Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Crespo-Ramos SM, Biernacka A. Pleomorphic invasive lobular carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantlobularpleomorphic.html. Accessed April 1st, 2025.
Definition / general
- Morphologic variant of invasive lobular carcinoma (ILC); retains the distinctive lobular growth pattern but exhibits a greater degree of cellular pleomorphism
Essential features
- Rare subtype of ILC with pleomorphic cytologic features
- Cellular discohesion and histologic architecture similar to classical ILC
- Marked cellular atypia and nuclear pleomorphism (nuclei ≥ 4 times the size of lymphocytes), equivalent to high grade ductal carcinoma in situ (DCIS)
- Molecular profile and immunostaining pattern (including loss of E-cadherin) support a common origin with the lobular family of breast lesions
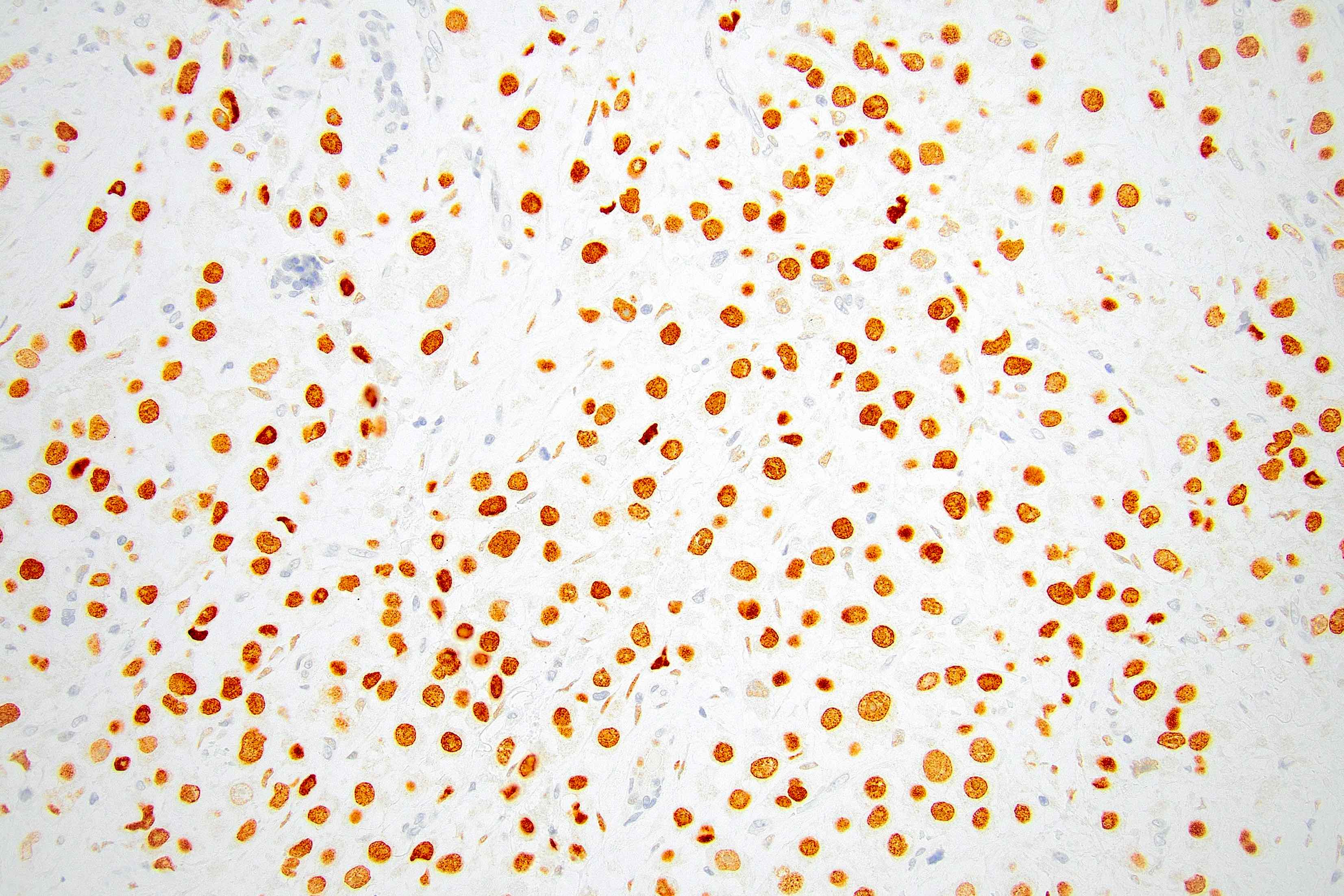
- Acquisition of further alterations, more typical of the high grade ductal carcinomas (e.g., p53 and HER2 positivity), likely drives more aggressive biology
Terminology
- Invasive lobular carcinoma, pleomorphic variant (may be denoted as PLC, PILC)
- Infiltrating pleomorphic lobular carcinoma
- First documented by Martinez and Azzopardi in 1979, subsequently reported by Dixon et al. in 1982 and formally introduced by Page et al. in 1987 (Histopathology 1979;3:467, Histopathology 1982;6:149, Page: Diagnostic Histopathology of the Breast, 1987)
- It was further characterized by Weidner and Semple and Eusebi et al. in 1992 and officially recognized as a subtype of ILC by the World Health Organization (WHO) in 2003 (Hum Pathol 1992;23:1167, Hum Pathol 1992;23:655)
ICD coding
- ICD-11: 2C61.2 & XH9HB7 - invasive pleomorphic lobular carcinoma of breast & lobular carcinoma, pleomorphic
Epidemiology
- 15% of ILC and < 1% of all breast cancers (Arch Pathol Lab Med 2013;137:1688)
- Postmenopausal women, aged 60 - 80 (older than classic ILC); very rare in men (Breast Cancer Res 2021;23:7)
- More commonly represented in BRCA2 carriers, in whom it presents at a much younger age (Clin Breast Cancer 2015;15:421, Cancer 1998;83:2335)
- In patients with E-cadherin (CDH1) germline mutations (hereditary diffuse gastric cancer), the risk of ILC development is 40 - 50% by the age of ~80 years (J Med Genet 2018;55:431)
- Most patients with ILC have somatic, not germline, E-cadherin gene mutations
Sites
- Breast
- Can occur in axillary accessory breast tissue
Pathophysiology
- Belongs to the lobular family of breast lesions defined by loss of cell to cell adhesion and actin cytoskeleton dysregulation
- Hallmark alteration is the loss or downregulation of E-cadherin, a transmembrane protein with a pivotal role in cellular junctions
- Intracellular domain of E-cadherin binds to the actin cytoskeleton, forming a complex with catenins (i.e., p120, alpha, beta and gamma catenin)
- Biallelic silencing of CDH1 gene (16q22.1); 2 hit hypothesis (first allele inactivated by somatic mutation; second allele inactivated by loss of heterozygosity), homozygous deletions or promoter methylation (Mod Pathol 2003;16:674)
