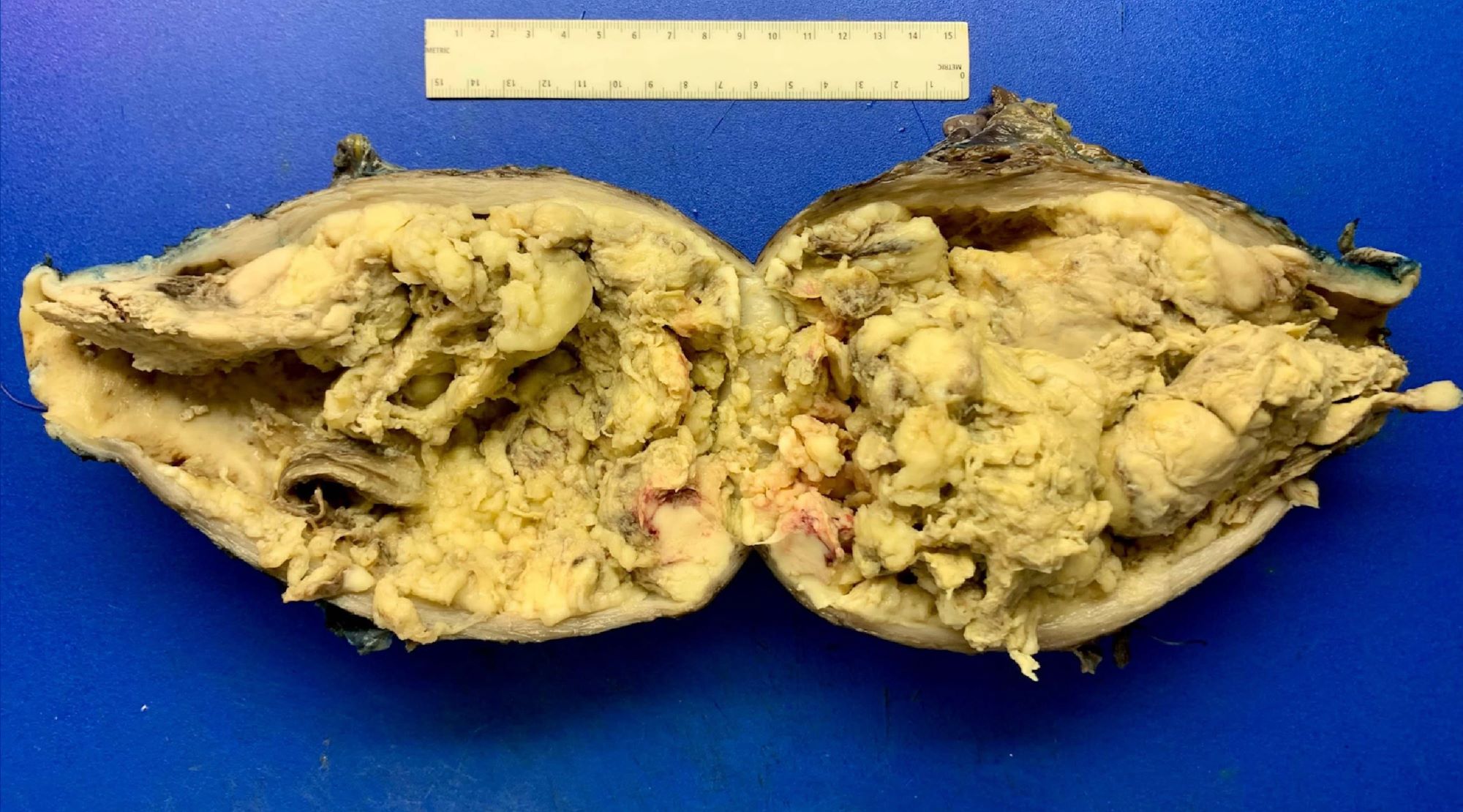
Gross description
- Often a solitary, bulky, fleshy mass within the myometrium - intramural (majority), submucosal, subserosal or pedunculated:
- Hemorrhagic, necrotic and cystic areas upon sectioning
- Grossly invasive / infiltrative
- Rarely may arise in the cervix (5%)
- Average diameter is 10 cm:
- ~25% are < 5 cm
- Myxoid leiomyosarcoma:
- Gelatinous cut surface
- Friable
- References: Oncol Res Treat 2018;41:680, Arch Pathol Lab Med 2008;132:595
Gross images
Frozen section description
- Not usually performed
