Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Agarwal I, Blanco L. Pseudoangiomatous stromal hyperplasia. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastpash.html. Accessed March 27th, 2025.
Definition / general
- Benign stromal myofibroblastic proliferation forming clefts, simulating a vascular lesion
- First described in 1986 (Hum Pathol 1986;17:185)
Essential features
- Benign stromal myofibroblastic proliferation
- Usually an incidental finding but may produce palpable or mammographic mass
- Complex interanastomosing spaces in dense collagenous, keloid-like stroma resembling blood vessels
- Some of these spaces have spindle shaped myofibroblasts at their margins, simulating endothelial cells
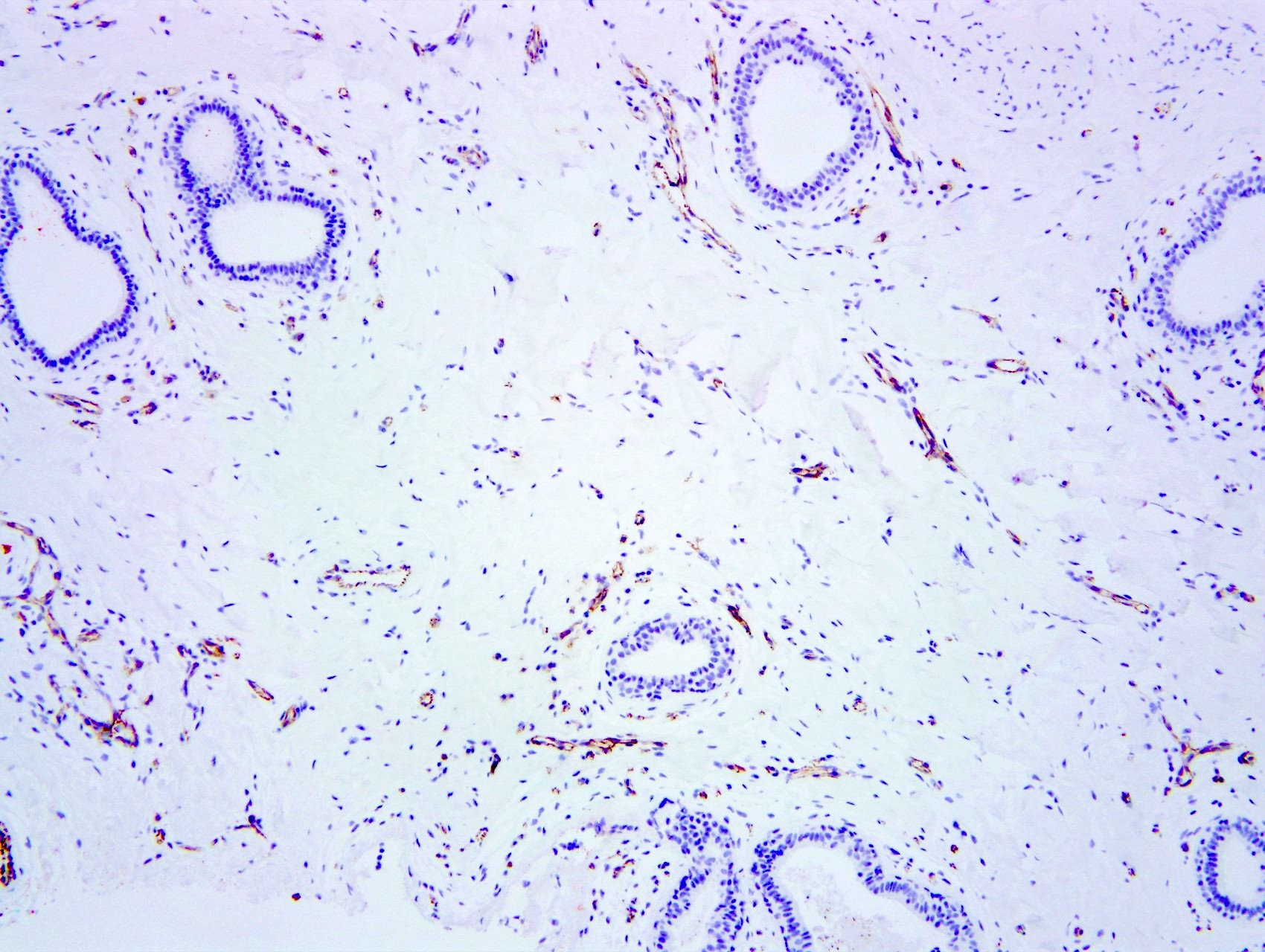
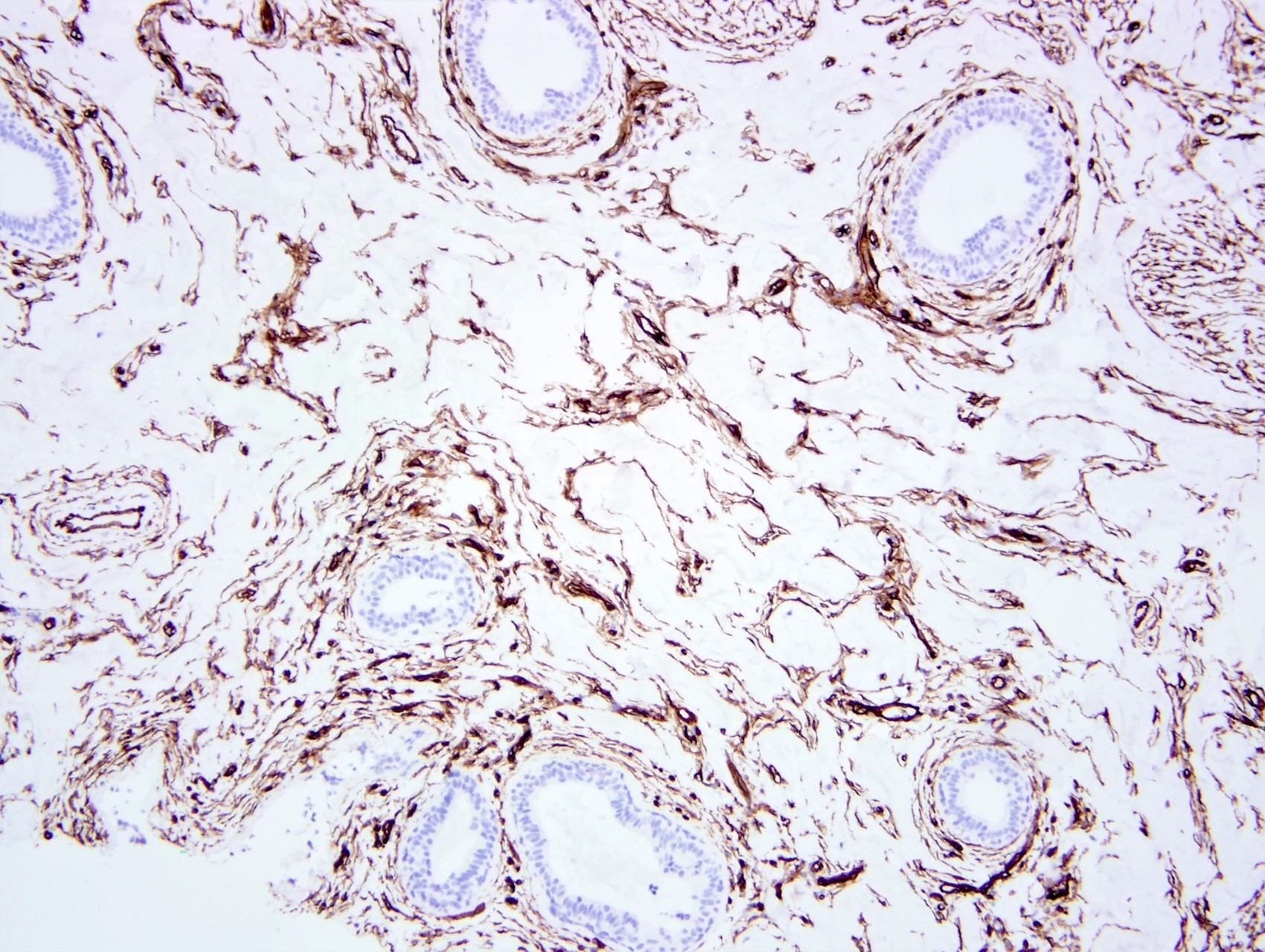
