Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Positive stains | Negative stains | Electron microscopy description | Electron microscopy images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Li JJX, Tse GM. Neuroendocrine tumor. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantneuroendocrine.html. Accessed April 1st, 2025.
Definition / general
- Primary, low to intermediate grade neoplasm of the breast with histological features of neuroendocrine differentiation and supported by immunoreactivity to neuroendocrine markers
Essential features
- Uncommon, primary neuroendocrine neoplasm of the breast of low to intermediate grade
- Morphology is the primary feature for classification, requires > 90% neuroendocrine tumor (NET) pattern
- Neuroendocrine markers helpful for diagnosis
- Not to be confused with distinct breast neoplasms that exhibit neuroendocrine differentiation (e.g., solid papillary carcinoma, mucinous carcinoma of the breast, invasive ductal carcinoma, no special / specific type [IDC-NST] with neuroendocrine differentiation)
Terminology
- Carcinoid tumor, atypical carcinoid, not recommended
- Prior to WHO 5th edition, often referred to as the category of tumors with neuroendocrine differentiation; the current WHO 5th terminology is neuroendocrine neoplasms, which includes neuroendocrine tumors (NET), neuroendocrine carcinomas (small cell and large cell NEC)
ICD coding
- ICD-O:
- ICD-10:
- C50.9 - malignant neoplasm of breast of unspecified site
- ICD-11:
Epidemiology
- Accounts for < 1% of primary breast cancers (Am J Surg 2006;191:799)
- Age of presentation commonly > 65 years (Eur J Surg Oncol 1995;21:609)
Pathophysiology
- Unconfirmed at this time but proposed theories include:
- Neoplastic transformation from native neuroendocrine cells in the breast (J Clin Pathol 2012;65:699)
