Table of Contents
Definition / general | Indications | Tissue type | Freezing tissue | Cryosectioning | Staining slides | Troubleshooting | Microscopic (histologic) images | Videos | Additional referencesCite this page: Wallace J. Frozen section procedure. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastfrozenprocedure.html. Accessed April 2nd, 2025.
Definition / general
- A frozen section (cryosection) is a pathological laboratory technique used for rapid microscopic analysis / diagnosis of a specimen / disease
- Usually used with oncologic surgery
- Rapid diagnosis can guide intra-operative patient management
Indications
- Use frozen section to
- Provide rapid gross or microscopic diagnosis to identify an unknown pathologic process, identify extent of disease / evaluate margins, identify metastases or simply identify a tissue
- Process tissue to provide appropriate and accurate diagnosis, prognosis and to adhere to research and special study protocols
- Confirm that pathological tissue is present for diagnosis on permanent sections
- Do not use if
- Frozen section diagnosis has no immediate implications for decision making
- Tissue is needed for permanent processing (is unique or small or requires extensive study for diagnosis)
- Consider not freezing tissue if
- Frozen section is known to produce severe artifacts that hinder proper interpretation
- Tissue is heavily ossified / calcified
- Risk of serious infection (HIV, TB, hepatitis B or C)
- Tissue is fatty
Tissue type
- Tissue should be received fresh, otherwise it will not stay on slide
- At time of receipt of tissue, decide whether to obtain smears or touch preps and whether to freeze all or part of it
- Touch preps and smears are often performed on lymph nodes suspicious for lymphoma
- Some primary small lesions should not be entirely submitted for frozen section
- There is debate on whether sentinel nodes should be entirely or representatively submitted for frozen section
- Fixed tissue:
- There are special slides to keep tissue affixed to slide
- To freeze fixed tissue, make sure it has been preserved in formalin and not alcoholic fixatives like Carnoy's, because tissue fixed in alcohol is harder to freeze
- Avoid freezing tissue fixed with heavy metal salts such as B5 and Helly's (Zenker’s formal solution), which can denature proteins and shrink the tissue
- Avoid hard tissues like bone and cartilage that require decalcification
- Avoid tissues with a lot of fat
- Avoid tissues from patients with known TB or other infection (if absolutely necessary, wear appropriate protection)
- Avoid freezing tissue that will be needed to make a permanent diagnosis
Freezing tissue
- OCT (optimal cutting temperature) or similar embedding media like TBS or Cryogel should be placed on an appropriate sized chuck that has been precooled in a cryostat
- The chuck should be clean
- A toothbrush is useful to remove tissue and OCT
- Dipping the chuck in methanol removes ice crystals
- Place the chuck into a -20 to -15 degree (optimal) cryostat; note that the OCT media should not be frozen completely
- It is better to have a semisolid consistency; this will alleviate tissue artifact
- Tissue size should be no greater than 3mm - 5mm in greatest dimension (thinner specimens have shorter freezing time and minimal ice crystal artifact formation)
- The smaller the tissue, the more even and thorough the freeze
- Place the tissue on the semisolid chuck and add more media rapidly over the tissue, covering it entirely but avoiding overflow
- Place chuck quickly back into the cryostat
- Apply heat sink or CO2 aerosol (optional) to rapidly freeze or use "quick freeze" option on cryostat
- Histobath: being phased out
- Cryowells: useful in keeping all tissue on an even plane; also helpful in eliminating loss of smaller tissues that are frozen with larger ones, although recommended to not freeze different sizes together
- Aerosol sprays: often canned CO2 (but may aerosolize infectious diseases)
- Liquid nitrogen
- Isopentane based workflow (Virchows Arch 2008;452:305)
Cryosectioning
- Once the chuck is in position, there should be a manual or an automatic advance option to move the block close to the cutting blade
- Fully face the tissue by using a trim setting on your cryostat; if you do not have this setting, then an advance button should be available, which should be pressed each time before one full revolution of the instrument's wheel
- If wells are used to freeze the blocks, then the tissue should be on an even plane and the tissue will be faced faster
- To polish the tissue, avoid advancing the cryostat or deselect the trim setting on the cryostat and turn 10 - 15 times
- As you cut the tissue, anchor the tissue to prevent folding or curling; this can be done with an anti roll bar (a plastic plate attached to cryostat) or by using a precooled paintbrush with stiff bristles and a wide gripping surface
