Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Jorns JM. Microglandular adenosis. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmicroglandularadenosis.html. Accessed March 31st, 2025.
Definition / general
- Rare benign lesion composed of haphazard, irregularly distributed, small, uniform, rounded, open glands with eosinophilic secretions
- Mimics invasive carcinoma due to infiltrative stromal growth and single epithelial layer devoid of myoepithelium
Essential features
- Disordered proliferation of small open glands comprised of bland epithelial cells
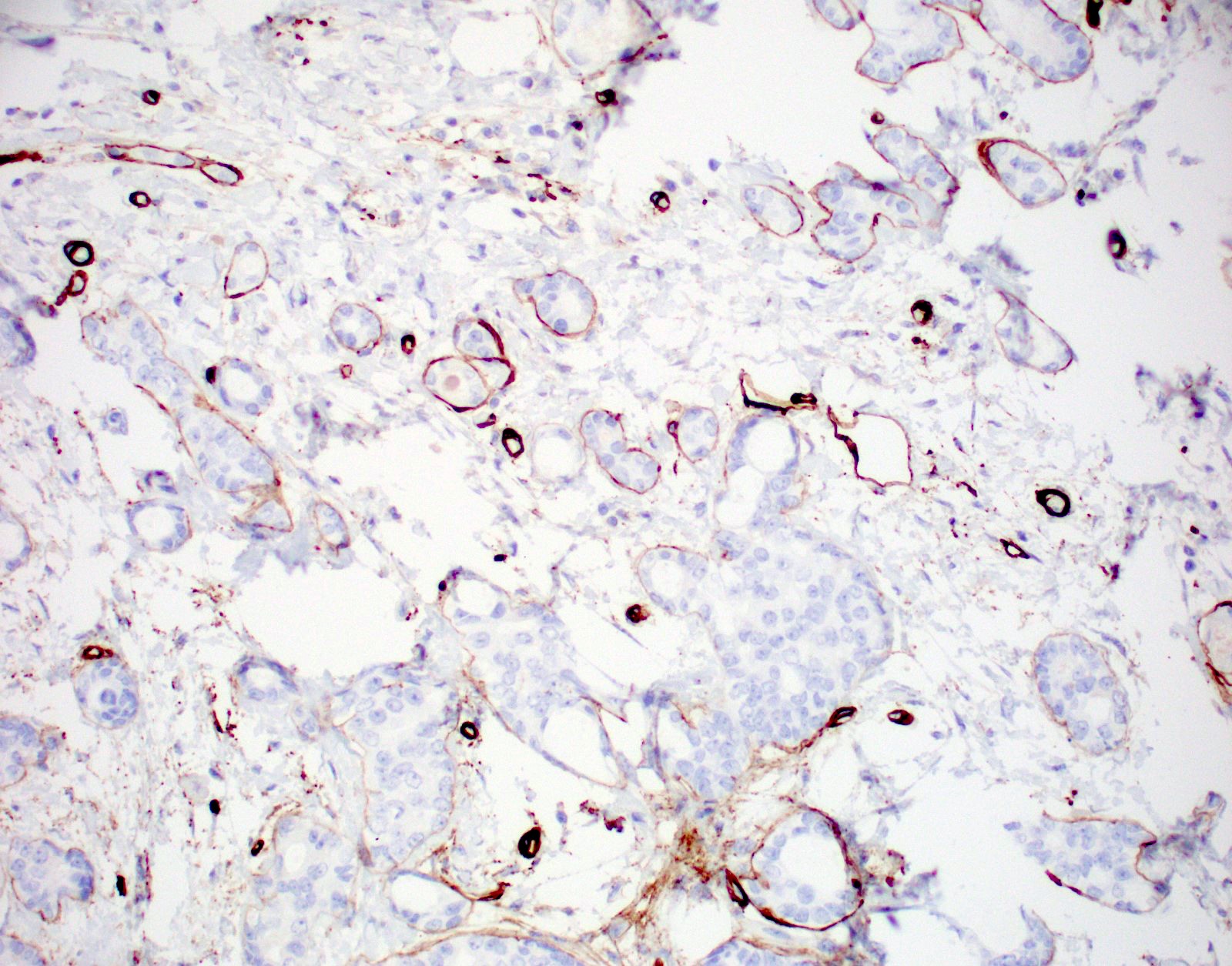
- Retains basement membrane but lacks myoepithelium
- Infiltrates adipose and fibrous stroma without a stromal response
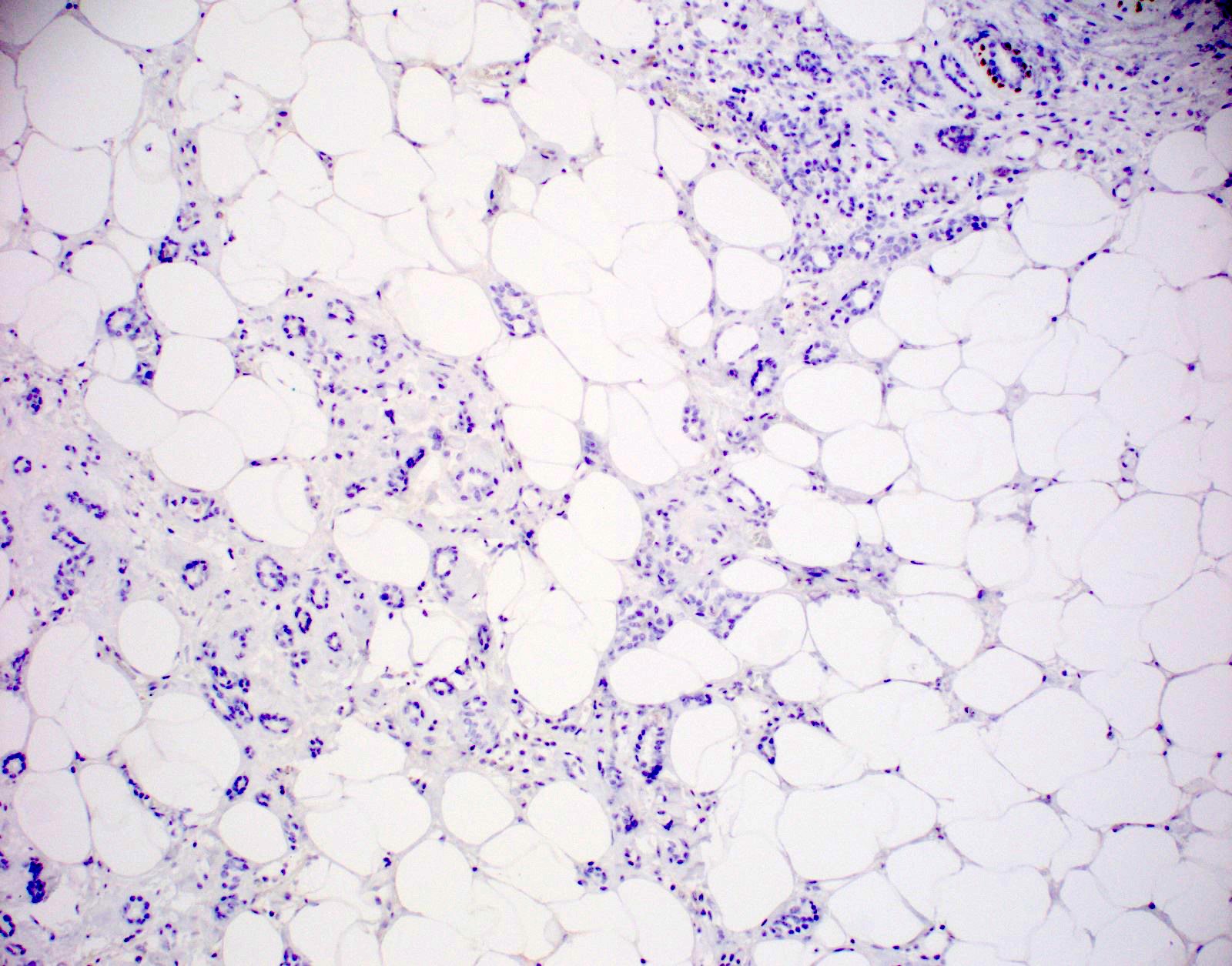
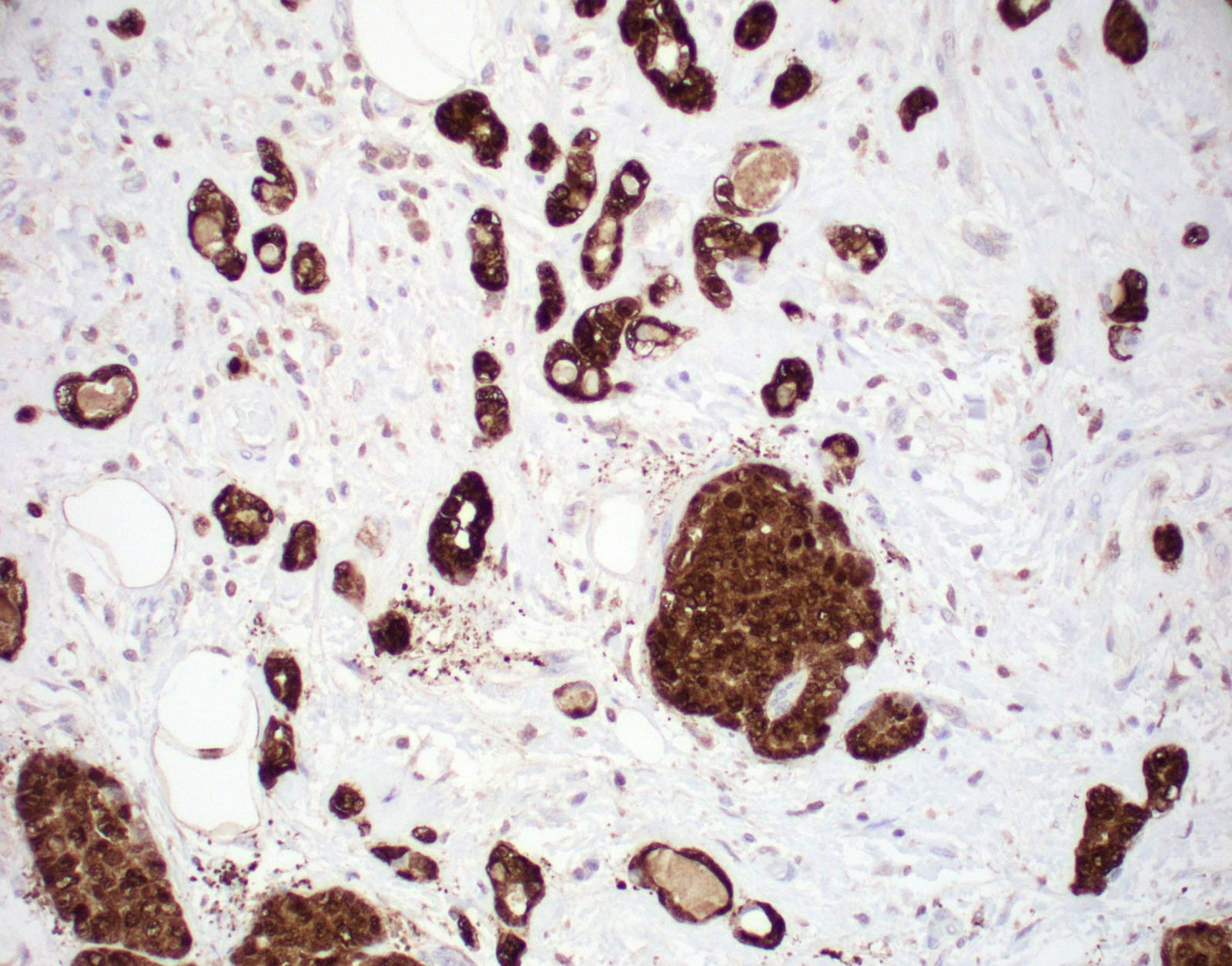
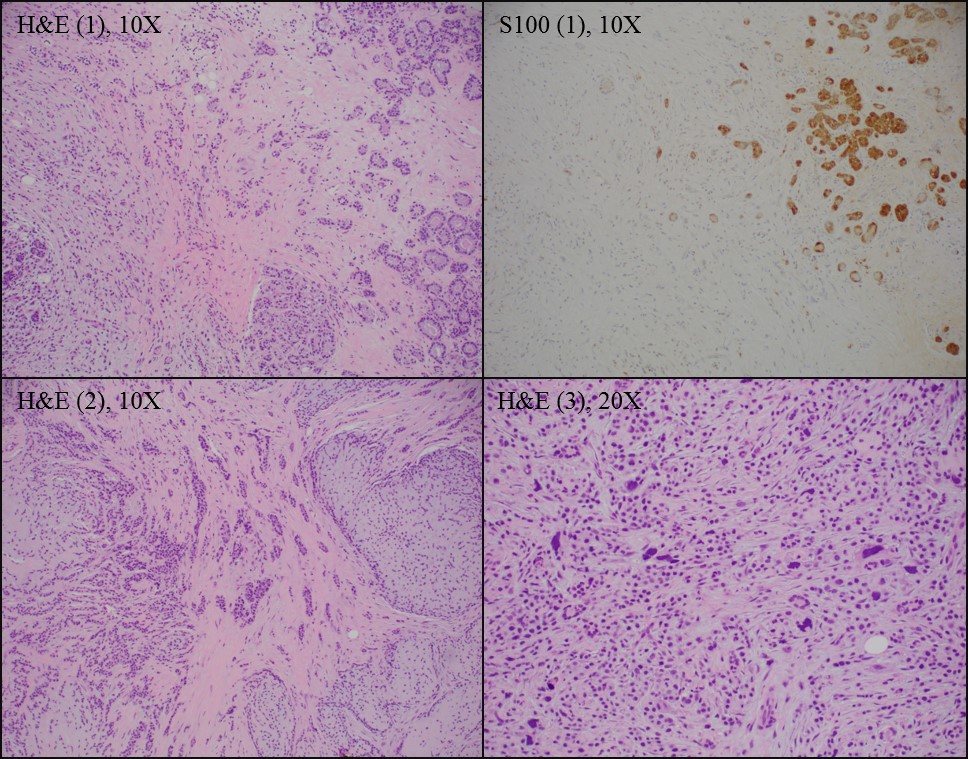
- S100+, ER-, PR-
- Potential precursor to triple negative invasive breast cancer
Terminology
- Microglandular adenosis (MGA)
- Less frequently referred to as:
- Microglandular hyperplasia
- Microglandular adenoma
ICD coding
Epidemiology
- Rare, represented in case reports and series and small studies
- No specific known epidemiologic associations (reported in women over a wide age range)
Sites
- No specific location in breast
Pathophysiology
- Gain of somatic mutations including TP53, PIK3CA pathway related and tyrosine kinase receptor signaling elated genes have been identified in MGA and MGA associated triple negative breast cancers, supporting a role as a nonobligate cancer precursor (J Pathol 2016;238:677)
Etiology
- Unknown
Clinical features
- Can present as a thickening, palpable mass or no clinical abnormality (seen on imaging only or incidental finding)
Diagnosis
- Histologic examination of tissue often with immunostains performed
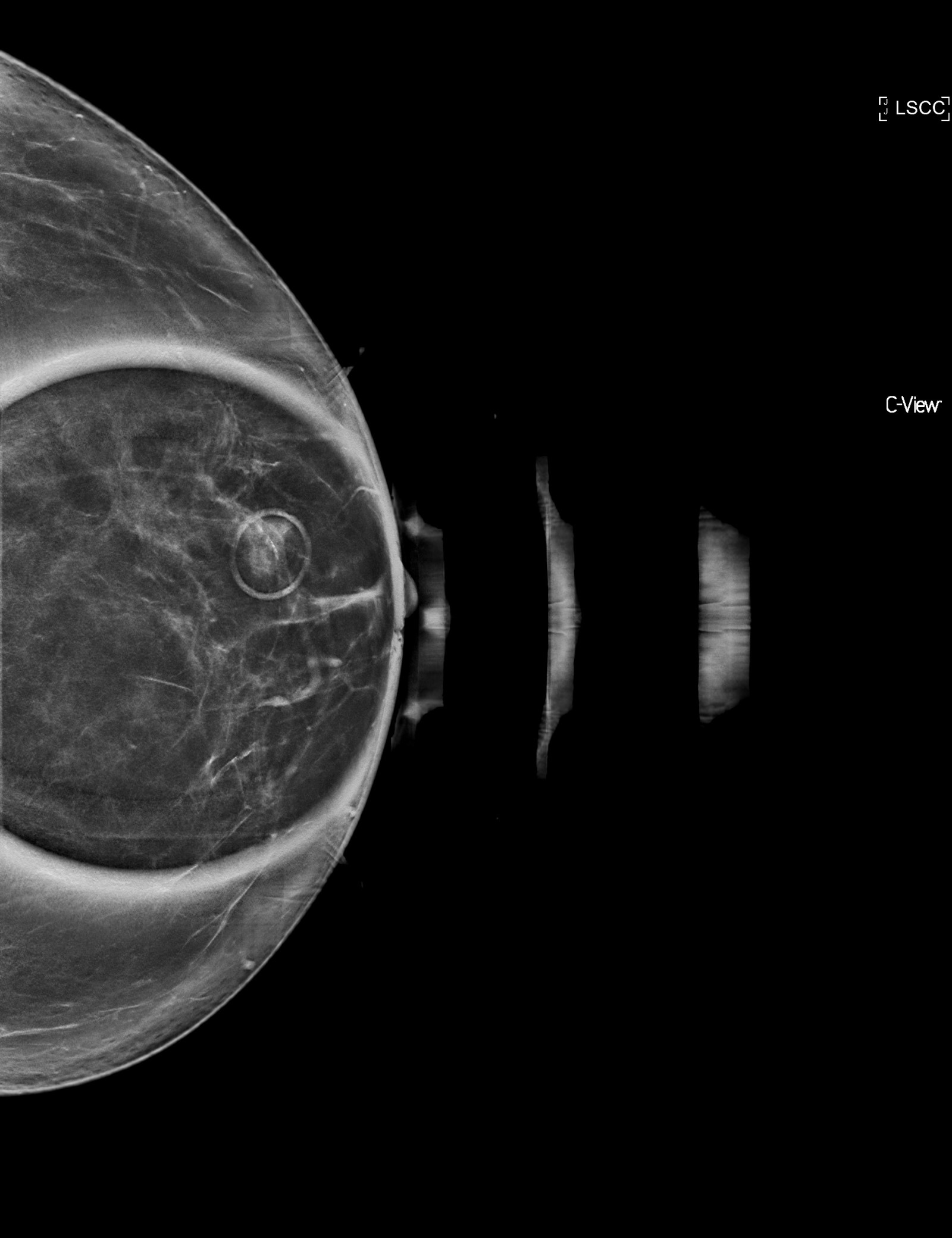
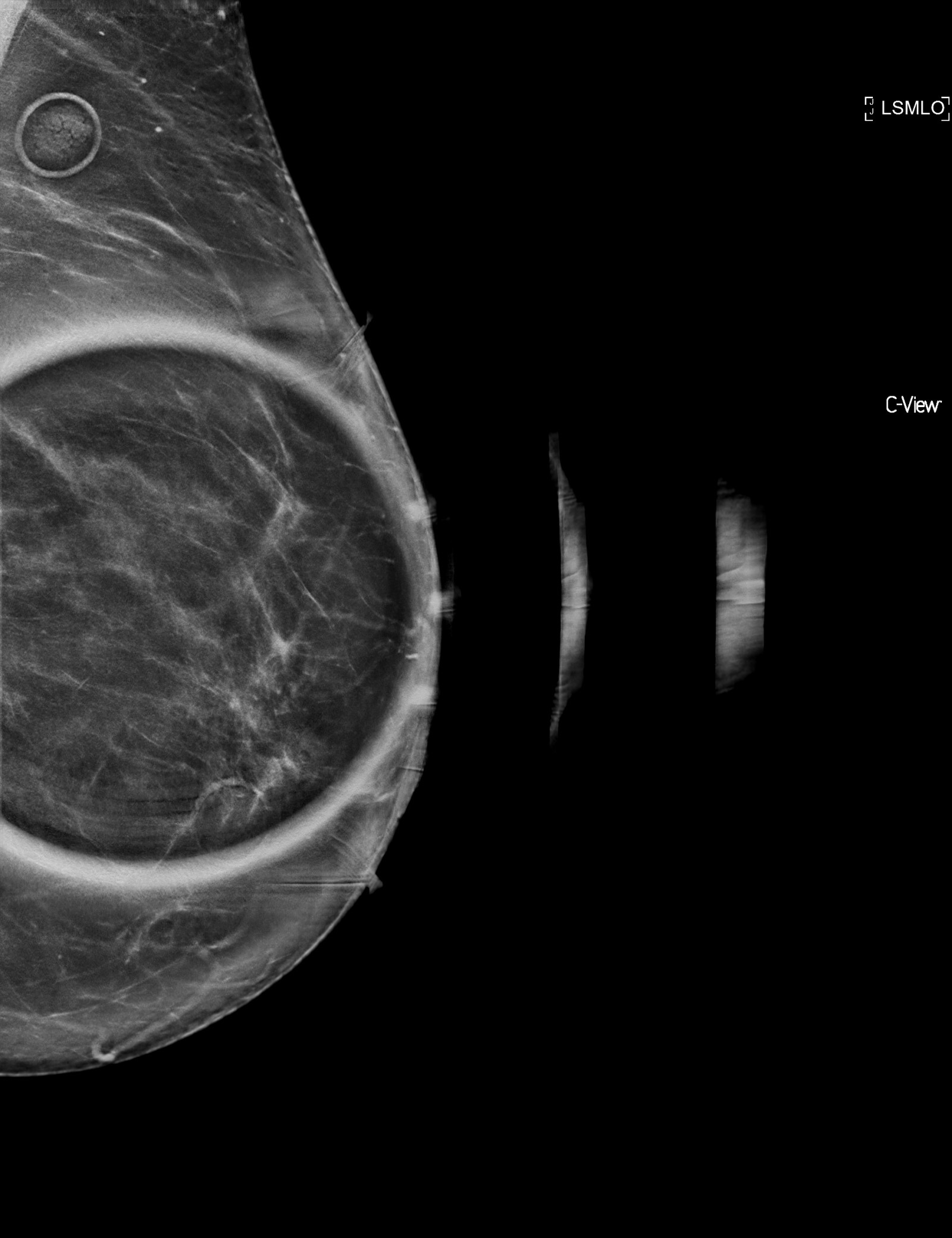
Radiology description
- Mammographic density or calcifications
Prognostic factors
- Benign although has been associated with carcinoma in 27% of cases (Am J Surg Pathol 1986;10:237)
