Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Krystel-Whittemore M, Wen HY. Adenomyoepithelioma & malignant adenomyoepithelioma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastadenomyo.html. Accessed March 30th, 2025.
Definition / general
- Biphasic tumor composed of variable number of myoepithelial cells surrounding epithelial lined spaces
- Usually expanded and prominent myoepithelial component
- First recognized in the breast by Hamperl (Curr Top Pathol 1970;53:161)
Essential features
- Biphasic tumor with epithelial and myoepithelial components
- Benign to low grade malignant behavior and a propensity for recurrence
- Either epithelial or myoepithelial component can show malignant transformation so thorough evaluation recommended
- Wide surgical excision with appropriate margins recommended to prevent recurrence
- Microscopy shows tubular or lobulated structures with epithelial and myoepithelial components
- Epithelial component: cytokeratin+, EMA+, CEA+; myoepithelial component: S100+, SMA+, SMMHC+, p63+
Terminology
- WHO (2019) divides adenomyoepithelioma into benign and malignant tumors
- Malignant adenomyoepithelioma = adenomyoepithelioma with carcinoma, which can arise in epithelial or myoepithelial component or in both components (J Clin Pathol 2013;66:465)
ICD coding
- Adenomyoepithelioma
- ICD-O: 8983/0 adenomyoepithelioma
- ICD-11: 2F30.Y & XH2V57 - other specified benign neoplasm of breast and adenomyoepithelioma, benign