- Absent or altered E-cadherin disrupts adherens junctions, resulting in the morphologic appearance of dyscohesive cells and diffuse infiltration pattern
- Cadherin catenin complex gets interrupted (membranous beta catenin is lost), whereas p120 catenin is upregulated and localized to the cytoplasm (rather than the membrane)
- In rare cases lacking CDH1 genetic / epigenetic alterations, the lobular phenotype may be driven by alterations of other cell adhesion genes, such as CTNNA1 (alpha catenin) or CTNND1 (p120) (J Pathol 2018;245:456, NPJ Precis Oncol 2024;8:33)
- Develops through molecular pathways similar to classical ILC with the acquisition of further molecular alterations, more typical of high grade ductal carcinomas
- Pleomorphic lobular carcinoma in situ (PLCIS) and classic LCIS frequently coexist with PLC
- All show remarkable similarities at the morphological, immunohistochemical and molecular level; all harbor hallmark genetics of lobular neoplasia (loss of 16q and gain of 1q) and lack functional E-cadherin, suggesting an early event in the development or evolution from the same precursor (J Pathol 2005;207:1)
- Additional molecular alterations, such as gains of HER2 / neu, c-MYC and p53 positivity, more typical of the high grade ductal pathway, arise during tumor progression (likely at the in situ stage), with an increasing level of genomic complexity and accumulation of mutations (Future Oncol 2009;5:233, J Pathol 2008;215:231)
- Rare example of dedifferentiation, an infrequent phenomenon in breast cancer, when a low grade lesion progresses to a high grade lesion (typically low and high grade lesions evolve through distinct molecular pathways) (J Pathol 2005;205:248)
- Dual knockout of CDH1 and TP53 in mouse mammary epithelium leads to the formation of mammary tumors resembling human pleomorphic ILC (Dis Model Mech 2011;4:347)
- Pleomorphic lobular carcinoma in situ (PLCIS) and classic LCIS frequently coexist with PLC
Etiology
- No well known causative factors specific to PLC
- Likely multifactorial, including lifestyle (e.g., alcohol), hormonal (including HRT) and genetic components (Breast Cancer Res 2015;17:37)
- LCIS, classic type, is considered a risk factor and a nonobligate precursor
- PLCIS is regarded as a genetically and biologically more advanced risk factor and possibly a direct precursor (Am J Surg Pathol 2019;43:399)
Clinical features
- Most patients have a poorly defined palpable breast mass
- Presents at a more advanced stage (large tumors and axillary lymph node metastasis) than patients with invasive ductal carcinoma (IDC) (J Breast Cancer 2012;15:313)
- More frequently multifocal or multicentric, bilateral and has more nipple areolar complex involvement than patients with IDC (J Breast Cancer 2012;15:313)
Diagnosis
- Diagnosis is made on core needle biopsy
- Based on the H&E appearance, negative E-cadherin is helpful but not required (and in some cases, positive staining occurs and does not preclude diagnosis)
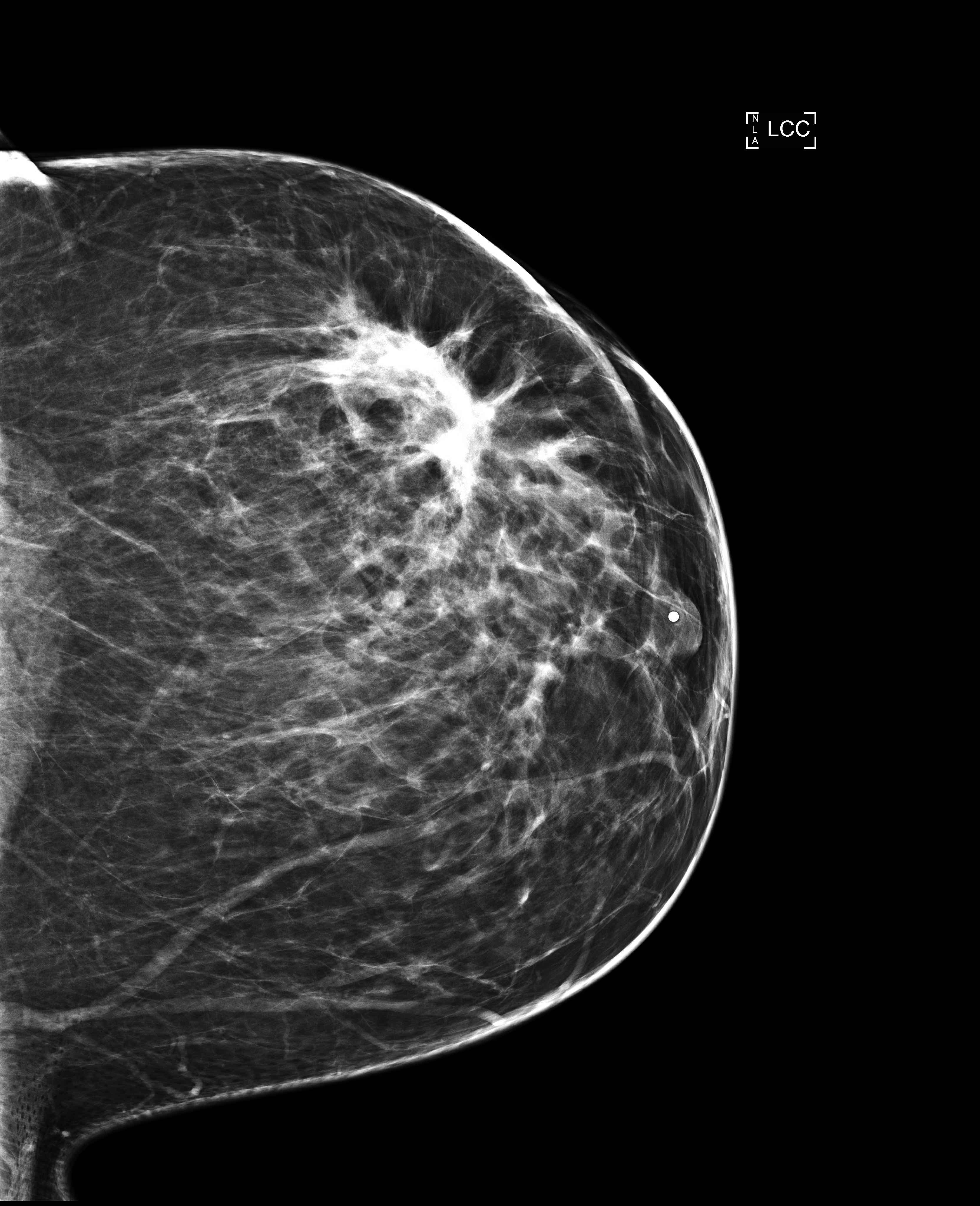
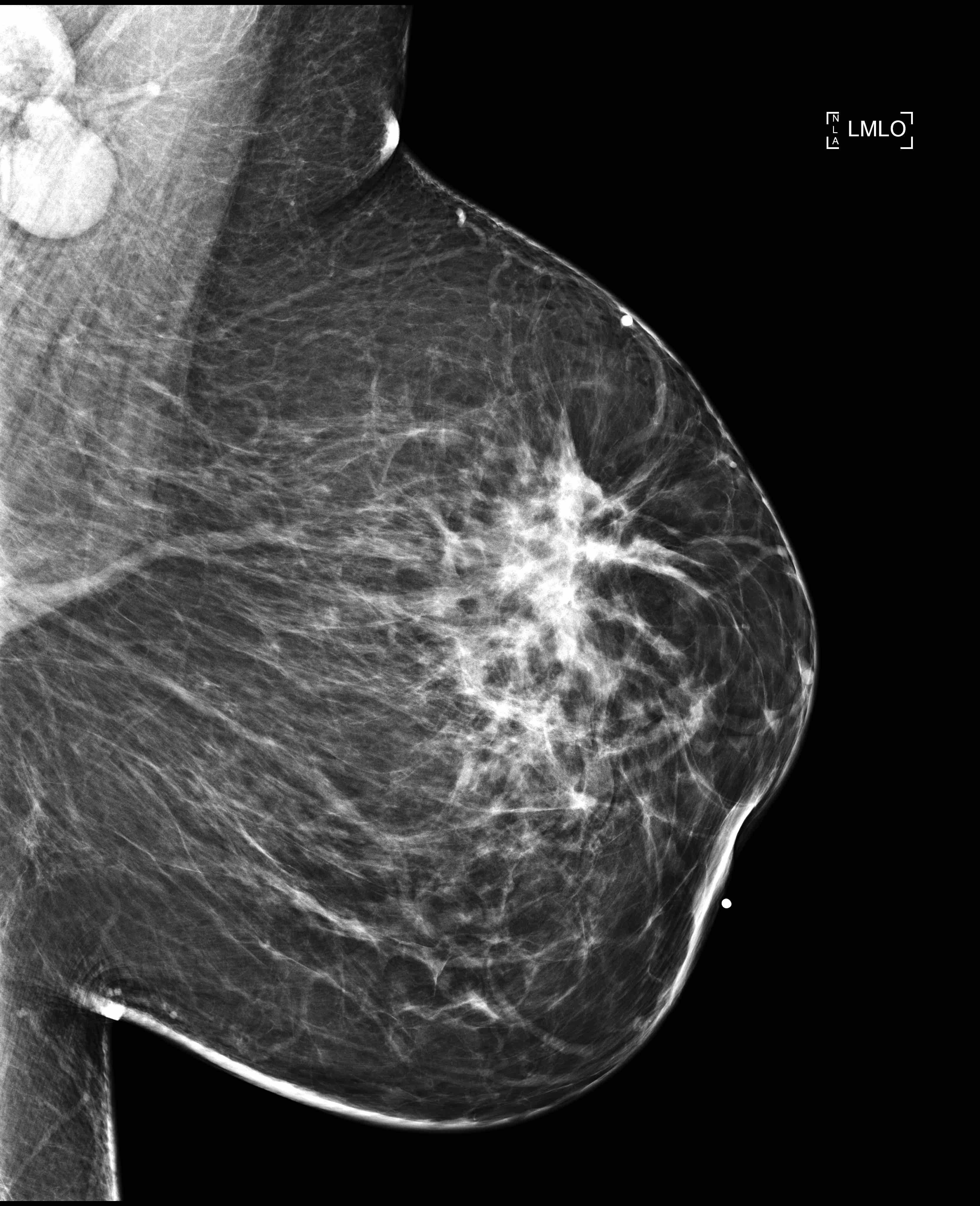
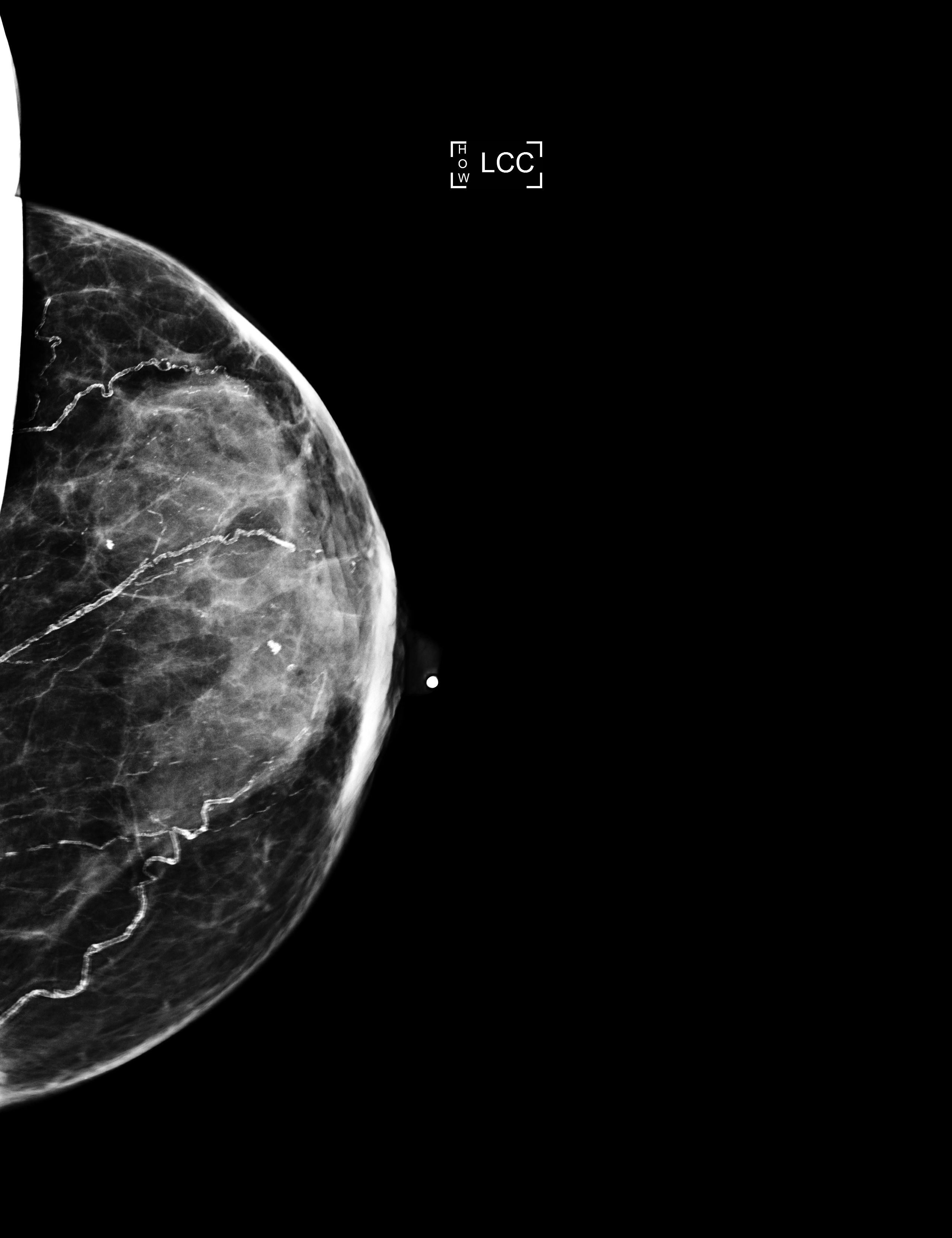
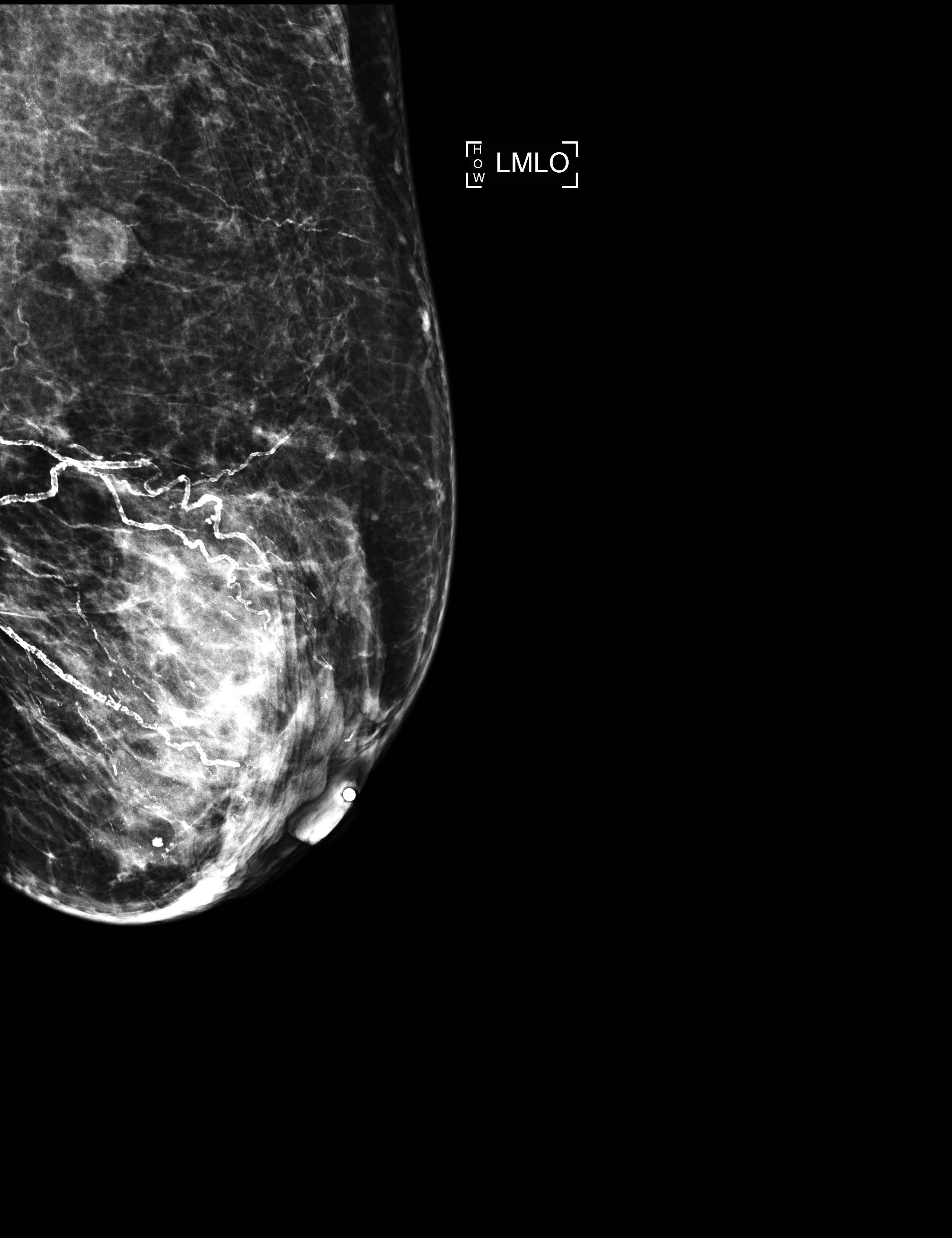
Radiology description
- No significant differences between classical ILC and PLC in radiologic studies (Breast 2013;22:324)