- Divergent neuroendocrine differentiation of neoplastic stem cells (Arch Pathol Lab Med 2017;141:1577)
Etiology
- Unknown at this time
Clinical features
- Typically presents as a mass lesion
- ~10% are bilateral (Am J Surg 2006;191:799)
- Tumor size ranges from 1 - 5 cm (Eur J Surg Oncol 1995;21:609)
- Nodal and systemic metastasis not uncommon on presentation (Eur J Surg Oncol 1995;21:609)
- Carcinoid syndrome not reported in neuroendocrine tumors of the breast (Am J Surg 2006;191:799)
- Published data suggests worse prognosis compared to IDC-NST (Breast Cancer Res Treat 2021;186:403)
Diagnosis
- Requires histomorphologic evidence of neuroendocrine differentiation
- Supplemented by positivity to neuroendocrine immunohistochemical markers
- Radiology and serology not sensitive nor specific in diagnosis
Laboratory
- Serum chromogranin (neuroendocrine secretory protein) can be elevated (Cureus 2021;13:e16860)
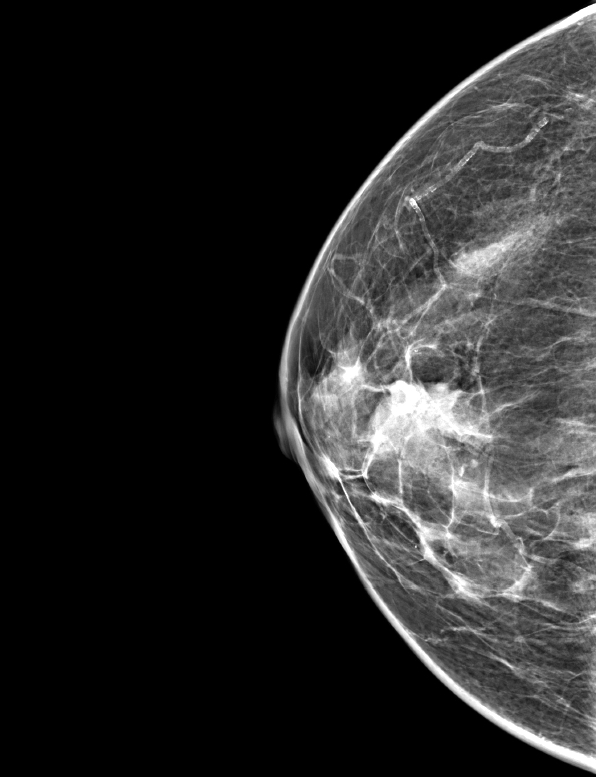
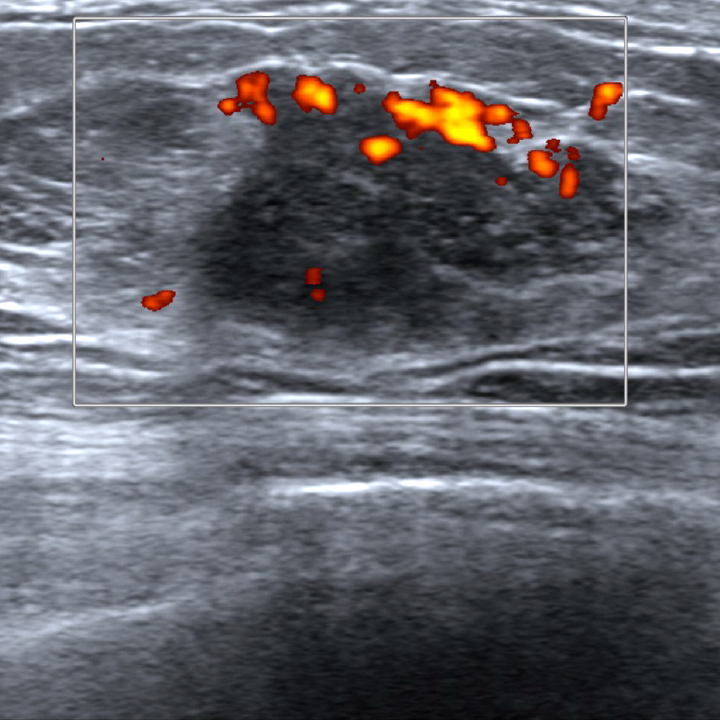
Radiology description
- Mammography (Eur J Surg Oncol 1995;21:609)
- Lobular, well defined dense mass
- Not associated with microcalcification
- Ultrasound (Radiat Med 2008;26:28)
- Mixed hypo and hyperechogenicity
- Increased vascularity
Radiology images
Prognostic factors
- Tumor stage (Histopathology 2014;64:647)
- Histological grade
- Most neuroendocrine tumors of the breast are Nottingham grades 1 and 2
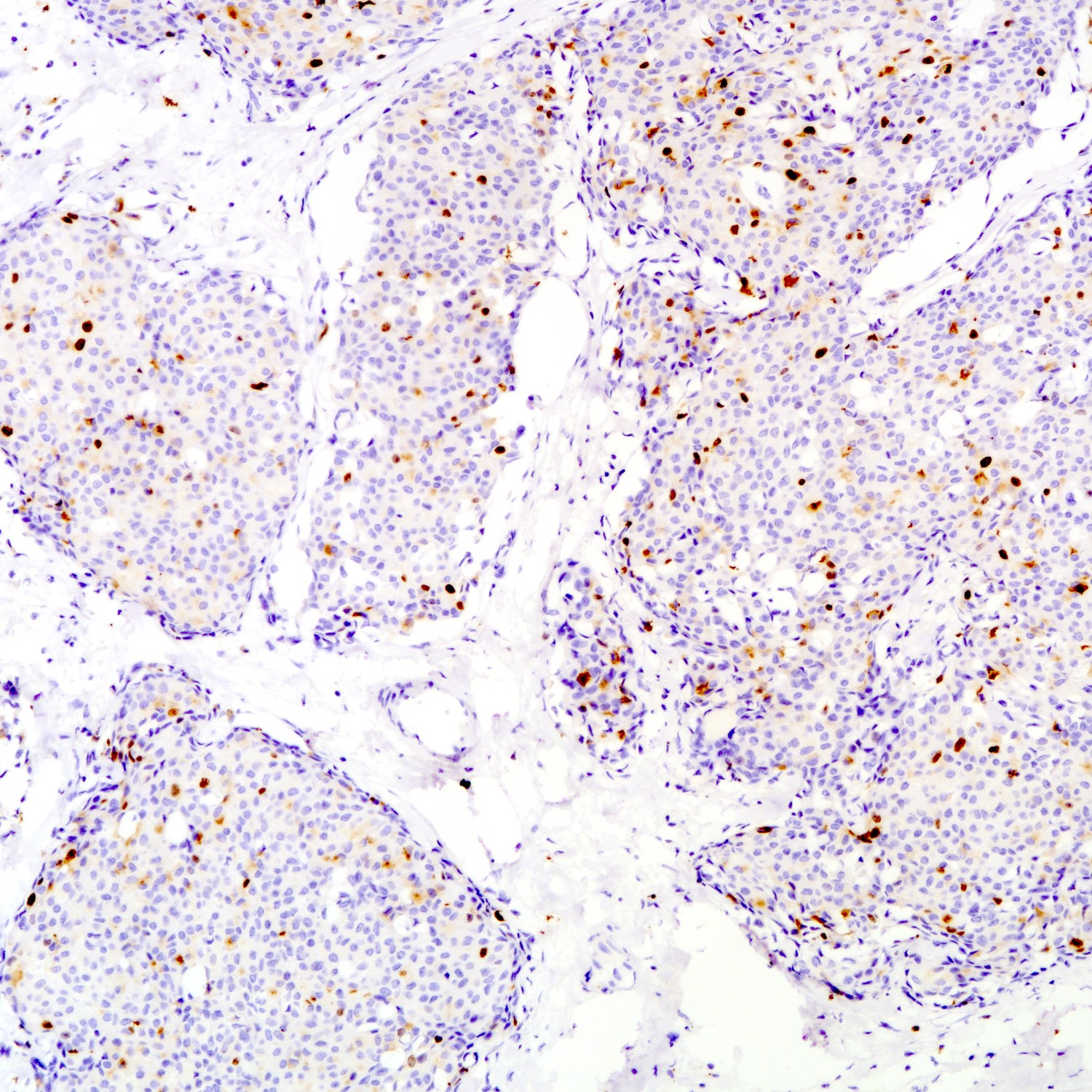
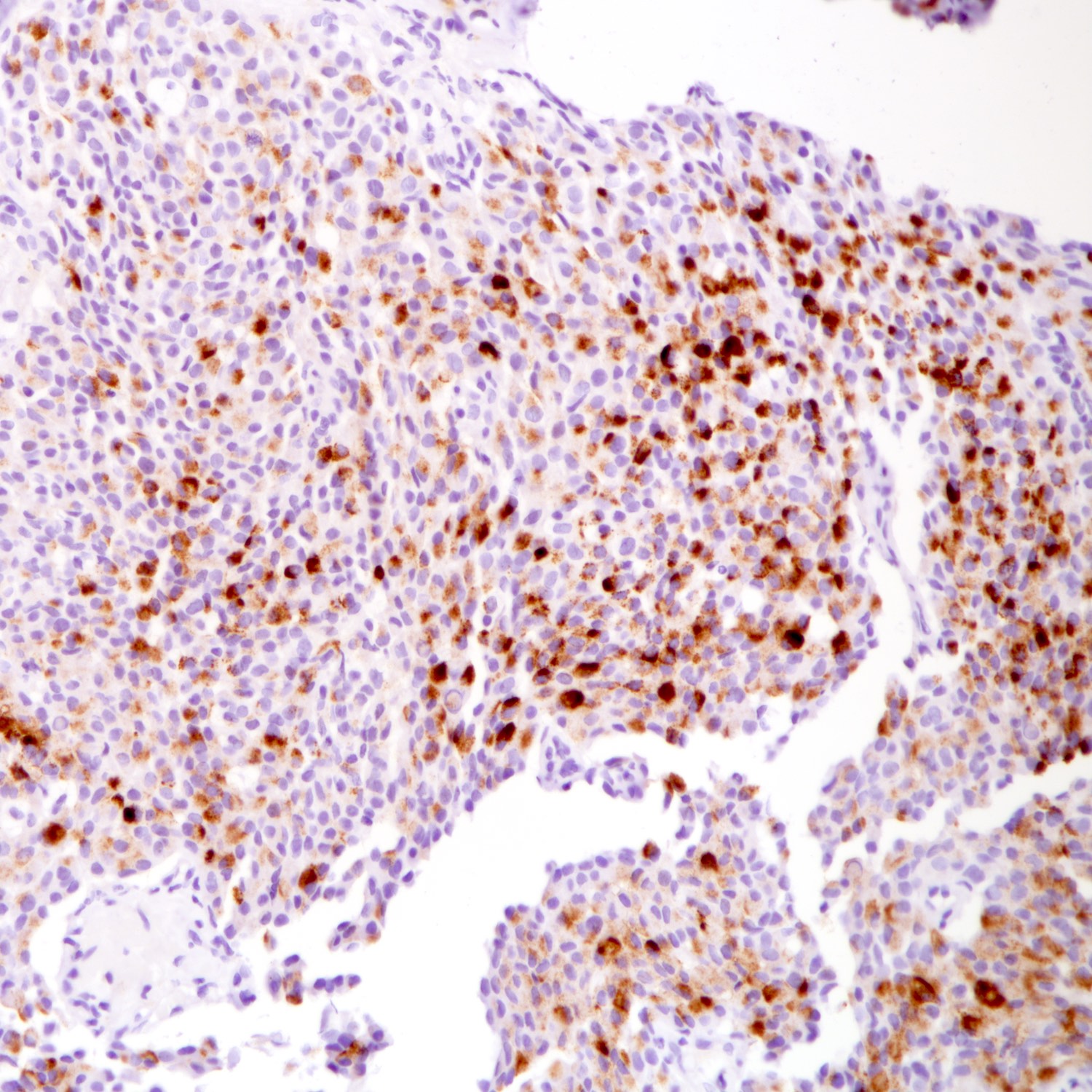
- Ki67 proliferation index
- High Ki67 proliferative index may indicate tumor aggressiveness
- No formal grading system defined
Case reports
- 42 year old woman with mixed neuroendocrine tumor and large cell carcinoma of the breast (Korean J Radiol 2013;14:395)