- Malignant adenomyoepitheliomas
- ICD-O: 8562/3 epithelial-myoepithelial carcinoma
- ICD-11: 2C6Y & XH7TL5 - malignant neoplasms of breast and adenomyoepithelioma with carcinoma
Epidemiology
- Uncommon, median age 67 years (Breast J 2020;26:653)
- F > M
Sites
- Usually occurs in the peripheral portion of the breast but can be central or areolar region (Hum Pathol 1987;18:1232)
- No predilection for either breast
- Malignant adenomyoepitheliomas can be seen in any location (Histopathology 2020;77:181)
Clinical features
- Usually presents as a solitary palpable mass or screen detected on breast imaging
- Most are benign, although may recur locally; malignant transformation can occur (Hum Pathol 1987;18:1232, Am J Surg Pathol 1992;16:868, Arch Pathol Lab Med 2006;130:1349)
- Associated carcinomas may be low or high grade and may metastasize to lung, brain or liver (Virchows Arch 1995;427:243, Virchows Arch 2003;442:504, J Cardiothorac Surg 2016;11:121, Arch Pathol Lab Med 2000;124:632)
Diagnosis
- Well circumscribed mass, infrequently associated with tenderness and nipple discharge
- Diagnosis on core biopsy specimen can be challenging
- Presence of tightly aggregated glands arranged in compact nodules and prominent clear cell or spindle cell myoepithelium are clues to the diagnosis (Breast J 2004;10:522)
- Immunohistochemical stains for myoepithelial markers are useful to highlight the myoepithelial component (Breast J 2004;10:522)
