Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Vickery J, Biernacka A. LCIS pleomorphic. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantpleolcis.html. Accessed April 2nd, 2025.
Definition / general
- A morphologic subtype of lobular carcinoma in situ (LCIS) composed of a noninvasive neoplastic proliferation of large dyscohesive cells with marked nuclear pleomorphism (nuclei are > 4 times the size of a lymphocyte, equivalent to the cells of high grade DCIS)
- With or without apocrine features (Histopathology 2020;77:181)
Essential features
- Lobulocentric proliferation of cells which are significantly more pleomorphic and larger when compared to cells of classic LCIS
- Lobular acini involved by pleomorphic LCIS and often substantially expanded
- Cytologic features more closely resemble high grade DCIS rather than classic LCIS
- Large nuclei (≥ 4x larger than a lymphocyte)
- Pleomorphism: 2 - 3x variation in nuclear size and shape
- May be associated with necrosis, including comedonecrosis
- Other features overlap with classic LCIS
- Loss / dysfunction of E-cadherin
- Cellular dyscohesion
- Lack of cell polarity
- Intracytoplasmic lumina, may have signet ring cells
- Pagetoid spread
- Regarded as a genetically and biologically more advanced lesion than classic LCIS and is a direct precursor to invasive carcinoma
Terminology
- First described by Frost et al. in 1996 (AJSP Rev Rep 1996;1:27)
- Pleomorphic LCIS (may be denoted as PLCIS, PL LCIS or P LCIS) is considered a morphologic variant of classic LCIS (CLCIS)
- Although it falls under the category of lobular neoplasia, the terms nonclassic lobular carcinoma in situ and variant lobular carcinoma in situ are not preferred because they do not distinguish between PCLIS and another morphologic subgroup called florid LCIS (FLCIS)
- In the past, some have referred to what is now PLCIS as pleomorphic ductal lobular carcinoma in situ (PL / DLCIS), which is no longer used
- The terms lobular carcinoma in situ with pleomorphic morphology or lobular carcinoma in situ with pleomorphic features are not preferred as they are more vague than using pleomorphic lobular carcinoma in situ
- While there is no established cutoff, some have suggested that lobular lesions be considered pleomorphic when at least 10% of the tumor cells show high grade nuclei (Histopathology 2014;64:981)
- Lobular acini involved by pleomorphic lobular cells are often substantially expanded but they may be only mildly distended or show no distension; the latter still qualify as pleomorphic LCIS since there is no recognized category of pleomorphic atypical lobular hyperplasia (ALH) (analogous to not having a high grade atypical ductal hyperplasia)
- Currently there is no term for a lesion with both the nuclear morphology of pleomorphic LCIS and architectural features of florid LCIS
ICD coding
- ICD-O:
- ICD-11:
- 2E65.0 & XH6EH0 - lobular carcinoma in situ of breast & lobular carcinoma in situ, NOS
Epidemiology