- Mammography
- Spiculated mass, asymmetry or architectural distortion with or without calcifications
- Findings may be seen on the craniocaudal (CC) view only (possibly related to greater breast compression as compared with the mediolateral oblique [MLO] view)
- May present with more aggressive features, resulting in the reported higher detection rate of PLC compared with classical ILC on mammography (J Breast Cancer 2012;15:313)
- May be mammographically occult
- Lower sensitivity for detection (57 - 79%) compared with other invasive carcinomas (Breast Cancer Res 2015;17:94)
- False negative rates as high as 19% compared with other invasive carcinomas (AJR Am J Roentgenol 1993;161:957)
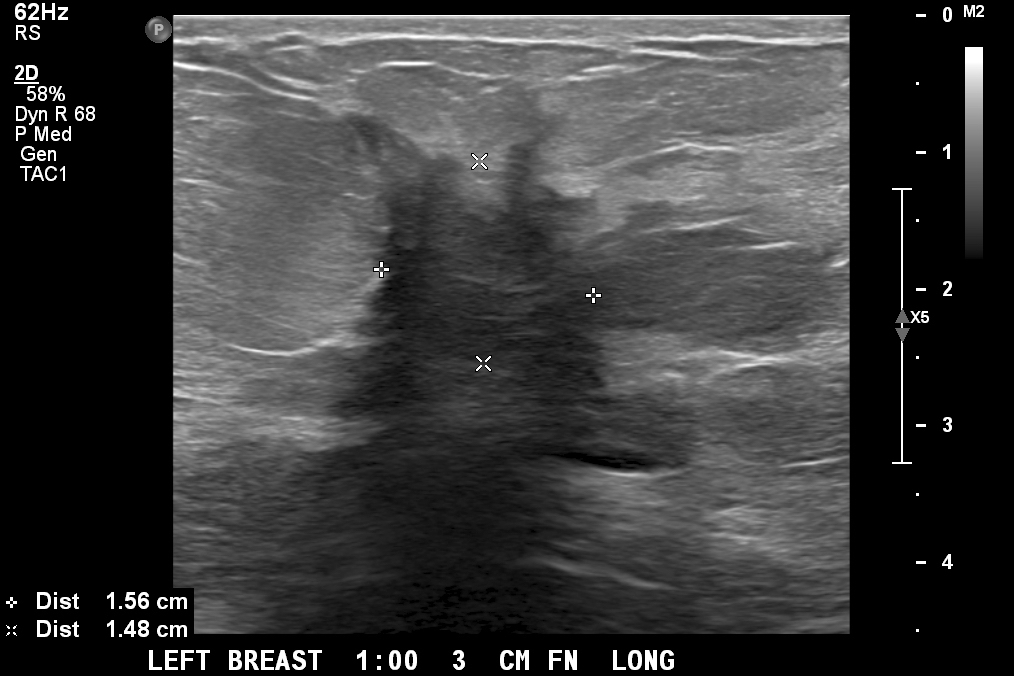
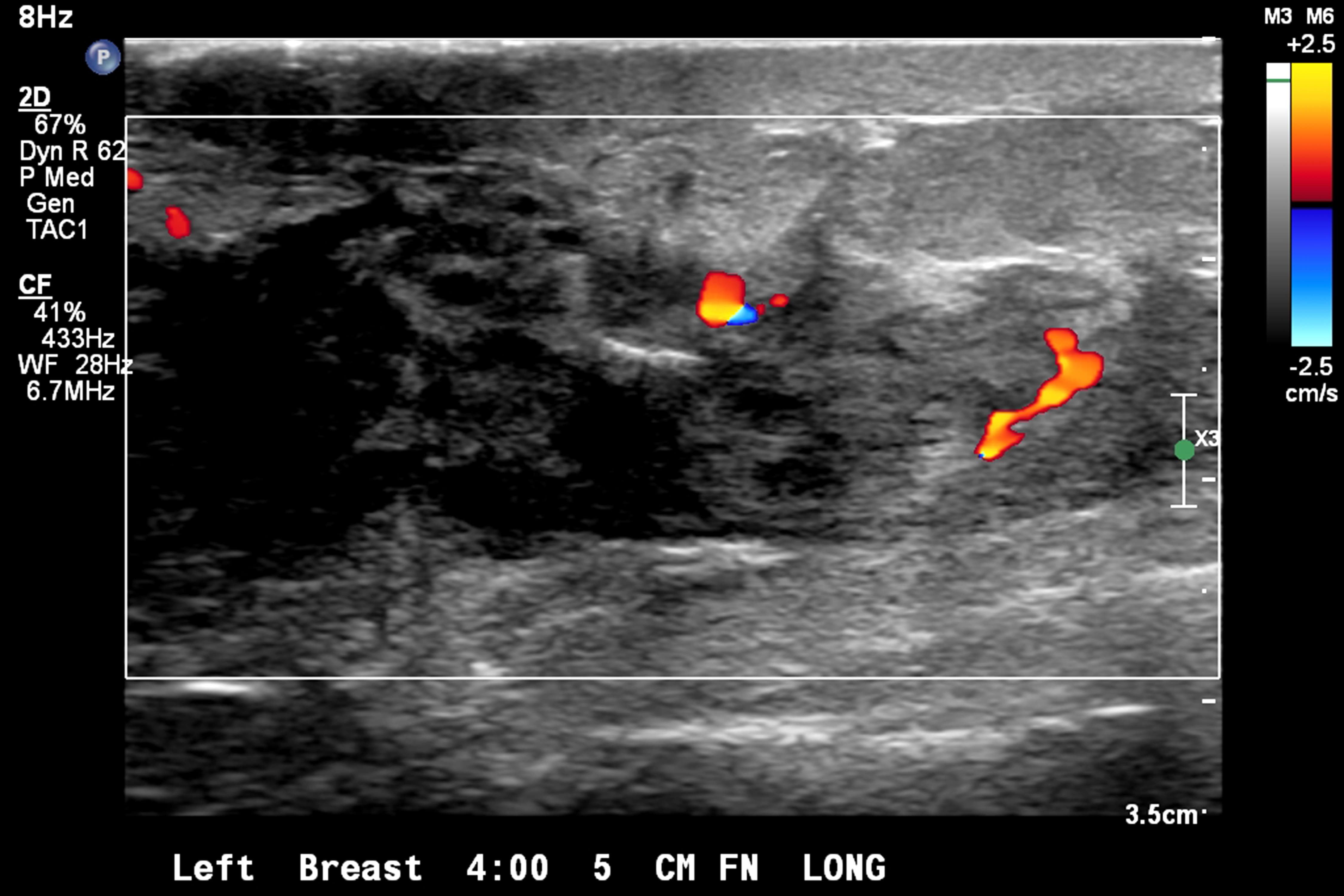
- Ultrasound
- Irregular mass or ill defined hypoechoic area with diffuse posterior shadowing and internal vascularity
- Lower sensitivity (68 - 92%) compared with other imaging modalities (Healthcare (Basel) 2023;11:746)
- Tumor size is underestimated in 18 - 53% of cases (Healthcare (Basel) 2023;11:746)
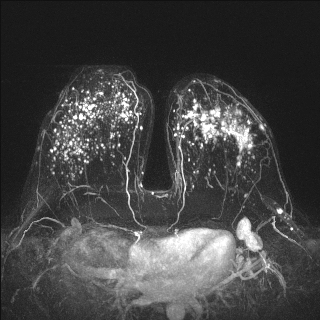
- Contrast enhanced MRI
- Increasingly important in the detection of multifocal, multicentric or contralateral disease but can lead to false positives and overestimation of tumor size (Eur Radiol 2004;14:1209)
Radiology images
Prognostic factors
- PLC, like other morphologic variants, seems more aggressive relative to the classical type (Breast Cancer Res Treat 2012;133:713)
- More likely to have a higher histologic grade, extracapsular extension, lymphovascular invasion, T3 - 4 tumors, lymph node and distant metastases or ER negative and HER2 positive disease (Breast 2019;43:67, Am Surg 2017;83:359, Ann Diagn Pathol 2012;16:185)
- Most common metastatic sites involve bone, liver, lung and peritoneum (Histopathology 2012;61:365)
- Conflicting data regarding the prognostic value of the PLC morphology by itself
- Several studies associated PLC with poor prognosis and decreased survival (Hum Pathol 1992;23:1167, Hum Pathol 1992;23:655, Breast 2019;43:67)
- Unclear whether pleomorphic histology alone predicts worse survival or whether it is due to other known adverse prognostic indicators commonly present in PLC (Ann Diagn Pathol 2015;19:64)
- Other studies demonstrated improved outcomes after adjustment for clinicopathological features and concluded that PLC subclassification does not provide valuable prognostic information in addition to advanced AJCC stage or high histologic grade (Clin Breast Cancer 2018;18:114, J Breast Cancer 2012;15:313, Ann Diagn Pathol 2015;19:64)
- Current evidence suggests that nuclear pleomorphism is not an independent prognostic factor, the most important discriminator being overall grade or mitotic count (Mod Pathol 2013;26:496)
- Mitotic count is the most useful predictor of outcome (J Breast Cancer 2012;15:313)
- When compared with IDC, the prognosis of PLC is similar to high grade tumors (J Breast Cancer 2012;15:313)
Case reports
- 42 year old woman with PLC and multiple metastases (Int J Surg Case Rep 2021;80:105581)