- Excision is necessary for thorough evaluation of atypia or carcinoma arising in an adenomyoepithelioma
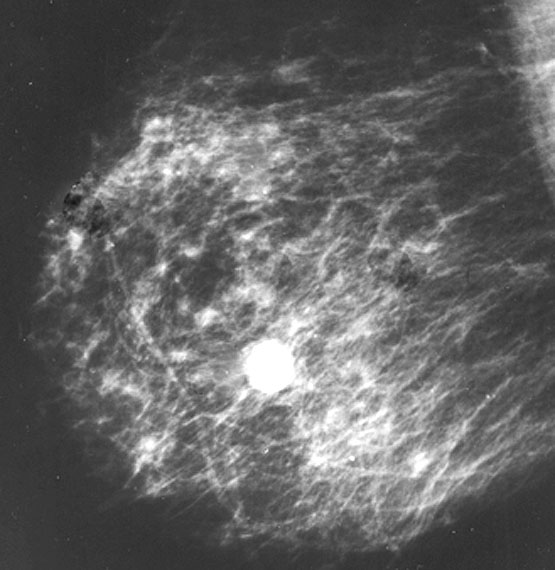
Radiology description
- Ultrasound: irregular / angulated or oval / circumscribed, hypoechoic mass (Korean J Radiol 2010;11:522, J Clin Ultrasound 2013;41:218)
- Mammography: oval, equal density mass (J Clin Ultrasound 2013;41:218, Breast 2016;29:132)
- Appears dense, mostly circumscribed, can have focally indistinct margins
- Calcifications and cystic change are not typical
- MRI: round, lobulate or oval masses with clear or shaded borders (Clin Radiol 2016;71:235)
- Malignant: irregular shapes with spiculate margins
Radiology images
Prognostic factors
- Tubular type of adenomyoepithelioma, intraductal extension along periphery of lesion, incomplete excision and cytologic atypia are associated with local recurrence (Am J Surg Pathol 1991;15:554)
- High mitotic rate, atypia, necrosis, cellular pleomorphism and infiltrative borders favor malignancy (Am J Surg Pathol 1992;16:868)
- Malignant adenomyoepithelioma has prognosis dependent on histological subtype and grade of the malignant component
Case reports
- 28 year old woman and 42 year old woman with tubular variant of adenomyoepithelioma (Cytojournal 2017;14:29)
- 47 year old man with adenomyoepithelioma (Hum Pathol 1993;24:678)
- 47 year old woman with malignant adenomyoepithelioma (J Breast Cancer 2019;23:93)
