Table of Contents
Apocrine adenosis / atypical apocrine adenosis | Apocrine adenoma | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Roychowdhury M, Jorns J. Apocrine adenosis / apocrine adenoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastapocrineadenosis.html. Accessed April 2nd, 2025.
Apocrine adenosis / atypical apocrine adenosis
Definition / general
Essential features
Terminology
ICD coding
Epidemiology
Sites
Pathophysiology
Clinical features
Diagnosis
Radiology description
Prognostic factors
Case reports
Treatment
Gross description
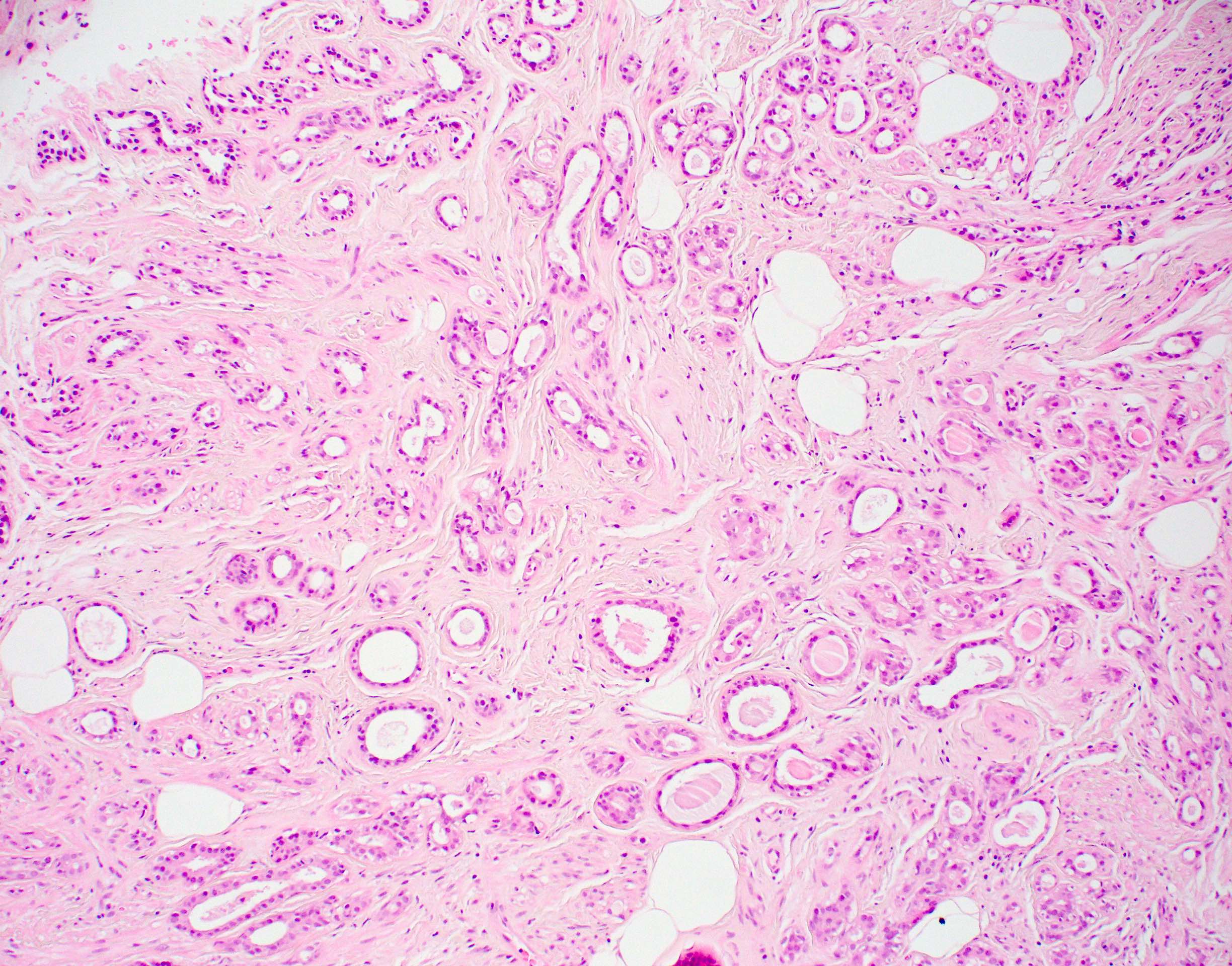
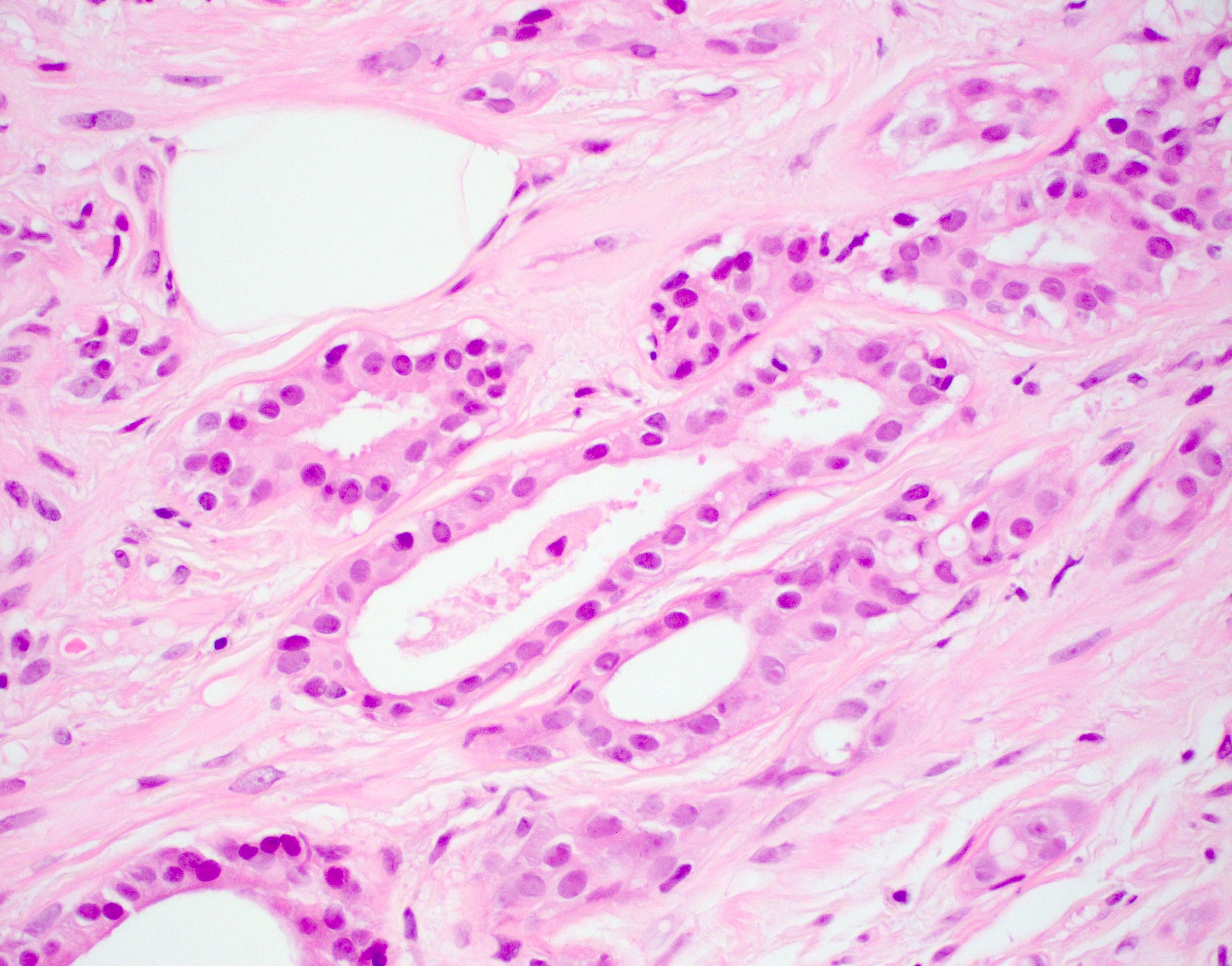
Microscopic (histologic) description
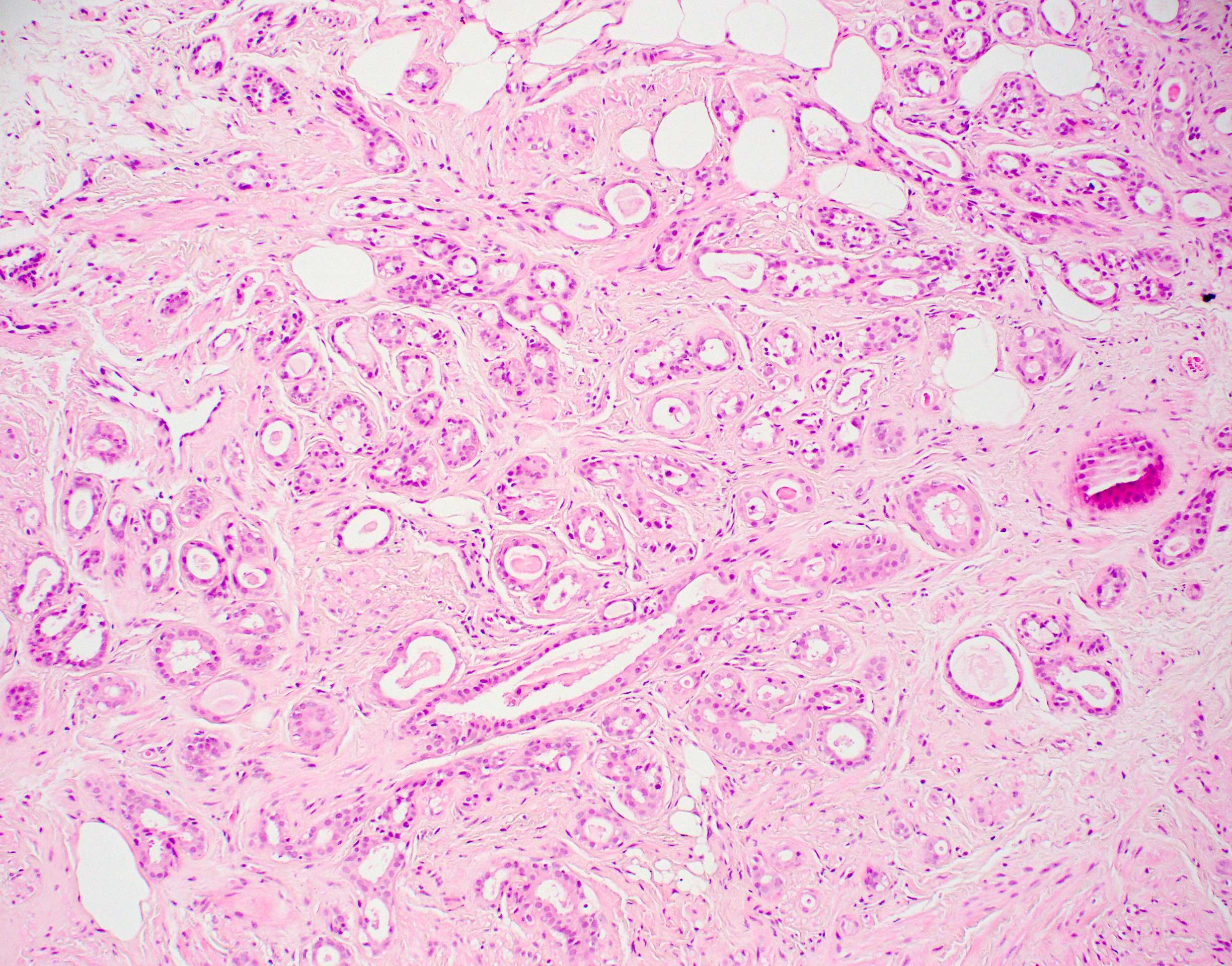
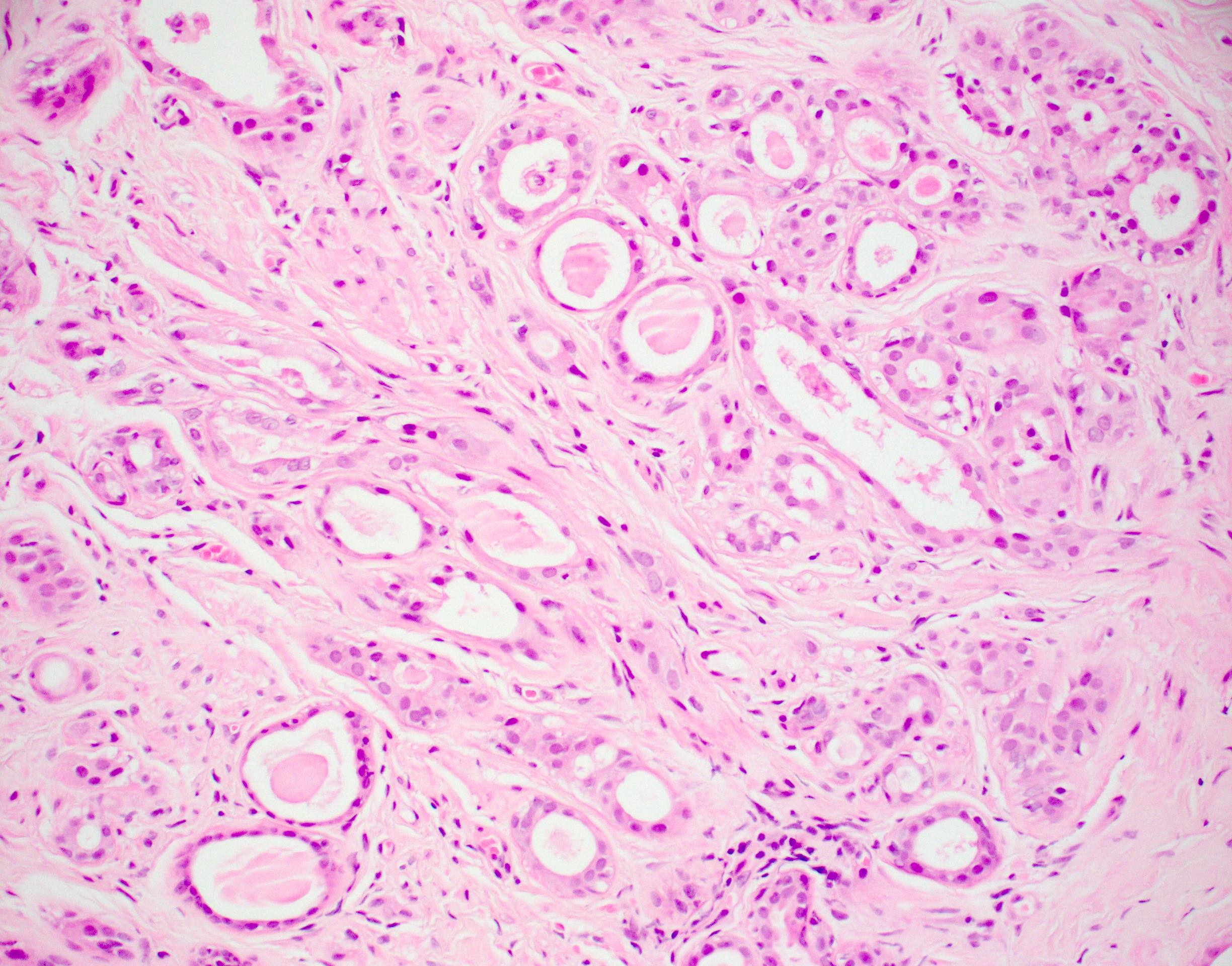
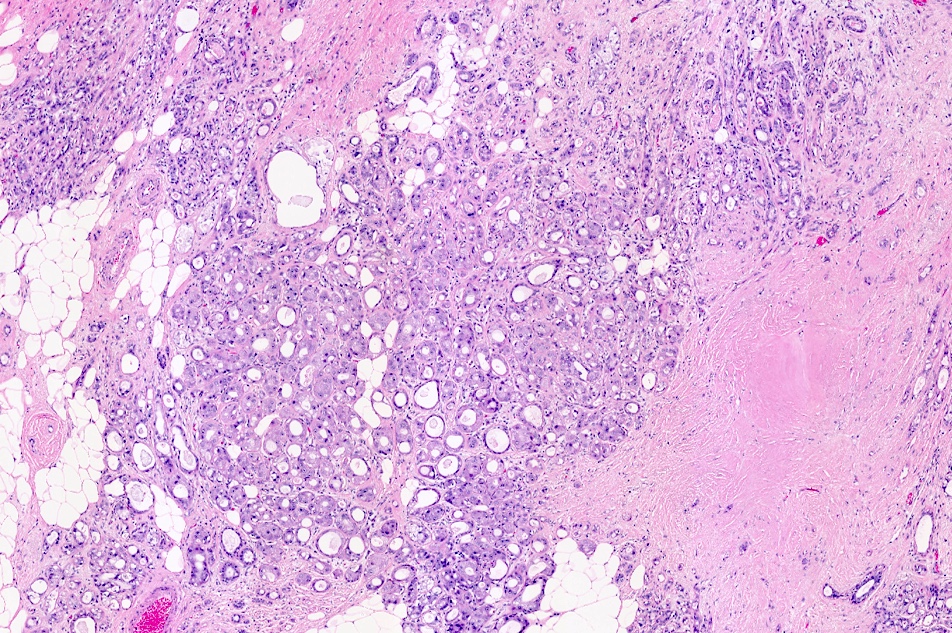
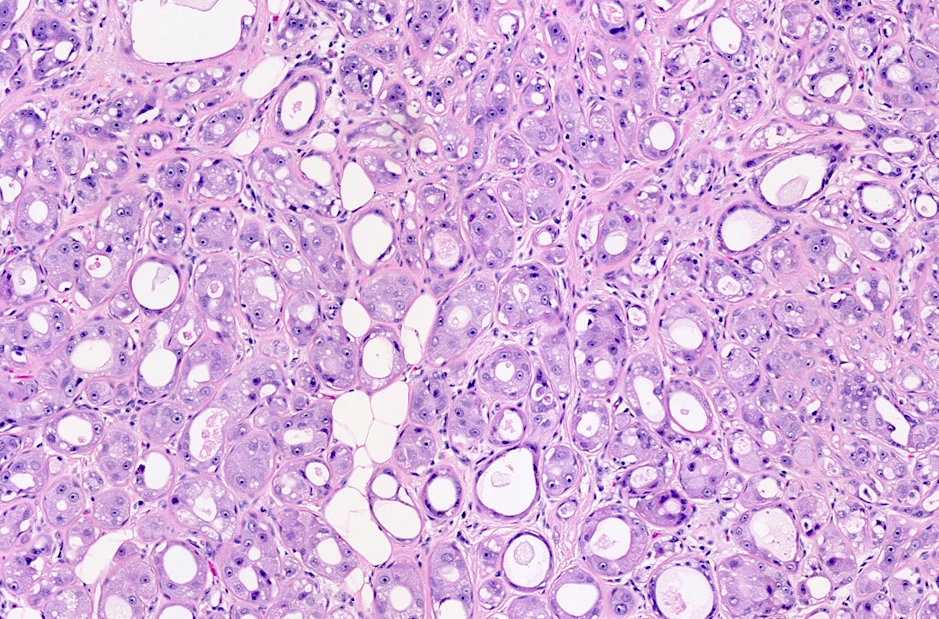
Microscopic (histologic) images
Contributed by Julie M. Jorns, M.D.
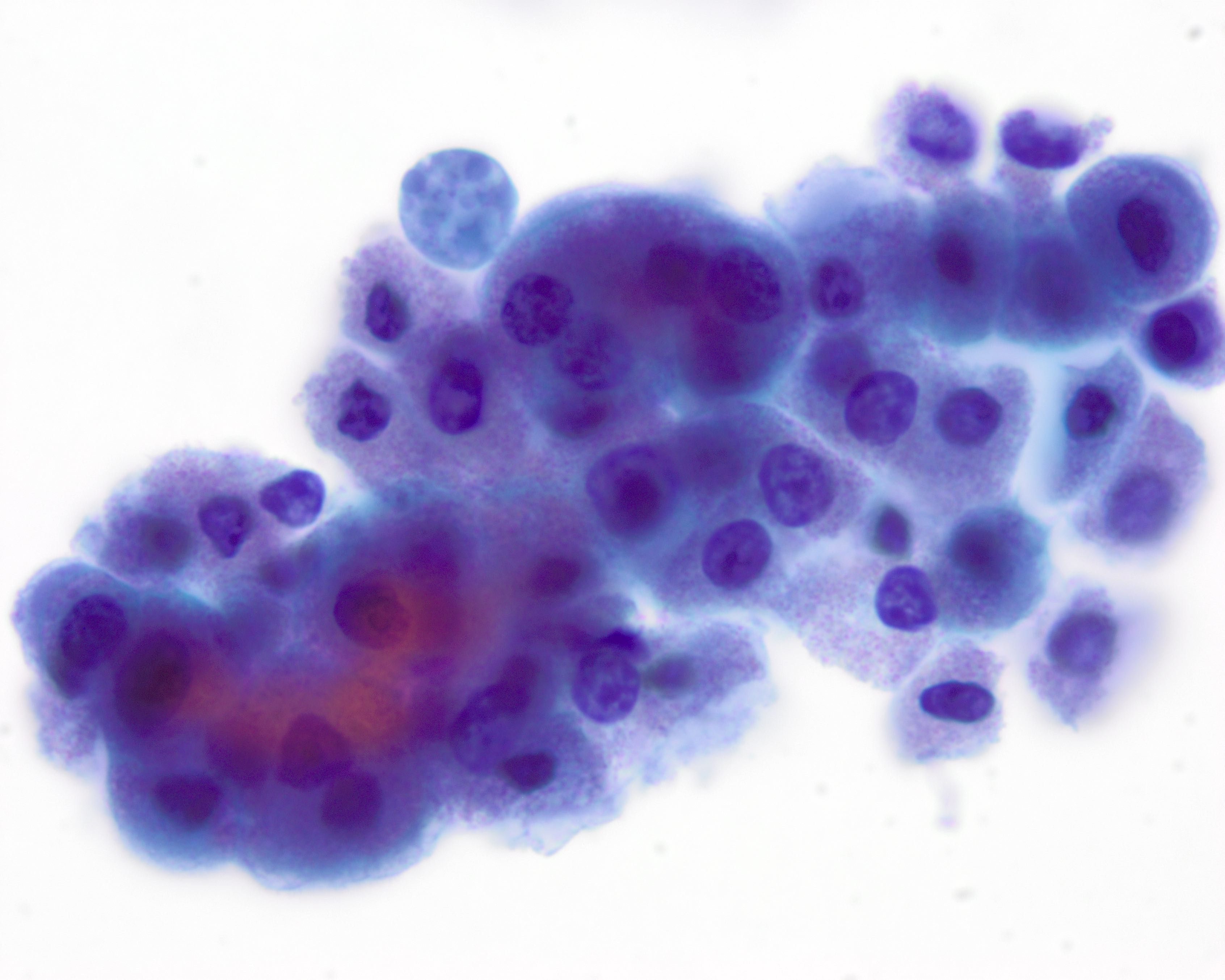
