Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Tozbikian G. DCIS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastmalignantDCIS.html. Accessed January 21st, 2025.
Definition / general
- Ductal carcinoma in situ (DCIS) is a neoplastic proliferation of mammary ductal epithelial cells confined to the ductal-lobular system without evidence of invasion through the basement membrane into the surrounding stroma (Arch Pathol Lab Med 2009;133:15)
- Is a nonobligate precursor lesion of invasive breast cancer (Breast Cancer Res Treat 2010;123:757, Cancer 2005;103:2481)
- Encompasses a heterogeneous group of lesions in terms of histomorphology, underlying genetic alterations, biomarker expression profile and biologic potential for progression to invasive carcinoma
Essential features
- Neoplastic proliferation of mammary ductal epithelial cells that is confined within ducts and lobules
- Nonobligate precursor of invasive breast disease
- Heterogenous entity with wide range of histologic appearances, growth patterns, genetic abnormalities and clinical behavior
Terminology
- Ductal carcinoma in situ (DCIS)
- Intraductal carcinoma
- Ductal intraepithelial neoplasia: terminology never widely adopted and removed from the 4th Edition WHO classification of mammary neoplasms (International Agency for Research on Cancer: WHO Classification of Tumours of the Breast, 4th Edition, 2012)
- Mammary intraepithelial neoplasia: a general term to include both ductal and lobular intraepithelial neoplasia but never adopted, terminology should not be used (Am J Surg Pathol 1991;15:209, Hum Pathol 2002;33:620, Am J Surg Pathol 1980;4:241)
- Extensive DCIS / extensive intraductal component: terminology should only be used to describe DCIS that is associated with invasive carcinoma in which the DCIS component constitutes > 25% of the entire area of invasive carcinoma and DCIS extends beyond the invasive carcinoma into the surrounding breast tissue (Pathol Annu 1988;23:1)
- Multicentric DCIS: no uniform definition, generally refers to DCIS that involves multiple breast quadrants and different distinct regions of the breast
ICD coding
Epidemiology
- Similar epidemiologic risk factors to invasive breast cancer, including older age, family history of breast cancer, nulliparity, late age at first birth, late menopause, elevated body mass index, high mammographic breast density, postmenopausal hormonal therapy use and genetic risk factors (e.g. BRCA1/2) (Mol Oncol 2010;4:357, Clinics (Sao Paulo) 2013;68:674, J Transl Med 2015;13:335)
- Incidence of DCIS increased significantly from 1983 - 1992 due to greater mammographic screening, particularly in patients younger than 50 years old (JAMA 1996;275:913, J Natl Cancer Inst Monogr 1997:151)
- Represents 20 - 25% of newly diagnosed breast cancers in the United States (CA Cancer J Clin 2017;67:7, Am Surg 2018;84:1)