- If done, assess:
- Cellularity
- Significant cytologic atypia
- Number of unequivocal mitotic figures
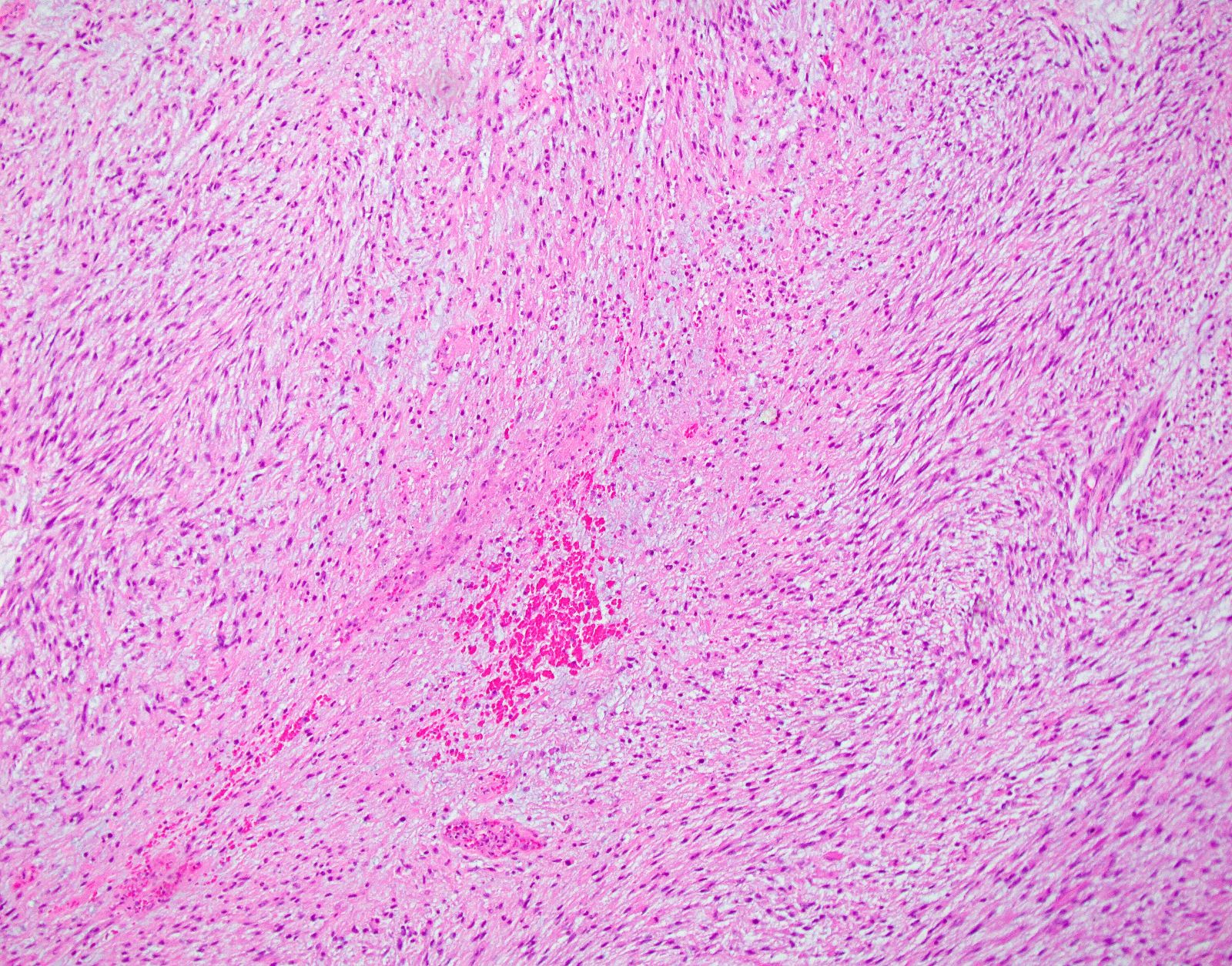
- Tumor cell necrosis:
- Karyorrhexis, perivascular cuffs of viable tumor cells in a background of necrosis and ghost cells are diagnostic clues for tumor cell necrosis (Am J Surg Pathol 2021;45:1179)
- If malignant criteria are met:
- Best practice is to call "malignant mesenchymal tumor”
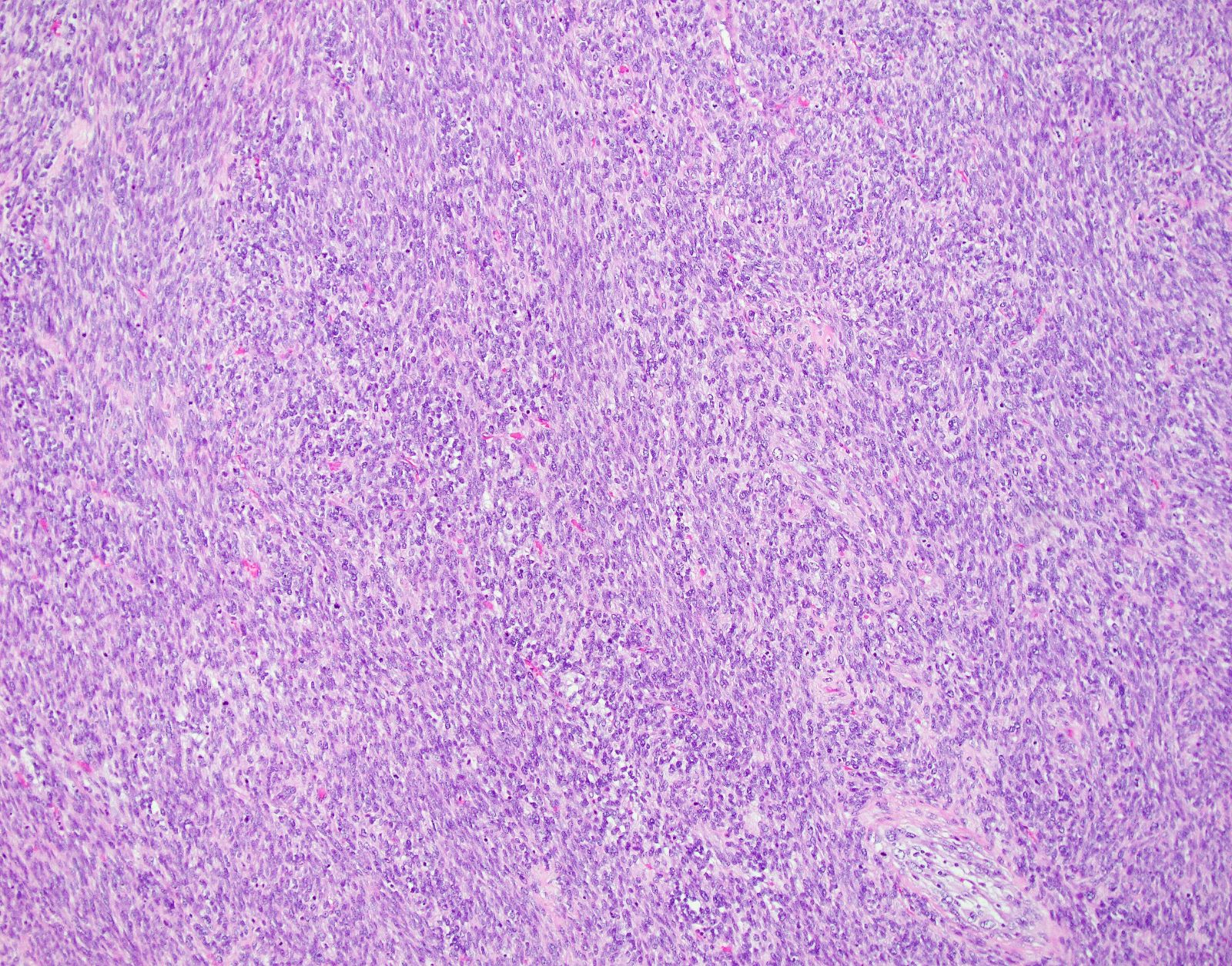
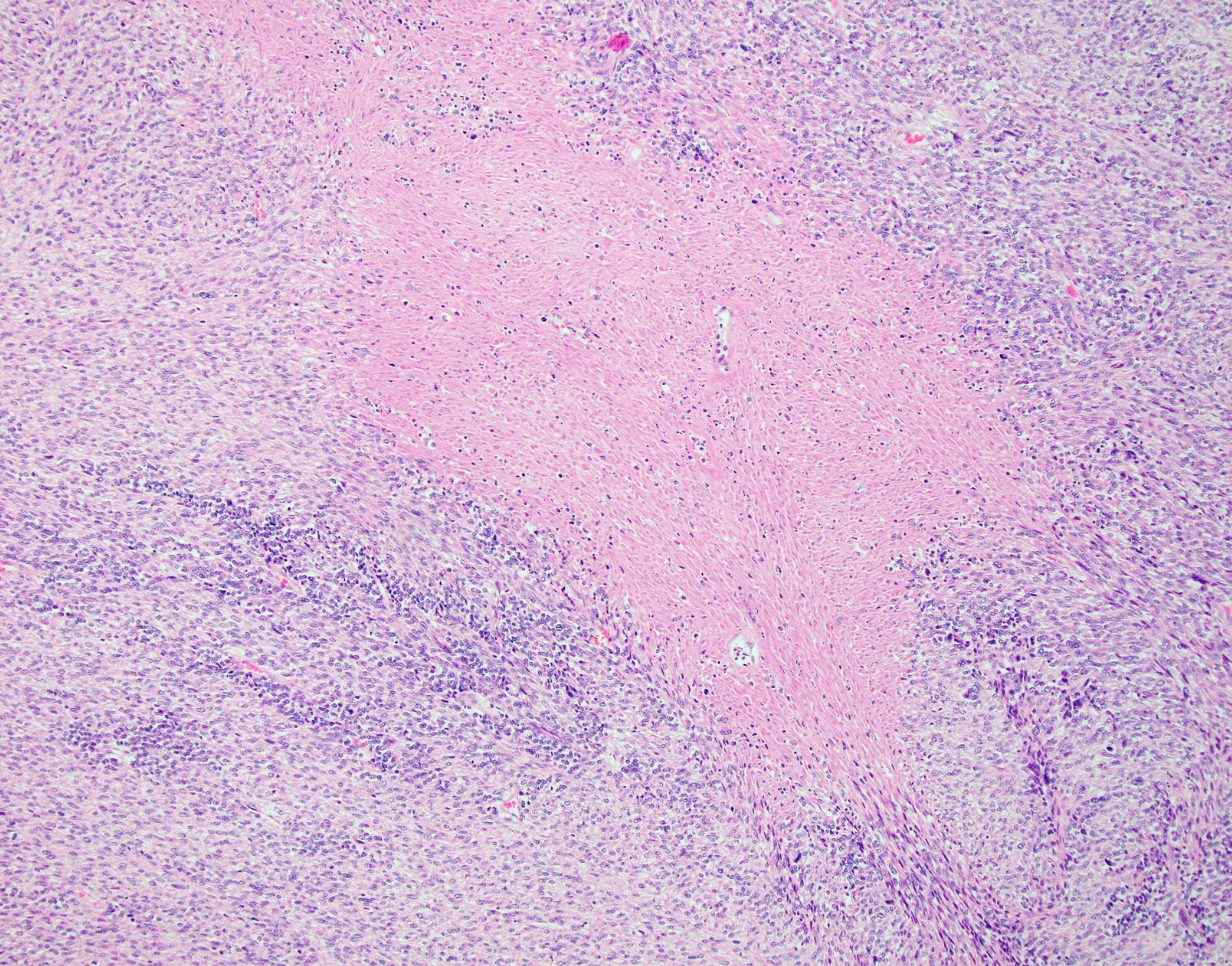
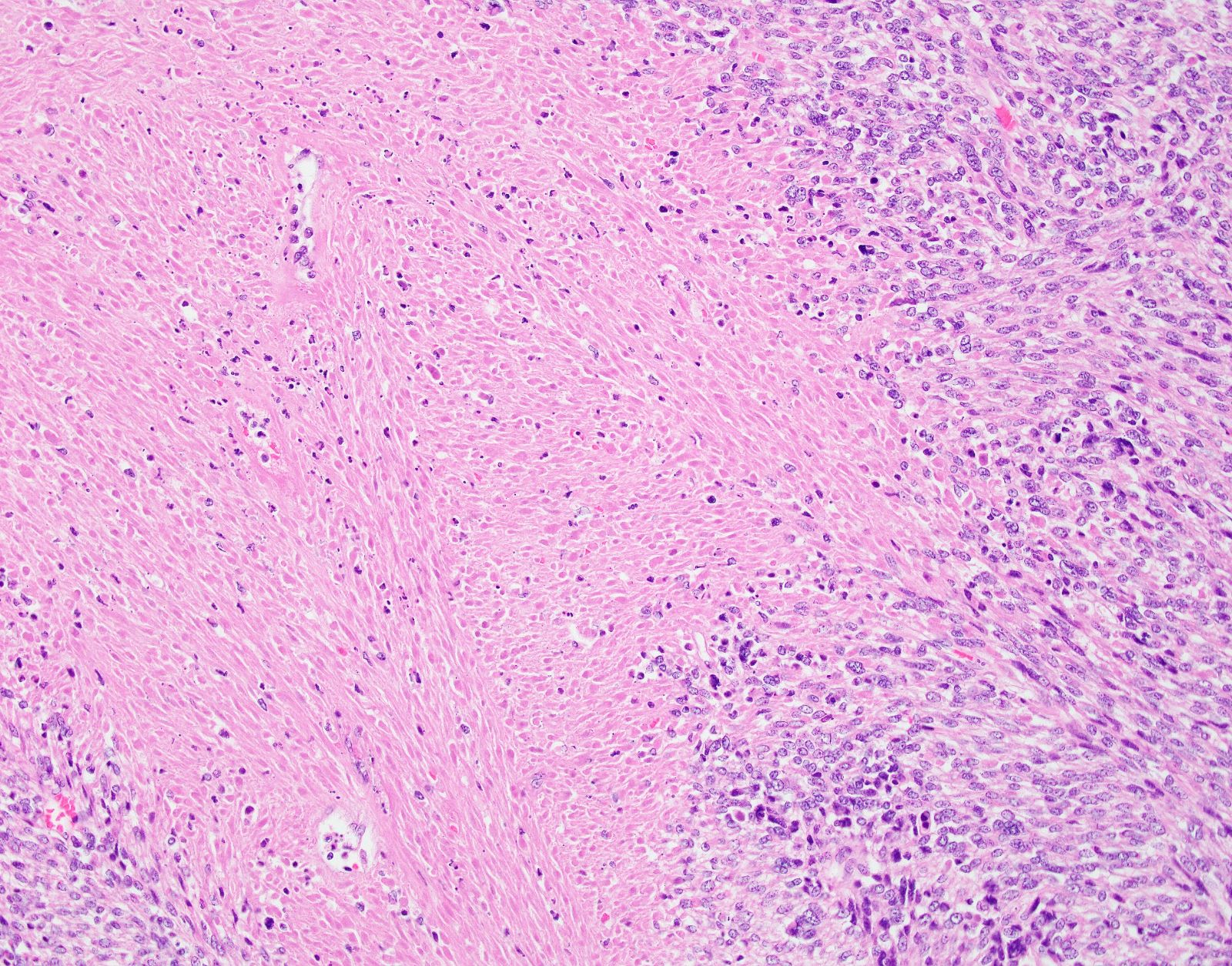