- 48 year old woman with pancreatic metastases from PLC (Diagn Pathol 2017;12:52)
- 49 year old woman with multiple vertebral metastases from PLC (Spine J 2016;16:e303)
- 57 year old woman with HER2 / neu positive PLC (Cureus 2020;12:e11108)
- 68 year old man with PLC and axillary lymph node involvement (BMC Clin Pathol 2014;14:16)
- 72 year old woman with PLC with osteoclast-like giant cells (Diagn Pathol 2018;13:62)
- 86 year old woman with benign osseous metaplasia infiltrated by PLC (Int J Surg Case Rep 2022;99:107716)
- 89 year old woman with PLC (Int J Surg Pathol 2018;26:434)
Treatment
- Similar to other invasive carcinomas of analogous grade, stage and hormone receptor status (guidelines specific to the management of patients with PLC are currently lacking due to rarity)
- Neoadjuvant therapy
- Studies suggest a worse response to neoadjuvant chemotherapy compared with IDC (Clin Breast Cancer 2015;15:421)
- Neoadjuvant endocrine therapy may be offered in hormone receptor positive cases
- Surgical resection
- Conservative surgery is favored for patients with localized disease
- More likely to require mastectomy due to multifocality, positive margins on excision and risk of local recurrence (Clin Breast Cancer 2015;15:421)
- Adjuvant therapy
- Endocrine therapy is usually recommended; most PLCs are ER positive
- Chemotherapy, in general, is less beneficial but offered to patients with adverse pathologic factors
- Anti-HER2 therapies, given a higher frequency of HER2 amplification / overexpression as well as activating HER2 mutations, sensitive to HER2 inhibitors (Breast Cancer Res Treat 2015;150:447, J Clin Oncol 2008;26:5823, Cancers (Basel) 2019;11:74)
- Androgen receptor (AR) may be a therapeutic target, given molecular apocrine / luminal AR features (PLoS One 2020;15:e0235790)
- Radiation: used after breast conservation for carcinomas of no special type
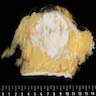
Gross description
- Irregular and poorly circumscribed mass or irregular fibrotic area
- Due to the diffuse growth pattern, there may be no mass but only a slightly firmer area on palpation (Cancer 1994;73:1673)
- Tumor size can be difficult to determine and requires a correlation of imaging, gross and microscopic findings (Cancer 1994;73:1673)
Gross images
Frozen section description
- More cellular than classic lobular; large tumor cells with cytoplasmic vacuoles and pleomorphic nuclei, single filing (Cancer 1997;81:29)
- May present with signet ring, apocrine or histiocytoid features
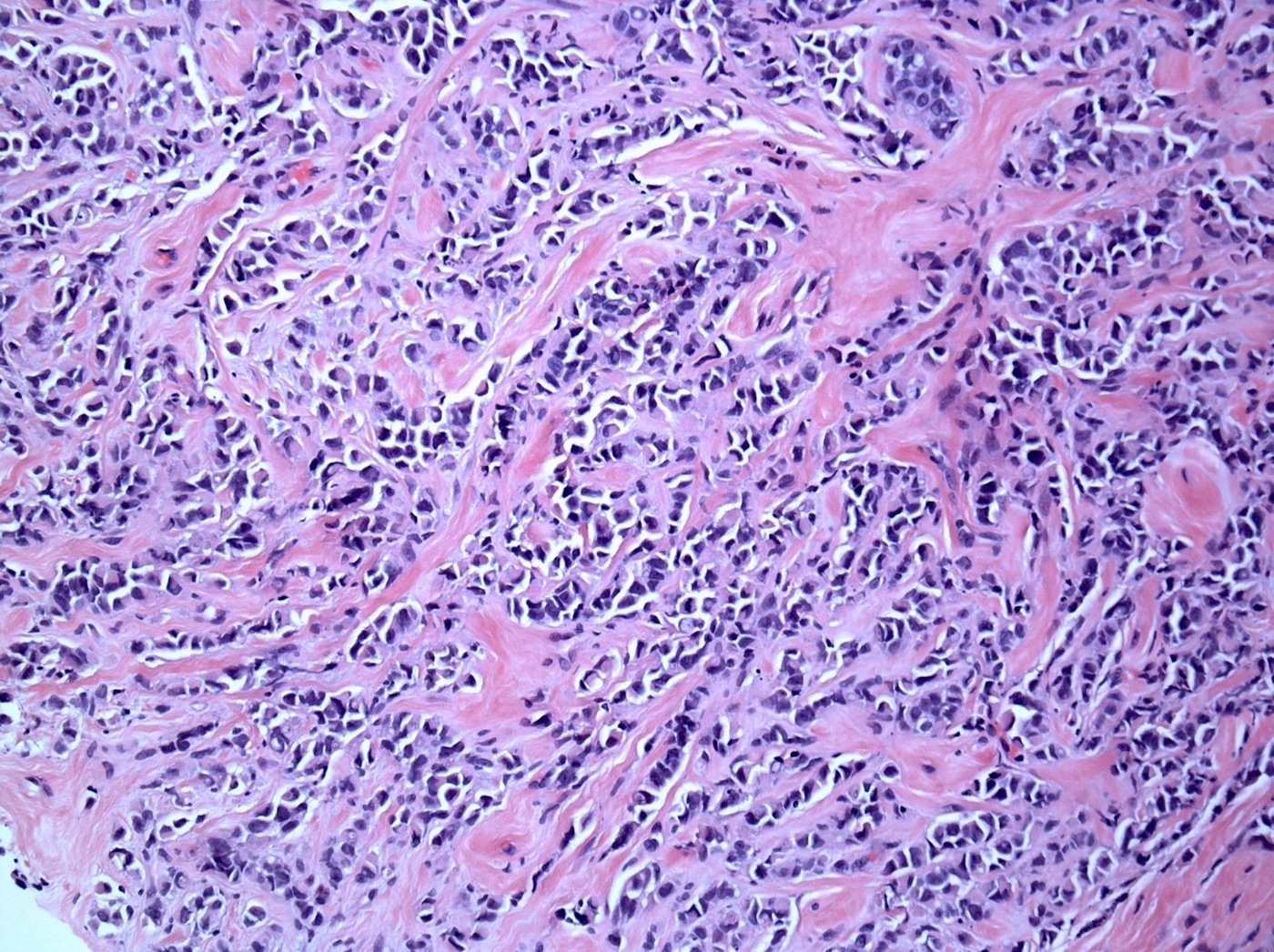
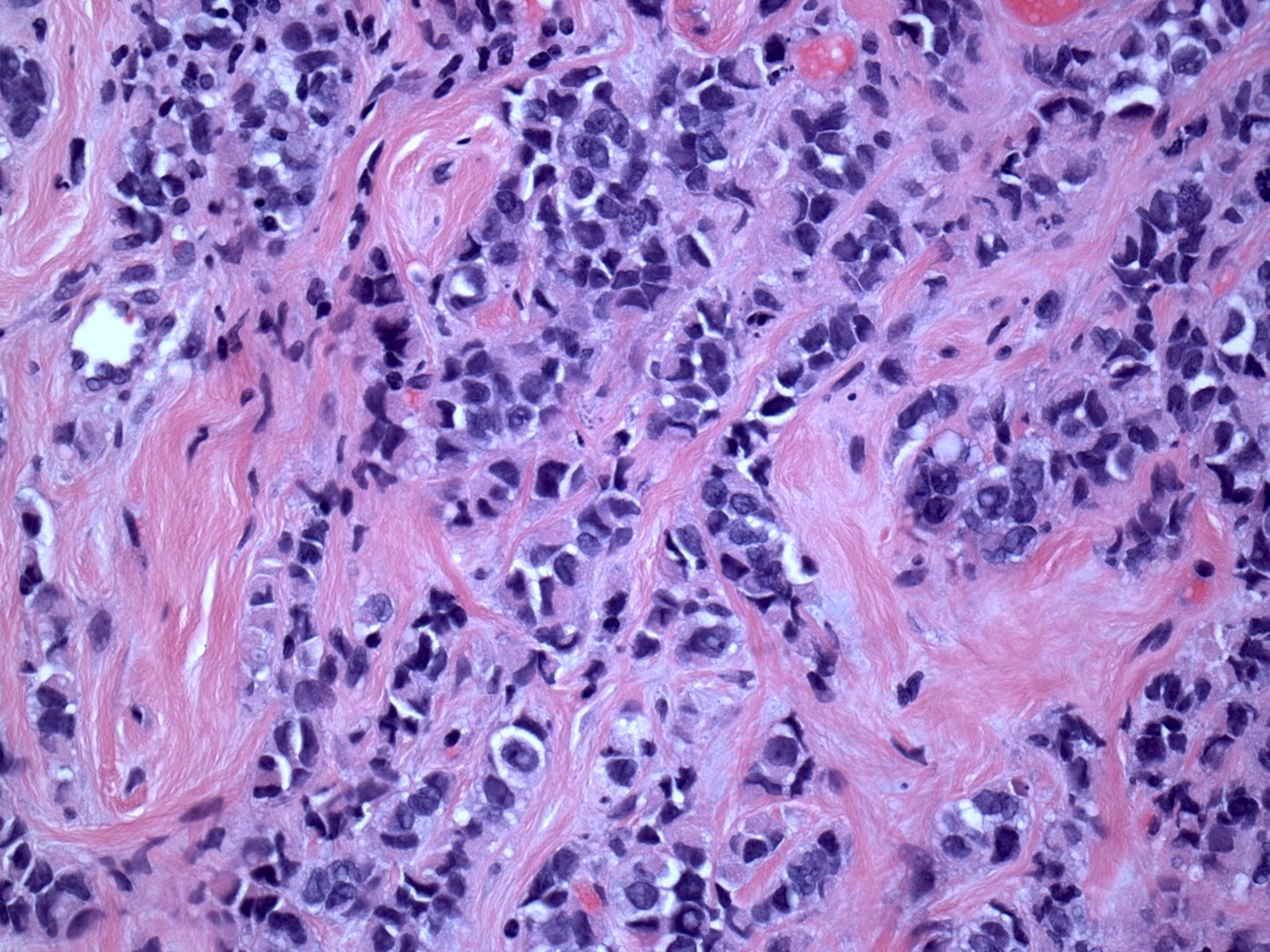
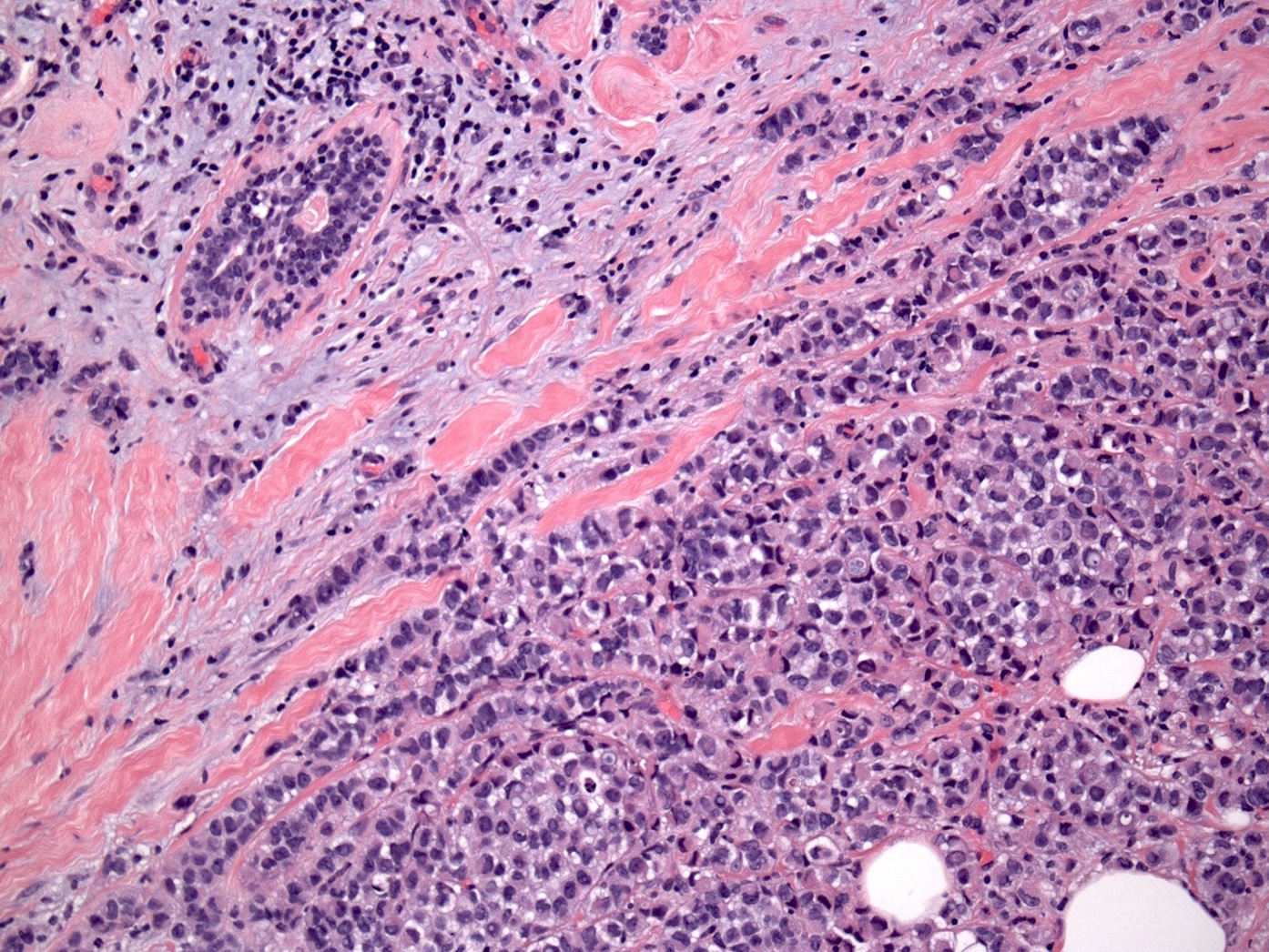
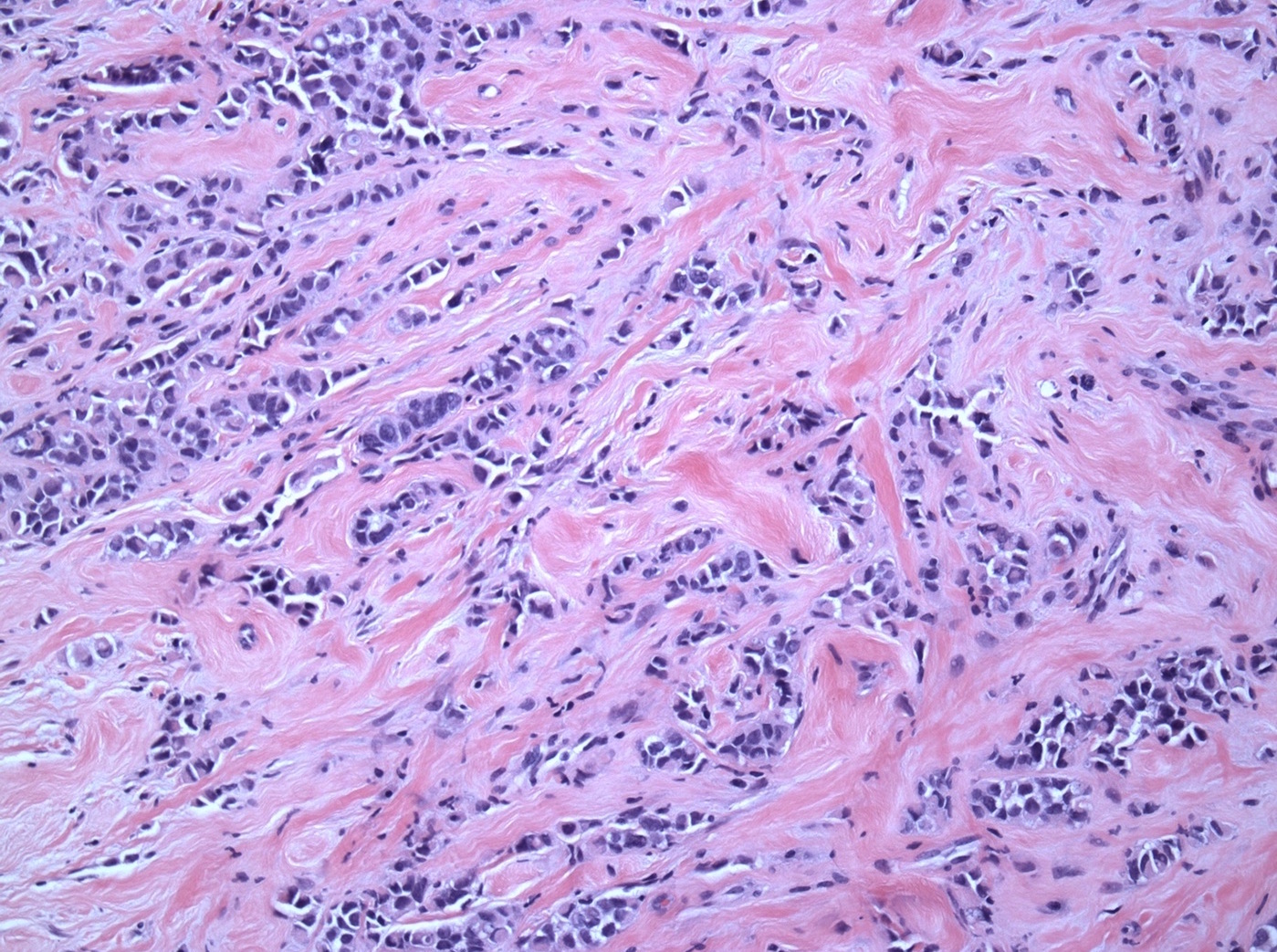
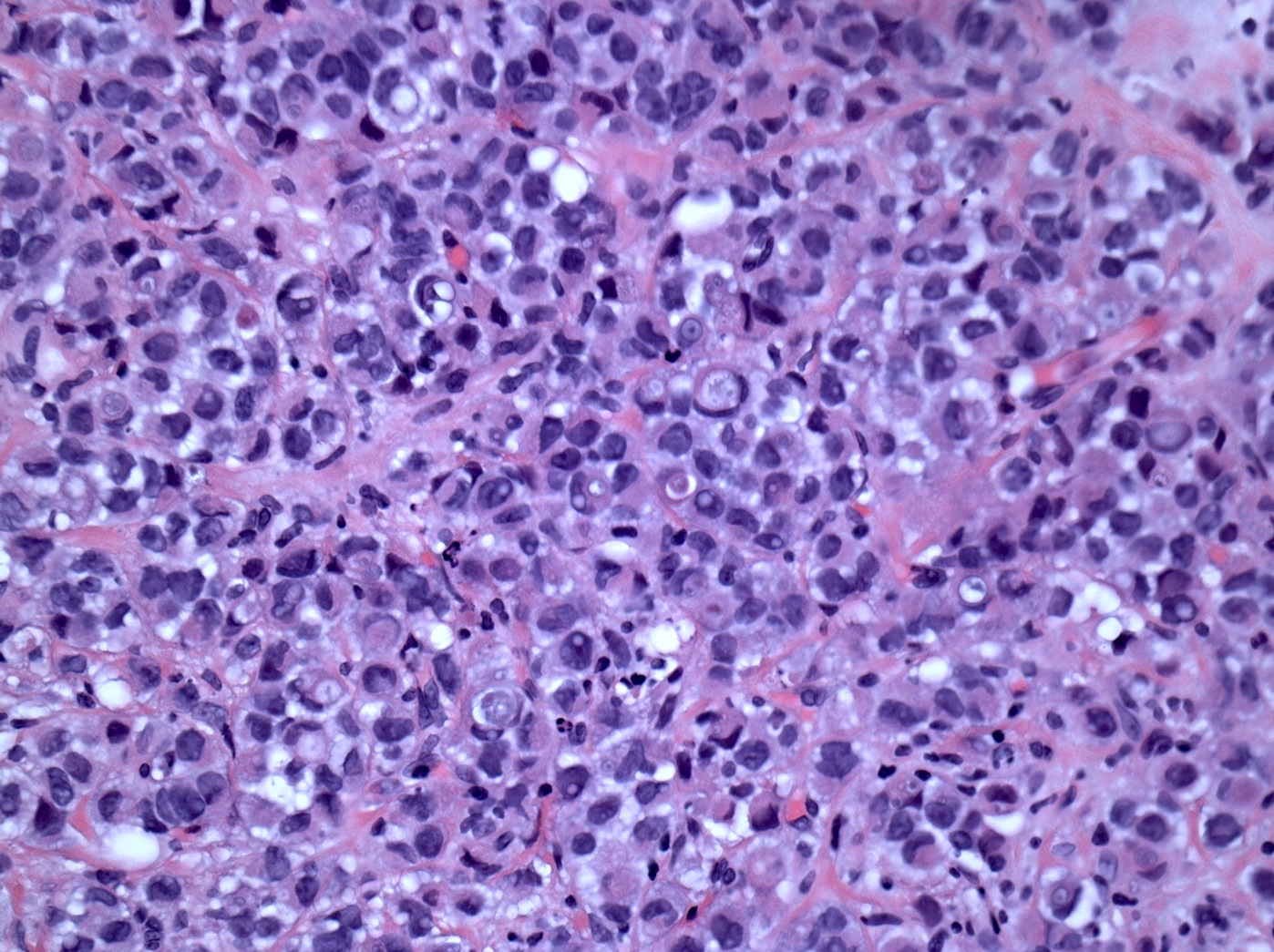
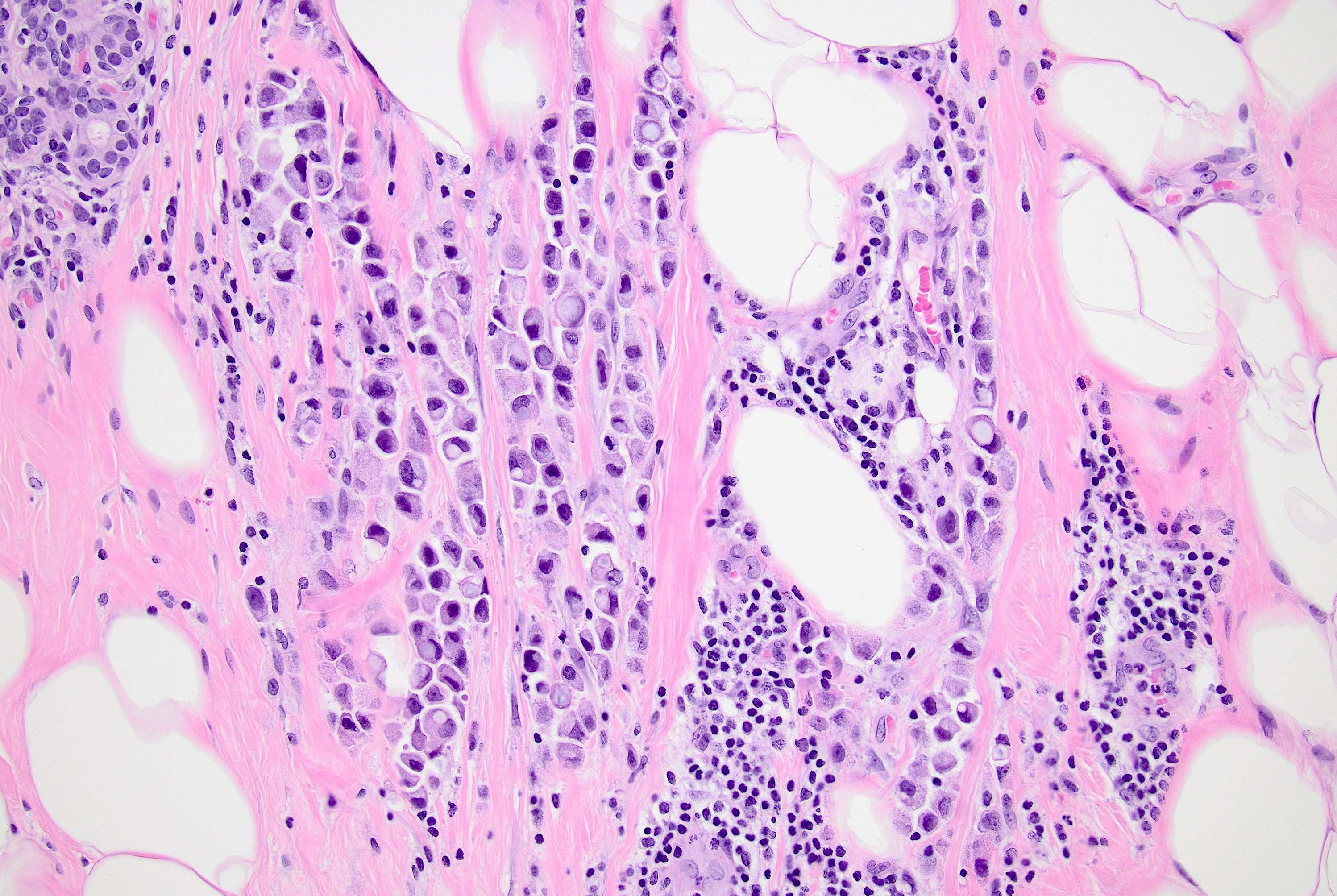
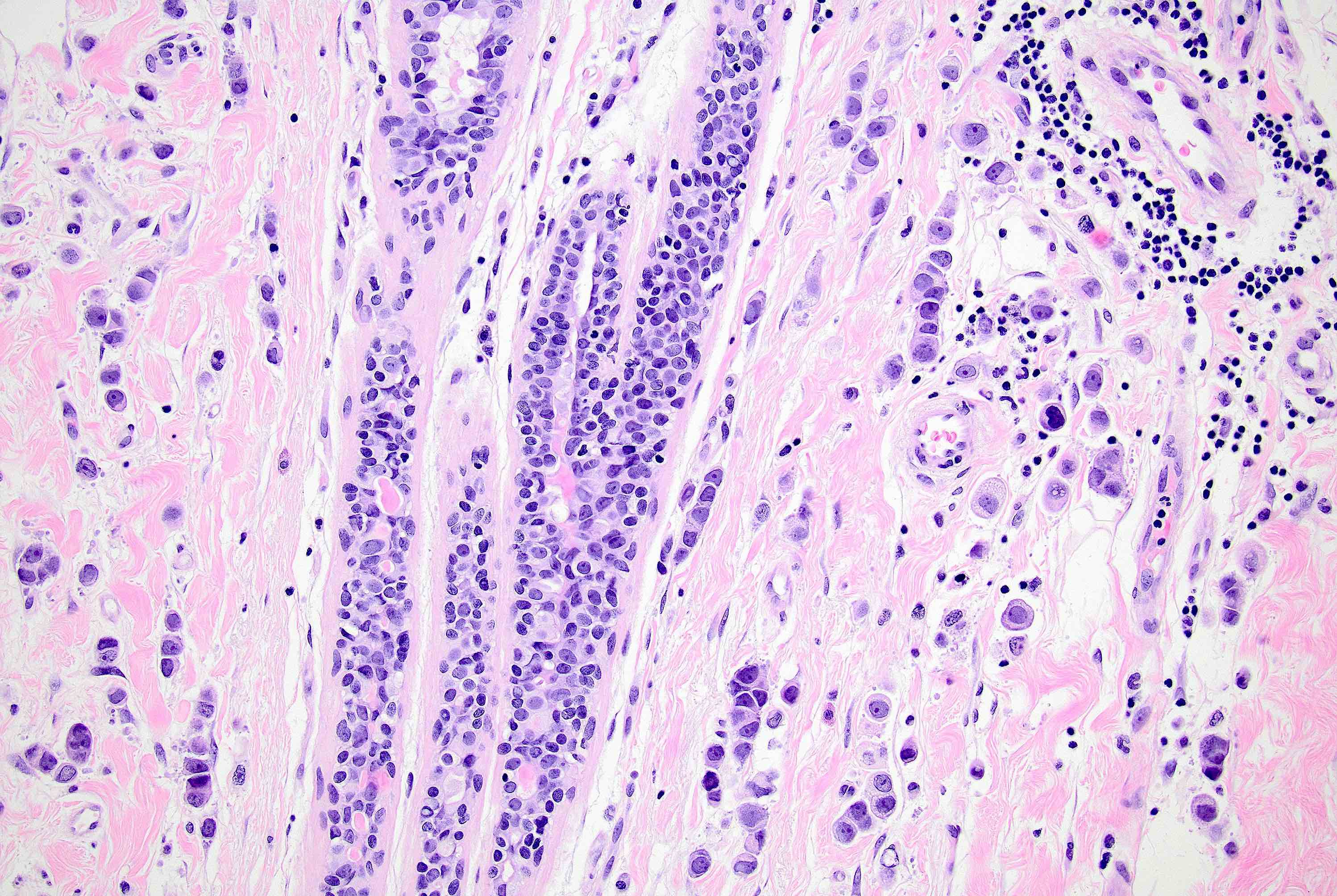
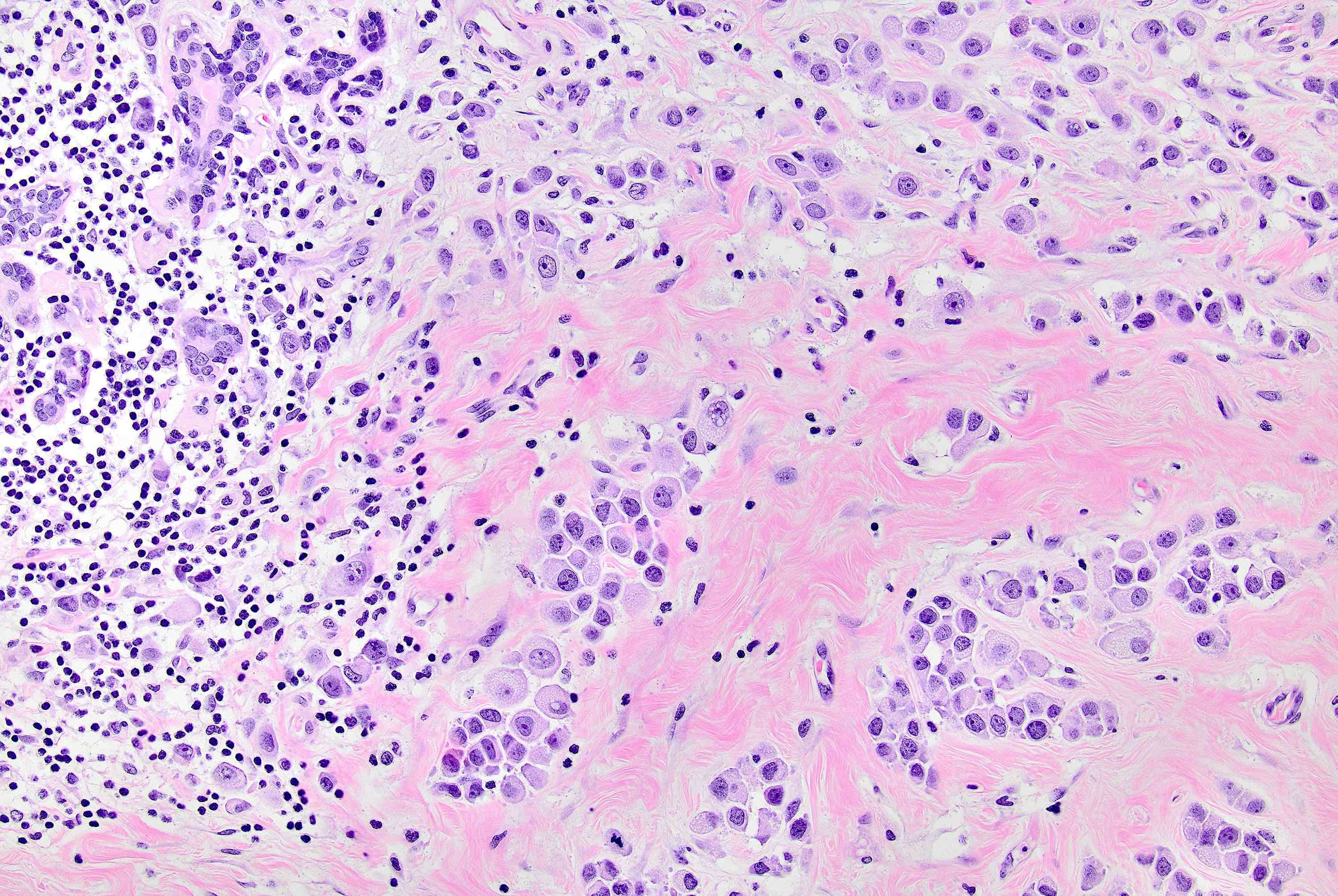
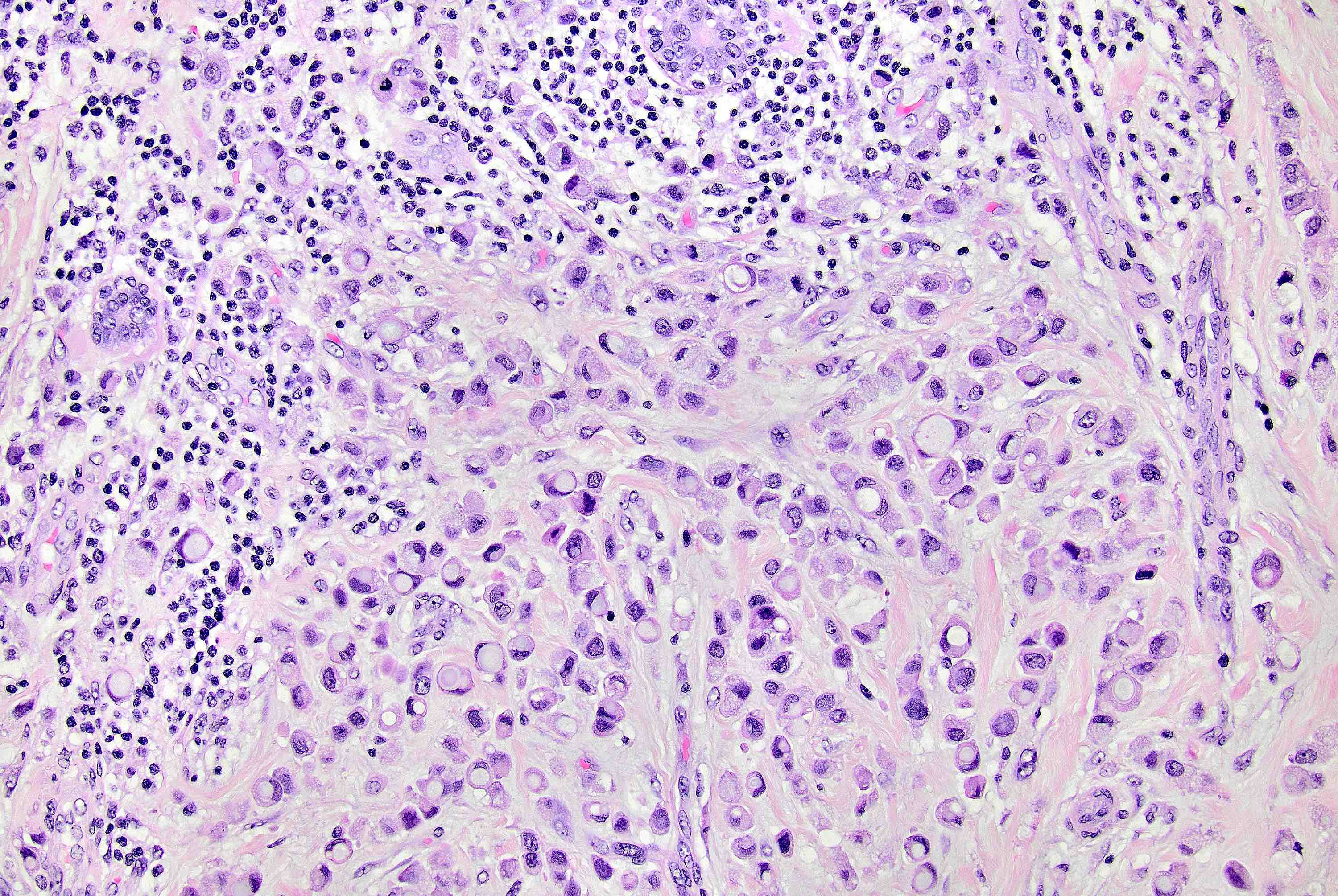
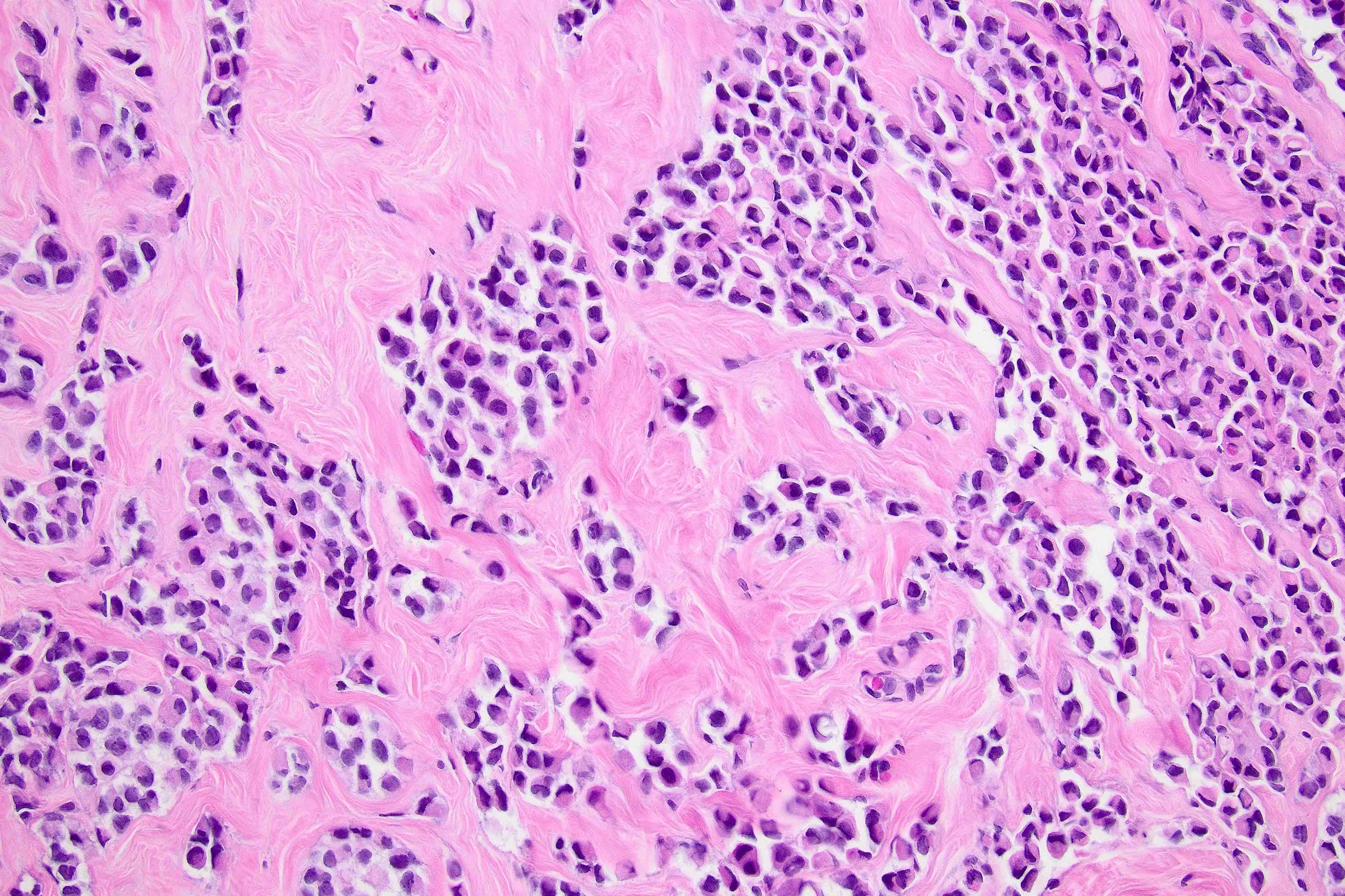
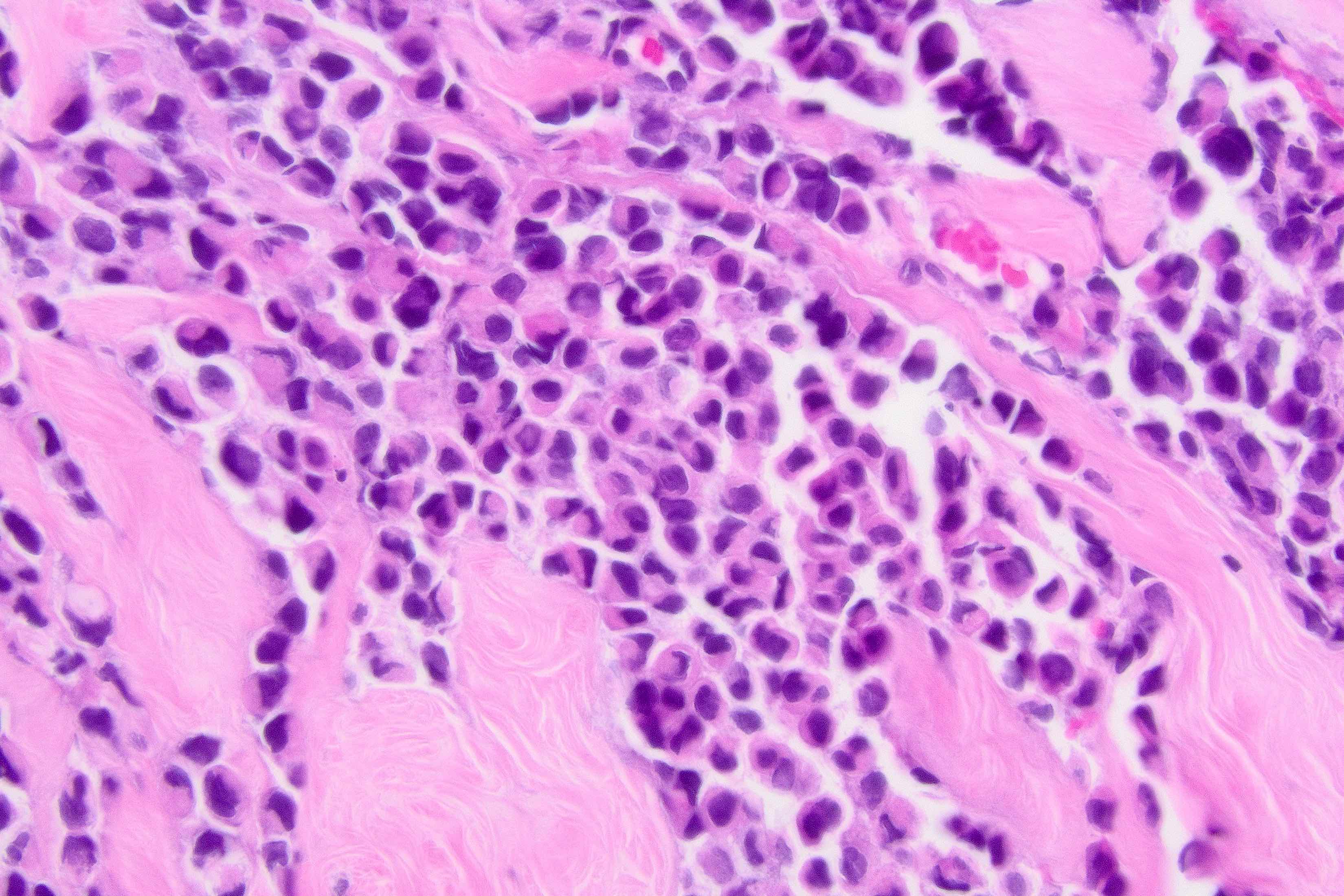
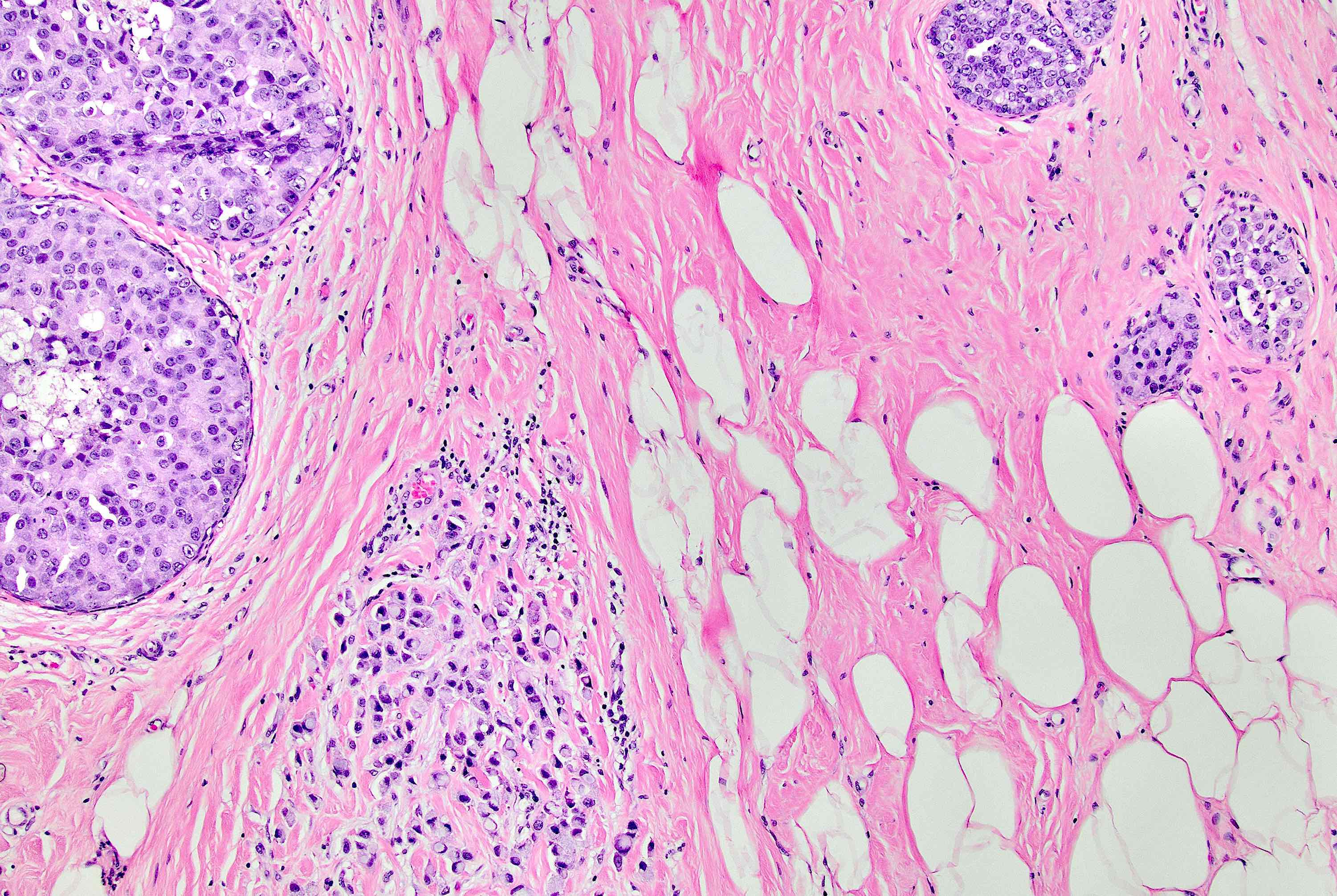
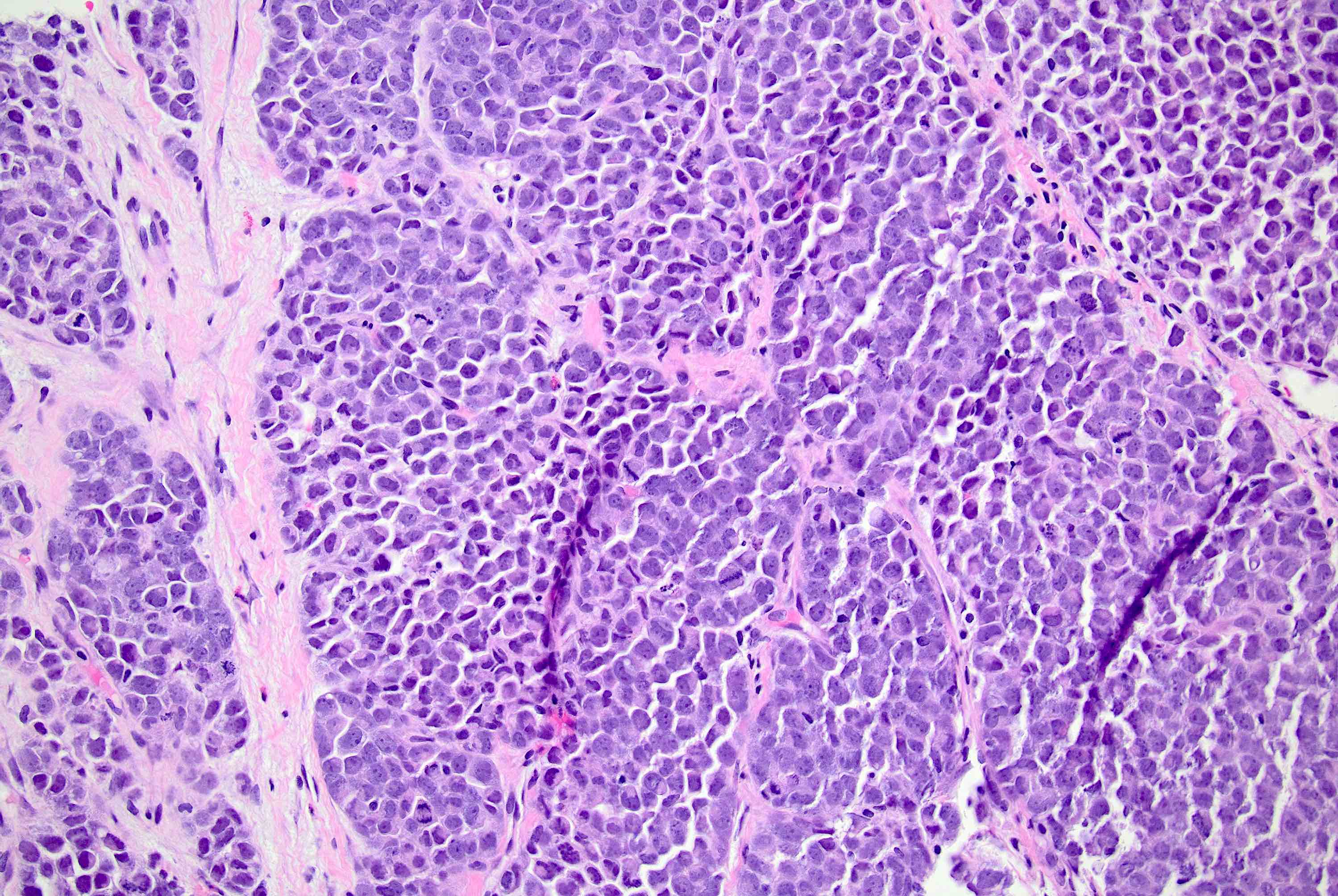
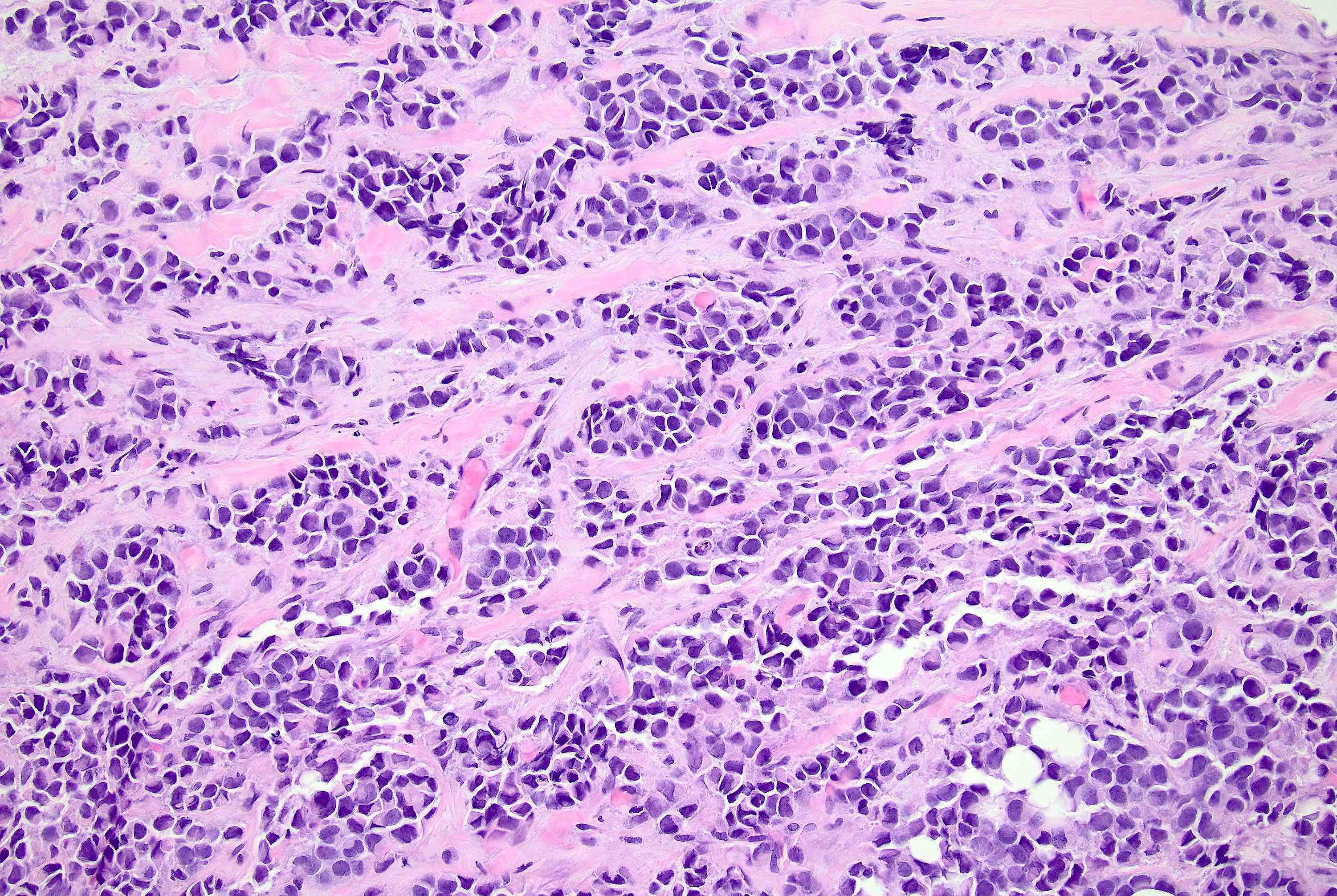
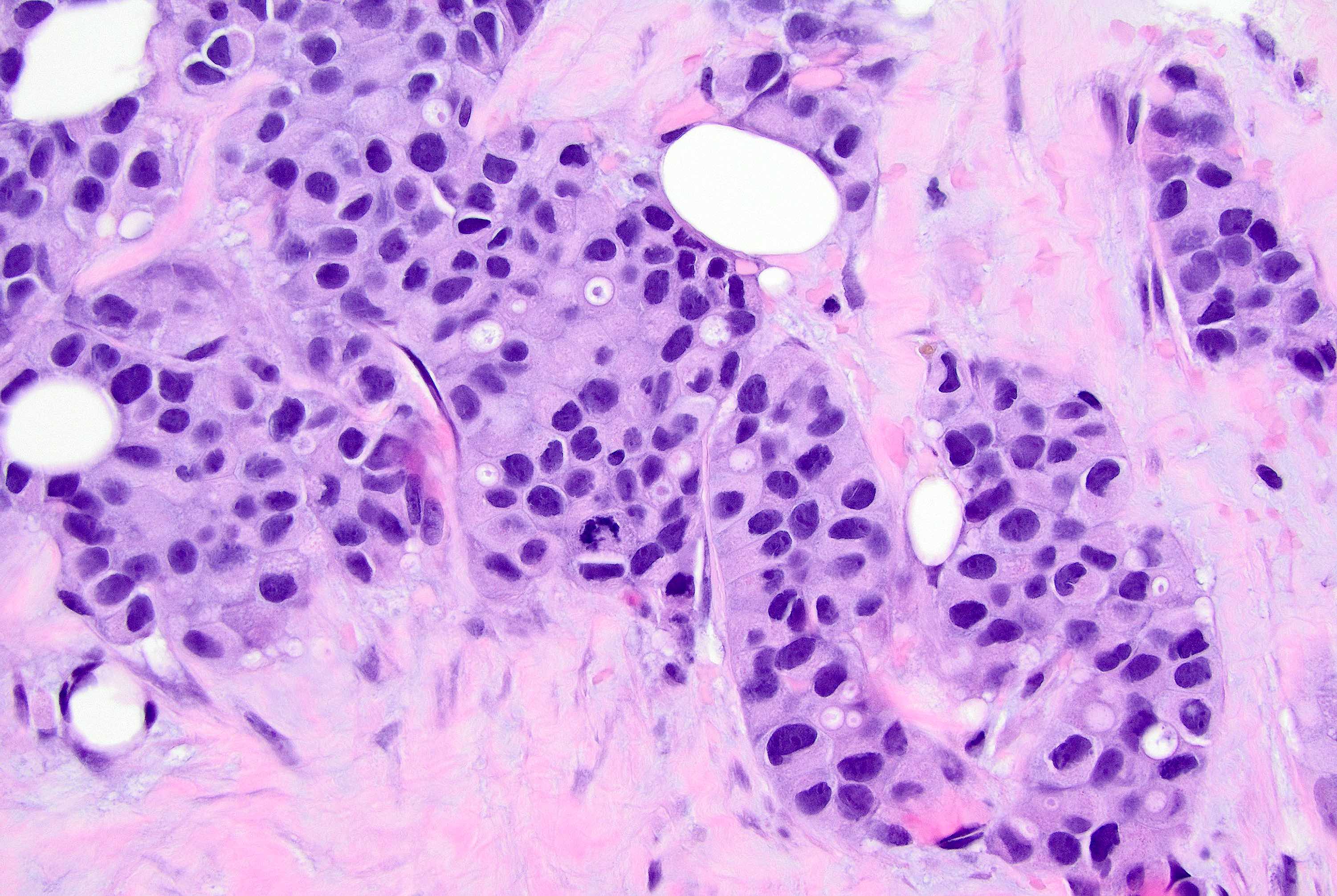
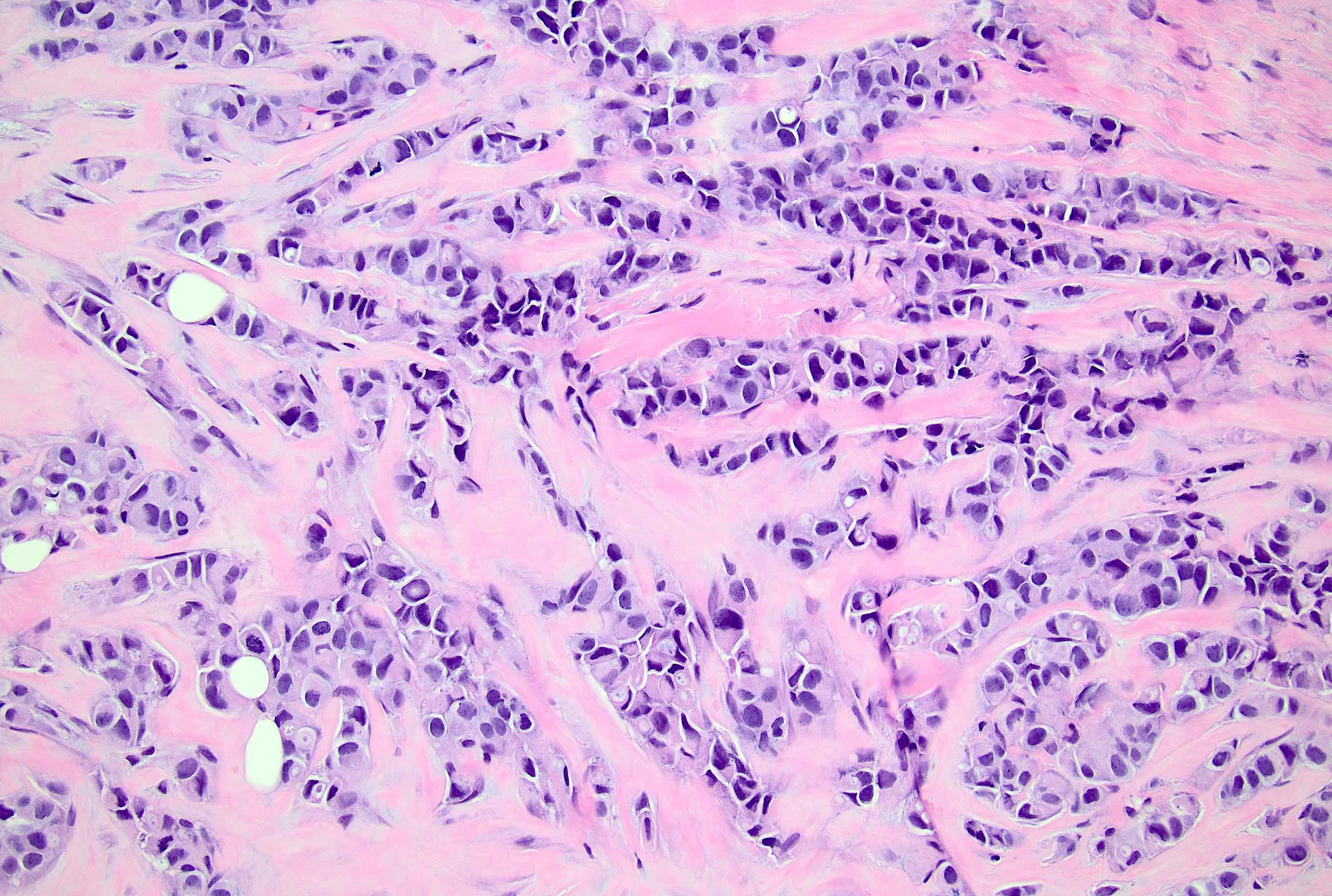
