Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Cite this page: Vickery J, Biernacka A. Lobular carcinoma in situ (LCIS) classic. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantlcis.html. Accessed April 3rd, 2025.
Definition / general
- Noninvasive, neoplastic proliferation of small, uniform, dyscohesive cells, which originates in the terminal duct lobular unit (TDLU) and fills and distends most of the acini of the involved lobule (Histopathology 2020;77:181)
Essential features
- Lobulocentric proliferation of monomorphic cells, which expands lobular units; with or without pagetoid involvement of terminal ducts
- Hallmark feature is loss of cellular cohesion due to dysfunctional E-cadherin catenin axis
- Loss of E-cadherin protein is the most common etiology (> 95%) and can be caused by mutations, deletions or methylation
- Aberrant E-cadherin expression can be observed in some cases
- Characterized by uniform, loosely cohesive and evenly spaced cells (marbles in a bag); cells are slightly larger than normal, with indistinct cell borders and pale cytoplasm, small uniform nuclei, evenly distributed chromatin and inconspicuous nucleoli
- Constitutes both a risk factor and nonobligate precursor of invasive breast cancer
- No longer classified as a tumor in situ (Tis) because it is considered a risk factor, not a malignancy (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
Terminology
- Lobular neoplasia (LN) of the breast includes atypical lobular neoplasia (ALH) and lobular carcinoma in situ (LCIS)
- Distinction between ALH and LCIS is the percentage of the TDLU involved (> 50% in LCIS and < 50% in ALH) with acini that are filled and expanded to qualify as LCIS
- Some authors use LN terminology to denote the histologic overlap and frequent coexistence of ALH and LCIS (Cancer 1978;42:737)
- This approach is not recommended as it combines into one category lesions, which (although parts of a spectrum) differ in the magnitude of the associated breast cancer risk (Tokai J Exp Clin Med 1989;14:361)
- Classic LCIS (CLCIS) consists of type A or B epithelial cells, which have different morphologic characteristics
- Other morphologic subtypes of LCIS that are nonclassical include pleomorphic and florid
ICD coding
Epidemiology
- Identified in 0.5 - 3.6% of benign breast biopsies and in 1.8 - 2.5% of all breast biopsies (Surg Pathol Clin 2018;11:123, Histopathology 2020;77:181)
- Incidentally found in 0.04 - 1.5% of reduction mammoplasty specimens (Breast Cancer Res Treat 2019;173:201, Hum Pathol 2013;44:1877)
- Incidence has varied over time (Ann Surg Oncol 2013;20:3240, Breast Cancer Res Treat 2002;75:259)
- Rapid increase after the introduction of mammography (1978 - 1998)
- Sharp decline associated with decreased use of hormone replacement therapy (2001 and 2004)
- In 2009, the estimated rate was 2.75 per 100,000
- Full age range of affected women is broad (27 - 83 years) but it is most often diagnosed in premenopausal women (J Clin Oncol 2015;33:3945)
- Mean age at diagnosis is 49 - 53.7 years (Ann Surg Oncol 2017;24:2509, AJR Am J Roentgenol 1991;157:257)
Sites
- Frequently found as multiple foci within the same or both breasts
- Multicentric in the same breast in up to 70% of patients (AJR Am J Roentgenol 1991;157:257)
- Bilateral in 20 - 60% of patients (Ann Surg 1972;176:178, Cancer 1984;53:798)
- May arise in ectopic breast tissue but this is incredibly rare (Gynecol Oncol 1999;73:155)
Pathophysiology