- 53 year old woman with adenomyoepithelioma with associated lobular neoplasia (Ann Diagn Pathol 2015;19:20)
- 56 year old woman with adenomyoepithelioma and late pulmonary metastases (J Cardiothorac Surg 2016;11:121)
- 56 year old woman with intracystic adenomyoepithelioma (Breast Cancer 2007;14:429)
- 58 year old woman with adenomyoepithelioma with myoepithelial carcinoma (Clin Case Rep 2019;7:930)
Treatment
- Complete wide excision with negative margins is standard treatment to prevent local recurrence
- Mastectomy with or without axillary node dissection - only needed if malignant transformation
- Reference: AJR Am J Roentgenol 2003;180:799
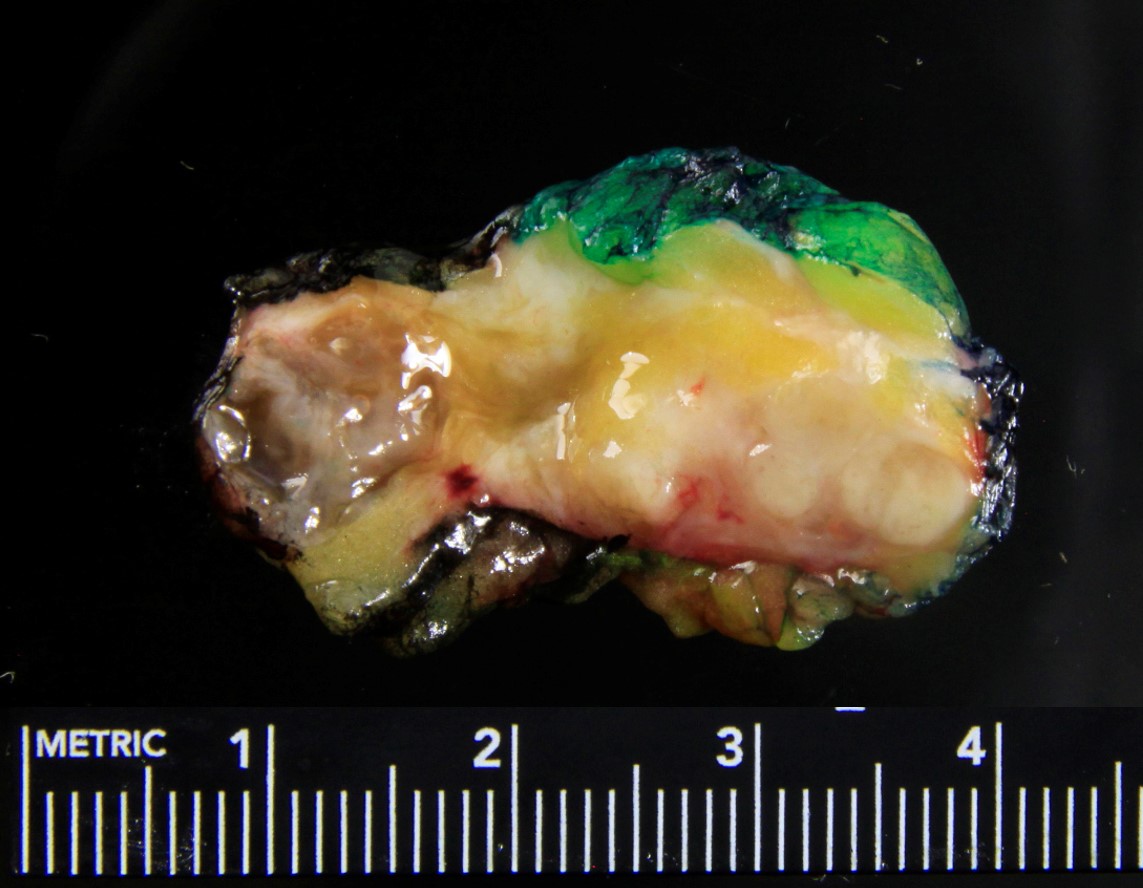
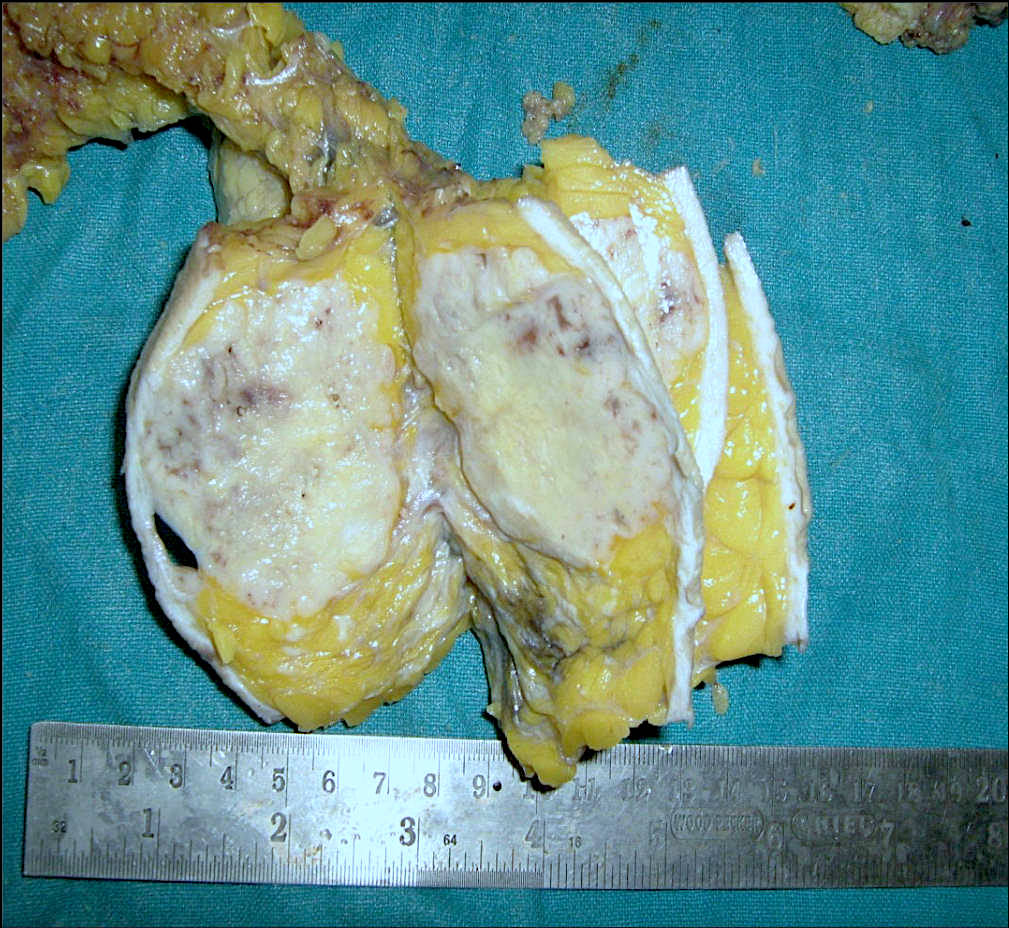
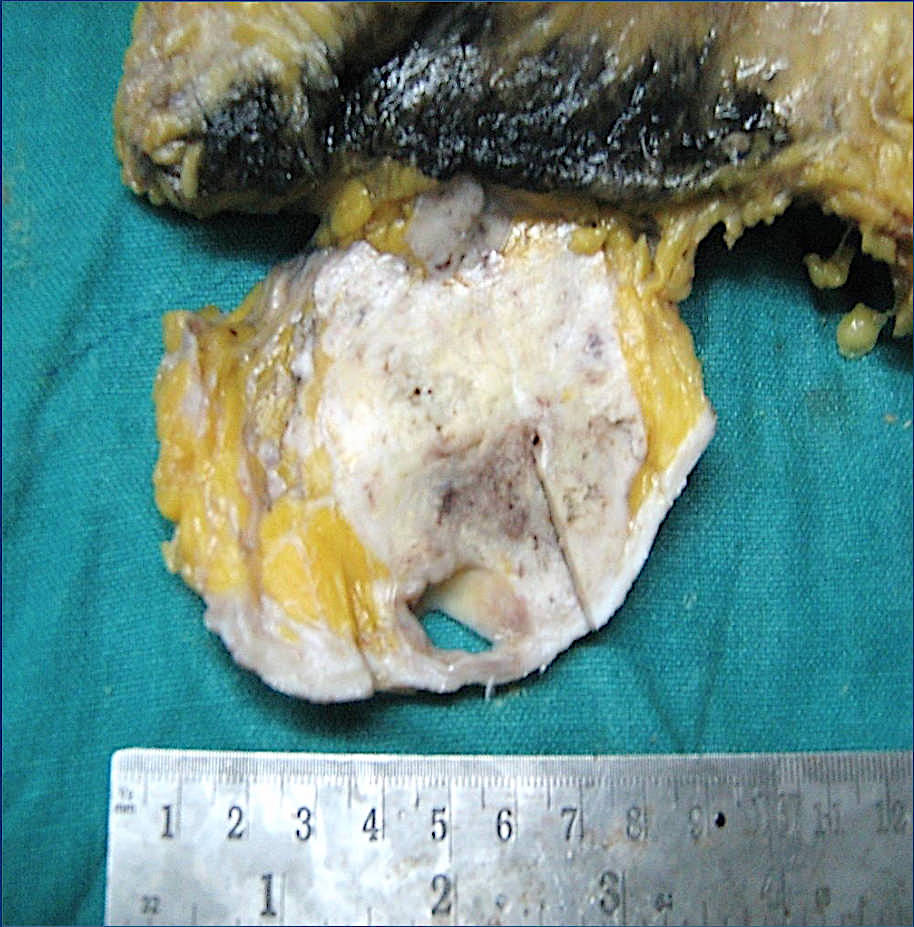
Gross description
- Usually solitary nodule, median size: 2 cm, can be up to 8 cm (Breast J 2020;26:653)
- Recurrent tumors usually larger
- Sectioning reveals well circumscribed, firm, pink-white to gray-tan lesion
- Can have focal cystic changes or necrosis
- Malignant adenomyoepithelioma can show infiltrative borders (J Clin Pathol 2011;64:477)
Gross images
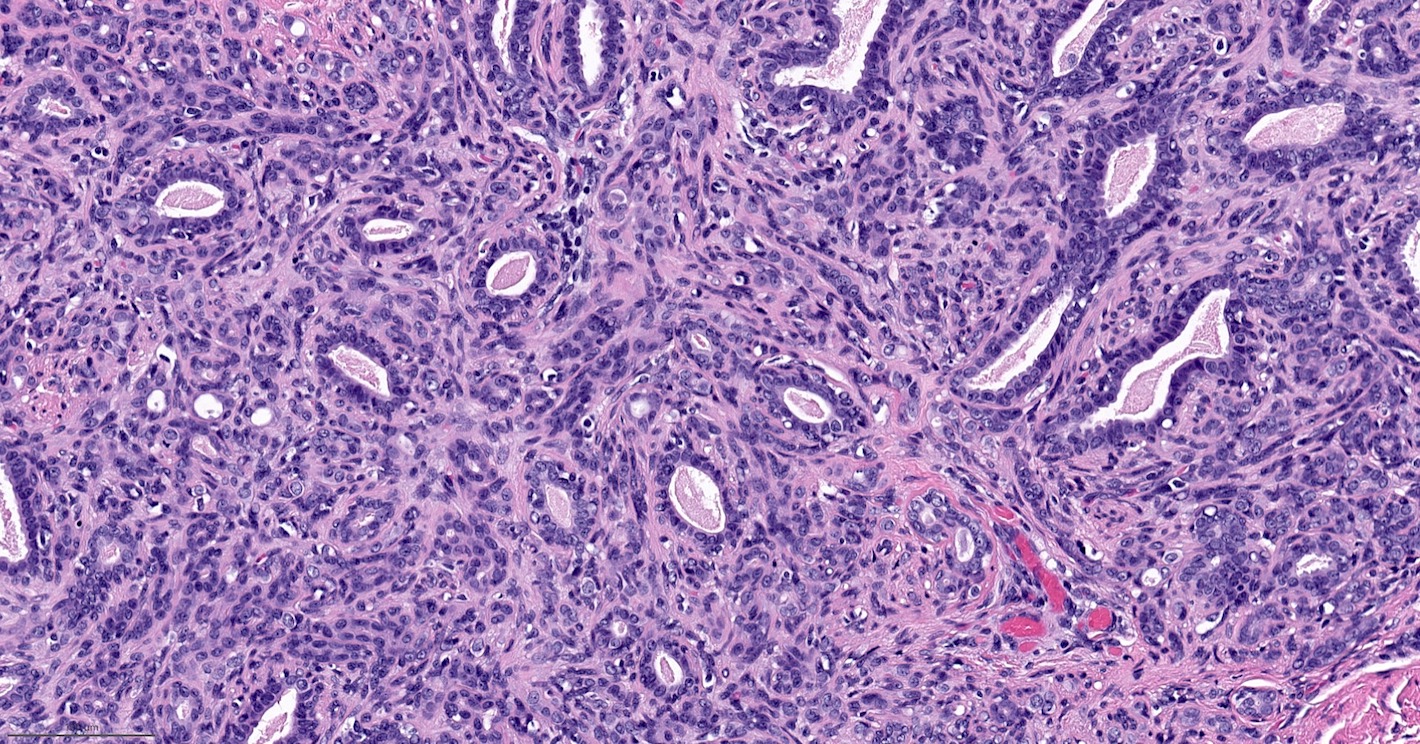
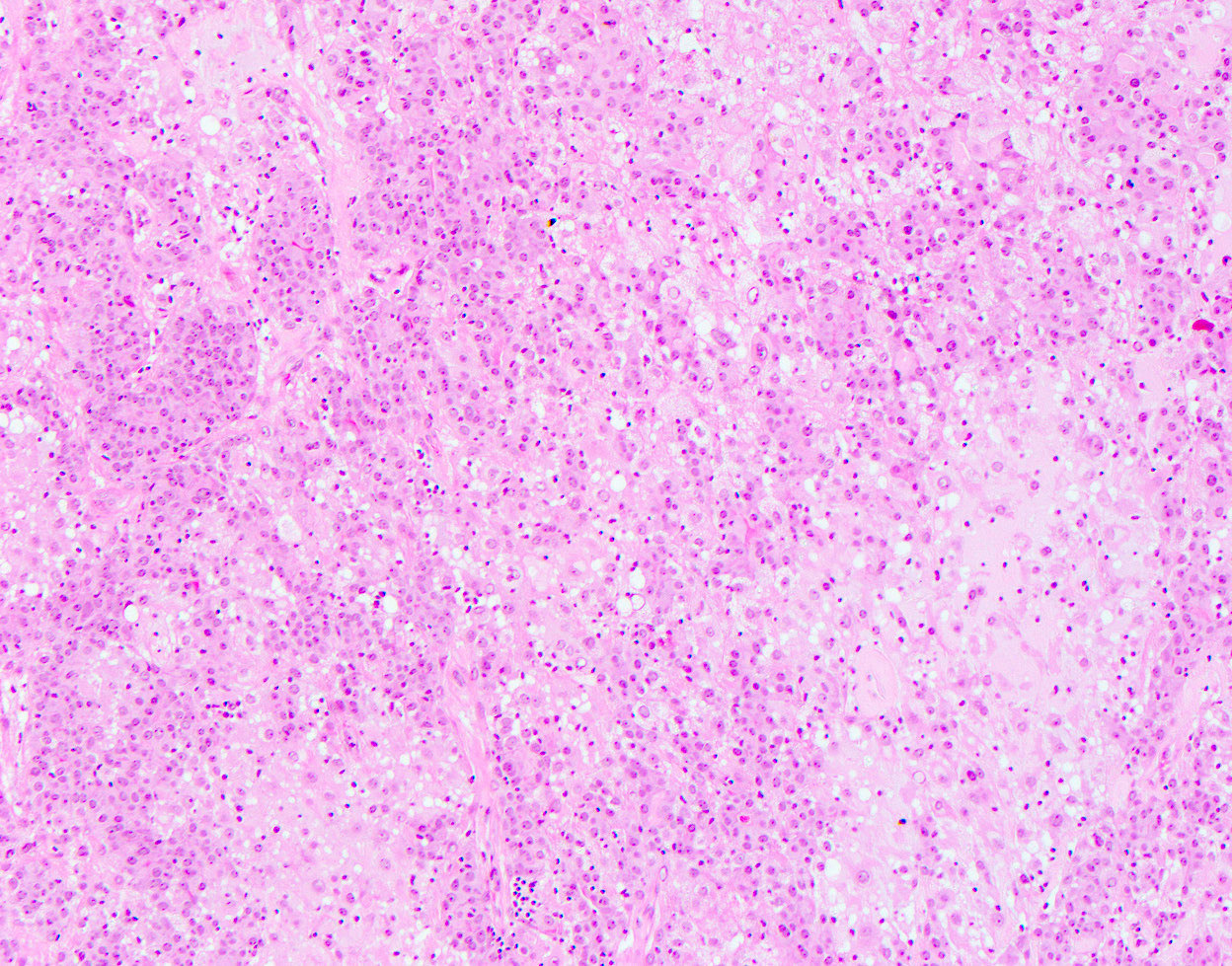
Microscopic (histologic) description
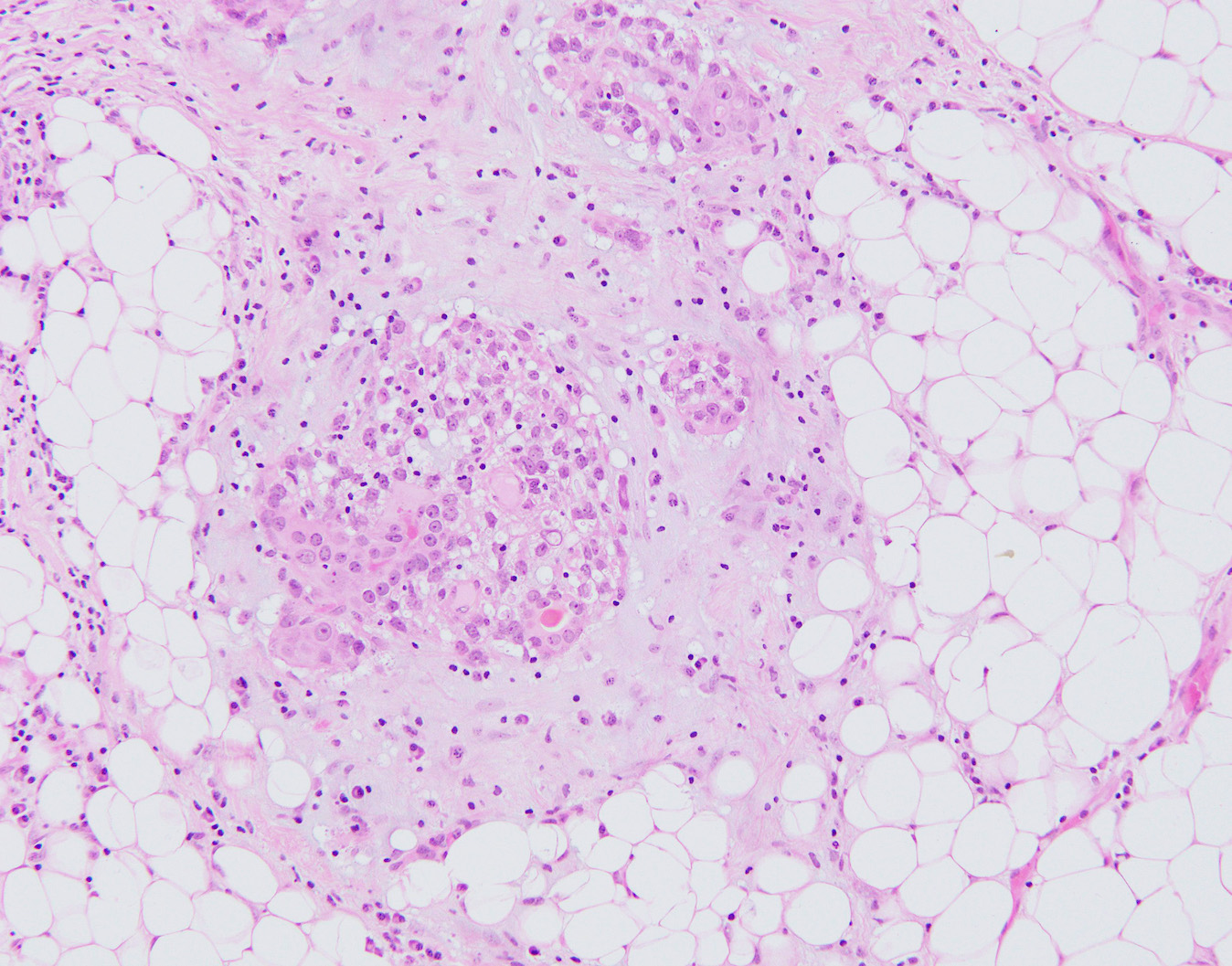
- Well circumscribed, may be encapsulated or multinodular and lobulated
- Biphasic proliferation of epithelial and myoepithelial cells
- Epithelial cells usually form glandular spaces; can show apocrine, sebaceous or squamous metaplasia
