Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Li JJX, Tse GM. Neuroendocrine carcinoma (NEC). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/breastNEC.html. Accessed April 2nd, 2025.
Definition / general
- Invasive carcinoma with characteristic high grade neuroendocrine features and supported by immunoreactivity to neuroendocrine markers
Essential features
- Neuroendocrine markers helpful for diagnosis
- Not to be confused with distinct breast neoplasms that exhibit neuroendocrine differentiation (e.g., solid papillary carcinoma, mucinous carcinoma of the breast)
- Morphologically indistinguishable from neuroendocrine carcinomas (small cell carcinoma and large cell carcinoma) of other organ primaries
Terminology
- Small cell neuroendocrine carcinoma
- Large cell neuroendocrine carcinoma
- Oat cell carcinoma (for small cell carcinoma, not recommended by the WHO)
ICD coding
- ICD-O:
- ICD-10: C50.9 - malignant neoplasm of breast of unspecified site
- ICD-11:
Epidemiology
- Small cell carcinoma of the breast:
- 0.1% of primary breast cancers (Springerplus 2015;4:138)
- 4% of extrapulmonary small cell carcinomas (J Surg Oncol 2011;104:604)
- More commonly presents in postmenopausal age (Springerplus 2015;4:138)
- Large cell carcinoma of the breast:
- < 0.1% of primary breast cancers; rarer than small cell carcinoma of the breast (Breast Care (Basel) 2015;10:281)
Pathophysiology
- Unconfirmed at this time but proposed theories include:
- Neoplastic transformation from native neuroendocrine cells in the breast (J Clin Pathol 2012;65:699)
- Divergent neuroendocrine differentiation of neoplastic stem cells (Arch Pathol Lab Med 2017;141:1577)
- Invasive and in situ ductal carcinomas occasionally identified in small cell carcinomas of the breast (Am J Surg Pathol 2000;24:1231)
- May suggest acquisition of neuroendocrine features and transformation from invasive or in situ breast carcinomas
Etiology
- Unknown at this time
Clinical features
- Presents as a mass lesion (J Clin Pathol 2005;58:775):
- Tumor size ranges from 1 - 11 cm (AJR Am J Roentgenol 2014;203:W221)
- Higher risk of presenting with distant disease than primary breast cancers overall (31% versus 5%) (BMC Cancer 2015;15:185, JAMA 2015;313:165)