- 3.3 - 5.9% of women with DCIS carry a germline mutation in BRCA1 or BRCA2 and prevalence of BRCA mutation is significantly greater in women diagnosed with DCIS before the age 50 and personal or family history of breast cancer (JAMA 2005;293:964, Cancer Prev Res (Phila) 2010;3:1579, J Clin Oncol 2002;20:1480)
Sites
- Breast
- Can occur in axillary breast tissue
Clinical features
- Majority are non-palpable and detected mammographically as microcalcifications (70 - 80%) (Am Surg 2018;84:1, Curr Treat Options Oncol 2013;14:75)
- Less frequently presents as palpable mass, nipple discharge, Paget disease of the nipple
- 2 - 8.6 times higher risk for subsequent development of invasive breast carcinoma of compared general population (N Engl J Med 1985;312:146, Ann Surg 1997;225:69, Lancet 2000;355:724, Int J Cancer 1998;77:392)
- Uncommon in women younger than 30 years, mean age 50 - 59 years (Arch Surg 1992;127:1392, Eur J Surg Oncol 1990;16:220)
- Rate of DCIS increases with age (0.6 per 1000 screening examinations in women aged 40 - 49 years versus 1.3 per 1,000 in women aged 70 - 84 years) (J Natl Cancer Inst Monogr 2010;2010:139)
Diagnosis
- Calcifications or distortion on imaging raises concern and need for obtaining tissue
- Diagnosis is made by histologic examination of tissue obtained via needle core biopsy, lumpectomy or mastectomy
Radiology description
- Mammography is highly sensitive, microcalcifications found in 72 - 98% of DCIS (Radiology 1989;170:411, Curr Treat Options Oncol 2013;14:75)
- Microcalcifications are usually the only radiologic finding; less frequently DCIS presents as mass or architectural distortion without calcifications (Eur J Radiol 2005;54:55)
- Pleomorphic calcifications are often associated with high grade DCIS; while fine or granular calcifications are more common in low grade DCIS, the distribution is generally clustered or linear (J Natl Cancer Inst Monogr 2010;2010:214)
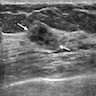
- Ultrasound generally has limited utility in detecting DCIS
- MRI may be a useful adjunct to detect DCIS that lacks calcifications, multifocal DCIS, contralateral disease and measure extent of disease (Breast J 2005;11:382, Radiology 2007;243:670)
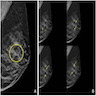
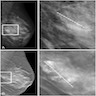
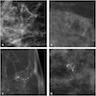
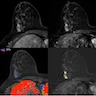
Radiology images
Contributed by Azadeh Khayyat, M.D. and Julie M. Jorns, M.D. (Case #533)
Images hosted on other servers:
Prognostic factors
- Women diagnosed with DCIS have 10 times higher risk of developing ipsilateral invasive breast if untreated (N Engl J Med 2004;350:1430, Cancer 1982;49:751)
- 36 - 53% of low grade DCIS lesions progress to invasive lesions if untreated (Mod Pathol 2015;28:662, Cancer 1980;46:919)
- Risk of progression of untreated high grade DCIS to invasive carcinoma is not well characterized as most clinically detected high grade DCIS is excised surgically (N Engl J Med 2004;350:1430)