- PLCIS is relatively rare, with an estimated incidence of < 5% of all LCIS (Histopathology 2014;64:981)
- Prevalence may be underestimated given the similarities of PLCIS to DCIS, especially high grade DCIS and probable misdiagnoses as such in the past
- Most patients with PLCIS are White females (Ann Surg Oncol 2015;22:4263)
- Compared to CLCIS, patients with PLCIS are typically older and postmenopausal
- Multiple studies have found PLCIS patients have a mean age of ~55 years (range: 36 - 86 years) (Am J Surg Pathol 2009;33:1683, Mod Pathol 2002;15:1044)
- One study found this age difference was mostly attributed to apocrine PLCIS, which was diagnosed primarily in postmenopausal women with a mean age of 60 years, whereas the mean age for nonapocrine PLCIS was 51 years (Am J Surg Pathol 2009;33:1683)
Sites
- Occurs in the breast with no specific laterality predilection
- PLCIS has been noted to be more unifocal and continuous in distribution than CLCIS, which is typically multicentric and bilateral (Am J Surg Pathol 2019;43:399)
Pathophysiology
- CDH1 inactivation, which leads to loss or impaired function of E-cadherin, is an early event and hallmark of lobular lesions, including PLCIS (Histopathology 2020;77:181)
- E-cadherin plays a major role in intercellular adhesion and cell polarity
- 1q gain and 16q loss combined with the lack of E-cadherin expression observed in PLCIS support a lobular lineage
- PLCIS accumulates additional alterations and generally exhibits greater genomic instability than classic LCIS (J Pathol 2005;207:1, Mod Pathol 2020;33:1287)
- Whether PLCIS arises from CLCIS and represents a genetically more advanced lesion or if PLCIS originates from a separate disease process is not entirely clear; however
- PLCIS and pleomorphic invasive lobular carcinoma (ILC) are frequently associated with foci of classic ILC and areas showing a morphological continuum between PLCIS and CLCIS have also been described (J Pathol 2005;207:1)
- Synchronous CLCIS and apocrine PLCIS share similar chromosomal changes with additional changes present in the apocrine PLCIS, suggesting evolution from the same precursor or through the same genetic pathway (Am J Surg Pathol 2009;33:1683)
Etiology
- No well known causative factors specific to PLCIS
- Etiologic factors associated with the development of CLCIS currently have insufficient data for being considered etiologic factors for PLCIS
- A study found that in their cohort of patients with nonclassic LCIS, which also included florid LCIS and LCIS with pleomorphic features, none had a personal history of breast cancer nor were known BRCA1 / 2 carriers but 41.7% (10/24) had a first degree family history of breast cancer (Surg Oncol 2019;28:190)
Clinical features
- Patients are usually asymptomatic
- The majority of patients (up to 87%) present with abnormal screening detected mammographic findings, such as calcifications (Am J Surg Pathol 2009;33:1683)
- Some cases of PLCIS lack specific radiologic features and may be an incidental microscopic finding in breast biopsies
