Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Interpretation | Uses by pathologists | Prognostic factors | Microscopic (histologic) images | Virtual slides | Positive staining - normal | Positive staining - disease | Negative staining | Sample pathology report | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Caraccio C, Bruehl F. CD68. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cdmarkersCD68.html. Accessed April 2nd, 2025.
Definition / general
- Lysosomal associated transmembrane glycoprotein that is present in a variety of normal and neoplastic cell types and is primarily used as a marker to identify histiocytes and histiocytic tumors
Essential features
- Lysosomal marker used to identify histiocytic and monocytic cells with limited specificity
- KP1 and PGM1 are the 2 most commonly used antibody clones, with PGM1 being the slightly more specific marker
Terminology
- Lysosomal associated membrane protein 4 (LAMP4)
- Gp110
- Scavenger receptor class D member 1 (SCARD1)
- Macrosilian (murine analog)
- KP1 (common antibody clone)
- PGM1 (common antibody clone)
- EBM11 (less commonly used antibody clone)
- KiM6 and KiM7 (less commonly used antibody clones)
Pathophysiology
- Mucin rich transmembrane glycoprotein (110 kDa), encoded by a gene on chromosome 17p13 (Blood 1993;81:1607, Lab Invest 2017;97:4)
- CD68 primarily localizes to lysosomes and endosomes; a smaller fraction circulates to the cell surface (Lab Invest 2017;97:4)
- Macrophage CD68 is upregulated in response to inflammatory stimuli (Proc Natl Acad Sci U S A 1996;93:14833, Atheroscler Thromb 2002;9:57)
- CD68 can bind apoptotic cells, phosphatidylserine and oxidized low density lipoprotein (Proc Natl Acad Sci U S A 1996;93:14833, Proc Natl Acad Sci U S A 28;92:1396, Proc Natl Acad Sci U S A 1995;92:1391)
Interpretation
- Dot-like granular or diffuse cytoplasmic stain (Mod Pathol 2011;24:390, Scand J Immunol 2020;92:e12889, Melanoma Res 2019;29:237)
Uses by pathologists
- Histiocytic malignancies are usually CD68 positive (Am J Clin Pathol 2004;122:794)
- CD68 is used to diagnose histiocytic sarcoma, which usually expresses at least 2 of the following markers: CD68, CD163, CD4, lysozyme (Blood 2016;127:2672)
- Myeloid / monocytic sarcoma is usually CD68 positive (Leukemia 2007;21:340)
- PGM1 is more specific to monocyte / macrophages than KP1, which is widely reactive (Ann Rheum Dis 2004;63:774)
- Renal cell carcinoma with t(6;11) is consistently KP1 positive and PGM1 negative, whereas epithelioid angiomyolipoma is KP1 and PGM1 positive (Mod Pathol 2018;31:474)
- Double staining of CD68 and CD31 can aid in the diagnosis of antibody mediated rejection by labeling intracapillary macrophages (Am J Transplant 2019;19:3149)