- Time interval to the development of recurrence varies, low grade DCIS has longer interval (> 15 years), shorter interval observed in high grade DCIS (< 5 years) (Cancer 2005;103:1778)
- Long term outcome meta analysis study of DCIS showed a 15 year total local recurrence rate of 40% with 28% recurring as invasive disease (BMC Cancer 2015;15:890)
- Positive surgical margins, high nuclear grade, large lesion size, comedo necrosis and young age (< 45 years) reported as the most important clinical pathologic predictors associated with local recurrence and prognosis (J Clin Oncol 1996;14:754, Int J Radiat Oncol Biol Phys 2001;50:991, Cancer 1999;85:616, J Clin Oncol 2006;24:3381, Cancer 2005;103:2481, Breast Cancer Res Treat 2008;109:405, Eur J Cancer 2007;43:291, Breast J 2005;11:242)
- A nomogram for predicting recurrence risk after breast conserving surgery based on clinical, pathologic and treatment variables has shown utility in providing individualized estimates of recurrence risk (J Clin Oncol 2010;28:3762)
- Predictive and prognostic biomarkers and ancillary testing in DCIS:
- Estrogen receptor
- Currently the only immunohistochemical biomarker recommended for routine clinical use; progesterone receptor is considered optional (J Clin Oncol 2020:JCO1902309)
- Determines potential benefit of endocrine therapies (J Clin Oncol 2020:JCO1902309, Lancet 1999;353:1993)
- ER negative status correlates with ipsilateral recurrence risk (Breast Cancer Res Treat 2018;167:751)
- Oncotype DX for DCIS® assay is a genomic test that predicts radiation therapy benefit and the likelihood of 10 year breast cancer local recurrence risk in patients with DCIS treated with breast conserving surgery (J Natl Cancer Inst 2013;105:701, Breast Cancer Res Treat 2015;152:389, J Natl Cancer Inst 2017;109)
- Estrogen receptor
- Novel biomarkers not used in routine clinical practice
- Recent studies correlated presence of associated tumor infiltrating lymphocytes (TIL) in periductal stroma and TIL immune phenotype to high risk features and Oncotype DX® Breast DCIS Score (Ann Oncol 2017;28:321, Breast Cancer Res Treat 2017;161:17, Mod Pathol 2015;28:1167)
- High Ki67 proliferation index has reported to correlate with increased recurrence risk (Appl Immunohistochem Mol Morphol 2016;24:20, Clin Breast Cancer 2018;18:157, J Breast Cancer 2016;19:185)
- HER2 (epidermal growth factor receptor family member 2) more frequently expressed (~ 40%) in DCIS than invasive carcinoma and reported to correlate with increased recurrence risk (J Cancer 2011;2:232, Breast Cancer Res Treat 2016;158:179)
- Several novel biomarkers such as COX-2, FOXA1, SIAH2 and p16 have been evaluated in DCIS with regard to radiotherapy benefit and recurrence risk (Breast Cancer Res Treat 2016;157:351)
Case reports