- Less commonly, patients can present with a palpable mass or nipple discharge (Histopathology 2014;64:981, Surg Oncol 2019;28:190)
- Patients who present with a palpable mass usually have PLCIS involving other lesions (e.g., a fibroadenoma) or have associated invasive carcinoma (AJR Am J Roentgenol 2018;211:462)
- Only 15 - 52.2% of patients have pure PLCIS alone on final surgical pathology on core biopsy or excision (Ann Surg Oncol 2015;22:4263, Am J Surg Pathol 2019;43:399)
- In the majority of cases (up to 77%), there is an associated invasive carcinoma, usually consisting of pleomorphic ILC but classic ILC or other histologic types may occur (Am J Surg Pathol 2019;43:399)
Diagnosis
- Most patients have imaging abnormalities detected on mammogram or ultrasound, leading to image directed core needle biopsies
- In a subset of patients, PLCIS may be detected at the time of excisional biopsy or operations for other lesions (Ann Surg Oncol 2018;25:3064)
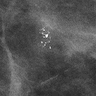
Radiology description
- Most patients present with suspicious mammographic calcifications similar to those associated with DCIS (AJR Am J Roentgenol 2012;199:929, AJR Am J Roentgenol 2001;176:1255)
- Rarely a patient may have a circumscribed mass identified on a diagnostic mammogram or ultrasound (AJR Am J Roentgenol 2018;211:462)
- The following imaging findings (from most to least common) were described in one study: fine pleomorphic grouped calcifications on mammograms, mammographic distortion, amorphous calcifications or regional clumped nonmass enhancement at screening MRI (AJR Am J Roentgenol 2018;211:462)
- Other studies using advanced breast imaging with MRI found nonmass-like enhancement (37.5 %), no MRI abnormality (31.3 %), mass / space occupying lesion (25.0%) or focal < 5 mm area of enhancement (6.3%) (Ann Surg Oncol 2015;22:4263)
Radiology images
Prognostic factors
- Pure PLCIS is relatively rare and the natural history of PLCIS not associated with invasive carcinoma remains largely unknown
- PLCIS is considered both a genetically advanced risk factor for and direct precursor to ILC / pleomorphic ILC
- PLCIS is associated with a high incidence of associated invasive carcinoma either on core needle biopsy or excision
- Reported numbers vary; one study cohort found 46 of 93 (49%) PLCIS patients had concurrent invasive disease on excision specimen analysis (World J Clin Oncol 2014;5:546)
- Given the increased genomic instability of PLCIS, the risk of subsequent breast cancer is suspected to be higher than CLCIS but further studies with more patients and longer follow up are necessary
- PLCIS is more likely to be ER or PR negative and HER2 positive when compared with CLCIS and FLCIS (Breast 2013;22:194)
- Up to 18% of patients may be triple negative (Mod Pathol 2020;33:1287)
- PLCIS also frequently has a high Ki67 labeling index and accumulation of p53 protein by immunohistochemistry, both features indicative of aggressive behavior (Breast 2013;22:194)