- CD68 can aid in identification of chronic granulomatous disease (CGD) in patients with colonic inflammation; CGD patients have a significantly smaller average of CD68 positive cells per mm2 of lamina propria (242.3 cells/mm2) than patients with Crohn’s disease (1,104.2 cells/mm2) and the control group (565.4 cells/mm2) (Inflamm Bowel Dis 2009;15:1213)
- CD68 can aid in the differential diagnosis between cellular fibrous histiocytoma (83% KP1 positive) and dermatofibrosarcoma protuberans (6% KP1 positive) (J Cutan Pathol 2006;33:353)
- Primary cutaneous acral CD8 positive T cell lymphoproliferative disorder shows dot-like CD68 positivity, allowing it to be distinguished from other primary or secondary cutaneous CD8 positive T cell lymphomas (Br J Dermatol 2015;172:1573)
Prognostic factors
- Elevated numbers of CD68 positive tumor associated macrophages (TAMs) correlate with negative survival outcomes in many cancer types; TAMs are predominantly M2 macrophages, which can promote tumor growth / spread through the secretion of MMP9, VEGF, EGF and TGF1 (Clin Transl Oncol 2016;18:251)
- Anaplastic large cell lymphoma (ALCL): increased intratumoral TAMs correlate with an adverse outcome in anaplastic lymphoma kinase negative ALCL (Histopathology 2014;65:490)
- Breast cancer: increased TAMs predict worse cancer specific survival and a shorter disease free interval; patients with lower TAM levels showed improved metastasis free survival (J Clin Pathol 2012;65:159, Int J Cancer 2012;131:426)
- Diffuse large B cell lymphoma: TAMs are associated with a favorable prognosis when patients are treated with rituximab in addition to multiagent chemotherapy and with a poor outcome when rituximab is not given (Haematologica 2015;100:143)
- Classical Hodgkin lymphoma: studies have suggested elevated TAM expression as a negative indicator of survival in classical Hodgkin lymphoma; however, this association has been disputed (Leuk Lymphoma 2011;52:1913, N Engl J Med 2010;362:875, Diagn Pathol 2020;15:10, BMC Med 2016;14:159, Ann Oncol 2012;23:736)
- Follicular lymphoma: TAMs are associated with adverse outcomes in chemotherapy treated patients; in contrast, after a rituximab and chemotherapy combination regimen, high TAM content is correlated with longer overall survival (Clin Cancer Res 2007;13:5784)
- Hepatocellular carcinoma: density of peritumoral TAMs is associated with poor recurrence free survival and poor overall survival (PLoS One 2013;8:e59771)
- Squamous cell carcinoma of the head and neck: increased TAMs are associated with worse relapse free survival, worse disease specific survival and worse overall survival (Head Neck 2016;38:1074, Oral Oncol 2015;51:90)
- Myxoid liposarcoma: increased TAMs are associated with poorer overall survival rate (Br J Cancer 2015;112:547)
- Urothelial carcinoma: a high CD68/CD3 positive ratio identifies a bad prognosis group among muscle invasive cases (Urol Oncol 2014;32:791)