- 58 year old woman with liver metastasis (Cureus 2021;13:e16860)
Treatment
- Mainstay of treatment is surgical resection, with or without lymphadenopathy (Eur J Surg Oncol 1995;21:609)
- Report of good response in metastatic disease with combination of traditional chemotherapy and somatostatin analog (Cureus 2021;13:e16860)
Gross description
- Well circumscribed, nonencapsulated tumors (Eur J Surg Oncol 1995;21:609)
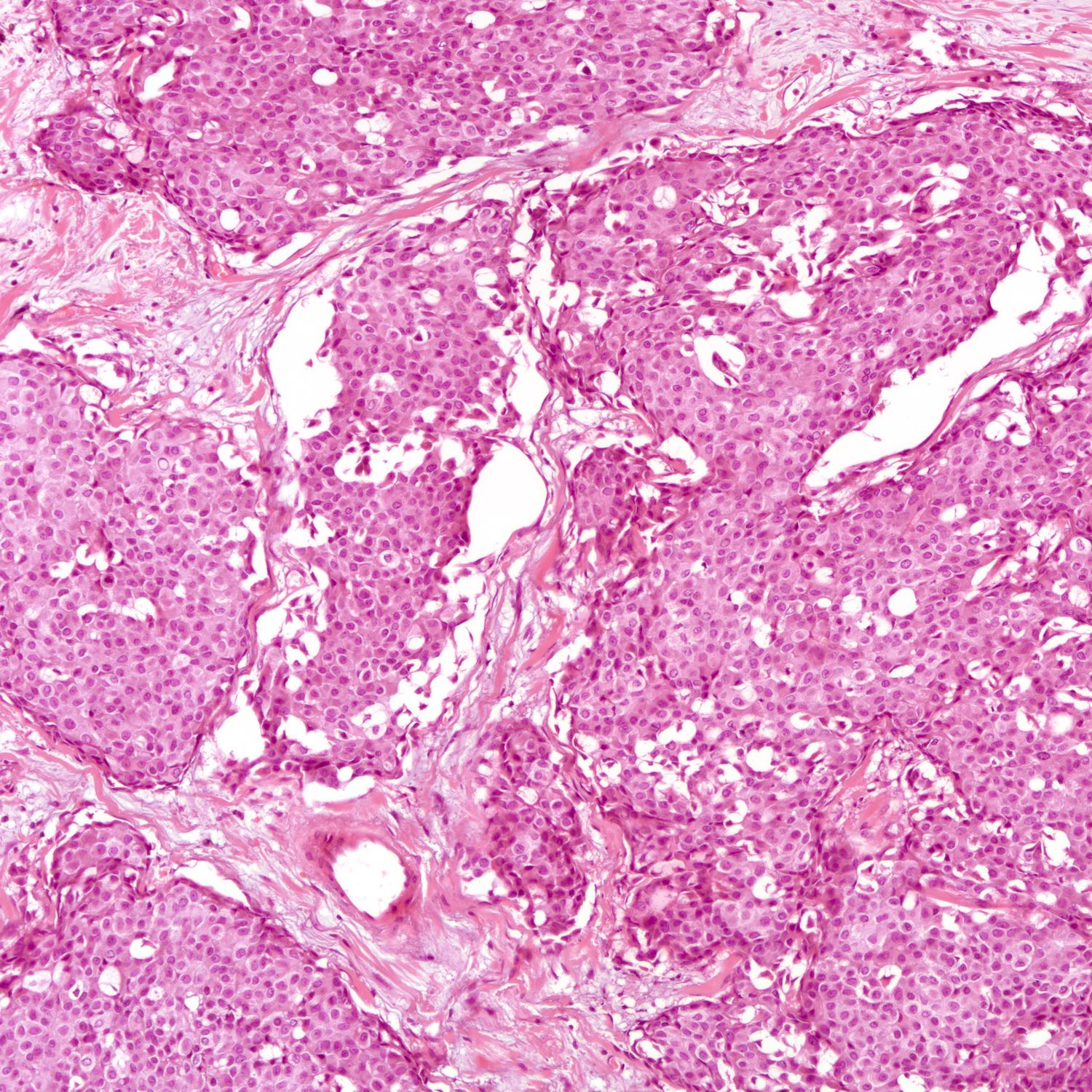
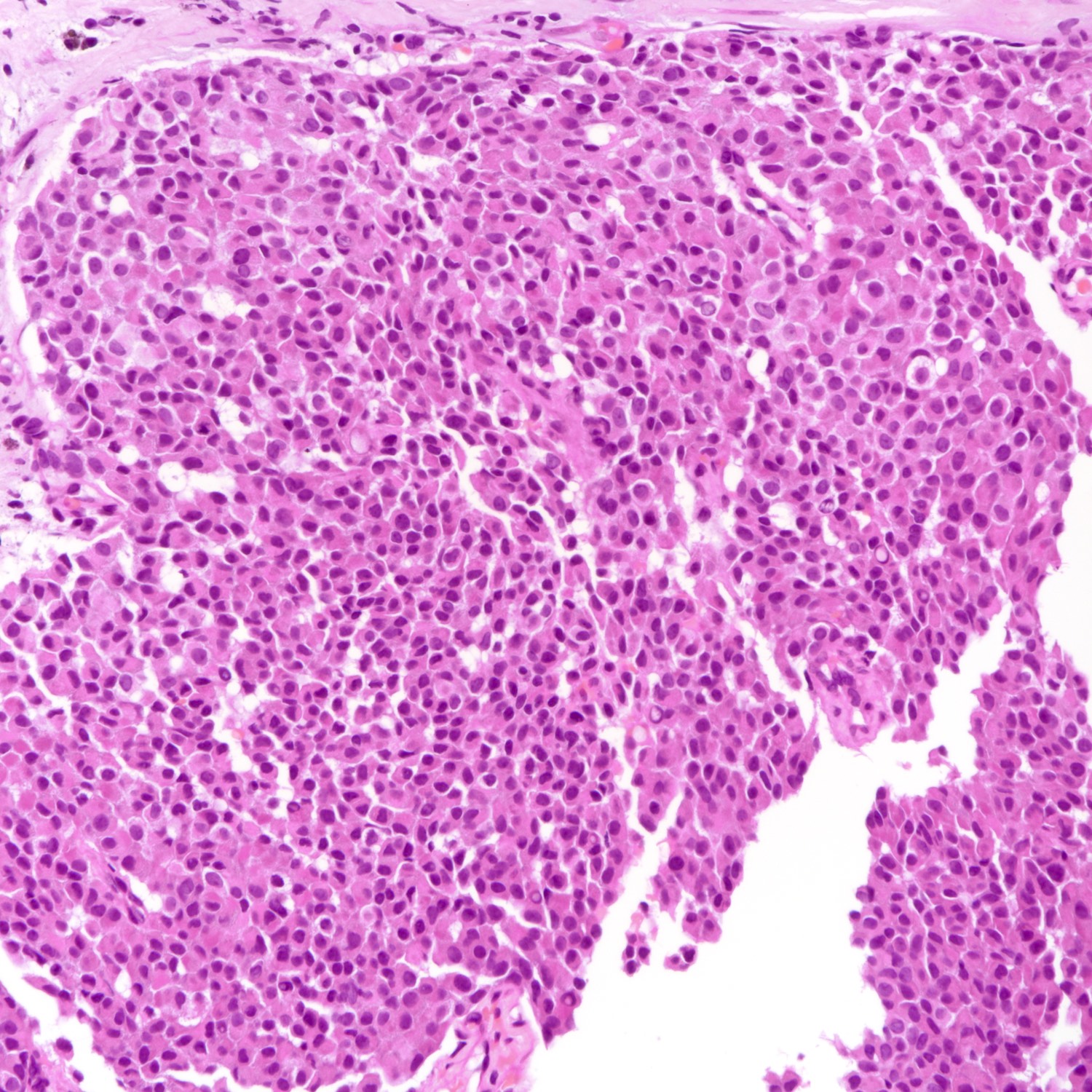
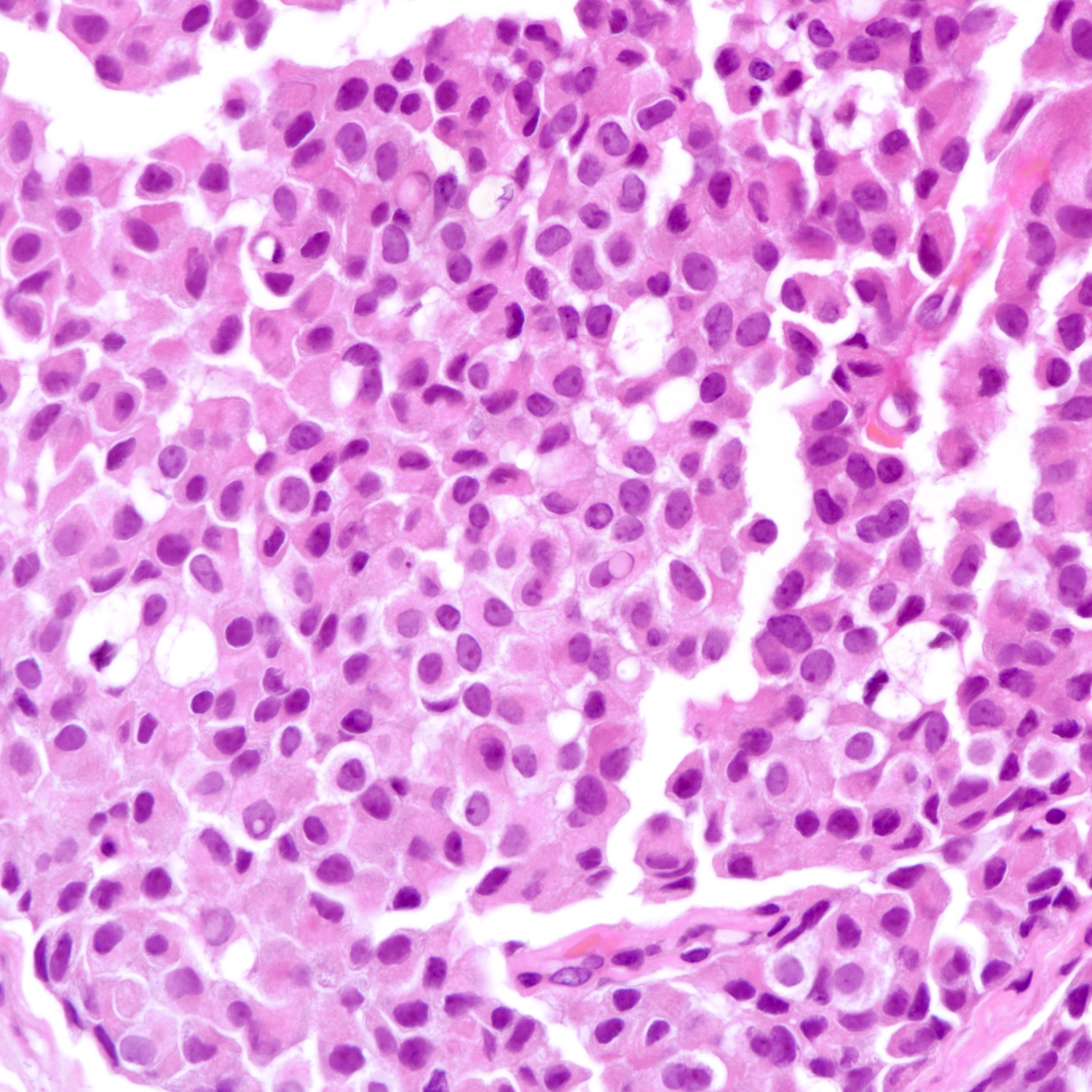
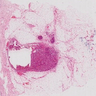
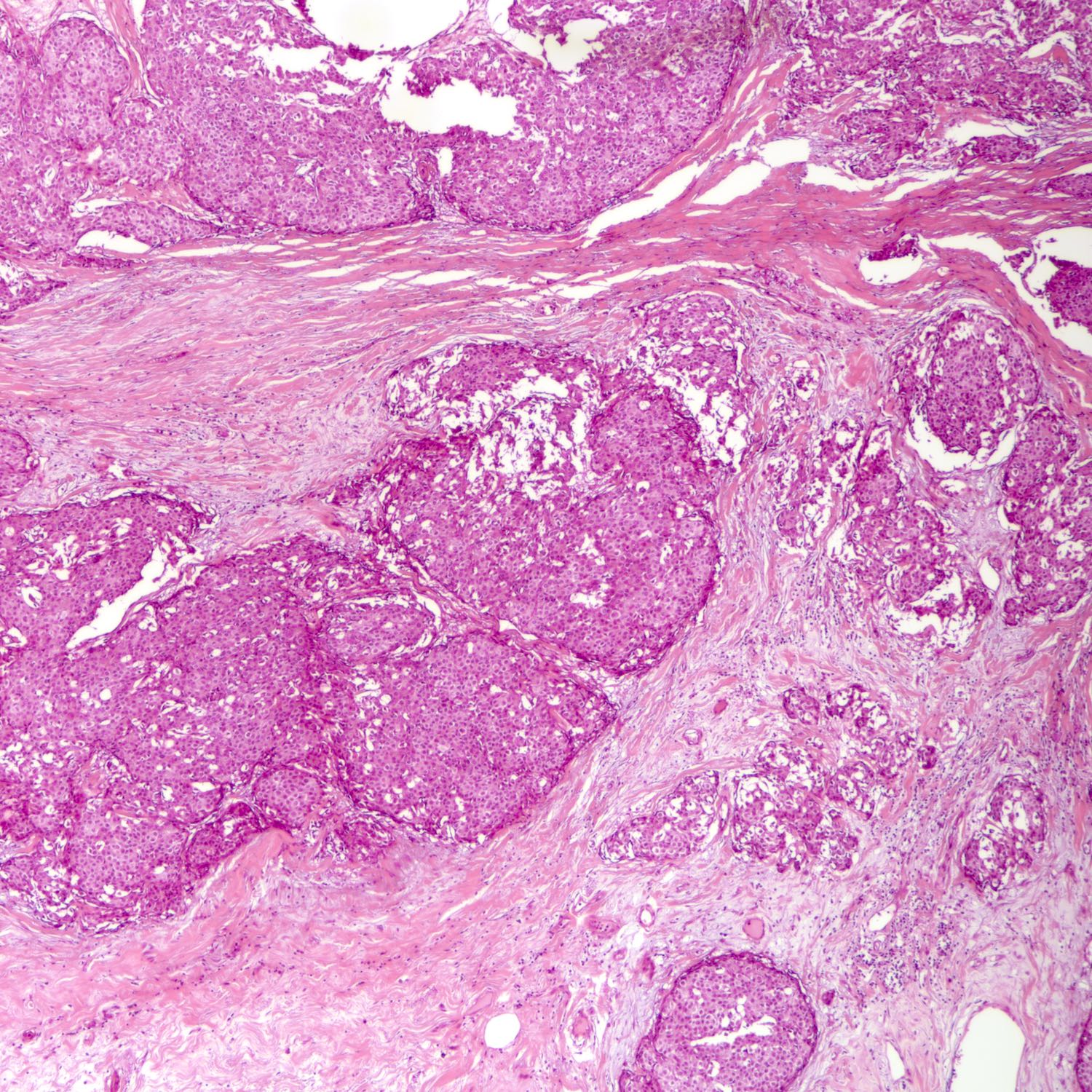
Microscopic (histologic) description
- Requires morphologic threshold of > 90% of NET pattern, consistent with the criteria defining other special subtypes of the breast
- Densely cellular, insular, organoid nests and trabeculae with delicate intervening fibrovascular stroma (Arch Pathol Lab Med 2017;141:1577, Korean J Radiol 2013;14:395)