- 19 year old woman with DCIS arising in phyllodes tumor (Ann R Coll Surg Engl 2018;100:e97)
- 30 year old woman with BRCA2 mutation undergoes prophylactic mastectomy (Cureus 2019;11:e5311)
- 43 year old woman with metastases after mastectomy for DCIS (BMC Cancer 2019;19:844)
- 58 year old man with left breast mass (Eur J Breast Health 2019;16:77)
- 73 year old woman with palpable breast mass (Int J Surg Case Rep 2018;45:9)
- Premenopausal woman with family history of breast cancer was found to have a subcentimeter mass with calcifications on mammogram (Case #533)
Treatment
- Breast conserving surgery alone, breast conserving surgery with radiotherapy or mastectomy
- Excision to negative margins (≥ 2 mm) for patients treated with breast conserving surgery (Pract Radiat Oncol 2016;6:287)
- Possible sentinel lymph node sampling if patients are planned for mastectomy or who have DCIS size > 5 cm or at high risk of microinvasive carcinoma (Ann Surg Oncol 2016;23:2229, Am J Surg 2008;196:81, Breast J 2008;14:135)
- Radiation reduces the risk of local recurrence by 50% in patients undergoing breast conserving therapy (J Clin Oncol 2008;26:1247)
- Tamoxifen therapy further decreases the risk of ipsilateral and contralateral recurrence by 30% in patients with estrogen receptor positive DCIS (Semin Oncol 2006;33:647, Breast Cancer Res Treat 2002;75:S7)
- Aromatase inhibitors with similar overall effectiveness to tamoxifen, with different safety profile (Lancet 2016;387:866, Lancet 2016;387:857)
- Omission of radiotherapy may be considered if DCIS is small, low grade and has clear margins (J Clin Oncol 2009;27:3211)
- Mastectomy may be recommended for multicentric DCIS, inability to attain clear surgical margins, large span or diffuse microcalcifications on imaging studies
Gross description
- Usually no gross lesion, but high grade DCIS may present as firm gritty mass with multiple yellow punctate flecks on the cut surface representing comedonecrosis
- Serial sequential sampling with mammographic correlation is useful to estimate DCIS size (Arch Pathol Lab Med 2009;133:31, Arch Pathol Lab Med 2009;133:26)
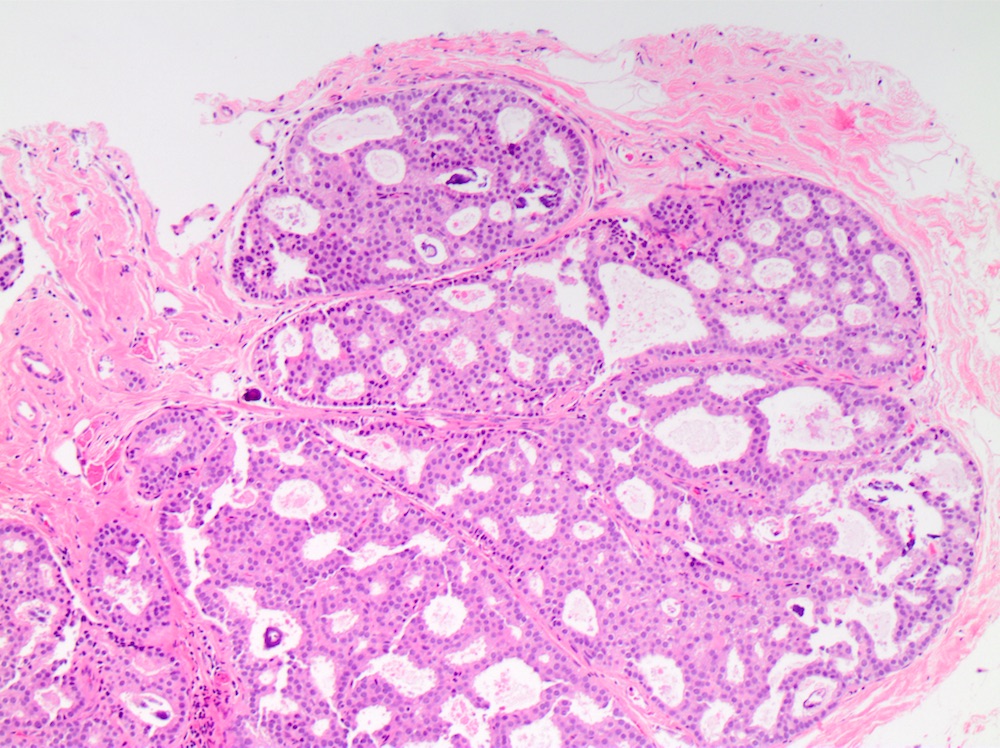
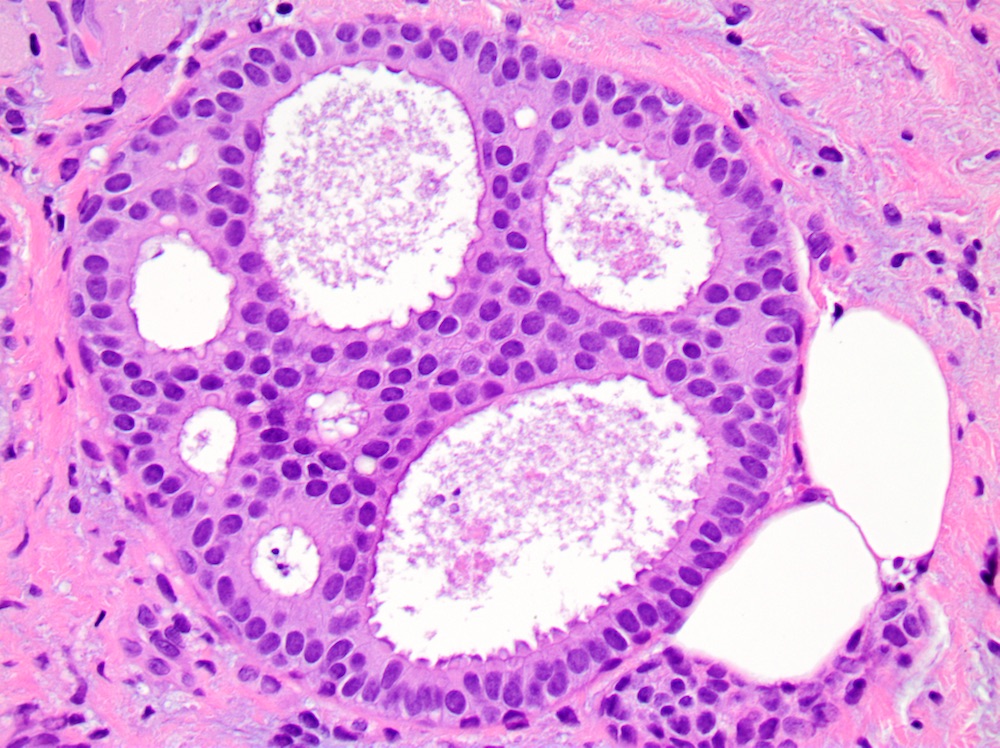
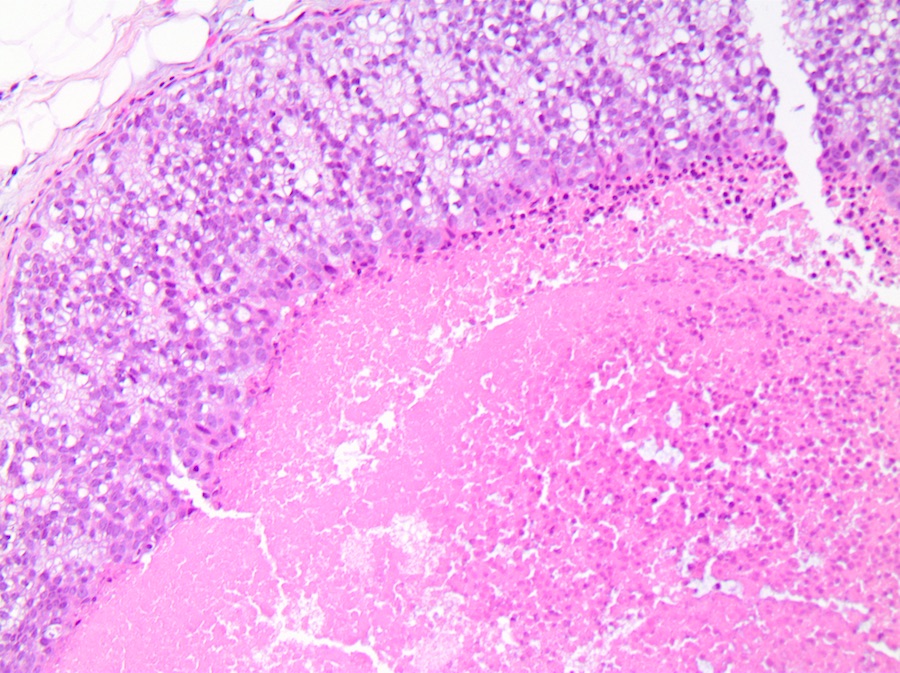
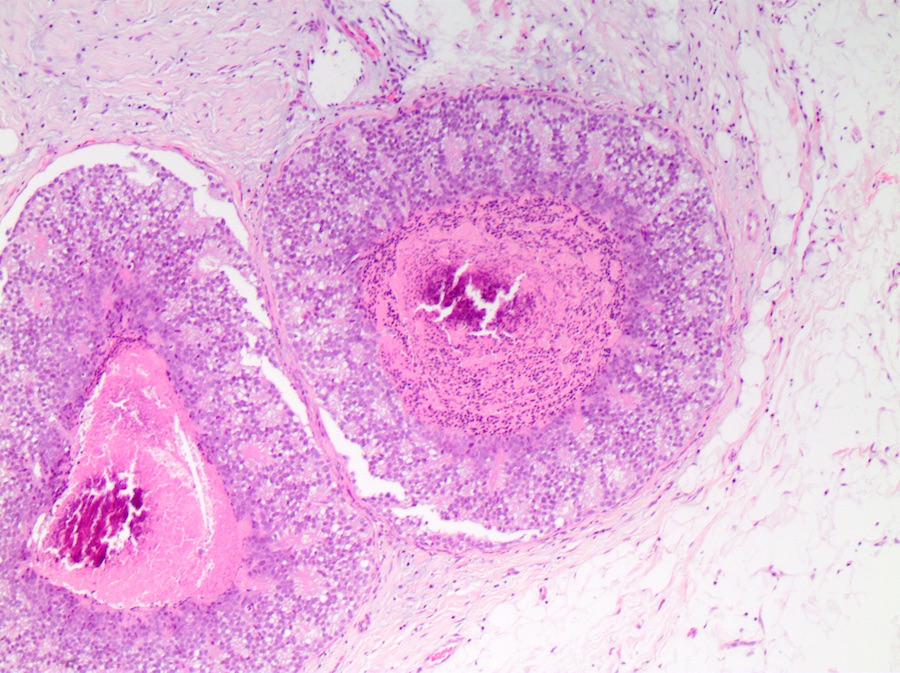
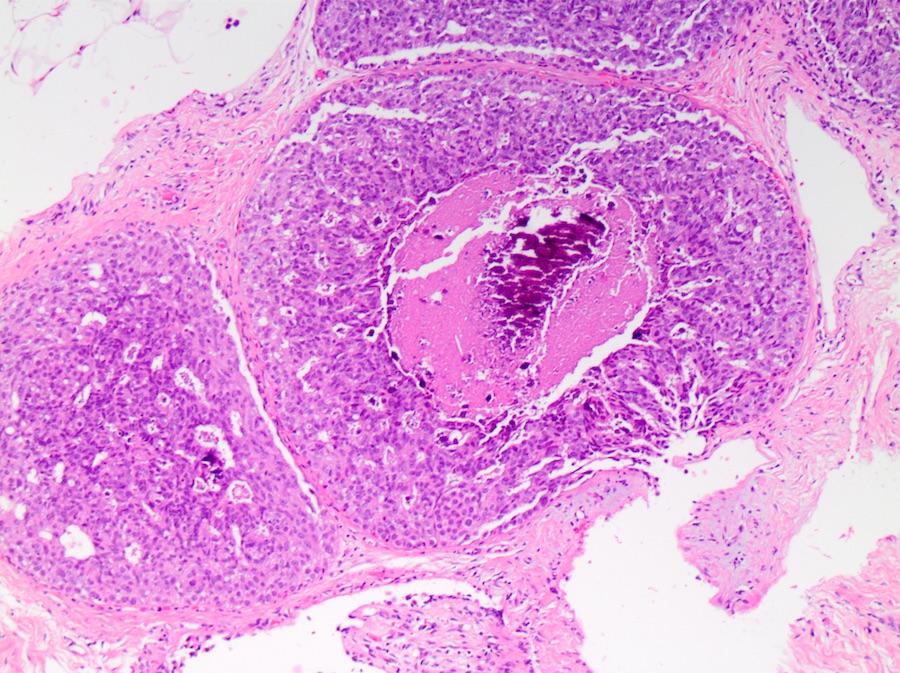
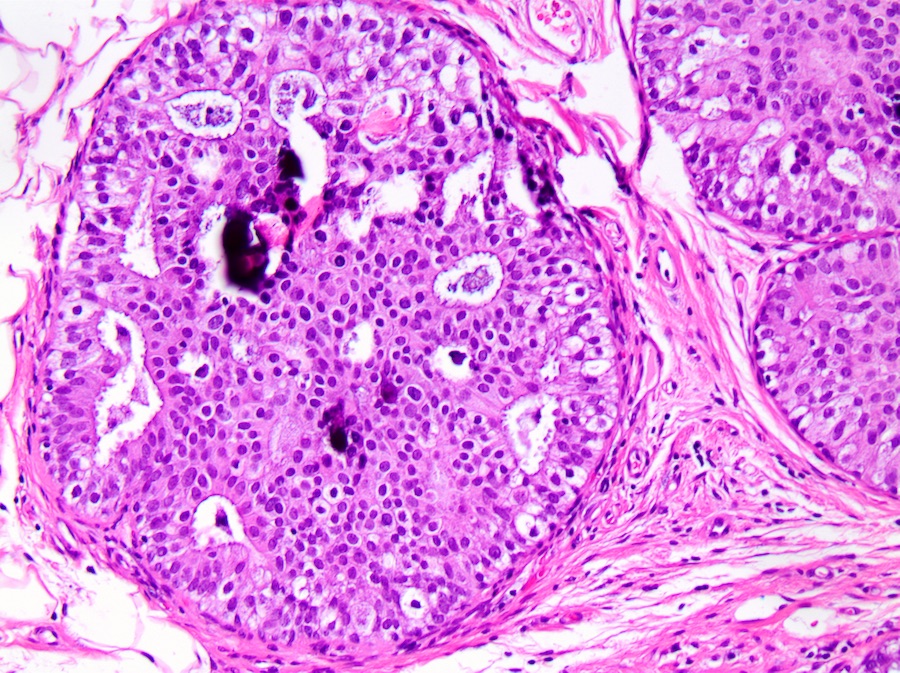
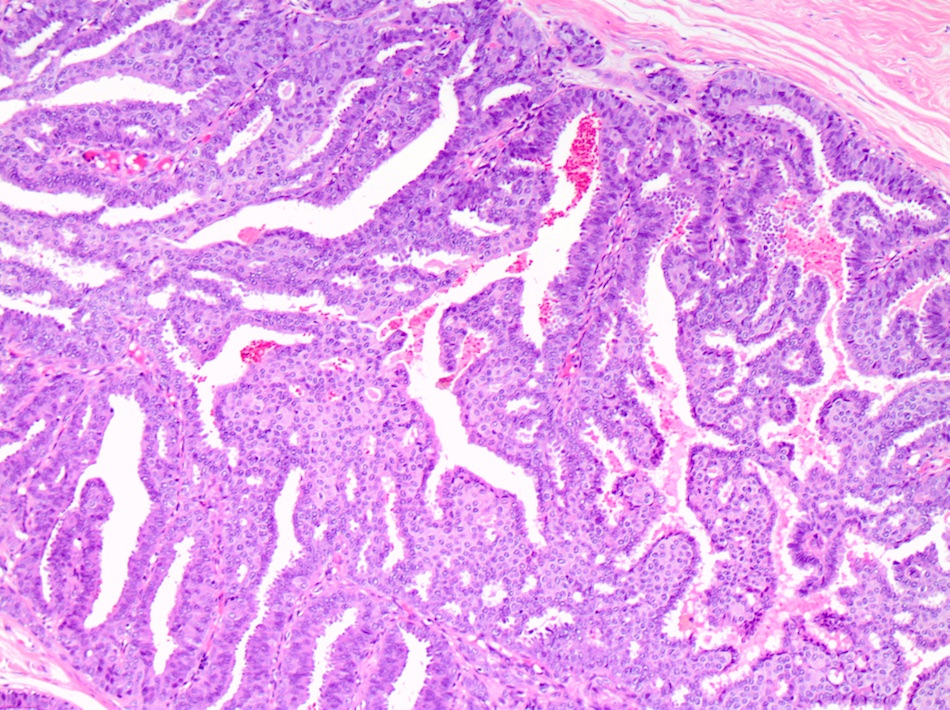
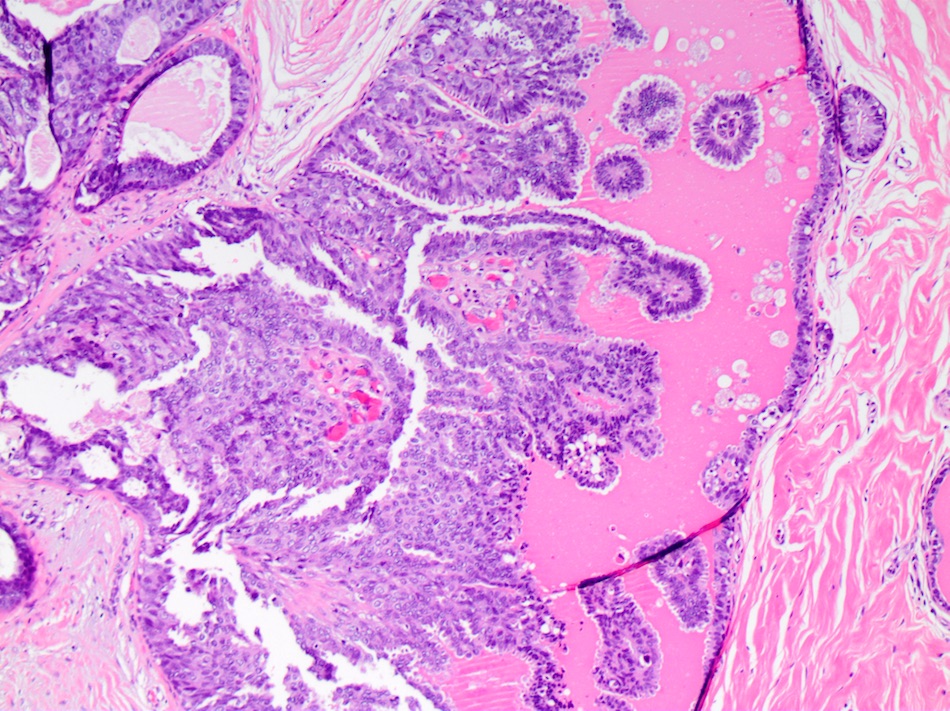
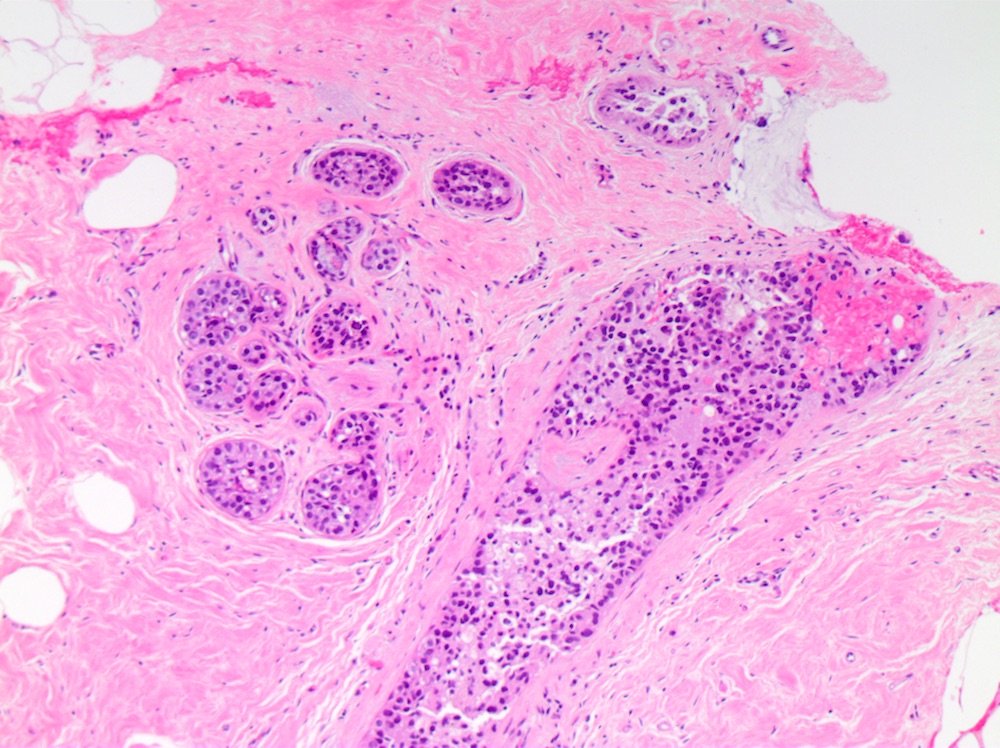
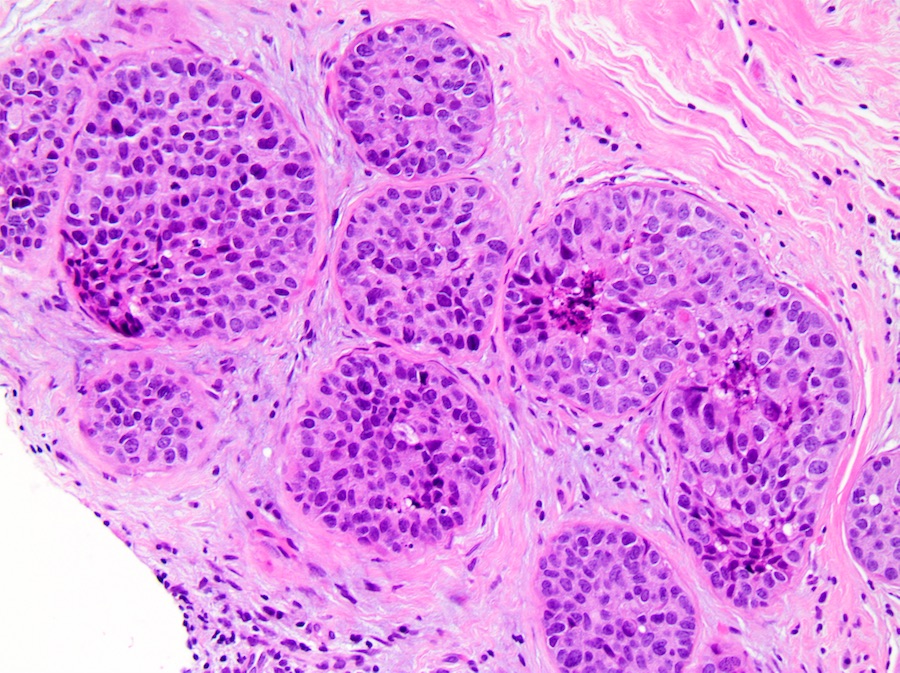
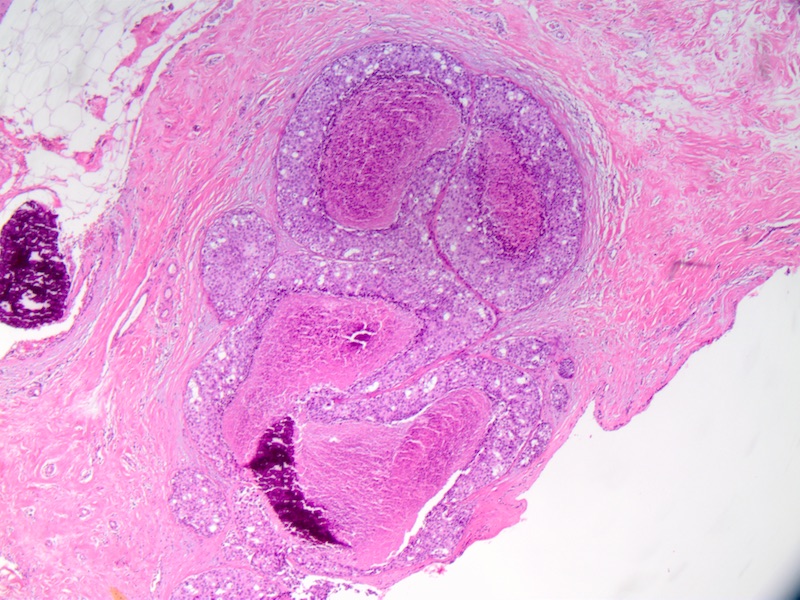
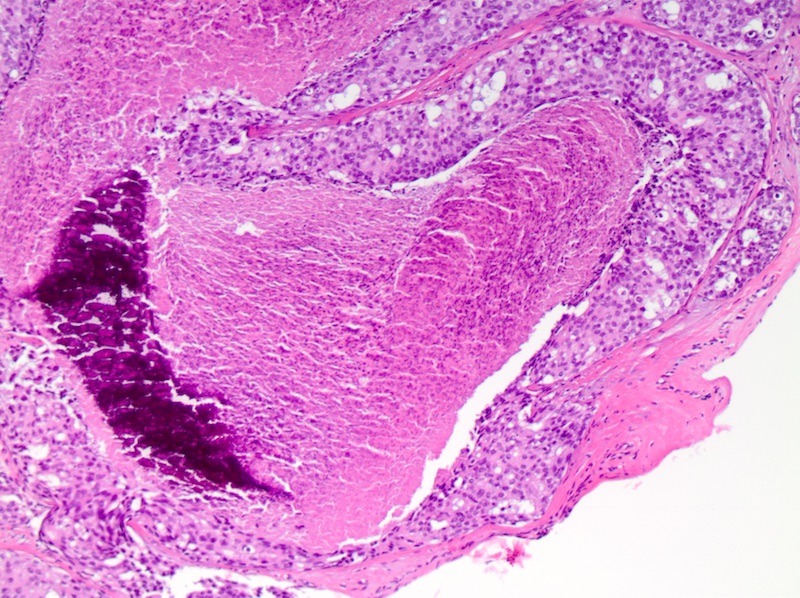
Microscopic (histologic) description
- DCIS traditionally classified based on architectural growth pattern (no clinical relevance other than comedo pattern):
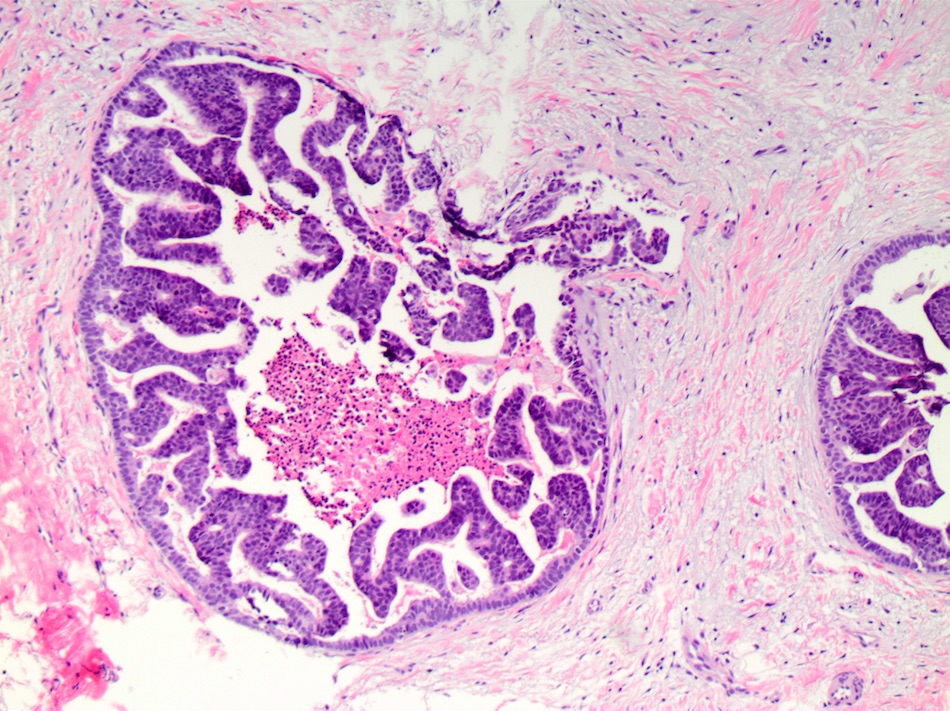
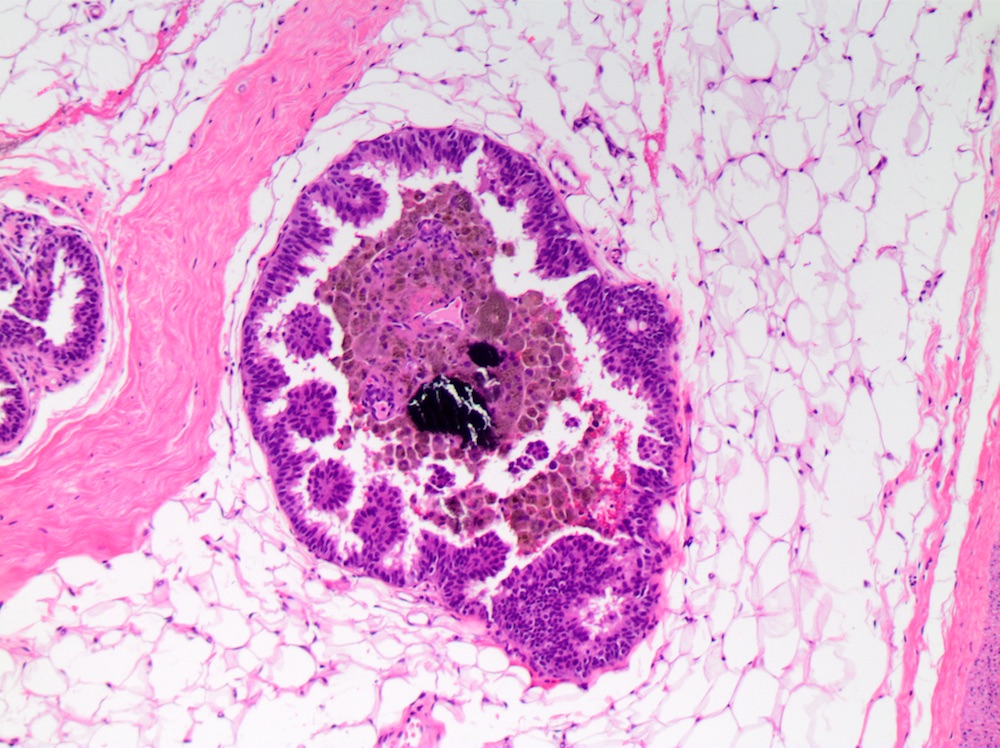
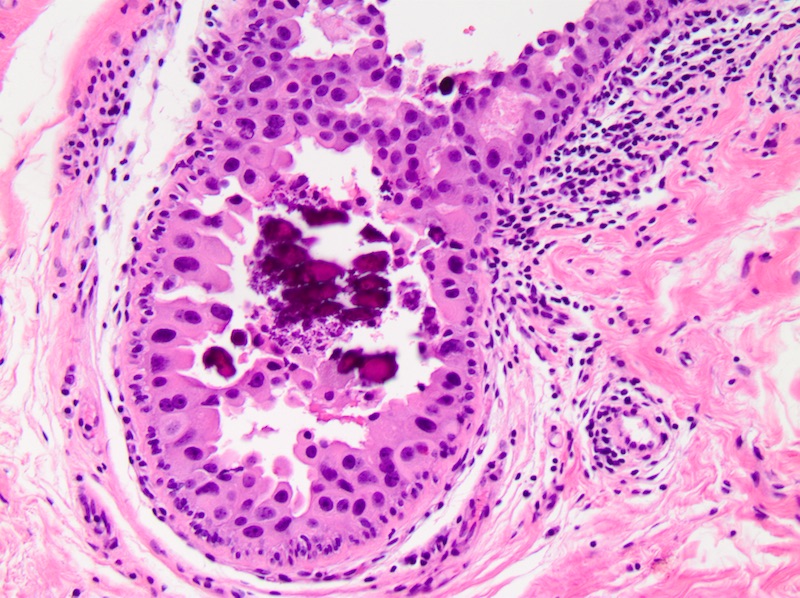
- Cribriform:
- Fenestrated proliferation with multiple, round, rigid extracellular lumens with punched out appearance
- Neoplastic cells are frequently evenly distributed equidistant and polarized with long axis of cell perpendicular to the central lumen
- Trabecular bars comprised of rigid rows of cells with long axes perpendicular or at least not parallel to the long axis of the bar
- Roman bridges comprised of curvilinear trabecular bars connecting two portions of the epithelial lining
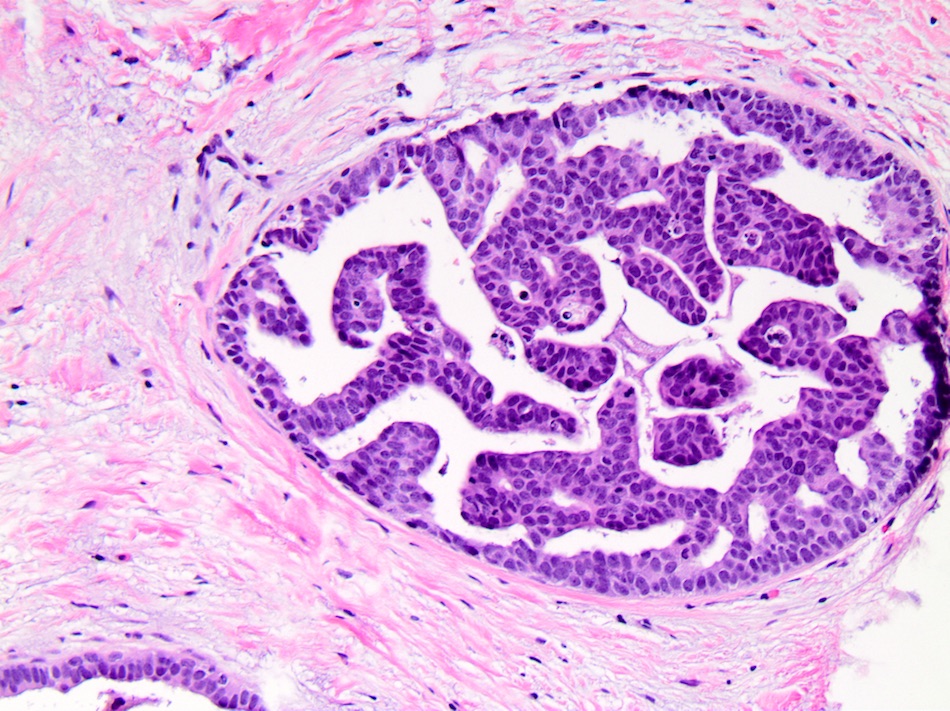
- Micropapillary:
- Papillary fronds and tufts lacking fibrovascular cores projecting into duct lumen
- Papillae often have club shaped cells comprising the micropapillae are uniform in appearance
- Tips of fronds may fuse, forming bridges and arcades
- Papillary:
- Papillary fronds containing prominent fibrovascular septa projecting into duct lumen, papillary cores generally lack myoepithelial cell layer
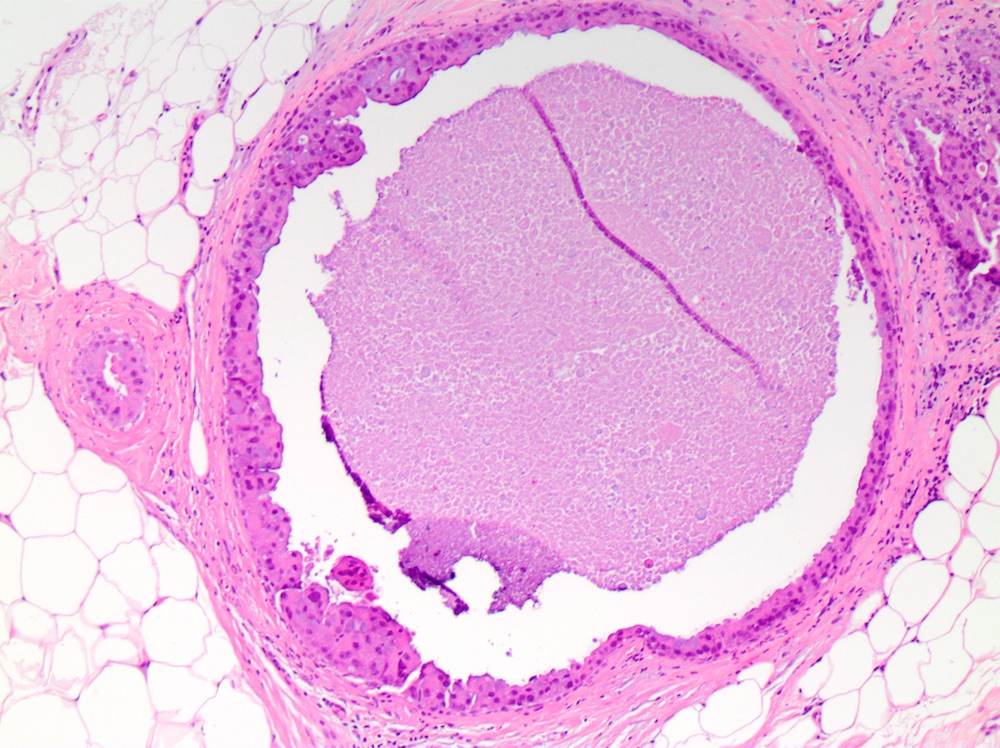
- Solid:
- Lumen of ducts or lobules filled with sheets of cohesive cells
- Cells are evenly spaced especially in low or intermediate grade DCIS
- Flat or clinging:
- 1 - 2 layers of generally high grade malignant cells lining a gland with a large empty lumen
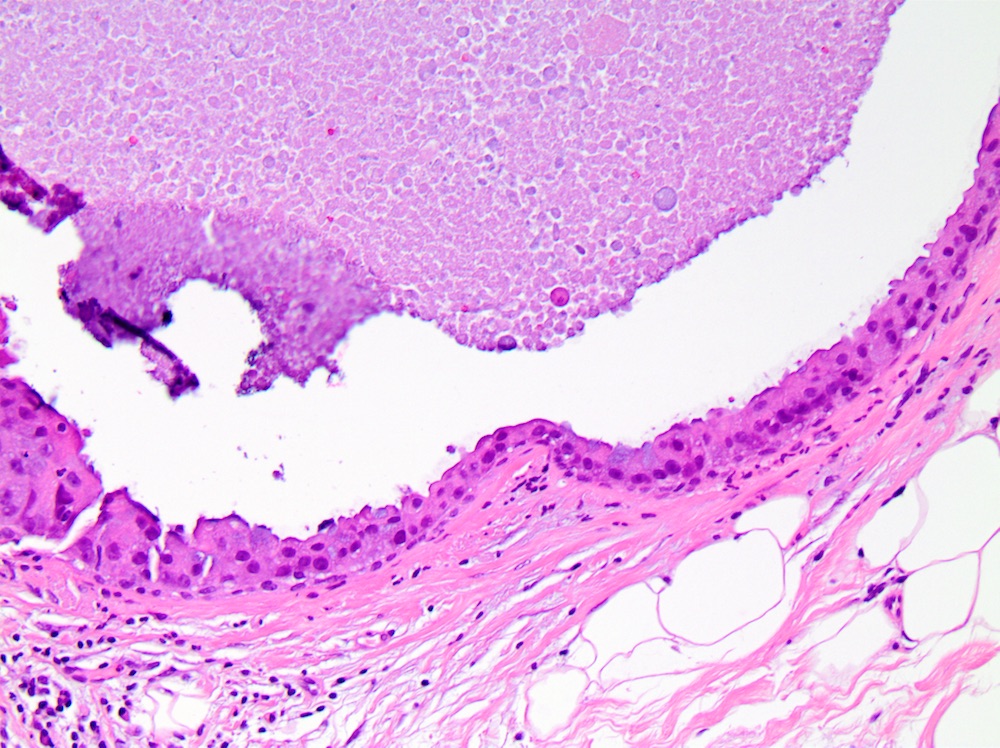
- Comedo:
- Central expansile necrosis containing cellular debris, generally associated with high grade DCIS, frequently associated with coarse microcalcifications
- Cribriform:
- No universally accepted grading system for DCIS but more recently endorsed classification systems stratify DCIS by nuclear grade (low, intermediate, high) and presence or absence of necrosis (Lancet 1995;345:1154, Hum Pathol 1998;29:1056, Cancer 1997;80:1798, Surg Clin North Am 1990;70:853)
- Nuclear grade:
- Low grade:
- Monotonous, round nuclei with smooth contours, small size nuclei (size of normal ductal epithelial cell or 1 - 1.5 diameter of normal red blood cell)
- Diffuse fine chromatin, absent or indistinct nucleoli, no or rare mitotic figures, necrosis is uncommon but does not preclude the diagnosis of low grade DCIS
- Intermediate grade:
- Moderate pleomorphism, mild to moderate variability in nuclear size
- Variably coarse chromatin, occasional nucleoli, infrequent mitoses, features in between low and high grade DCIS
- Loss of monotony can mimic usual ductal hyperplasia
- High grade:
- Prominent pleomorphism, large size nuclei (> 2.5 size of normal ductal epithelial cell)
- Vesicular chromatin with irregular distribution, prominent nucleoli, frequent mitoses, comedo necrosis frequent but not required
- Low grade:
- Necrosis:
- College of American Pathologists DCIS protocol recommends reporting necrosis as not present or present and if present must be differentiated as focal or comedo
- Protocol for the examination of specimens from patients with ductal carcinoma in situ (DCIS) of the breast (College of American Pathologists: Protocol for the Examination of Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast [Accessed 6 March 2020])