- Brain tumors: CD68 TAMs are associated with higher tumor grade in astrocytoma (Clin Cancer Res 2013;19:3776)
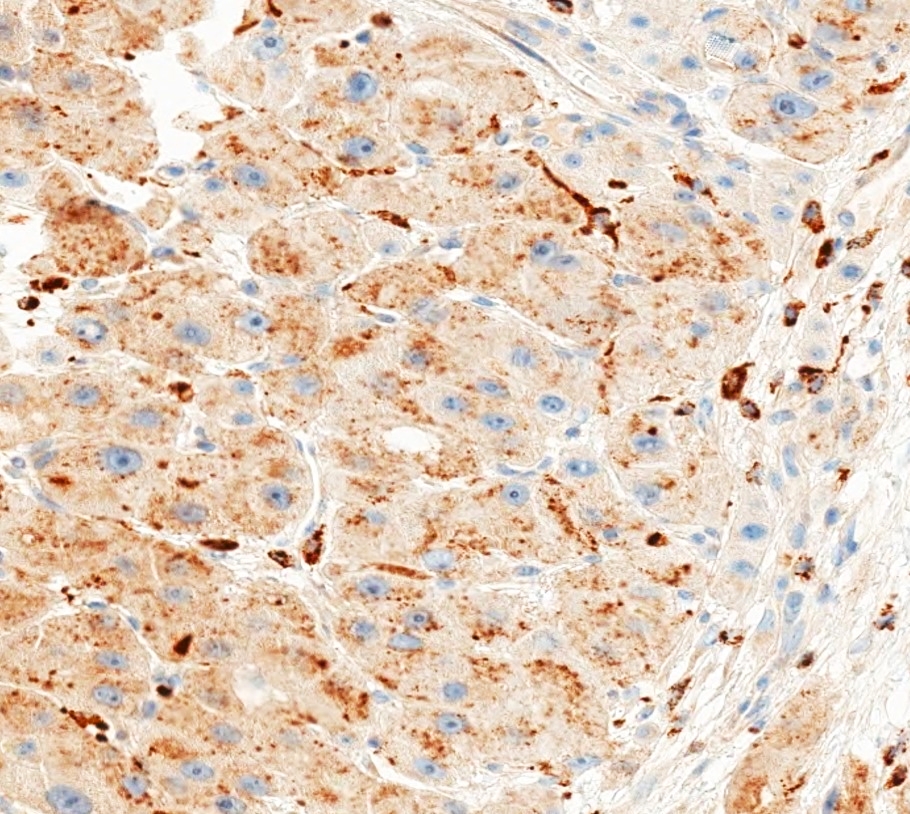
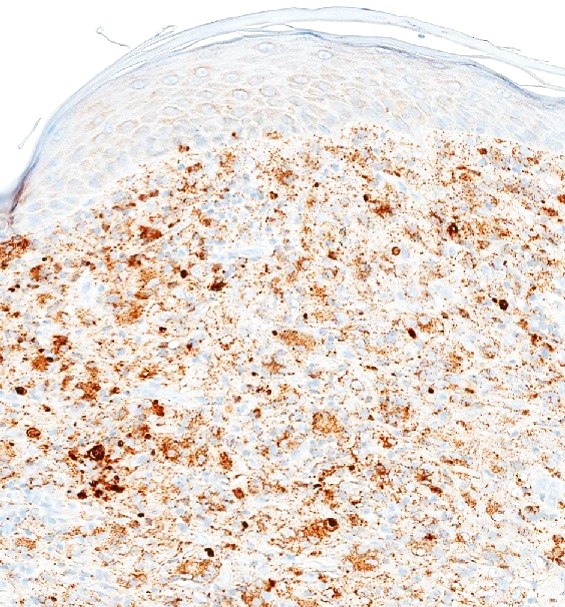
Microscopic (histologic) images
Virtual slides
Positive staining - normal
- Highly expressed in cells of mononuclear phagocyte lineage, including macrophages, microglia, osteoclasts, tissue histiocytes, Kupffer cells (IHC KP1), multinucleated giant cells (IHC KP1), Langerhans cells (IHC KP1), Hofbauer cells (IHC PGM1) (J Clin Pathol 1989;42:414, J Leukoc Biol 2012;92:723, Head Neck Pathol 2009;3:95, Am J Clin Pathol 1989;92:273, Pediatr Dev Pathol 2010;13:300)
- Comparably high expression reported in fibroblasts (IHC KP1, EBM11) and endothelial cells (IHC KP1, FCM KP1) (Ann Rheum Dis 2004;63:774, Scand J Immunol 2008;67:453, Verh Dtsch Ges Pathol 2003;87:215)
- Lower expression in neutrophils (ICC KP1), CD34 positive progenitor cell subsets (IHC KiM7), adipocytes (IF and FCM), activated T and B cells, gamma delta T cells and NK cells (IHC KP1, EMB11; FCM KP1, EMB11) (Int Immunol 1990;2:973, Blood 1995;86:4115, FEBS Lett 2005;579:5631, Hum Pathol 1994;25:872, Scand J Immunol 2008;67:453)
- Other myeloid cells such as basophils (ICC KP1) and mast cells (IHC KP1) (Int Immunol 1990;2:973, J Clin Pathol 1990;43:719)
- Intimal smooth muscle cells of human arteries (ICC KP1), fetal small intestinal inclusions (Atherosclerosis 1997;135:19, Cesk Patol 2001;37:7)
Positive staining - disease
- Benign tumors:
- Calcifying aponeurotic fibroma (KP1: 100%) (Hum Pathol 1998;29:1504)
- Cellular fibrous histiocytoma (KP1: 83%) (J Cutan Pathol 2006;33:353)
- Chondroid lipoma (KP1: 82%) (Am J Surg Pathol 1993;17:1103)
- Angiomyolipoma (PGM1 and KP1) (Mod Pathol 2012;25:100)
- Epithelioid angiomyolipoma (PGM1 in 40 - 80% of cells, 100% of cases; KP1 in 50 - 90% of cells, 100% of cases in one study; PGM1 and KP1 in 100% of cases in another study) (Mod Pathol 2012;25:100, Mod Pathol 2018;31:474)
- Giant cell tumor of the bone (strong positivity in osteoclast-like giant cells in 100% of cases, weaker and less frequent positivity in stromal cells in one study; positivity in osteoclast-like giant cells in another study) (Hum Pathol 1996;27:754, J Clin Orthop Trauma 2016;7:109)
- Giant cell tumor of soft tissue (strong positivity in osteoclast-like giant cells in 87% of cases, positivity in mononuclear cells in 73% of cases) (Am J Surg Pathol 2000;24:386)
- Granular cell tumor (KP1 strong positivity: 100%) (Arch Pathol Lab Med 2004;128:771)
- Malignant granular cell tumor of soft tissue (KP1: 65%) (Am J Surg Pathol 1998;22:779)
- Granuloma annulare (KP1: 86%; PGM1: 100%) (J Cutan Pathol 2002;29:590)
- Juvenile xanthogranuloma (100% in one study; KP1: 100% in another study) (Am J Surg Pathol 2003;27:579, Am J Dermatopathol 2001;23:104)