- Can have papillary epithelial proliferation
- Myoepithelial cells usually dominant and may be polygonal shaped with clear cytoplasm or spindled
- Variants:
- Spindle cell: spindle myoepithelial cells proliferation, epithelial lined spaces may be sparse
- Tubular: proliferation of rounded tubules, ill defined margins
- Lobulated: nests of myoepithelial cells surround compressed epithelial lined spaces
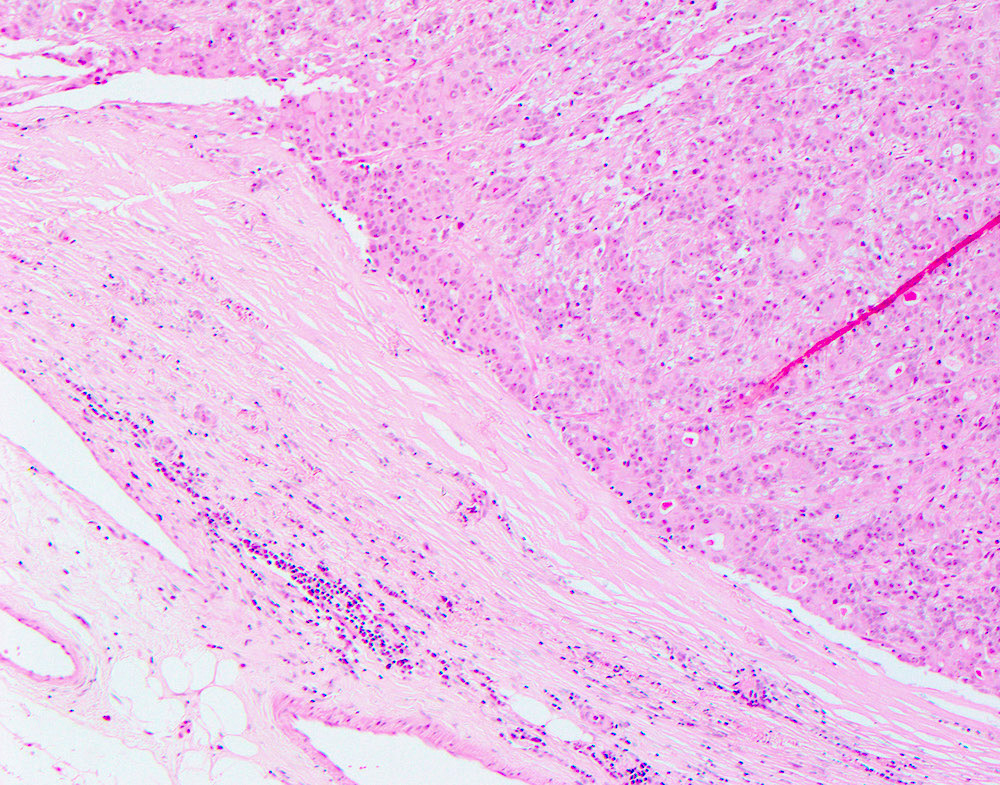
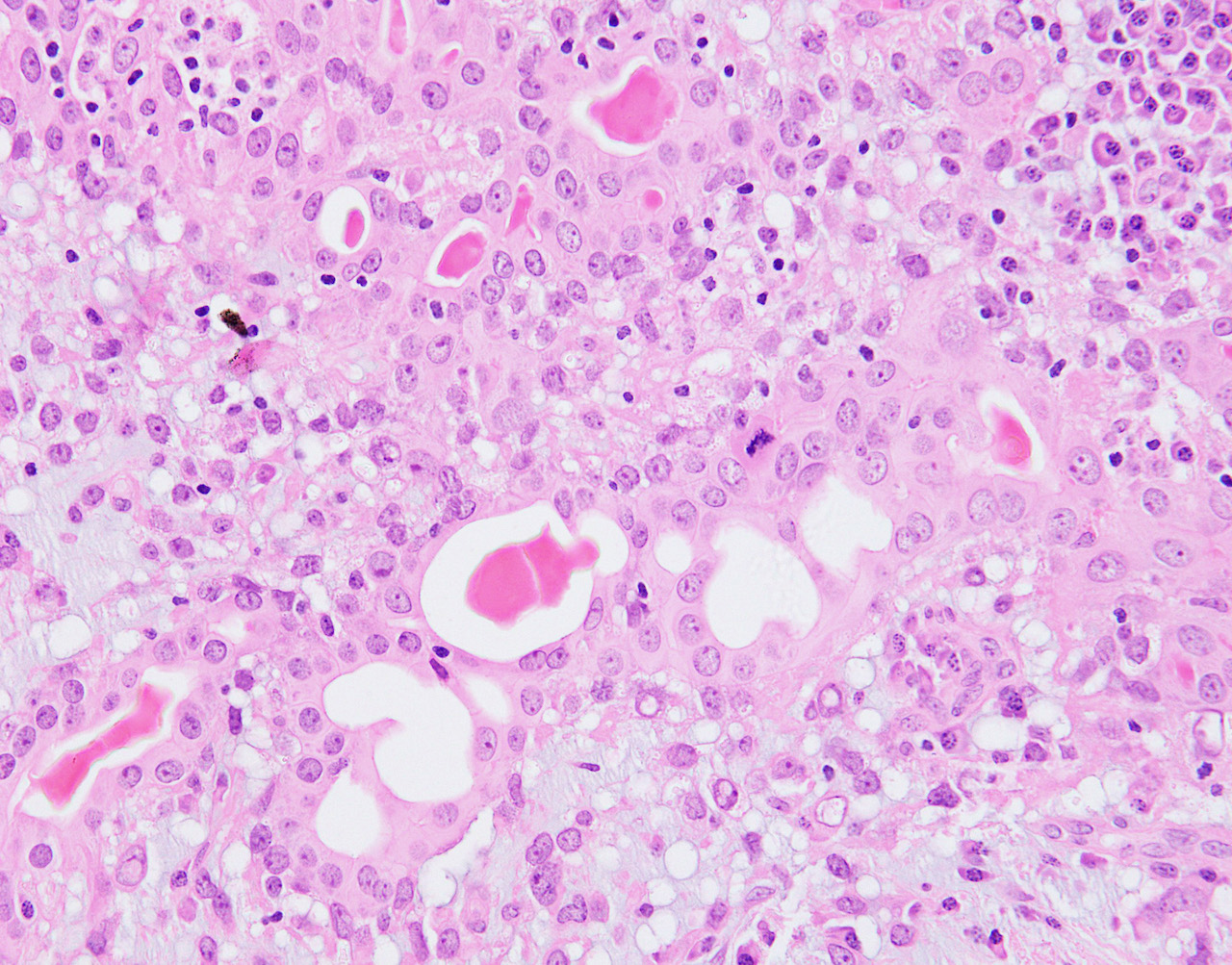
- Malignant cases have infiltrative growth pattern, high mitotic rate or severe atypia which can be seen in the epithelial or myoepithelial component or in both components (Arch Pathol Lab Med 2000;124:632, Am J Surg Pathol 1992;16:868, Virchows Arch 1998;432:123)
- Malignant transformation of epithelial component can have features of invasive carcinoma no special type, invasive lobular carcinoma, metaplastic carcinoma, including squamous cell carcinoma, spindle cell carcinoma or matrix producing carcinoma, low grade adenosquamous carcinoma or adenoid cystic carcinoma (Breast 2016;29:132, Pathol Int 2009;59:179, Virchows Arch 1995;427:243, Pathol Res Pract 2007;203:599, Virchows Arch 1998;432:123, Am J Surg Pathol 1998;22:631, Breast J 2019;25:731, Diagn Pathol 2014;9:148)
- Malignant transformation of myoepithelial component shows features of myoepithelial carcinoma including overgrowth of myoepithelial cells, nuclear atypia and mitotic activity (Pathol Int 2006;56:211, Breast J 2007;13:203, J Clin Pathol 2011;64:477)