- E-cadherin gene mutations and loss of the wild type allele by loss of heterozygosity (LOH) is an early genetic event that plays a critical role in pathogenesis (Br J Cancer 1997;76:1131)
- E-cadherin mediates intracellular adhesion and cell polarity and plays a key role in the maintenance of lobular architecture; it can also inhibit the growth and invasion of breast cancer cells (Breast Cancer Res 2001;3:289)
- Defective E-cadherin results in loss of cell to cell cohesion, increased cell proliferation and altered organization of the lobules, giving rise to the characteristic appearance of lobular neoplasia
Etiology
- Many risk factors for LCIS are similar to those established for DCIS and invasive breast carcinoma
- Exposure to hormones likely plays a role in etiology; the majority of LCIS shows a strong diffuse expression of ER
- Increased risk of LCIS is associated with a family history of breast cancer, a previous benign breast biopsy, nulliparity, older age at first full term birth, older age at menopause and high mammographic density (J Natl Cancer Inst 2001;93:1811, Cancer 2015;121:1369)
- In postmenopausal women, the risk of LCIS increases with the use of unopposed estrogen replacement therapy (Am J Epidemiol 2017;186:1329)
Clinical features
- No specific reproducible clinical or imaging findings
- Usually identified in biopsy or surgical excision specimen performed for other indications (Am J Pathol 1941;17:491)
- Rare cases (< 2%) of classic LCIS can be targeted for biopsy due to associated imaging abnormalities; this includes grouped amorphous or granular calcifications on mammography, a shadowing, avascular, irregular, hypoechoic mass on the ultrasound or heterogeneous nonmass-like enhancement with persistent enhancement kinetics on MRI (Breast 2016;27:109, Acad Radiol 2013;20:463)
- Uncommonly, after biopsy for a palpable mass or nipple discharge, patients may be found to have LCIS (J Clin Oncol 2015;33:3945)
Diagnosis
- Diagnosis of classic LCIS is usually an incidental finding in a breast biopsy performed for other indications, including screen detected calcifications or mass producing lesions (Breast Cancer Res 2003;5:258)
- Columnar cell lesions (frequently associated with LCIS) often have calcifications; these are the targets on mammography leading to the discovery of LCIS (Am J Surg Pathol 2007;31:417)
- Cases of LCIS or DCIS are distinguished based on histomorphology or by using E-cadherin and p120 catenin immunostains (Int J Clin Exp Pathol 2014;7:2551)
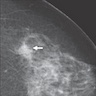
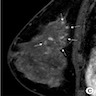
Radiology description
- There are no radiologic features specific to classic LCIS
- In a retrospective radiologic review of patients that were diagnosed pathologically with only classic LCIS, the majority had no abnormalities on mammography; few patients had masses or calcifications (J Clin Ultrasound 2011;39:59)
- Generally, the sensitivity of ultrasound is low; however, when present, associated sonographic findings include an avascular, irregularly shaped, ill defined, hypoechoic mass with posterior shadowing (Acad Radiol 2013;20:463)
- When detected via MRI, the most common finding was heterogeneous nonmass-like enhancement with delayed persistent enhancement kinetics (Acad Radiol 2013;20:463)
- Microcalcifications are not commonly seen in LCIS but can be present (AJR Am J Roentgenol 2001;176:1255)
- In one study, small focal calcifications were associated with LCIS on biopsy in up to 20% of patients (Cancer 2013;119:1073)