Cytology description
Cytology images
Contributed by Volodymyr Shponka, M.D.
Positive stains
Negative stains
Electron microscopy description
Sample pathology report
Differential diagnosis
Additional references
- Defined as the presence of apocrine cytology in a recognizable terminal duct lobular unit (TDLU) associated with sclerosing adenosis (J Clin Pathol 2007;60:1313)
- Preserved 2 cell layer (inner epithelial and outer myoepithelial cells) in which the epithelial layer has prominent apocrine features
Essential features
- Lobulocentric proliferation with distortion by stromal fibrosis / sclerosis and apocrine cytology
Terminology
- Recommended by WHO
- Apocrine adenosis
- Not recommended by WHO
- Sclerosing adenosis with apocrine metaplasia
- Nodular adenosis with apocrine metaplasia
- Terminology apocrine adenosis has also been used to refer to adenomyoepithelial adenosis, a different lesion
- Atypical apocrine adenosis (AAA): rare, defined as ≥ threefold variation in nuclear size
- Previous (historical) names for AAA
- Atypical apocrine hyperplasia
- Atypical apocrine metaplasia
ICD coding
- ICD-10
- ICD-11
Epidemiology
- Most frequent in third to fourth decades but occurs over a wide age range
- Atypical apocrine adenosis: rare - 0.4% of 9,340 cases in the Mayo Benign Breast Disease Cohort; average age is 58 years (Arch Pathol Lab Med 2012;136:179, Mod Pathol 1991;4:1)
Sites
- Terminal duct lobular unit; otherwise, no specific location within the breast
Pathophysiology
- Loss of heterozygosity in several loci (1p, 3, 11q, 16 and 17q)
- Accumulation of losses and gains if progression through apocrine DCIS and apocrine carcinoma
Clinical features
- Considered a part of the spectrum of fibrocystic changes
- Often an incidental finding or detected by screening
- Can present as a palpable mass if nodular adenosis / adenosis tumor
Diagnosis
- Histologic examination of tissue with or without immunohistochemistry