- Transition to invasive carcinoma (with immunohistochemical profile similar to microglandular adenosis)
- Associated carcinomas include adenoid cystic, secretory and squamous, matrix producing and triple negative, basal-like carcinomas (Int J Surg Pathol 2000;8:303, Am J Surg Pathol 2009;33:496, Am J Surg Pathol 2003;27:1052, Hoda: Rosen's Breast Pathology, 4th Edition, 2014, Hum Pathol 2019;85:65, Histopathology 2009;55:732, J Pathol 2016;238:677)
- Higher risk of progression to carcinoma if atypical features are present
Case reports
- 57 year old woman with atypical microglandular adenosis postneoadjuvant chemotherapy (Pathology 2016;48:S75)
- 73 year old woman with mammary carcinoma arising in microglandular adenosis (Arch Pathol Lab Med 2003;127:77)
- Microglandular adenosis of the breast: fine needle aspiration biopsy of 2 cases (Diagn Cytopathol 1993;9:72)
Treatment
- Complete excision to rule out invasion and close followup, particularly if atypical features (Am J Surg Pathol 2008;32:544, Am J Surg Pathol 1983;7:137)
- May recur if incompletely excised
- Wide excision with documented negative margins is advised in atypical microglandular adenosis (Arch Pathol Lab Med 2007;131:1397)
Gross description
- Indiscernible from surrounding tissue
- Mass / nodule: more frequently identified with coexisting carcinoma
- Ill defined margins
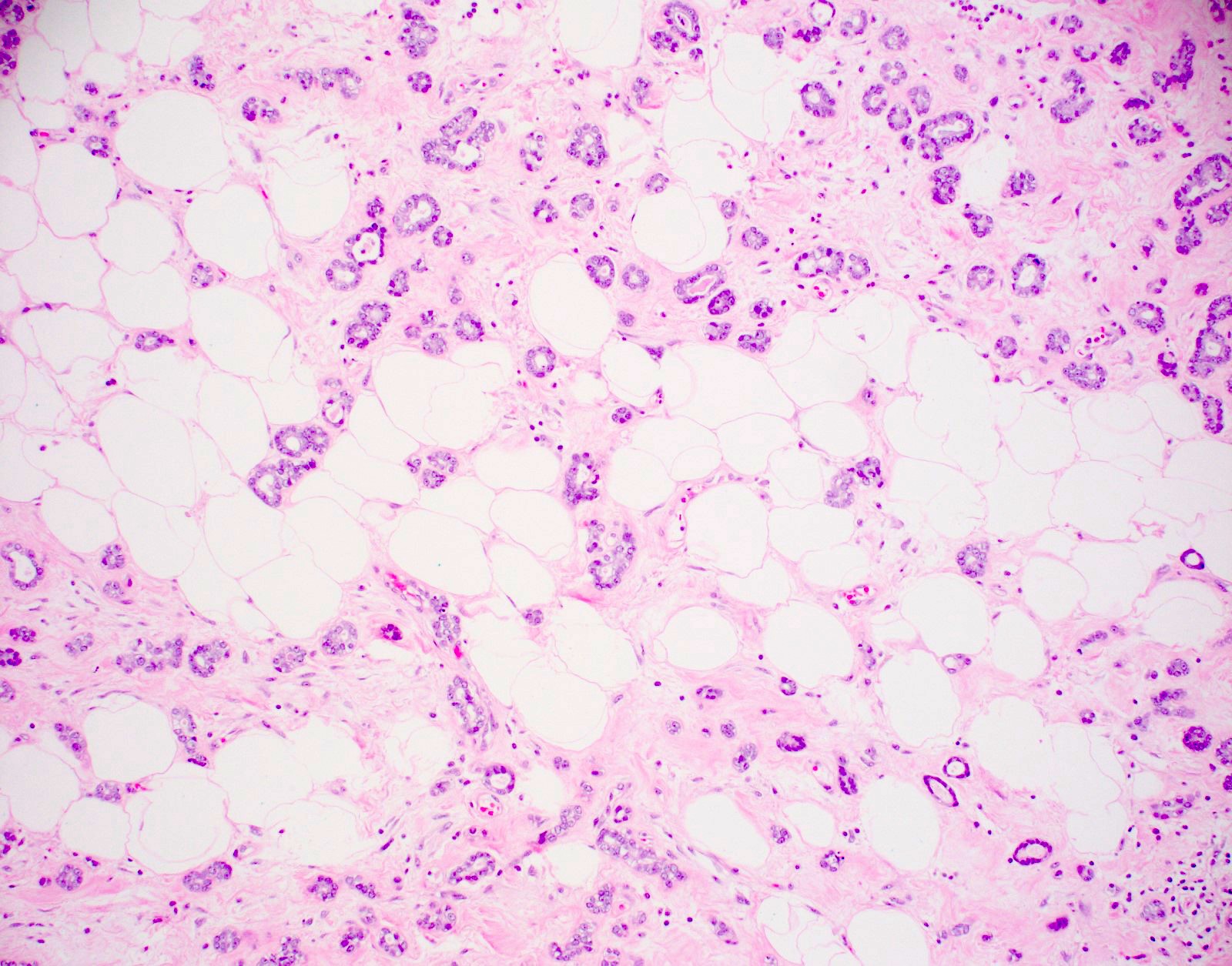
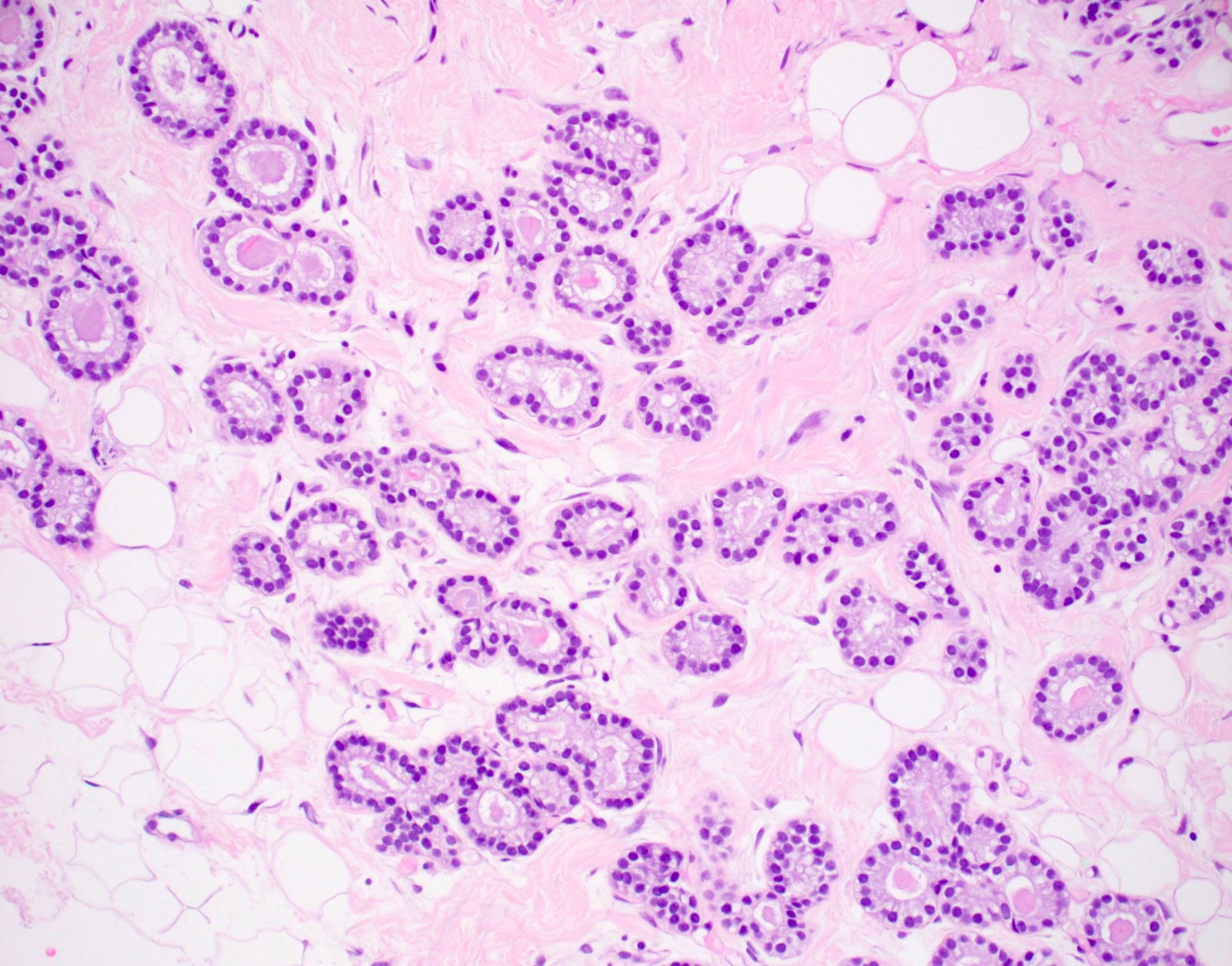
Microscopic (histologic) description
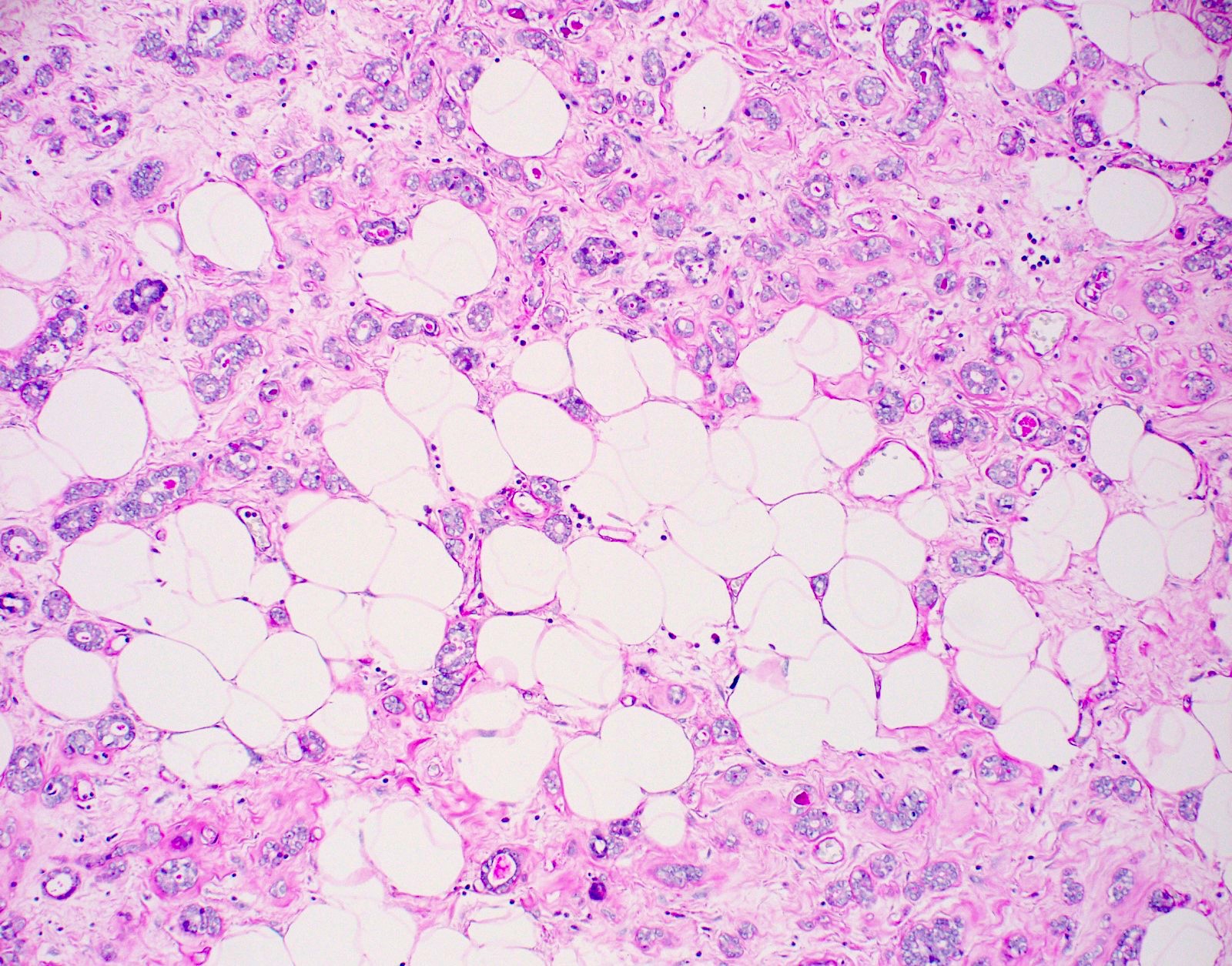
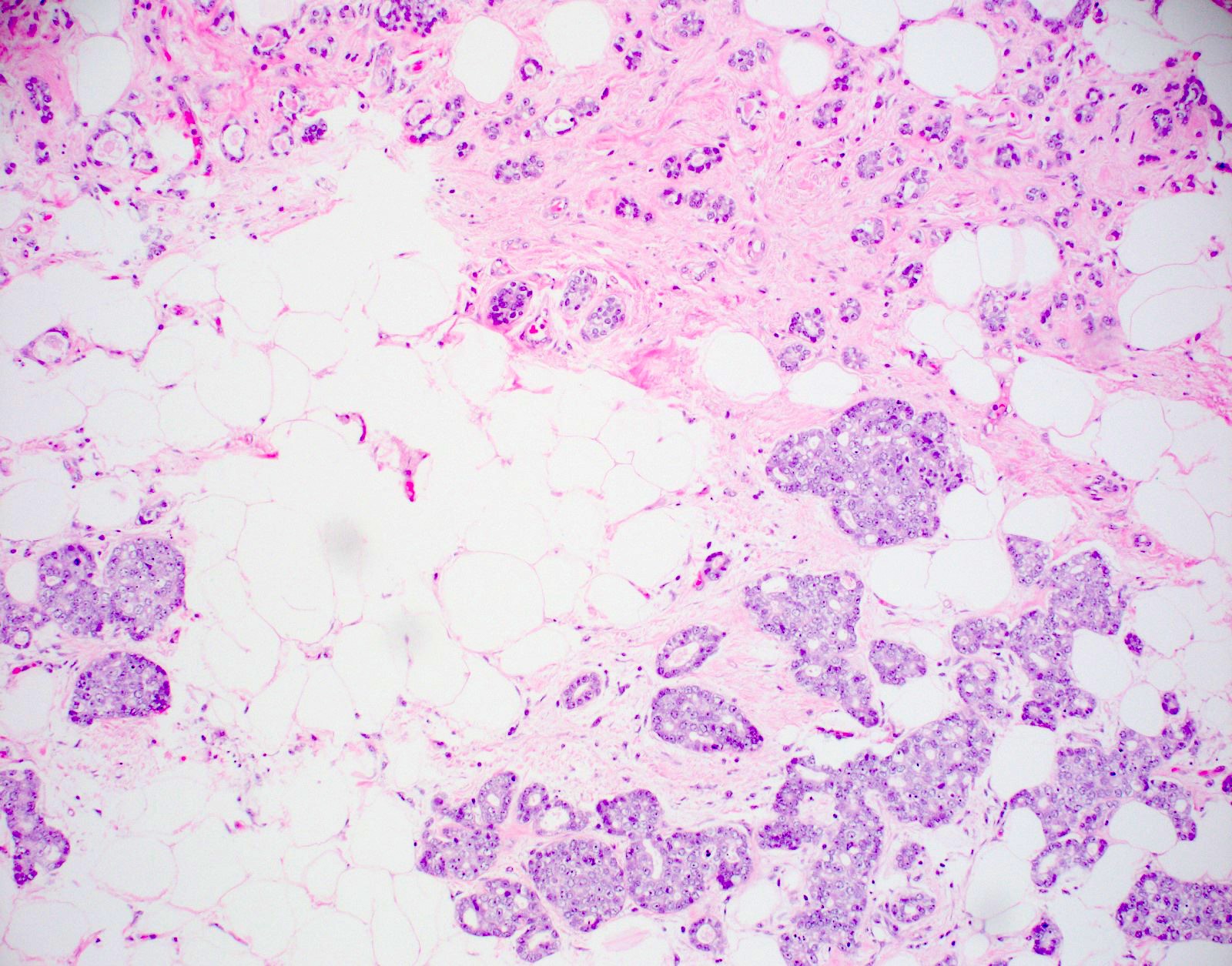
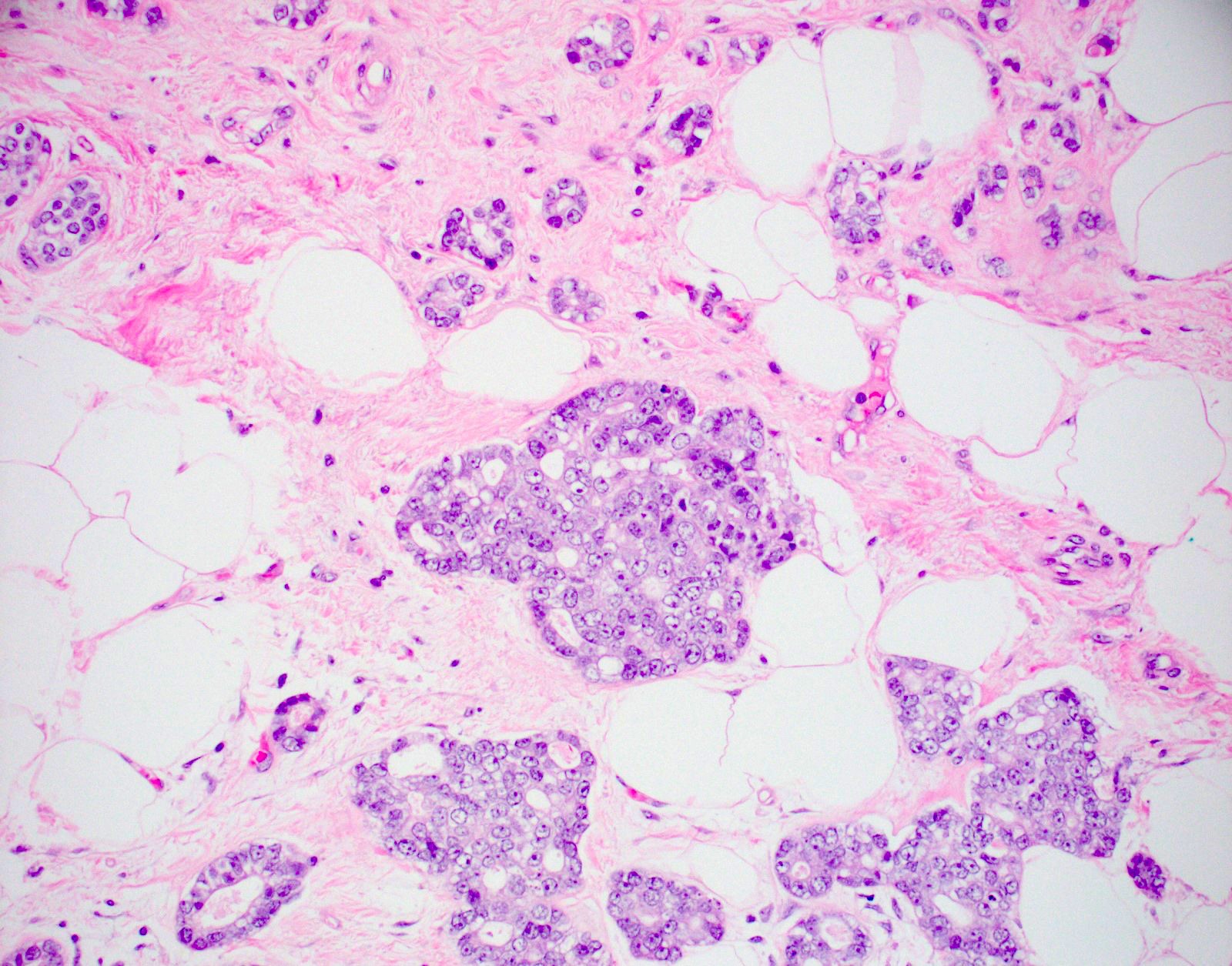
- Haphazardly infiltrating collection of small, uniform, rounded, open glands with eosinophilic secretions, irregularly distributed in fibrous or adipose tissue
- Glands lined by single layer of cuboidal / flat cells with vacuolated / granular cytoplasm and bland nuclei
- No apocrine snouts, no nucleoli, no myoepithelial layer but thick basement membrane
- Atypical microglandular adenosis:
- Increased complexity including multilayered epithelium, fused glandular units, luminal bridging, cribriform architecture
- Mild cytologic atypia, hyperchromatic nuclei, prominent nucleoli, occasional mitotic figures (WHO Classification of Tumours Editorial Board: Breast Tumours (Medicine), 5th Edition, 2019)
Microscopic (histologic) images
Cytology description
- Sparse cellularity, monotonous population of medium sized cells with vacuolated clear cytoplasm, round and uniform nuclei, small nucleoli
- Also clear cells that are solitary or clustered with spindly fibroblasts (Diagn Cytopathol 1993;9:72)
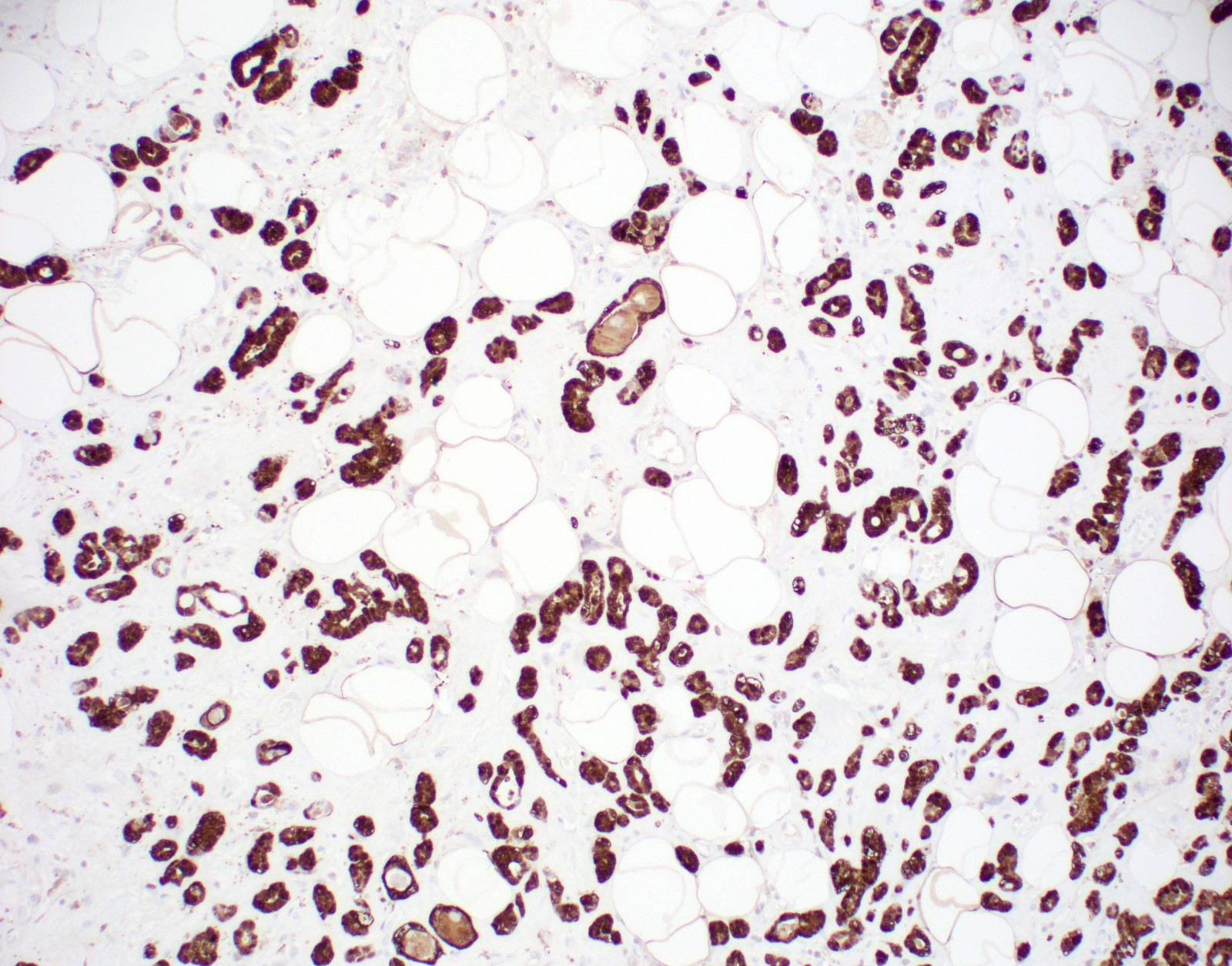
Positive stains
- CAM 5.2, AE1, S100, p63 (secretory epithelium) (Arch Pathol Lab Med 2007;131:1397, Histopathology 2005;47:611)
- CK8 / CK18 and EGFR (Am J Surg Pathol 2008;32:544)
- PAS+ diastase resistant secretions
- Variable smooth muscle actin, vimentin, type IV collagen and laminin (around glands)
Negative stains
Electron microscopy description