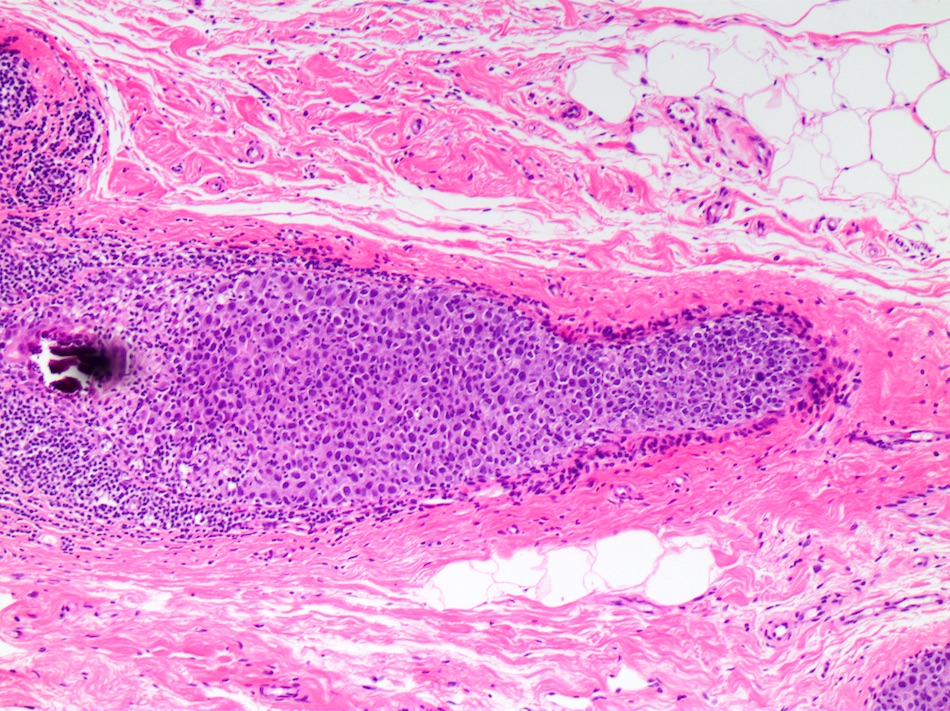
- Myoepithelial cell layer surrounding ducts spaces containing DCIS is intact, but may be attenuated especially in high grade DCIS
- Associated stromal reaction (chronic inflammatory infiltrate, fibrosis / sclerosis) may be prominent in areas surrounding DCIS, especially in high grade DCIS and does not indicate invasion
- Cancerization of lobules, extension of DCIS into acini of terminal duct lobular unit
- Variants include apocrine, cystic hypersecretory, squamous, spindle cell, clear cell, signet ring cell, mucinous, small cell, others
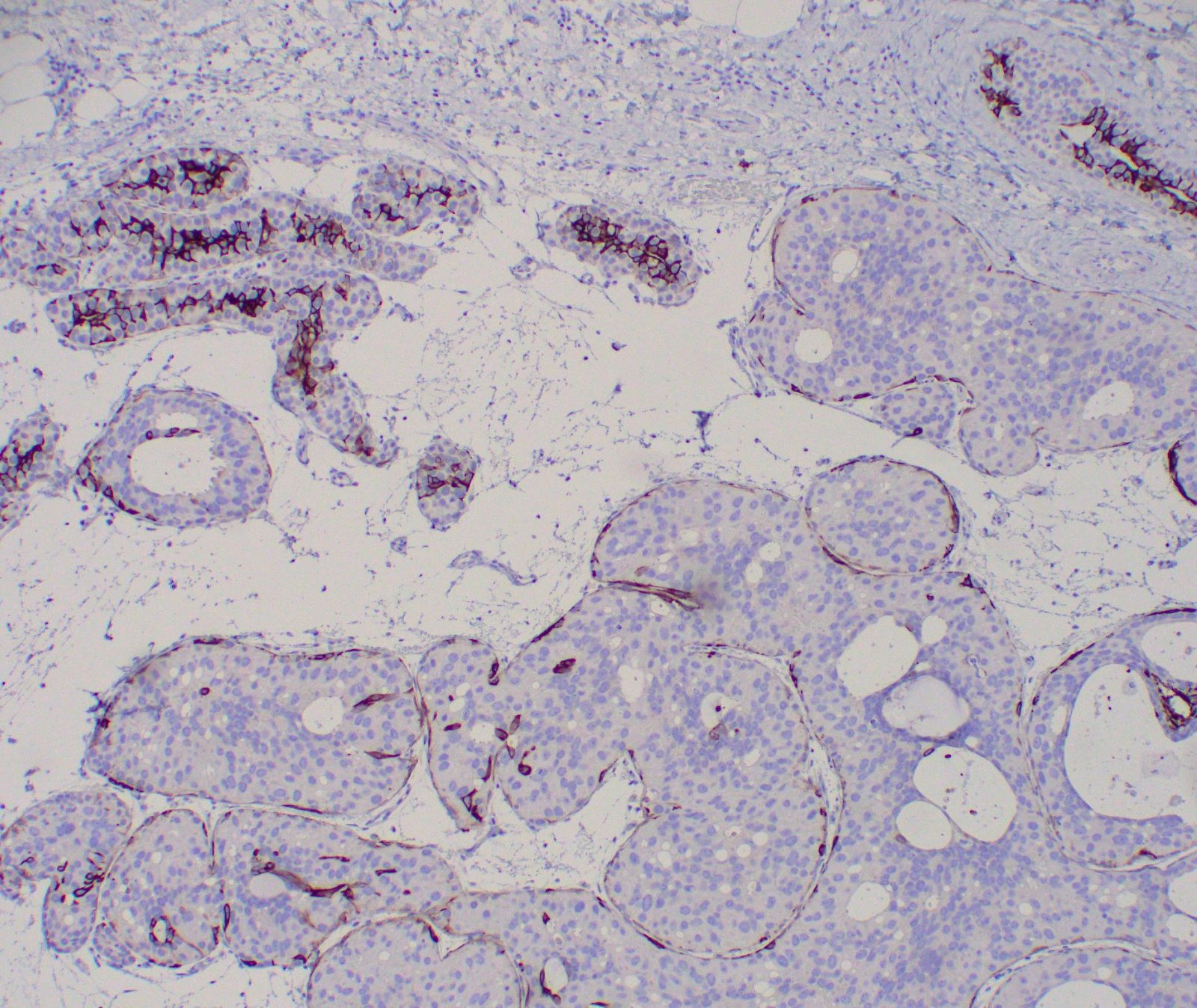
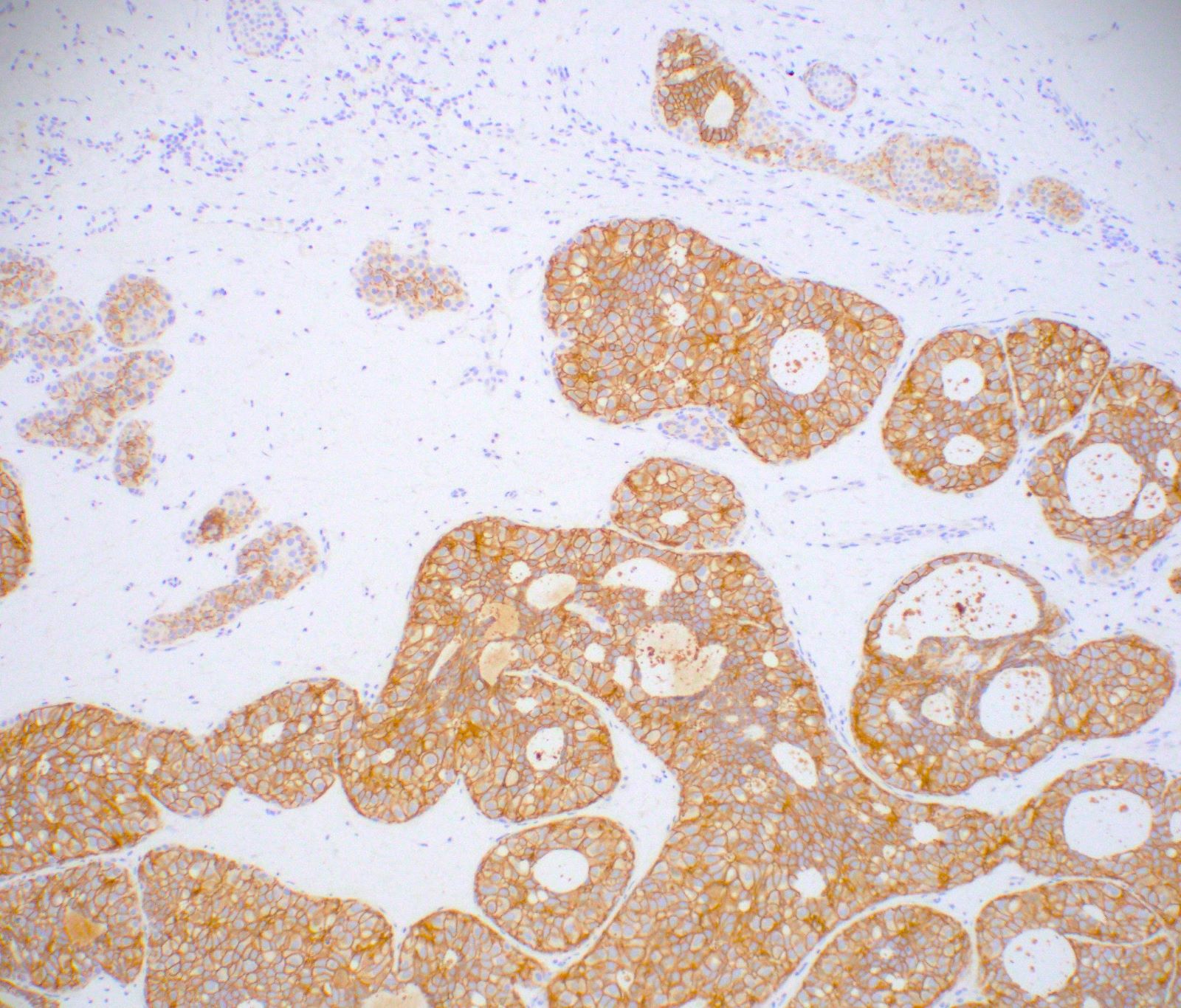
Microscopic (histologic) images
Cytology description
- Non high grade DCIS: cohesive sheets and aggregates, balls or papillary fragments, mild to variable atypia, some debris, often microcalcifications (Lancet 1995;345:1154, Cancer 2005;105:21)
- High grade DCIS: large pleomorphic cells, irregular aggregates and single cells, necrotic debris, individual cell necrosis, microcalcifications (Lancet 1995;345:1154, Cancer 2005;105:21)
- Core needle biopsy is preferred to fine needle aspiration cytology (FNAC) due to the uncertainty in differentiating between in situ and invasive carcinoma by aspiration (Cancer 1998;84:186, Diagn Cytopathol 1993;9:713, Am J Clin Pathol 1989;92:736)
Positive stains
- Myoepithelial cells:
- p40, p63, SMMS, calponin, CK5/6 and CK5 highlight surrounding intact myoepithelial cell layer (but may be attenuated compared to normal mammary ductal-lobular structures, especially in high grade DCIS) (Am J Surg Pathol 2009;33:227)
- Acinar cells:
- E-cadherin (strong membranous) (Am J Surg Pathol 2001;25:229)
- p120 (strong membranous)
- Estrogen receptor and progesterone receptor, overall ER positive rate ~69%, expression is correlated with nuclear grade (strongly positive in low grade DCIS, frequently negative in high grade DCIS) (J Cancer 2011;2:232, Br J Cancer 2008;98:137)
Negative stains
- Acinar cells:
- CK5/6 and CK5, CK14, 34betaE12 usually negative, however may be positive if high grade with basal-like phenotype or squamous differentiation and should not be mistaken for heterogenous pattern of expression that is seen in usual type ductal hyperplasia (Hum Pathol 2002;33:620, Am J Surg Pathol 1999;23:1048, Mod Pathol 2010;23:S8)
Molecular / cytogenetics description
- Low grade DCIS and high grade DCIS are genetically distinct
- Low grade has frequent chromosomal losses at 16q and 17p and gains at 1q
- High grade has frequent losses at 8p, 11q, 13q and 14q, and gains at 5p, 8q and 17q (J Pathol 2005;205:248)
- High grade DCIS has similar molecular profile as invasive breast cancer (Cancer Res 2015;75:3980)
Sample pathology report
- Right breast, 10:00 calcifications with ribbon clip, stereotactic core needle biopsy:
- Ductal carcinoma in situ (DCIS), intermediate nuclear grade, solid and cribriform patterns with comedo necrosis and microcalcifications
- Immunohistochemical biomarker results: Estrogen receptor positive (90%, strong intensity), Progesterone receptor positive (40%, moderate intensity)
- Ductal carcinoma in situ (DCIS), intermediate nuclear grade, solid and cribriform patterns with comedo necrosis and microcalcifications
Differential diagnosis
- Usual type ductal hyperplasia:
- Heterogenous proliferation of cells with variable size/shape, that are irregularly distributed with cohesive or syncytial growth, haphazard orientation of nuclei, nuclear overlap, nonrigid peripheral slit-like fenestrations with nuclei parallel to the lumina
- Heterogeneity can mimic intermediate grade DCIS
- Variable or mosaic pattern of expression of CK5/6 and CK5 and estrogen receptor
- Atypical ductal hyperplasia:
- Architectural and cytologic features suggestive of but not diagnostic for a diagnosis of low grade DCIS, partial involvement of ducts, limited quantitative extent
- Lobular carcinoma in situ:
- Low grade discohesive cells, often feathery clear space between cells, with solid growth pattern, intracytoplasmic vacuoles, lack of polarization around luminal spaces
- Reduced or absent membranous expression of E-cadherin, cytoplasmic reactivity for p120
- Invasive cribriform carcinoma:
- Infiltrative growth pattern, stromal reaction
- Lack circumscription by myoepithelial markers (p40 / p63 / SMMS / calponin / CK5/6 and CK5)
- Adenoid cystic carcinoma:
- Infiltrative growth pattern, dual cell population comprised of ductal epithelial cells and basal or myoepithelial cells, pseudolumens containing basement membrane material
- Myoepithelial markers (p40 / p63 / SMMS / calponin / CK5/6 and CK5) highlight myoepithelial cell component
- Negative for estrogen receptor and progesterone receptor
- Positive for CD117/KIT and MYB
- Collagenous spherulosis:
- Pseudolumens containing nodules of flocculent blue-grey basement membrane enclosed by cuticle of basement membrane and surrounded by stellate myoepithelial cells
- Lacks true cribriform spaces with ductal epithelial cells polarized around true lumens
- Can be involved by atypical lobular hyperplasia or lobular carcinoma in situ
- Myoepithelial markers (p40 / p63 / SMMS / calponin / CK5/6 and CK5) highlight myoepithelial cell component
- Lymphovascular invasion:
- Gynecomastia:
- Micropapillae have tapered (nonclubbed) configuration, features of usual type ductal hyperplasia (cellular heterogeneity, haphazard, nonuniform distribution)
- Solid papillary carcinoma:
- Solid growth pattern punctuated by delicate fibrovascular cores, perivascular pseudorosettes, neuroendocrine features, extracellular mucin
- Lack circumscription by myoepithelial markers (p40 / p63 / SMMS / calponin / CK5/6 and CK5) at periphery of nodules