- Kikuchi disease (Kikuchi-Fujimoto disease of the lymph node) (KP1: 100%; PGM1: 100%) (Am J Surg Pathol 1999;23:1040, Am J Surg Pathol 1995;19:798, Am J Pathol 2001;159:915)
- Mycobacterial pseudotumor (Am J Surg Pathol 1999;23:656, Iran J Med Sci 2018;43:94)
- Multicentric reticulohistiocytosis (KP1) (Am J Dermatopathol 1994;16:577)
- Cellular neurothekeoma (KP1: 46 - 59%) (Am J Surg Pathol 2007;31:1103, Mod Pathol 2014;27:701)
- Nodular fasciitis (KP1: 92%) (Am J Surg Pathol 1991;15:942)
- Pleomorphic xanthoastrocytoma (Folia Neuropathol 2003;41:89)
- Schwannoma (KP1: 80%) (Int Pharmacopsychiatry 1977;12:72, Mod Pathol 1993;6:463)
- Splenic littoral cell angioma (KP1: 100%) (Am J Surg Pathol 1997;21:827, Am J Surg Pathol 2006;30:1036)
- Solitary reticulohistiocytoma of the skin (KP1) (Am J Surg Pathol 2006;30:521)
- Tenosynovial giant cell tumor (Hum Pathol 2003;34:670)
- Localized type (previously giant cell tumor of the tendon sheath) (Hum Pathol 1995;26:771, Tumorit 1997;83:841)
- Diffuse type (previously pigmented villonodular synovitis) (Hum Pathol 2003;34:65, Hum Pathol 1995;26:771)
- Carcinoma:
- Fibrolamellar carcinoma of the liver (KP1 strong positivity: 97%) (Mod Pathol 2011;24:390)
- Osteoclast-like giant cells in tumors of pancreas and urinary tract (KP1: 100%) (Arch Pathol Lab Med 1998;122:266, Mod Pathol 2006;19:161)
- Renal cell carcinoma with t(6,11) (KP1: 100%) (Mod Pathol 2018;31:474)
- Hematologic malignancies:
- Acute myeloid leukemia (AML): KP1 in most AML subtypes (Am J Clin Pathol 2000;113:814, Am J Clin Pathol 2004;122:794)
- Chronic myeloid leukemia (chronic myelogenous leukemia) (KP1 and PGM1: 100%) (Am J Clin Pathol 2004;122:794, Mod Pathol 2006;19:1536)
- Chronic myelomonocytic leukemia (KP1 and PGM1: 100%) (Am J Clin Pathol 2004;122:794, Mod Pathol 2006;19:1536)
- Hairy cell leukemia (KP1: 78 - 100%) (Am J Pathol 1989;135:1089, Pathol Oncol Res 2015;21:203, Mod Pathol 1996;9:982)
- Myelodysplasia bone marrow nodules (KP1 and PGM1 in one study; PGM1: 100% in another study) (Am J Clin Pathol 2003;120:874, Am J Hematol 2005;79:329)
- Small lymphocytic lymphoma (KP1: 83 - 87%) (Hum Pathol 1993;24:886, Am J Clin Pathol 1995;103:425)
- Erdheim-Chester disease (KP1: 100%) (Am J Surg Pathol 1999;23:17)
- Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) (KP1 in one study; KP1 and PGM1: 100% in another study) (Mod Pathol 2001;14:172, Am J Clin Pathol 2004;122:794)
- Langerhans cell histiocytosis (100% in one study; KP1 and PGM1: 96% in another study) (Blood 2001;97:1241, Histopathology 2002;41:1)
- Primary cutaneous acral CD8+ T cell lymphoma (dot-like positivity) (J Am Acad Dermatol 2021;85:1073)
- Histiocytic sarcoma (KP1: 86 - 100%; PGM1: 100%) (Am J Surg Pathol 2004;28:1133, Mod Pathol 2005;18:693, Cancer Cytopathol 2017;125:604)