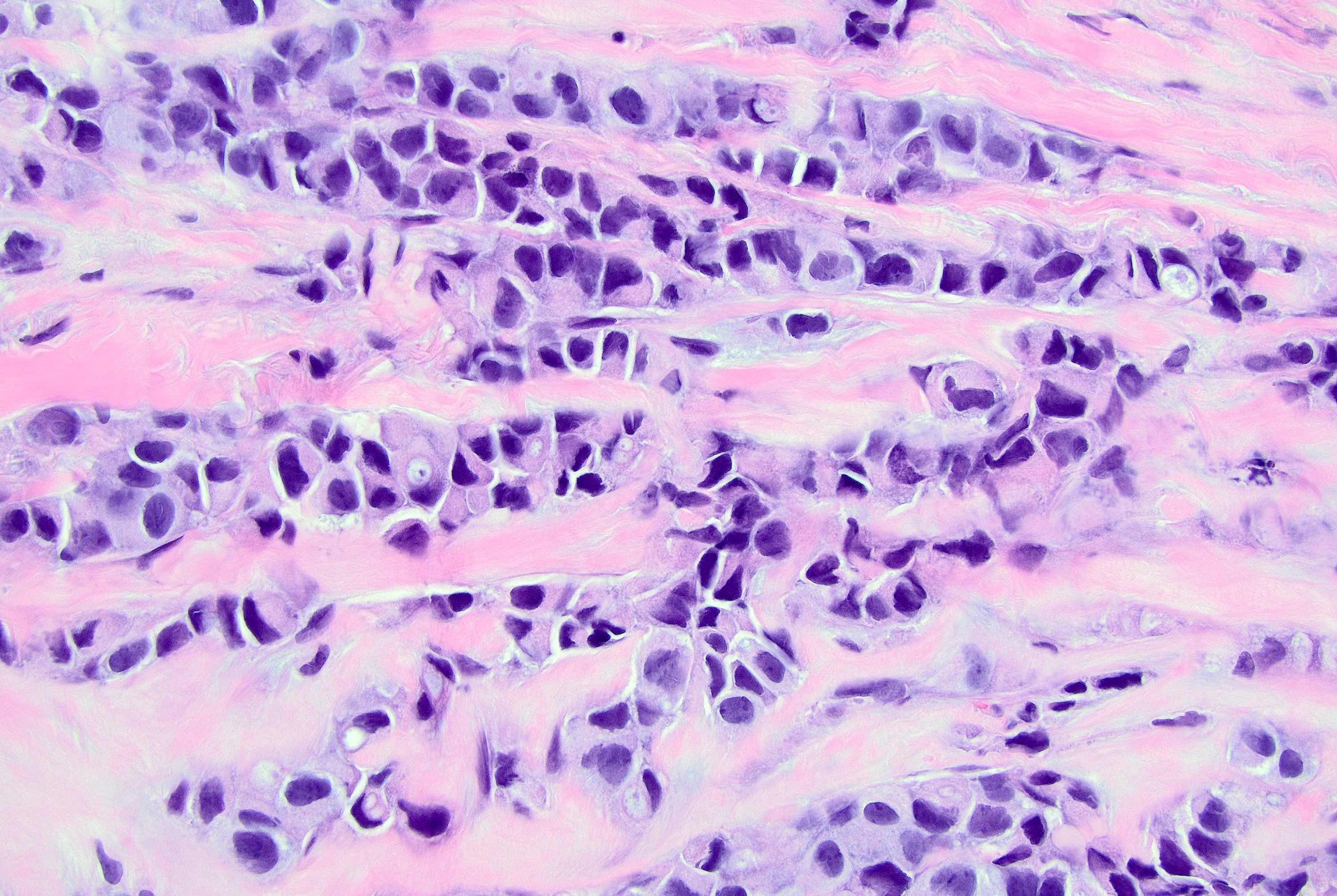
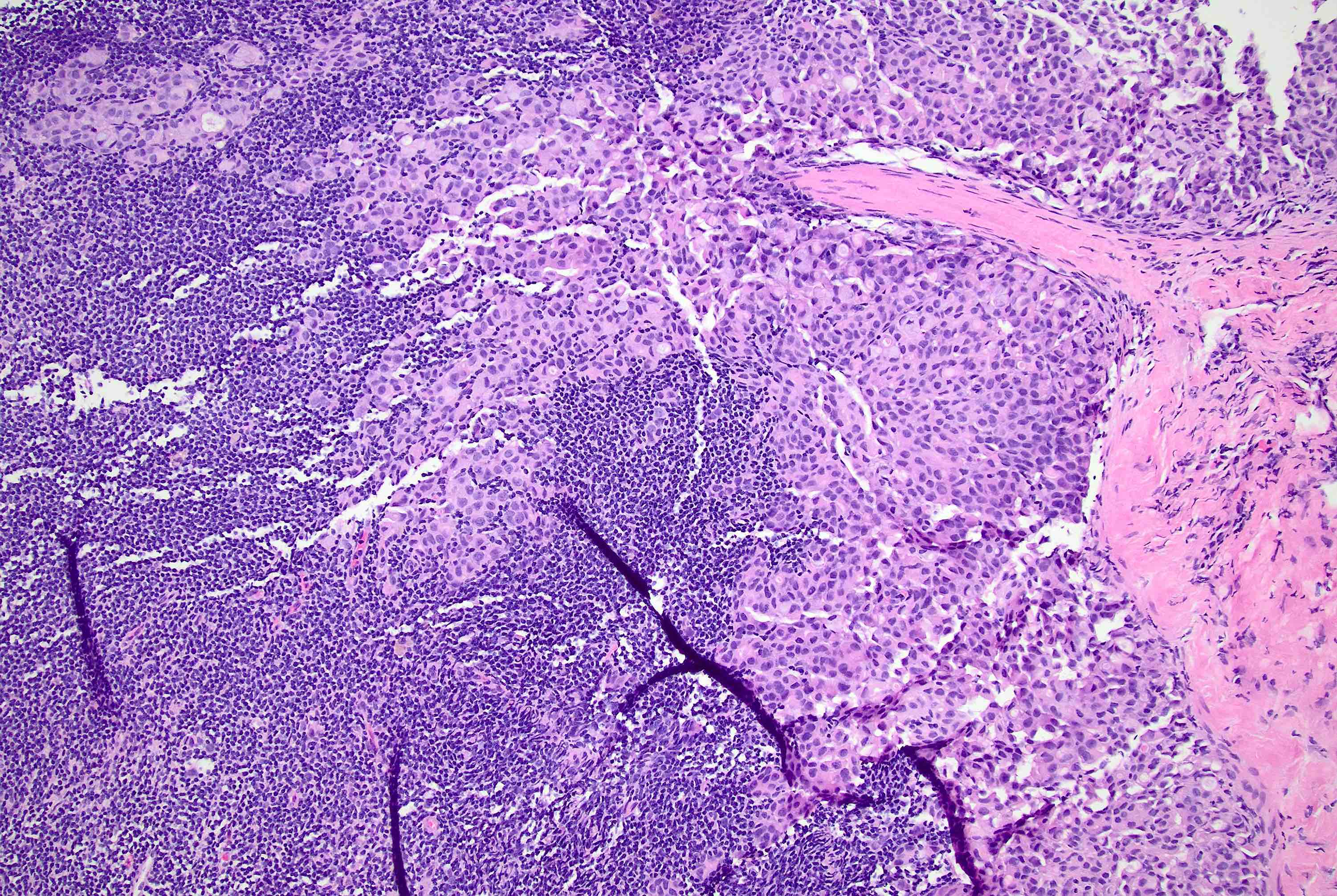
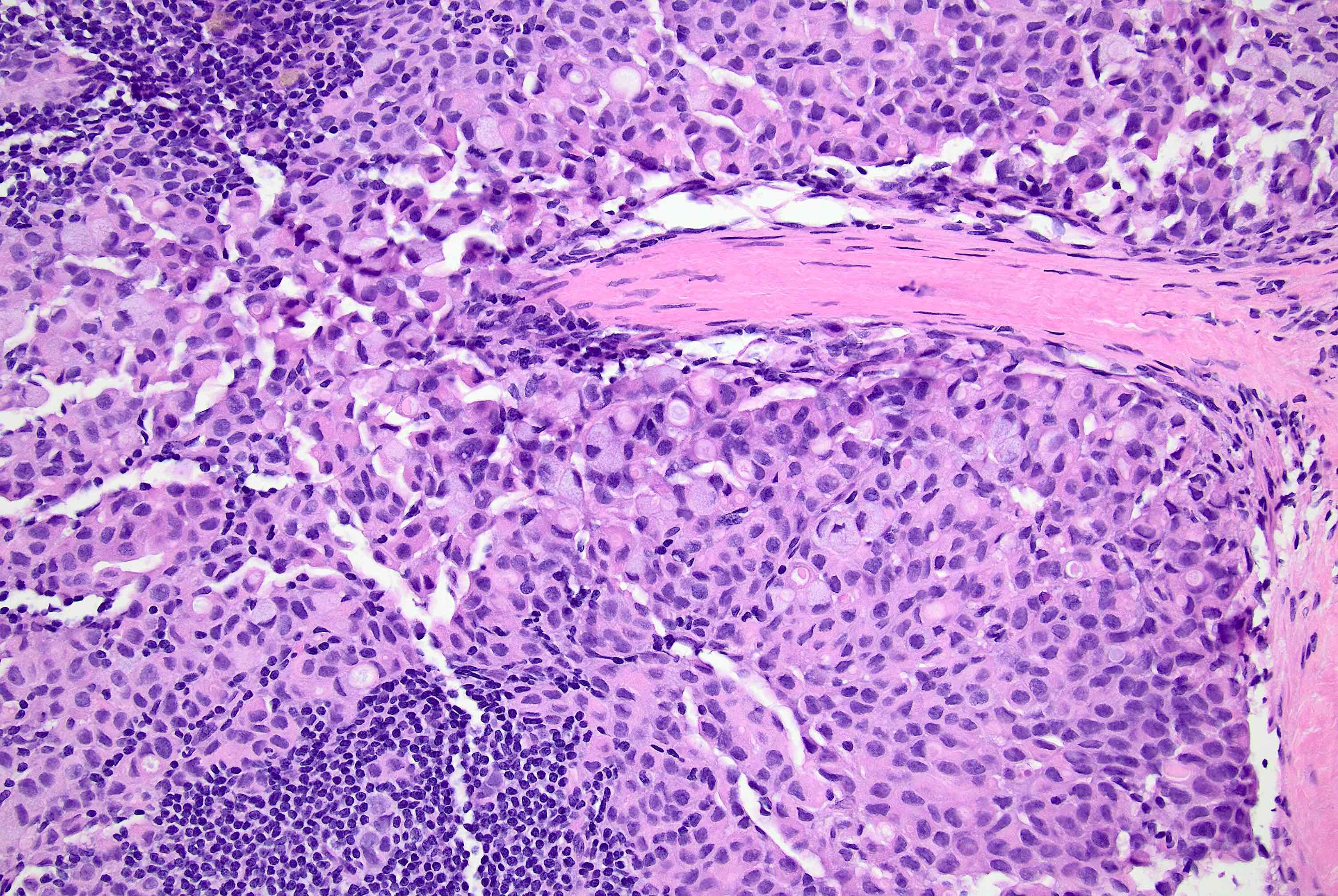
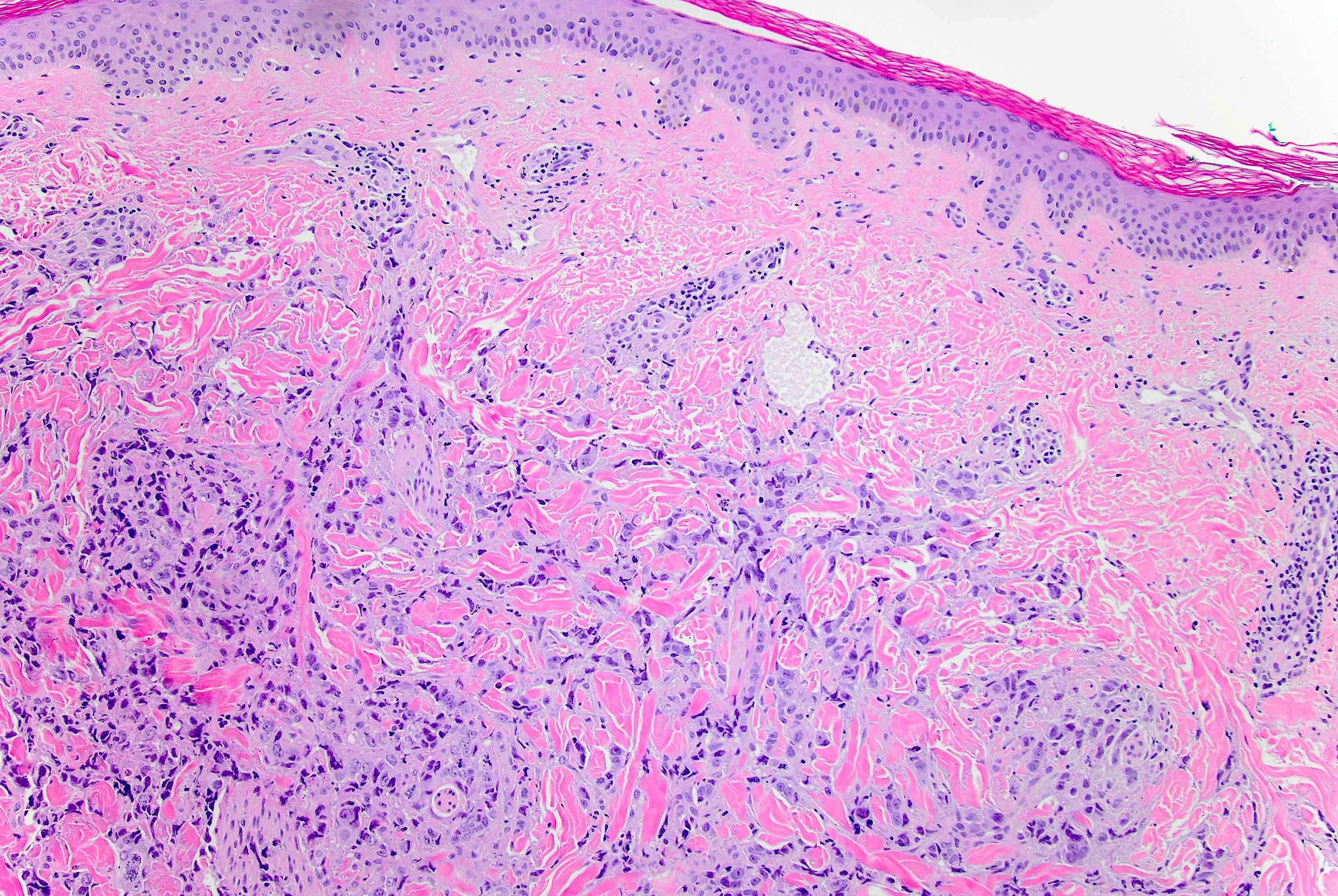
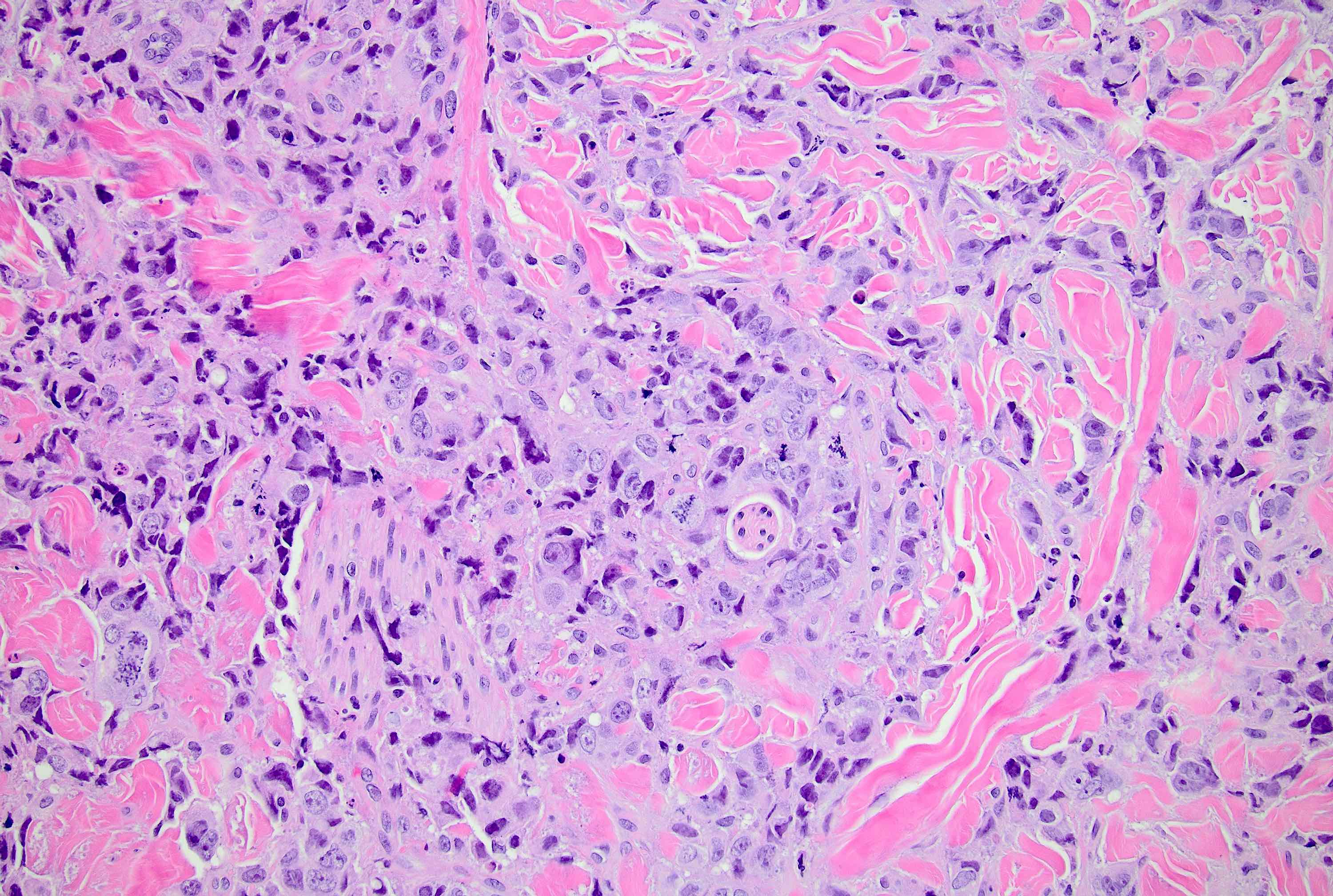
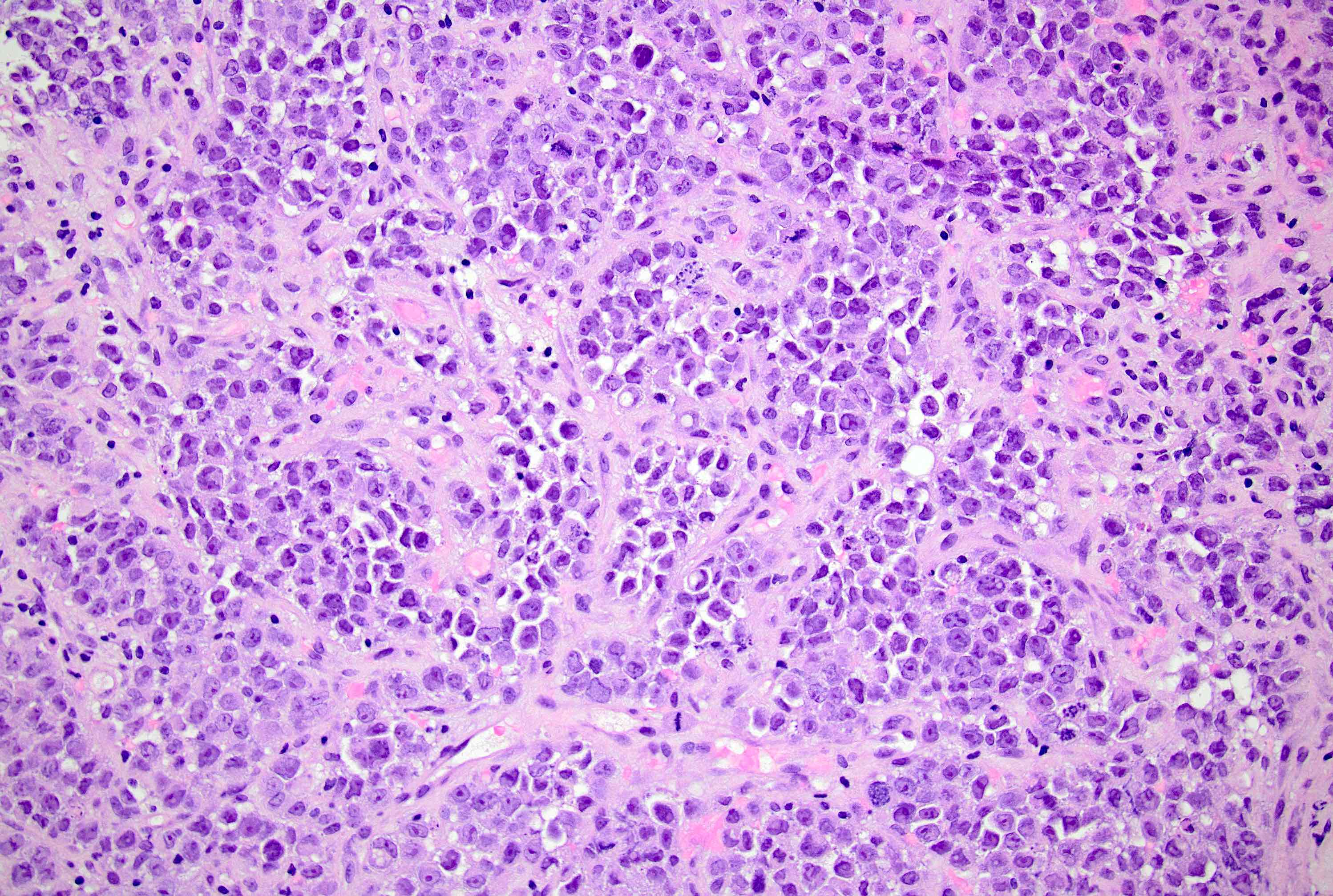
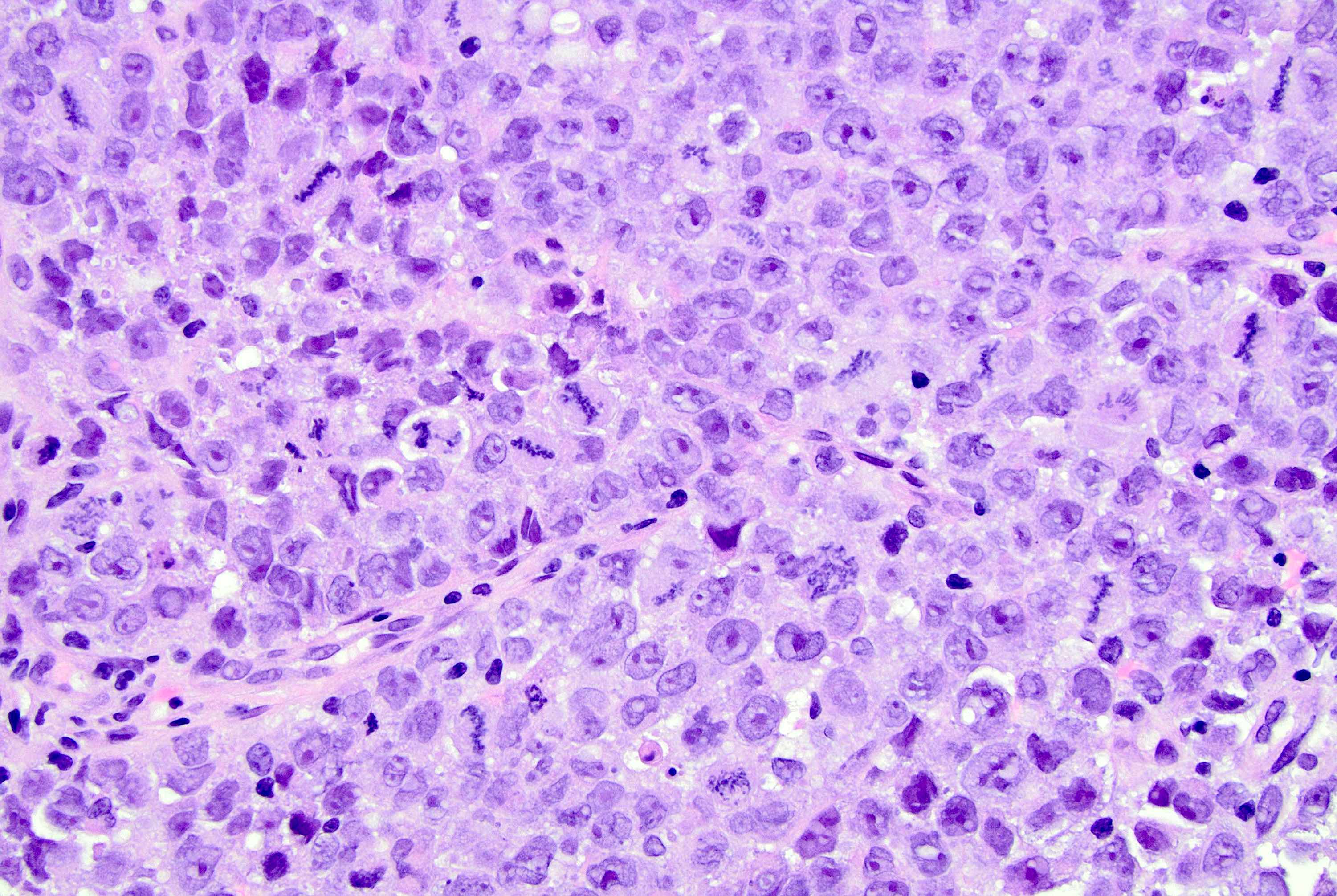
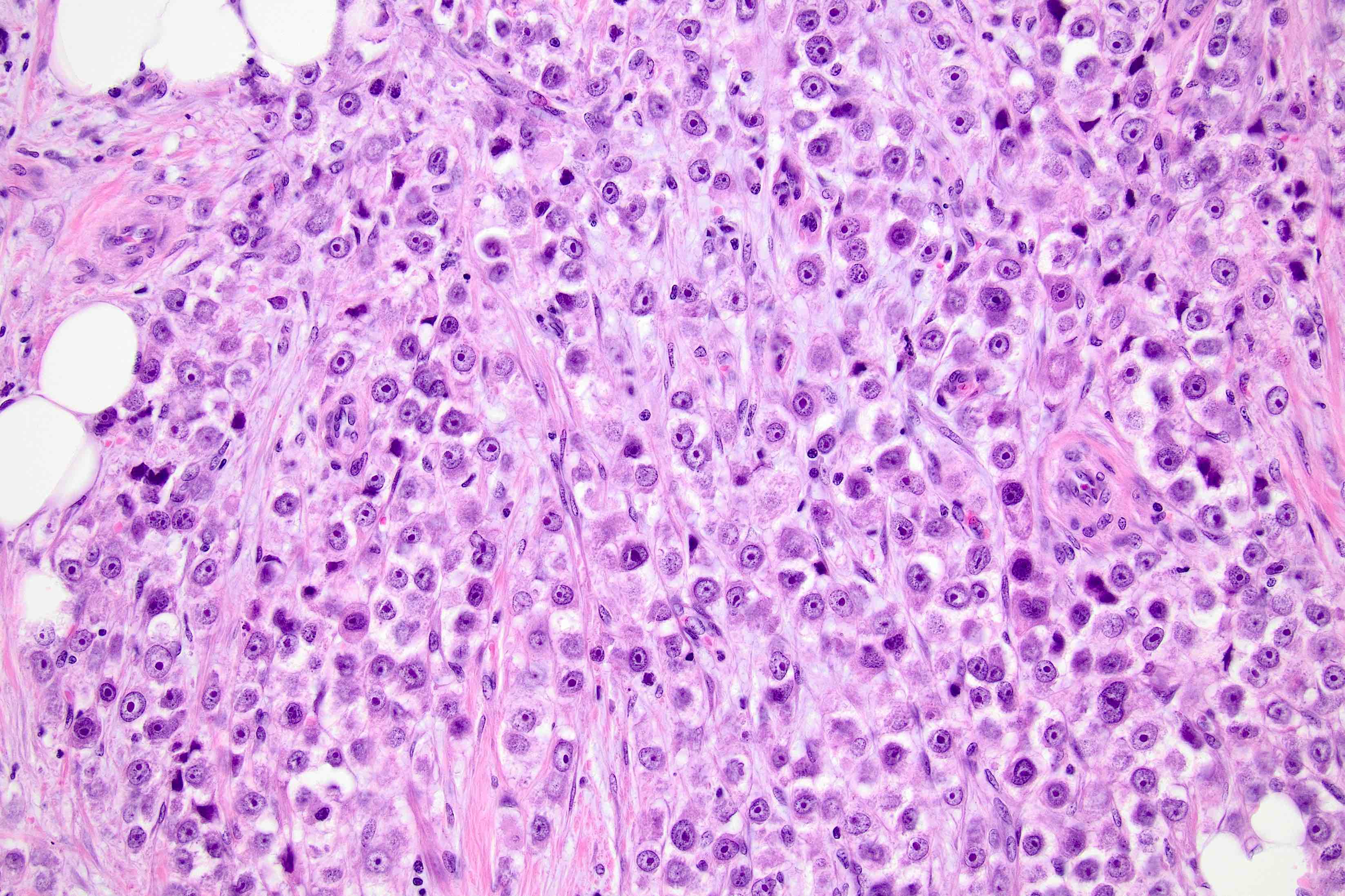
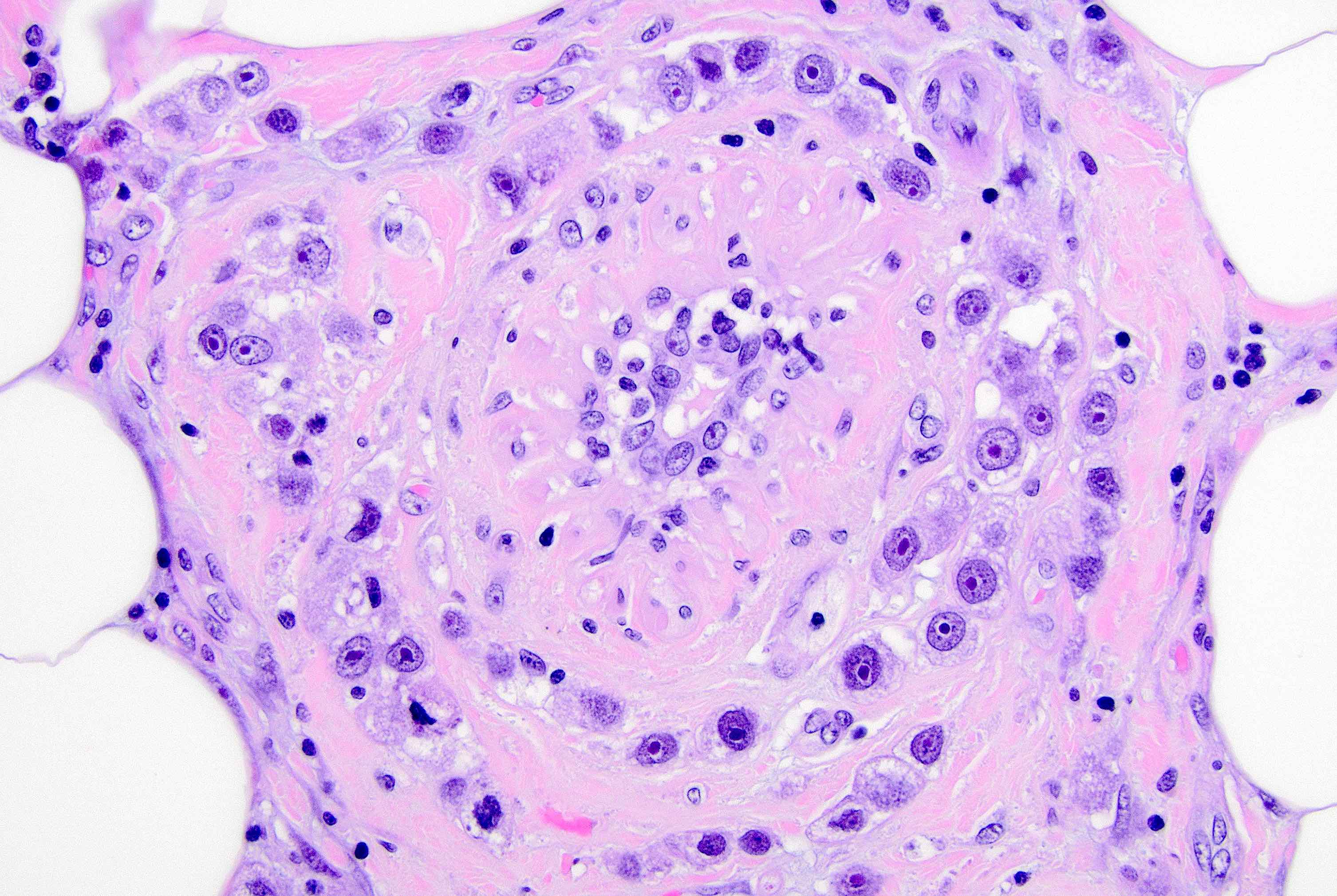
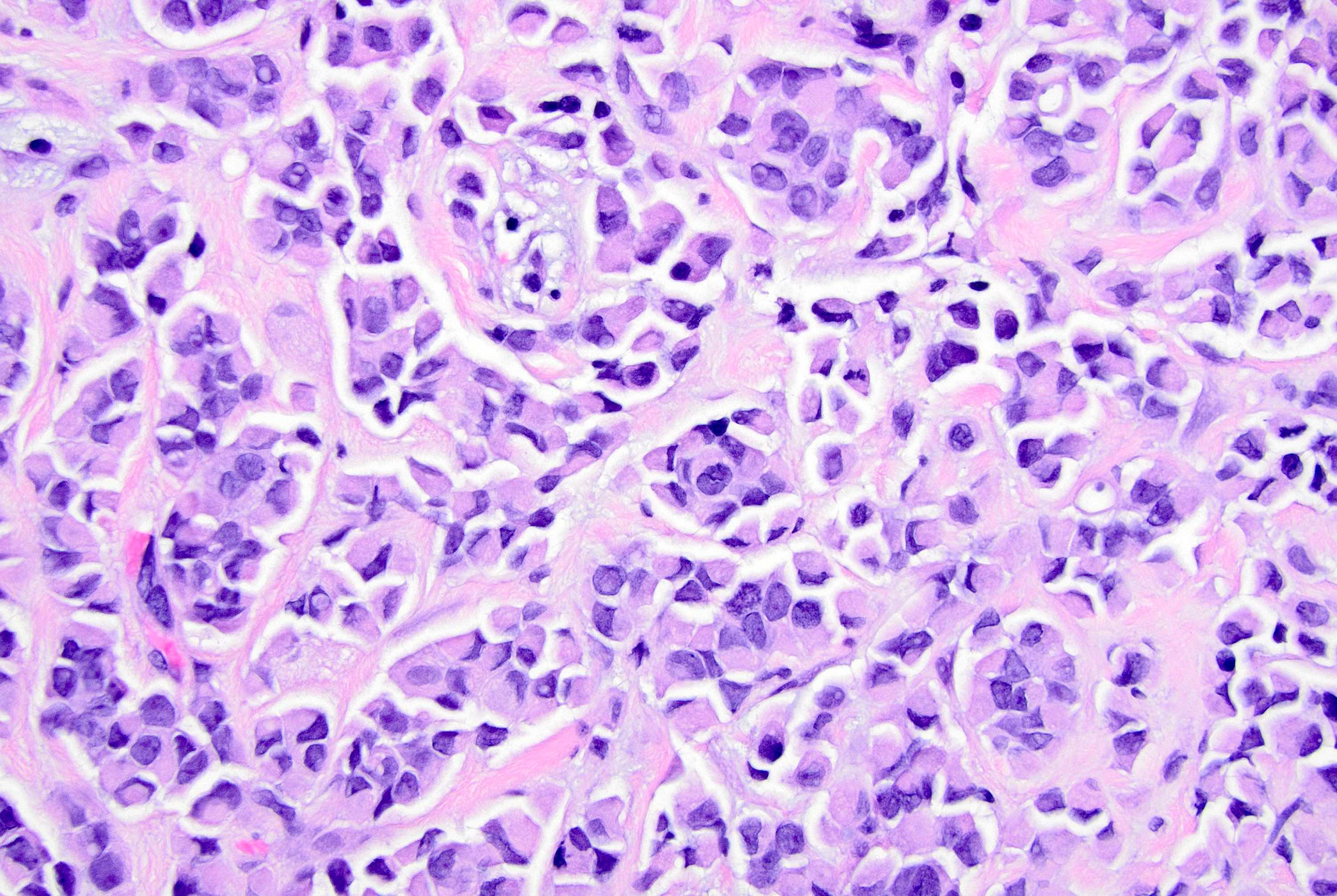
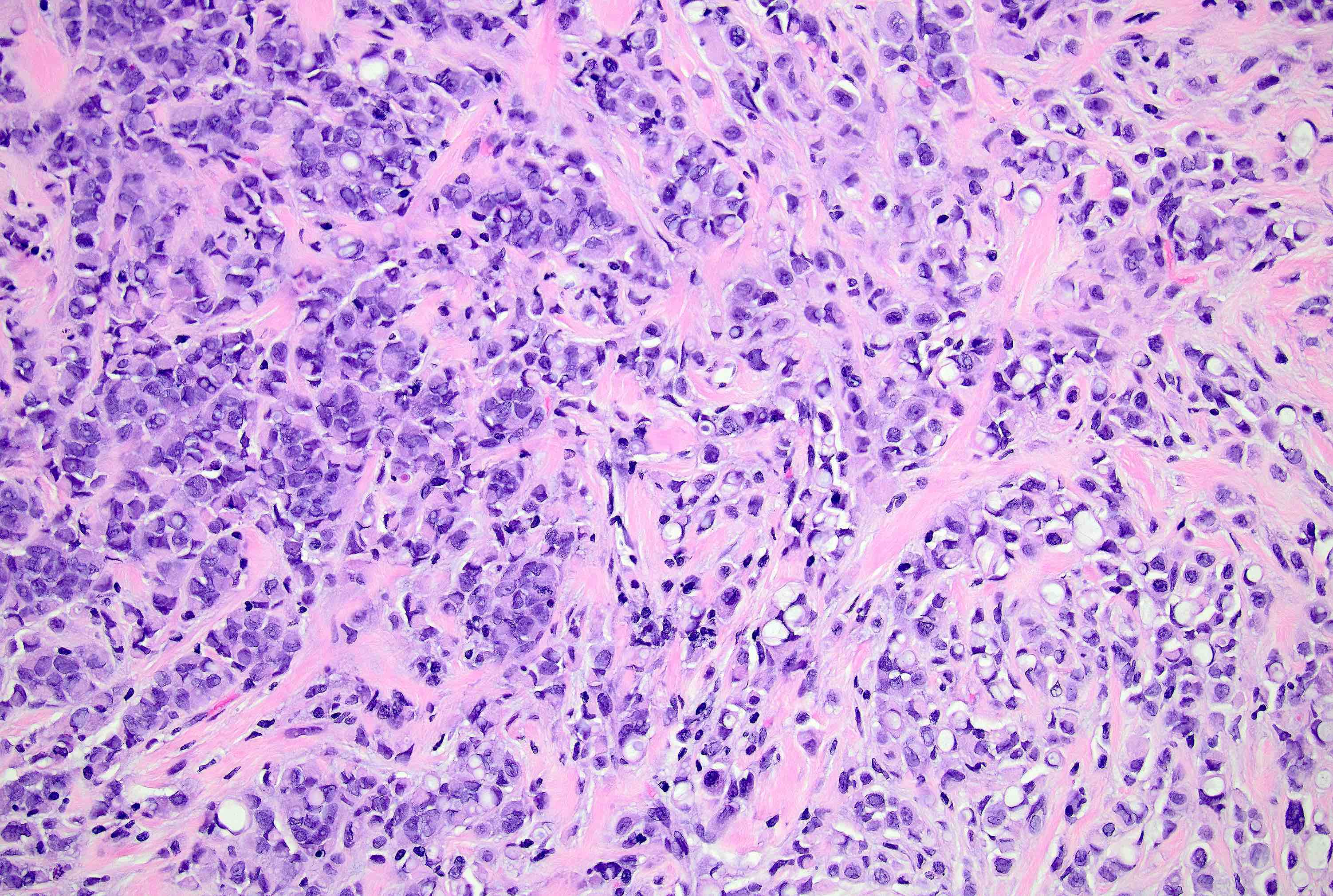
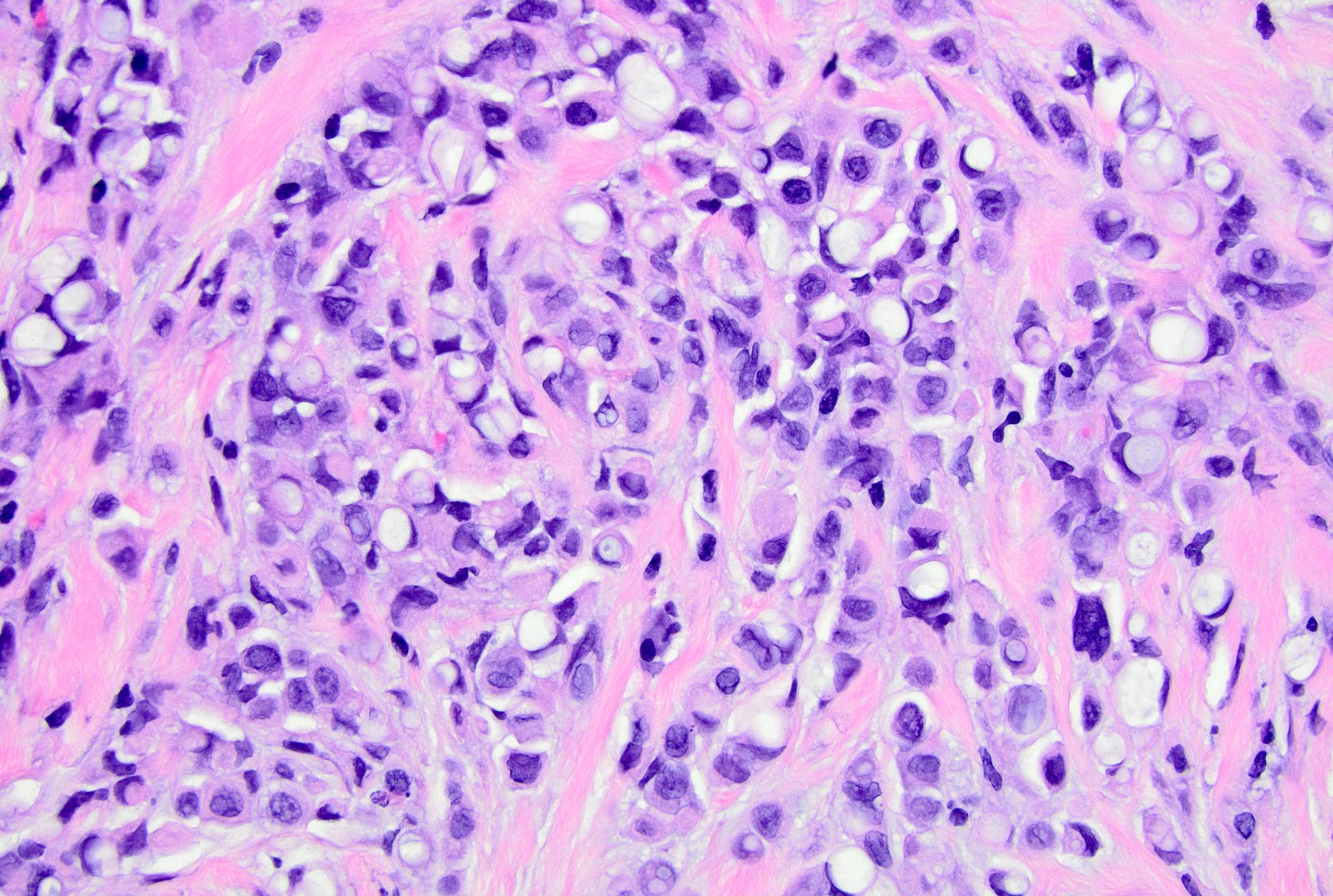
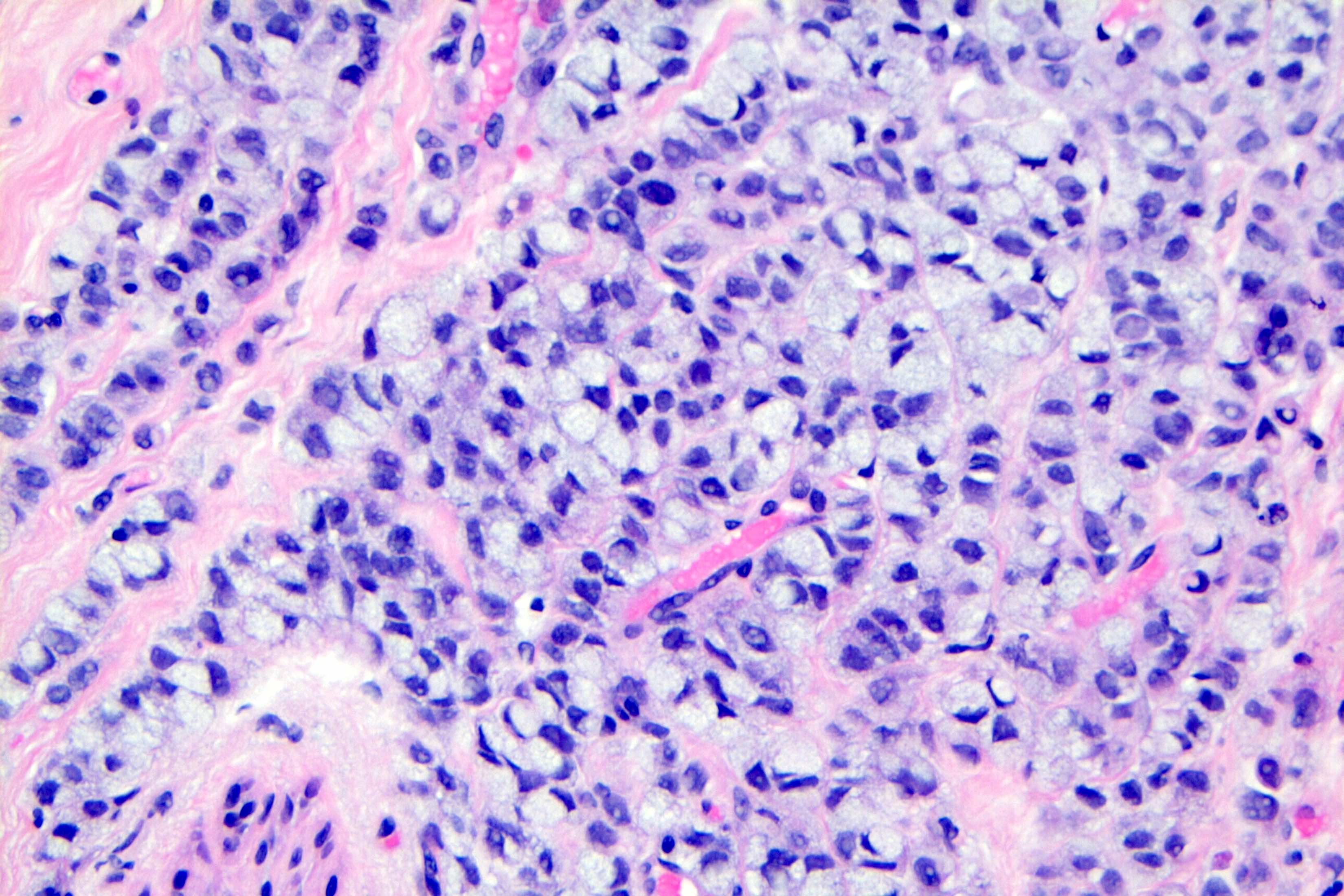
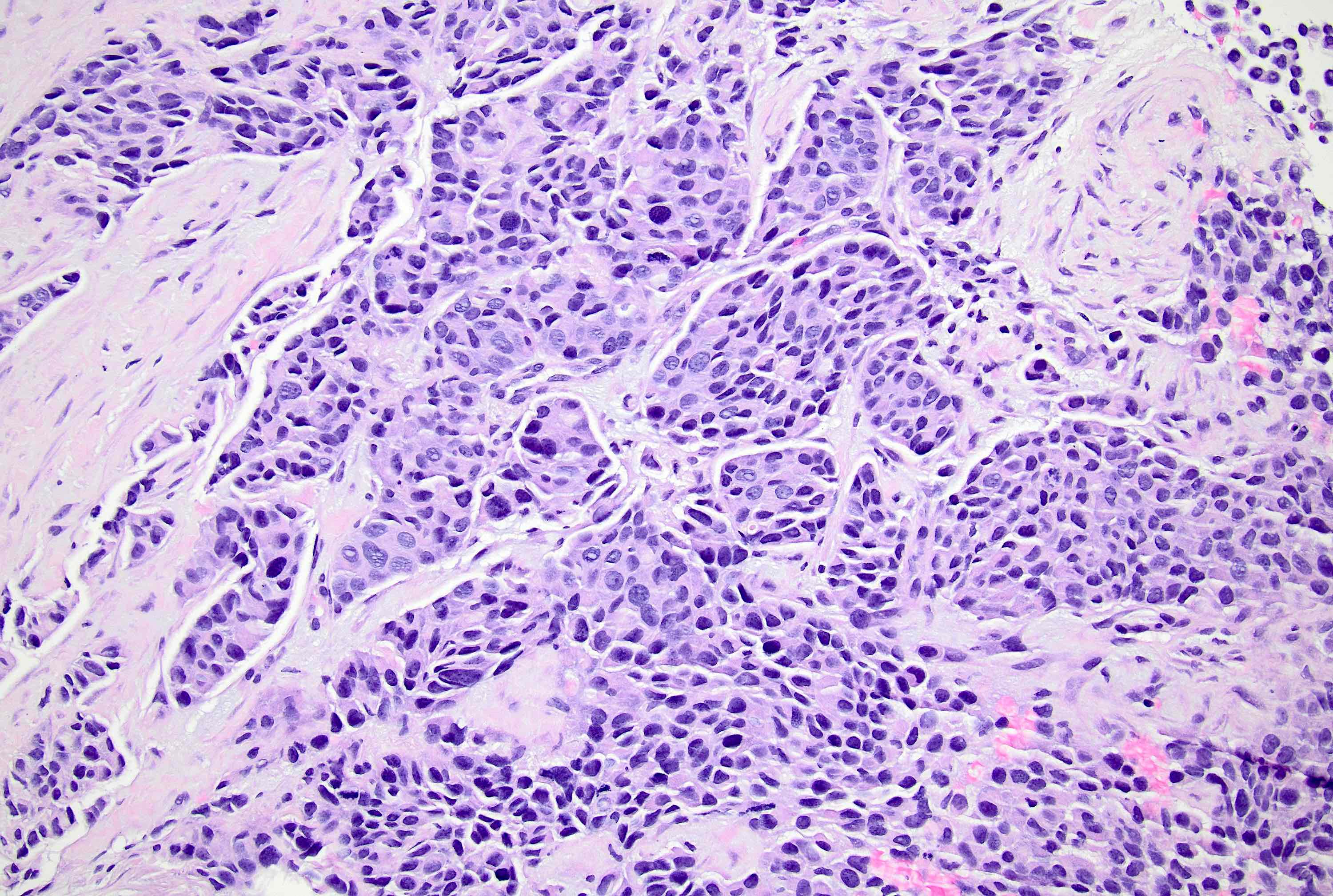
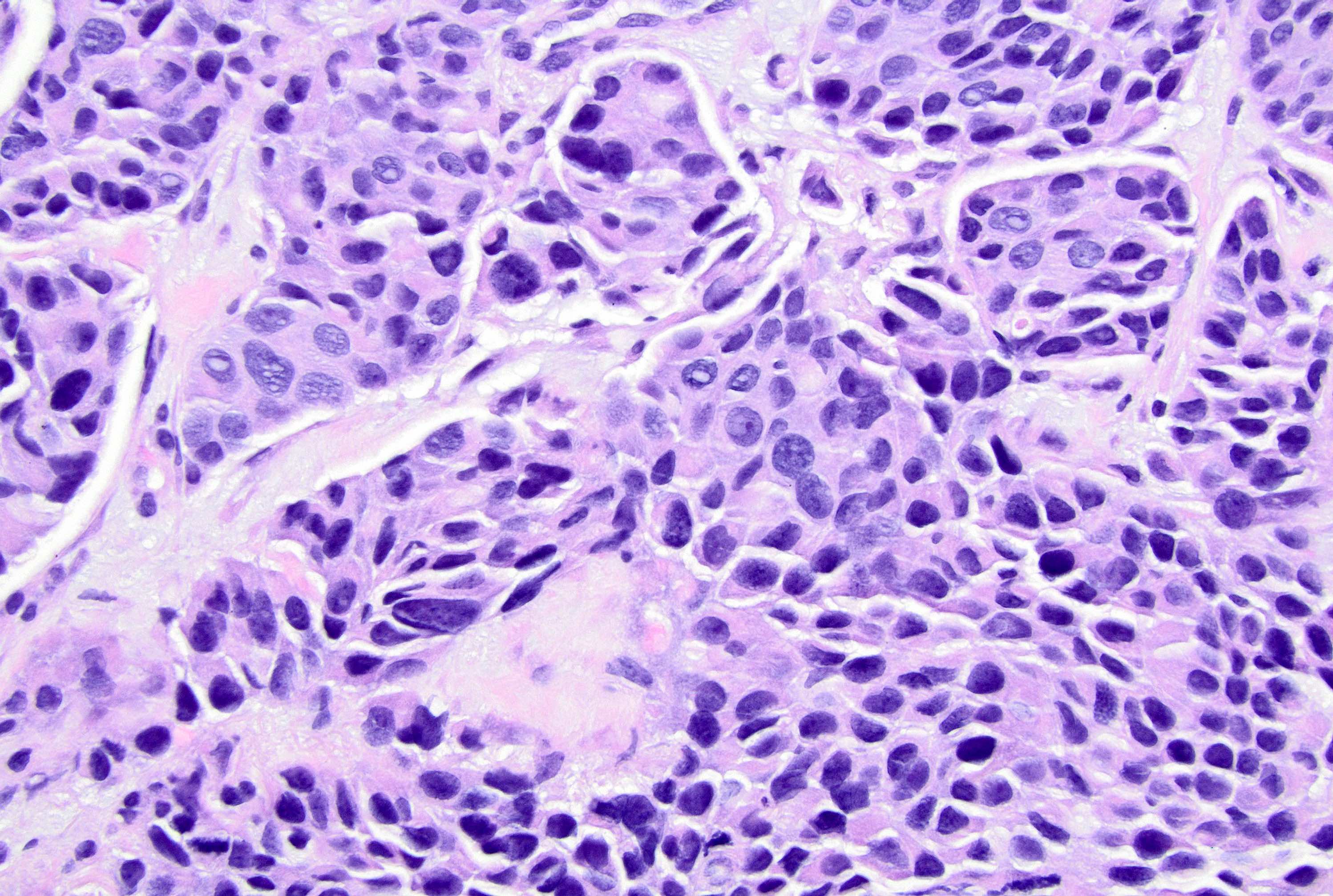
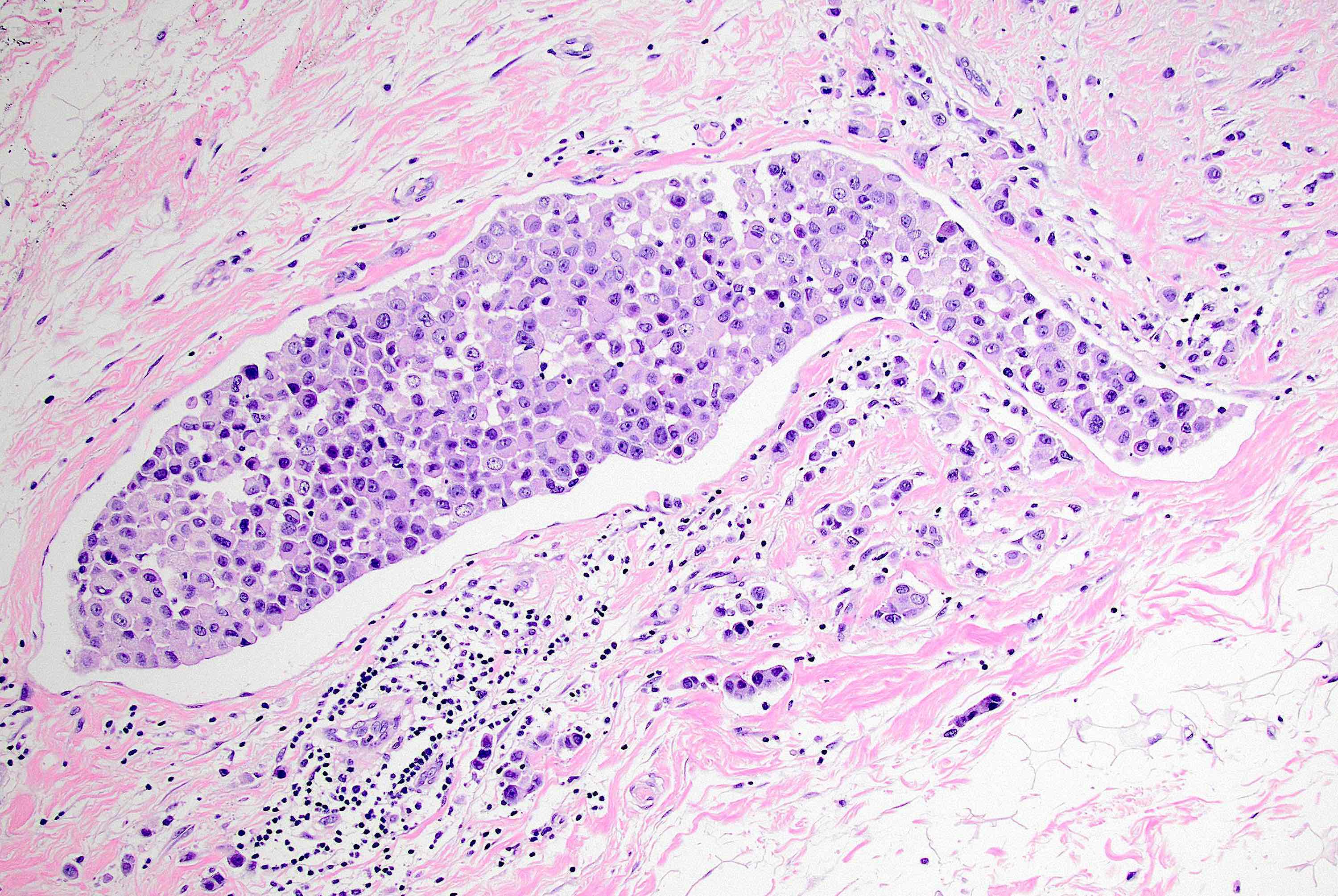
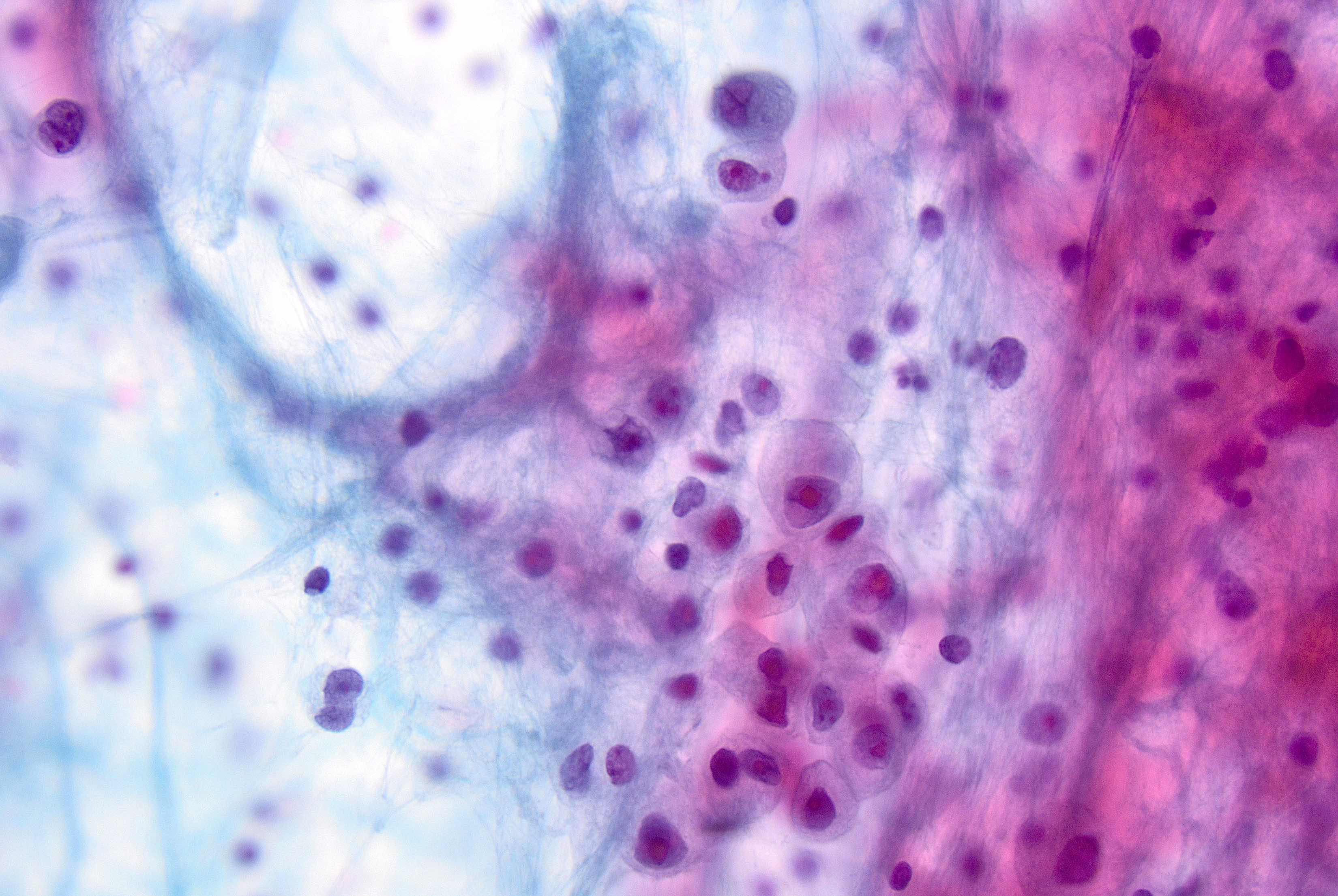
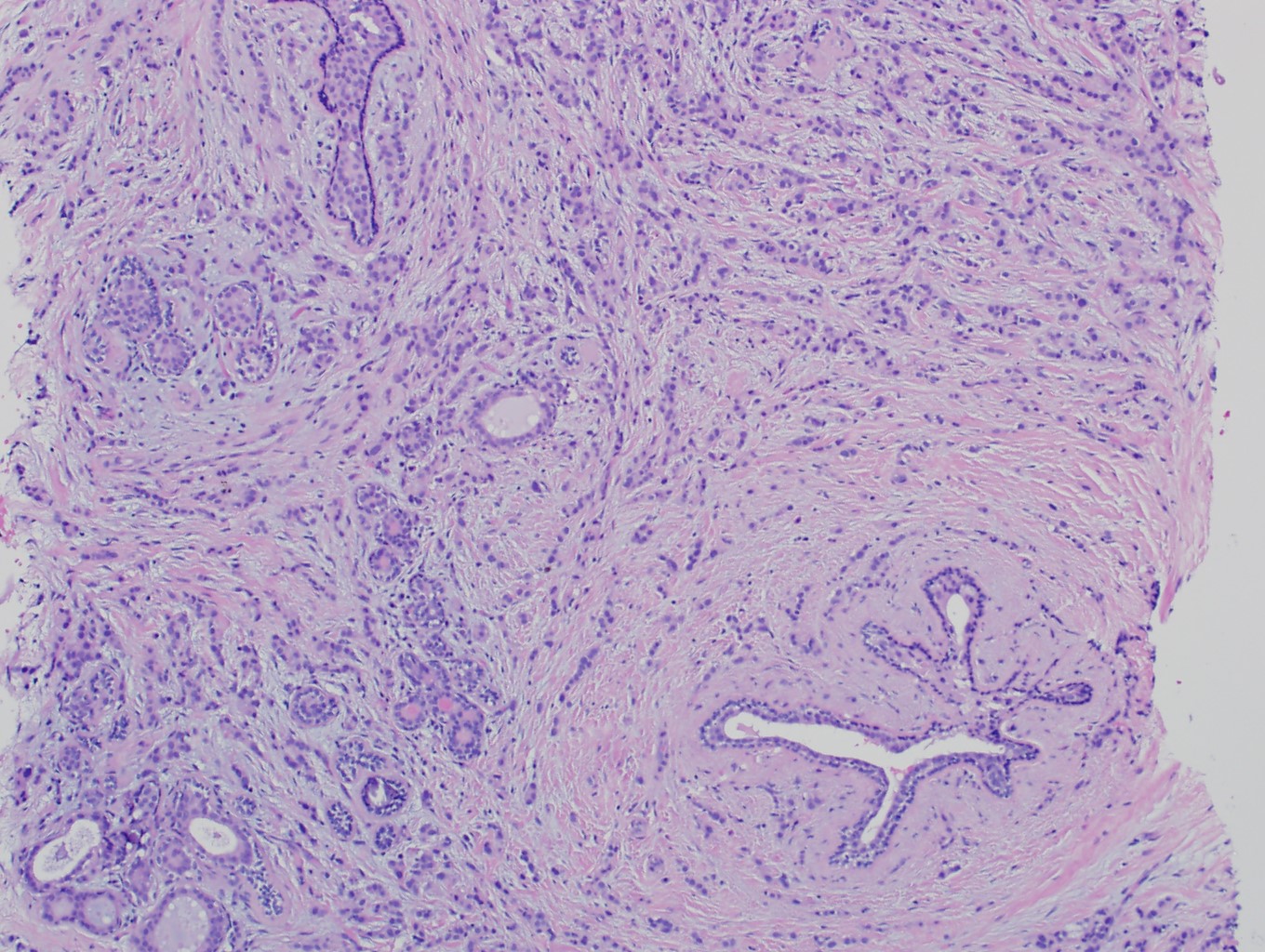
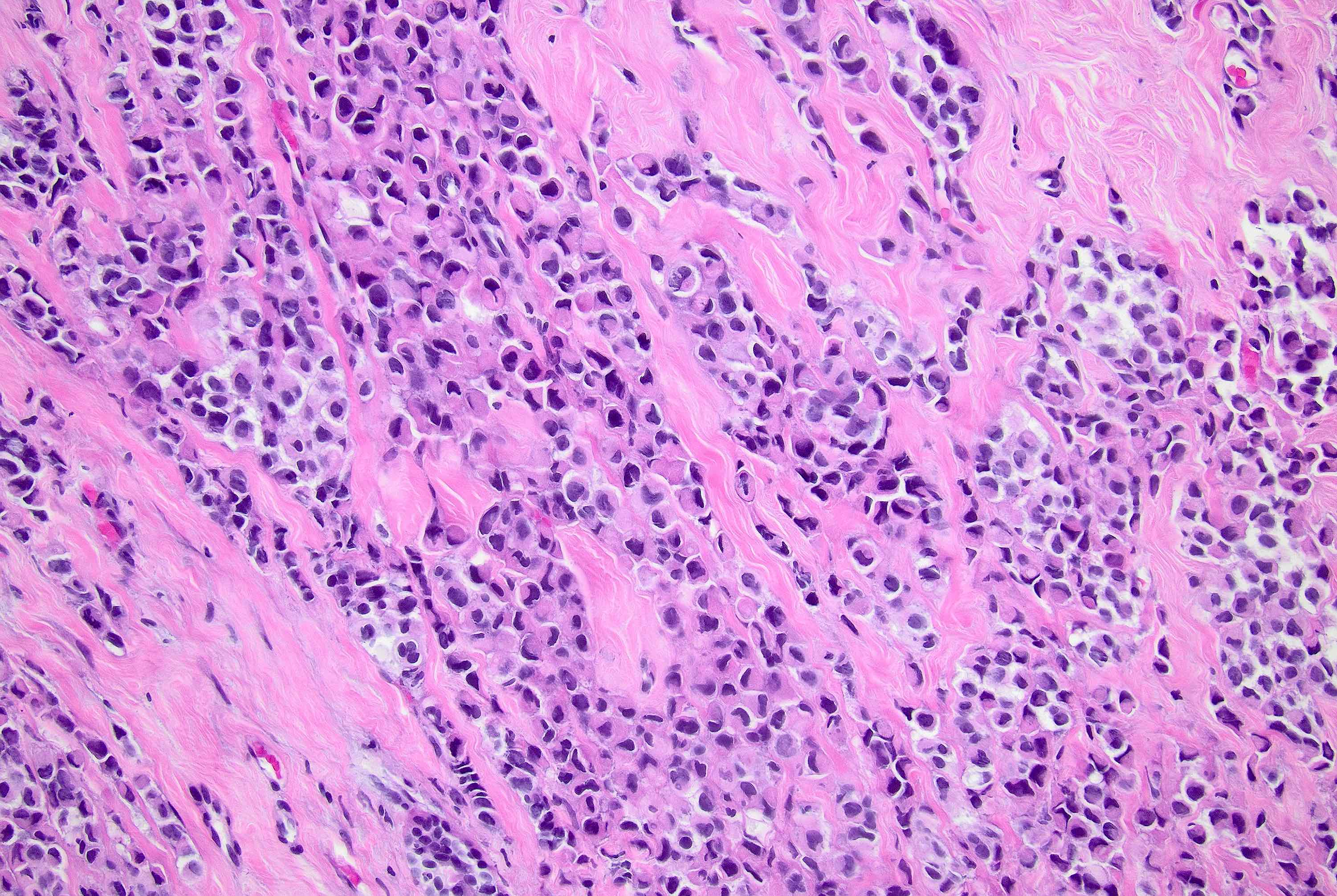
Microscopic (histologic) description
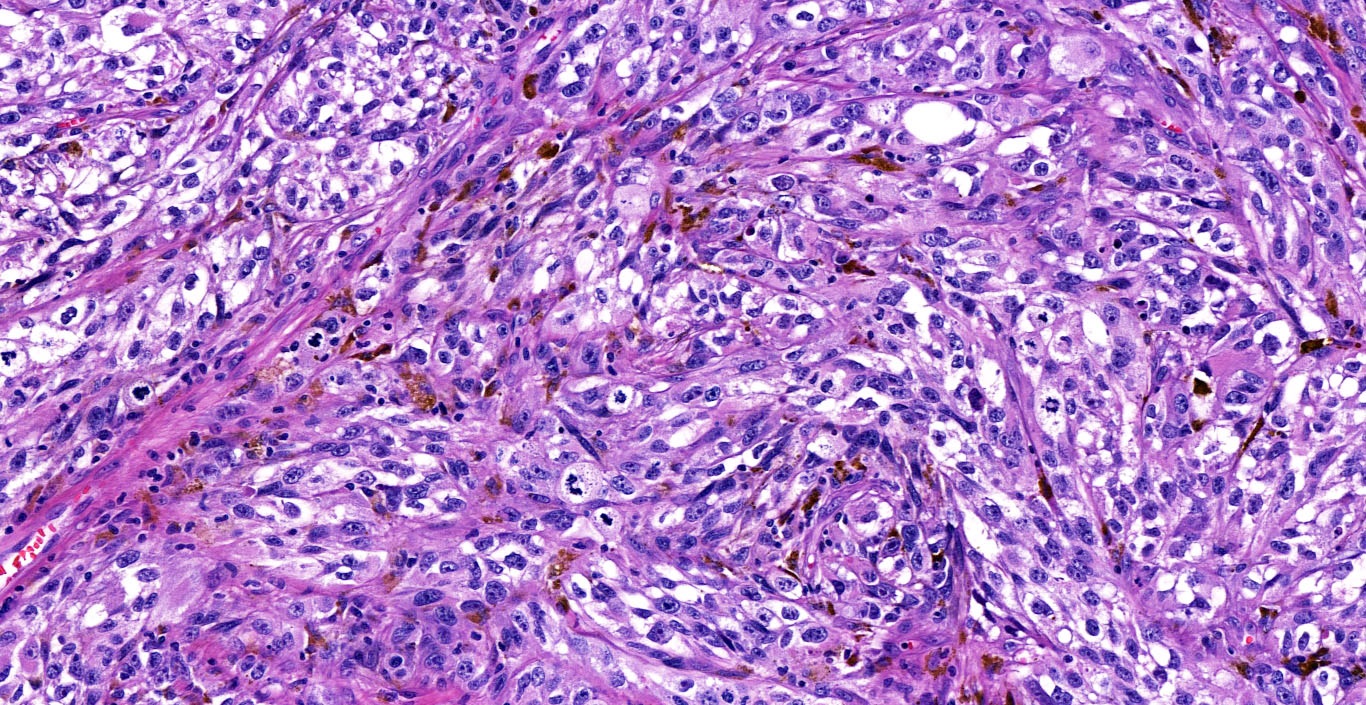
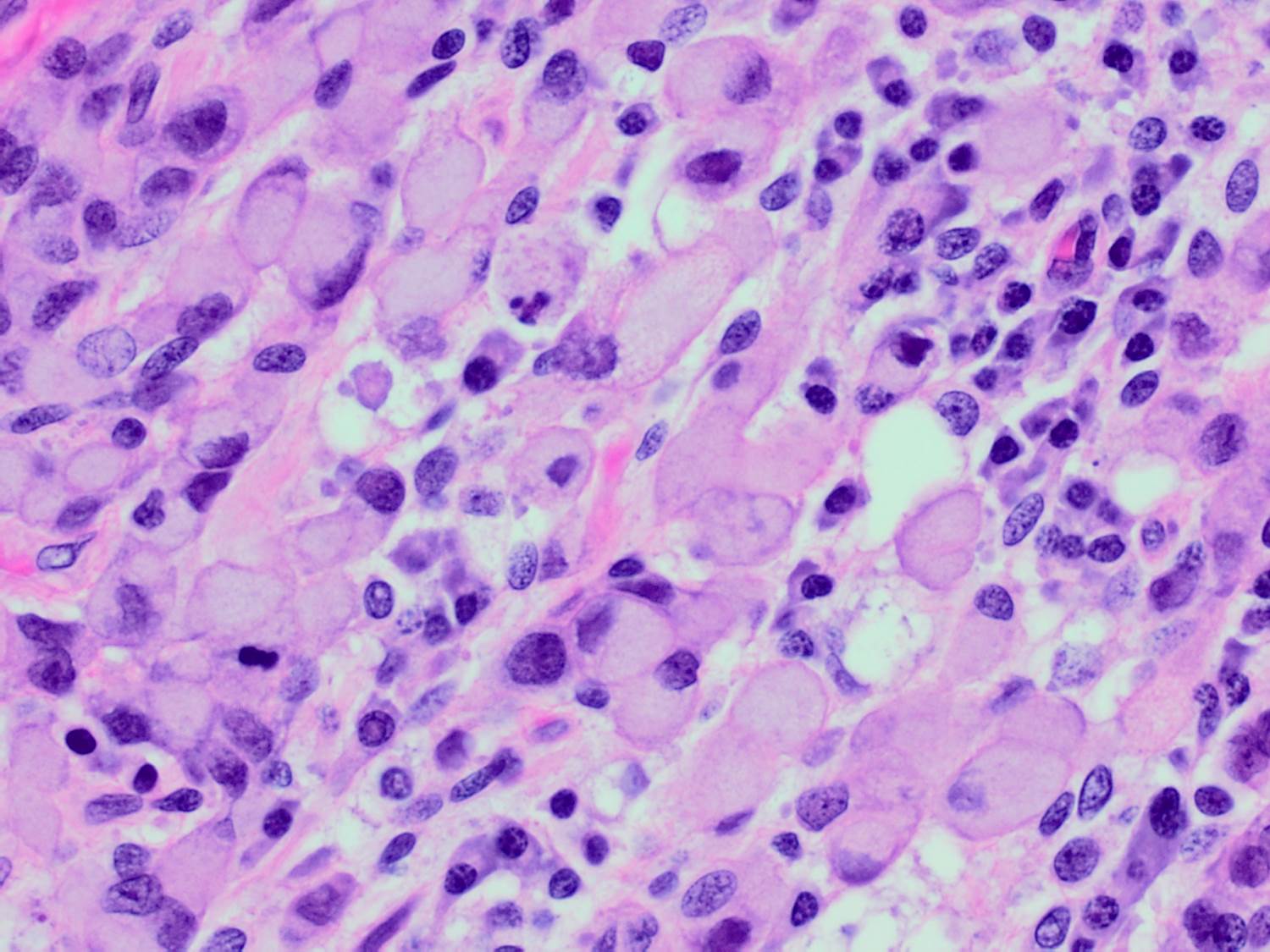
- Original morphologic criteria are still applied to identify PLC
- Defects in cell adhesion produce unique morphology (genotype phenotype correlation): tumor cells are rounded, dyscohesive and lack polarity (Diagnostics (Basel) 2022;12:2658)
- Compared with classic lobular, PLC cells are larger, with greater cytonuclear atypia and higher mitotic count
- Nuclear abnormalities include enlargement (nuclei ≥ 4x the size of lymphocytes), variable size and shape (nuclear pleomorphism / anisonucleosis), abnormal shapes (such as bilobed, multilobed or multinucleation), contour irregularities, aberrant chromatin patterns (hyperchromasia, vesicular or coarse chromatin) and prominent single or multiple nucleoli
- Cytoplasm tends to be more abundant: eosinophilic, granular, foamy or vacuolated
- Atypical mitotic figures, apoptosis and necrosis may be seen
- May show apocrine, histiocytoid and signet ring features (Hum Pathol 1992;23:655, Am J Surg Pathol 2000;24:1650)
- Apocrine: abundant eosinophilic coarsely granular or vacuolated cytoplasm, usually with distinct cell borders, enlarged nuclei with prominent eosinophilic nucleoli, resembling apocrine sweat glands (Hum Pathol 1992;23:655)
- Histiocytoid: ample, pale, delicate, finely vacuolated to granular cytoplasm, small, dark to vesiculated and eccentrically placed nuclei and inconspicuous nucleoli; resembling histiocytes (Am J Surg Pathol 1995;19:553, Ann Diagn Pathol 2002;6:141, Histopathology 1989;14:515)
- Signet ring: intracytoplasmic mucin; single large mucin vacuole with a central eosinophilic globule (most common) or multiple vacuoles giving a foamy appearance (similar to gastric signet ring cell carcinomas), nuclei are indented, lobulated and eccentric
- Plasmacytoid cytomorphology is also seen: round cells with a nucleus located to the side (eccentrically placed) and abundant cytoplasm, coarse chromatin and inconspicuous nucleoli, resembling plasma cells
- Polygonal cells with eosinophilic cytoplasm reminiscent of rhabdomyoblasts are also occasionally found
- In classical growth, tumor cells are dispersed individually and arranged in single files
- Invasion proceeds along the path of least resistance between collagen bundles (linear pattern) and along fibrovascular septa or around ducts and lobules (targetoid pattern)
