Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Hart J, Alexiev BA. Osteosarcoma, NOS. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/boneosteosarcomageneral.html. Accessed April 2nd, 2025.
Definition / general
- Malignant tumor of connective tissue (mesodermal) origin within which the tumor cells produce bone or osteoid (tumor bone or tumor osteoid) (Eur J Cancer 2002;38:1218, Am J Clin Pathol 2006;125:555)
Essential features
- Malignant bone tumor with compatible imaging (Eur J Cancer 2002;38:1218)
- Bone production by tumor cells (Am J Clin Pathol 2006;125:555)
- Permeative and destructive growth pattern
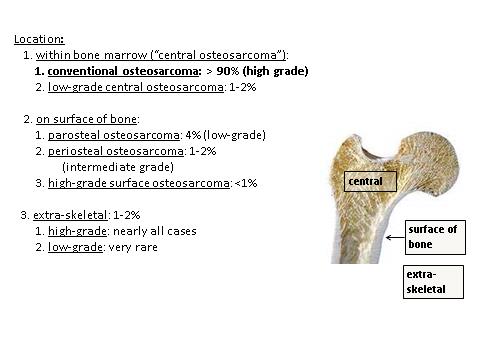
- Conventional central osteosarcoma:
- Intramedullary sarcoma
- Tumor cells with high grade atypia; atypical mitotic figures are frequently present
- Low grade central osteosarcoma:
- Intramedullary location
- Predominantly fibroblastic osteosarcoma with mild nuclear atypia and well formed neoplastic bony trabeculae
- MDM2 amplification
- Paraosteal osteosarcoma:
- Paraosteal location
- Low grade spindle cell tumor with woven bone formation
- MDM2 amplification
- Periosteal osteosarcoma:
- Surface location under the periosteum
- Intermediate grade, often with chondroblastic features
- High grade surface osteosarcoma:
- Histologically high grade osteosarcoma
- Arising on the surface of the bone without a substantial intraosseous component
- No MDM2 amplification
- Secondary osteosarcoma:
- Known history of Paget disease of bone, previous radiation therapy, bone infarction, osteomyelitis, orthopedic implants
- Osteosarcoma histology
Terminology
- Primary osteosarcoma: a bone forming sarcoma arising in otherwise normal bone
- Conventional central osteosarcoma: an intramedullary high grade bone forming sarcoma
- Low grade central osteosarcoma: a low grade malignant bone forming sarcoma that originates within the medullary cavity
- Parosteal osteosarcoma: a low grade malignant bone forming sarcoma that arises on the cortical surface of a bone
- Periosteal osteosarcoma: an intermediate grade, predominantly chondroblastic, bone forming sarcoma arising on surface of the bone, typically underneath the periosteum
- High grade surface osteosarcoma: a high grade malignant bone forming sarcoma arising on the cortical surface of a bone
- Extraskeletal osteosarcoma: a bone forming sarcoma that arises without connection to the skeletal system
- Secondary osteosarcoma: a bone forming sarcoma arising in abnormal bone (prior radiation therapy, coexisting Paget disease of bone, infarction, etc.)
- See Diagrams / tables
ICD coding
- ICD-O: 9180/3 - osteosarcoma, NOS
- ICD-10: C41.9 - malignant neoplasm of bone and articular cartilage, unspecified
- ICD-11: 2B51.Z & XH1XF3 - osteosarcoma of bone and articular cartilage of unspecified sites & osteosarcoma, NOS
Epidemiology