- Overall, due to its unfavorable immunoprofile and a higher likelihood of being associated with invasive disease, it is regarded as having a worse prognosis than CLCIS
Case reports
- 44 year old woman with PLCIS of the breast composed almost entirely of signet ring cells (Pathol Int 2006;56:683)
- 52 year old woman with metastatic invasive pleomorphic lobular carcinoma with PLCIS / high grade DCIS-like morphology in affected lymph nodes (Int J Surg Pathol 2020;28:436)
- 68 year old woman with apocrine differentiation in invasive pleomorphic lobular carcinoma arising in a background of multiple in situ lesions, including apocrine PLCIS, classic LCIS and DCIS (Pathol Oncol Res 2002;8:151)
- 79 year old woman with apocrine PLCIS associated with microglandular adenosis and metaplastic matrix producing carcinoma (Diagnostics (Basel) 2022;12:1458)
Treatment
- There are no well defined guidelines for the treatment of PLCIS
- It is currently debated whether it is necessary to achieve negative margins and if there is any potential benefit of adjuvant radiation
- WHO Classification of Tumors Editorial Board recommends excision for PLCIS diagnosed on core needle biopsy (Histopathology 2020;77:181)
- In a resection specimen, the margin status of CLCIS is not reported but many recommend it should be reported for PLCIS to help inform subsequent management decisions (Arch Pathol Lab Med 2011;135:737)
- For management guidelines, many organizations suggest considering excision of PLCIS with negative margins (World J Clin Oncol 2014;5:546)
- Others recommend PLCIS should be treated similarly to DCIS; surgical excision with negative margins with or without other adjuvant treatments (World J Clin Oncol 2014;5:546)
- As with high grade DCIS, some suggest that a sentinel lymph node biopsy may be considered at the time of PLCIS excision (World J Clin Oncol 2014;5:546)
- Reported recurrence rates of PLCIS treated with conservative surgery with or without antiestrogen therapy vary widely from 0% to as high as 57% (Histopathology 2020;77:181)
- It is unclear whether positive margin status affects the likelihood of recurrence
- One study evaluating only PLCIS at or near an excision margin found an overall recurrence rate of 3.8% regardless of treatment (Arch Pathol Lab Med 2011;135:737)
- Chemoprevention can be considered in ER positive PLCIS patients
Gross description
- Not associated with any specific grossly recognizable abnormalities
- Cut surface of breast tissue harboring PLCIS may have a faintly granular appearance when viewed with tangential light because affected lobules may be sufficiently enlarged to be visible and microcalcifications are almost always present
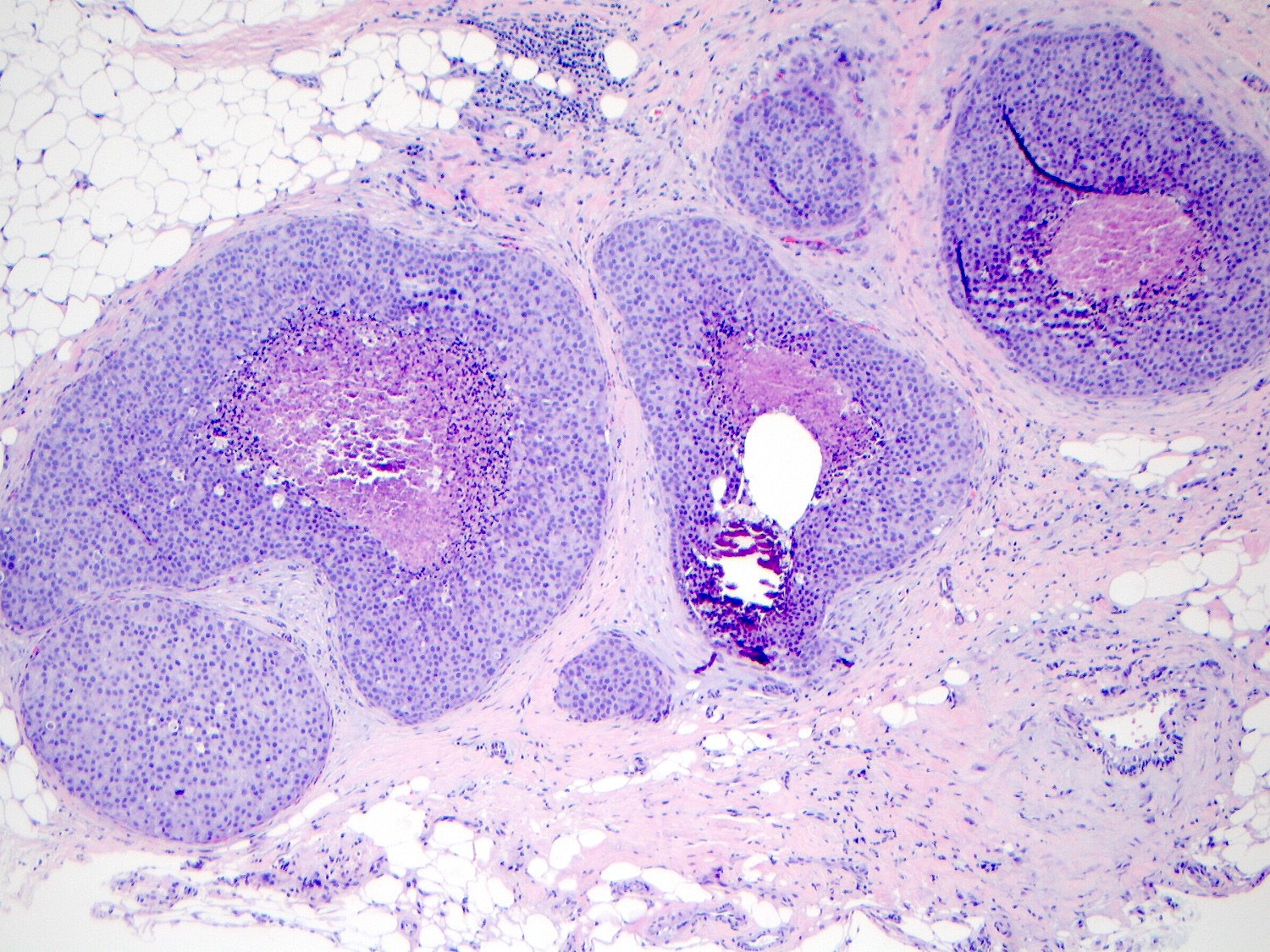
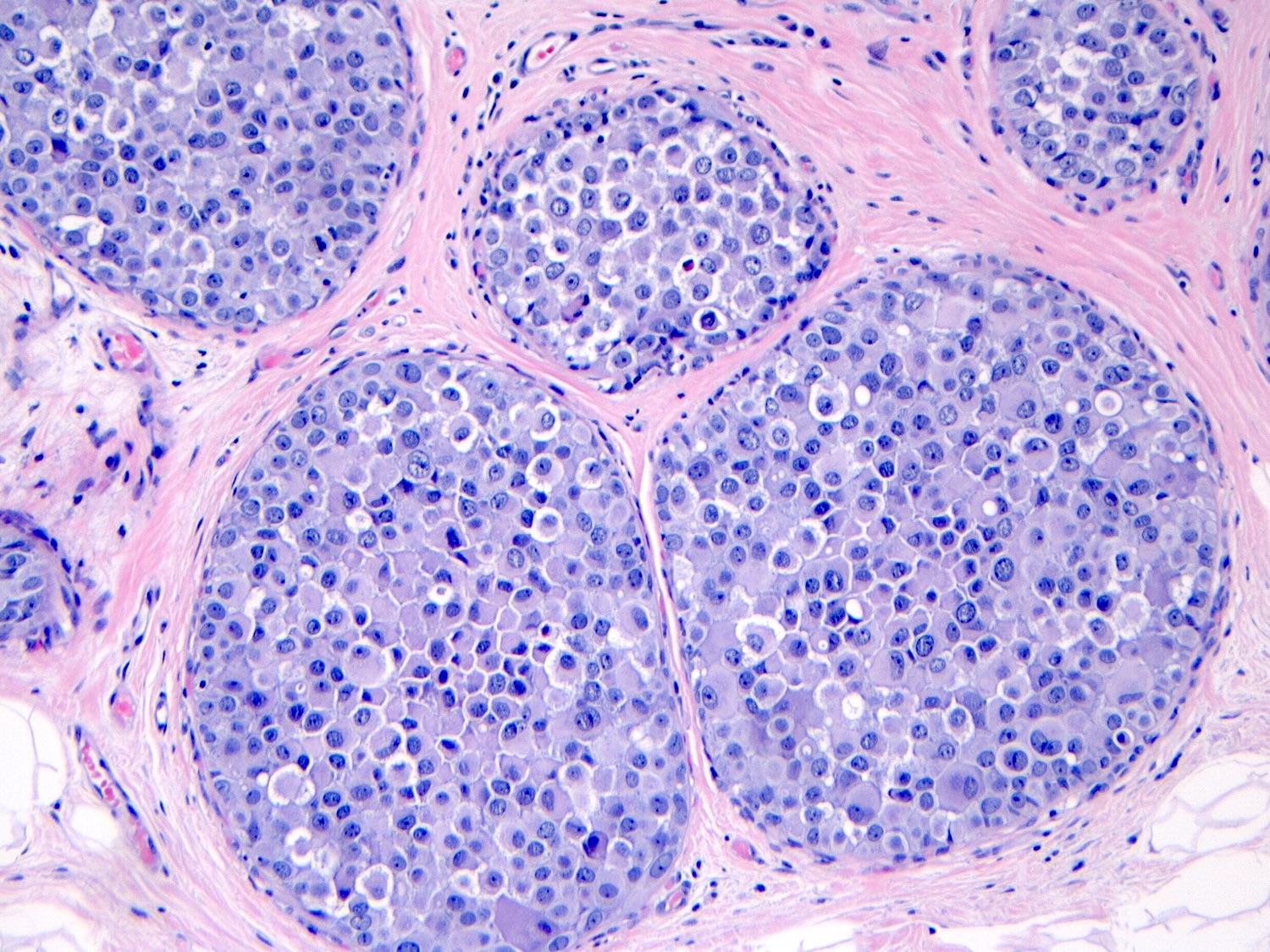
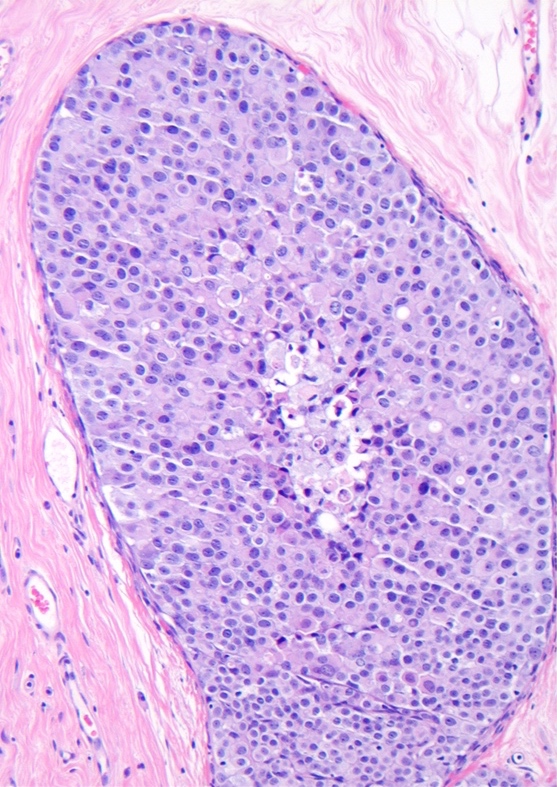
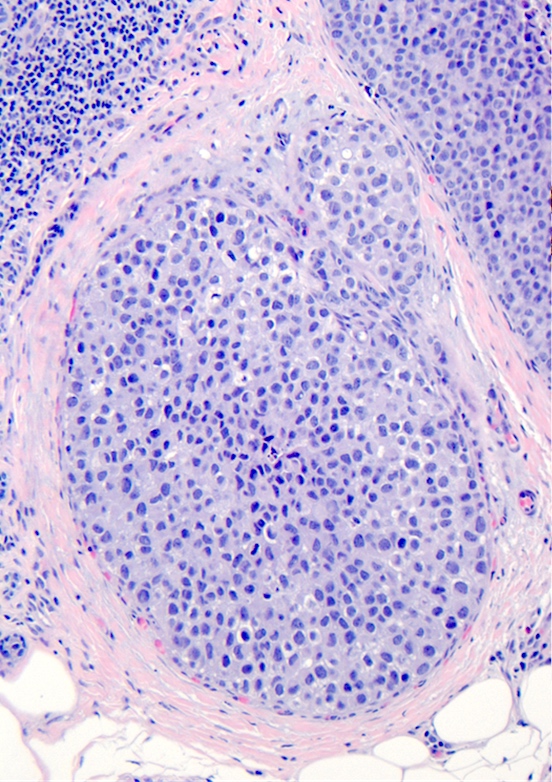
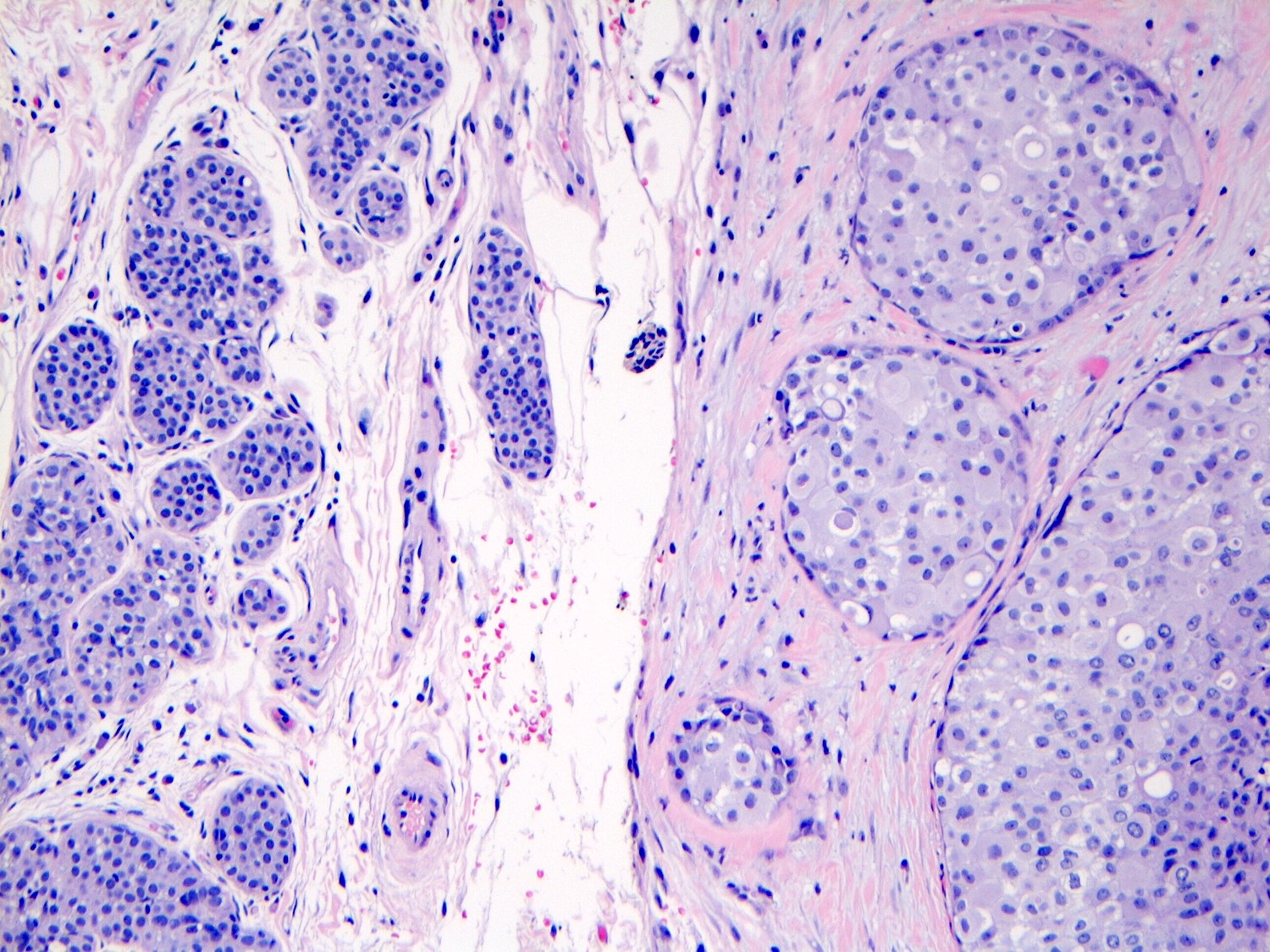
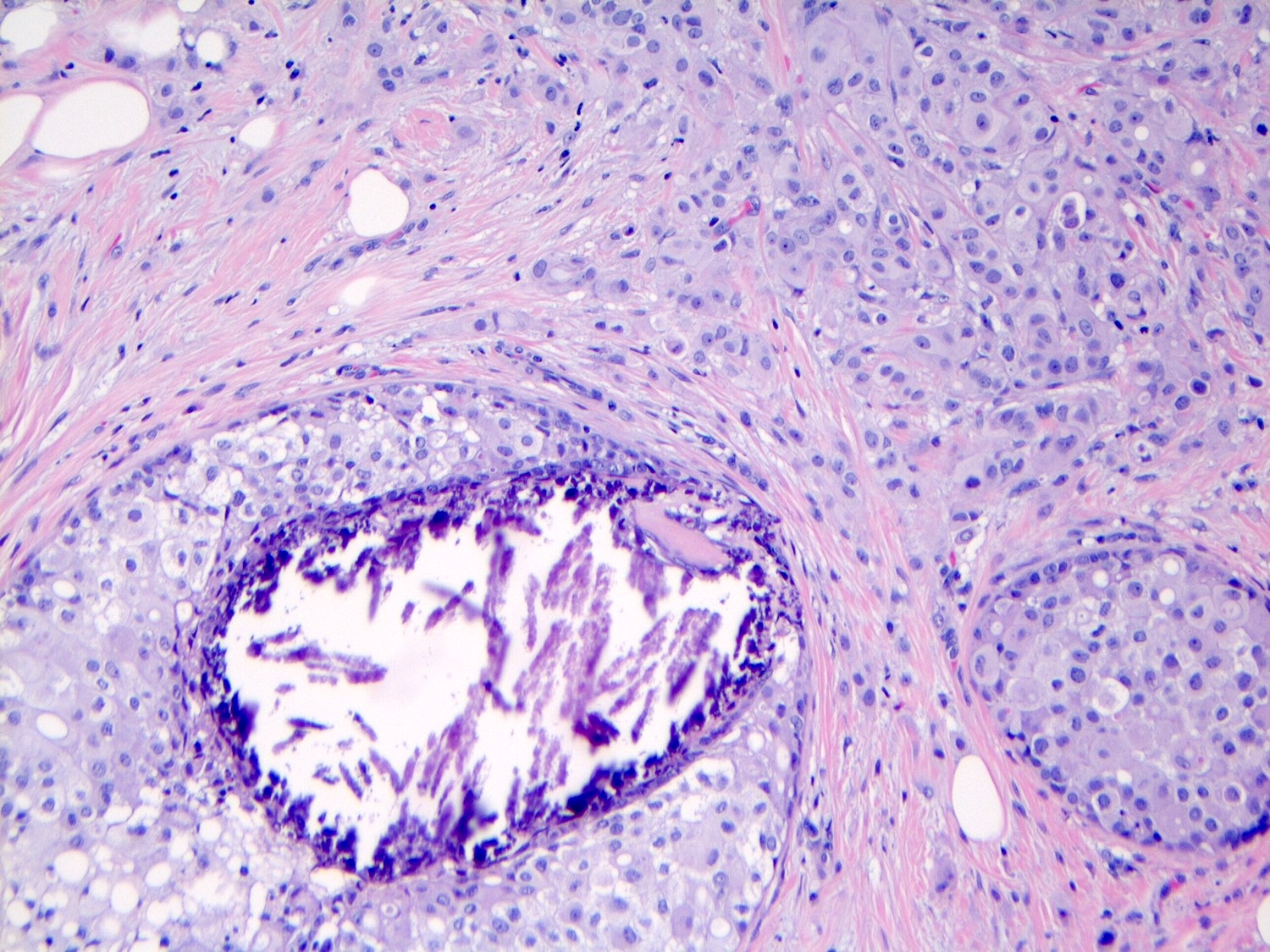