- Biphasic malignant tumors (of epithelial and myoepithelial components) can be seen, with myoepithelial cells being the predominant component (Breast J 2019;25:1273)
- Usually multilobulated or multinodular and can show a distinct transition from benign to malignant components
- Malignant changes must be seen in both epithelial and myoepithelial cell types, specifically including increased mitotic activity
- Malignant adenomyoepithelioma can be ER positive or negative but the carcinoma component is most commonly ER / PR / HER2 negative (Nat Commun 2018;9:1816)
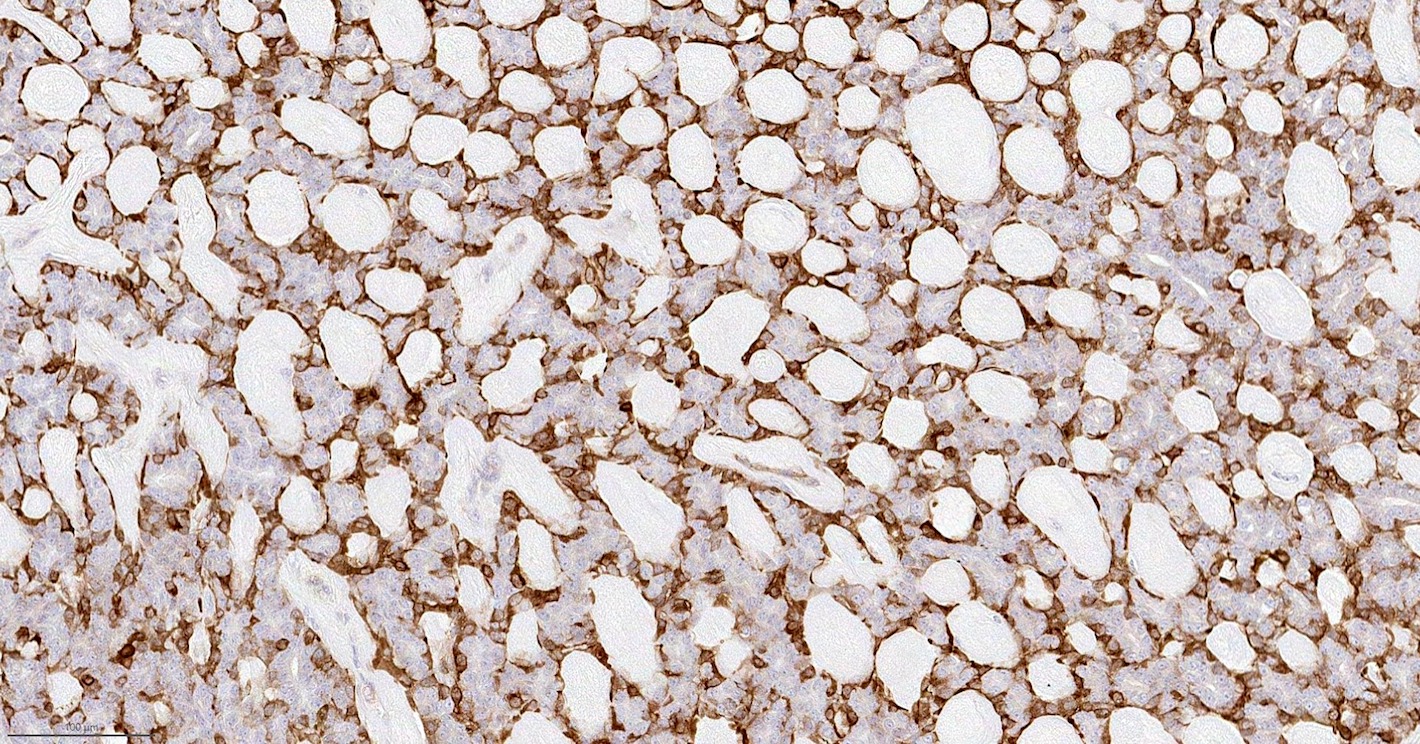
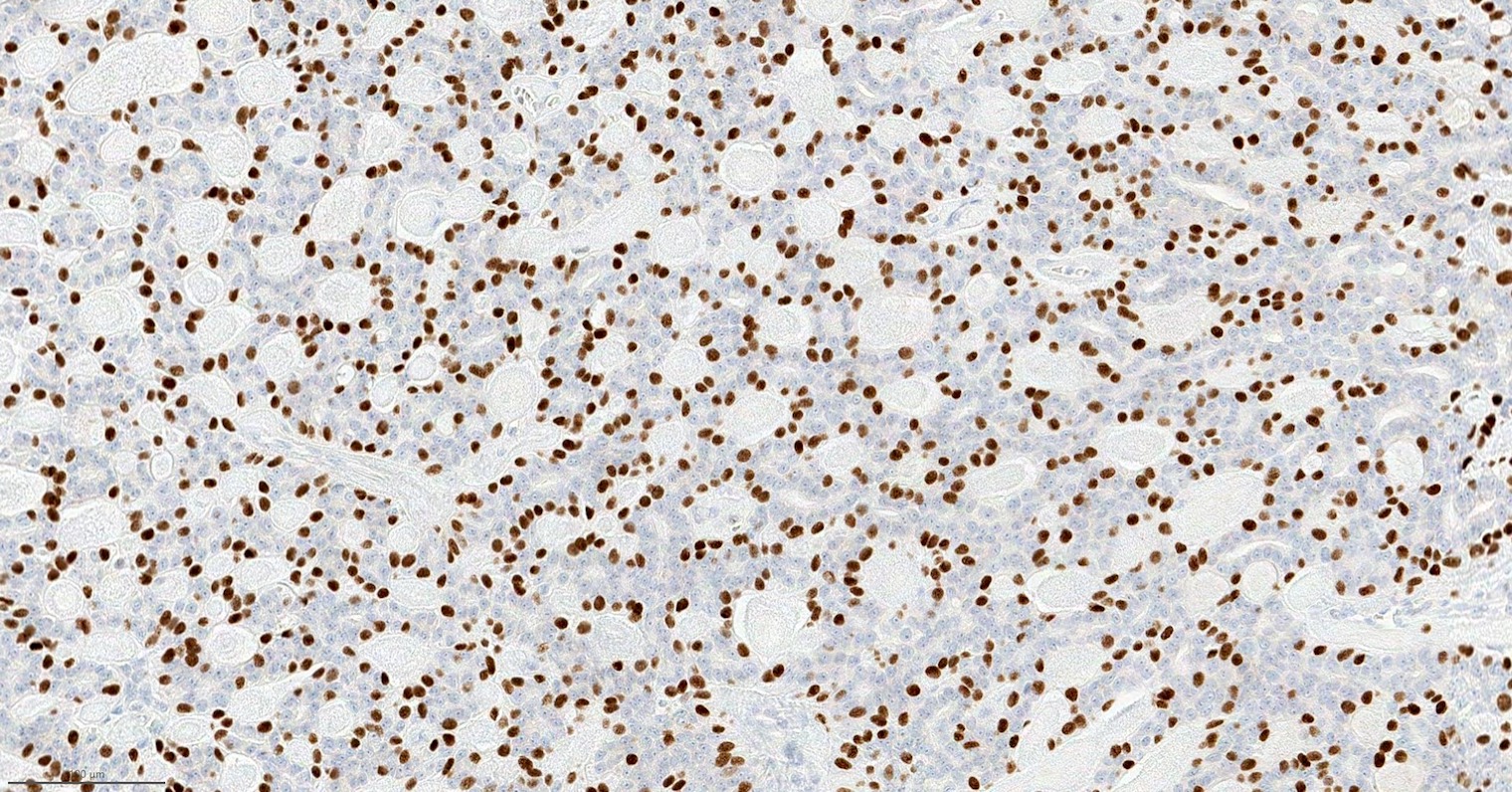
Microscopic (histologic) images
Contributed by Hannah Y. Wen, M.D., Ph.D.
Contributed by Fresia Pareja, M.D., Ph.D.
Cytology description
- Moderate to highly cellular with large clusters of epithelium and myoepithelium
- Tubular structures occasionally found
- Myoepithelium appears as small clusters or dispersed cells with epithelioid morphology, intranuclear or intracytoplasmic vacuoles, often naked bipolar nuclei
- Mild to moderate nuclear atypia present
- Metachromatic fibrillary stroma occasionally found
- No mitotic figures, no necrosis
- Often classified incorrectly as fibroadenoma, suspicious for malignancy or malignant (Cancer 2006;108:250)
- Malignant adenomyoepithelioma is highly cellular with neoplastic appearing cells