Diagnosis
- Largely dependent on histologic evidence of neuroendocrine differentiation and small cell / large cell features (see Microscopic description)
- Supplemented by positivity to neuroendocrine markers
- Clinical exclusion of metastasis to breast from a nonmammary primary site is essential
- Identification of in situ component is the most helpful feature for confirmation of the breast as the primary site of origin (e.g. gastrointestinal tract, pulmonary) (Am J Case Rep 2021;22:e932274)
Laboratory
- Serum chromogranin A not demonstrated to be useful in detection and disease monitoring in a large case control cohort (Int J Biol Markers 2001;16:268)
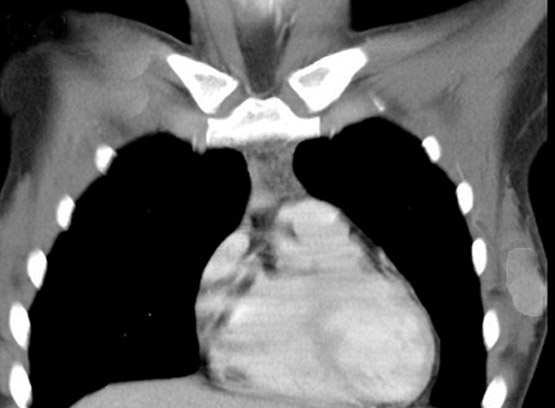
Radiology description
- Mammogram (AJR Am J Roentgenol 2014;203:W221):
- High density
- Round, oval or lobular mass
- Nonspiculated margins
- Ultrasound:
- Hypoechoic
- Irregular with indistinct margins
- With or without posterior acoustic enhancement (Appl Radiol 2016;45:27)
- MRI:
- Irregular mass
Radiology images
Prognostic factors
- Histological grade:
- Small cell carcinomas show worse survival than other neuroendocrine neoplasms of the breast (Breast Cancer Res Treat 2014;148:637)
- Neuroendocrine carcinomas are most commonly Nottingham grade 3, whereas neuroendocrine tumors of the breast are usually grades 1 and 2 (Case Rep Pathol 2016;2016:6762085)
- Tumor stage:
- Small cell and large cell carcinomas of the breast demonstrate unfavorable prognosis (Breast Cancer Res Treat 2014;148:637)
- Neuroendocrine marker expression:
- Low synaptophysin and chromogranin expression correlates with poor disease free survival (Oncologist 2020;25:e1318)
- INSM1 expression associated with better survival (Pathology 2021;53:170)
Case reports
- 38 year old woman with self detected breast mass (Proc (Bayl Univ Med Cent) 2017;30:200)
- 39 year old woman presenting with left breast refusal (Am J Case Rep 2021;22:e932274)
- 42 year old woman with mixed neuroendocrine tumor and large cell carcinoma of the breast (Korean J Radiol 2013;14:395)
- 50 year old woman with small cell carcinoma of breast showing TTF1 positivity (Front Endocrinol (Lausanne) 2020;11:228)
- 64 year old woman with rapidly progressive fungating breast mass (Case Rep Oncol 2021;14:761)
Treatment
- Mainstay of treatment is surgical resection, usually with lymphadenectomy (Am J Surg Pathol 2000;24:1231)
- Adjuvant radiotherapy not associated with improved prognosis (Springerplus 2015;4:138)
- Hormonal therapy given for chemoprevention and palliation with reports of good outcome (Am J Surg Pathol 2000;24:1231, Clin Breast Cancer 2012;12:226)
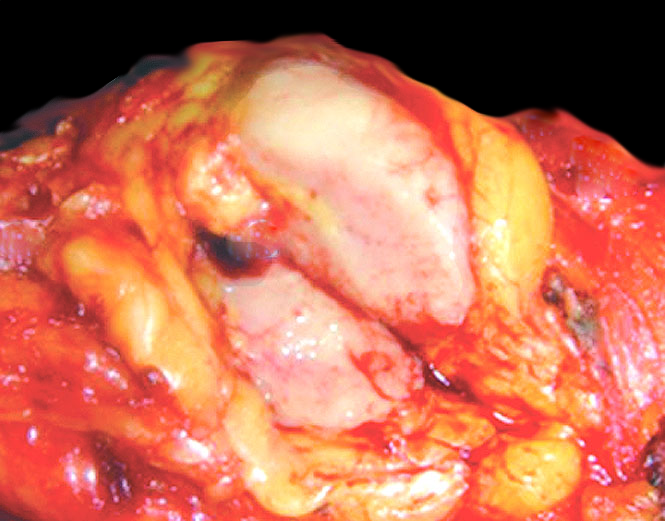
Gross description
- Ill defined firm mass (Am J Surg Pathol 2000;24:1231)
- Tan or fleshy cut surface
- Calcification, hemorrhage and necrosis uncommon
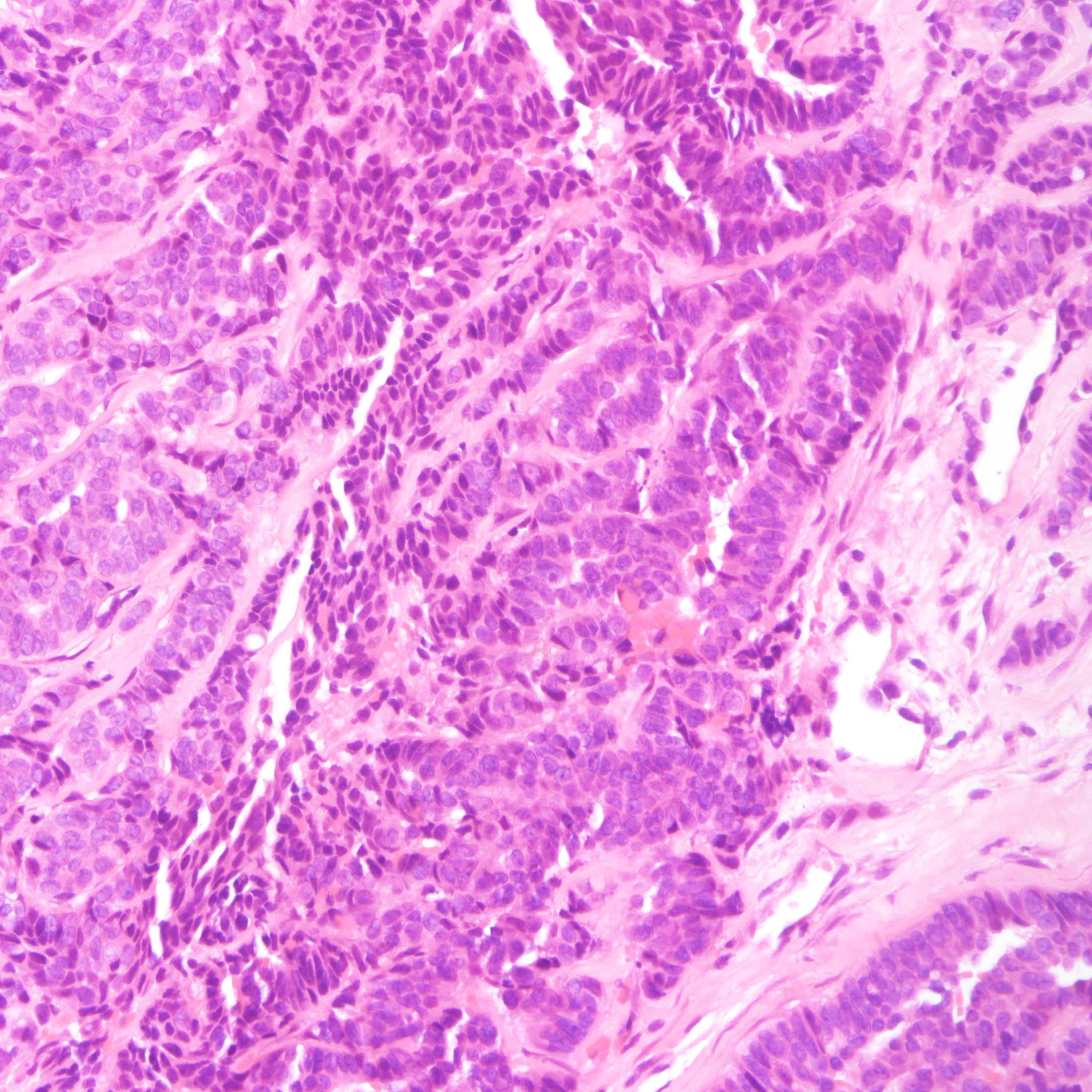
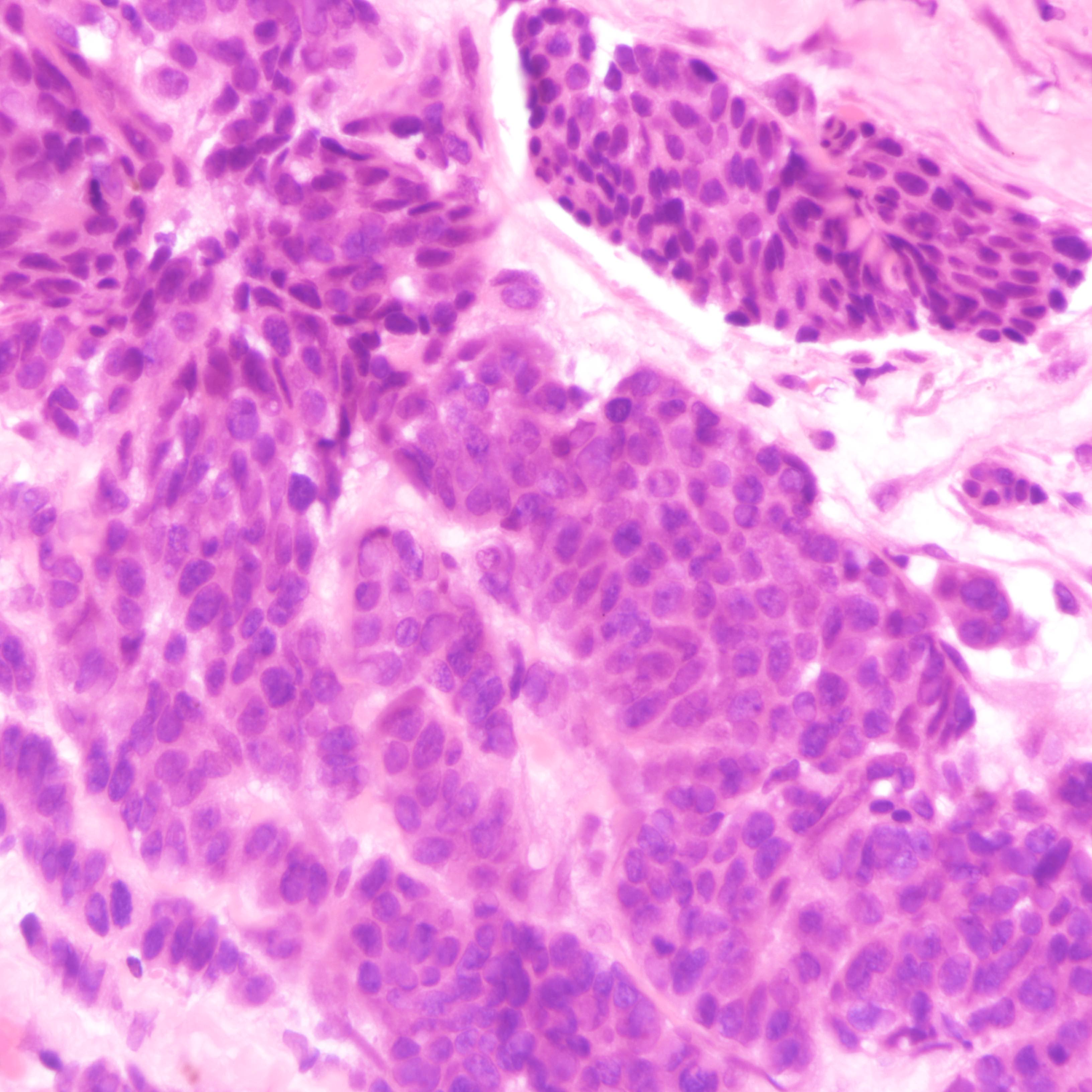
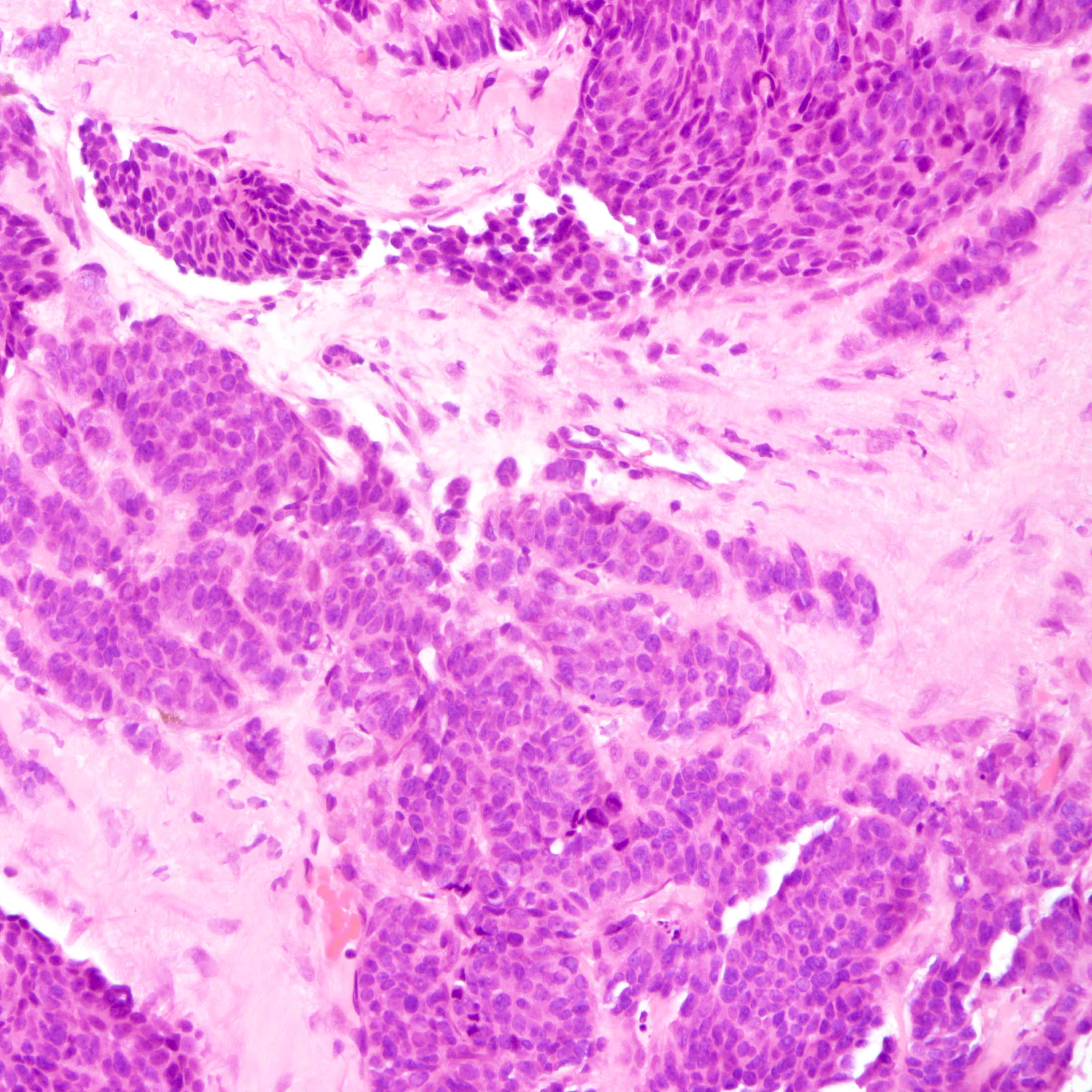
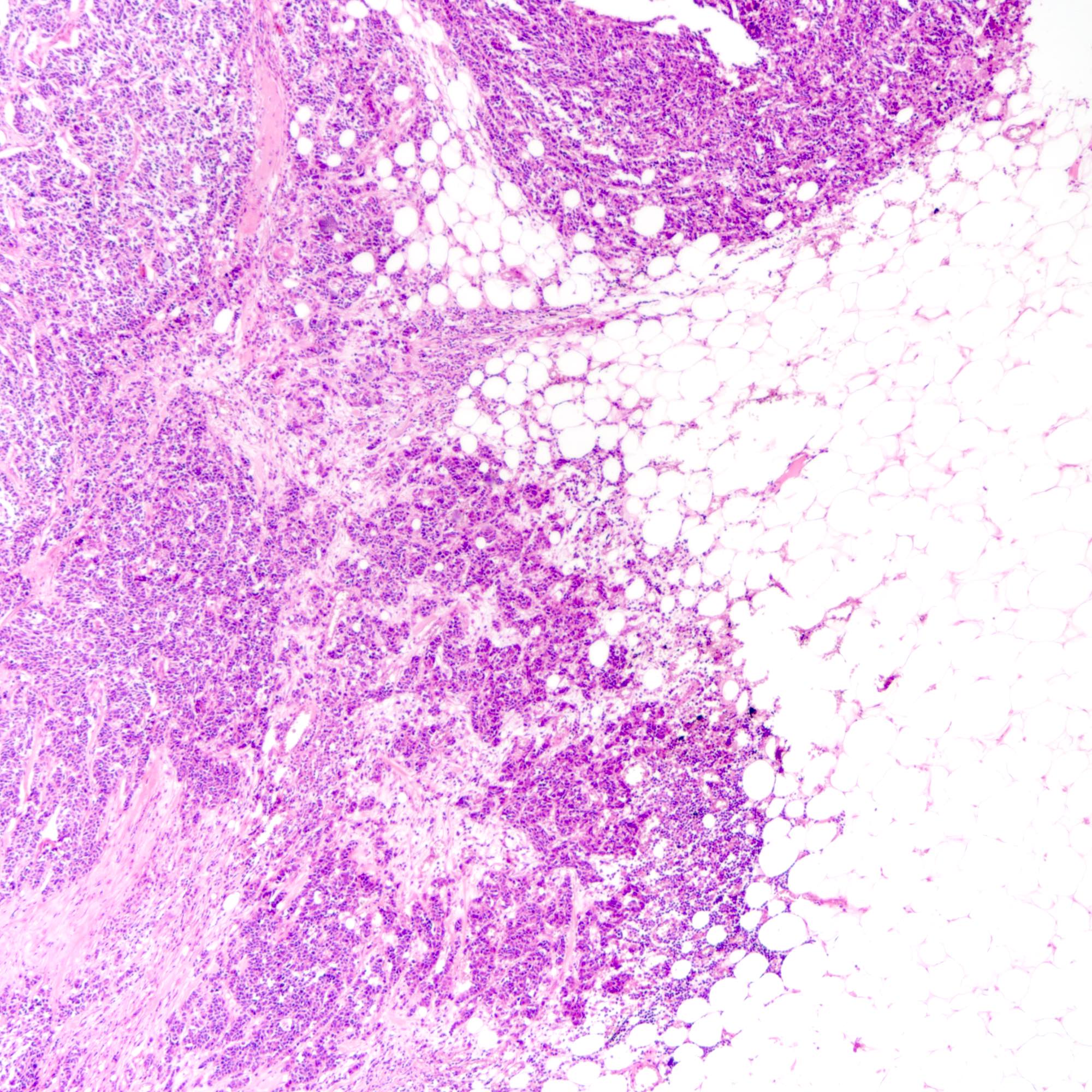
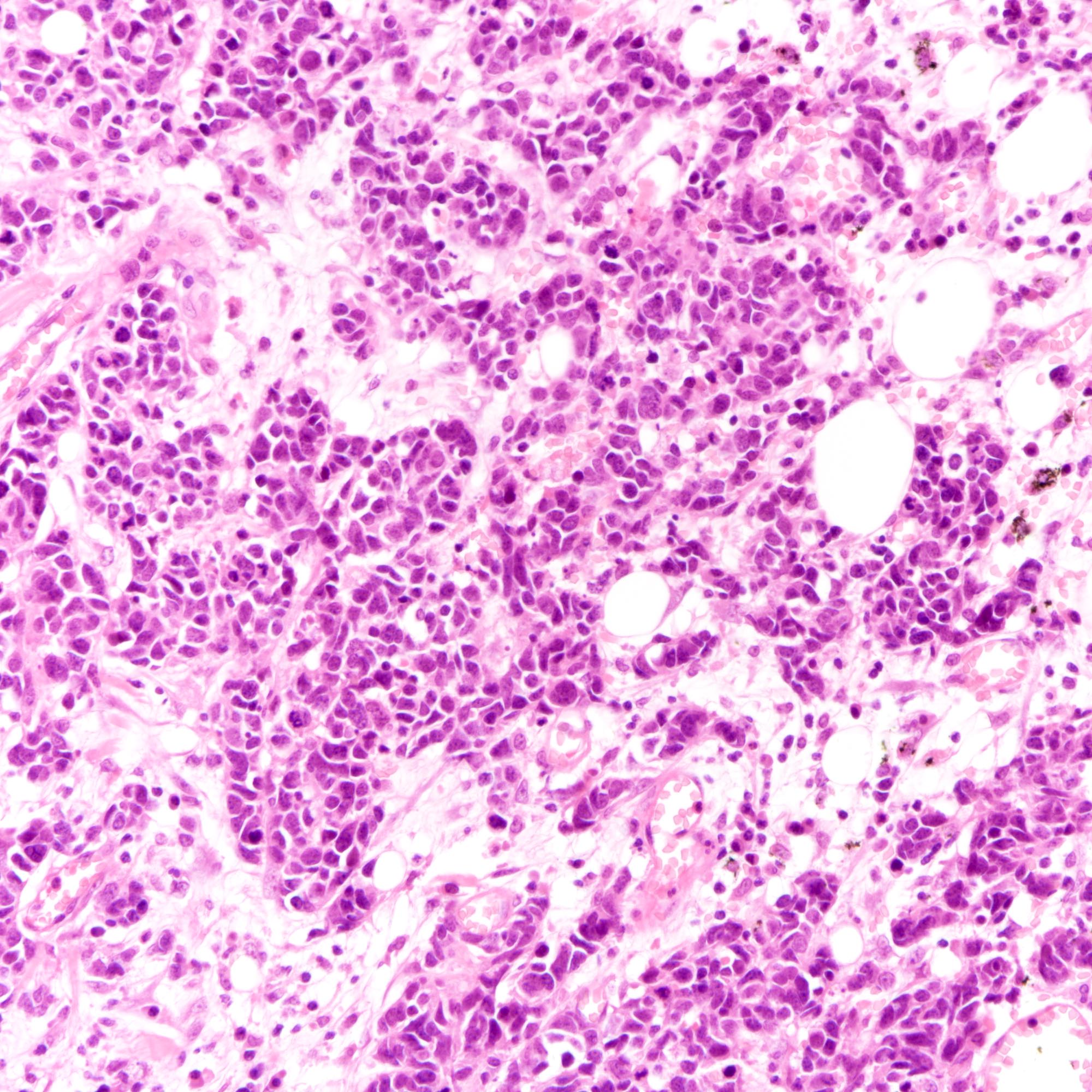
Microscopic (histologic) description
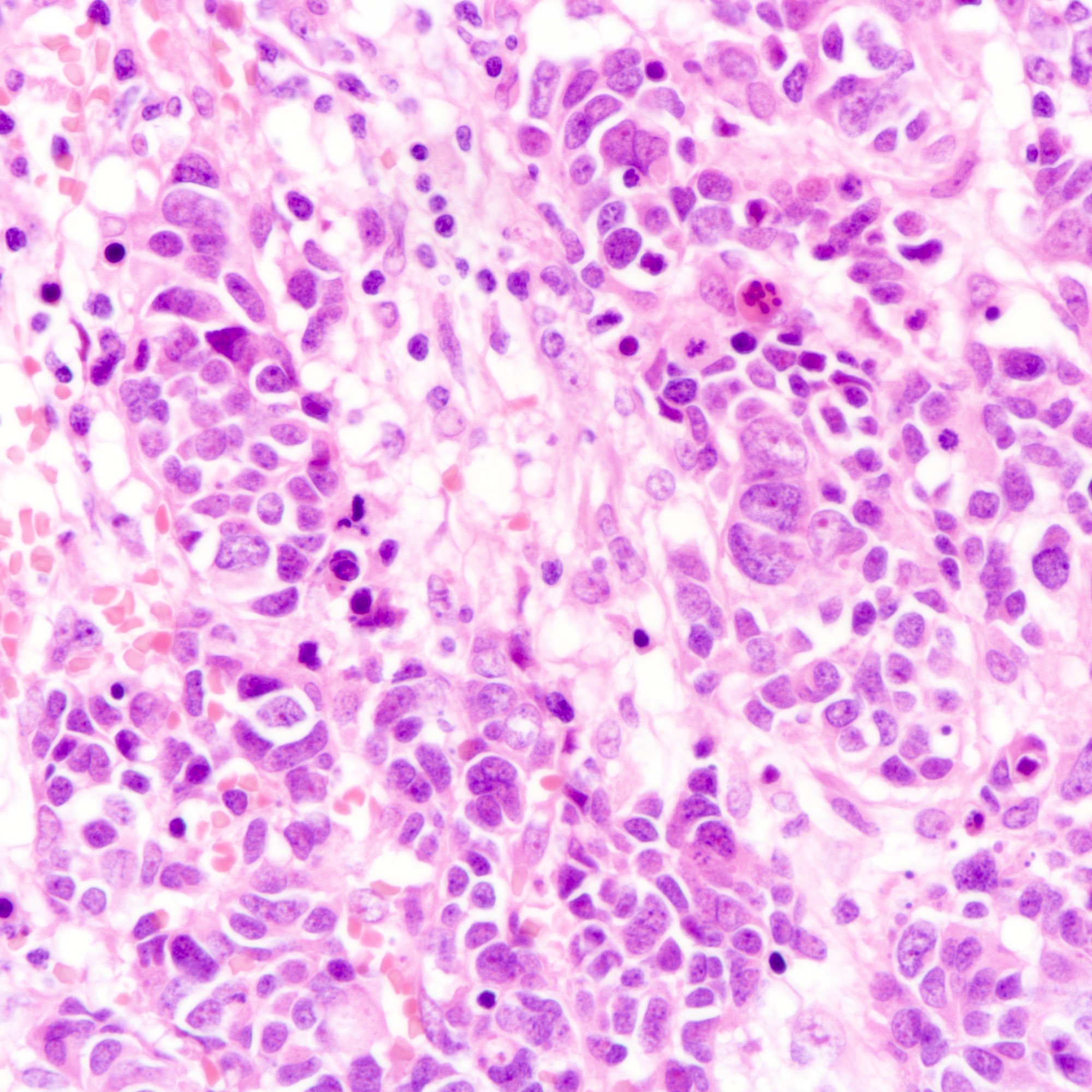
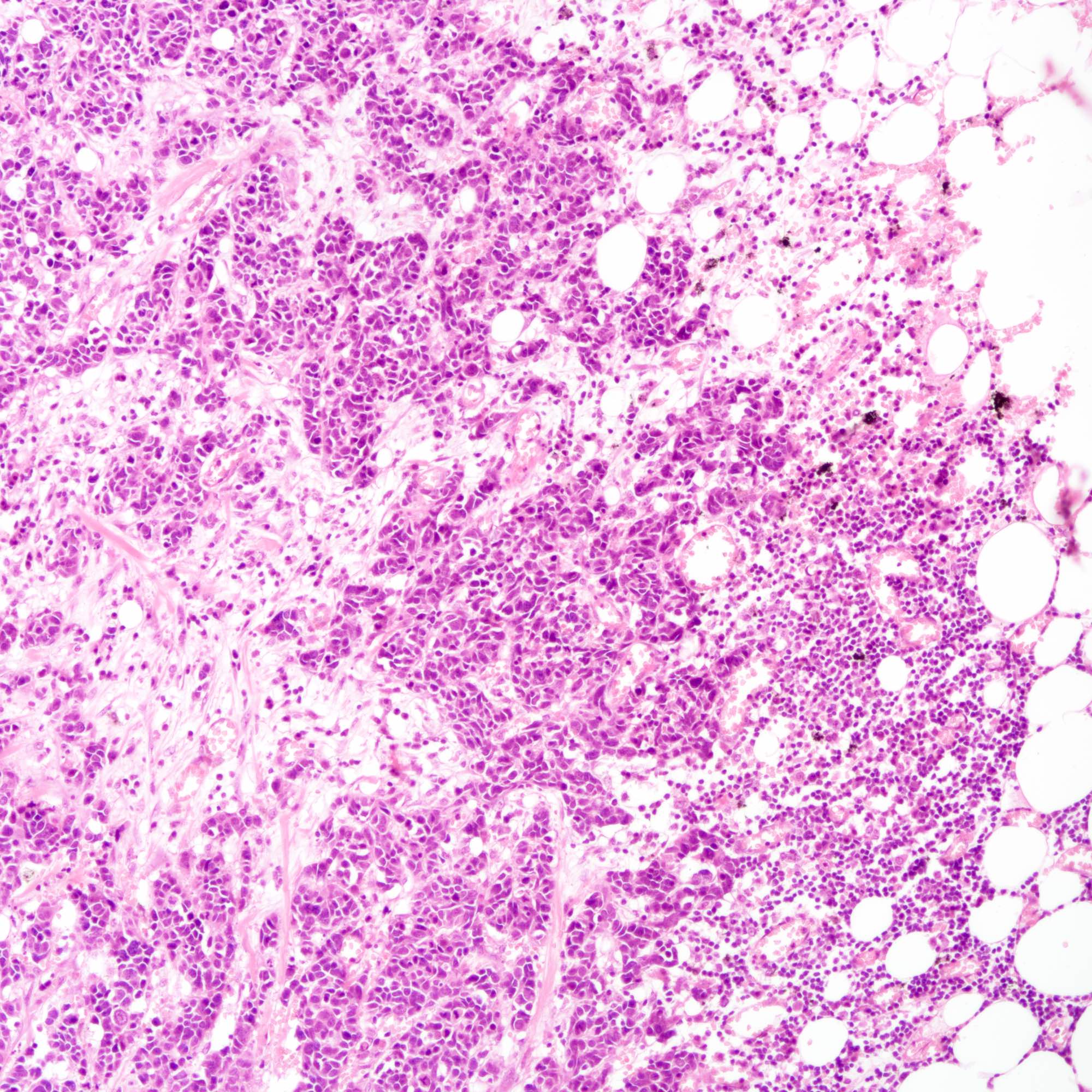
- Small cell carcinoma:
- Infiltrative growth pattern
- Crush artifact frequent
- Necrosis and lymphovascular invasion common
- Tumor cells densely packed with ill defined cell borders