Microscopic (histologic) description
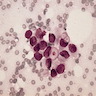
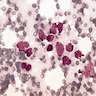
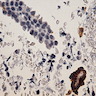
- Pleomorphic LCIS has architectural features similar to CLCIS, characterized by solid proliferation of dyscohesive neoplastic cells within terminal duct lobular units (TDLUs)
- Pagetoid spread to adjacent ducts may be seen, similar to CLCIS
- Differs from classic LCIS with regard to the degree of nuclear atypia or lobular acinar expansion
- Unlike CLCIS, PLCIS has more nuclear enlargement and variability
- Lobular acini involved by pleomorphic lobular cells are often substantially expanded but they may be only mildly distended or show no distension
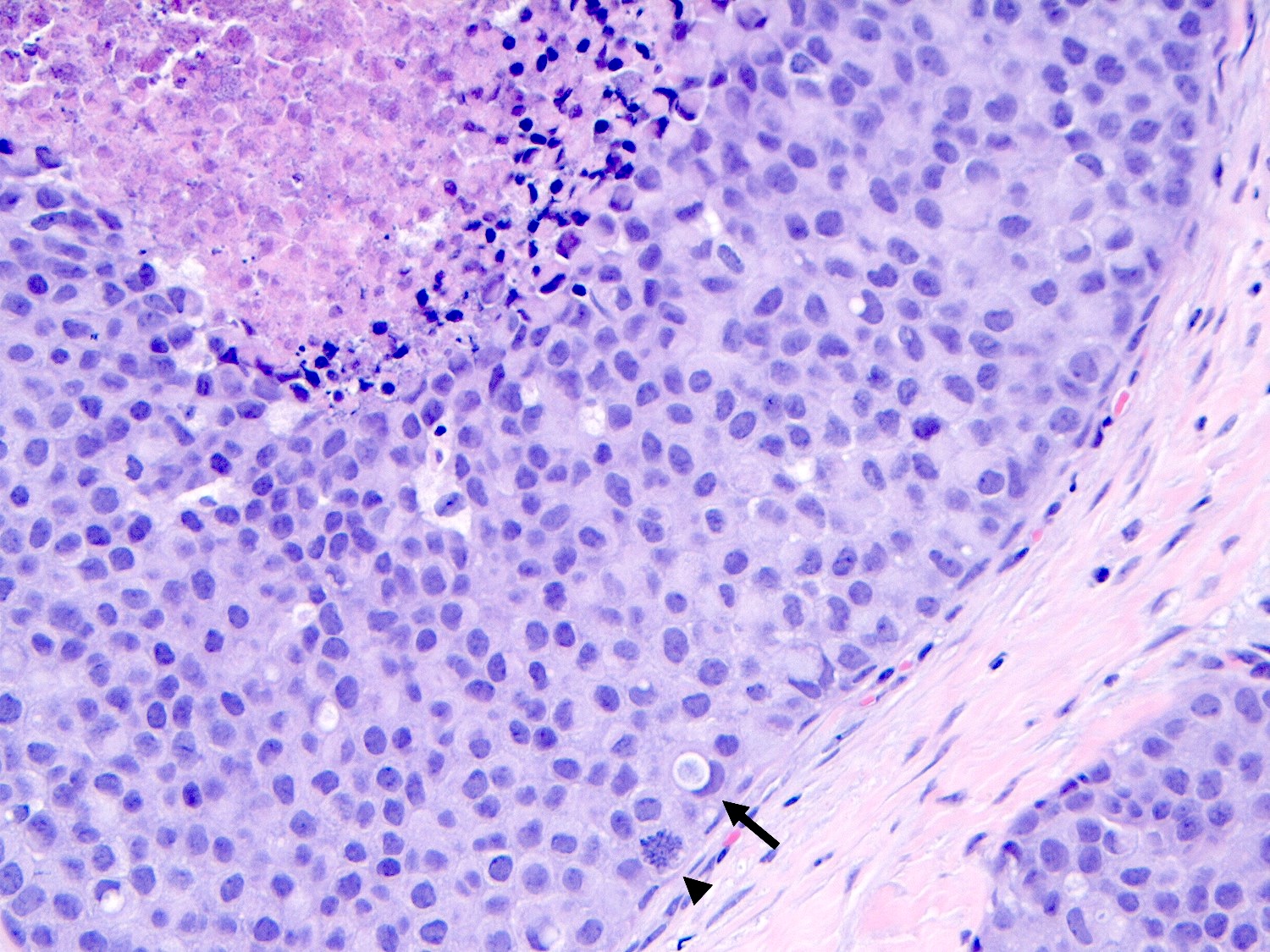
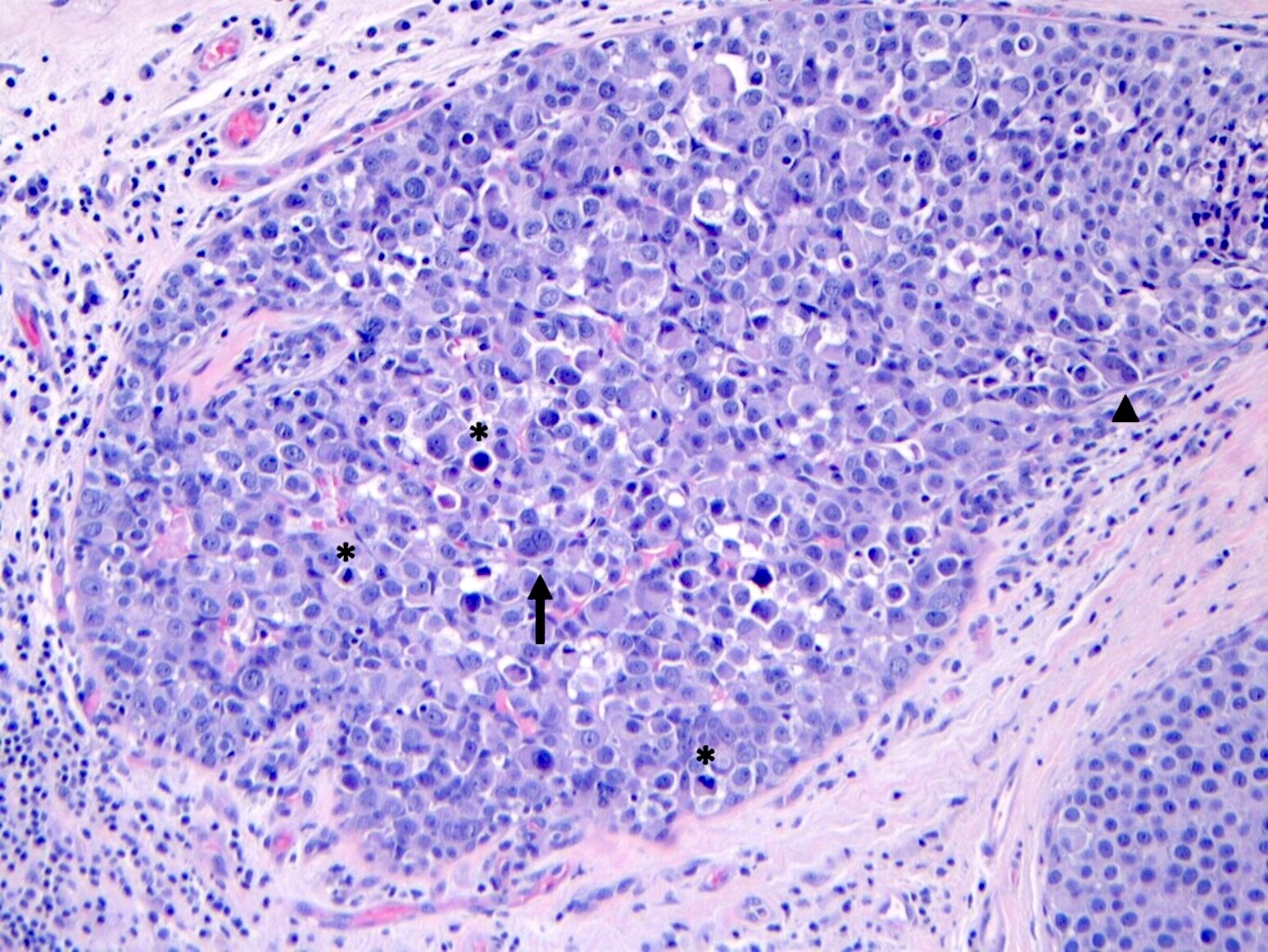
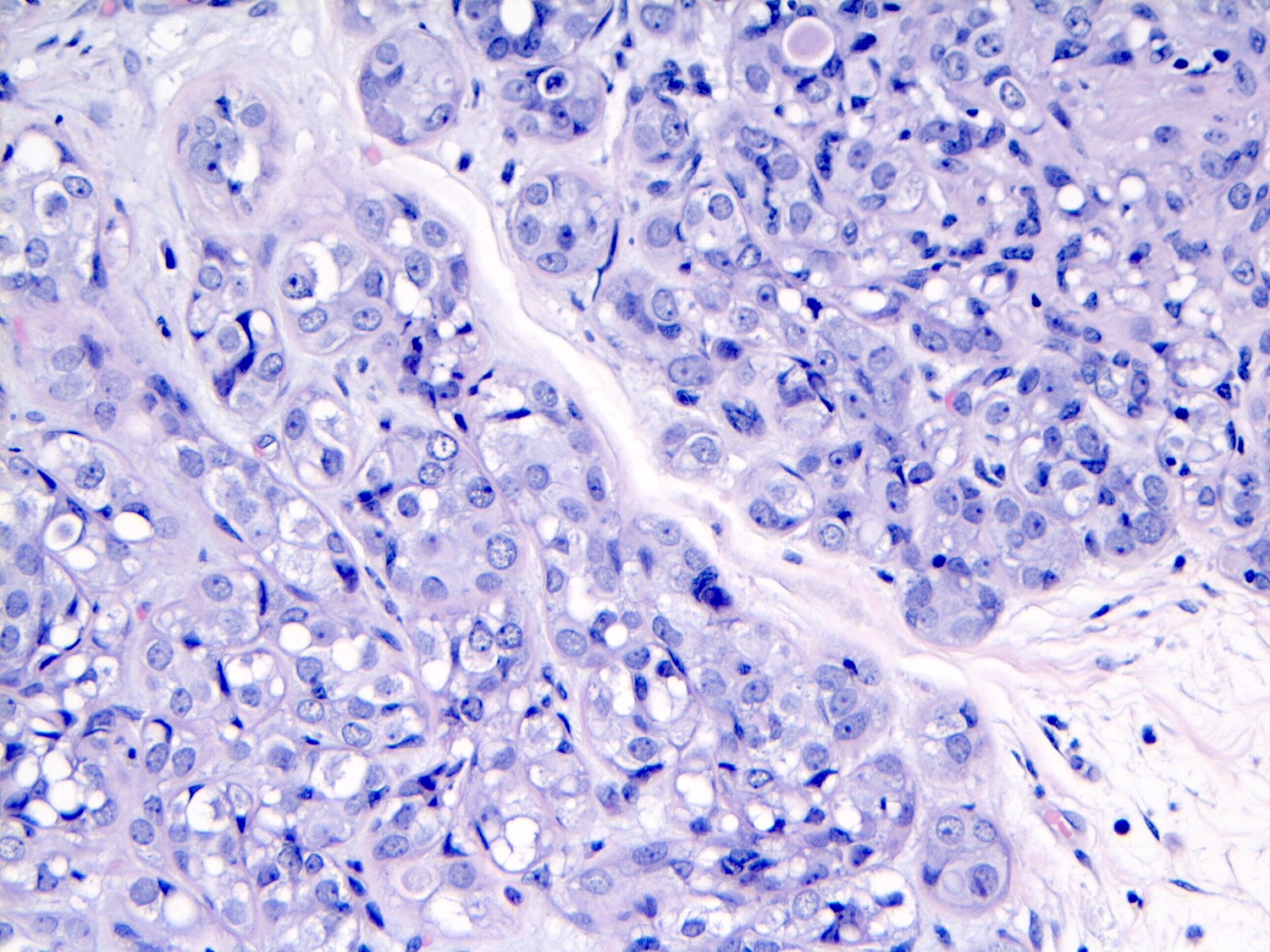
- Individual cells are medium to large with high N:C ratios, PLCIS nuclei are larger (≥ 4x the size of a lymphocyte), often exhibit moderate to marked nuclear pleomorphism (> 2 - 3x variation in nuclear size) and have distinct small to prominent nucleoli (Mod Pathol 2002;15:1044)
- Nuclei are often eccentrically placed and binucleated / multinucleated cells are frequently seen (Am J Clin Pathol 2015;144:722)
- Chromatin is coarse and mitotic figures can be seen
- Cytoplasm of PLCIS cells is moderate to abundant, dense eosinophilic or granular
- Intracytoplasmic vacuoles are often present with or without mucin; may be large enough to produce signet ring cell forms
- Apocrine differentiation is observed in a subset of pleomorphic LCIS, referred to as apocrine PLCIS
- This subset is characterized by abundant eosinophilic cytoplasm, cytoplasmic granules and prominent nucleoli
- Central, comedo type necrosis is often present but not required for diagnosis
- Microcalcifications are a common associated finding
- PLCIS coexists with classic LCIS in > 40% of cases (Mod Pathol 2002;15:1044)
- Associated with invasive carcinoma, most commonly pleomorphic ILC but may be of any histologic type, careful assessment of surrounding tissue is essential
Comparison of cytomorphologic features
| Type of carcinoma | Nuclear sizea | Nuclear pleomorphism | Nucleoli | Cytoplasm | Dyshesion | Central necrosis and calcifications |
| PLCIS | ≥ 4x | Moderate / marked | Small (occasionally prominent) | Moderate / abundant | Yes (often) | Yes (common) |
| Classic LCIS type A | 1.5x | Absent | Indistinct | Scant | Absent | Absent |
| Classic LCIS type B | 2x | Mild / moderate | Indistinct | Moderate | Yes (common) | Absent |
| FLCIS | 1.5 - 2x | Absent to moderate | Indistinct | Scant to moderate | Occasional | Yes (common) |
- Adapted and modified from Mod Pathol 2002;15:1044
- aSize in comparison with a mature lymphocyte
- Florid LCIS (FLCIS) cells show the cytological features of classic LCIS
- FLCIS can be composed of type A or type B LCIS cells and is defined based on architectural not cytologic features (Mod Pathol 2002;15:1044)