Radiology description
- Variable depending on the size / extent of breast involvement
- If focal, may not be visualized (i.e., incidental finding on histologic examination)
- Amorphous or pleomorphic clustered microcalcifications; architectural distortion or circumscribed to spiculated mass on mammogram
- Irregular, noncircumscribed mass on ultrasound is associated with upgrade to malignancy on resection (J Ultrasound Med 2020;39:1517, Breast J 2017;23:569)
Prognostic factors
- Increased risk of cancer of 1.5 - 2 times, as seen with proliferative fibrocystic changes
- Atypical apocrine adenosis (AAA): conflicting evidence of increased cancer risk
- Study of 37 patients showed no increased risk for carcinoma (Arch Pathol Lab Med 2012;136:179)
- Study of 37 patients showed the relative risk of developing carcinoma was 5.5 (95% CI 1.9 - 16), with age association - all patients developing cancer were > 60 years at diagnosis of AAA (Cancer 1996;77:2529)
- Upgrade risk: surgical resection showed carcinoma only in patients with coexisting carcinoma present on core biopsy (N = 10) in a study of 41 patients with apocrine adenosis (N = 29) and atypical apocrine adenosis (N = 12) (Ann Diagn Pathol 2016;24:4)
Case reports
- 37 year old woman with atypical apocrine adenosis diagnosed via fine needle aspiration (Acta Cytol 2002;46:369)
- 54 year old woman with atypical apocrine adenosis diagnosed via incidental discovery on coronary angiogram CT (Cureus 2020;15:e8624)
- 66 year old woman with nodular atypical apocrine adenosis with monoclonality via restriction fragment length polymorphism (RFLP) (Histopathology 2001;38:221)
Treatment
- Presence of apocrine adenosis alone in a core biopsy does not require surgical excision
- Coexisting atypia typically prompts surgical consultation, although excision is controversial based on limited prognostic data
Gross description