- Calcifications are more common in lesions that may coexist with LCIS, such as sclerosing adenosis, columnar cell change / hyperplasia, atrophic lobules and ducts and collagenous spherulosis
Radiology images
Prognostic factors
- LCIS is both a risk factor for and a nonobligate precursor of invasive breast carcinoma
- Compared with the general population, women with LCIS have a 7 - 10 fold increase in breast cancer risk (N Engl J Med 1985;312:146)
- Absolute risk is ~1 - 2% per year for lifetime risk of 30 - 40%
- Slightly greater risk to ipsilateral than contralateral breast
- Time to cancer from LCIS diagnosis ranges 15 - 30 years
- Subsequent invasive carcinomas are 3x more likely to be lobular (than in the general population) but most commonly are of no special type
- Clinical and histologic features that may identify patients with LCIS that are likely to develop invasive breast cancer have not been consistently identified
- Presence of LCIS at (or close to) the margin of resection is not associated with increased local recurrence (Ann Surg Oncol 2008;15:2263)
- Concurrent lobular neoplasia increases the risk of ipsilateral breast cancer recurrence in patients with ductal carcinoma in situ treated with breast conserving therapy (Cancer 2009;115:1203)
Case reports
- 45 year old woman with LCIS within a fibroadenoma of the breast (Postgrad Med J 1999;75:293)
- 47 year old woman with axillary lymph node metastasis of breast cancer after risk reducing mastectomy, in which only LCIS was present on histopathology (J Surg Case Rep 2012;2012:2)
- 64 year old woman with LCIS presenting as a solid mass (Br J Radiol 2011;84:e48)
Treatment
- Excision is not recommended after incidental diagnosis of classic LCIS by percutaneous image guided core needle biopsy, as the risk of finding invasive carcinoma on excision is very low (< 5%) (Radiology 2013;269:340, Mod Pathol 2008;21:1208, Cancer 2013;119:1073, Cancer 2008;112:2152, Ann Surg Oncol 2016;23:722, Ann Surg Oncol 2012;19:3131)
- Surgical biopsy is recommended when core needle biopsy contains classic LCIS in addition to other high risk proliferative lesions (e.g., radial scar or ADH or there is discordance between histology and imaging) (AJR Am J Roentgenol 1999;173:291, Clin Breast Cancer 2016;16:507)
- Management options for classic LCIS include active surveillance and chemoprevention with anti estrogens (J Clin Oncol 2013;31:2942)
- Clinical trials with anti estrogens have shown a 56% reduction in risk of developing subsequent carcinoma in patients with LCIS and other risk factors (e.g., positive family history) (Recent Results Cancer Res 2009;181:113)
- Identification of nonclassical LCIS in a needle core biopsy specimen mandates surgical excision (Ann Surg Oncol 2019;26:55, Am J Surg Pathol 2019;43:399, Arch Pathol Lab Med 2010;134:1024)
- Mirror biopsy of the contralateral breast, once advocated for treatment of LCIS, is no longer routinely performed
- Obtaining negative surgical margins in excision specimens for classic LCIS is not necessary or recommended
- In patients who are BRCA1 or BRCA2 gene mutation carriers, prophylactic mastectomy is the most effective single intervention for overall survival (Avicenna J Med 2018;8:67)
Gross description
- Classic LCIS by itself does not cause any grossly visible / palpable alteration in breast tissue
- May have abnormal findings secondary to the coexisting lesions / proliferative changes (e.g., cysts, firm / hard tissue or nodularity, which are not attributable to LCIS)