- Myeloid / monocytic sarcoma (KP1: 100%; PGM1: 51%) (Leukemia 2007;21:340)
- Mastocytosis and mast cell disease (KP1: 100%) (Am J Surg Pathol 2000;24:703)
- Sarcoma:
- Angiosarcoma (KP1: 60%) (Virchows Arch 1994;424:635)
- Angiomatoid fibrous histiocytoma (65 - 95%) (Pathology 2014;46:199, Pediatr Dev Pathol 2012;15:181)
- Phyllodes tumor (benign and malignant, stromal component, KP1: 75%) (Virchows Arch 1994;424:635)
- Follicular dendritic cell sarcoma (92%; KP1: 100% in one study; KP1 and PGM1: 62% in another study) (Diagn Pathol 2010;5:67, Am J Surg Pathol 1998;22:1048, Histopathology 2002;41:1)
- Inflammatory myofibroblastic tumor (previously inflammatory pseudotumor) - hepatic (KP1: 100%); splenic (KP1: 60%) (Mod Pathol 2007;20:884, Arch Pathol Lab Med 2001;125:379)
- Interdigitating dendritic cell sarcoma (92%) (Diagn Pathol 2010;5:67)
- Leiomyosarcoma (KP1: 89%; some PGM1) (Virchows Arch 1994;424:635)
- Malignant peripheral nerve sheath tumor (neurofibrosarcoma) (KP1: 67%) (Virchows Arch 1994;424:635)
- Myxoinflammatory fibroblastic sarcoma (KP1 and PGM1: 68 - 80%) (Ann Diagn Pathol 2002;6:272, Am J Surg Pathol 1998;22:911, J Cutan Pathol 2008;35:186)
- Undifferentiated embryonal sarcoma of the liver (KP1: 67%) (Am J Surg Pathol 1991;15:615)
- Undifferentiated pleomorphic sarcoma (malignant fibrous histiocytoma) (KP1: 72 - 79%; of the breast, KP1: 71%) (Am J Clin Pathol 1992;97:759, Am J Clin Pathol 1995;103:425, Ann Diagn Pathol 2011;15:407)
- Other:
- Chronic intervillositis (positivity in 90% of mononuclear cells in intervillous spaces, in 100% of cases) (Hum Pathol 2000;31:1389)
- Crystal storing histiocytosis (KP1: 100%) (Histopathology 2016;68:482)
- Desquamative interstitial pneumonia (diffuse and extensive intra-alveolar deposition of alveolar CD68 positive macrophages) (KP1) (Am J Respir Crit Care Med 1997;156:2003)
- Diffuse alveolar damage (DAD) (positive association of PGM1 macrophage infiltrates with DAD) (Nat Commun 2020;11:5086)
- Gaucher disease (100%) (Am J Clin Pathol 2004;122:359, Acta Med Croatica 2001;55:131)
- Malakoplakia (Diagn Pathol 2020;15:97)
- Plexiform fibrohistiocytic tumor (KP1: 55 - 100%) (Am J Surg Pathol 1999;23:662, Histopathology 1991;19:503, Am J Surg Pathol 2009;33:905)
- Rheumatoid nodules (KP1: 93%; PGM1: 100%) (J Cutan Pathol 2002;29:590)
- Steatohepatitis (Mod Pathol 2002;15:699)
- Whipple disease (Hum Pathol 2003;34:589)
- Xanthogranulomatous pyelonephritis (Arch Pathol Lab Med 2005;129:e209)
- Xanthoma (Hum Pathol 2003;34:814)
Negative staining
- Anaplastic large cell lymphoma (KP1: 10%) (Hum Pathol 1993;24:886)
- Atypical fibrous histiocytoma (Am J Surg Pathol 2002;26:35)
- Hemangiopericytoma (KP1: 50%) (Virchows Arch 1994;424:635)
- Liposarcoma (KP1: 33%) (Virchows Arch 1994;424:635, Am J Clin Pathol 1995;103:425)