- Papillary and insular patterns and alveolar-like structures may be seen
- Tumor cells display classical neuroendocrine morphology:
- Spindle, plasmacytoid or polygonal cells
- Eosinophilic granular cytoplasm
- Eccentric nuclear placement
- Salt and pepper stippled chromatin may be present, often not obvious
- Intranuclear inclusions
- Mammary NET often lacks classic carcinoid-like features (ribbons, cords and rosettes) (Histopathology 2015;66:761)
- Majority Nottingham grade 1 or 2
Microscopic (histologic) images
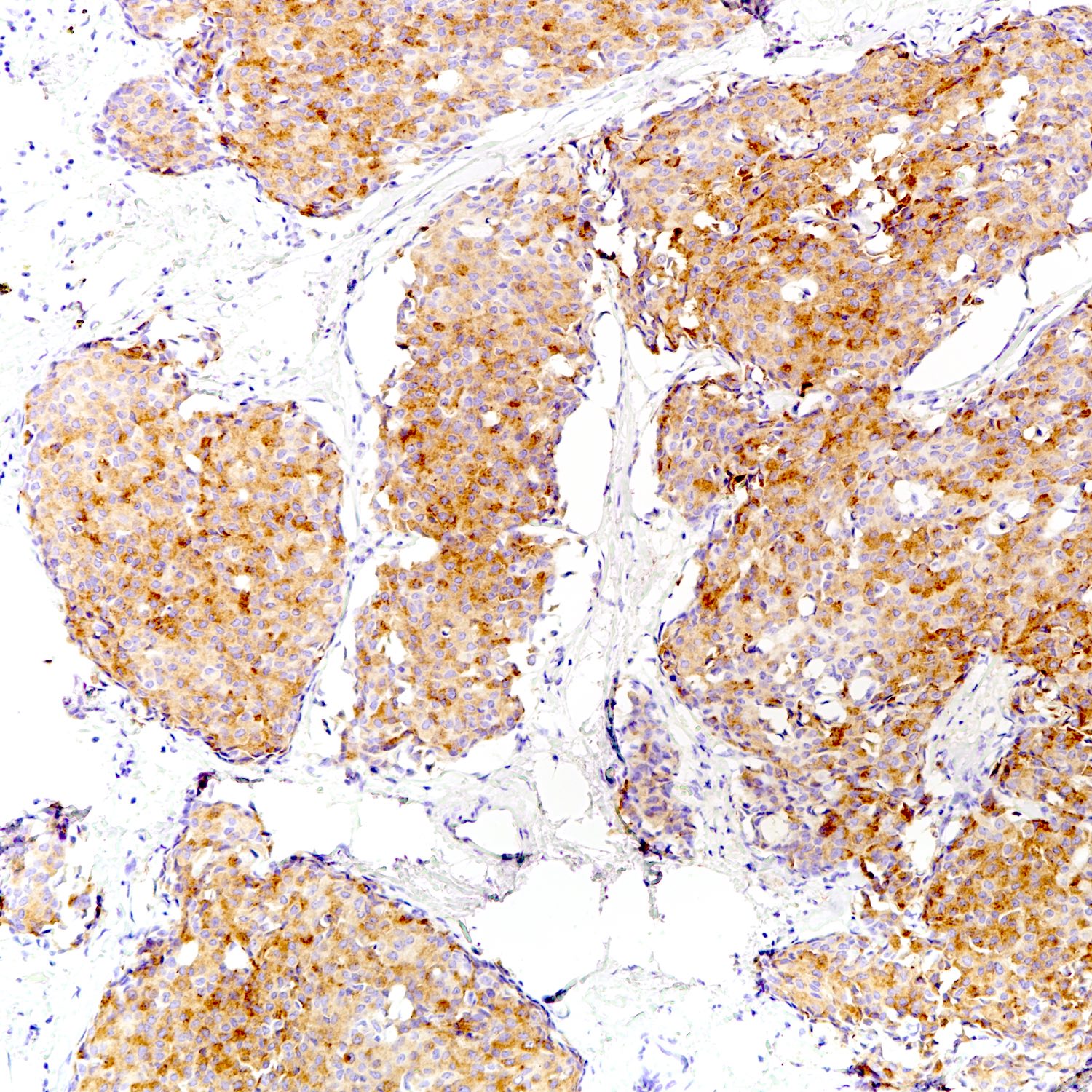
Positive stains
- Neuroendocrine markers:
- Hormonal markers:
- ER, PR and AR (Mod Pathol 2001;14:768)
- Other markers:
Negative stains
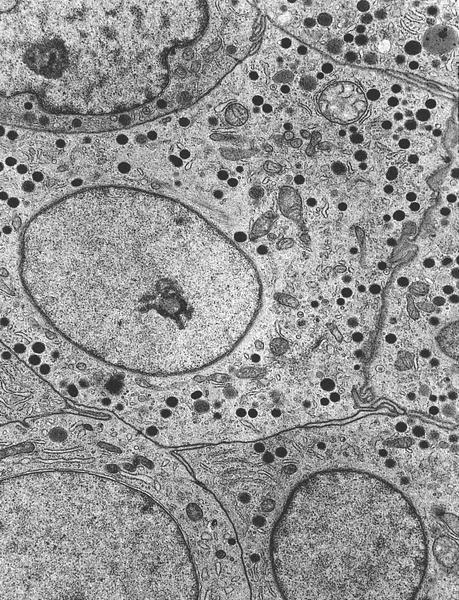
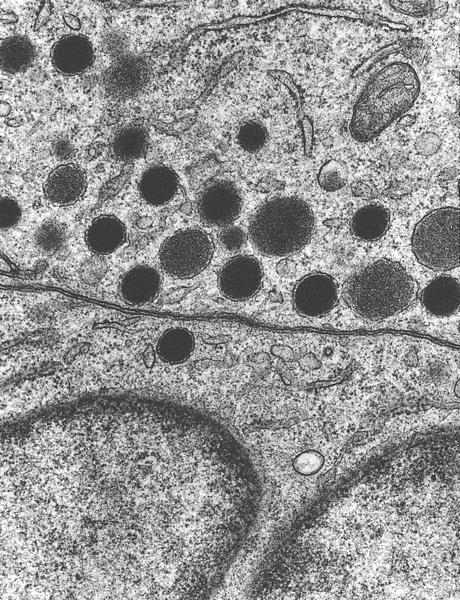
Electron microscopy description
- Dense core vesicles and presynaptic vesicles
Molecular / cytogenetics description
- ARID1A, ATRX, FOXA1, GATA3 and PIK3CA mutations reported (J Pathol 2017;241:405, Mod Pathol 2018;31:68)
- TP53 mutation more common in (high grade) neuroendocrine carcinomas (Mod Pathol 2018;31:68)
Sample pathology report
- Left breast, local excision:
- Neuroendocrine tumor, grade __, measuring ___ cm (see comment and synoptic report)
- Comment: Sections show breast tissue infiltrated by groups and rounded nests of tumor cells. The tumor cells possess low grade nuclei that are rounded to ovoid, scanty to moderate amount of cytoplasm and some of them show plasmacytoid features with stippled chromatin. The mitotic count is at __ per 10 high power fields. The features are those of neuroendocrine tumor, grade __. The tumor measures __ in maximal dimension and is clear from (specify if < 1 cm __) / involves the resection margins.