Frozen section description
- Due to the relatively bland cytological features of classic LCIS frozen section, diagnosis is generally not performed
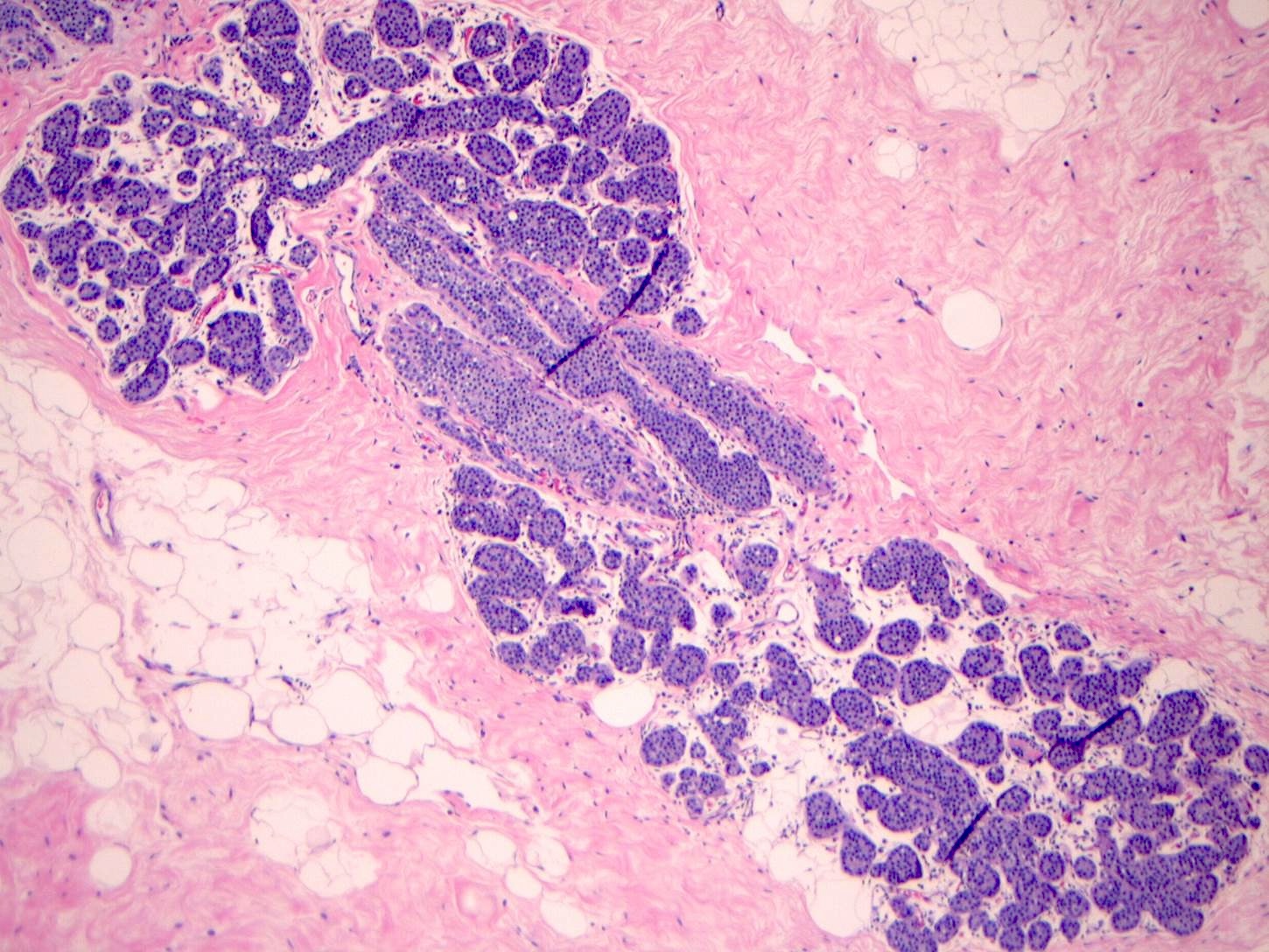
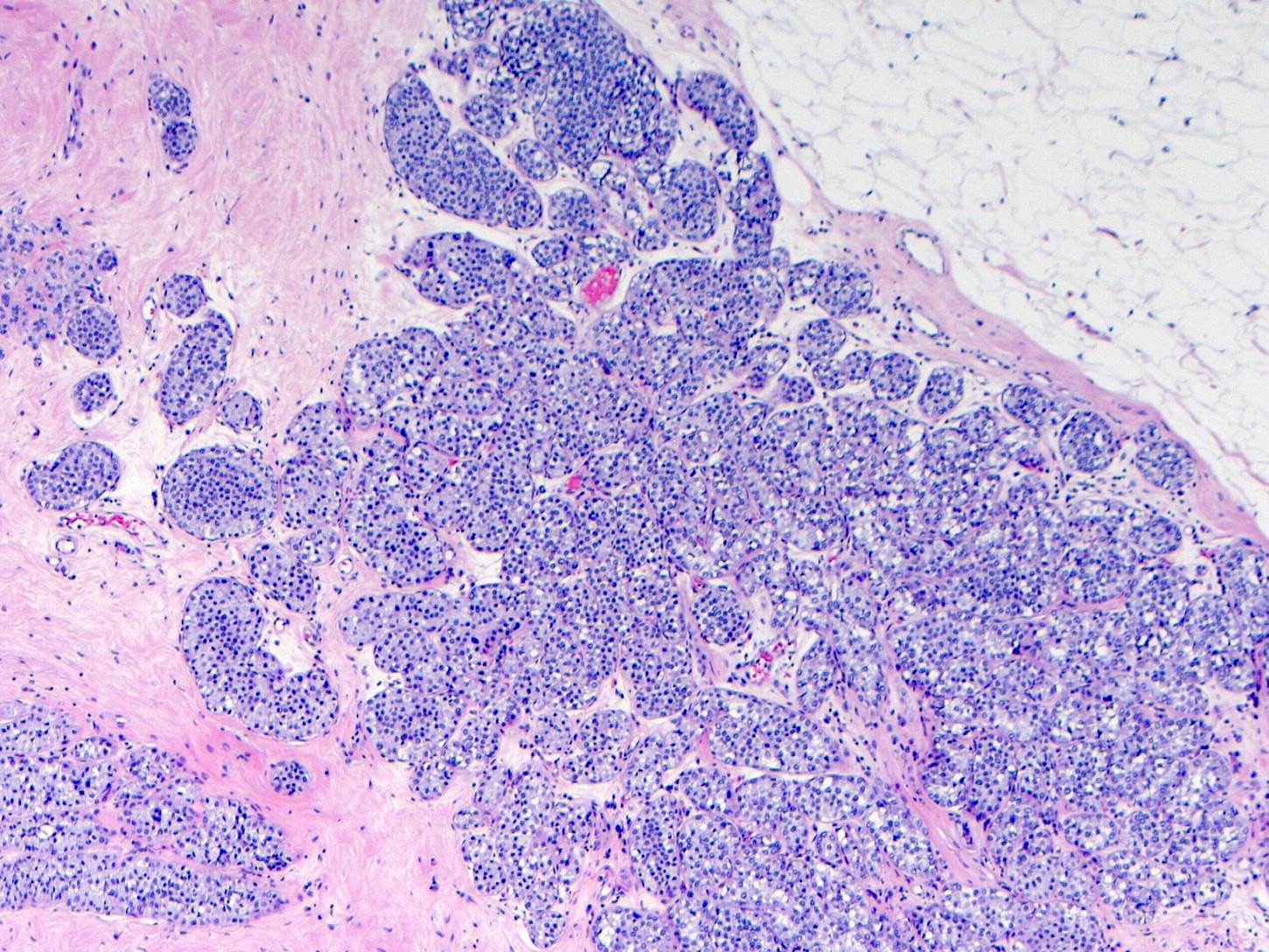
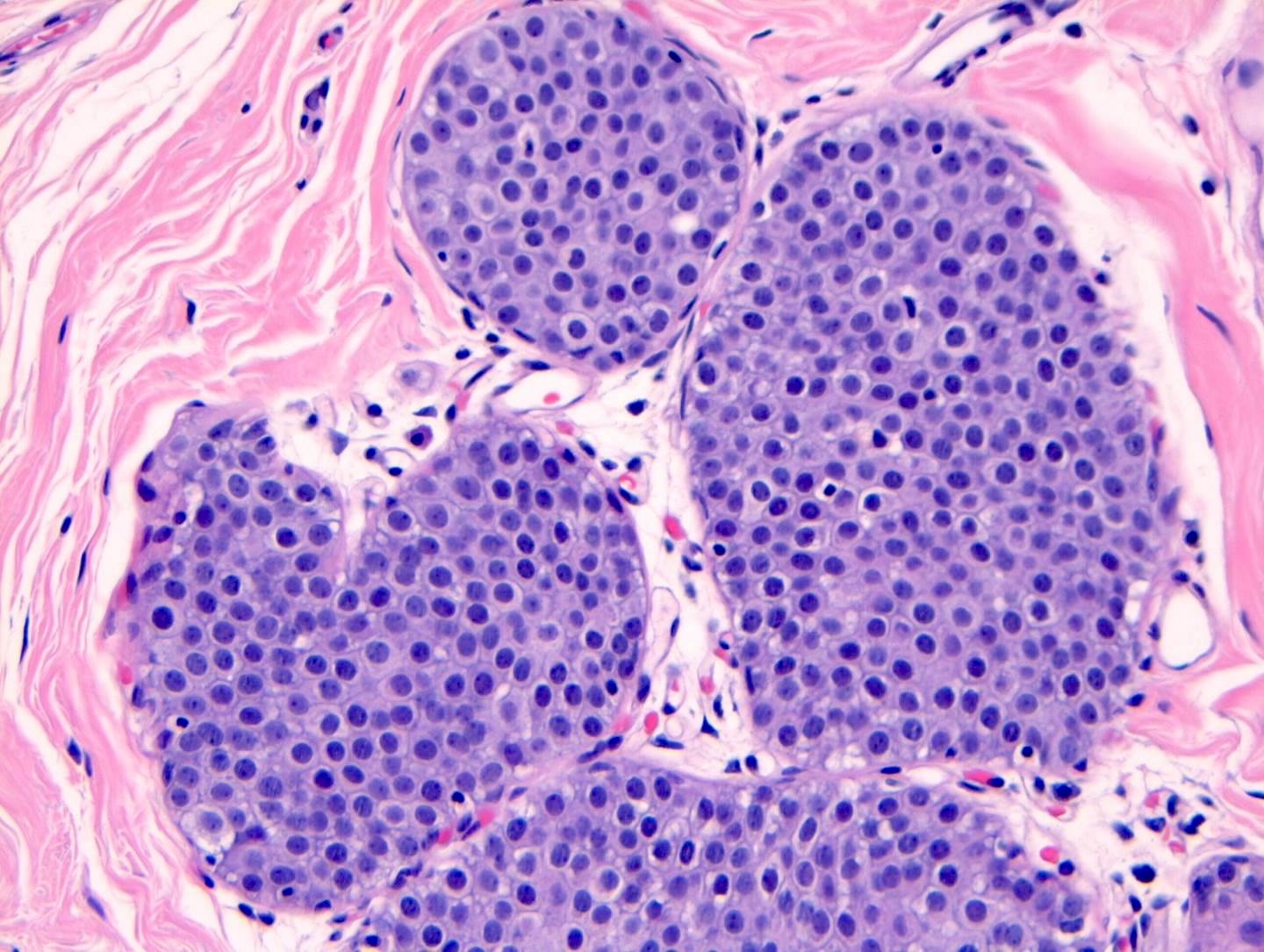
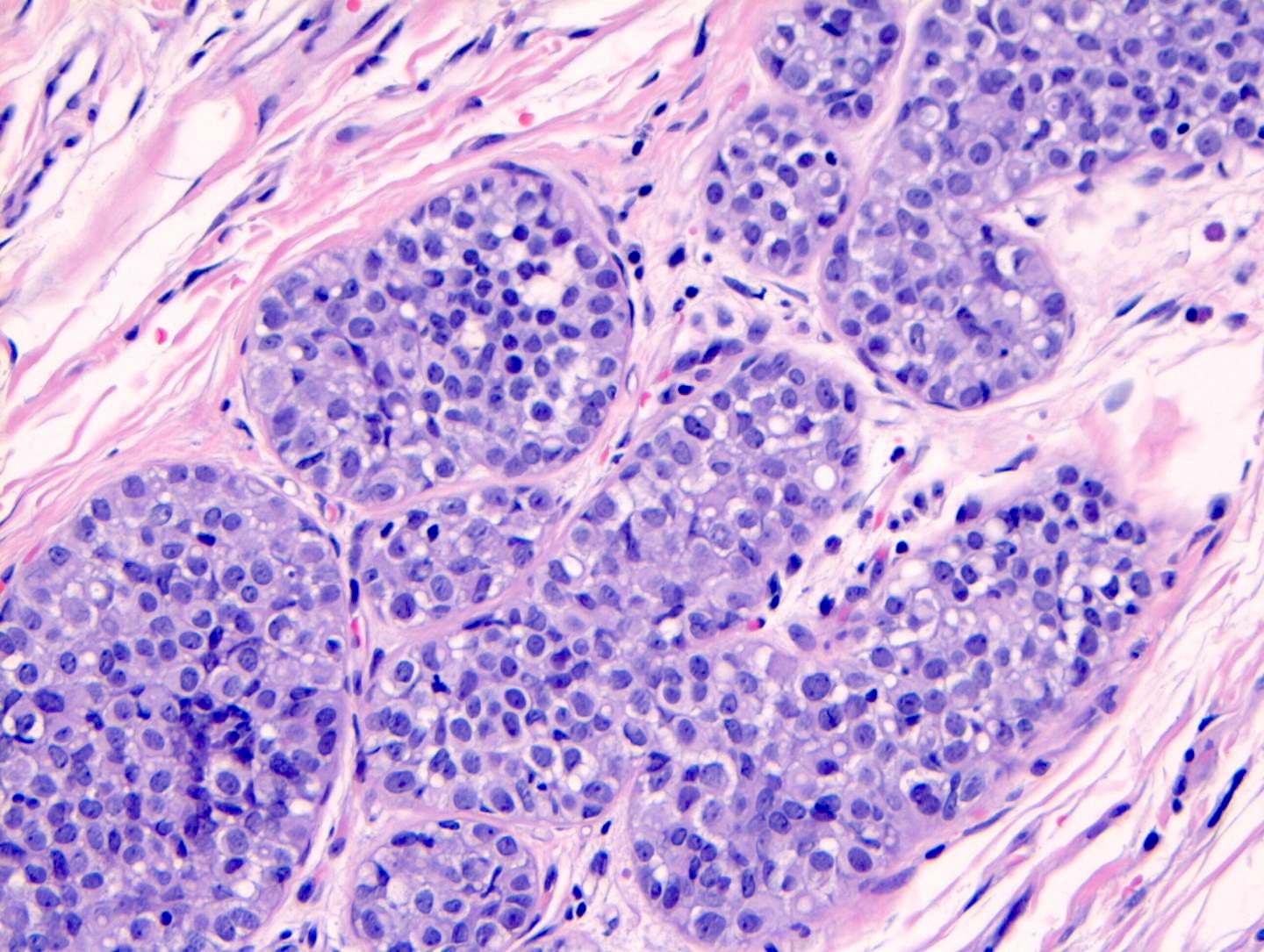
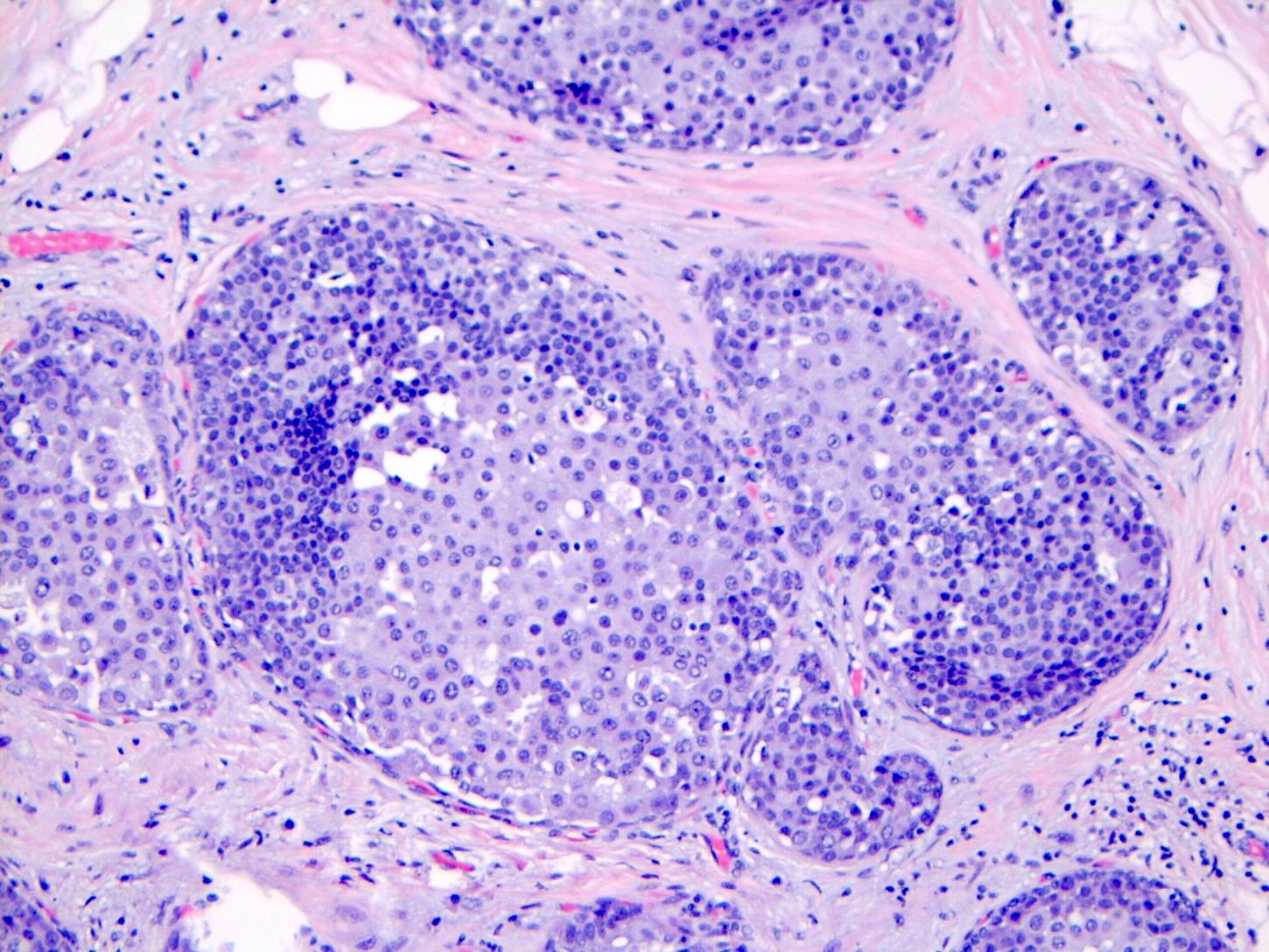
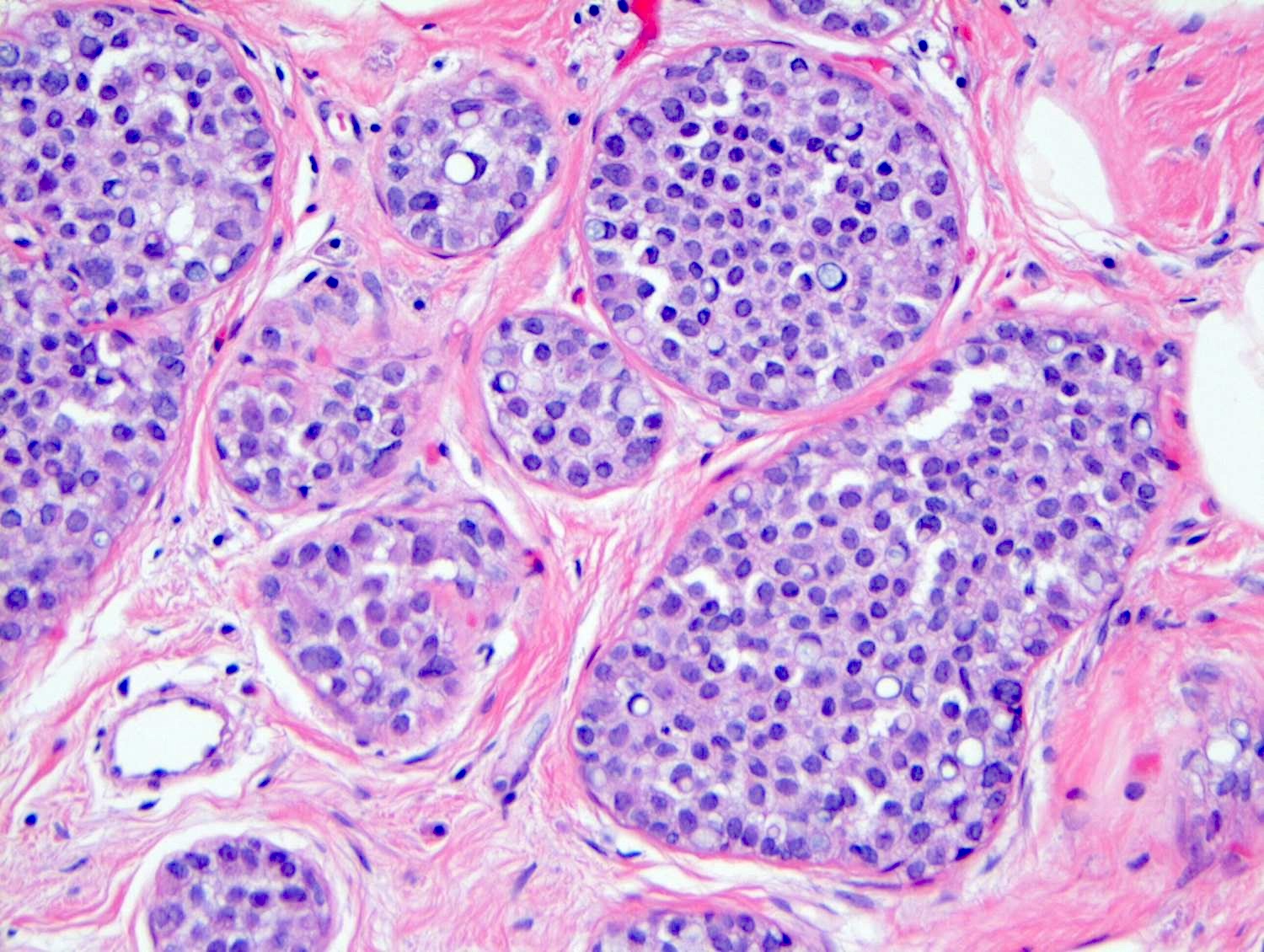
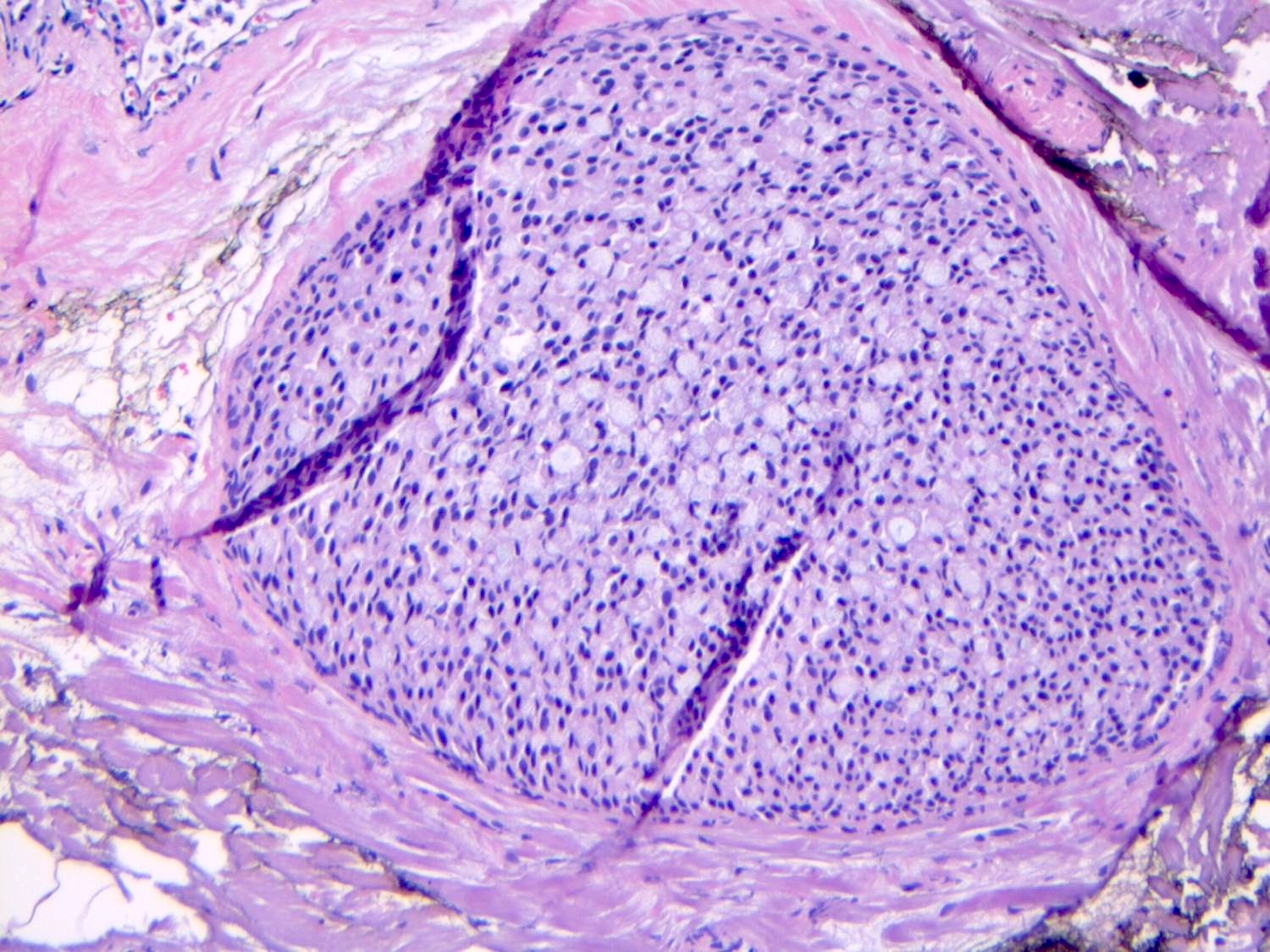
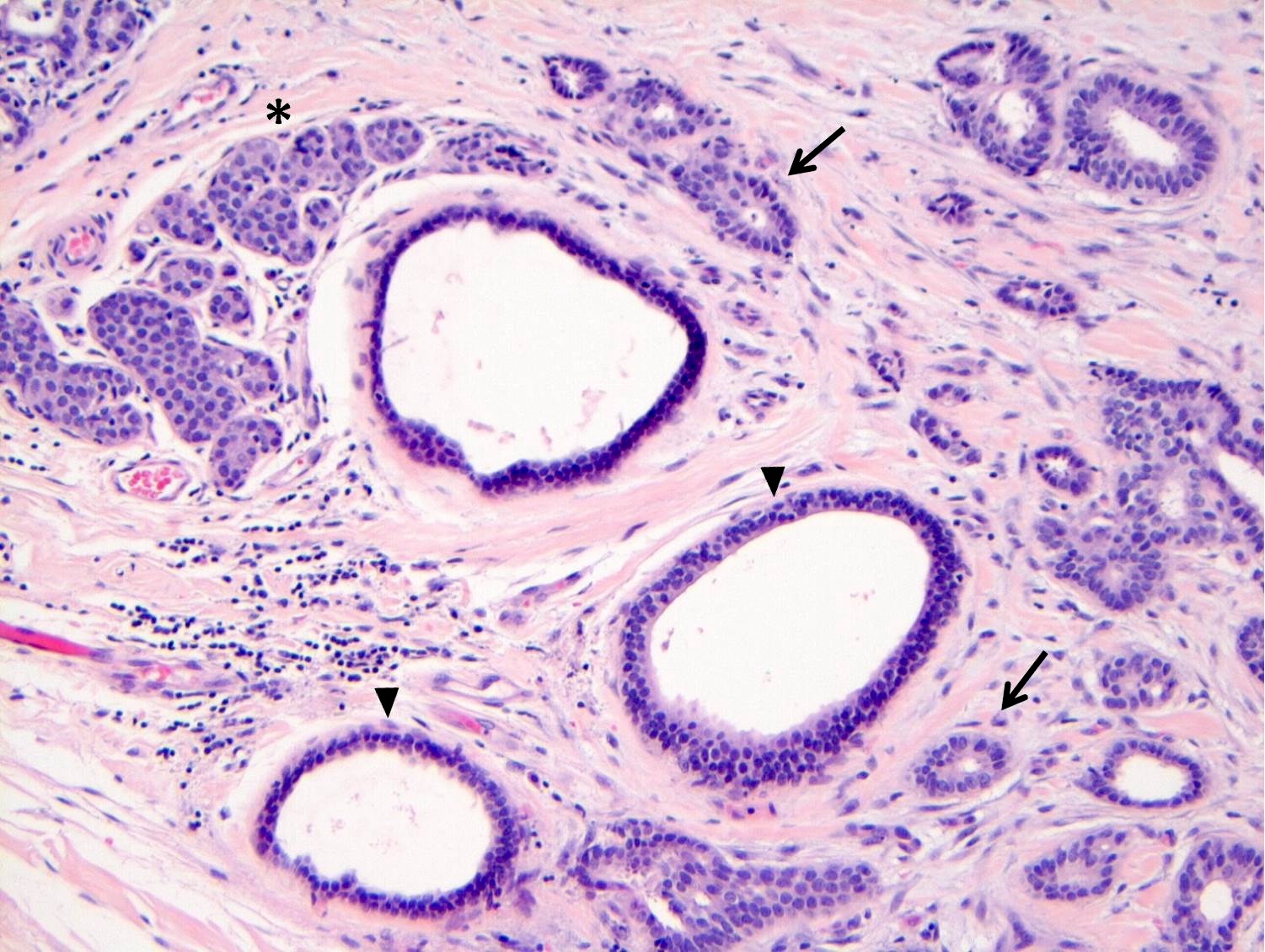
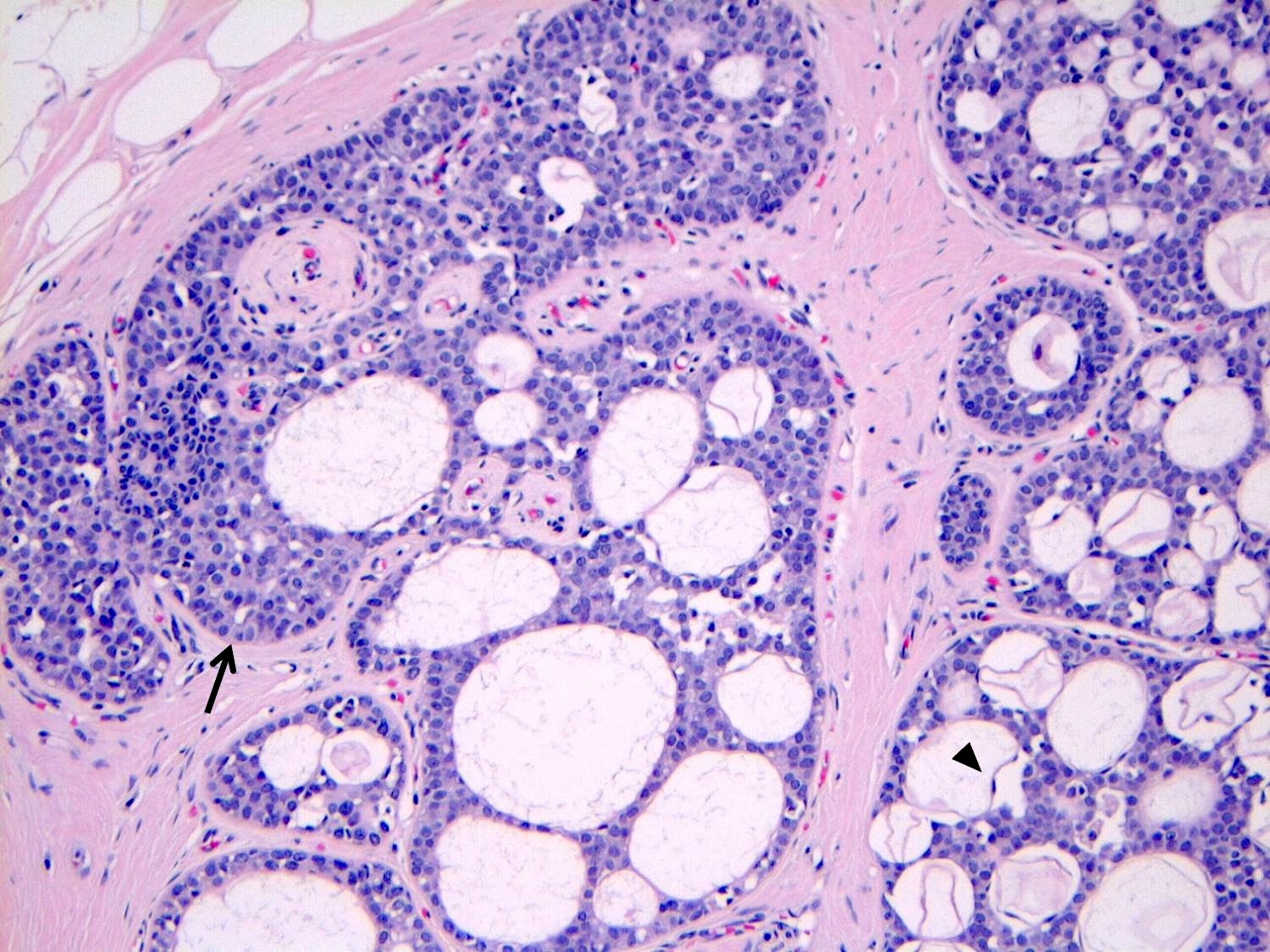
Microscopic (histologic) description
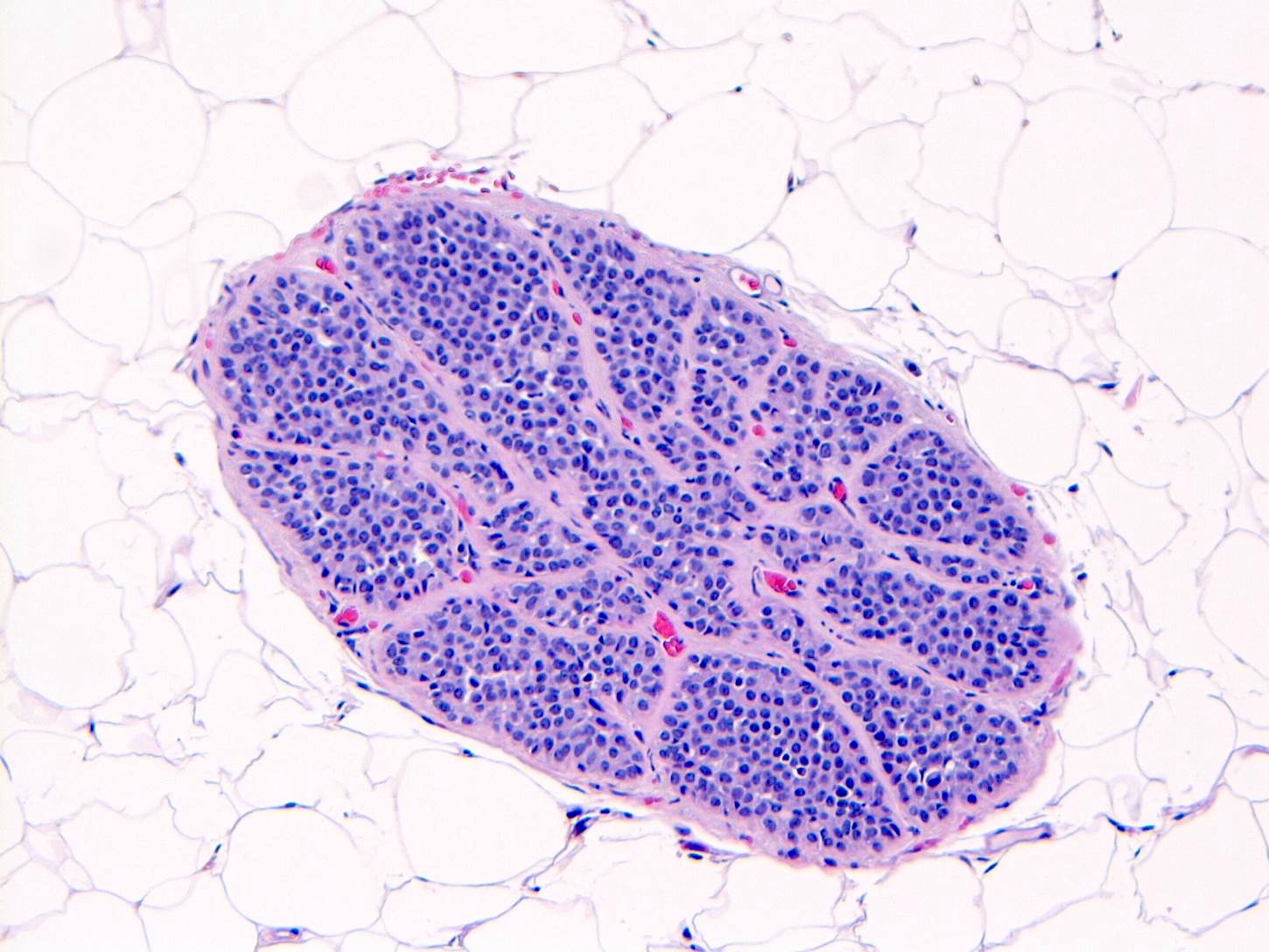
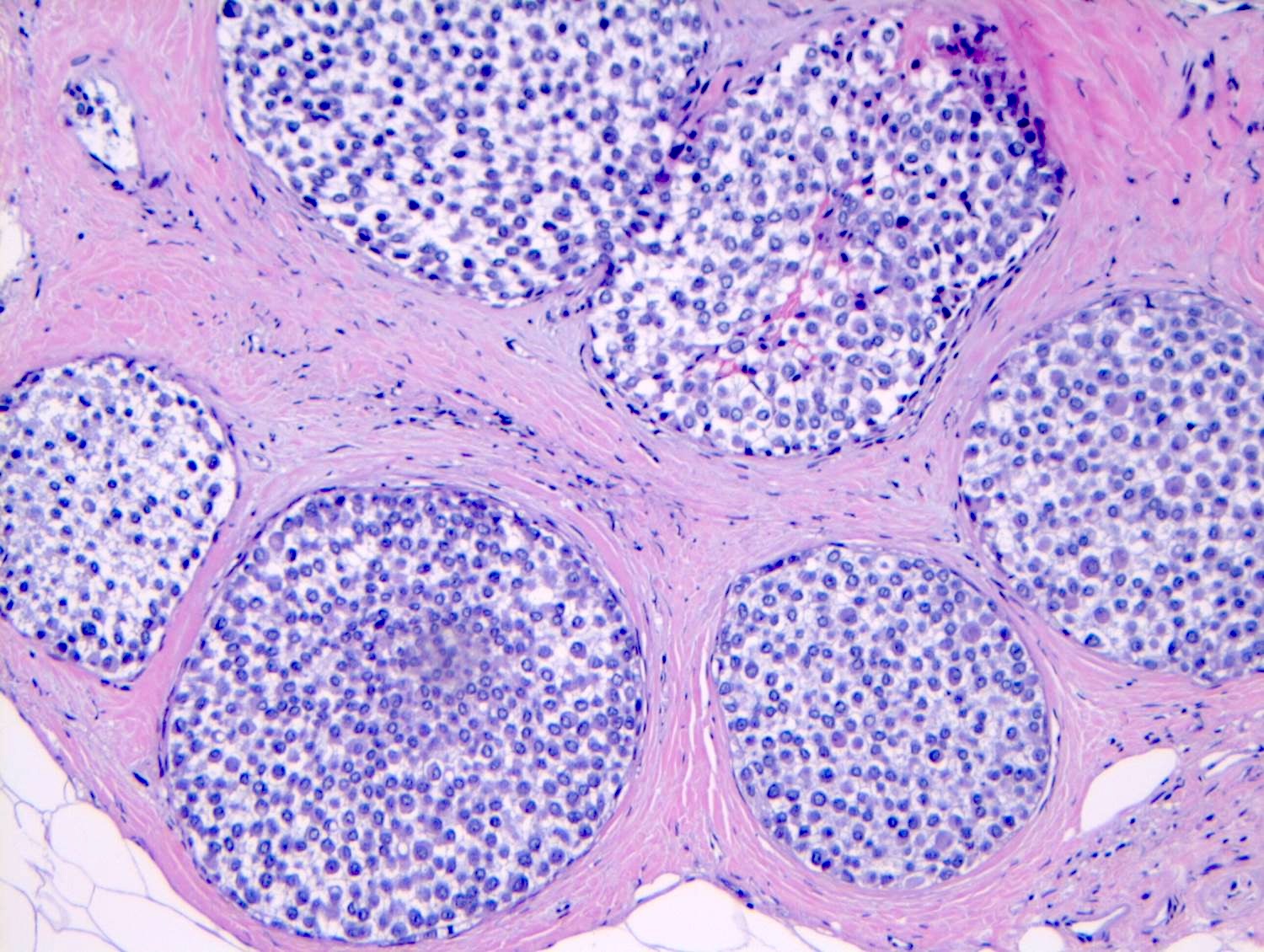
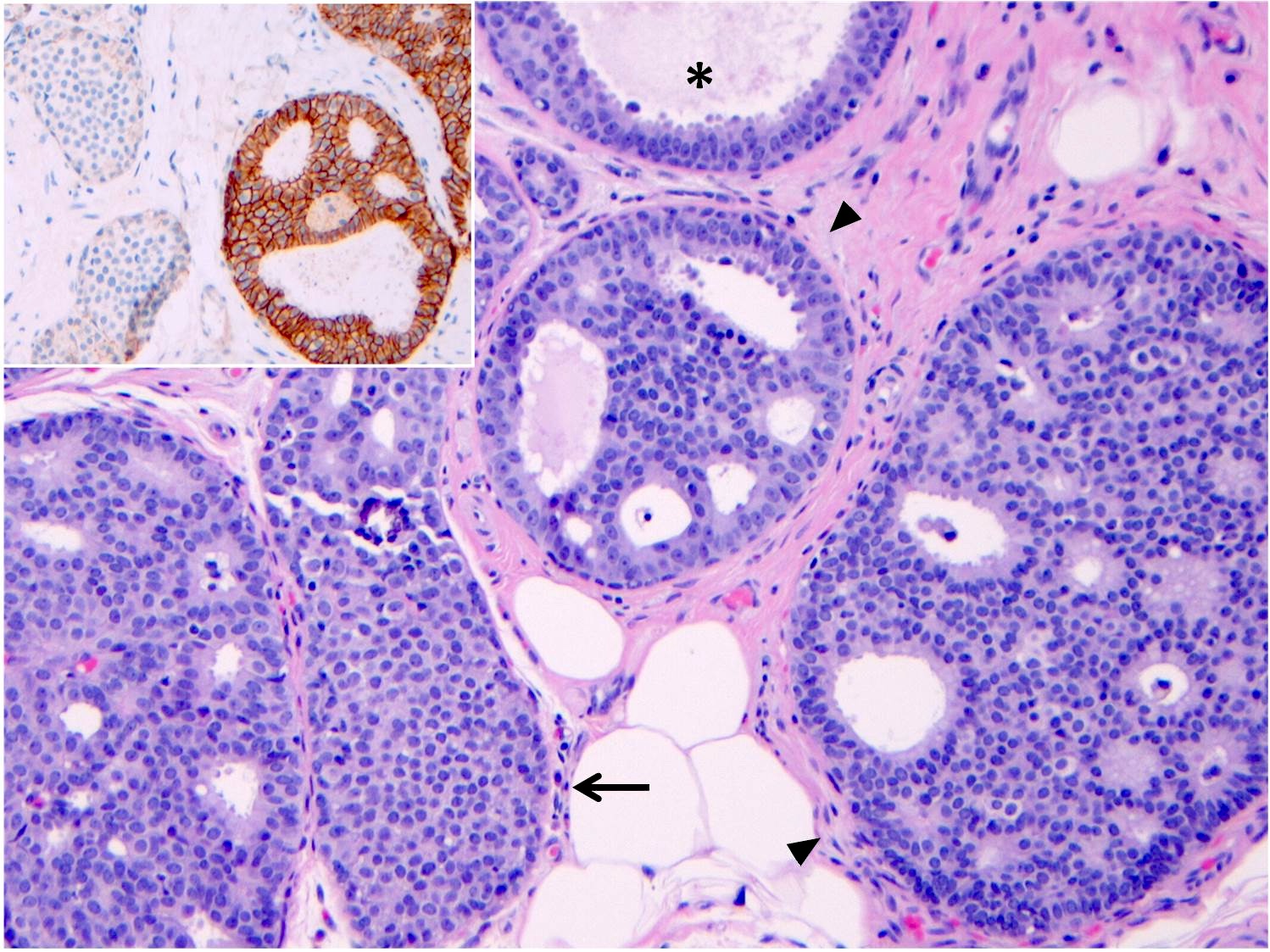
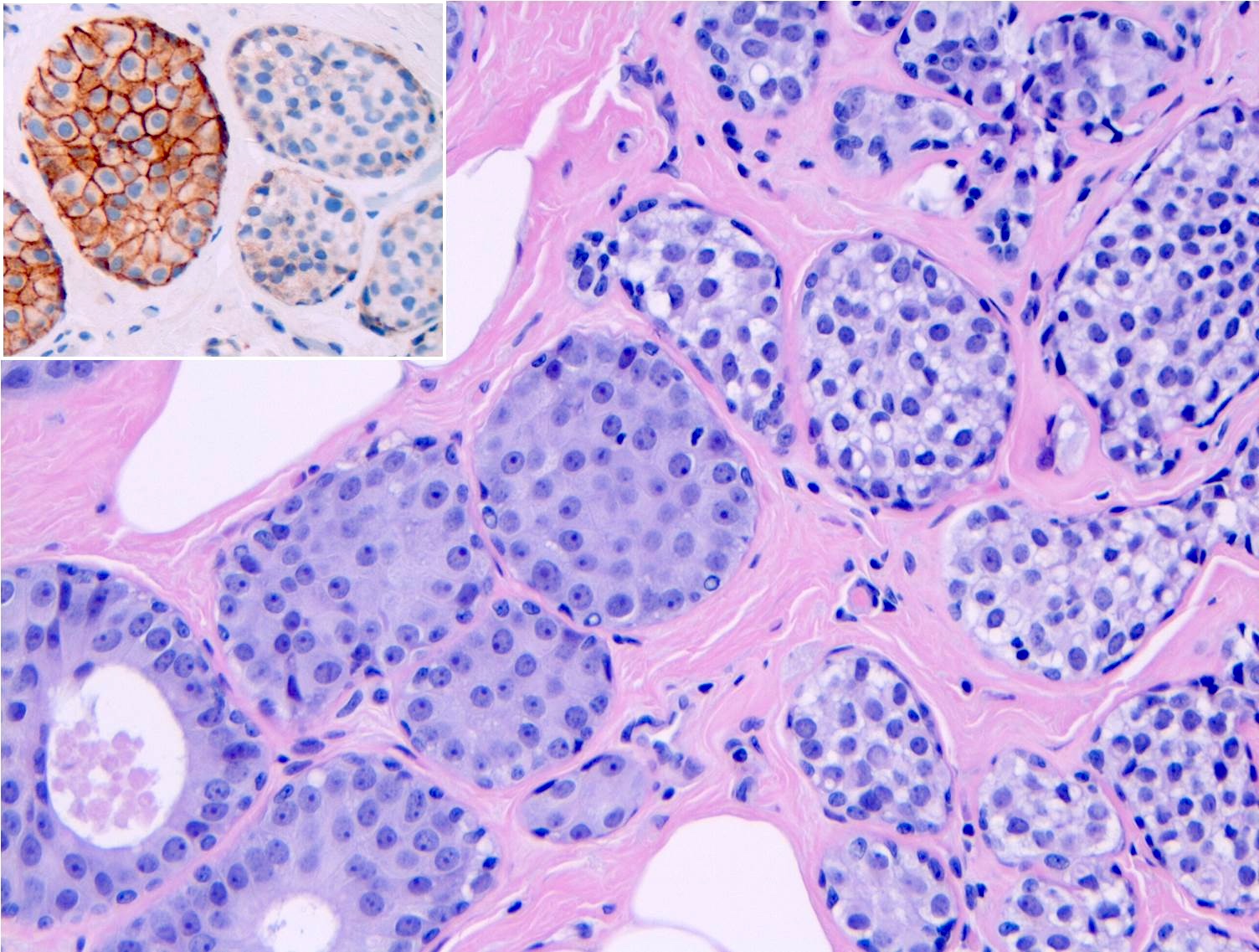
- LCIS involves the terminal duct lobular unit (TDLU), filling and distending acini
- > 50% of the acini in a TDLU must be filled and expanded to qualify as LCIS; otherwise, called atypical lobular neoplasia
- Lobular distention is defined as the presence of ≥ 8 cells in the cross sectional diameter of an acinus
- Involved lobules may be compared with uninvolved lobules to estimate the degree of distension
- ALH only partially involves the lobule by filling up the acini without significant distention
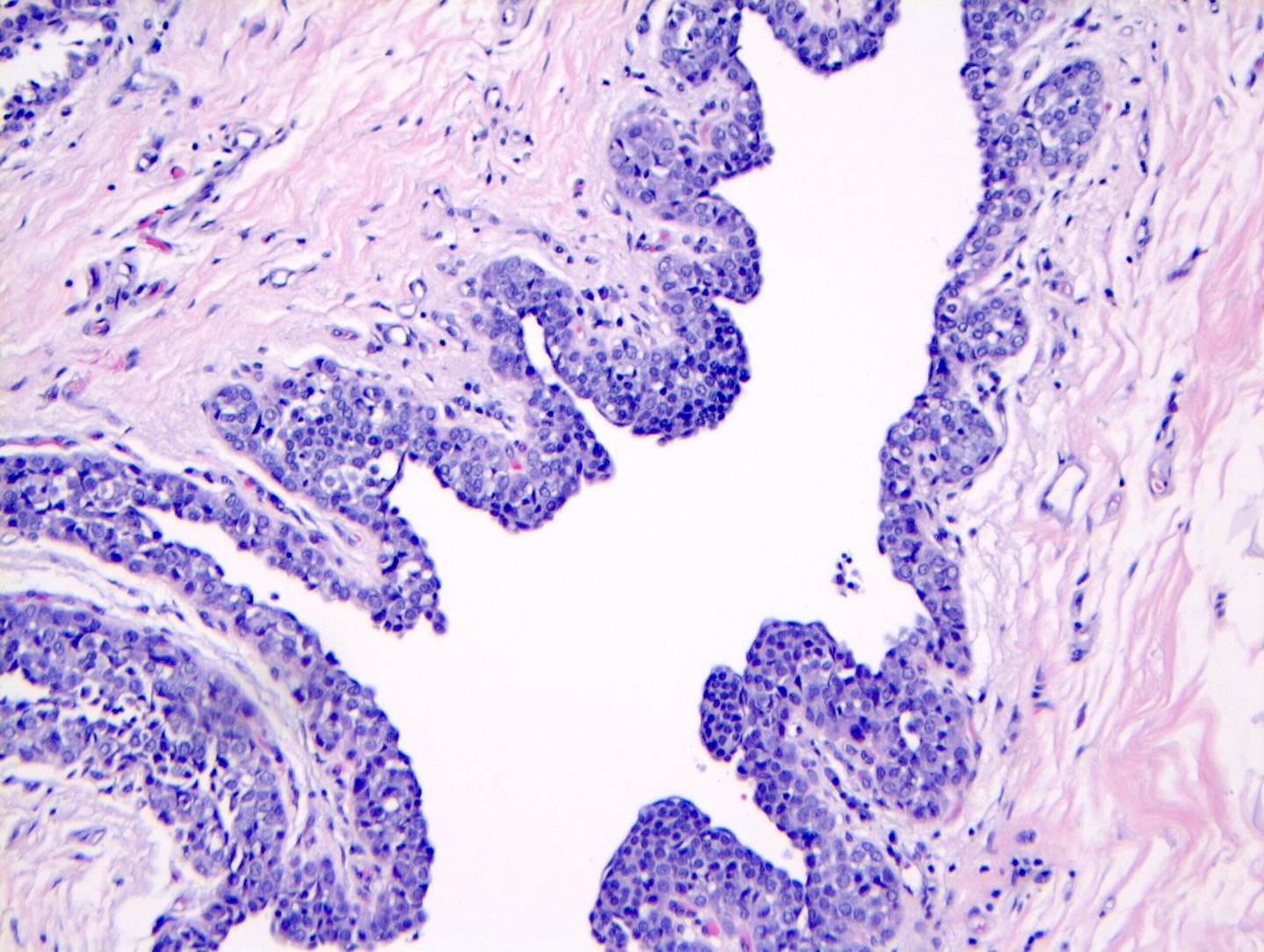
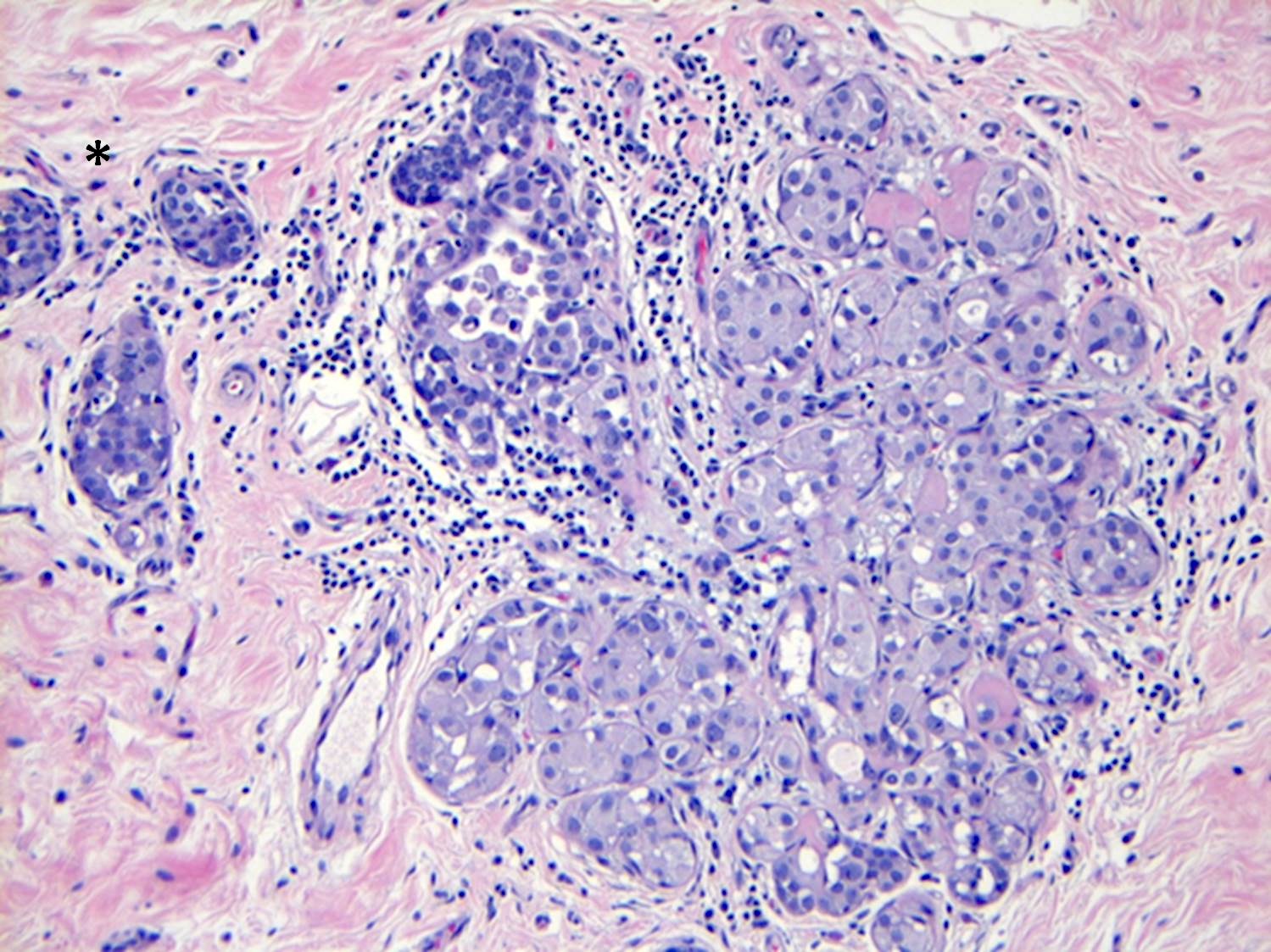
- LCIS most often involves lobules but may also grow along the basement membrane of ducts (i.e., pagetoid spread)
- Pagetoid spread in ducts is the characteristic growth of cells between luminal and myoepithelial layers of a duct without destroying ductal epithelium or filling up ductal lumina
- Often makes the ducts appear convoluted; this is called a cloverleaf pattern
- LCIS may secondarily involve (or arise in) sclerosing adenosis, radial scar, fibroadenoma, collagenous spherulosis or papilloma
- Rosen triad: the presence of tubular carcinoma, columnar cell lesions and LCIS / ALH
- Initial morphologic observation of lesions that are genetically linked
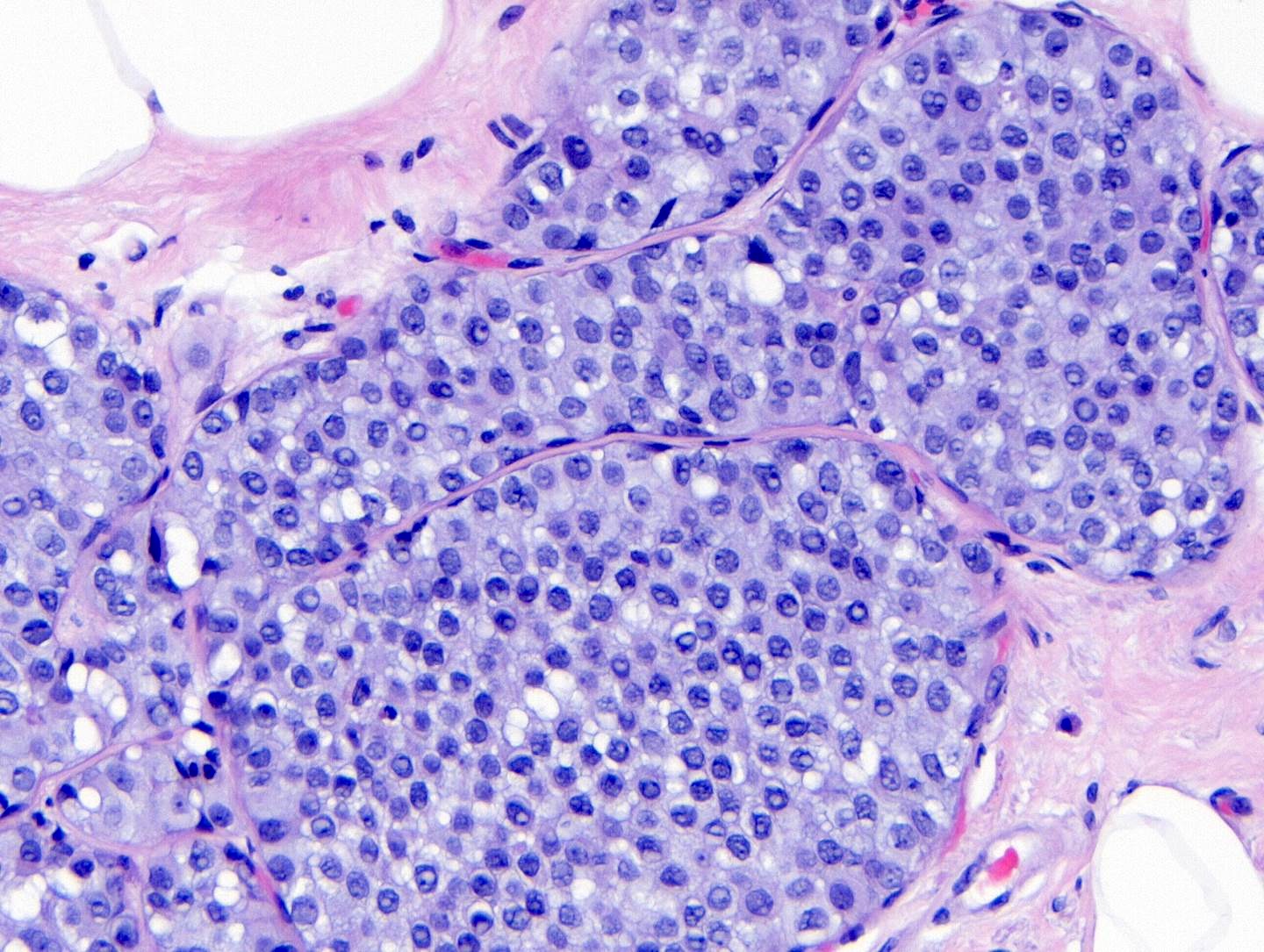
- Classic LCIS cells are monomorphic, evenly spaced, loosely cohesive and do not show polarization or gland formation
- 2 types of cells are described:
- Type A: nuclei are small to slightly enlarged (1 - 1.5x size of lymphocyte) with uniform round nuclei and inconspicuous nucleoli
- Type B: nuclei larger (2x size of lymphocyte), more abundant cytoplasm and more prominent nucleoli
- Type A and B cells can coexist in the same lesion
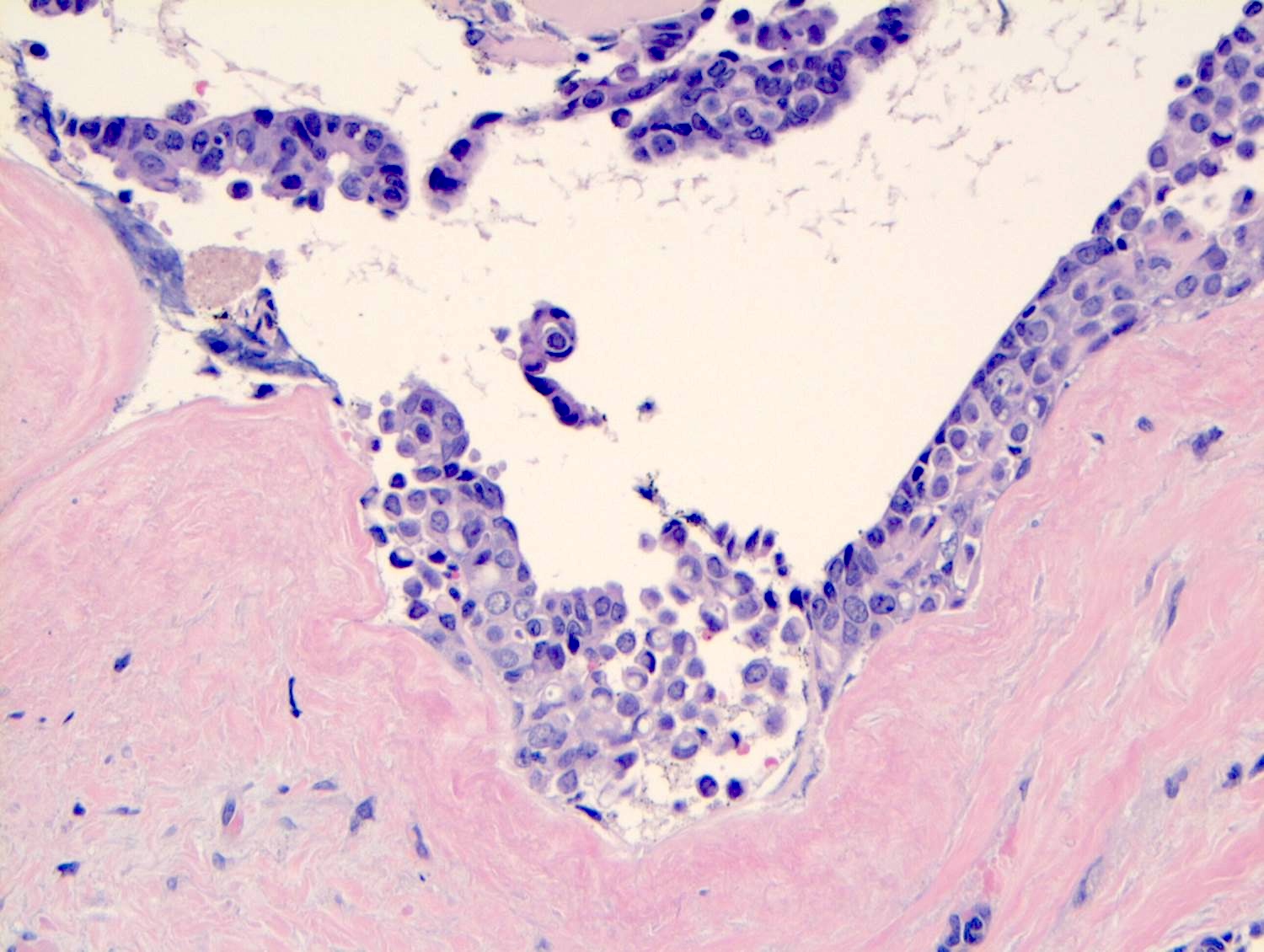
- Cytoplasm of LCIS cells is typically pale to lightly eosinophilic with indistinct cell borders
- In almost all cases of LCIS, at least some cells contain intracytoplasmic vacuoles or lumina, which may contain an eosinophilic globule; this feature is not specific to LCIS
- Vacuoles may be subtle such that special histochemical stains for mucin are required in order to demonstrate; alternatively, they may be large enough to push the nucleus against the cell membrane and produce signet ring cell forms
- Outer layer of myoepithelial cells is retained in the acini and ducts involved but it may be attenuated
- In some cases, scattered myoepithelial cells can be admixed with the neoplastic epithelial cells within the involved spaces
- Myoepithelial cells show weak positivity for E-cadherin due to the cross reactivity of P-cadherin