- Background stroma shows dense fibrosis; there may be no / minimal host response
- Frequent variations in growth include nonclassical (solid, alveolar and mixed) patterns in a single tumor; nonclassical growth patterns are more frequent in tumors with marked nuclear pleomorphism
- May show areas of classical ILC
- Frequently associated with LCIS with classic and pleomorphic features (Am J Surg Pathol 2000;24:1650)
- Usually scored as grade 2 or 3 within the Nottingham grading system, depending on mitotic activity
- Lymphovascular invasion is common
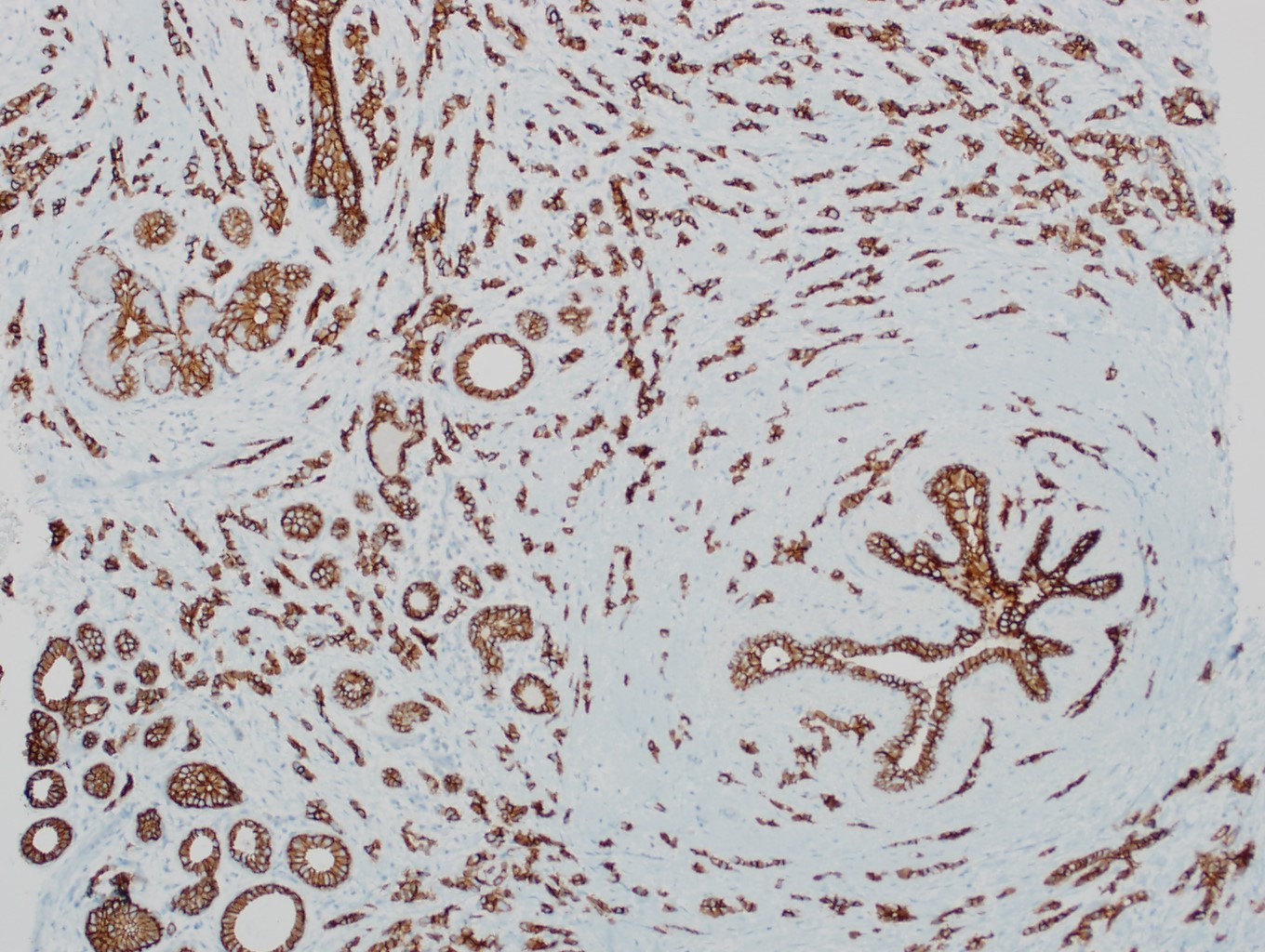
Microscopic (histologic) images
Contributed by Mirna B. Podoll, M.D., Susanne M. Crespo-Ramos, M.D. and Anna Biernacka, M.D., Ph.D.
Virtual slides
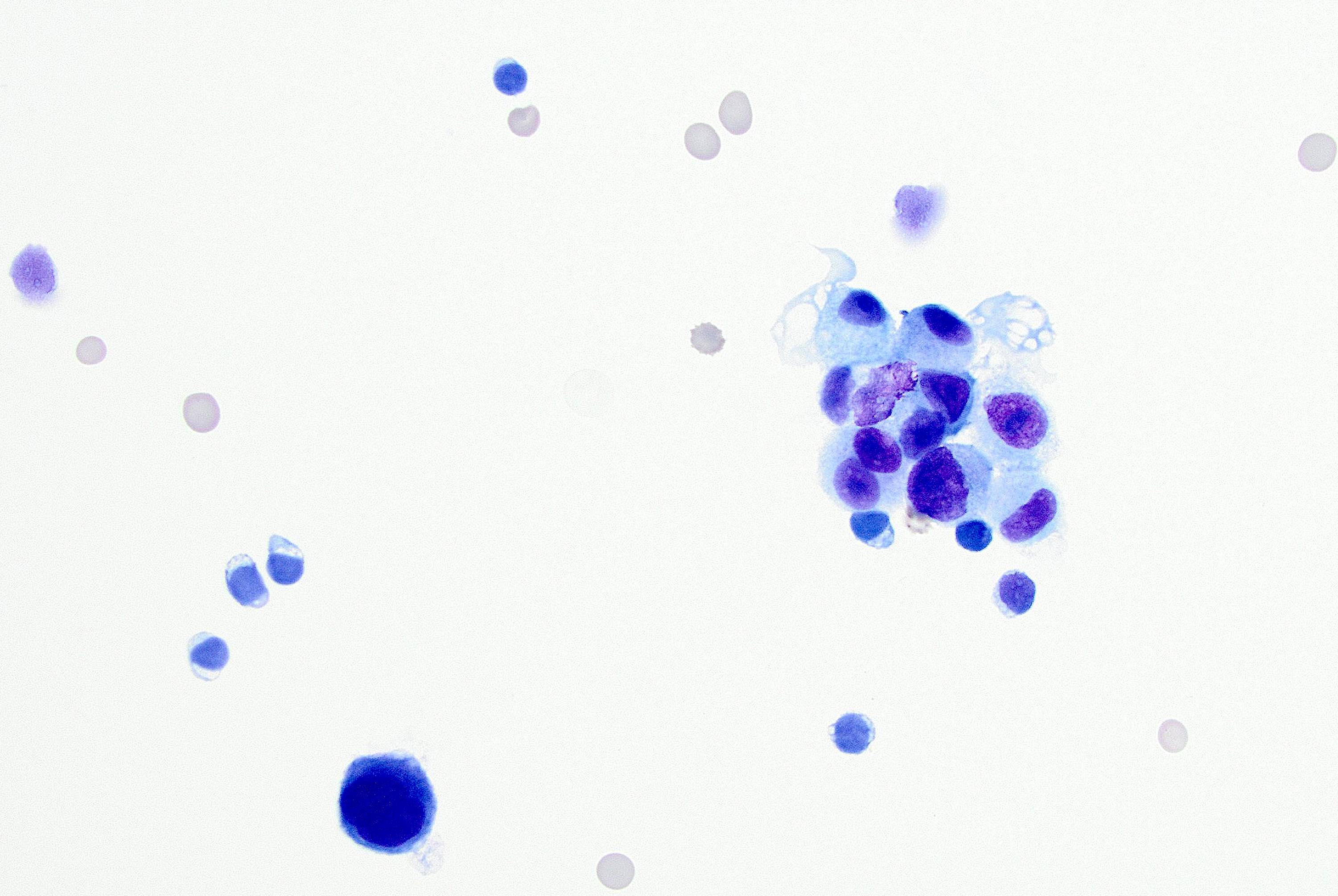
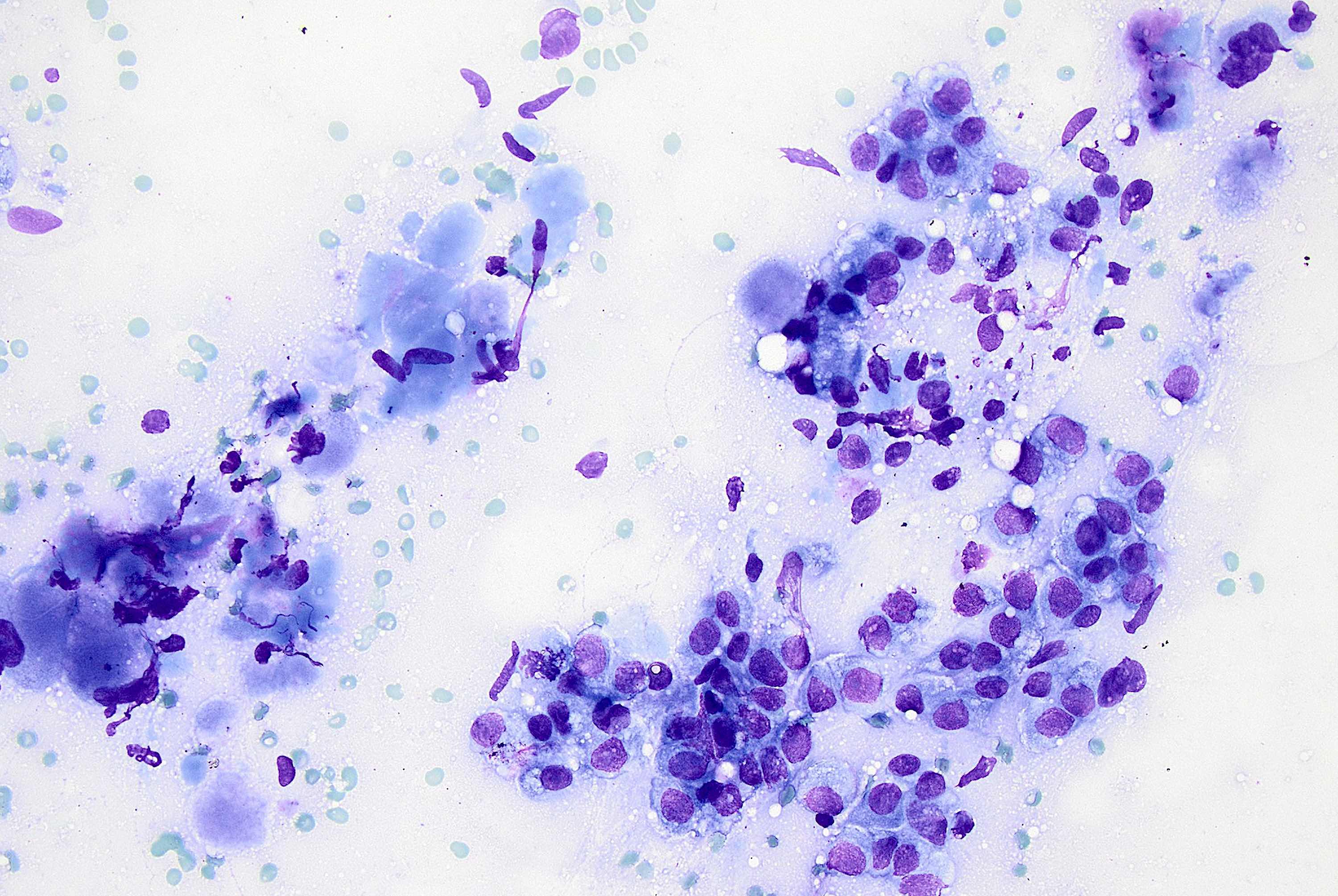
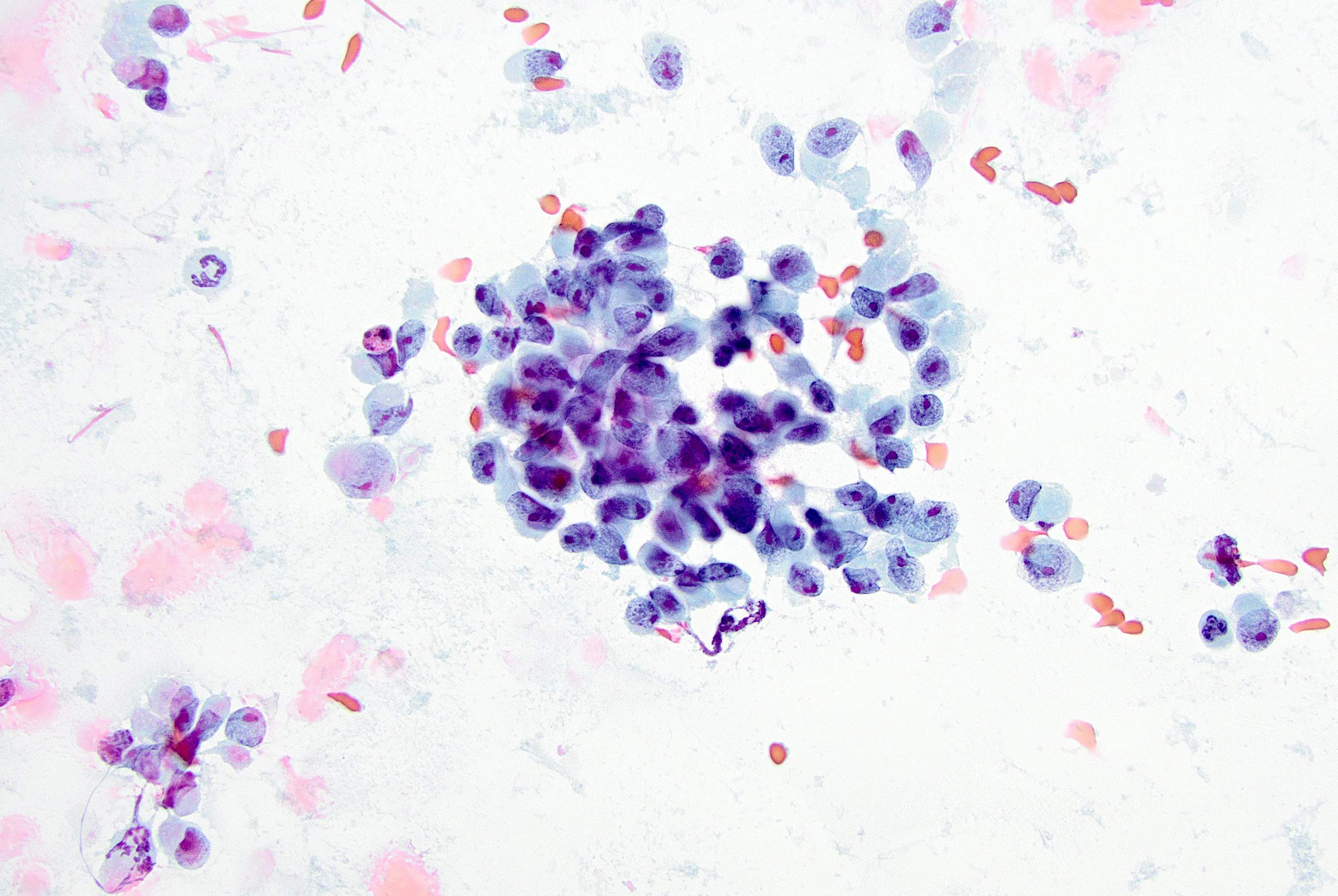
Cytology description
- More cellular smears than classic lobular; large tumor cells with pleomorphic nuclei, prominent nucleoli and cytoplasmic vacuoles (Cancer 1997;81:29)
- Predominantly dyshesive pattern, occasionally forming small, loosely cohesive aggregates
- Cytologic features overlap with high grade IDC (difficult to make a diagnosis prospectively on cytology alone) (Acta Cytol 2002;46:909)
- Tumor cells with apocrine features may resemble atypical mesothelial cells in body fluids (Diagn Cytopathol 2008;36:657)
- Ductal lavage: similar features, although less striking, including epithelial cells in small clusters, single file or isolated; also nuclear atypia, cytoplasmic vacuoles and signet ring features (Acta Cytol 2008;52:207)
Cytology images
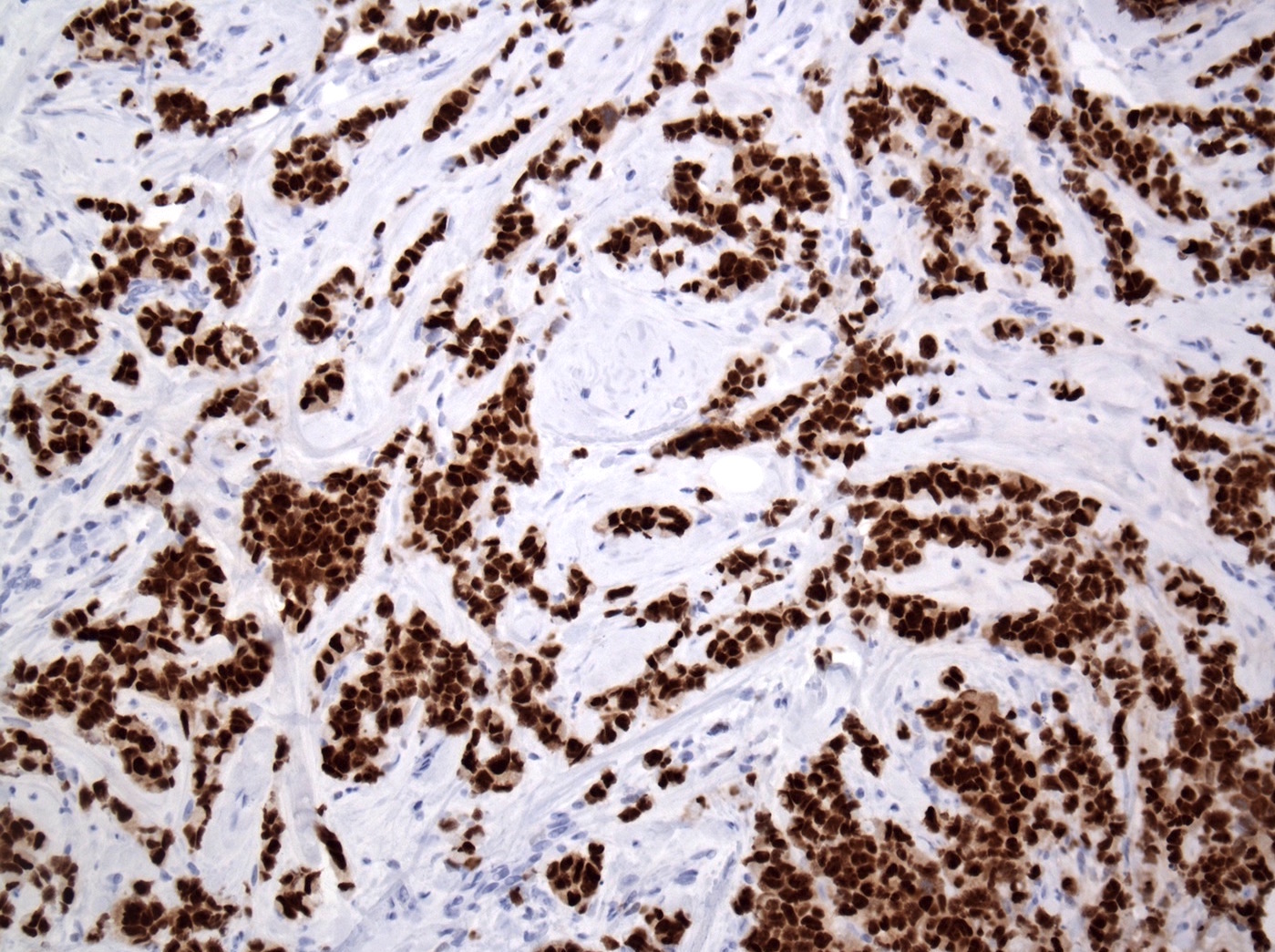
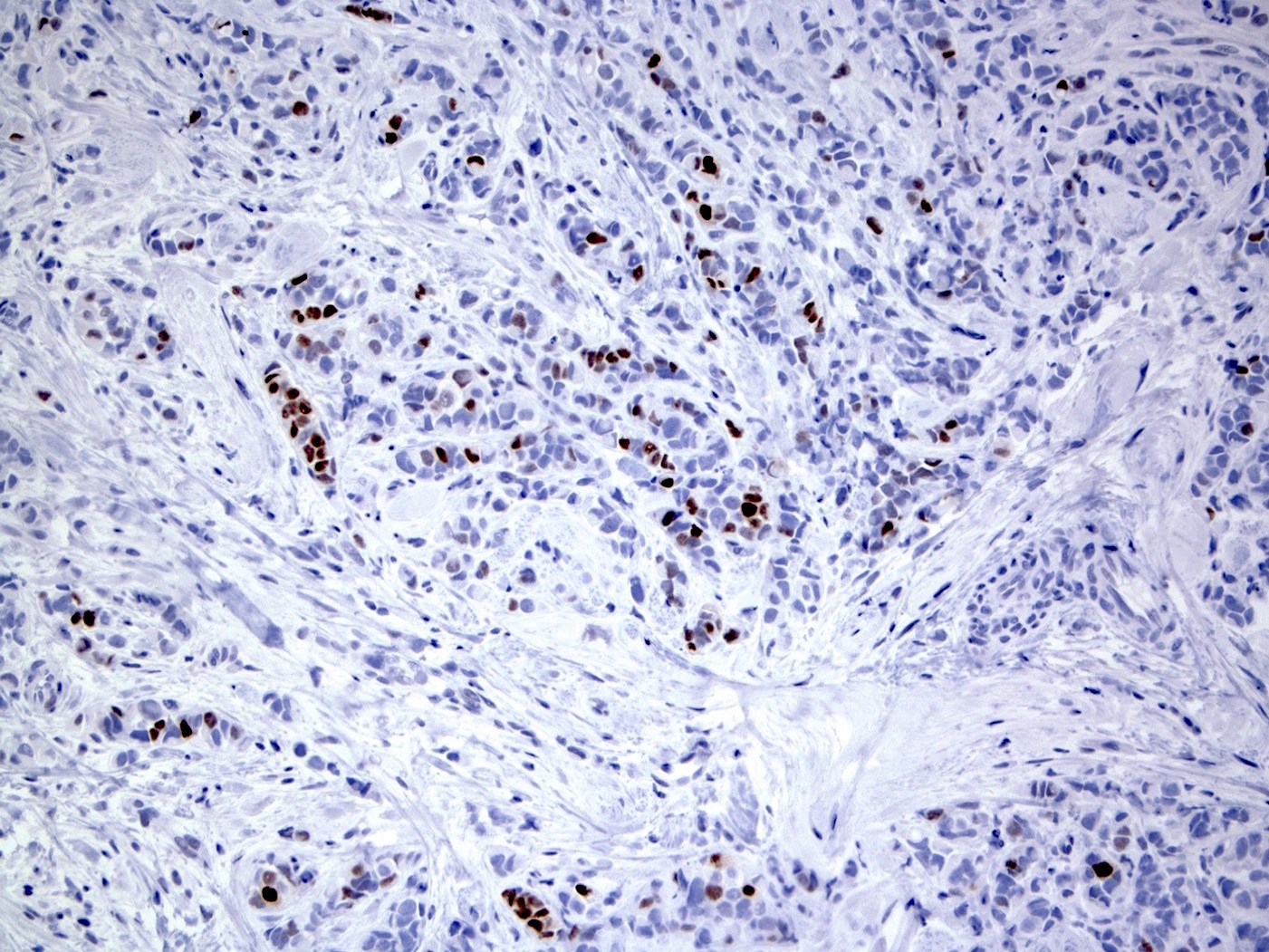
Positive stains
- Variable ER and PR (~5 - 10% are hormone receptor negative)
- HER2: overexpression in 20 - 30%
- Higher Ki67 proliferative index (Ann Diagn Pathol 2012;16:185, Int J Breast Cancer 2020;2020:8816824)
- p53: 20 - 60%
- GCDFP-15: 71%, due to apocrine nature (includes apocrine and histiocytoid morphology) (Hum Pathol 1992;23:655, Virchows Arch 1994;425:459, Ann Diagn Pathol 2002;6:141)
- GATA3 (Diagn Pathol 2017;12:52)
- AR (PLoS One 2020;15:e0235790)
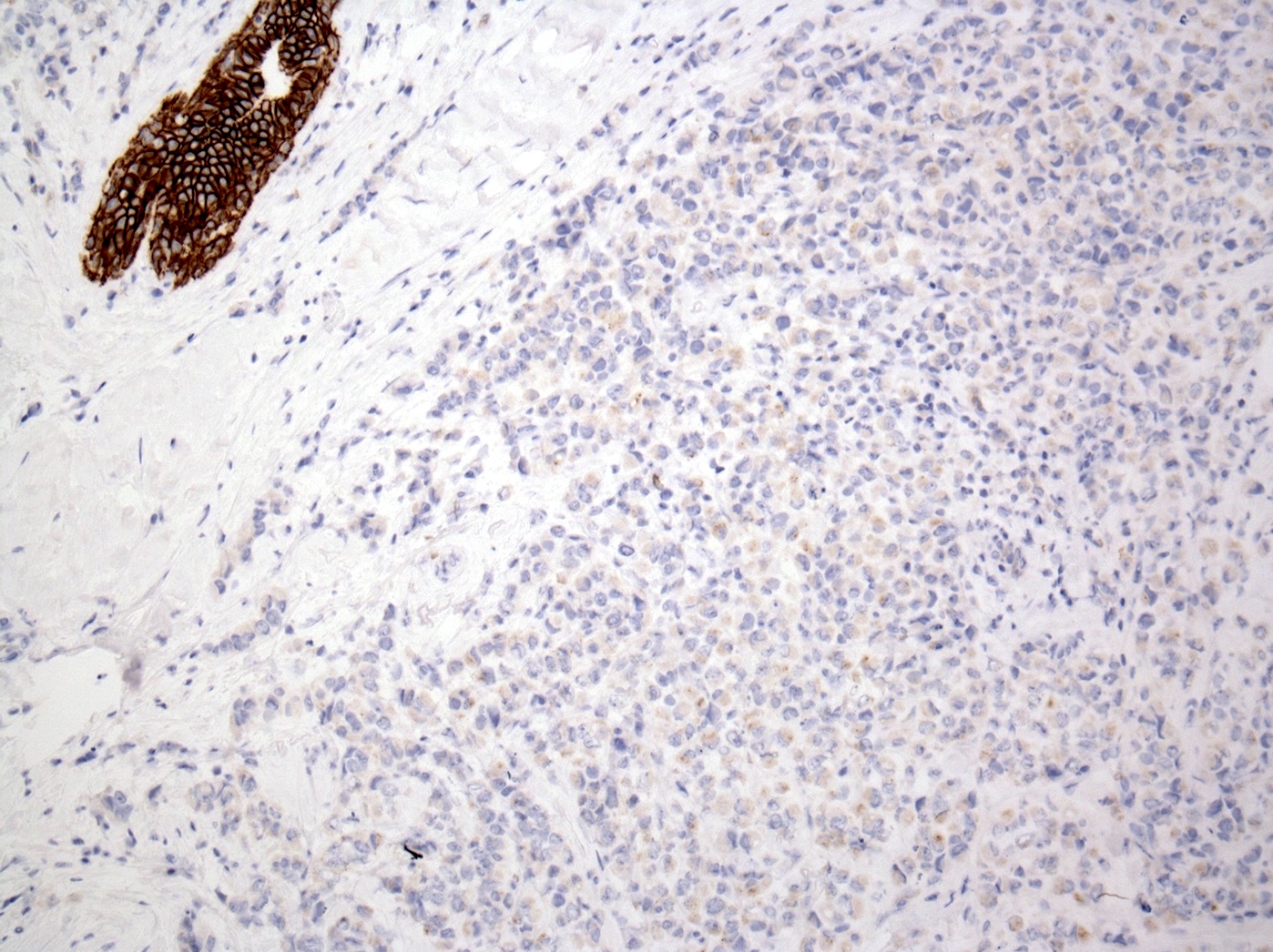
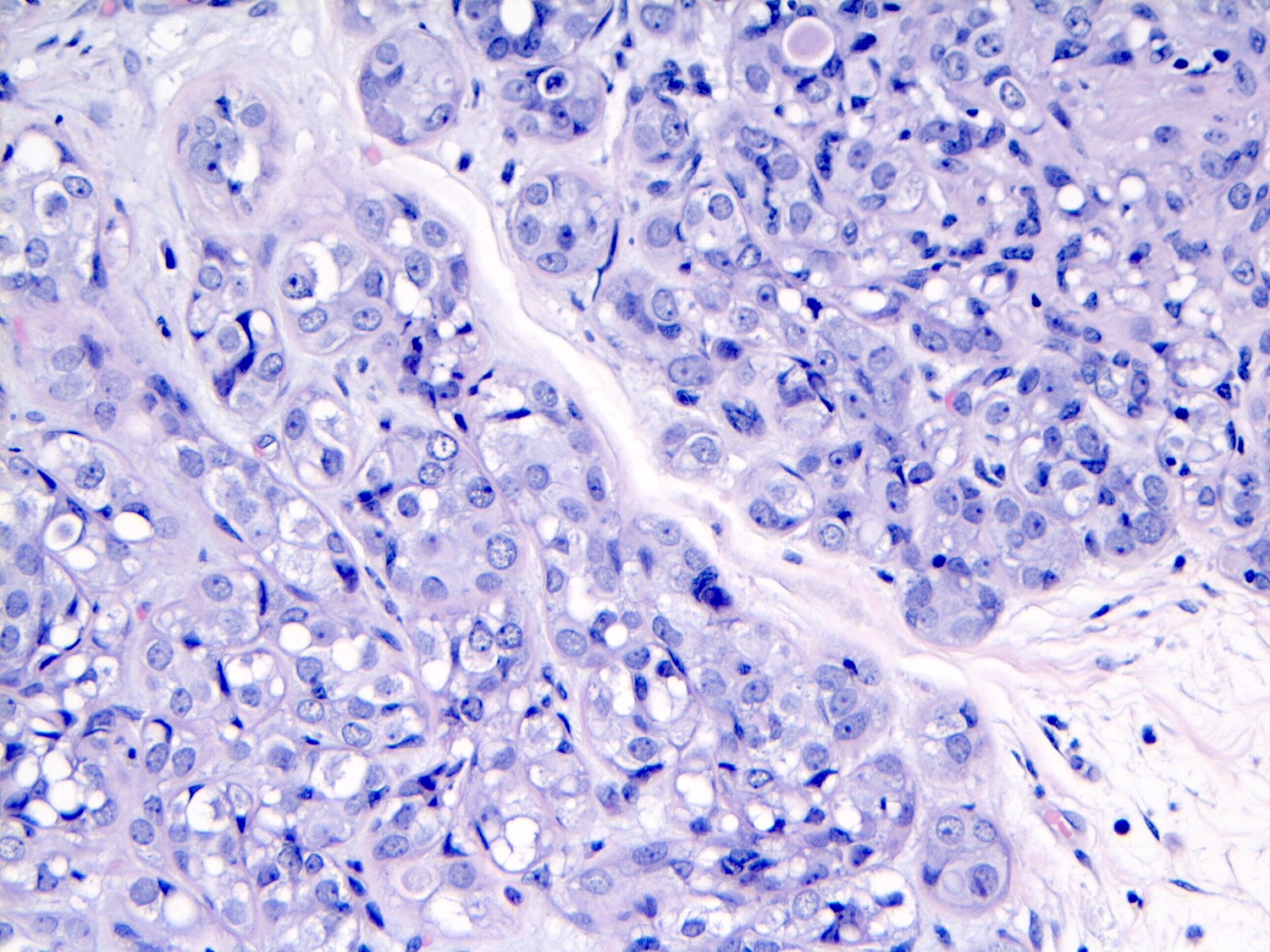
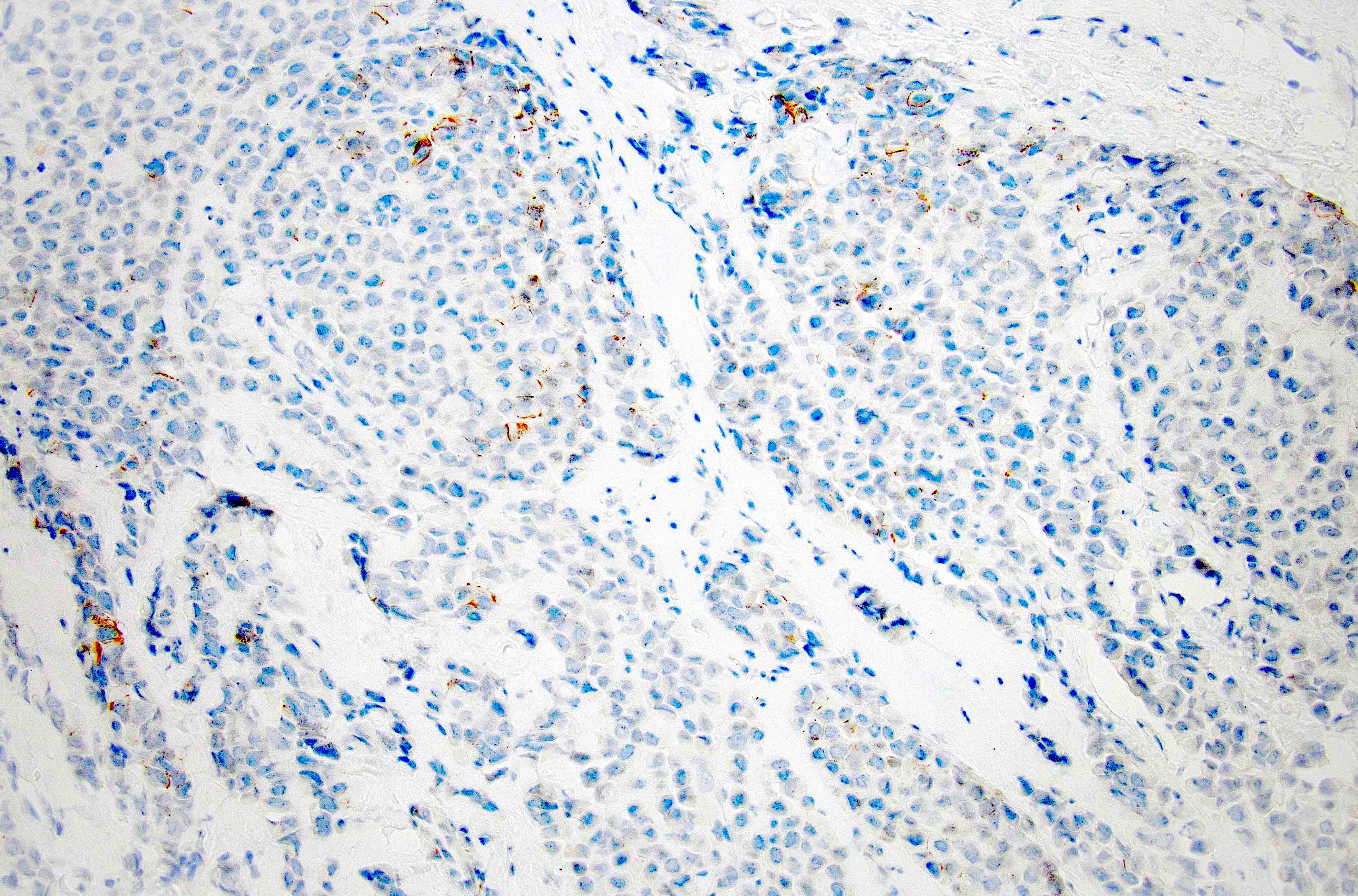
Negative stains
- E-cadherin: ~15% are positive; may be partial / granular on the membrane, cytoplasmic (diffuse or dot-like) or heterogeneous
- Positive staining should not be used to reclassify a lobular lesion as ductal (Am J Surg Pathol 2008;32:773)
- Cases with aberrant or unusual E-cadherin, beta catenin or p120 catenin staining are useful
- Beta catenin (membrane staining absent) (Appl Immunohistochem Mol Morphol 2007;15:260)
- p120: cytoplasmic in most cases (Appl Immunohistochem Mol Morphol 2007;15:260)
- IHC staining can also be useful in the following cases
- 13% are triple negative (Histopathology 2012;61:365)
- Myoepithelial markers (p63, smooth muscle myosin heavy chain, calponin) absent, supporting invasion
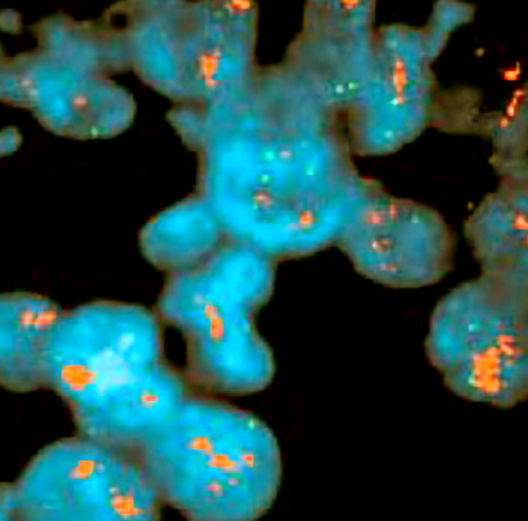
Molecular / cytogenetics description