- Osteosarcoma (KP1: 33%) (Am J Clin Pathol 1995;103:425)
- Rhabdomyoma (adult and fetal) (ORL J Otorhinolaryngol Relat Spec 1998;60:178, Hum Pathol 1993;24:754)
- Synovial sarcoma (KP1: 14%, weak staining) (Virchows Arch 1994;424:635)
- Dermatofibrosarcoma protuberans (KP1: 6%) (J Cutan Pathol 2006;33:353)
Sample pathology report
- Skin, biopsy:
- Diagnosis: myeloid / monocytic sarcoma (see comment)
- Comment: The skin biopsy shows a dense infiltrate of intermediate sized cells with open and dispersed chromatin, irregular nuclear contours, variable amounts of cytoplasm and frequent mitotic figures. Areas of tumoral necrosis are present. Immunohistochemical stains show that the atypical infiltrate are positive for CD4, CD33, CD43, CD68 (KP1) and CD68 (PGM1). The atypical cells are negative for CD3, CD20, PAX 5, CD34, CD56, CD123 and muramidase. A Ki67 stain is positive in 90% of tumor cell nuclei. In summary, these findings represent involvement by myeloid / monocytic sarcoma.
Board review style question #1
Which of the following marker combinations fits the immunoprofile of Langerhans cell histiocytosis (LCH)?
- CD68 negative, CD1a negative, CD207 (langerin) negative
- CD68 negative, CD1a positive, CD207 (langerin) positive
- CD68 positive, CD1a negative, CD207 (langerin) negative
- CD68 positive, CD1a positive, CD207 (langerin) positive
Board review style answer #1
D. CD68 positive, CD1a positive, CD207 (langerin) positive. LCH cells are positive for CD68, a traditional histiocyte marker, as well as CD1a and CD207 (langerin), typical Langerhans cell markers. CD1a and CD207 positivity (as opposed to answer choice C) can distinguish surrounding CD68 positive macrophages from LCH cells.
Comment Here
Reference: CD68
Comment Here
Reference: CD68
Board review style question #2
CD68 IHC can be helpful in which of the following differential diagnostic scenarios?
- Chronic granulomatous disease versus Whipple disease
- Clear cell renal cell carcinoma versus chromophobe renal cell carcinoma
- Reticulohistiocytoma versus neurothekeoma
- Rhabdomyoma versus granular cell tumor
- Rheumatoid nodules versus granuloma annulare
Board review style answer #2
D. Rhabdomyoma versus granular cell tumor. Granular cell tumors are diffusely CD68 positive whereas rhabdomyomas are CD68 negative. Chronic granulomatous disease and Whipple disease are both marked by an abundance of CD68 positive macrophages in the lamina propria (A). Clear cell renal cell carcinoma and chromophobe renal cell carcinoma are CD68 negative (B). Reticulohistiocytoma is uniformly CD68 positive, while (cellular) neurothekeoma may be positive in over half of cases (C). Rheumatoid nodules and granuloma annulare both contain many CD68 positive macrophages (E).
Comment Here
Reference: CD68
Comment Here
Reference: CD68