- Spindle cells usually positive for ER, PR and CD34 but negative for other vascular markers (e.g., CD31) (Breast J 2012;18:242)
- Pseudoangiomatous stromal hyperplasia (PASH) is an incidental microscopic finding in up to 23% of breast surgical resections (Int J Surg 2011;9:20)
Terminology
- Also called pseudoangiomatous hyperplasia of mammary stroma
ICD coding
- ICD-11: GB20.Y - other specified benign breast disease
Epidemiology
- Mostly occurs in pre and perimenopausal women but can occur in males too (Radiol Case Rep 2022;17:2919)
- Also occurs in children (Pediatr Dev Pathol 2009;12:450)
Sites
- Breast stroma
Etiology
- Myofibroblastic origin, postulated role of hormonal factors (Breast J 2012;18:242)
Clinical features
- Incidental finding, can occur in both females and males
- Can be associated with other benign and malignant lesions
Diagnosis
- Histologic examination of resected or biopsied tissue
Radiology description
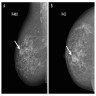
- Incidental but may present as a mass, well defined or irregular (SA J Radiol 2018;22:1366)
- Nonspecific findings on magnetic resonance imaging (MRI), ranging from an enhancing mass to clumped, nonmass-like enhancement (Clin Radiol 2010;65:145)
Prognostic factors
- Nonneoplastic but mass forming lesion may rarely recur, especially in younger patients
Case reports
- 13 year old girl with a giant mass in the left breast for 3 months (J Pediatr Adolesc Gynecol 2021;34:209)
- 14 year old premenarchial girl with PASH presenting as bilateral gigantomastia (J Breast Cancer 2023;26:391)