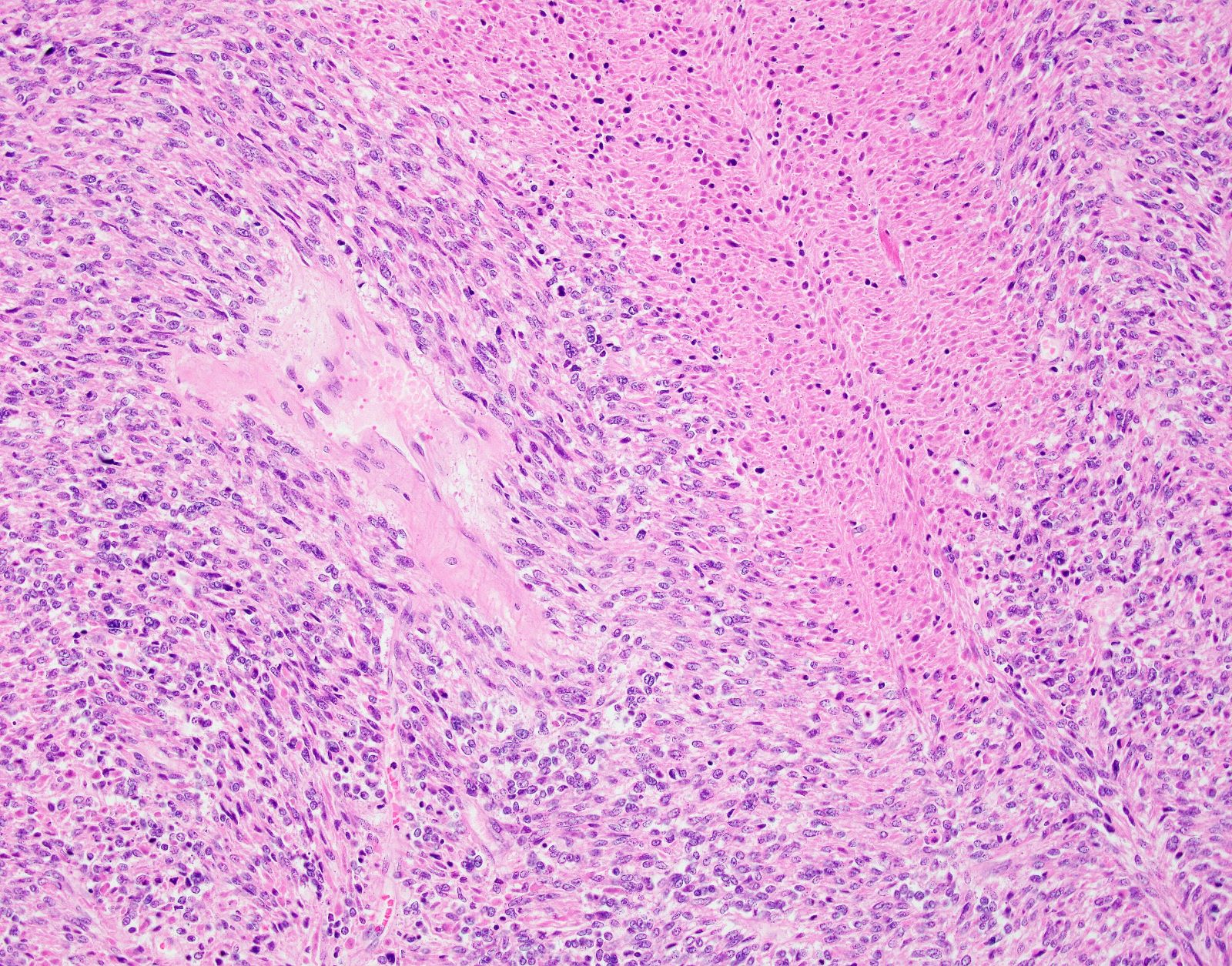
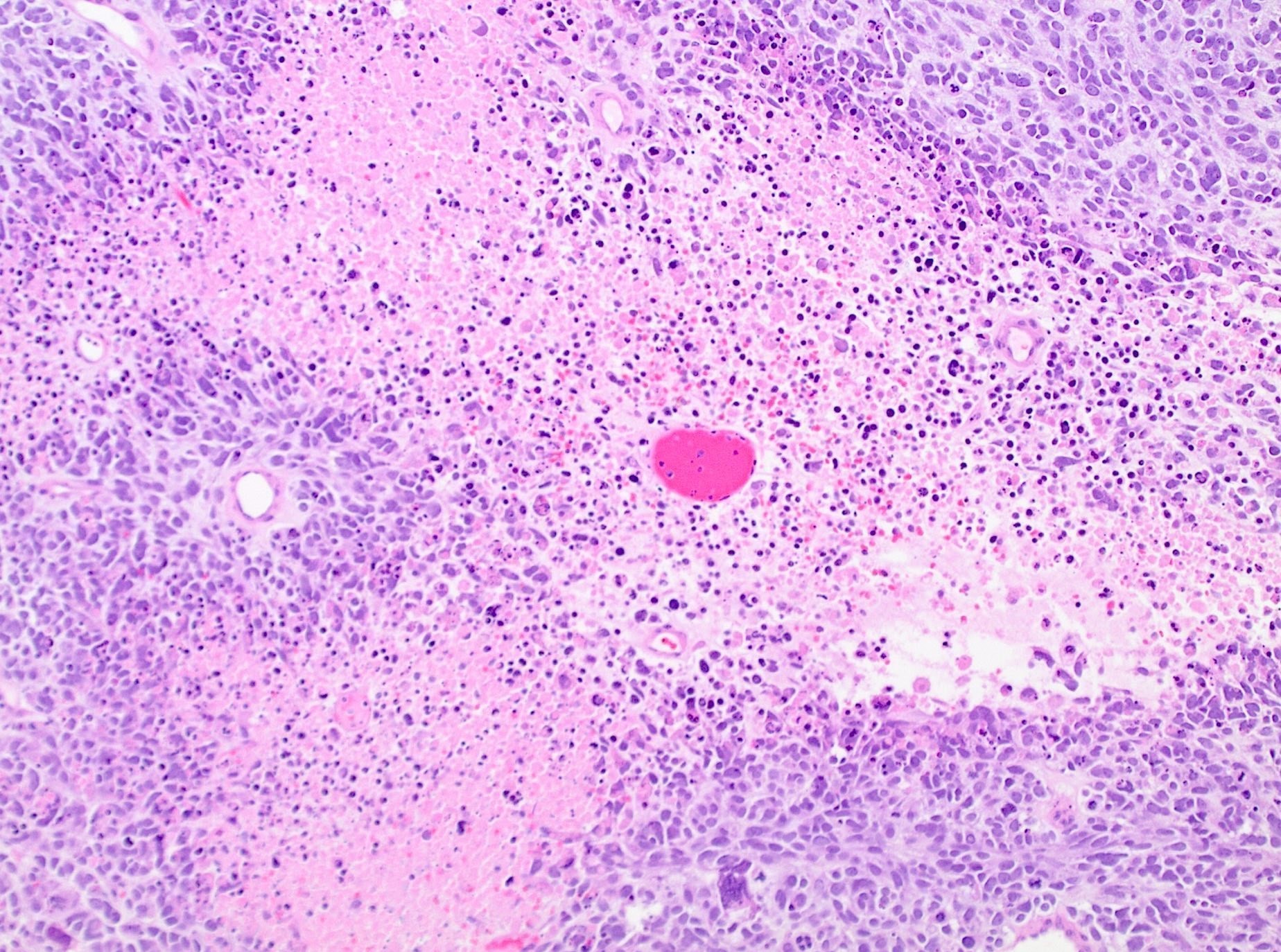
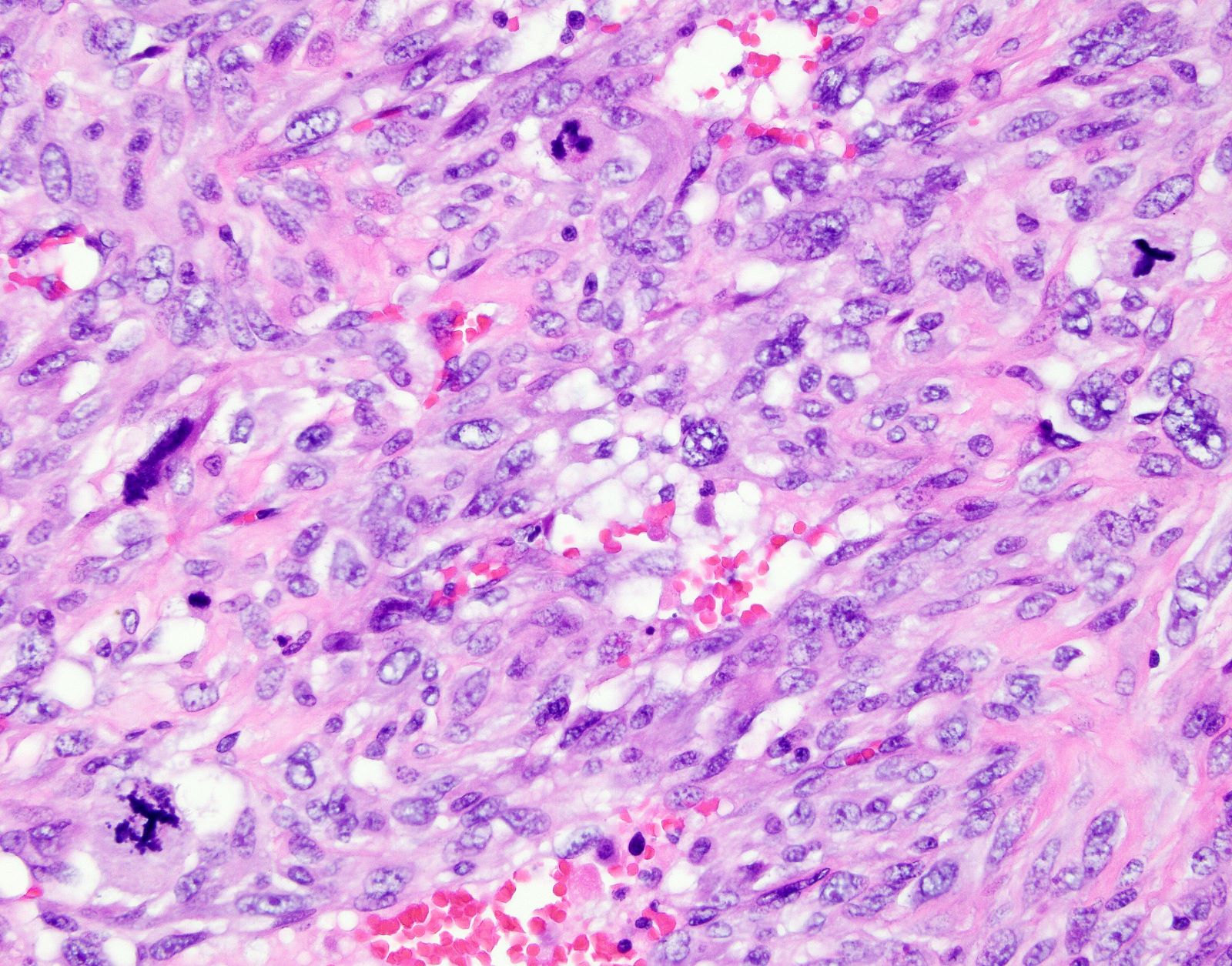
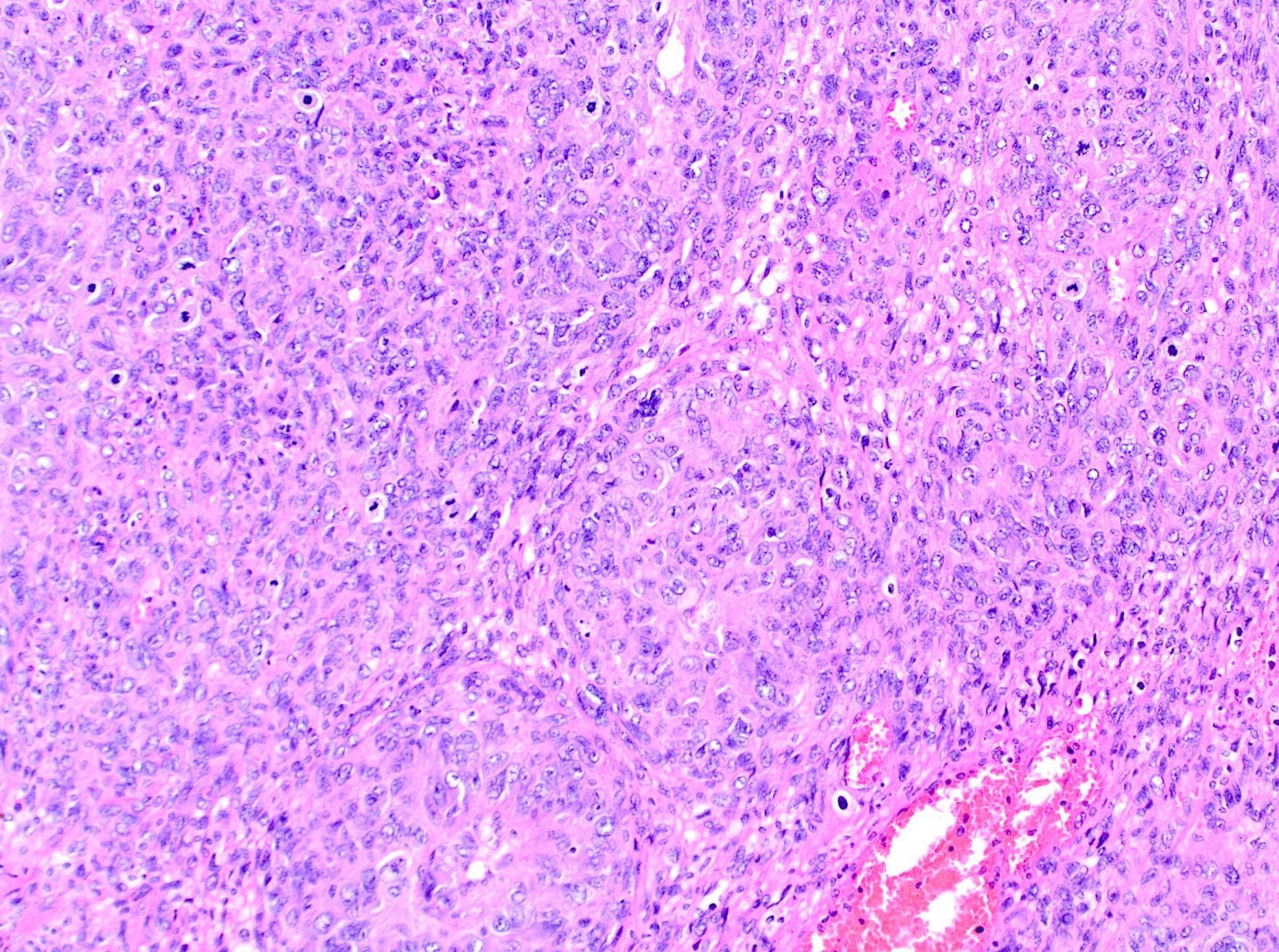
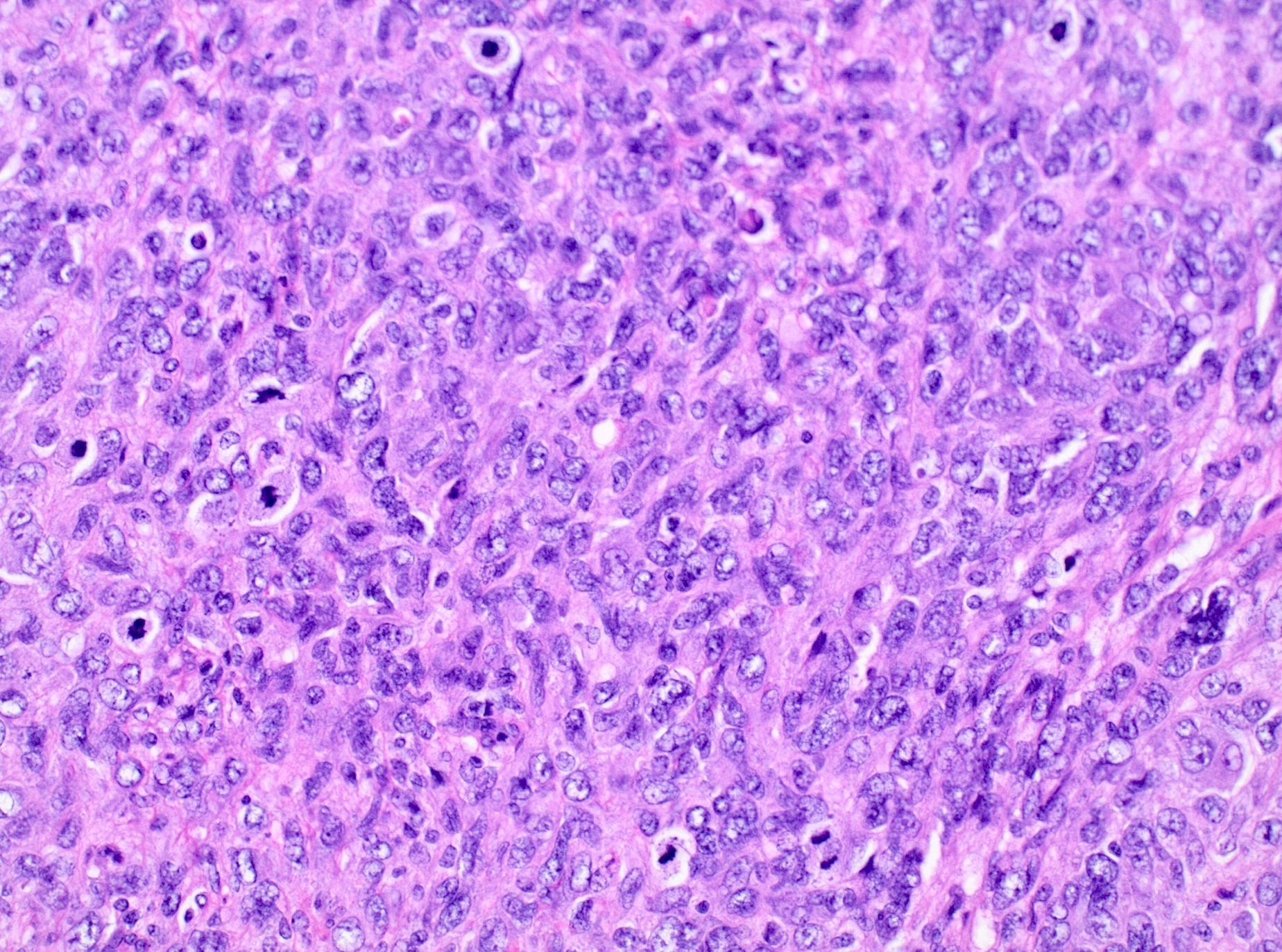
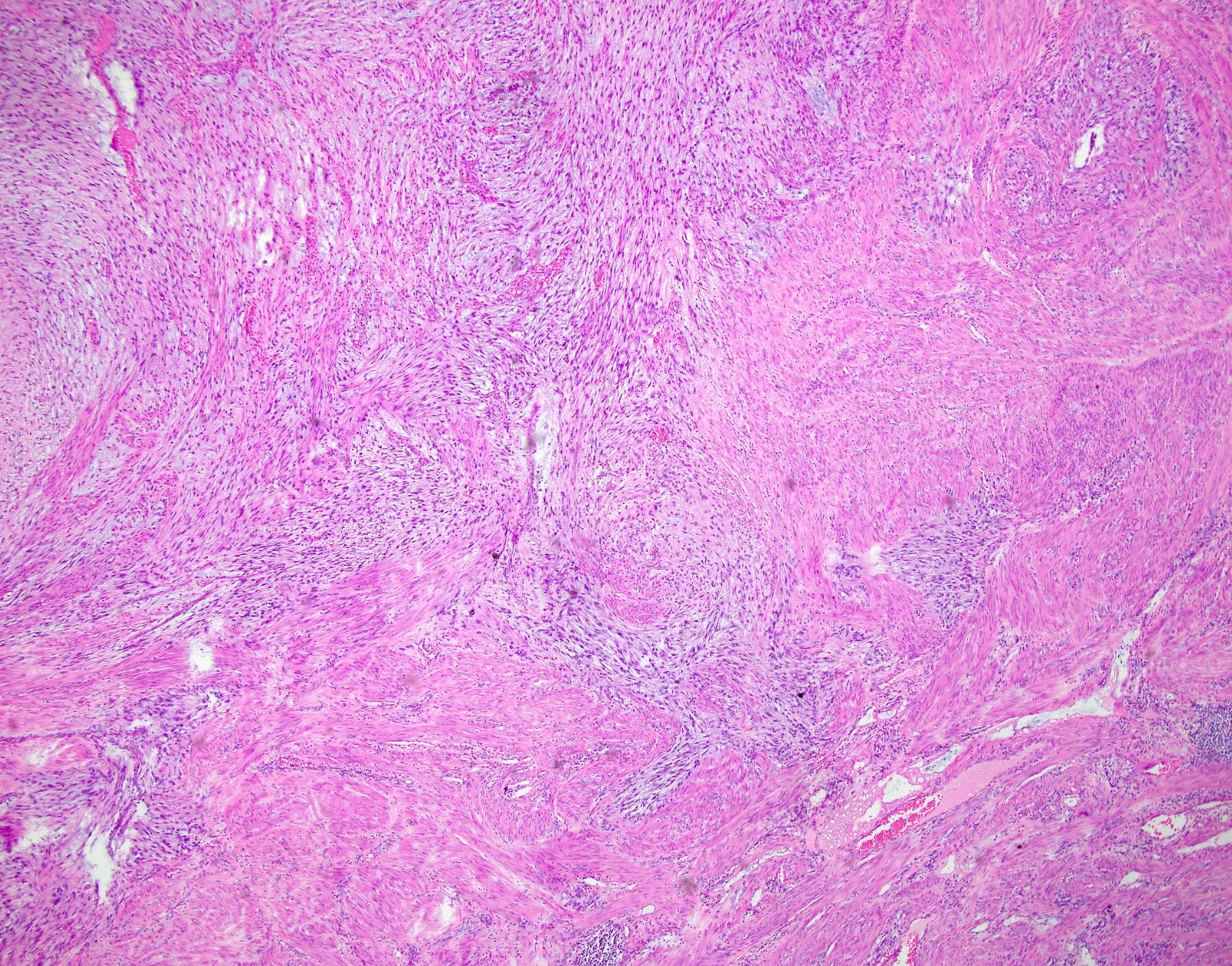
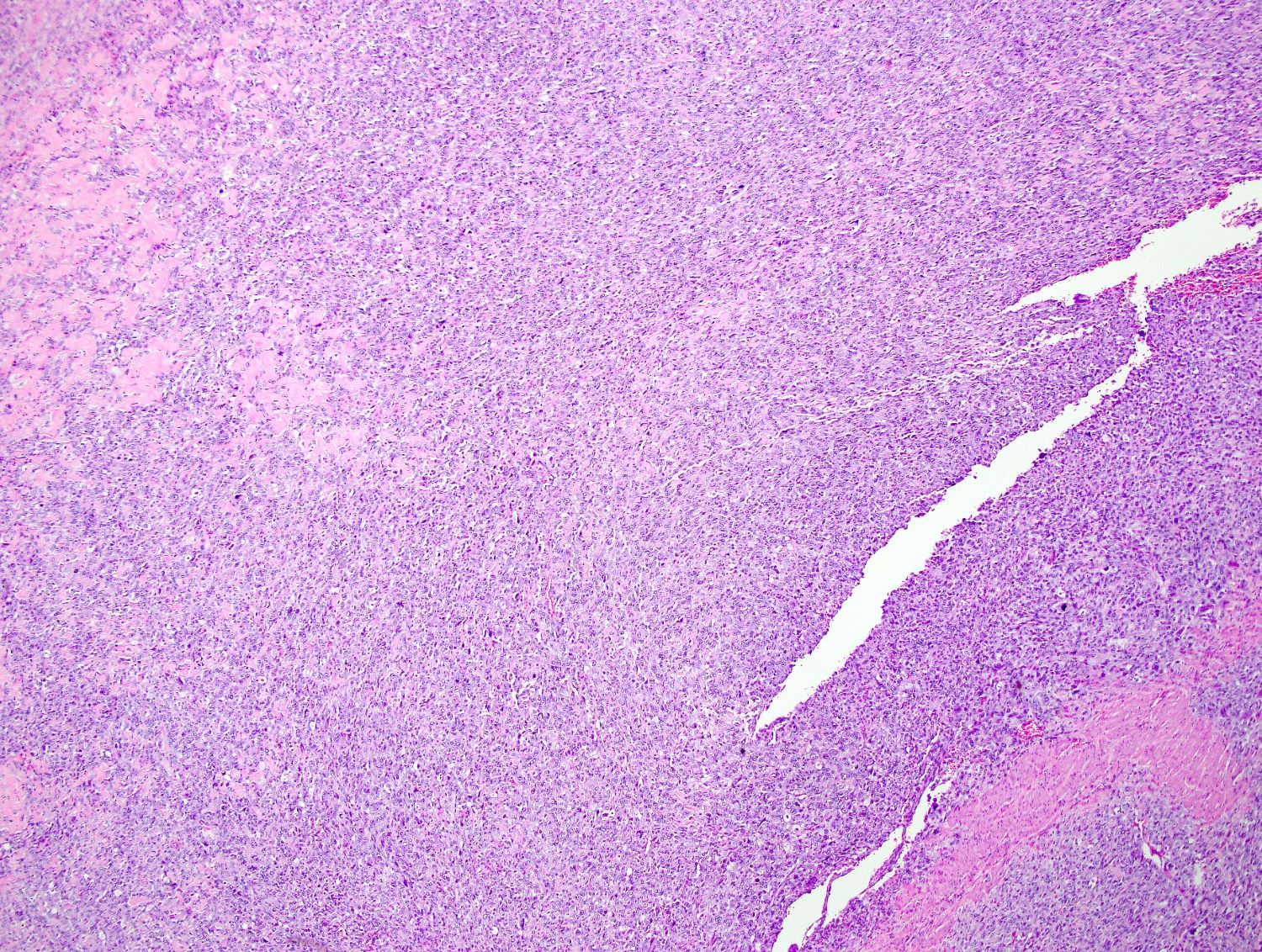
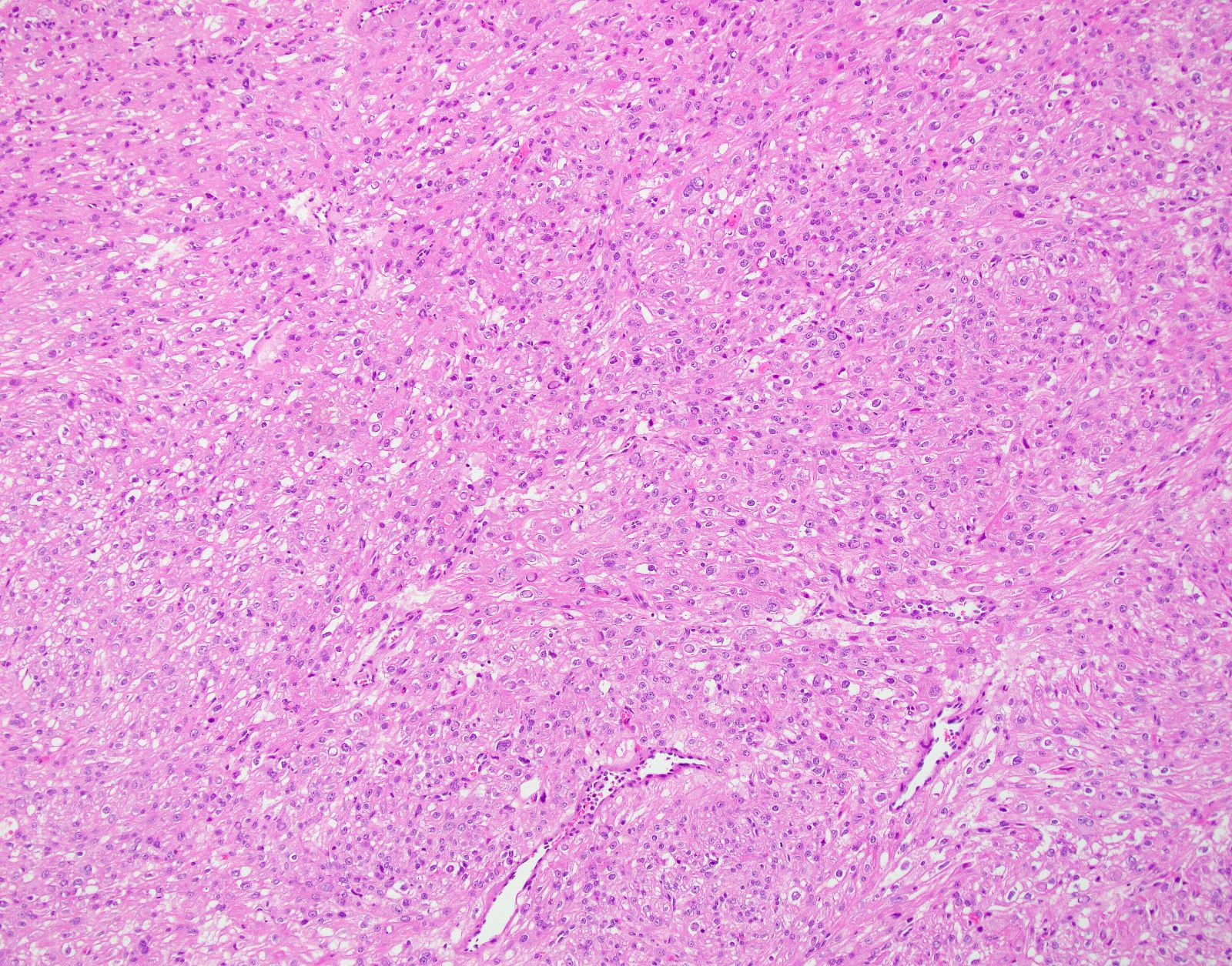
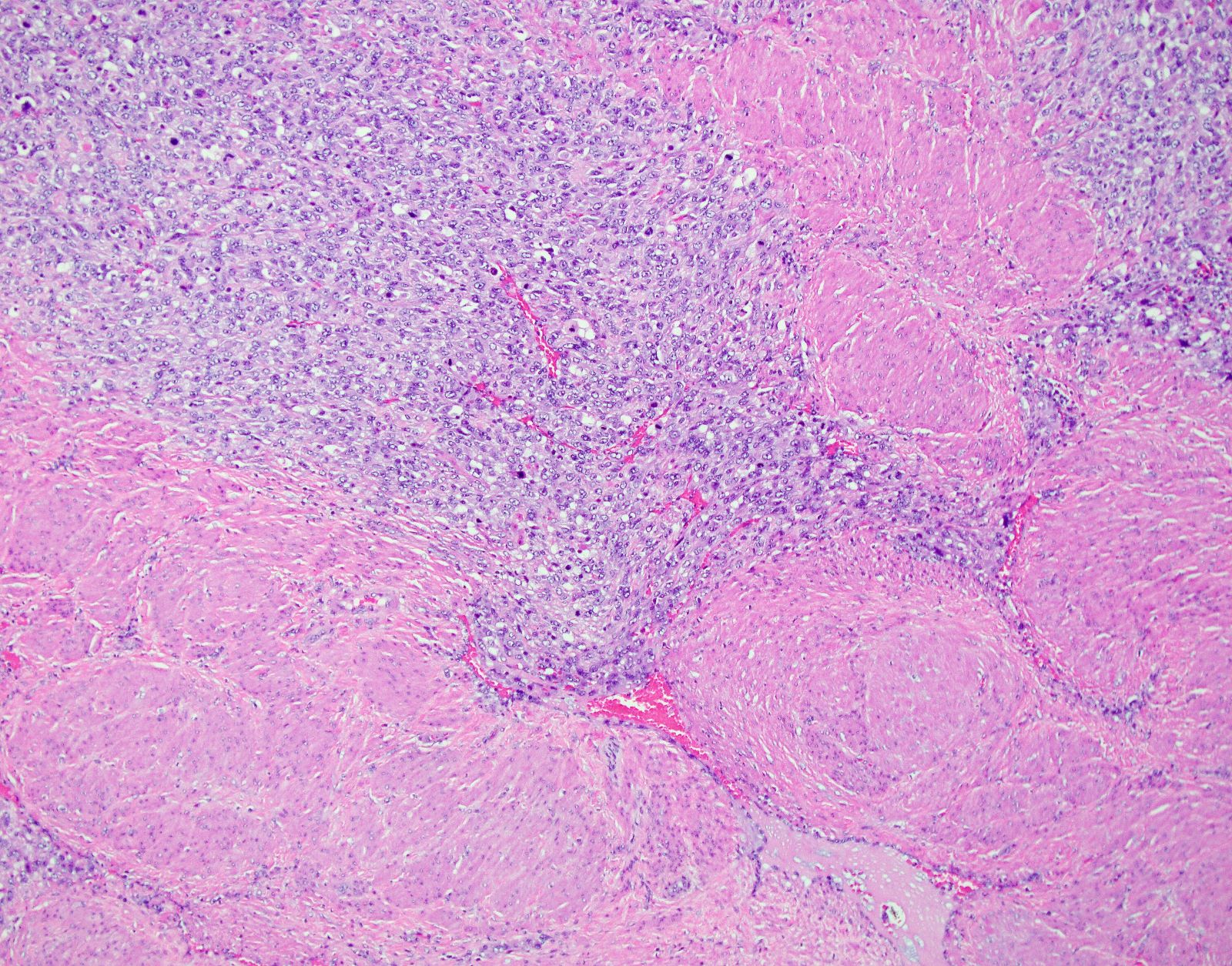
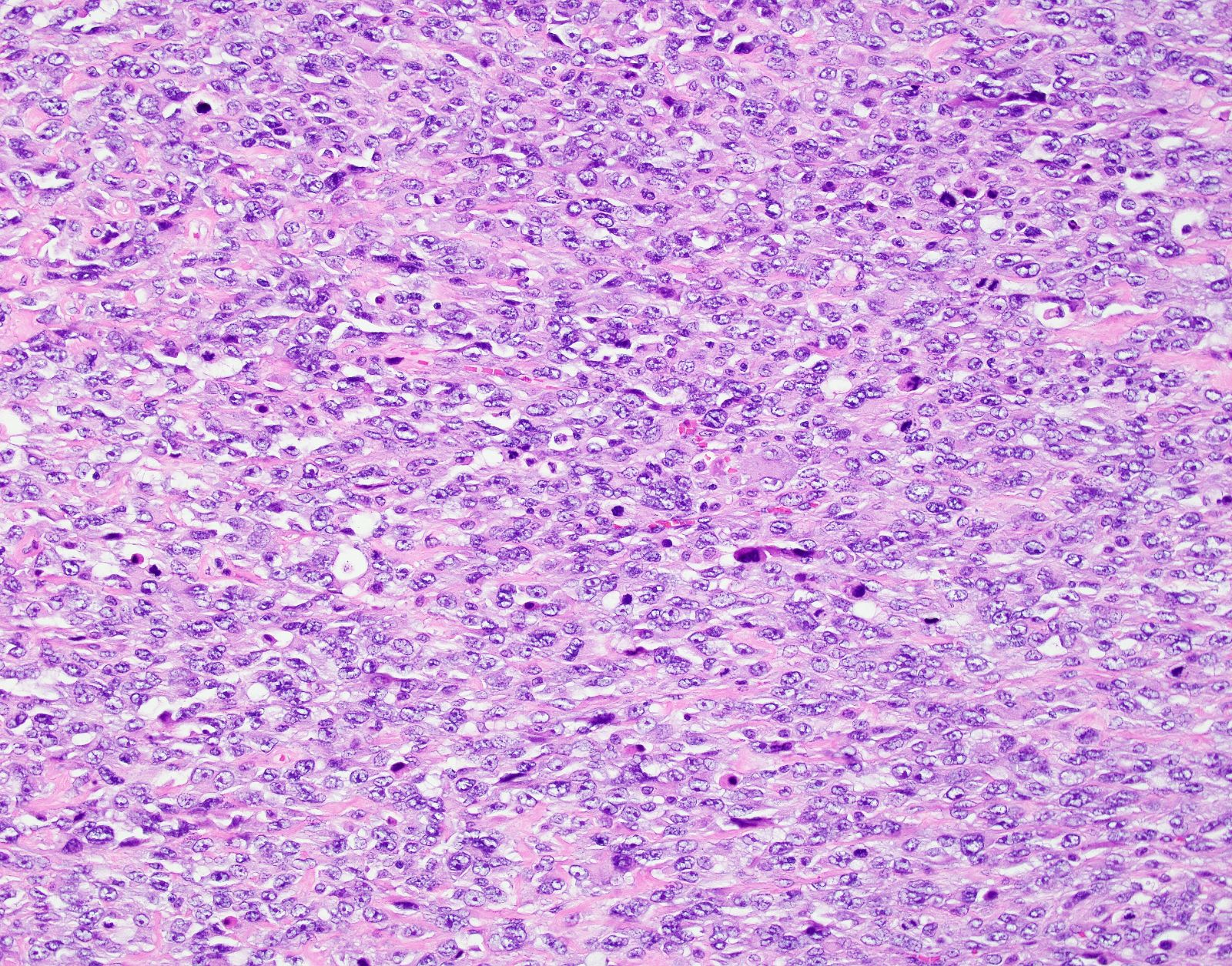
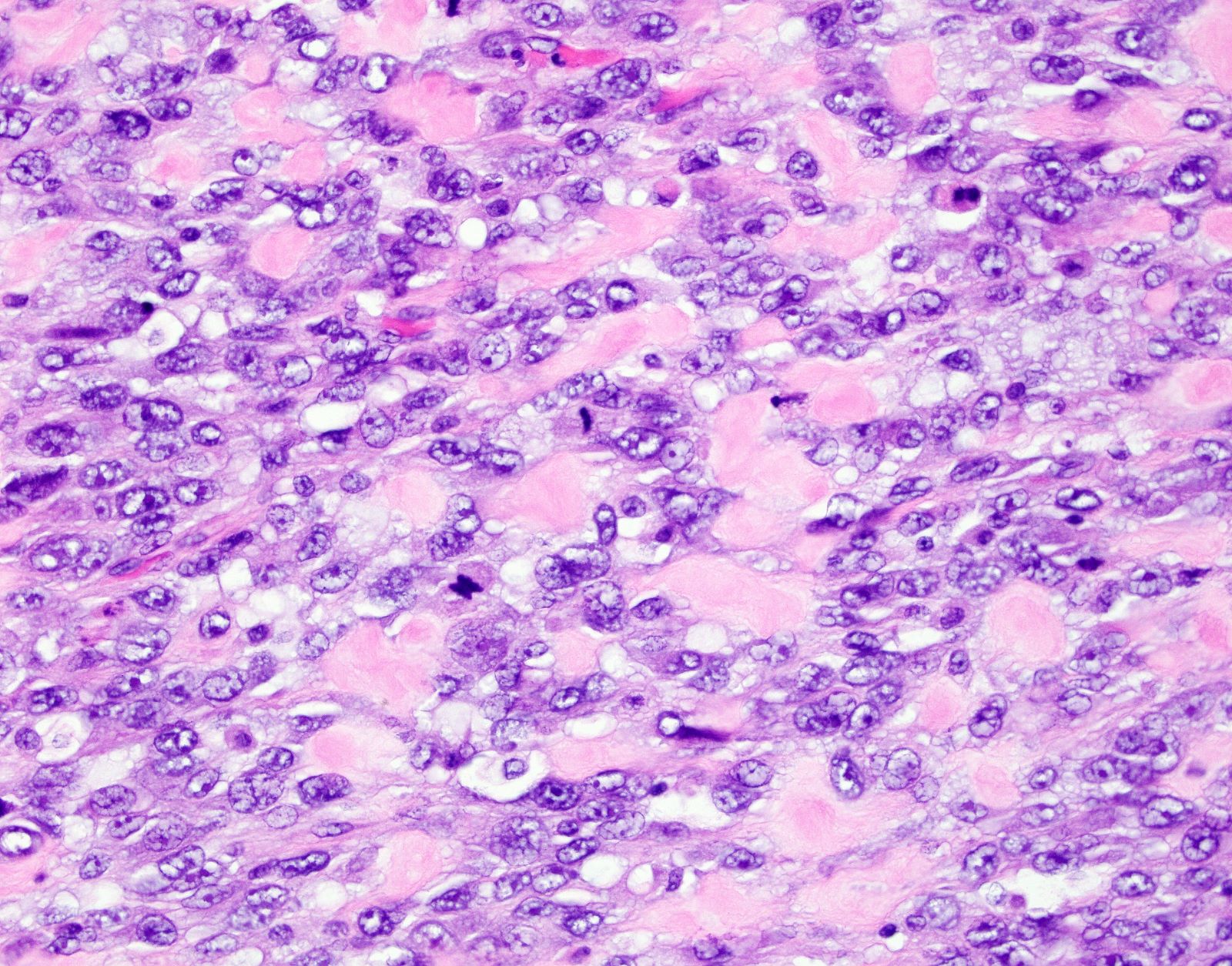
Microscopic (histologic) description
- Conventional / spindle cell type:
- Essential diagnostic criteria:
- Requires 2 of 3 histologic features:
- Marked cytologic atypia
- ≥ 10 mitoses / 10 high power fields
- Tumor cell necrosis
- Identified by abrupt transition from viable tumor cells to necrotic cells (ghost cells, apoptotic bodies may be seen)
- Granulation tissue surrounding necrosis is absent
- Requires 2 of 3 histologic features:
- Growth pattern:
- Cellular tumor comprised of long intersecting or haphazard fascicles
- Infiltrative border (common)
- Rarely, may arise from background leiomyoma
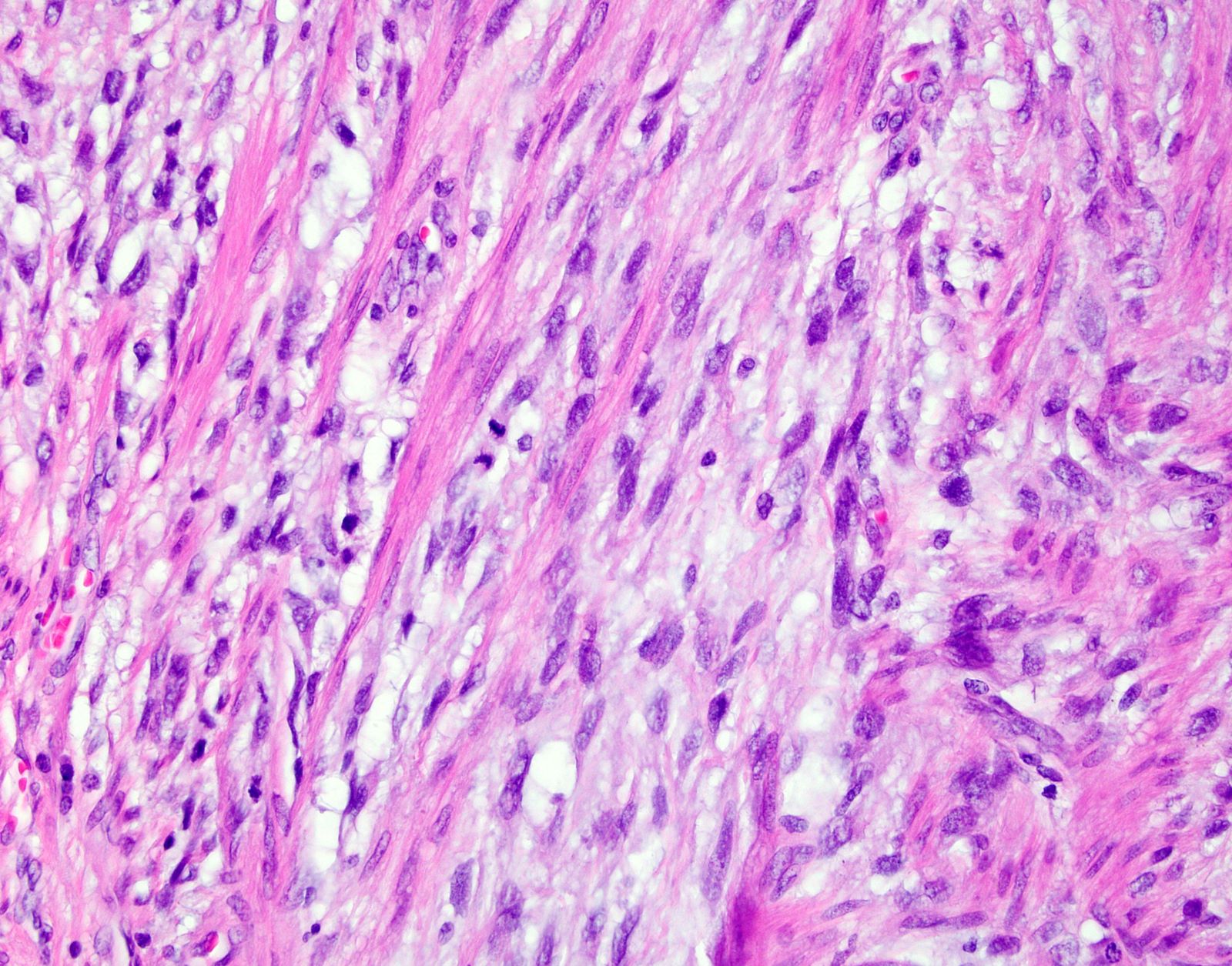
- Cytologic features:
- Spindle / elongated cells
- Eosinophilic cytoplasm
- Hyperchromatic nuclei often with moderate to severe nuclear pleomorphism (can be deceptively bland and uniform)
- Atypical mitoses are frequently identified
- Multinucleated and osteoclast-like giant cells may be seen
- Essential diagnostic criteria:
- Myxoid leiomyosarcoma:
- Diagnosis based on ≥ 1 of the following:
- Moderate to severe cytologic atypia
- Coagulative tumor cell necrosis
- ≥ 2 mitosis / 10 high power fields
- Infiltrative borders / irregular margins
- Growth pattern:
- Hypocellular tumor with abundant myxoid stroma
- Fascicular or nodular patterns are uncommon
- Myxoid stroma may be difficult to differentiate from hydropic change in small / limited samples
- Extensive sampling is generally required for diagnosis
- Diagnosis based on ≥ 1 of the following:
- Epithelioid leiomyosarcoma:
- Diagnosis based on ≥ 1 of the following:
- Moderate to severe cytologic atypia
- Tumor cell necrosis
- ≥ 4 mitoses / 10 high power fields
- Growth pattern:
- Arranged in nests, cords or sheets
- May show pseudoglandular spaces
- Cytologic features:
- > 50% of round or polygonal cells with eosinophilic or clear cytoplasm
- Rarely, extensive hyalinization
- Alternative criteria include ≥ 2 of the following features: moderate or severe atypia, ≥ 4 mitoses / 2.4 mm2 and tumor cell necrosis (Am J Surg Pathol 2022;46:464)
- Diagnosis based on ≥ 1 of the following:
- Classification systems used:
- FIGO
- TNM
- References: Arch Pathol Lab Med 2008;132:595, StatPearls: Leiomyosarcoma [Accessed 26 October 2022]
Microscopic (histologic) images
Contributed by Ashley Monsrud, M.D. and Paulette Mhawech-Fauceglia, M.D.