- The brush should be held like a pen with your left hand at an angle
- You can rest your fifth finger on the stage for stabilization
- Cutting the brushes' bristles at an angle can aid in the brush meeting the tissue flat over its length because you will hold it at an angle
- Turn the wheel with your right hand in a continuous motion without stopping; avoid speeding up or slowing down
- Avoid stopping the wheel at the beginning of the section, slowly grabbing the tissue and then resuming wheel revolutions; this can cause artifacts such as variation in section thickness and tissue folding
- Move the brush as the chuck moves
towards the blade; your brush should move down in
pace with the chuck
- You can rest your brush softly on the very bottom of your chuck avoiding tissue contact
- Pull the brush away easily as the chuck meets the blade
- The downward motion of the brush allows you to keep a continuous motion as you take your section
- A glass slide is gently laid upon the tissue section
- The tissue section should melt onto the slide
- Prepared slides should immediately go into formal alcohol, 95% alcohol (methanol/ethanol) or formalin while awaiting the stain line; if you delay this step, drying artifact will occur
- You can take a deeper level after approximately 20 turns (multiple levels may be needed for breast or prostate biopsies)
- Optimal cutting thickness is 4 - 7 microns for sectioning and 20 - 40 microns for trimming
Staining slides
- Keep all stains and solutions fresh and well maintained
- Dip slide in reagents in this order for H&E staining:
- After obtaining frozen section, IMMEDIATELY fix in 95% ethanol (even 15 seconds of delay can cause significant artifact)
- Formal alcohol, formalin or 95% alcohol: 45 - 60 seconds
- Water: 5 - 7 seconds
- Hematoxylin: 60 seconds
- Lithium carbonate or 0.2 % aqueous ammonia (Bluing): 15 - 20 seconds
- Eosin: 20 - 60 seconds
- 95% alcohol: 10 seconds
- 100% alcohol: 10 seconds
- Xylene, toluene, limonene derivatives and Clearite: 10 seconds
- Then add mounting media for cover slipping
Troubleshooting
- Ice crystal artifacts
- Due to slow freezing of tissue
- Solution: Freeze fast (flash / snap); the faster the freeze, the smaller the ice crystals, the less tissue damage (best freezing method is arguably liquid nitrogen)
- Smaller tissues yield less artifact - optimally tissue should be 0.5 x 0.5 x 0.3 cm or less
- Never freeze fragments larger than the diameter of the chuck
- Avoid freezing fat around tissue
- Blot the outer surface of the tissue dry with gauze before making your block
- Knife artifact
- A nicked cutting blade will produce a split / tear in your section
- Solution: change your blade every few cases; some institutions use a new blade for each case
- Overfreezing
- Can cause section to have holes
- Solution: polish block with a couple extra turns of the blade to create friction and warm up block by pressing on it with your finger (5 - 10 seconds)
- Underfreezing
- Underfreezing can be troublesome for fatty tissue
- Solution: add heat sink to block or select rapid freeze setting on your cryostat (if available)
- Staining issues
- Dirty "stain line" can cause floaters (extraneous foreign tissue) to adhere to slides; overly diluted stains and alcohols can diminish slide quality
- Poor staining hinders frozen section diagnoses, as nuclear detail is compromised
- Solutions: (a) maintain a clean stain line by frequent solution changes; (b) follow recommended staining times; (c) don't rush
- Note: brain tissue may stain best in eosin for 60+ seconds
- Water: should be changed after each frozen section
- Alcohols and stains: change at least weekly, alcohols may need to be changed more frequently depending on work load
- Fatty tissue
- Includes lymph nodes, breast, skin; may be too soft to cut
- Solution: maintain an extremely cold cutting temperature (-20C)
- Firm lymph nodes, spleen, brain and liver cut better at -10C; tissue may shatter if sectioning is performed at lower temps
- Air bubbles
- May be trapped under cover slips, which can cause the underlying tissue to dry out
- Solution: make sure an appropriate amount of resin (2 drops) is applied; gently move air bubbles off the slide with finger or tweezers; do not press on the slide too hard or it will break
- Overly thick sections
- May cause tissue to fall off slide
- Solution: reduce the cryostat's sectioning thickness
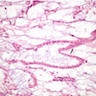
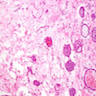
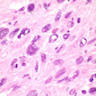
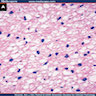
Microscopic (histologic) images
Videos
Brush technique
Embedding small specimens
Speed embedding
Additional references