- 46 year old woman with bilateral marked breast enlargement (BMJ Case Rep 2015;2015:bcr2014204343)
Treatment
- Local excision needed only in symptomatic mass forming lesions
- If diagnosed on core needle biopsy, no surgical excision is required, provided the diagnosis is concordant with radiologic findings
Gross description
- Usually unilateral, well circumscribed, firm rubbery mass (Am J Surg Pathol 1995;19:270)
- Cut surface is firm, gray-white
Microscopic (histologic) description
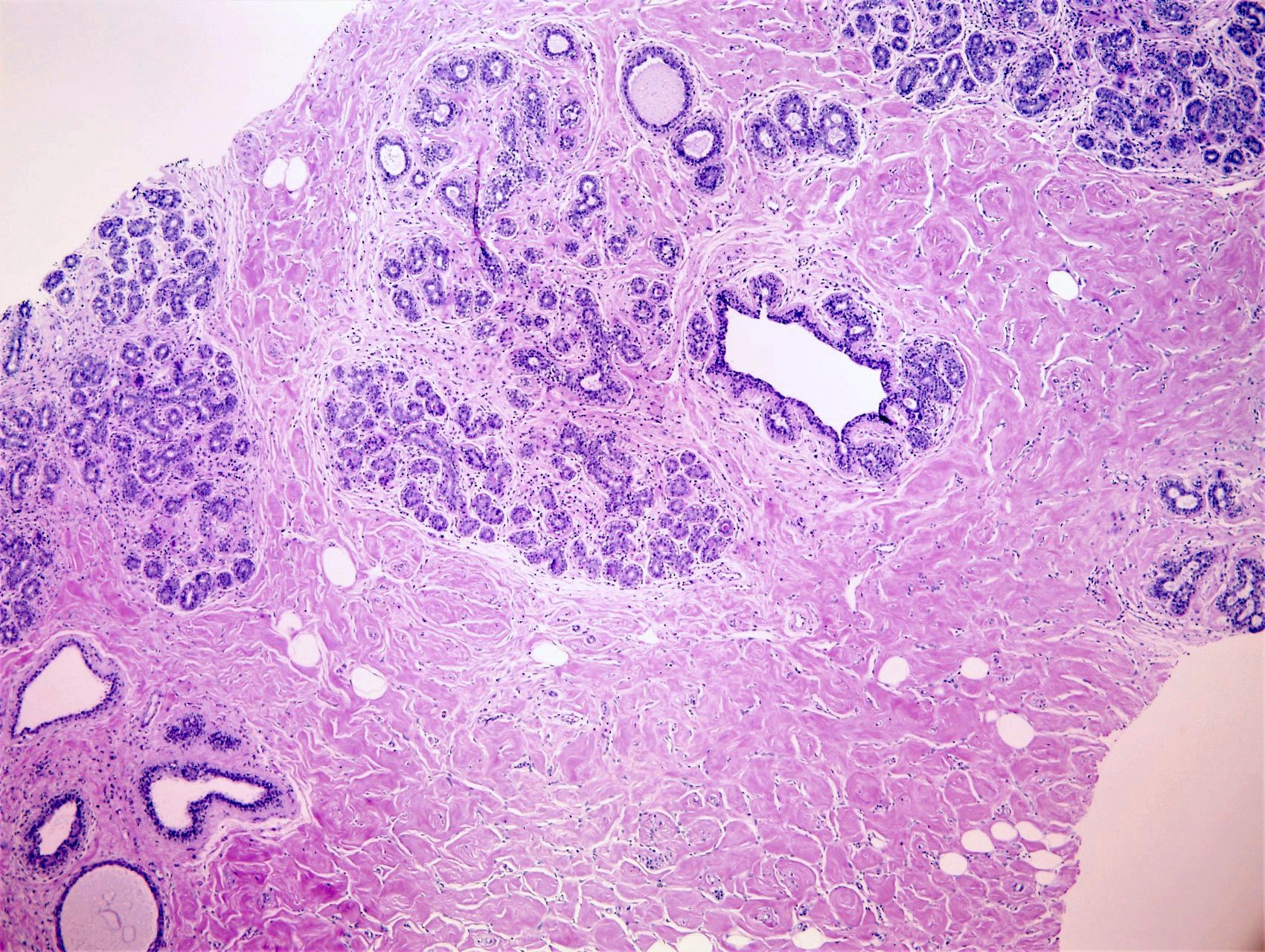
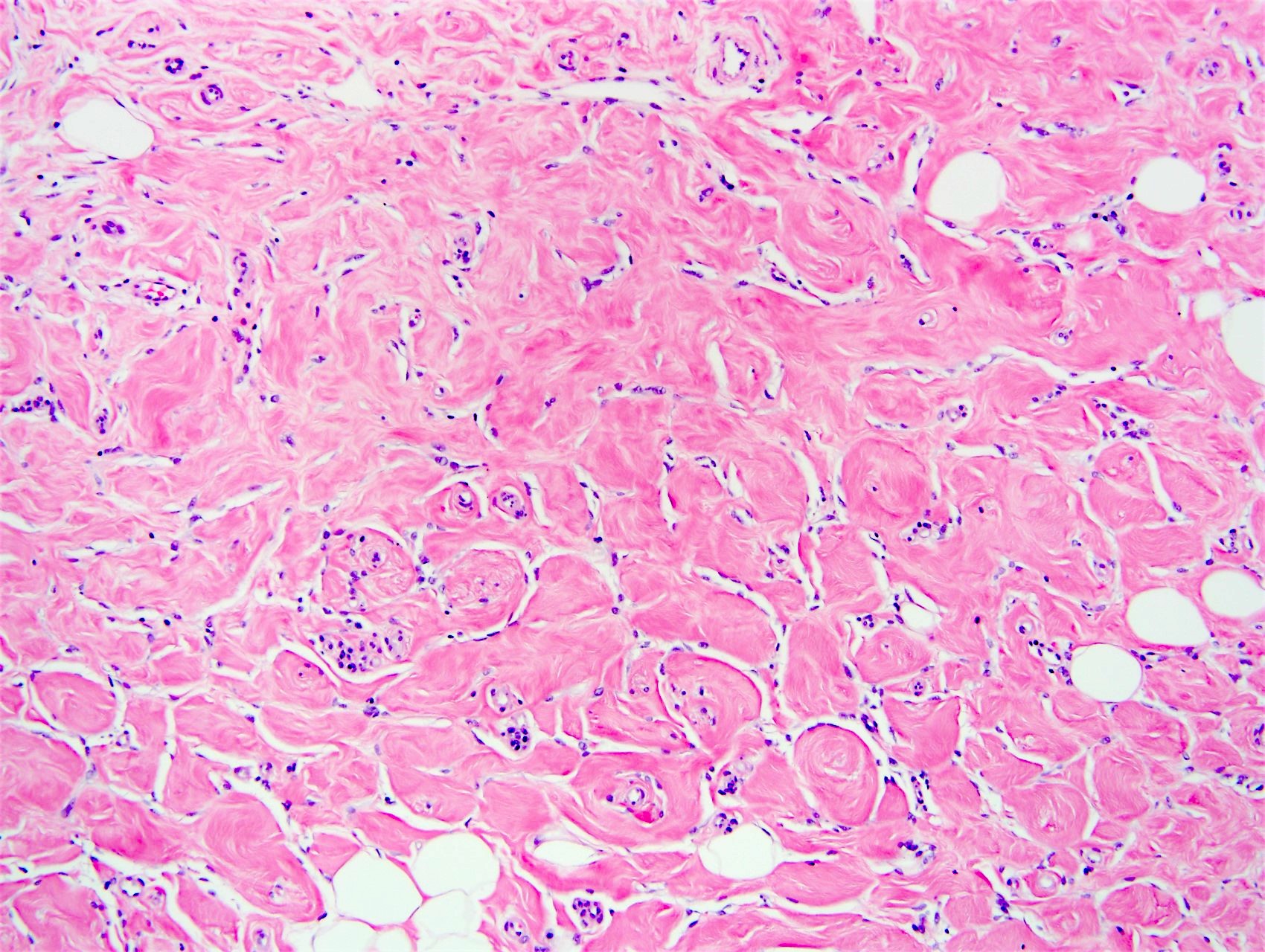
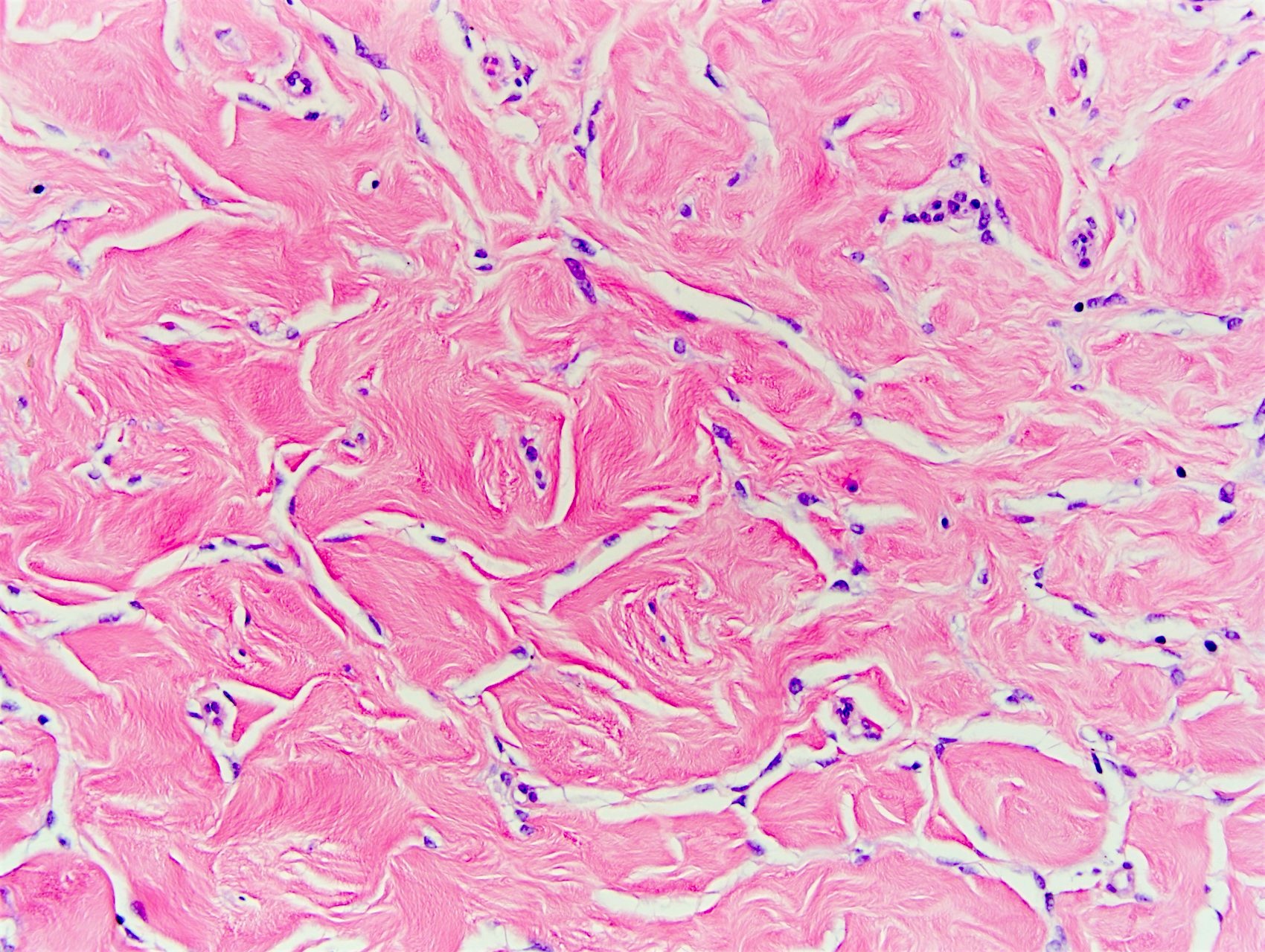
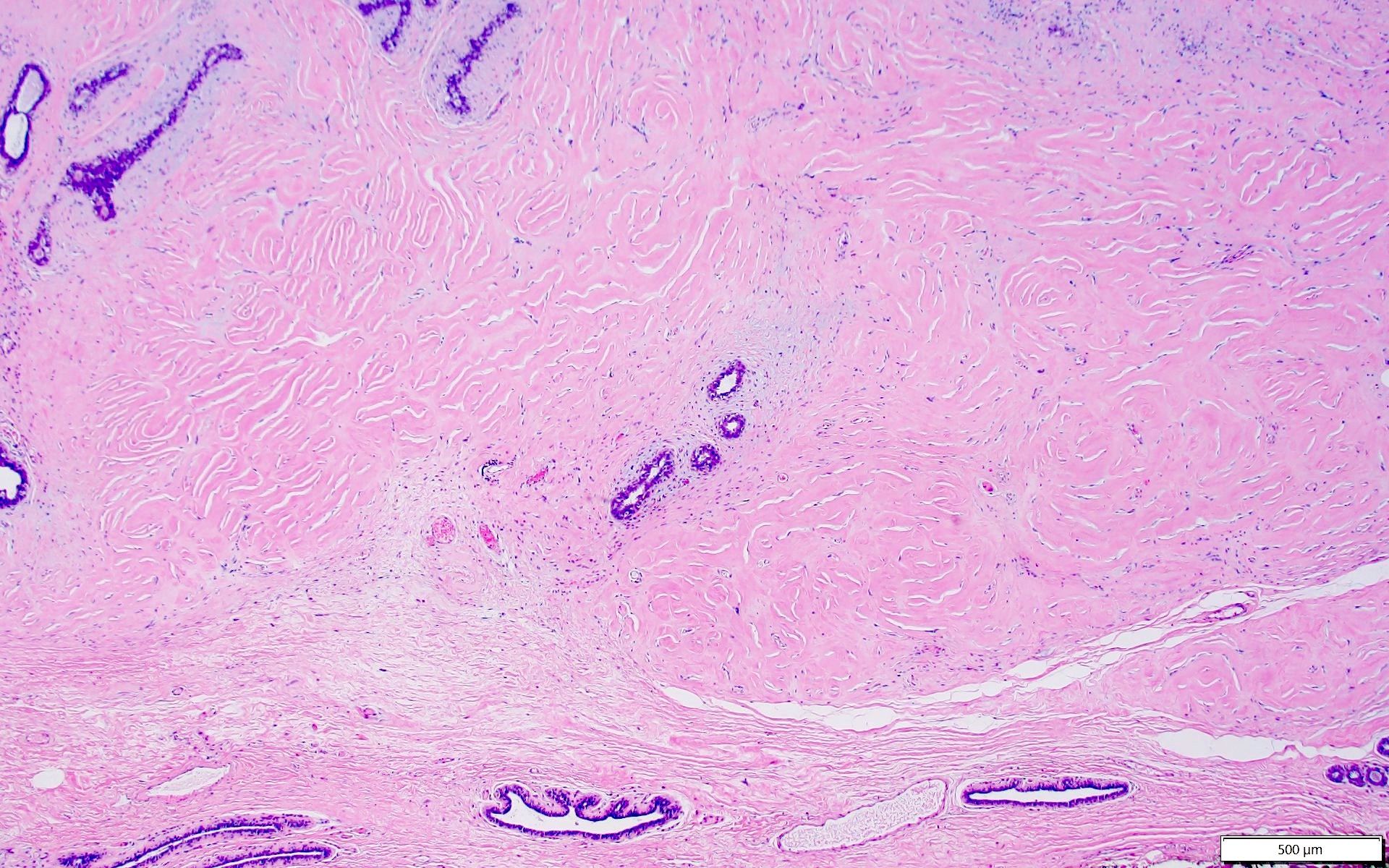
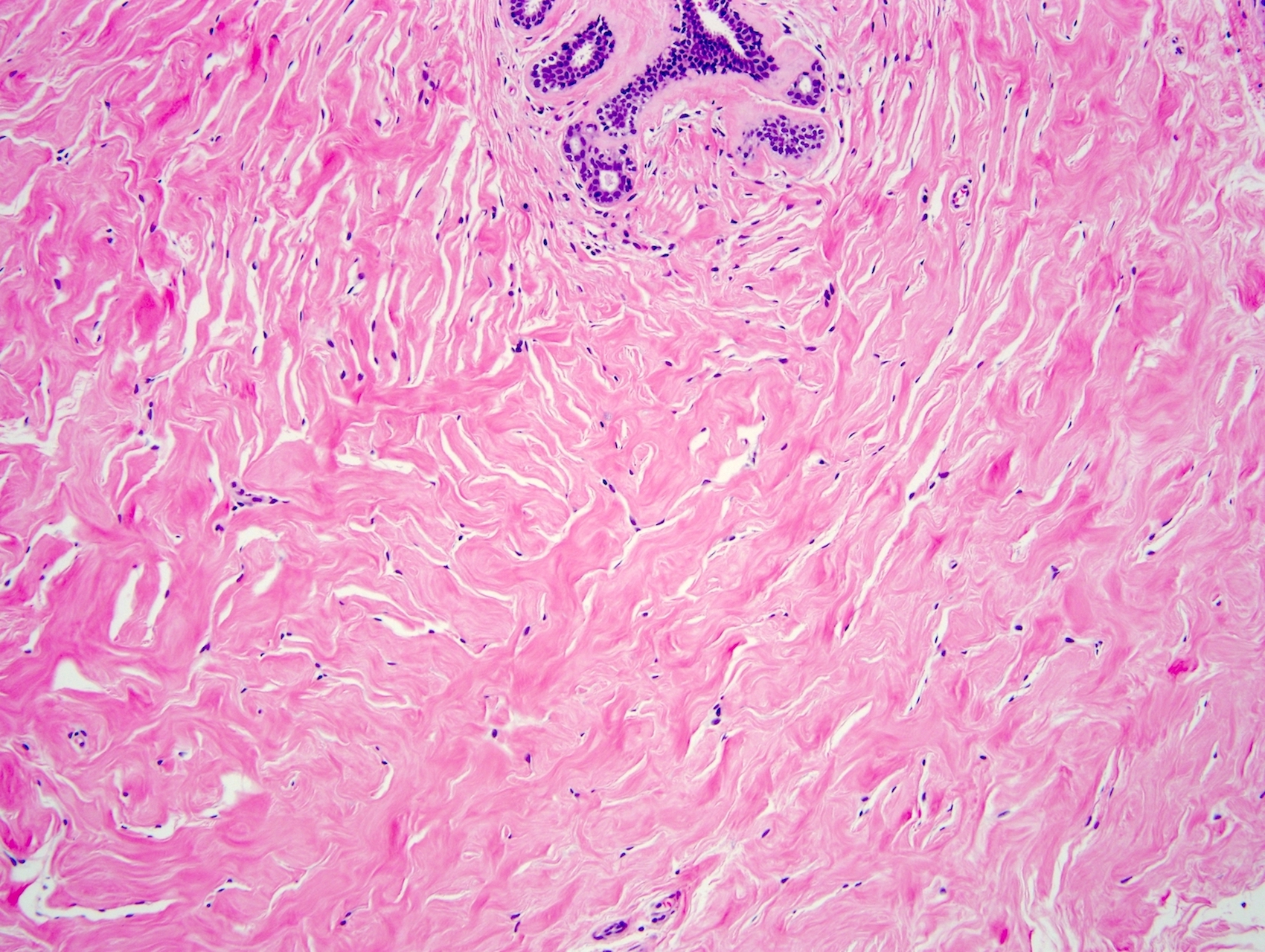
- Complex interanastomosing spaces in dense collagenous, keloid-like stroma
- Some of these spaces have spindle shaped myofibroblasts at their margins that simulate endothelial cells
