Table of Contents
Definition / general | Trade name | Pathophysiology | Diagrams / tables | Clinical information | Uses by pathologists | Side effects | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Yeh YA. Pertuzumab. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastpertuzumab.html. Accessed December 26th, 2024.
Definition / general
- Humanized IgG1 kappa monoclonal antibody targets extracellular domain of HER2 tyrosine kinase receptor
- Discovered and developed by Genentech, Inc., South San Francisco, CA
- Synonym: 2C4 (monoclonal antibody)
- rhuMAb-2C4
Trade name
- Perjeta®
Pathophysiology
- HER2, a member of HER family, does not have a receptor specific ligand binding site
- HER2/neu signaling in cancer cells (N Engl J Med 2007;357:39)
- HER2 heterodimerizes with HER1, HER3 or HER4, phosphorylates and activates intracellular tyrosine kinase domain
- The activated tyrosine kinase on HER2 activates PI3K-Akt pathway and induces cellular survival
- The activated tyrosine kinase activates SOS, induces a cascade of activation of RAS-RAF-MAPK-MEK and MAPK, eventually promotes cellular proliferation
- Cleavage of HER2 extracellular domain produces phosphorylated P95 that could activate downstream signal transduction
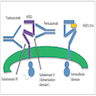
- Mechanism of action of pertuzumab (Oncology (Williston Park) 2014;28:186)
- Upon binding the extracellular dimerization subdomain II of HER2, pertuzumab reduces activation of HER2 by disrupting dimerization of HER2 with HER1, HER3 or HER4
- See Diagrams / tables, figure 7
Diagrams / tables
Clinical information
- Approved by United States Food and Drug Administration (FDA) on June 8, 2012 (Clin Cancer Res 2019;25:2949)
- Used in combination with trastuzumab and docetaxel or other drugs to treat HER2 positive breast cancer (National Cancer Institute: Pertuzumab [Accessed 17 February 2020]):
- Metastatic breast cancer that has not been treated with either hormone or chemotherapy
- Locally advanced, inflammatory or early stage breast cancer
- Early breast cancer with high risk of tumor recurrence
- Clinical pharmacokinetics: (Ann Oncol 2013;24:273)
- Route of administration: intravenous infusion
- Elimination: metabolized through proteolytic catabolism
- Half life: 10 days
- Mechanism of drug resistance:
- HER2 positive cancer cells with S310F mutation might disrupt the interaction between pertuzumab and HER2 (Onco Targets Ther 2019;12:11597)
- Pertuzumab costs about $5,900 a month, about $71,000 a year or about $180,000 for a course of treatment (Drugs.com: Perjeta Prices, Coupons and Patient Assistance Programs [Accessed 18 February 2020])
- Comparisons between pertuzumab and trastuzumab (Ann Oncol 2013;24:273)
- See Diagrams / tables, figure 8
Uses by pathologists
- Identify HER2 positive breast adenocarcinomas
- HER2 testing needed on all newly diagnosed breast adenocarcinomas
- Metastatic diseases (test performed in a metastatic site)
- HER2 immunohistochemistry in breast adenocarcinoma (see Diagrams / tables, figure 1)
- ASCO-CAP HER2 Test 2013 and 2018 Guideline Recommendation (Arch Pathol Lab Med 2014;138:241, Arch Pathol Lab Med 2018;142:1364)
- Negative (score 0):
- No staining
- Incomplete faint membrane staining ≤ 10% invasive tumor cells
- Negative (score 1+):
- Incomplete faint membrane staining > 10% invasive tumor cells
- Equivocal (score 2+): perform HER2 ISH (see Diagrams / tables, figure 2)
- Incomplete, weak / moderate membrane staining > 10% invasive tumor cells
- Complete, intense membrane staining ≤ 10% invasive tumor cells
- Positive (score 3+):
- Complete, intense circumferential membrane staining > 10% invasive tumor cells
- Negative (score 0):
- ASCO-CAP HER2 Test 2013 and 2018 Guideline Recommendation (Arch Pathol Lab Med 2014;138:241, Arch Pathol Lab Med 2018;142:1364)
- HER2 single probe in situ hybridization (ISH) in breast adenocarcinoma (see Diagrams / tables, figure 2)
- ISH negative (not amplified)
- Single probe HER2 copy number < 4.0
- ISH positive (amplified)
- Single probe HER2 copy number ≥ 6.0 signals/cell
- ISH equivocal: perform HER2/CEP17 dual probe ISH (see Diagrams / tables, figure 3)
- Single probe HER2 copy number ≥ 4.0 and < 6.0 signals/cell
- ISH negative (not amplified)
- HER2 dual probe ISH in breast adenocarcinoma (see Diagrams / tables, figure 3)
- Negative:
- Dual probe HER2/CEP17 < 2.0, HER2 copy number < 4.0 signals/cell
- Positive:
- Dual probe HER2/CEP17 ≥ 2.0, HER2 copy number ≥ 4.0 signals/cell
- Equivocal (see Diagrams / tables, figures 4, 5 and 6):
- HER2/CEP17 ≥ 2.0, with HER2 copy number < 4.0 signals/cell (see Diagrams / tables, figure 4)
- HER2/CEP17 < 2.0, HER2 copy number ≥ 6.0 signals/cell (see Diagrams / tables, figure 5)
- HER2/CEP17 < 2.0, HER2 copy number ≥ 4.0 and < 6.0 signals/cell (see Diagrams / tables, figure 6)
- Negative:
Side effects
- Common side effects (> 30% of patients) when given in combination with trastuzumab and docetaxel (MedWatch: The FDA Safety Information and Adverse Event Reporting Program [Accessed 17 February 2020]):
- Diarrhea
- Hair loss
- Leukopenia
- Nausea
- Fatigue
- Rash
- Peripheral neuropathy
Additional references
Board review style question #1
- Which of the following drugs target HER2 / neu on cancer cells?
- Dabrafenib
- Larotrectinib
- Pertuzumab
- Trametinib
Board review style answer #1
Board review style question #2
- Which of the following results is interpreted as positive staining (score 3+) for HER2 in
breast adenocarcinoma?
- Complete, intense basolateral membranous staining > 10% of tumor cells
- Complete, intense circumferential membranous staining > 10% of tumor cells
- Complete, intense luminal membranous staining > 10% of tumor cells
- Complete, intense nuclear and cytoplasmic staining > 10% of tumor cells
Board review style answer #2
B. Complete, intense circumferential membranous staining > 10% of tumor cells
Comment Here
Reference: Pertuzumab
Comment Here
Reference: Pertuzumab