- Cytologic or architectural features of LCIS may deviate from the classical appearances and exhibit distinct cell membranes, clear cell change or apocrine change, or have a myoid appearance with dark, eccentric nuclei and dense eosinophilic cytoplasm similar to rhabdomyoblasts
- Classic LCIS does not show significant nuclear pleomorphism or mitosis
- Classic LCIS may rarely display single cell apoptosis or minute foci of necrosis but typically does not show comedo type necrosis
- Rarely, comedo necrosis can be present in classic LCIS that has typical cytological and architectural features; this (by itself) should not be diagnosed as pleomorphic LCIS (Am J Surg Pathol 2006;30:1445)
- Pleomorphic LCIS is defined as having large cells with enlarged pleomorphic nuclei; additionally, central necrosis and apocrine features can be present
- Florid LCIS, with or without central necrosis, refers to lesions with cytologic features of classic LCIS but with marked duct distension in a solid architectural pattern
- LCIS lesions, which are borderline between classic LCIS composed of type B cells and pleomorphic LCIS, should be categorized as classic LCIS composed of type B cells
- Lesions with cytological features of classic LCIS, which show distension of the acini borderline between classic LCIS and florid LCIS, should be designated as classic LCIS, even when LCIS is extensive
Microscopic (histologic) images
Contributed by Anna Biernacka, M.D., Ph.D.
Cytology description
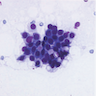
- Fine needle aspiration (FNA) of classic LCIS shows loosely cohesive groups of small, uniform and cytologically bland neoplastic cells (Am J Clin Pathol 1989;92:22, Diagn Cytopathol 1993;9:713, Diagn Cytopathol 2002;27:22)
- Nuclei are mildly enlarged and eccentrically placed and may show small but prominent nucleoli (Diagn Cytopathol 2005;32:276)
- Cytoplasm may have occasional intracytoplasmic lumina
- Nonatypical spindled myoepithelial cells may be present in association with classic LCIS cells
- Classic LCIS may be suspected in an FNA sample if signet ring cells are identified and associated with detached fragments of the lobular epithelium; however, definitive diagnosis in these sparely cellular samples is rarely possible
- There are no reliable cytological criteria for distinguishing between classic LCIS and invasive lobular carcinoma (ILC) (Diagn Cytopathol 1993;9:713, Diagn Cytopathol 2002;27:22)
- FNA samples of classic LCIS are usually cellular, whereas samples of ILC are often scant due to prominent desmoplasia that surrounds the infiltrative tumor cells (Diagn Cytopathol 2002;27:22)
- ILC samples generally have a higher proportion of noncohesive cells with more nuclear atypia and pleomorphism (Am J Clin Pathol 1989;92:22)
- > 50% of aspirates from classic LCIS are determined to be benign or nondiagnostic (Diagn Cytopathol 2002;27:22)
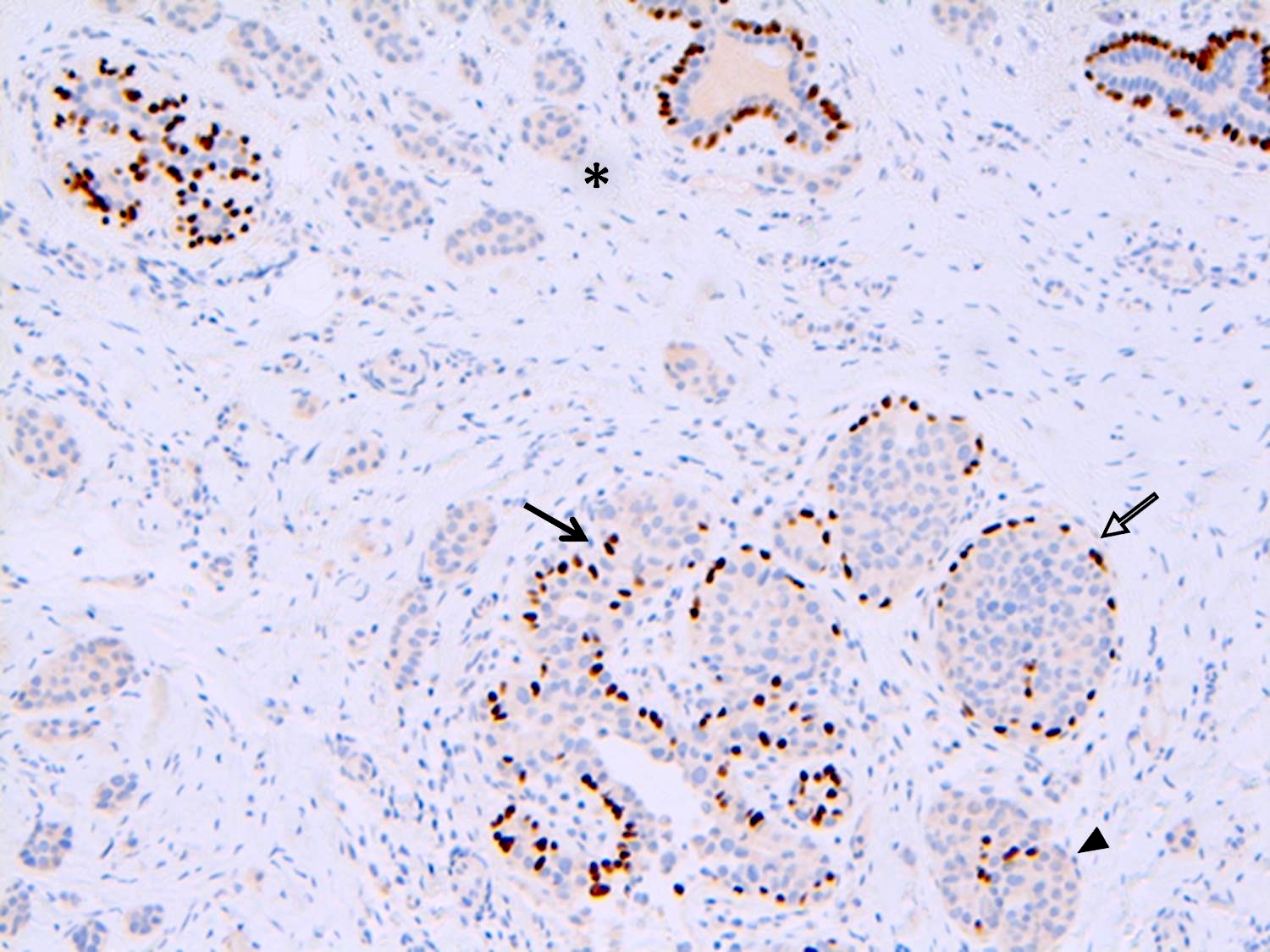
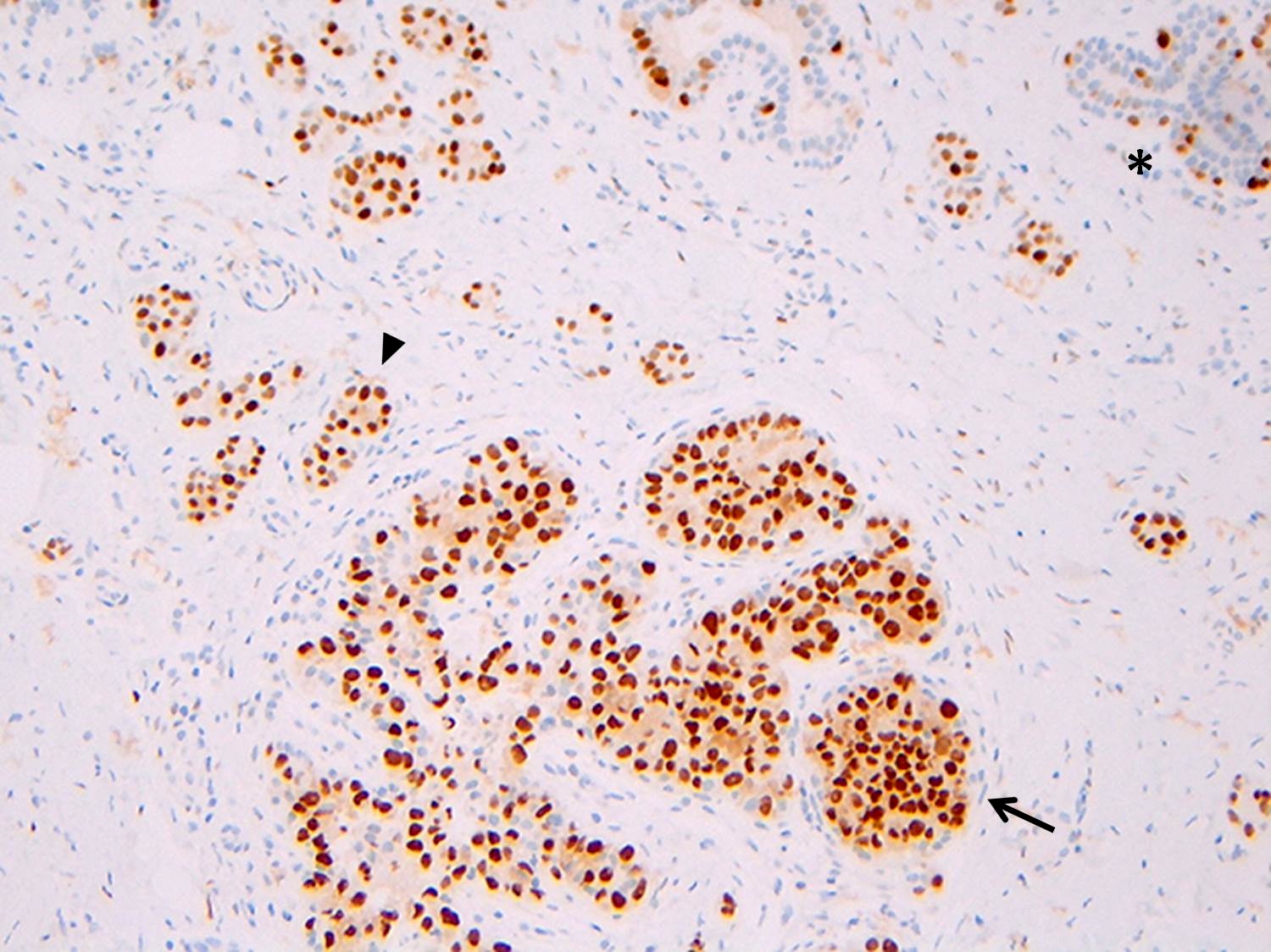
Positive stains
- Strong and diffuse nuclear ER and PR, low Ki67 proliferation index (Cancer 1996;78:1403)
- Mucin, Alcian blue, mucicarmine in cells with cytoplasmic vacuoles
- p120 catenin in a cytoplasmic pattern (Arch Pathol Lab Med 2017;141:1668, Int J Clin Exp Pathol 2014;7:2551)
Negative stains
- E-cadherin: most cases of classic LCIS demonstrate complete loss of E-cadherin staining
- In some cases of classic LCIS, attenuated or aberrant E-cadherin expression is observed, with scattered cells that show dot-like discontinuous / weak membranous staining or patchy cytoplasmic staining