- Shares molecular alterations with classical lobular and high grade ductal cancers (Future Oncol 2009;5:233)
- Overall, genetically more closely related to lobular lineage and low grade, ER positive neoplasia pathway; acquires high grade alterations during tumor progression
- Similar to classical ILC: ER / PR+, E-cadherin-, CDH1 gene mutation / methylation, highly recurrent gains (1q+ and 16p+) and losses (11q- and 16q-), der(1;16) / der(16)t(1;16) rearrangement (J Pathol 2008;215:231)
- Similar to high grade IDC: HER2+, p53+, frequent gains (8q+ and 17q24-q25+), losses (13q-) and amplification of 8q24 (MYC), 12q14 (MDM2), 17q12 (HER2) and 20q13 (ZNF217) (J Pathol 2008;215:231)
- More frequent TP53 mutations than classic lobular (Cell Oncol (Dordr) 2012;35:111)
- Loss of BRCA2 detected in ~40% of PLC (J Pathol 2008;215:231)
- High frequency of ERBB2 activating mutations and amplifications and activating PIK3CA mutations suggesting potential efficacy of HER2 and PI3K inhibitors (Breast Cancer Res 2021;23:7, Cancers (Basel) 2019;11:74)
- HER2 mutations and amplification / overexpression are mutually exclusive and more frequent in apocrine and ER negative tumors (Breast Cancer Res Treat 2015;150:447)
- Mutations in IRS2 (30% of PLCs) may contribute to an enhanced invasion (JCI Insight 2018;3:e97398)
- Classified less often as luminal A and more often as luminal B, HER2 enriched and triple negative subtype compared with classical ILC and other variants (J Clin Transl Res 2022;8:523, Diagnostics (Basel) 2024;14:660, Breast Cancer Res Treat 2012;133:713)
- Cases with apocrine features and AR positive and ER negative receptor status show molecular apocrine gene signature and belong to molecular apocrine or luminal AR (LAR) breast cancer (Oncogene 2005;24:4660, J Pathol 2008;216:141)
- Oncotype Dx (Breast J 2015;21:514, Int J Breast Cancer 2020;2020:8816824)
- Although PLC has higher recurrence scores (RS) than classical ILC, the majority of cases fall into the intermediate risk category with a small subset of cases showing a high range RS
- In contrast, most classical ILCs are distributed in low risk groups, while the remaining cases have an intermediate risk
- These results suggest that both would likely not derive significant benefit from adjuvant chemotherapy
Sample pathology report
- Breast, right, partial mastectomy:
- Pleomorphic invasive lobular carcinoma, grade III, measuring 0.7 cm in greatest dimension, surgical margins negative (see comment)
- Comment: Immunohistochemical stains are performed and show the tumor cells to be negative for E-cadherin and beta catenin, consistent with a lobular phenotype, supporting the diagnosis.
Differential diagnosis
- Invasive ductal carcinoma with lobular features:
- E-cadherin strongly positive (although ~15% of pleomorphic ILC are E-cadherin positive)
- Lobular carcinoma in situ, pleomorphic:
- Myoepithelial cell layer present (myoepithelial markers positive at the periphery)
- Central comedo type necrosis
- Melanoma:
- Melanin pigment may be present
- Melanocytic markers positive: HMB45, MelanA / MART1
- Cytokeratin negative
- One of the most common metastases to the breast
- Lymphoma:
- Cytokeratin negative
- Lymphoid markers positive