- Pleomorphic LCIS and florid LCIS often demonstrate comedonecrosis and calcifications
Microscopic (histologic) images
Contributed by Anna Biernacka, M.D., Ph.D.
Cytology description
- Samples are typically highly cellular
- Cells are dyscohesive, predominately single but may form small, loosely cohesive aggregates
- Cells have abundant dense cytoplasm and may have signet ring morphology
- Nuclei are large, pleomorphic and frequently have nucleoli
- Cells may be bi or multinucleated, lobulated, indented or eccentrically placed
- Cells can resemble those of DCIS
- Intracytoplasmic mucin or signet ring cells can be present, which can help to distinguish from DCIS
- Microcalcifications or necrosis may be seen in the background
- In ductal lavage (DL), the degree of cytologic atypia is lower than that seen in fine needle aspiration (FNA) (Acta Cytol 2008;52:207)
- There are no reliable cytologic criteria for distinguishing between PLCIS and ILC
- Reference: Cancer 2008;114:111
Positive stains
- ER and PR are frequently positive
- In PLCIS
- ER is positive in 72 - 100% of cases
- PR is positive in 50 - 100% of cases
- HER2 is overexpressed in 1 - 41% of cases (Surg Pathol Clin 2018;11:123)
- Notably, PLCIS is more likely to be ER negative than CLCIS
- CLCIS are typically diffusely and strongly positive for ER and PR (> 90% of cases) and very rarely show ERBB2 (HER2) overexpression or gene amplification (0 - 4%) (Histopathology 2020;77:181)
- In a study comparing PLCIS and classic lobular with pleomorphic features (borderline CLCIS lesions with focal atypia), expression of ER was identified in 81% of PLCIS cases and 100% of CLCIS with pleomorphic features (Breast Cancer Res Treat 2017;165:411)
- Apocrine PLCIS appears to be a biologically and immunohistochemically distinct subtype
- It is mostly (~80%) negative for ER and PR, has HER2 gene amplification in 33% of cases and has elevated Ki67 (> 10%) (Am J Surg Pathol 2009;33:1683)
- Although AR expression in a study (see table below) was identified in all LCIS lesions, nonapocrine PLCIS had significantly lower AR levels than both CLCIS and apocrine PLCIS (Am J Surg Pathol 2009;33:1683)
| Comparison of biomarkers among lobular carcinoma in situ (LCIS) subtypes | ||||||||
| ER | PR | AR | AR intensity | Mean Ki67 | HER2 | GCDFP-15 | CK5/6 | |
| CLCIS | 100% | 100% | 100% | Moderate | 4.20% | 0% | Not done | 19% |
| All PLCIS | 66% | 62% | 100% | Variable | 11.5% | 13% | 74% | 23% |
| Nonapocrine PLCIS | 100% | 94% | 100% | Weak | 9.9% | 0% | 50% | 28% |
| Apocrine PLCIS | 23% | 17% | 100% | Strong | 13.9% | 31% | 100% | 17% |
- Adapted from Am J Surg Pathol 2009;33:1683
Negative stains
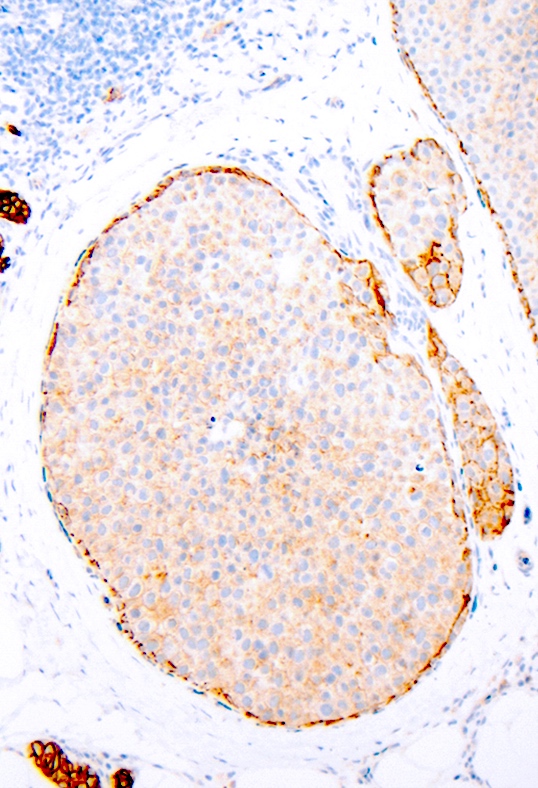
- Loss of membranous expression of E-cadherin is the defining immunohistochemical feature of lobular differentiation including PLCIS
- Inactivation of E-cadherin usually leads to loss of p120 and beta catenin expression on cell membranes and aberrant accumulation of p120 in the cytoplasm (Arch Pathol Lab Med 2017;141:1668)