- The tumor cells are diffusely positive for synaptophysin and focally positive for chromogranin. Ki67 proliferative index is approximated at __%.
Differential diagnosis
- Metastatic neuroendocrine tumors of other primaries:
- Cannot be reliably distinguished by morphology
- Dependent on history of neuroendocrine tumors in other sites, correlation with clinical history and imaging is required
- Positivity to hormonal markers and breast markers; negativity to markers associated with other primaries (e.g., TTF1, glypican 3) may be helpful
- Identification of an in situ component can help distinguish primary breast from metastatic nonmammary tumors
- Breast neoplasms with neuroendocrine differentiation:
- Neuroendocrine carcinomas:
- Small cell carcinoma:
- Small hyperchromatic nuclei, nuclear molding, often crushed
- Very high nuclear / cytoplasmic ratio
- Mitotic count and Ki67 proliferative index high
- Necrosis
- Large cell carcinoma:
- Grade 3, large pleomorphic nuclei with coarse chromatin
- Mitotic count and Ki67 proliferative index high
- Often necrosis
- Small cell carcinoma:
- Invasive carcinoma with neuroendocrine differentiation:
- If an NEN pattern is present in 10 - 90% of a malignancy, the terminology for mixed invasive (NST or other special type) and NET may be used, with an estimation of the percent of each component reported
- Negativity to neuroendocrine markers, in particular synaptophysin, may help identify nonneuroendocrine areas
- IBC-NSTs can be immunoreactive for neuroendocrine markers, expression of neuroendocrine markers alone is not sufficient for a diagnosis of mammary NET
- Solid papillary carcinoma:
- Nuclear and cytologic features can be similar
- Fibrovascular cores with palisading, when identified, are helpful for diagnosing solid papillary carcinoma
- Majority of solid papillary carcinomas are positive for neuroendocrine markers (Am J Surg Pathol 2016;40:1334)
- Mucinous carcinoma:
- Nuclear and cytologic features can be similar
- > 90% mucinous morphology (e.g. extracellular mucin pools)
- Mucinous carcinomas can be positive to neuroendocrine markers (Mod Pathol 2004;17:568)
- Positivity to neuroendocrine markers more common in type B than type A mucinous carcinoma (Front Oncol 2021;10:558760)
- Neuroendocrine carcinomas:
- Apocrine carcinoma:
- Abundant eosinophilic cytoplasm may resemble neuroendocrine differentiation
- Prominent nucleoli and vesicular chromatin distinguish apocrine from neuroendocrine differentiation
- Apocrine carcinomas are negative to neuroendocrine markers
- Lobular neoplasia:
- Monomorphic low grade nuclei in lobular neoplasia can be confused with neuroendocrine differentiation
- In situ lesions may appear similar to the nested pattern in neuroendocrine tumors
- Genuine neuroendocrine nuclear features are not observed in lobular neoplasia
- Lobular neoplasia is noninvasive with preserved myoepithelial cells
- Loss of E-cadherin staining confirmatory of lobular neoplasia
Additional references
Board review style question #1
Which of the following clinicopathological features best supports the diagnosis of a neuroendocrine tumor of breast primary (as compared to neuroendocrine tumors metastasizing to the breast)?
- Absence of carcinoid syndrome
- Negative imaging and endoscopy findings
- Negativity to TTF1
- Positivity to CK7
- Positivity to ER and PR
Board review style answer #1
B. Negative imaging and endoscopy findings. CK7 is also positive in neuroendocrine tumors of the lung. ER and PR positivity are not uncommon in neuroendocrine tumors of other primaries such as the lung and pancreas. TTF1 negative tumors are less likely of lung origin but do not exclude other primaries. Carcinoid symptoms are not reported in neuroendocrine tumors of the breast but do not exclude metastasis. Negative imaging and endoscopy findings best support the diagnosis of a primary neuroendocrine tumor of the breast by excluding metastasis from other sites (Hum Pathol 2001;32:1087, Arch Pathol Lab Med 2008;132:1889, Neuroendocrinology 2011;93:249, Am J Surg 2006;191:799).
Comment Here
Reference: Neuroendocrine tumor of breast
Comment Here
Reference: Neuroendocrine tumor of breast
Board review style question #2
In which of the following breast tumors are neuroendocrine markers most likely to be negative?
- Grade 1 neuroendocrine tumor
- Grade 2 neuroendocrine tumor
- Mucinous carcinoma, type A
- Mucinous carcinoma, type B
- Small cell carcinoma
Board review style answer #2
C. Mucinous carcinoma, type A. Neuroendocrine tumors and small cell carcinomas are neuroendocrine neoplasms of the breast. Solid papillary carcinomas and mucinous carcinomas are specific breast cancer subtypes with frequent neuroendocrine differentiation and positivity to neuroendocrine markers. Positivity to neuroendocrine markers is more common in type B than type A mucinous carcinoma (Front Oncol 2021;10:558760).
Comment Here
Reference: Neuroendocrine tumor of breast
Comment Here
Reference: Neuroendocrine tumor of breast