- Metachromatic matrix material can be seen around nests of neoplastic cells (J Clin Pathol 2011;64:477)
Positive stains
- Epithelial component:
- Keratin (AE1 / AE3), CAM 5.2, CK7, EMA; variable ER
- Myoepithelial component:
Electron microscopy description
- Myoepithelial features (classic) include myofibrils with dense bodies, pinocytotic vesicles, desmosomes or tight junctions, patchy basement membrane
Molecular / cytogenetics description
- ER positive adenomyoepitheliomas: PIK3CA or AKT1 activating mutations (Nat Commun 2018;9:1816, Mod Pathol 2020;33:1764)
- ER negative adenomyoepitheliomas: Q61R HRAS hotspot mutations with concurrent PIK3CA or PIK3R1 mutations (Nat Commun 2018;9:1816)
- IHC analysis of RAS Q61R for detection of HRAS Q61R mutations has been shown to be a useful marker in ER negative adenomyoepitheliomas (Histopathology 2020;76:865)
- Case report with t(8;16)(p23;q21) (Cancer Genet Cytogenet 2005;156:14)
- Malignant adenomyoepithelioma displays similar mutational profile as that of benign (Nat Commun 2018;9:1816, Mod Pathol 2020;33:1764)
- Homozygous CDKN2A homozygous deletions, TERT promoter mutations or MYC amplifications may be associated with tumor progression (Nat Commun 2018;9:1816, J Breast Cancer 2019;23:93)
Sample pathology report
- Breast, left, excision:
- Adenomyoepithelioma, 3.2 cm
- Surgical margins negative for tumor
Differential diagnosis
- Sclerosing adenosis:
- Proliferation of epithelial glands with stromal sclerosis, which can cause architectural distortion of the glands
- Less commonly mass forming
- No prominent myoepithelial component
- Intraductal papilloma:
- Papillary lesion comprised of epithelial proliferation with fibrovascular cores
- No prominent myoepithelial component
- Invasive carcinoma (on core biopsy):
- Nipple adenoma:
- Epithelial proliferation arising in the collecting ducts of the nipple
- No prominent myoepithelial component
- Tubular adenoma:
- Very well circumscribed
- Proliferation of tubules with only single layer of myoepithelial cells surrounding the epithelial component without expansion of myoepithelial component
Additional references
Board review style question #1
A 58 year old woman presents with a 2 cm nontender, firm nodule in the upper outer quadrant of her left breast. A core biopsy and subsequent resection of the lesion is performed. Representative microscopic images from the excision specimen are shown, which revealed an adenomyoepithelioma. Which of the following is true?
- Chemotherapy is the standard treatment of this lesion
- This lesion is usually HER2 positive and treated with anti-HER2 therapy
- This is a malignant lesion
- Without negative surgical margins, there is a risk of local recurrence of the lesion
Board review style answer #1
D. Without negative surgical margins, there is a risk of local recurrence of the lesion
Comment Here
Reference: Adenomyoepithelioma
Comment Here
Reference: Adenomyoepithelioma