- Variable depending on extent of involvement and calcifications
- May be indistinguishable from surrounding breast tissue
- Multinodular, ill defined cuts with increased resistance due to fibrosis
- Gritty due to frequent calcifications but no chalky yellow white foci or streaks as seen in fat necrosis
- Circumscribed to ill defined white, fibrotic mass if nodular adenosis / adenosis tumor
Microscopic (histologic) description
- Cells with apocrine metaplasia have abundant eosinophilic cytoplasm with bright eosinophilic granules that are PAS positive
- Apocrine cells have round nuclei and may show nuclear pleomorphism (≥ threefold in atypical apocrine adenosis); a central, round, eosinophilic nucleolus is generally seen
- Rare multinucleation may be observed and is not considered atypical
- Rare to no mitosis
- No necrosis
Microscopic (histologic) images
Contributed by Julie M. Jorns, M.D.
Cytology description
- Often highly cellular
- Cells have apocrine metaplasia with prominent nucleoli and pleomorphism, possibly resembling carcinoma but minimal hyperchromasia
- Naked nuclei are present (Diagn Cytopathol 2007;35:296)
Cytology images
Contributed by Volodymyr Shponka, M.D.
Positive stains
- EMA, GCDFP-15 (Am J Surg Pathol 1993;17:99)
- Androgen receptor
- PAS for basement membrane (J Clin Pathol 1999;52:838, Mod Pathol 1993;6:318)
Negative stains
Electron microscopy description
- Distinct basal lamina present
Sample pathology report
- Left breast, core biopsy:
- Fibrocystic changes, including apocrine adenosis with microcalcifications
Differential diagnosis
- Apocrine carcinoma:
- Infiltrative growth
- Associated desmoplasia
- Lack of myoepithelium
- Cancerization of lobule by apocrine DCIS:
- Architectural atypia: expansile glandular involvement by atypical, monotonous cells
- Other architectural patterns of DCIS are typically also present (e.g., comedo, cribriform, etc.)