- Rarely attenuated E-cadherin expression (scattered cells showing dot-like discontinuous and weak membranous staining or patchy cytoplasmic staining) is seen but this does not preclude the diagnosis of PLCIS if typical morphology is present
Molecular / cytogenetics description
- The molecular hallmark of LCIS, including PLCIS, is the loss of expression or function of membranous E-cadherin, a transmembrane glycoprotein encoded by the CDH1 gene on the long arm of chromosome 16 (16q)
- Overall, PLCIS and CLCIS share similar genomic alterations: both groups are characterized by 1q gain (75% in PLCIS versus 69% in CLCIS) and 16q loss (85% versus 76%, for PLCIS and CLCIS, respectively) (Am J Surg Pathol 2009;33:1683)
- PLCIS is thought to evolve through a molecular genetic pathway similar to that of CLCIS but then undergoes additional genetic events
- PLCIS exhibits greater genomic instability than CLCIS, with increased copy number changes, gene amplifications and additional mutations
- Mutations (and amplification) of ERBB2 (HER2) are frequent molecular alterations in PLCIS
- Amplification of HER2 is found in ~15 - 40% of cases
- HER2 mutations in ~20% of cases (more frequent in apocrine PLCIS)
- Mutations and amplifications are mutually exclusive
- HER2 alterations are more common in ER- lesions
- At least some PLCIS are nonobligate precursors of pleomorphic ILC, analogous to high grade DCIS and invasive ductal carcinoma
- These lesions show additional molecular genetic changes, including gains of c-myc (8q24) and HER2 (17q12), gains on 8p, 8q and 13q and losses on 1p, 8p, 12p, 14q, 18q, 19p and 19q (J Pathol 2005;207:1)
- When separating apocrine and nonapocrine PLCIS, one study found that the overall extent of genomic alterations was similar between CLCIS and nonapocrine PLCIS; additional genomic changes were observed only in the apocrine PLCIS (Am J Surg Pathol 2009;33:1683)
Videos
Lobular carcinoma in situ and invasive lobular carcinoma including variants
Sample pathology report
- Breast, left, seed localized partial mastectomy:
- Pleomorphic lobular carcinoma in situ, largest focus measuring 1.5 cm in greatest dimension, surgical margins negative (see comment)
- Comment: Immunohistochemical stains are performed and show the tumor cells to be negative for E-cadherin and beta catenin consistent with a lobular phenotype supporting the diagnosis above.
Differential diagnosis
- Florid LCIS:
- Has nuclear features of CLCIS and lacks the nuclear pleomorphism of PLCIS
- FLCIS shows marked distention of ducts and lobules with little residual stroma between the expanded spaces
- It may or may not have necrosis or calcifications (Am J Surg Pathol 2019;43:399)
- Classic LCIS with a predominance of type B cells:
- Type B cells of CLCIS have slightly larger vesicular nuclei with mild variability in size and shape and with small nucleoli when compared to type A cells
- They lack the marked nuclear pleomorphism of PLCIS
- The distinction may be difficult in some cases