- Less cytoplasm
- Chemotherapy and radiation treatment effect:
- Hypereosinophilic cytoplasm with vacuolization, nuclear enlargement, multinucleation and vesicular chromatin (Schnitt: Biopsy Interpretation of the Breast, 3rd Edition, 2018)
- Gastric signet ring cell carcinoma:
- Can be identical in appearance
- Individuals with germline E-cadherin mutations are at risk for both types of carcinoma (~40 - 50% lifetime risk of breast cancer)
- CDX2 and HepPar1+ and ER, GCDFP-12 and MUC1-
Additional references
Board review style question #1
A 63 year old woman had a mammogram revealing left breast architectural distortion and a left breast biopsy showing the histologic features in the image seen above. An E-cadherin staining was performed and it was negative. Which of the following is the correct diagnosis?
- Classic invasive lobular carcinoma
- Invasive ductal carcinoma
- Mucinous carcinoma
- Pleomorphic invasive lobular carcinoma
- Tubular carcinoma
Board review style answer #1
D. Pleomorphic invasive lobular carcinoma. The tumor cells lack cohesion and invade the fibrotic stroma as single files and single cells. The cells show marked nuclear pleomorphism, similar to that seen in high grade ductal carcinoma and brisk mitotic activity. There is abundant eosinophilic cytoplasm with some intracytoplasmic mucin vacuoles. Answer B is incorrect because E-cadherin is negative and there is no gland formation. Answer E is incorrect because the nuclei are high grade and there are no well formed tubules with pointed ends. Answer A is incorrect because the tumor cells are larger and show more nuclear pleomorphism. Answer C is incorrect because there are no small pools of extracellular mucin.
Comment Here
Reference: Pleomorphic lobular carcinoma
Comment Here
Reference: Pleomorphic lobular carcinoma
Board review style question #2
Which of the following is true regarding the pleomorphic variant of invasive lobular carcinoma (ILC)?
- Aggressive nature of these tumors is reflected by the pleomorphic variant designation
- Do not show HER2 overexpression
- Frequency of p53 expression is similar to that of classic ILC
- High histologic grade contributes to poor patient outcomes
- Usually hormone receptor negative
Board review style answer #2
D. High histologic grade contributes to poor patient outcomes. Studies show that the pleomorphic variant of ILC is likely to have a higher histologic grade, extracapsular extension and lymphovascular invasion, leading to poorer patient outcomes. Answer A is incorrect because the aggressive nature of the pleomorphic variant of ILC and poorer outcomes are related to the high histologic grade of this variant rather than the pleomorphic designation itself. Answer E is incorrect because while these tumors may be ER and PR negative in ~5 - 10% of cases, the majority are hormone receptor positive. Answer B is incorrect because HER2 expression can be seen in ~20% of cases. Answer C is incorrect because the pleomorphic variant of ILC more commonly shows p53 expression compared with the classic type ILC.
Comment Here
Reference: Pleomorphic lobular carcinoma
Comment Here
Reference: Pleomorphic lobular carcinoma