- High N/C ratio with scanty cytoplasm
- Small dark hyperchromatic nuclei and inconspicuous nucleoli
- Mitotic count high
- Associated with proliferative changes, in situ carcinomas and invasive carcinomas (Am J Surg Pathol 2000;24:1231)
- In situ small cell carcinoma present in 5 out of 9 cases in a case series
- In situ / invasive ductal, lobular and squamous neoplastic components occasionally identified
- Infiltrative growth pattern
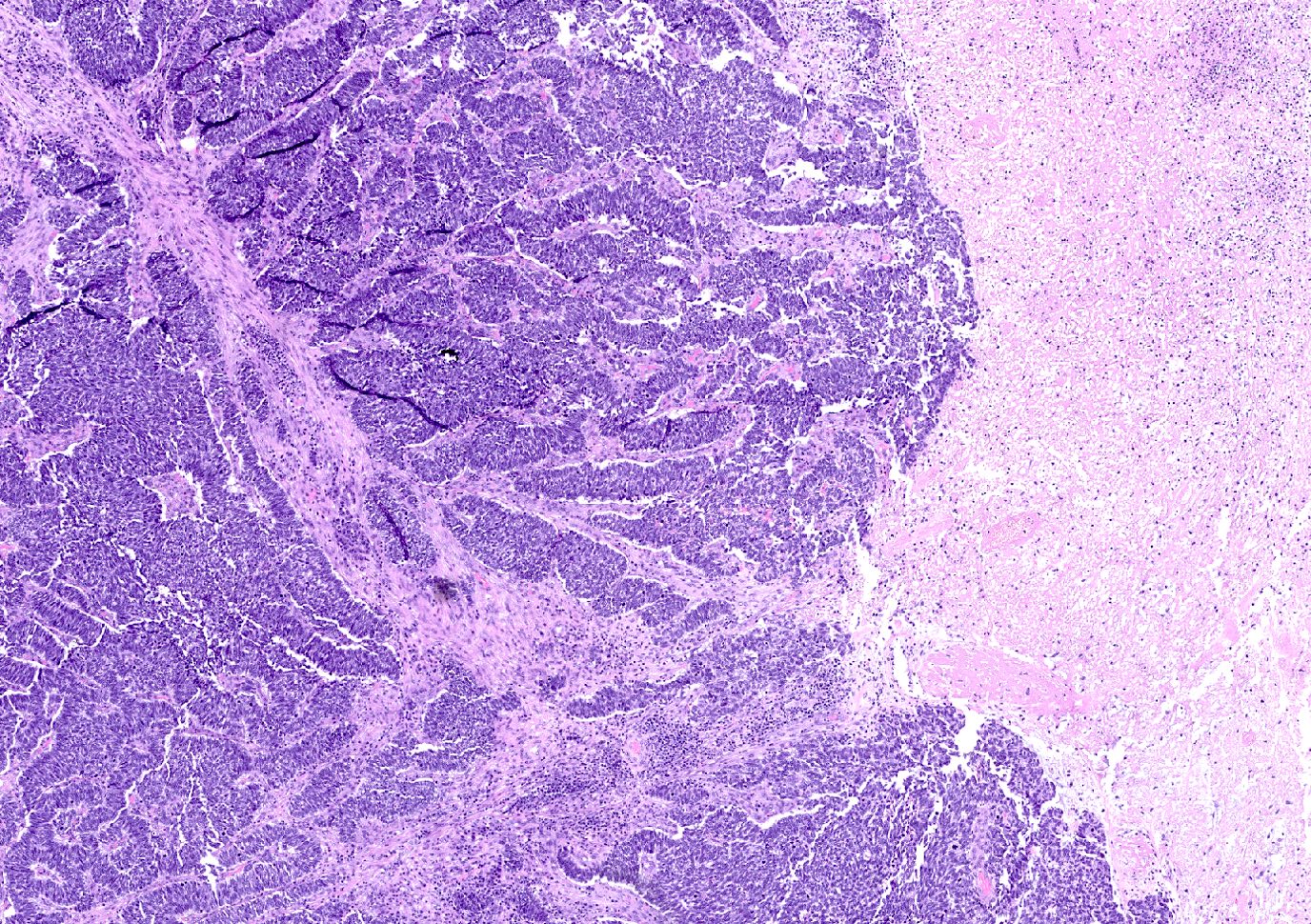
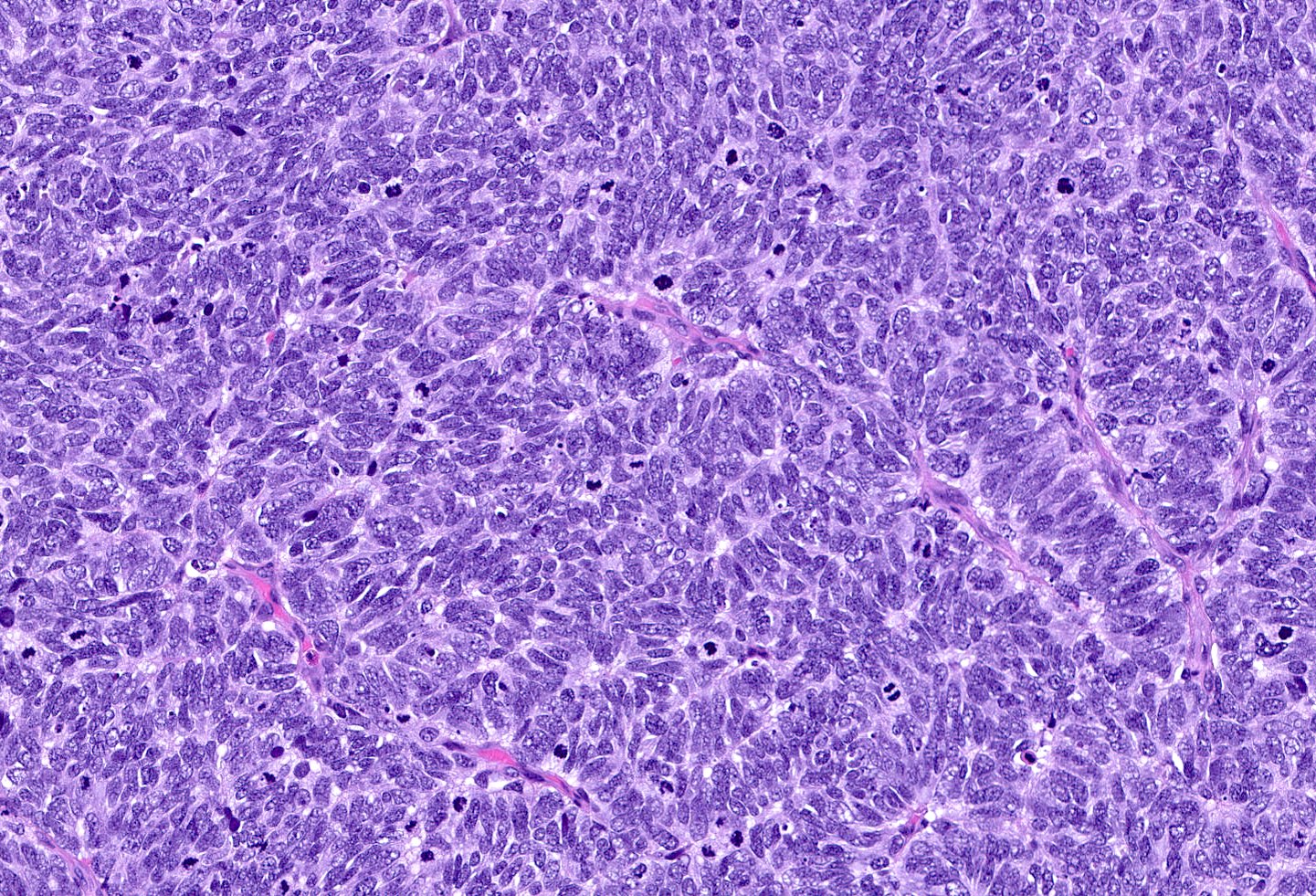
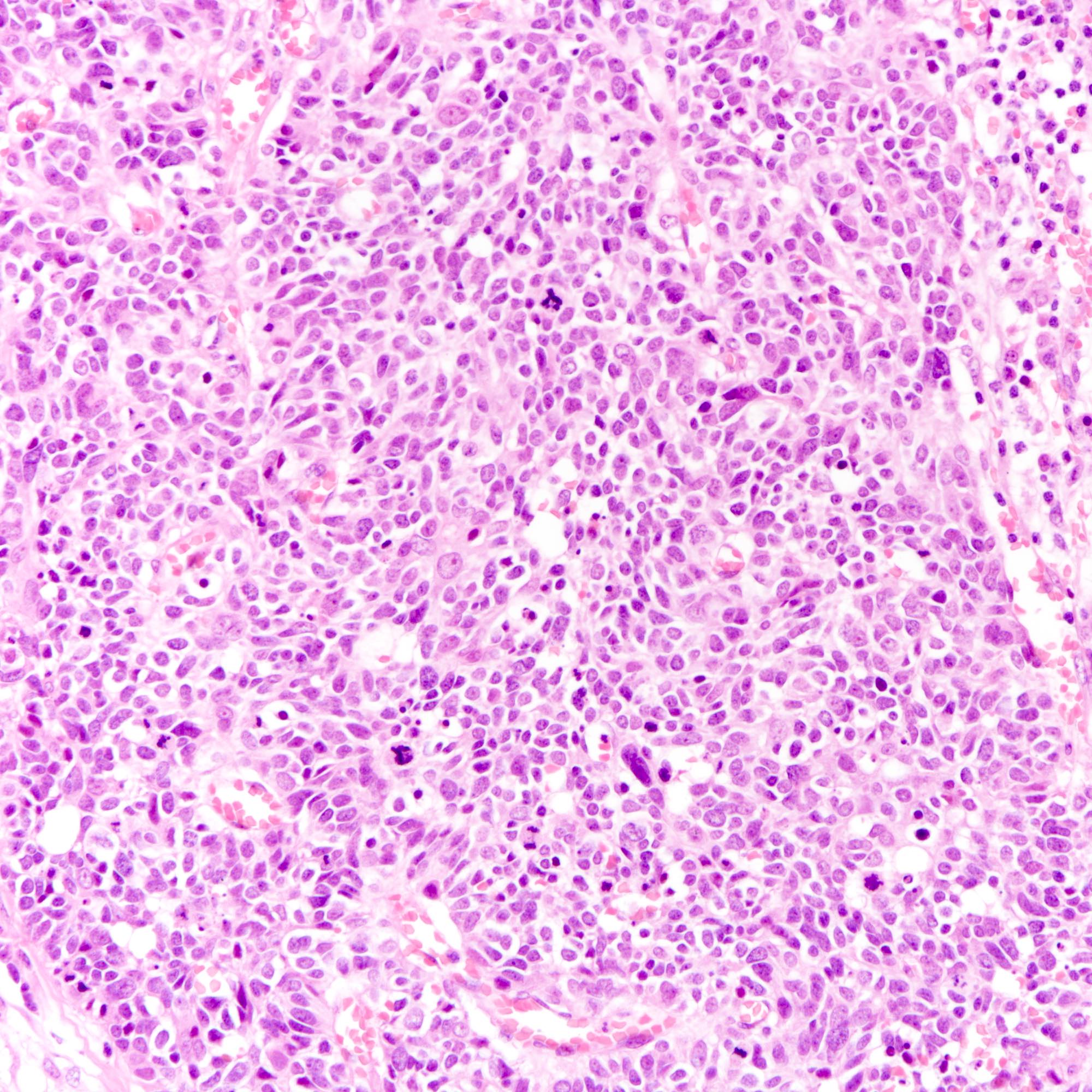
- Large cell carcinoma:
- High grade features (frequent necrosis, lymphovascular invasion and mitosis) similar to small cell carcinoma
- Tumor cells have a larger amount of cytoplasm
- Chromatin pattern coarse with occasional distinct nucleoli
- Neuroendocrine carcinomas of the breast histologically indistinguishable from their counterparts of other primaries
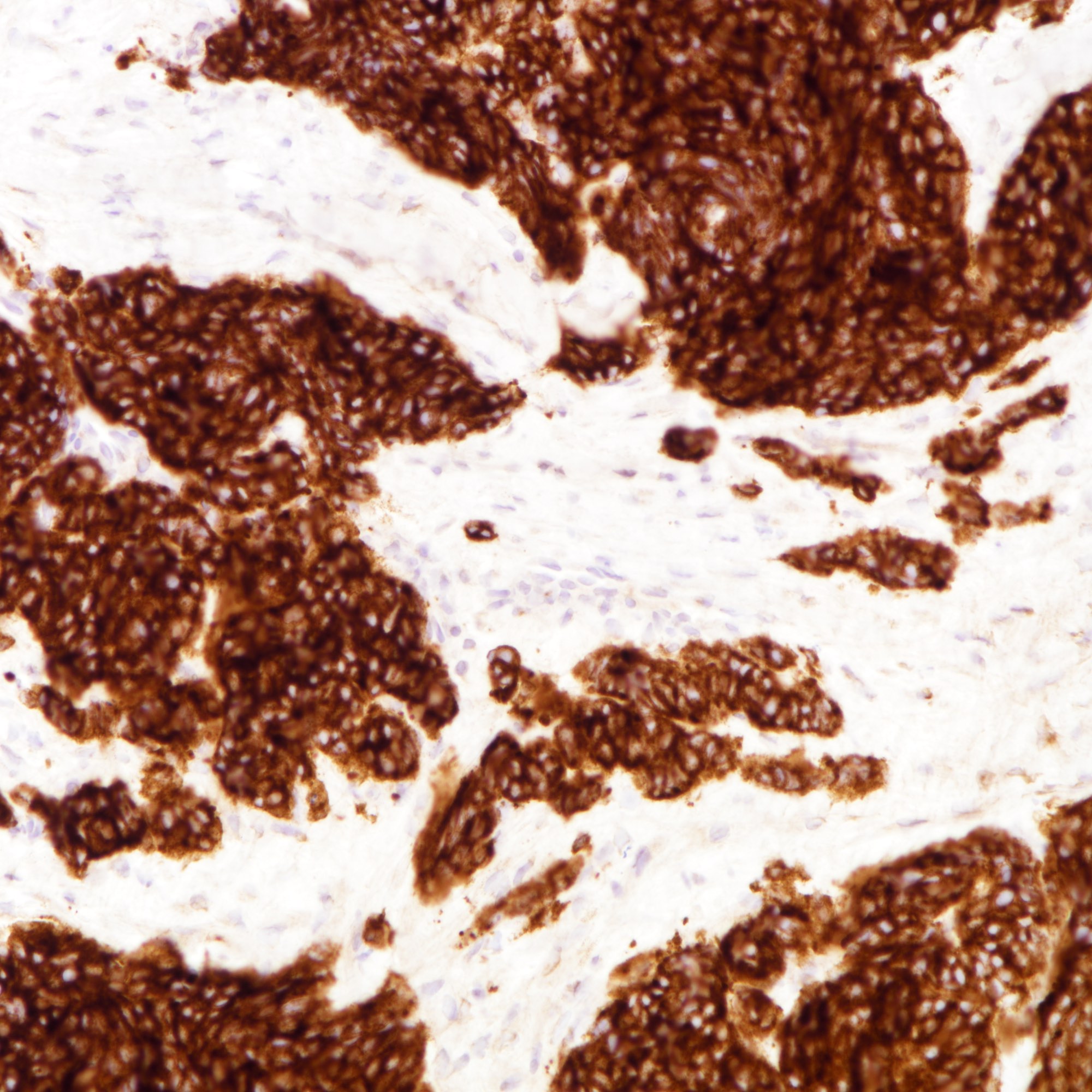
Microscopic (histologic) images
Contributed by Joshua J.X. Li, M.B.Ch.B., Gary M. Tse, M.B.B.S. and Kristen E. Muller D.O.