- Many authors and the current WHO recommend LCIS lesions that are borderline between CLCIS composed of type B cells and PLCIS should be categorized as CLCIS composed of type B cells (Histopathology 2020;77:181, Ann Diagn Pathol 2020;45:151481)
- High grade ductal carcinoma in situ:
- No intracytoplasmic vacuoles, mucin or signet ring cells
- E-cadherin positive
- Apocrine intraductal carcinoma:
- Predilection for lobular involvement
- E-cadherin positive
Board review style question #1
The depicted image is of a breast lesion from a postmenopausal woman. The first image is stained with H&E. The second image is the same area as the first image, stained immunohistochemically with E-cadherin. Other areas (not depicted) had associated microcalcifications. What is the most likely diagnosis?
- Apocrine intraductal carcinoma
- Classic lobular carcinoma in situ
- High grade ductal carcinoma in situ
- Invasive ductal carcinoma
- Pleomorphic lobular carcinoma in situ
Board review style answer #1
E. Pleomorphic lobular carcinoma in situ, PLCIS. Answer B is incorrect because although the lesion has some similar characteristics to classic lobular carcinoma in situ (CLCIS), such as distension of an affected lobule and loss of membranous E-cadherin staining, the cells have enlarged markedly pleomorphic nuclei, which would not be seen in CLCIS. Also, the patient being postmenopausal and the lesion being associated with microcalcifications favors PLCIS rather than CLCIS. Answers A, C and D are incorrect because apocrine intraductal carcinoma, high grade ductal carcinoma in situ and invasive ductal carcinoma would not demonstrate loss of E-cadherin staining. Also, regarding answer D, PLCIS is an in situ lesion and not an invasive carcinoma.
Comment here
Reference: LCIS pleomorphic
Comment here
Reference: LCIS pleomorphic
Board review style question #2
Which of the following is true about pleomorphic lobular carcinoma in situ (PLCIS)?
- Displays positive beta catenin membranous staining by IHC
- Infrequently associated with central, comedo type necrosis
- Is a precursor of invasive carcinoma
- Most patients present with bloody nipple discharge
- The majority of patients are triple negative (estrogen receptor, progesterone receptor and HER2 negative)
Board review style answer #2
C. Is a precursor of invasive carcinoma. Although pleomorphic lobular carcinoma in situ (PLCIS) is an in situ lesion, it is considered a precursor of invasive carcinoma. Answer A is incorrect because beta catenin is part of the cadherin - catenin complex and PLCIS would lose membranous staining by immunohistochemistry. Answer B is incorrect because this lesion is frequently associated with central comedonecrosis. Answer D is incorrect because most patients present with a screening detected abnormality on imaging. Answer E is incorrect because ~10% of patients with PLCIS are triple negative.
Comment here
Reference: LCIS pleomorphic
Comment here
Reference: LCIS pleomorphic