Board review style question #1
- Which of the following statements regarding DCIS is correct?
- Adjuvant anti endocrine therapy reduces the risk of local recurrence in patients with DCIS who have undergone breast conserving surgery with radiation therapy
- DCIS is generally a clinically and mammographically occult lesion
- Low grade DCIS is a nonobligate precursor lesion to high grade DCIS
- Recommended standard biomarkers to evaluate and report in patients with DCIS include estrogen receptor, progesterone receptor, and HER2
- Sentinel lymph node sampling is not indicated in patients with DCIS only, as the disease is noninvasive and there is no risk of metastatic involvement of axillary lymph nodes
Board review style answer #1
A. DCIS is a nonobligate precursor lesion to invasive breast cancer. Molecular studies have demonstrated that low and high grade DCIS are genetically distinct biologic entities. DCIS most often presents mammographically as microcalcifications. Per ASCO/CAP guidelines, currently the only recommended biomarker for DCIS is estrogen receptor, while testing for progesterone receptor is optional. Unlike for invasive breast carcinoma, HER2 is not assessed in patients with DCIS only as anti HER2 therapy is not indicated. Sentinel lymph node sampling has a role in subset of DCIS only patients, the reported positive rate is 0.4 - 13.7% (mostly isolated tumor cell deposits and micrometastases) presumably due to missed or undersampled invasive disease. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial, tamoxifen reduced the likelihood of ipsilateral recurrence at five years from 9 - 6%, and also reduced the risk of a tumor in the contralateral breast. (J Clin Oncol 2014;32:1365, Lancet 1999;353:1993)
Comment Here
Reference: DCIS - general
Comment Here
Reference: DCIS - general
Board review style question #2
- A 55 year old woman undergoes a stereotactic core needle biopsy for mammographically detected microcalcifications. Which statement is true regarding the grade and expected immunohistochemical staining pattern of this lesion?
- Negative for circumferential calponin and p63
- Positive (Score 3+) for HER2 by immunohistochemistry
- Reduced or absent membranous reactivity for E-cadherin
- Strongly, diffusely positive for estrogen receptor
- The lesion is high grade
Board review style answer #2
D. The biopsy shows low grade DCIS with a cribriform pattern and associated microcalcifications. Loss of E-cadherin expression is seen lobular neoplasia. Myoepithelial markers such as calponin and p63 will show retained myoepithelial cell layer surrounding foci of DCIS. Assessment of HER2 status in DCIS is not indicated per current guidelines, however HER2 overexpression is more often observed in high grade DCIS. Low grade DCIS typically shows strong estrogen receptor expression.
Comment Here
Reference: DCIS - general
Comment Here
Reference: DCIS - general