- Mitosis and necrosis frequently identified
- Microglandular adenosis:
- Glands are smaller, more regular
- Lacks myoepithelial cells
- Tubular adenosis:
- Haphazard proliferation of elongated tubules
Additional references
Apocrine adenoma
Definition / general
Essential features
Terminology
Etiology
Clinical features
Prognostic factors
Case reports
Treatment
Microscopic (histologic) description
Cytology description
Positive stains
Negative stains
Differential diagnosis
- Adenoma with apocrine cytology throughout
- Rare adenoma that is:
- Composed exclusively of benign apocrine cells (homogeneous)
- Sharply demarcated from surrounding breast tissue
- Contains only epithelial proliferative elements
- Has minimal supportive stroma
Essential features
- Nodular mass composed exclusively of apocrine cells without cytological atypia
- Sharply demarcated from adjacent breast tissue and has minimal stromal component if any
- Considered benign, excision is curative
- Cytological atypia, necrosis and invasive features suggest atypical hyperplasia / apocrine malignancy (DCIS / invasive), recommend careful evaluation of the entire lesion
Terminology
- Pure breast adenomas with apocrine differentiation were first described by Hertel et al. in 1976 (Cancer 1976;37:2891)
Etiology
- May represent nodular sclerosing adenosis with apocrine differentiation
Clinical features
- Can present as a mass
Prognostic factors
- Generally accepted to be benign, however, the number of cases reported is not sufficient to determine the level of risk associated (J Clin Pathol 2007;60:1313)
Case reports
- 45 year old man with 3 mm tumor (Arch Pathol Lab Med 2003;127:1498)
- 47 year old woman with mammographically detected tumor (WV Med J 2008;104:16)
- 53 year old woman with coexisting invasive ductal carcinoma (Pathol Res Pract 2007;203:809)
Treatment
- Complete excision is curative
Microscopic (histologic) description
- By definition, composed exclusively of benign apocrine cells (homogeneous), sharply demarcated from surrounding breast tissue, containing only epithelial proliferative elements, with minimal supportive stroma
- Localized nodular focus of tubular, papillary and cystic apocrine metaplasia; benign glands have abundant granular eosinophilic cytoplasm, apical luminal blebs and decapitation secretion
- May contain calcifications (Pathology 2001;33:149)
Cytology description
- Can be cuboidal or flattened, two distinct types seen:
- Cytoplasm granular and strongly eosinophilic, supranuclear vacuole containing yellow brown pigment (rich in iron / hemosiderin)
- Globoid and pale nuclei with 1 - 2 prominent nucleoli (nuclei may become hyperchromatic in flattened epithelium as in tension apocrine cysts)
- Cytoplasm distinctly foamy with small vacuoles that may coalesce and show lipofuscin pigment in cytoplasm
- Central nuclei with 1 - 2 prominent nucleoli
- Cytoplasm granular and strongly eosinophilic, supranuclear vacuole containing yellow brown pigment (rich in iron / hemosiderin)
- Recommend caution to exclude apocrine DCIS or invasive apocrine carcinoma if necrosis / atypia / mitosis present
Positive stains
- PAS (with diastase), EMA, CK8 / 18, AR (androgen receptor), GCDFP15, GCDFP24 (apolipoprotein D), GCDFP44 (zinc alpha2 glycoprotein)
Negative stains
Differential diagnosis
- Apocrine DCIS:
- Extensive proliferation, marked nuclear pleomorphism, multiple prominent nucleoli and comedo type necrosis
- Atypical apocrine hyperplasia:
- Architectural atypia such as Roman bridges, cribriform patterns and multiple papillary fronds
- No connective cores or cytological atypia such as 3 fold variation in nuclear size and marked pleomorphism
- Fibroadenoma:
- Prominent proliferating stromal component, compressed epithelium in intracanalicular variant
- Prominent apocrine changes as a part of fibrocystic changes:
- Not nodular, no distinct mass
- Well differentiated apocrine carcinoma:
- Has obvious malignant changes
Board review style question #1
Board review style answer #1
D. Observation. The pictured lesion is apocrine adenosis with microcalcifications. There is no significant atypia present; thus, this biopsy is benign and concordant with imaging findings and the patient can be observed with routine screening. There is no need to rebiopsy, excise, do additional imaging or initiate any therapies.
Comment Here
Reference: Apocrine adenosis / apocrine adenoma
Comment Here
Reference: Apocrine adenosis / apocrine adenoma
Board review style question #2
What feature is present in apocrine ductal carcinoma in situ (DCIS) but is lacking in atypical apocrine adenosis (AAA)?
- Calcifications
- Cytologic atypia
- Epithelial expansion
- Myoepithelium
- Prominent nucleoli
Board review style answer #2
C. Epithelial expansion. Atypical apocrine adenosis (AAA) and apocrine ductal carcinoma in situ (DCIS) share features of apocrine cytology, including enlarged nuclei with prominent nucleoli and eosinophilic cytoplasm, as well as significant cytologic atypia and an intact myoepithelial cell layer. Both may also have calcifications; however, apocrine DCIS is differentiated from AAA by architectural atypia or epithelial expansion by clonal, monotonous cells.
Comment Here
Reference: Apocrine adenosis / apocrine adenoma
Comment Here
Reference: Apocrine adenosis / apocrine adenoma