Small cell carcinoma
Large cell carcinoma
Cytology description
- Neuroendocrine features, including nuclear molding and fine salt and pepper chromatin, may be identified in small cell carcinoma
- Necrosis can be present in the background (J Cytol 2011;28:91)
- Smear preparation can accentuate crush artifact
Cytology images
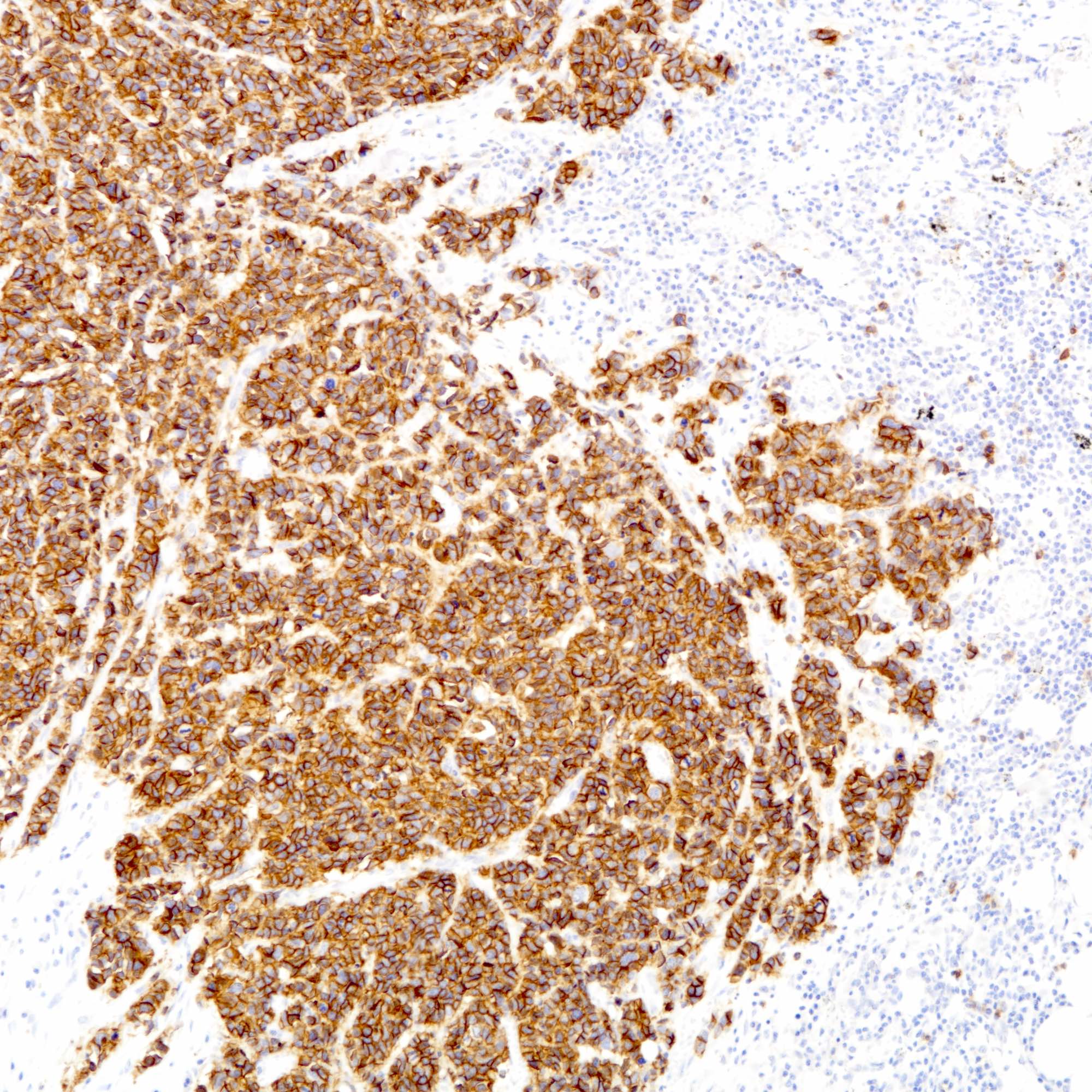
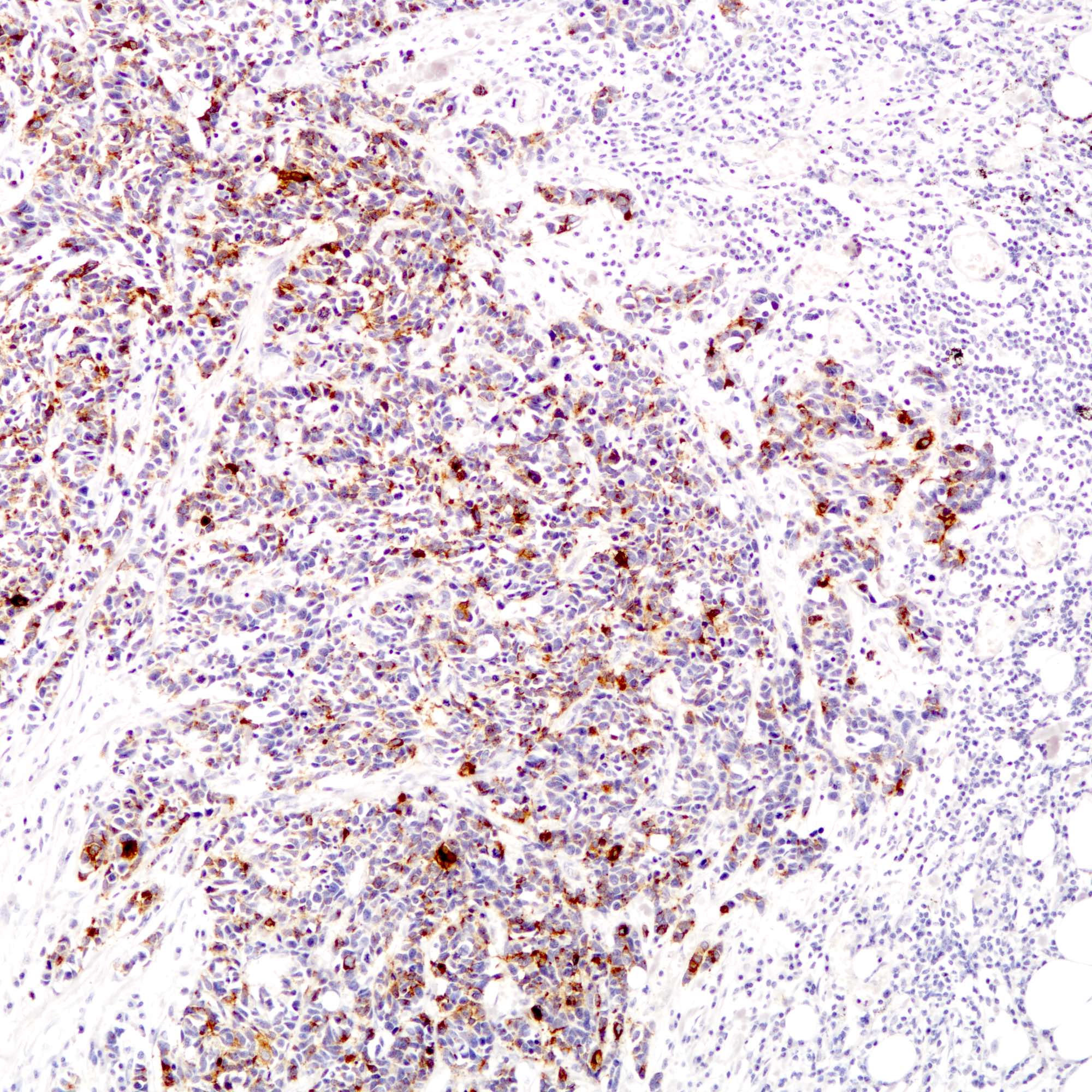
Positive stains
- Cytokeratins:
- Neuroendocrine markers:
- Hormonal markers:
- Estrogen receptor (30 - 67%), progesterone receptor (13 - 56%) (Am J Surg Pathol 2000;24:1231, Breast Cancer Res Treat 2016;158:195)
- Of note, pulmonary neuroendocrine carcinomas also not uncommonly express estrogen and progesterone receptor (Arch Pathol Lab Med 2008;132:1889)
- Other markers:
Negative stains
Molecular / cytogenetics description
- TP53 (75%) and PIK3CA (33%) mutations common in small cell carcinoma of the breast (Breast Cancer Res Treat 2016;158:195):
- PIK3CA mutation not present in small cell carcinoma of lung
- RB1 mutation present in small cell carcinoma of lung but not of breast primary
- Mutations in AKT1, ARID1A, CDH1, FOXA1, GATA3 and TBX3 also detected (J Pathol 2017;241:405)
- Compared to luminal breast cancers, PIK3CA mutations are less frequent, whereas FOXA1 and TBX3 mutations are more common
Sample pathology report
- Left breast, local excision:
- Small cell carcinoma (see comment)
- Comment: Sections show breast tissue infiltrated by cords, nests and sheets of tumor cells. The tumor cells possess small dark hyperchromatic nuclei and inconspicuous nucleoli with scanty cytoplasm. The mitotic count is at __ per 10 high power fields. The tumor measures __ in maximal dimension and is clear from (specify if < 1 cm __) / involves the resection margins.
- The tumor cells are diffusely positive for synaptophysin and focally positive for chromogranin.
- Right breast, local excision:
- Large cell carcinoma (see comment)
- Comment: Sections show breast tissue infiltrated by nests and sheets of tumor cells. The tumor cells possess enlarged nuclei with coarse chromatin pattern, distinct nucleoli and a moderate amount of cytoplasm. The mitotic count is at __ per 10 high power fields. The tumor measures __ in maximal dimension and is clear from (specify if < 1 cm __) / involves the resection margins.
- The tumor cells are diffusely positive for synaptophysin and focally positive for chromogranin.
Differential diagnosis
- Neuroendocrine carcinomas of other, nonmammary origin:
- Neuroendocrine carcinomas of the breast histologically indistinguishable from their counterparts of other primaries
- Background ductal in situ or invasive, when present, highly suggestive of breast primary (Am J Surg Pathol 2000;24:1231)
- Hormonal markers, GATA3 and TTF1 stains can be helpful but not entirely sensitive or specific (Mod Pathol 2016;29:788, Front Endocrinol (Lausanne) 2020;11:228)
- Correlation with clinical history and radiological findings often required
- Neuroendocrine tumors:
- Majority of neuroendocrine tumors are Nottingham grade 1 or 2 (low nuclear grade and infrequent mitosis)
- Necrosis and lymphovascular invasion not features of neuroendocrine tumors
- Ki67 index is lower in neuroendocrine tumors than neuroendocrine carcinomas
- Invasive carcinoma with neuroendocrine differentiation:
- Invasive carcinomas with neuroendocrine differentiation show neuroendocrine features in terms of nuclear morphology or positivity to neuroendocrine markers in 10 - 90% of the tumor
- Complete small cell or large cell nuclear features usually absent
- Negativity to neuroendocrine markers, in particular synaptophysin, may help identify nonneuroendocrine areas
- Adenoid cystic carcinoma (solid):
- Solid pattern of adenoid cystic carcinoma can resemble small cell carcinoma
- Focal cribriform pattern, tubular pattern and luminal secretion, if identified, aids in diagnosing adenoid cystic carcinoma
- Markedly hyperchromatic nuclei with crush artifact not a feature of adenoid cystic tumor
- Positivity to KIT, MYB and SOX10 with negativity to hormonal markers confirms a diagnosis of adenoid cystic carcinoma
- Lymphoma:
- Markedly hyperchromatic nuclei with crush artifact in small cell carcinoma can resemble lymphoma
- Lymphoma does not show cellular cohesion
- Cytokeratins, hormonal, neuroendocrine and lymphoid markers differentiate lymphomas from carcinomas
- Melanoma:
- Degree of nuclear atypia and nuclear features of melanoma can overlap with large cell carcinoma
- Melanin pigment, when identified, indicates melanoma
- Definitive confirmation may require melanocytic markers
Board review style question #1
Which of the following features distinguishes large cell carcinoma from small cell carcinoma of breast primary?
- Coarse chromatin pattern and distinct nucleoli
- Positive axillary lymph nodes
- Positivity to estrogen and progesterone receptors
- Positivity to synaptophysin but not chromogranin
- Presence of extensive tumor necrosis
Board review style answer #1
A. Coarse chromatin pattern and distinct nucleoli. Large cell carcinoma is distinguished from small cell carcinoma based on cellular morphologic features, including a coarse chromatin pattern, distinct nucleoli and a greater amount of cytoplasm. The immunoprofile of large cell and small cell carcinomas are similar (Breast Care (Basel) 2015;10:281). Other histological and clinical features are also not useful in distinguishing large cell carcinoma from small cell carcinoma.
Comment Here
Reference: Neuroendocrine carcinoma (NEC)
Comment Here
Reference: Neuroendocrine carcinoma (NEC)
Board review style question #2
Which of the following findings most favors small cell carcinoma of breast primary over metastatic small cell carcinoma from the lung?
- Positivity to estrogen and progesterone receptors
- Positivity to TTF1 stain
- Presence of adjacent ductal carcinoma in situ
- Presence of RB1 mutation
- Presence of TP53 mutation
Board review style answer #2
C. Presence of adjacent ductal carcinoma in situ. The majority of small cell carcinomas of the breast are associated with proliferative changes, in situ and invasive carcinomas (Am J Surg Pathol 2000;24:1231). TP53 mutation is detected in small cell carcinomas of both lung and breast primaries, whereas RB1 mutation was only present in small cell carcinomas of lung primary from a cohort described by McCullar et al. (Breast Cancer Res Treat 2016;158:195). TTF1, estrogen receptor and progesterone receptor expression do not accurately distinguish small cell carcinomas of breast and lung (Front Endocrinol (Lausanne) 2020;11:228, Am J Surg Pathol 2000;24:1231).
Comment Here
Reference: Neuroendocrine carcinoma (NEC)
Comment Here
Reference: Neuroendocrine carcinoma (NEC)