- Incidence: most common high grade sarcoma of skeleton
- Age (bimodal age distribution) (Cancer Treat Res 2009;152:3, Cancer 2009;115:1531):
- Most cases: 10 - 14 years old
- Second smaller peak: adults (> 40 year old); usually secondary osteosarcoma
- M:F = 1.3:1
- Paget disease of bone: ~1% (variable range among studies) of patients develop conventional osteosarcoma (55 - 70% arises in polyostotic Paget disease) (Bone 2009;44:431)
- Parosteal osteosarcoma: most common surface osteosarcoma
- Low grade central osteosarcoma: 1 - 2% of osteosarcoma
- Periosteal osteosarcoma: rare (less common than parosteal osteosarcoma)
- High grade surface osteosarcoma: rare (< 1% of osteosarcoma)
- Extraskeletal osteosarcoma: rare; usually occurs in adults
Sites
- See Diagrams / tables
- Osteosarcoma may arise in any bone
- Most common: long bones of extremities (near the most proliferative growth plates) (Cancer 2009;115:1531)
- Distal femur
- Proximal tibia
- Proximal humerus
- Metaphysis: 90%
- Diaphysis: 9%
- Epiphysis: 1%
- Older patients: jaw, pelvis, spine (Cancer 2009;115:1531)
- Multifocal osteosarcoma: may occur in the setting of Paget disease of the bone
- Very rare: small bones of hands and feet
- Parosteal osteosarcoma: arises on cortical surface, usually metaphysis
- Most common: distal posterior femur
- Other sites: proximal tibia, proximal humerus
- Uncommon: flat bones
- Low grade central osteosarcoma:
- Long bones: distal femur, proximal tibia (metaphysis and diaphysis); may fill the medullary space of the entire bone
- Uncommon: flat bones, small tubular bones of hands and feet
- Periosteal osteosarcoma: arises from periosteum, usually metaphyseal or metadiaphyseal (often with an anterior or medial epicenter; often wraps around bone)
- Common sites: long bones, pelvis
- Other less common sites: clavicle, ribs, cranial bones, jaw
- High grade surface osteosarcoma: arises on cortical surface (femur, tibia, humerus)
- Extraskeletal osteosarcoma:
- Most common: arises in the deep soft tissues (thigh, buttocks, shoulder girdle, trunk, retroperitoneum)
- Skin and subcutaneous tissue: ~10%
Pathophysiology
- Remains uncertain but a relationship seems to exist between rapid bone growth and the onset of this malignancy (JAAPA 2018;31:15)
Etiology
- In most patients, the etiology of osteosarcoma remains obscure (Ann Oncol 2010;21:vii320)
- The predilection of osteosarcoma for the age of pubertal growth spurt and the sites of maximum growth suggest a correlation with rapid bone proliferation (Ann Oncol 2010;21:vii320)
- Association between Paget disease of bone and osteosarcoma (J Bone Miner Res 2006;21:P58)
- A minority of osteosarcomas are caused by radiation exposure
- Most common mutated genes in sporadic osteosarcoma are TP53 in > 90% and RB1 in 56% of cases
- Osteosarcoma associated with genetic syndromes:
- Li-Fraumeni syndrome (TP53)