- Spaces are usually empty but may contain rare erythrocytes
- Cellular areas or plump spindle cells may obscure pseudoangiomatous structure
- Often gynecomastia-like changes (Mod Pathol 2008;21:201)
- Rarely multinucleated giant cells (Breast J 2007;13:568)
- No mitotic figures, no necrosis, no atypia
- Fascicular PASH: cellular variant in which myofibroblasts aggregate into fascicles with reduced or absent clefting, resembles myofibroblastoma (Am J Surg Pathol 1995;19:270)
Microscopic (histologic) images
Positive stains
- Spindle cells
- PR (intense), ER beta, androgen receptor, vimentin, CD34 (Am J Surg Pathol 1991;15:145, Am J Surg Pathol 1995;19:270)
- Variable desmin and actin
Negative stains
- Spindle cells
- Factor VIII, Ulex, CD31, keratin
Sample pathology report
- Left breast, at 5 o'clock and 4 cm from the nipple, ultrasound core needle biopsy:
- Breast tissue with pseudoangiomatous stromal hyperplasia (PASH)
Differential diagnosis
- Low grade angiosarcoma:
- Hemorrhagic, soft, interanastomosing vascular channels containing red blood cells with invasion into breast parenchyma
- Papillary endothelial growth and hyperchromatic endothelial cells
- CD31+, factor VIII+ (Breast J 2012;18:242)
- Myofibroblastoma:
- Absence of typical breast epithelial elements within myofibroblastoma
- Neoplastic clonal tumors with characteristic genetic change (del 13q14) (this can be demonstrated by loss of Rb protein immunohistochemistry in myofibroblastoma)
- Other spindle cell tumors (e.g., fibromatosis):
