Table of Contents
Definition / general | Trade name | Clinical information | Pathophysiology | Diagrams / tables | Uses by pathologists - general | Uses by pathologists - breast carcinoma | Uses by pathologists - gastric and gastroesophageal adenocarcinomas | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Yeh YA. Trastuzumab. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastherceptin.html. Accessed April 2nd, 2025.
Definition / general
- Humanized IgG1 kappa monoclonal antibody targets HER2 / neu receptor (human epidermal receptor 2)
- Discovered by scientists including Dr. Axel Ullrich and Dr. H. Michael Shepard at Genentech, Inc., South San Francisco, CA
- Synonym: anti-HER2 (N Engl J Med 2007;357:39)
Trade name
- Herceptin®
Clinical information
- Approved by U.S. Food and Drug Administration on September 25, 1998 (Oncology (Williston Park) 1998;12:1727)
- Biosimilars (Ther Adv Med Oncol 2019;11:1758835919887044):
- Trastuzumab-dkst (Ogivri): FDA approved, 2017
- Trastuzumab-pkrb (Herzuma): FDA approved, 2018
- Trastuzumab-dttb (Ontruzant): FDA approved, 2019
- Trastuzumab-qyyp (Trazimera): FDA approved, 2019
- Trastuzumab-anns (Kanjinti): FDA approved, 2019
- Indications and usage (National Cancer Institute: Trastuzumab [Accessed 9 February 2022]):
- Breast cancer with HER2 positive and hormone receptor negative: combination therapy or alone after combination chemotherapy including anthracycline
- Metastatic breast cancer: combined with paclitaxel as first line treatment or alone in patients with previous chemotherapy
- Gastric adenocarcinoma or gastroesophageal junctional adenocarcinoma with HER2 positive and metastasis: combination therapy with cisplatin and capecitabine or fluorouracil
- Clinical pharmacokinetics (Oncologist 2011;16:800):
- Route of administration: intravenous
- Elimination: predominantly in epithelial cells; renal elimination very low
- Half life: 28 days
- Side effects (Ann Pharmacother 2019 Oct 9 [Epub ahead of print]):
- Infusion related reactions, if occurring < 24 hours after first infusion: fever, chills, rash, headache, dizziness; (severe) angioedema, respiratory distress syndrome, severe hypotension, anaphylaxis (Oncologist 2007;12:601)
- Cardiotoxicity: hypotension, congestive heart failure, ventricular dysfunction
- Respiratory: dyspnea, bronchospasm, hypoxia, asthma
- Gastrointestinal: diarrhea, vomiting, dyspepsia
- Hematologic: anemia, leukopenia, thrombocytopenia
- Mechanism of drug resistance (Front Oncol 2012;2:62):
- Loss of phosphatase and tensin homologue (pTEN)
- Activation of the phosphoinositide 3 kinase pathway
- Overexpression of other surface receptors (insulin-like growth factor)
- Trastuzumab costs about U.S. $70,000 for a full course of treatment, U.S. $1,800 - $1,955 per 440 mg vial (Wikipedia: Trastuzumab [Accessed 9 February 2022])
Pathophysiology
- HER2, a member of HER family, does not have a receptor specific ligand binding site
- HER2 / neu signaling in cancer cells:
- HER2 heterodimerizes with HER1, HER3 or HER4, phosphorylates and activates intracellular tyrosine kinase domain
- Activated tyrosine kinase on HER2 activates PI3K-Akt pathway and induces cellular survival
- Activated tyrosine kinase activates SOS, induces a cascade of activation of RAS-RAF-MAPK-MEK and MAPK, eventually promotes cellular proliferation
- Cleavage of HER2 extracellular domain produces phosphorylated P95 that could activate downstream signal transduction
- Mechanism of action:
- Upon binding the extracellular domain of HER2, trastuzumab reduces cleavage of HER2 receptor, blocks the activation ability of P95 residue and eventually decreases signaling
- Binding of trastuzumab to extracellular domain of HER2 inhibits homo or heterodimerization of HER2 to HER1, HER2, HER3 or HER4 and reduces signaling
- Trastuzumab binds to immune effector cells and activates antibody dependent cell mediated cytotoxicity, eventually leads to tumor cell lysis
- Endocytosis of HER2 is increased and leads to intracellular HER2 degradation
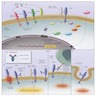
- Reference: N Engl J Med 2007;357:39, see Diagrams / tables, figure 3
Diagrams / tables
Uses by pathologists - general
- Identify HER2 positive tumors to identify candidates for treatment
- Primary breast carcinomas
- Gastric and gastroesophageal adenocarcinomas
- Metastatic diseases (test performed in a metastatic site)
Uses by pathologists - breast carcinoma
- HER2 immunohistochemistry in breast carcinoma:
- ASCO-CAP HER2 Test 2013 and 2018 Guideline Recommendation (Arch Pathol Lab Med 2014;138:241, Arch Pathol Lab Med 2018;142:1364)
- Negative (score 0):
- No staining
- Incomplete faint membrane staining in ≤ 10% invasive tumor cells
- Negative (score 1+):
- Incomplete faint membrane staining in > 10% invasive tumor cells
- Equivocal (score 2+): perform HER2 ISH
- Incomplete, weak / moderate membrane staining in > 10% invasive tumor cells