- Hereditary retinoblastoma syndrome (RB1)
- Bloom syndrome (RECQL3)
- Rothmund-Thompson syndrome (RECQL4)
- Werner syndrome (RECQL4)
Clinical features
- Patients typically present with a dull, aching pain of several months' duration that may suddenly become more severe (JAAPA 2018;31:15)
- Night pain is common
- Physical examination may reveal localized tenderness, swelling and restricted range of motion of the adjacent joint
- Presence of a mass or pathologic fracture
- Nearly 10 - 20% of patients are affected by measurable metastatic disease before actual onset, the most common site being the lungs (85%), followed by the bones (8 - 10%) and occasionally the lymph nodes
Diagnosis
- Imaging features of a bone tumor:
- For any suspected bone lesion, a preoperative imaging protocol should be followed, which includes taking at least 2 Xray views of the involved bone and the adjacent joints
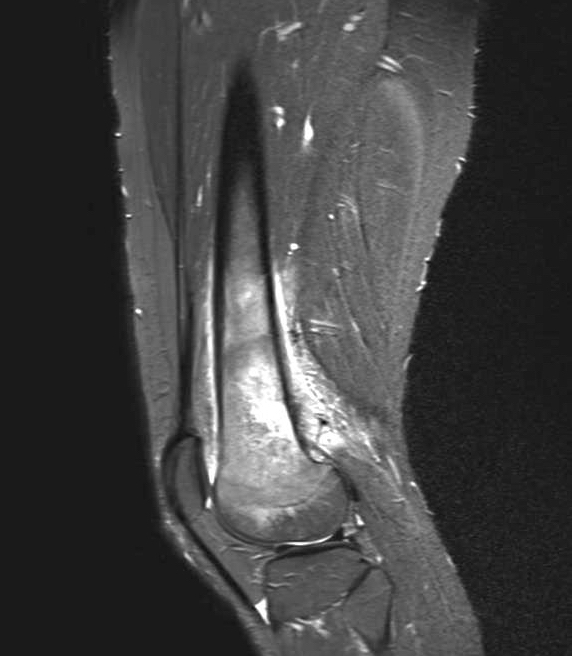
- Magnetic resonance imaging (MRI) is warranted to evaluate the lesion’s invasion into the soft tissue and neurovascular structures, level of bone marrow replacement, skip lesions and extension into the bordering joint (AJR Am J Roentgenol 1994;163:1171)
- Computed tomography (CT) scans are useful in defining cortical irregularities, fracture sites, mineralization and neurovascular involvement
- Positron emission tomography (PET) scans can be used to assess the primary lesion and to detect metastases in other organs
- Some suggest using PET scans to assess the response of the disease to chemotherapy as well as to predict progression free survival (Clin Adv Hematol Oncol 2010;8:705)
- Biopsy is essential in the diagnosis of osteosarcoma (SICOT J 2018;4:12)
- Both core needle and open biopsy provide adequate tissue for accurate analysis (Clin Adv Hematol Oncol 2010;8:705, Skeletal Radiol 2002;31:349)
- Core biopsy is preferred because there less risk of local contamination; this is important in patients who may have limb sparing surgery (Cancer 1993;71:3358)
- Fine needle aspiration should not be used, as it does not provide a large enough sample for a precise diagnosis (Skeletal Radiol 2002;31:349)
Laboratory
- There is no laboratory test that is diagnostic of osteosarcoma (SICOT J 2018;4:12)
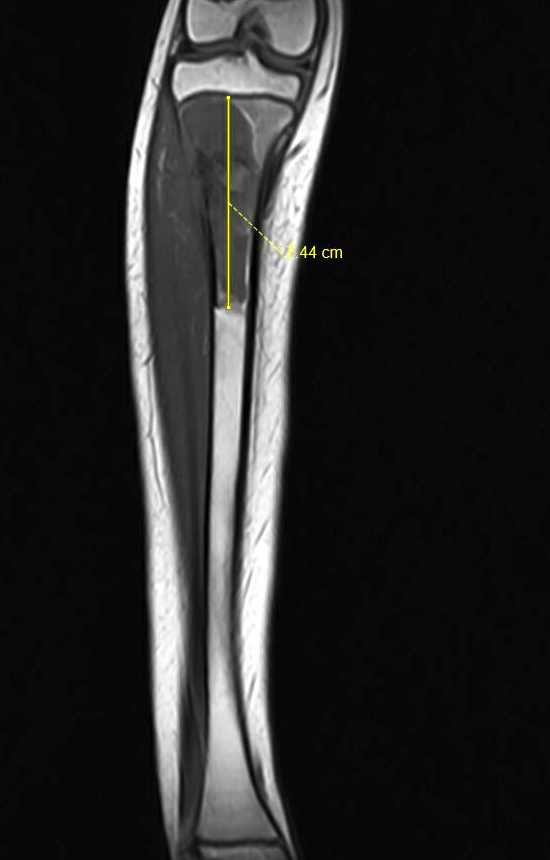
Radiology description
- Conventional central osteosarcoma (Radiographics 2010;30:1653):
- Medullary and cortical bone destruction
- Wide zone of transition, permeative appearance
- Aggressive periosteal reaction
- Sunburst type
- Codman triangle
- Lamellated (onion skin) reaction
- Tumor matrix ossification / calcification
- Soft tissue involvement
- Low grade central osteosarcoma (Radiographics 2010;30:1653, Skeletal Radiol 2004;33:373):
- Intracompartmental expansile lytic fibro-osseous lesion with varying amounts of septal ossification