Virtual slides
Positive stains
- Smooth muscle markers including h-caldesmon (more specific), desmin and SMA:
- May be weak or patchy, especially in myxoid leiomyosarcoma (Adv Anat Pathol 2017;24:354)
- p53 may show aberrant expression (Histopathology 2017;70:1138)
- p16 often shows diffuse expression (Histopathology 2017;70:1138)
- ER / PR
- WT1, keratins, EMA and CD10 are often positive (Am J Surg Pathol 2002;26:403)
- Myxoid subtypes: Alcian blue, colloidal iron and PLAG1 (~50%) (Am J Surg Pathol 2019;43:382, Mod Pathol 2019;32:1688)
- High Ki67 proliferation index (> 10% nuclear staining) can be helpful unless if differentiating from a leiomyoma with bizarre nuclei (Int J Gynecol Pathol 2020;39:354, Int J Gynecol Pathol 2021;40:257)
Negative stains
- HMB45, MelanA, TFE3, MITF and cathepsin K (usually negative or focally and weakly positive) (Int J Gynecol Pathol 2020;39:529, Am J Surg Pathol 2021;45:77, Am J Surg Pathol 2022;46:464)
- ALK
- Androgen receptor (AR) (may be positive)
- Cyclin D1 (rare exceptions)
- S100
Molecular / cytogenetics description
- No specific diagnostic / pathognomonic molecular alterations
- Substantial mutational heterogeneity, widespread DNA copy number alterations including chromothripsis and frequent whole genome duplication (Nat Commun 2018;9:144)
- Hallmarks of "BRCAness", including alterations in homologous recombination DNA repair genes, multiple structural rearrangements and enrichment of specific mutational signatures (Proc Natl Acad Sci U S A 2021;118:e2025182118, Nat Commun 2018;9:144)
- Alternative telomere lengthening (78%) (Nat Commun 2018;9:144)
- Somatic mutations (BMC Cancer 2017;17:639, Proc Natl Acad Sci U S A 2021;118:e2025182118, Nat Commun 2018;9:144)
- TP53 (30 - 60%)
- ATRX (24 - 30%)
- RB1 (27 - 61%)
- MED12 (~20%)
- PTEN (~31 - 57%)
- C-MYC (18%) and TERT (26%) amplifications (Proc Natl Acad Sci U S A 2021;118:e2025182118)
- NR4A3::PGR fusion or PGR rearrangements in 35% of epithelioid leiomyosarcomas (Am J Surg Pathol 2019;43:810)
- PLAG1 rearrangements in ~25% of myxoid leiomyosarcomas (Am J Surg Pathol 2019;43:382)
Videos
Review of uterine leiomyosarcoma
Sample pathology report
- Uterus and cervix, total hysterectomy:
- Myometrium:
- Leiomyosarcoma
- Tumor size: 15 cm
- Cytologic atypia: diffuse, marked
- Coagulative tumor cell necrosis: present
- Mitotic count: 25 per 10 high power fields
- Lymphovascular invasion: negative
- Margin status: negative
- Other findings: leiomyomata
- See synoptic report
- Endometrium: inactive
- Uterine serosa: benign
- Cervix: benign
- Myometrium:
Differential diagnosis
- Endometrial stromal sarcoma:
- Low grade endometrial stromal sarcoma:
- Tumor cells resemble proliferative type endometrial stroma
- Minimal cytologic atypia and low mitotic index
- Diffusely positive for CD10 and ER / PR but h-caldesmon typically negative or weak expression
- Cyclin D1 (focal)
- ~66% harbor gene fusions:
- JAZF1::SUZ12 (most common), followed by JAZF1::PHF1, EPC1::PHF1 and MEAF6::PHF1
- High grade endometrial stromal sarcoma:
- Round or spindle high grade cells with brisk mitoses and necrosis
- Infiltrative growth pattern
- Molecular alterations are: YWHAE::NUTM2A / YWHAE::NUTM2B fusion, ZC3H7B::BCOR and BCOR internal tandem duplication (ITD)
- Low grade endometrial stromal sarcoma:
- Leiomyoma variants:
- Mitotically active leiomyoma:
- No cytologic atypia or coagulative tumor cell necrosis
- 6 - 14 mitoses / 10 HPF
- Leiomyoma with apoplectic changes:
- Zonation phenomena (benign smooth muscle away from necrotic areas)
- Cellular leiomyoma:
- Increased cellularity but no cytologic atypia or tumor necrosis
- Leiomyoma with bizarre nuclei:
- Scattered or diffuse bizarre nuclei with adjacent areas of classic leiomyoma
- Myxoid leiomyoma:
- Usually focal myxoid change within a conventional leiomyoma, no coagulative tumor cell necrosis, cytologic atypia or mitotic activity
- Epithelioid leiomyoma:
-
No coagulative tumor cell necrosis, cytologic atypia or mitotic activity
- Mitotically active leiomyoma:
- Smooth muscle tumors of uncertain malignant potential (STUMP):
- Some but not all criteria are met for uterine leiomyosarcoma:
- Spindled smooth muscle tumors: focal / multifocal or diffuse cytologic atypia and 2 - 4 mitoses / mm2 (6 - 9 mitoses / 10 high power fields, 0.55 mm field of diameter, 0.24 mm2 in area) but lacking coagulative necrosis; unequivocal coagulative necrosis but lacking cytologic atypia or elevated mitoses; elevated mitoses at > 6 mitoses / mm2 or > 15 mitoses / 10 high power fields (FD = 0.55, 0.24 mm2 in area) but lacking coagulative necrosis or cytologic atypia; diffuse cytologic atypia and uncertain mitotic count, often due to prominent karyorrhexis but lacking coagulative necrosis
- Epithelioid smooth muscle tumors: epithelioid morphology with 2 - 3 mitoses / 10 high power fields (FD = 0.55, 0.24 mm2 in area) but lacking moderate to severe cytologic atypia and coagulative necrosis
- Myxoid smooth muscle tumors: myxoid morphology but lacking mitotic activity, moderate to severe cytologic atypia, coagulative necrosis and infiltrative / irregular borders
- Some but not all criteria are met for uterine leiomyosarcoma:
- Inflammatory myofibroblastic tumor:
- Spindle cell neoplasm with myxoid stroma and associated lymphoplasmacytic inflammatory cells
- Atypia can range from mild to severe
- ALK staining is seen in most tumors and ALK rearrangement is seen in 80% of cases (Mod Pathol 2017;30:1489)
- Perivascular epithelioid cell tumor:
- Composed of epithelioid or spindled cells with eosinophilic to clear cytoplasm with variable cytologic atypia and mitoses
- Cells may be organized in a perivascular fashion, short fascicles, sheets or nests
- Melanin rarely present
- Typically strongly HMB45 positive and often MelanA or MITF positive
- Cathepsin K staining
- A subset harbor TSC1 and TSC2 alterations or TFE3 fusion (Mod Pathol 2022;35:515)
Additional references
Board review style question #1
Board review style answer #1
C. ≥ 10 mitoses per 10 high power fields. Uterine spindle cell leiomyosarcomas are diagnosed based on the presence of 2 of 3 morphologic features: a mitotic count of ≥ 10 mitoses per 10 high power fields, moderate to marked cytologic atypia and coagulative tumor cell necrosis.
Comment Here
Reference: Leiomyosarcoma
Comment Here
Reference: Leiomyosarcoma
Board review style question #2
A 62 year old woman underwent a hysterectomy for a uterine mass. Immunohistochemical stains show that the tumor is positive for h-caldesmon and desmin and negative for CD10 and ALK. What is the most likely diagnosis?
- Endometrial stromal sarcoma
- High grade endometrial adenocarcinoma
- Inflammatory myofibroblastic tumor
- Leiomyosarcoma, myxoid subtype
Board review style answer #2
D. Leiomyosarcoma, myxoid subtype. The image shows a myxoid mesenchymal tumor with infiltrative borders. The morphologic findings combined with the immunophenotype is consistent with leiomyosarcoma, myxoid subtype.
Comment Here
Reference: Leiomyosarcoma
Comment Here
Reference: Leiomyosarcoma