- Atypical E-cadherin patterns or membranous positivity do not preclude the diagnosis of classic LCIS (Surg Pathol Clin 2018;11:123)
- Most cases show loss of expression of membrane beta catenin
- HER2 / neu
- See Diagrams / tables
Molecular / cytogenetics description
- Molecular data has shown that LCIS is a clonal neoplastic proliferation and an early lesion in the low grade neoplasia pathway
- Studies comparing LCIS and synchronous ILC highlight the shared copy number aberrations and somatic genetic mutations, indicating that LCIS is a nonobligate precursor to ILC (Genes Chromosomes Cancer 2010;49:463, Cancer 2004;100:2562, J Pathol 2005;207:1)
- LCIS associated with ILC has more genetic alterations than pure LCIS
- Most frequent copy number changes include loss of 16q and gain of 1q (Breast Cancer Res Treat 2005;90:249, Hum Pathol 2001;32:292, Cancer Res 1998;58:4721, Mol Pathol 2000;53:118)
- Loss of 16q and gain of 1q are also seen in other ER positive proliferations, including columnar cell lesions and well differentiated invasive ductal carcinoma, including tubular carcinoma and low grade DCIS
- This has led to an understanding that LCIS belongs to a group of lesions in a low grade, ER positive pathway of breast carcinogenesis (Histopathology 2011;59:549, Arch Pathol Lab Med 2017;141:1668)
- Although the target gene of 16q deletion in DCIS and IDC remains to be identified in lobular lesions, it involves the CDH1 tumor suppressor gene encoding for cell adhesion molecule E-cadherin (mapping at 16q22.1)
- Loss of 16q is often accompanied by CDH1 inactivating mutations, CDH1 homozygous deletions or CDH1 gene promoter methylation, which results in biallelic silencing of the gene
- Different combinations of alterations can result in different patterns of immunoreactivity
- Completely absent, weak and beaded, or aberrantly present in the cytoplasm in a dot-like pattern
- Rare cases (< 10%) may show membrane staining even though the protein is nonfunctional
- Other tumor suppressor genes located on chromosome 16 have been shown to be lost in LCIS:
- CCCTC binding factor (CTCF) gene, a transcriptional regulator of several genes linked to tumorigenesis
- Dipeptidase 1 (DPEP1) gene, which is involved in the metabolism of important glutathione and may have a role in the degradation of the surrounding extracellular matrix
- Somatic genetic mutations: CDH1 is the most frequently mutated gene in LCIS (in as many as 81% of cases), followed by PIK3CA (41%) and CBFB (12%); p53 and PTEN mutations are remarkably rare (Clin Cancer Res 2019;25:674, Mol Oncol 2016;10:360, Breast Cancer Res 2017;19:7)
- Germline mutations in CDH1, which are highly penetrant, are associated with an increased risk of hereditary diffuse gastric cancer and an increased risk of lobular carcinoma, including bilateral LCIS, with or without ILC (J Med Genet 2018;55:431, Br J Cancer 2014;110:1053)
- Variants of LCIS: pleomorphic and florid variants exhibit greater genomic instability than classic LCIS, with increased copy number alterations and gene amplification (Am J Surg Pathol 2009;33:1683)
Videos
Histopathology breast: lobular carcinoma in situ
Lobular carcinoma in situ and invasive lobular carcinoma
Sample pathology report
- Right breast, core needle biopsy:
- Lobular carcinoma in situ, classic type
- No longer classified as Tis because it is considered a risk factor, not a malignancy (Amin: AJCC Cancer Staging Manual, 8th Edition, 2016)
- Reporting on size or margin status is not necessary
Differential diagnosis
- Atypical lobular hyperplasia:
- Identical to classic LCIS morphologically but defined by limited extent
- < 50% of acini in a lobule are involved or all the acini are involved but are not distended
- Artifactual dyshesion:
- Poor tissue fixation may result in artifactual dyshesion of the epithelial cells lining normal ductal lobular structures or comprising benign lesions
- Appearance can mimic LCIS; however, diagnostic cytologic features of LCIS are not present
- Intraepithelial histiocytes:
- May be mistaken for pagetoid, classic LCIS cells
- Small nuclei, abundant foamy to granular cytoplasm and the lack of intracytoplasmic vacuoles distinguish these cells from classic LCIS
- IHC specific for histiocytes, such as CD68, can be used in these cases
- Myoepithelial hyperplasia:
- Myoepithelial cells surrounding normal lobular acini or ducts may assume an epithelioid appearance, with abundant pale to clear cytoplasm and prominent round nuclei
- Myoepithelial cell markers (calponin, smooth muscle myosin heavy chain and p63) are positive
- Clear cell change:
- Variant of apocrine metaplasia
- Pseudolactational hyperplasia:
- Vacuolated cytoplasm, apical apocrine tufts, hyperchromatic or atypical nuclei, psammoma-like calcifications, usually in postmenopausal women
- Invasive carcinoma:
- LCIS involving a pre-existing sclerosing lesion (e.g., sclerosing adenosis, radial scar or complex sclerosing lesion) may produce a pattern mimicking stromal invasion
- Solid or alveolar patterns of invasive lobular carcinoma (ILC) can be difficult to distinguish from LCIS
- Myoepithelial cell markers are retained in LCIS and absent in invasive carcinoma
- Pleomorphic LCIS:
- May be difficult to distinguish from LCIS composed of type B cells
- Nuclei of type B LCIS are larger and more variable than in type A LCIS
- Still, the nuclear size and shape variability is relatively subtle compared with pleomorphic LCIS
- In cases of uncertainty, the lesion should be categorized as classic LCIS with type B cells rather than as pleomorphic LCIS
- Ductal carcinoma in situ:
- Solid low grade DCIS:
- Cohesive cells, microacini with cells polarized around microlumen, no intracytoplasmic vacuoles, no pagetoid ductal involvement
- E-cadherin positive
- Cancerization of lobules by DCIS:
- Cellular cohesion, moderate to high grade cytology, necrosis or calcifications
- E-cadherin positive
- Cribriform DCIS and collagenous spherulosis involved by LCIS:
- Lumens of DCIS are formed by polarized epithelial cells and are empty or contain necrosis or calcifications
- Myoepithelial cells in DCIS surround the periphery of the involved lobule
- Lumens in collagenous spherulosis contain eosinophilic basement membrane material or myxoid material and are lined by myoepithelial cells
- Pagetoid LCIS versus pagetoid DCIS:
- Pagetoid ductal involvement is a common feature of LCIS, less commonly seen in DCIS
- Cellular dyshesion and the presence of intracytoplasmic vacuoles favor LCIS, cellular cohesion and moderate to high grade nuclear atypia favor DCIS
- LCIS with comedo necrosis versus DCIS with comedo necrosis:
- Some examples of LCIS composed of classical type A or type B cells may show comedo necrosis in the involved spaces and may simulate DCIS
- Recognition of characteristic cytologic features of LCIS (particularly dyshesion and intracytoplasmic vacuoles) and, in problematic cases, an immunostain for E-cadherin leads to the correct diagnosis
- In situ carcinoma with mixed ductal and lobular phenotype:
- Mixed architectural and cytological features; heterogeneous expression of E-cadherin
- Solid low grade DCIS:
Additional references
Board review style question #1
What is the Rosen triad?
- DCIS, LCIS, tubular carcinoma
- LCIS, flat epithelial atypia, columnar cell hyperplasia
- Tubular carcinoma, DCIS, apocrine metaplasia
- Tubular carcinoma, LCIS, columnar cell hyperplasia
- Tubular carcinoma, LCIS, flat epithelial atypia
Board review style answer #1
D. Tubular carcinoma, LCIS, columnar cell hyperplasia
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic
Board review style question #2
Which of the following is true of classical lobular carcinoma in situ (classic LCIS)?
- Classic LCIS belongs to group lesions in a low grade, ER positive pathway of breast carcinogenesis
- Classic LCIS increases the patients' risk only for subsequent unilateral breast cancer
- Classic LCIS is staged as Tis using the AJCC 8th edition cancer staging
- If present at a surgical margin, it should be noted in the final pathology report
- Loss of nuclear expression of E-cadherin is the defining immunohistochemical feature of classic LCIS
Board review style answer #2
A. Classic LCIS belongs to group lesions in a low grade, ER positive pathway of breast carcinogenesis
The presence of classic LCIS at or close to the margin of resection is not associated with increased local recurrence and does not need to be reported. Classic LCIS increases the patients' risk for breast cancer in either breast. E-cadherin typically displays a membranous staining pattern. Classic LCIS is no longer classified as Tis because it is considered a risk factor, not a malignancy.
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic
The presence of classic LCIS at or close to the margin of resection is not associated with increased local recurrence and does not need to be reported. Classic LCIS increases the patients' risk for breast cancer in either breast. E-cadherin typically displays a membranous staining pattern. Classic LCIS is no longer classified as Tis because it is considered a risk factor, not a malignancy.
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic
Board review style question #3
Board review style answer #3
B. Intracytoplasmic lumens / vacuoles
Intracytoplasmic lumens, vacuoles and even signet ring morphology are associated with the diagnosis of classic LCIS. Prominent nucleoli, microcalcifications and comedo type necrosis are morphologic features more commonly seen in DCIS or the pleomorphic variant of LCIS. Although type B LCIS cells may have nucleoli, they are typically multiple and scattered, with the lesional cells having uniform and smooth nuclear contours. Intranuclear inclusions are typically secondary to viral cytopathic effect and are not associated with breast lobular neoplasia.
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic
Intracytoplasmic lumens, vacuoles and even signet ring morphology are associated with the diagnosis of classic LCIS. Prominent nucleoli, microcalcifications and comedo type necrosis are morphologic features more commonly seen in DCIS or the pleomorphic variant of LCIS. Although type B LCIS cells may have nucleoli, they are typically multiple and scattered, with the lesional cells having uniform and smooth nuclear contours. Intranuclear inclusions are typically secondary to viral cytopathic effect and are not associated with breast lobular neoplasia.
Comment here
Reference: Lobular carcinoma in situ (LCIS) classic