- Another less common pattern is as a dense sclerotic lesion
- Cortical erosion and soft tissue extension are also common features
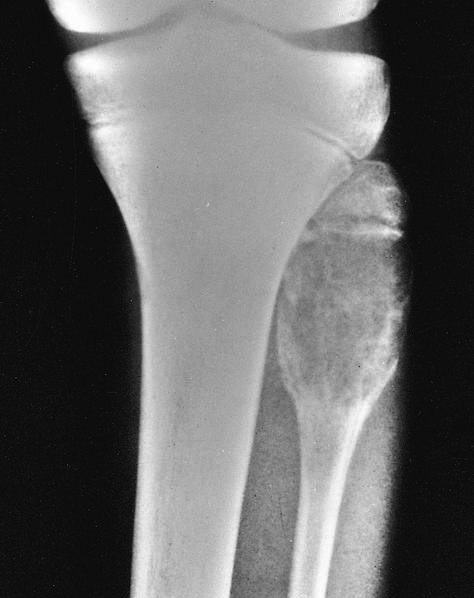
- Parosteal osteosarcoma (Radiographics 2010;30:1653, Radiology 2004;233:129):
- Usually located at the metaphysis, most commonly at the posterior aspect of the distal femur, followed by either end of the tibia and then the proximal humerus
- Lobulated, cauliflower-like surface mass with central dense ossification adjacent to the bone
- Tumor stalk
- Cortical thickening without aggressive periosteal reaction is often seen
- True marrow invasion is rare
- Periosteal osteosarcoma (Radiographics 2010;30:1653, Skeletal Radiol 2021;50:9):
- Lesions tend to be diaphyseal
- Lucent, fusiform mass on the surface of the bone with variable mineralization, frequently accompanied by focal clumps of calcifications
- Tumor incompletely encircles the bone
- Although the thickened underlying cortex is eroded by the mass, the endosteal cortex typically remains intact
- Periosteal reaction extending into the soft tissue component
- Arises from the cortex, intramedullary extension is rare
- Predominantly chondroid matrix
- High grade surface osteosarcoma (Radiographics 2010;30:1653, Cancer 2008;112:1592):
- Tumor arises from the bony surface
- Dense ossification
- Cortical erosions and medullary involvement (~50% of cases)
- Relatively high circumferential involvement
- Periosteal reaction uncommon
Radiology images
Prognostic factors
- Conventional (high grade) osteosarcoma:
- Aggressive local growth and early hematogenous dissemination (lung metastasis, skeletal metastases)
- Nonmetastatic disease: 60 - 70% 3 year event free survival with current treatment (neoadjuvant chemotherapy followed by resection) (Acta Orthop 2011;82:211)
- Poor prognostic factors:
- Metastatic tumor (lung, skeletal, pleura, heart) (J Clin Oncol 2002;20:776)
- Axial tumor site (opposed to extremities)
- Age < 14 years old, high serum alkaline phosphatase, tumor volume > 200 mL, older chemotherapy protocol, inadequate surgical margins and poor histologic response (good response: ≥ 90% treatment effect; poor response: < 90% treatment effect) (Cancer 2006;106:1154)
- Parosteal and low grade central osteosarcoma:
- 5 year overall survival: ≥ 90% (Clin Orthop Relat Res 2008;466:1318)
- Dedifferentiation (~15 - 25% of cases): poor prognosis (similar to conventional high grade osteosarcoma) (Clin Orthop Relat Res 2008;466:1318)
- Periosteal osteosarcoma:
- 5 year overall survival: ~89%
- 10 year overall survival: ~83% (Clin Orthop Relat Res 2006;453:314)
- Metastasis: ~15%
- High grade surface osteosarcoma: prognosis is similar to conventional (high grade, intramedullary) osteosarcoma
- Extraskeletal osteosarcoma:
- 5 year overall survival: ~25%
Case reports