- Thick basement membrane around epithelium; electron lucent cytoplasm, sparse organelles (Am J Surg Pathol 1983;7:731)
Molecular / cytogenetics description
- Clonal in some cases, array CGH and sequencing studies demonstrate molecular progression to matched invasive carcinoma, supports the role of MGA as a nonobligate precursor to triple negative breast cancer (Hum Pathol 2019;85:65, Histopathology 2012;60:E115)
- Mutations in PI3K pathway (PIK3CA, PTEN, INPP4B and BRCA1) in MGA and atypical MGA (Mod Pathol 2017;30:69)
Sample pathology report
- Right breast, core biopsy:
- Microglandular adenosis (MGA) (see comment)
- Comment: Immunohistochemistry shows the lesion to be S100 positive (diffusely, strongly), estrogen receptor negative and to lack myopithelium via p63 and calponin, supporting the diagnosis. Controls are appropriate.
Differential diagnosis
- Well differentiated invasive ductal carcinoma:
- Tubular carcinoma:
- Stellate growth pattern, desmoplastic stroma, glands vary in size and shape with angulated tear drop appearance
- Lined by cells with prominent apical snouts
- Also lacks a surrounding basement membrane
- EMA+, S100- (Arch Pathol Lab Med 2007;131:1397)
- ER+, PR+
- MGA associated invasive carcinoma:
- Infiltrative, irregular glands with moderate severe atypia and desmoplastic response
- S100 variable, ER-, PR-, HER2- (Arch Pathol Lab Med 2020;144:42)
- Apocrine adenosis:
- Larger glands lined by luminal tall columnar cells with apocrine features and surrounded by flat myoepithelial cells
- Sclerosing adenosis:
- Benign lobules with glands distorted by fibrosis but surrounded by a myoepithelial layer
- In situ carcinoma:
- Proliferation of atypical epithelial cells with preserved basement membrane and myoepithelial
Additional references
Board review style question #1
Board review style answer #1
A. ER-, PR-, HER2-. The pictured lesion is microglandular adenosis (MGA) with associated invasive carcinoma, which is supported by S100 positivity in the MGA. Both MGA and associated carcinomas are most frequently ER-, PR- and HER2- (triple negative).
Comment Here
Reference: Microglandular adenosis of breast
Comment Here
Reference: Microglandular adenosis of breast
Board review style question #2
- What is a feature that is characteristic of atypical microglandular adenosis (versus typical microglandular adenosis)?
- Bland cytology
- Fused / cribriform glands
- Intraluminal secretions
- S100 positivity
Board review style answer #2
B. Fused / cribriform glands. Typical microglandular adenosis (MGA) is characterized by haphazardly infiltrative tubules with characteristic luminal secretions and lined by a single layer of bland epithelial cells. Atypical forms have atypical cytology and architecture, including multilayered epithelium, gland fusion and cribriform architecture. Atypical MGA may not have as prominent secretions as in more typical forms. Both typical and atypical MGA are S100 (diffusely, strongly) positive.
Comment Here
Reference: Microglandular adenosis of breast
Comment Here
Reference: Microglandular adenosis of breast