- Tumor cells arranged in long fascicles without significant clefting, nuclear beta catenin staining and CD34-
Additional references
Board review style question #1
Board review style answer #1
E. No abnormality; incidental finding. Pseudoangiomatous hyperplasia is usually an incidental finding but may less commonly produce palpable or mammographic mass. Answers A, B, C and D are incorrect because the most common presentation is an incidental finding only.
Comment Here
Reference: Pseudoangiomatous stromal hyperplasia
Comment Here
Reference: Pseudoangiomatous stromal hyperplasia
Board review style question #2
Which of the following sets of immunohistochemical stains would be most suitable for the diagnosis of pseudoangiomatous hyperplasia (PASH)?
- CD34-, CD31-, ER-, nuclear beta catenin+
- CD34-, CD31-, nuclear beta catenin+, AE1 / AE3+
- CD34+, CD31-, ER+, factor VIII-
- CD34+, CD31+, ER-, factor VIII+
Board review style answer #2
C. CD34+, CD31-, ER+, factor VIII-. This immunoprofile is indicative of PASH.
Answer D is incorrect because the immunoprofile is most consistent with angiosarcoma or other vascular lesions, with positive vascular markers. Answer A is incorrect because it is the immunoprofile for fibromatosis with positive nuclear beta catenin. It is important to note that fibromatosis is negative for CD34 as it does not arise from myofibroblasts. Answer B is incorrect because it is the classic staining pattern for a spindle cell carcinoma with positive AE1 / AE3.
Comment Here
Reference: Pseudoangiomatous stromal hyperplasia
Comment Here
Reference: Pseudoangiomatous stromal hyperplasia