- Complete, intense membrane staining in ≤ 10% invasive tumor cells
- Positive (score 3+):
- Complete, intense circumferential membrane staining in > 10% invasive tumor cells
- Negative (score 0):
- ASCO-CAP HER2 Test 2013 and 2018 Guideline Recommendation (Arch Pathol Lab Med 2014;138:241, Arch Pathol Lab Med 2018;142:1364)
- HER2 in situ hybridization (ISH) (see Diagrams / tables, figure 1):
- The Panel recommends that concomitant IHC review should become part of the interpretation of single probe ISH results and the Panel preferentially recommends the use of dual probe instead of single probe ISH assays
- Positive:
- Single probe average HER2 copy number ≥ 6.0 signals/cell
- Dual probe HER2/CEP17 ratio ≥ 2.0 and an average HER2 copy number ≥ 4.0
- Additional workup required:
- If a case has a HER2/CEP17 ratio ≥ 2.0 but the average HER2 signals/cell is < 4.0, a definitive diagnosis will be rendered based on additional workup
- If a case has an average of ≥ 6.0 HER2 signals/cell with a HER2/CEP17 ratio of < 2.0, formerly diagnosed as ISH positive for HER2, a definitive diagnosis will be rendered based on additional workup
- If the case has an average HER2 signals/tumor cell of ≥ 4.0 and < 6.0 HER2 signals/cell and HER2/CEP17 ratio is < 2.0, formerly diagnosed as ISH equivocal for HER2, a definitive diagnosis will be rendered based on additional workup
- Additional workup steps:
- IHC testing for HER2 should be performed using sections from the same tissue sample used for ISH
- If the IHC result is 3+, diagnosis is HER2 positive
- If the IHC result is 2+, recount ISH by having an additional observer, blinded to previous ISH results, count at least 20 cells that include the area of invasion with IHC 2+ staining:
- If reviewing the count by the additional observer changes the result into another ISH category, the result should be adjudicated per internal procedures to define the final category
- If the count remains an average of < 4.0 HER2 signals/cell and HER2/CEP17 ratio is ≥ 2.0, the diagnosis is HER2 negative with a comment
- If the HER2/CEP17 ratio remains < 2.0 with ≥ 6.0 HER2 signals/cell, the diagnosis is HER2 positive
- If the count remains an average of ≥ 4.0 and < 6.0 HER2 signals/cell with HER2/CEP17 ratio < 2.0, the diagnosis is HER2 negative with a comment
- If the IHC result is 0/1+, diagnosis is HER2 negative with comment
- IHC testing for HER2 should be performed using sections from the same tissue sample used for ISH
Uses by pathologists - gastric and gastroesophageal adenocarcinomas
- HER2 immunohistochemistry testing in gastric and gastroesophageal adenocarcinoma (see Diagrams / tables, figure 2):
- 2017 CAP / ASCP / ASCO guidelines (J Clin Oncol 2017;35:446, Virchows Arch 2010;457:299):
- Representative surgical samples or at least 6 to 8 biopsy samples
- Score 0: negative
- No membranous staining or staining in < 10% of tumor cells (surgical specimen) or < 5 cohesive tumor cells (biopsy)
- Score 1+: negative
- Weak staining in only one part of the membrane in ≥ 10% of tumor cells (surgical specimen) or at least 5 cohesive tumor cells (biopsy)
- Score 2+: equivocal, perform HER2 ISH
- Moderate / weak complete or basolateral membranous staining in ≥ 10% of tumor cells (surgical specimen) or at least 5 cohesive tumor cells (biopsy)
- Score 3+: positive
- Strong or complete or basolateral membranous staining in ≥ 10% of tumor cells (surgical specimen) or at least 5 cohesive tumor cells (biopsy)
- 2017 CAP / ASCP / ASCO guidelines (J Clin Oncol 2017;35:446, Virchows Arch 2010;457:299):
- HER2 ISH in gastric and gastroesophageal adenocarcinoma (Mod Pathol 2012;25:637):
- Positive: HER2/CEP17 ratio ≥ 2.0
- Positive: HER2 copy number > 6.0 (using single probe)
- HER2 copy number 4 to 6: use dual probe testing and recount 20 cells
Additional references
- Am J Clin Pathol 2019;152:17, N Engl J Med 2019;381:1284, Cell 2019;179:8, J Hematol Oncol 2019;12:50, Cancer Treat Rev 2019;75:12, Br J Cancer 2019;121:199, J Clin Med 2019;8:E254, Exp Hematol Oncol 2019;8:25, Clin Cancer Res 2019;25:2033, Breast Cancer Res 2014;16:209, ISRN Oncol 2012;2012:428062, N Engl J Med 2012;366:664, N Engl J Med 2012;366:663, Hum Pathol 2010;41:304, Oncogene 2007;26:3637, Oncogene 2007;26:6577
Board review style question #1
Which of the following drugs target HER2 / neu on cancer cells?
- Dabrafenib
- Larotrectinib
- Trametinib
- Trastuzumab
Board review style answer #1
Board review style question #2
Which of the following results is interpreted as positive staining (score 3+) for HER2 in gastric and gastroesophageal adenocarcinoma?
- Intense nuclear and cytoplasmic staining > 10% of tumor cells
- Intense luminal membranous staining > 10% of tumor cells
- Intense basolateral membranous staining > 10% of tumor cells
- Intense luminal and lateral membranous staining > 10% of tumor cells
Board review style answer #2