- 9 and 11 year old girls with L5 osteosarcoma and 15 year old boy with L4 osteosarcoma (Rev Esp Cir Ortop Traumatol 2019;63:122)
- 23 year old woman with rare rib lesion due to parosteal osteosarcoma (J Med Case Rep 2019;13:19)
- 34 year old woman with parosteal osteosarcoma with focal fatty metaplasia (Radiol Case Rep 2018;14:200)
- 48 year old woman with primary osteosarcoma originating from kidney (Medicine (Baltimore) 2019;98:e14234)
- 3 patients with secondary osteosarcoma after brain injury and allogeneic bone marrow transplants (J Pediatr Hematol Oncol 2020;42:e100)
Treatment
- Conventional osteosarcoma treatment consists of a combination of neoadjuvant and adjuvant chemotherapy and surgery (J Clin Oncol 1987;5:21)
- Chemotherapy regimens for osteosarcoma based on high dose methotrexate and cisplatin with doxorubicin and ifosfamide (Cancer 2006;106:1154)
- Low grade osteosarcoma can typically be treated with excision alone and chemotherapy is avoided if final pathology confirms low grade (SICOT J 2018;4:12)
- National Comprehensive Cancer Network (NCCN) guidelines (NCCN Guidelines: Bone Cancer [Accessed 2 December 2022]):
- Low grade osteosarcomas (low grade central and parosteal osteosarcoma): wide excision
- If high grade dedifferentiation is identified on the excisional specimen, adjuvant chemotherapy is administered
- Periosteal osteosarcoma: wide excision
- In some cases, neoadjuvant chemotherapy may be administered
- High grade osteosarcoma (intramedullary and surface): neoadjuvant chemotherapy followed by wide excision
- Adjuvant therapy includes chemotherapy and depending on the final margin status (or if the tumor is unresectable), may include radiation
- Metastatic disease at presentation:
- If the clinical team chooses to resect the metastases, then resection and chemotherapy
- If the metastases are not resected, chemotherapy and radiation therapy are administered
- Extraskeletal osteosarcoma: treated as a soft tissue sarcoma
- Treatment depends on tumor location, stage and resectability
- Low grade osteosarcomas (low grade central and parosteal osteosarcoma): wide excision
- Assessment of preoperative chemotherapy:
- Preoperative chemotherapy is commonly used with limb salvage procedures for treatment of high grade sarcomas, particularly osteosarcoma and Ewing sarcoma
- It is generally required to quantify the extent of tumor necrosis as a percentage of the total tumor area
- For osteosarcomas, chemotherapy induced necrosis of ≥ 90% has a > 90% disease free survival, compared with < 50% in patients with < 90% tumor necrosis
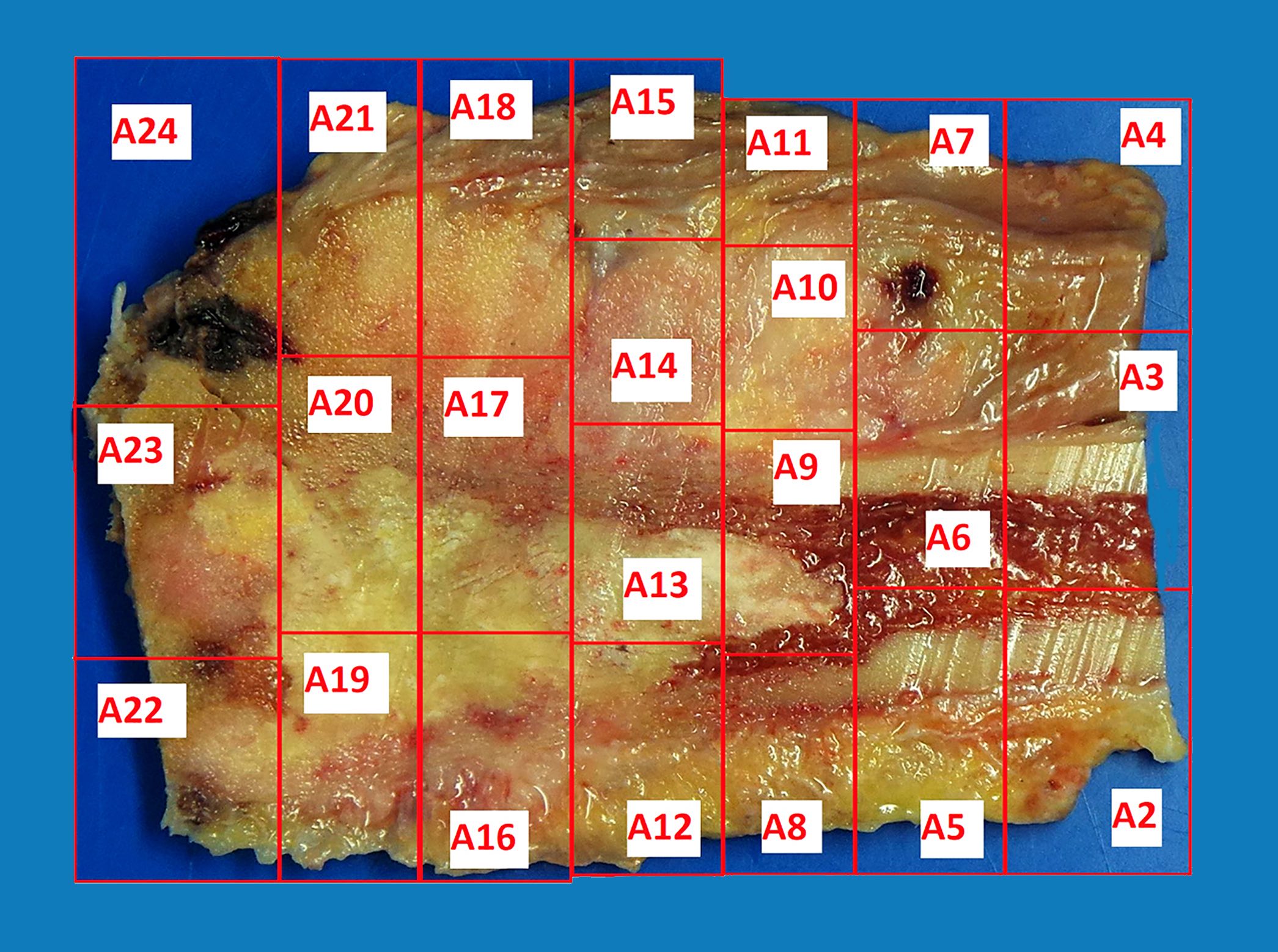
- To determine the extent of necrosis in an osteosarcoma or Ewing sarcoma specimen, the slab specimen of the resected bone containing tumor provides the template for histologic analysis
- Photograph of the slice is taken and the site of each numbered block is marked on a grid pattern diagram
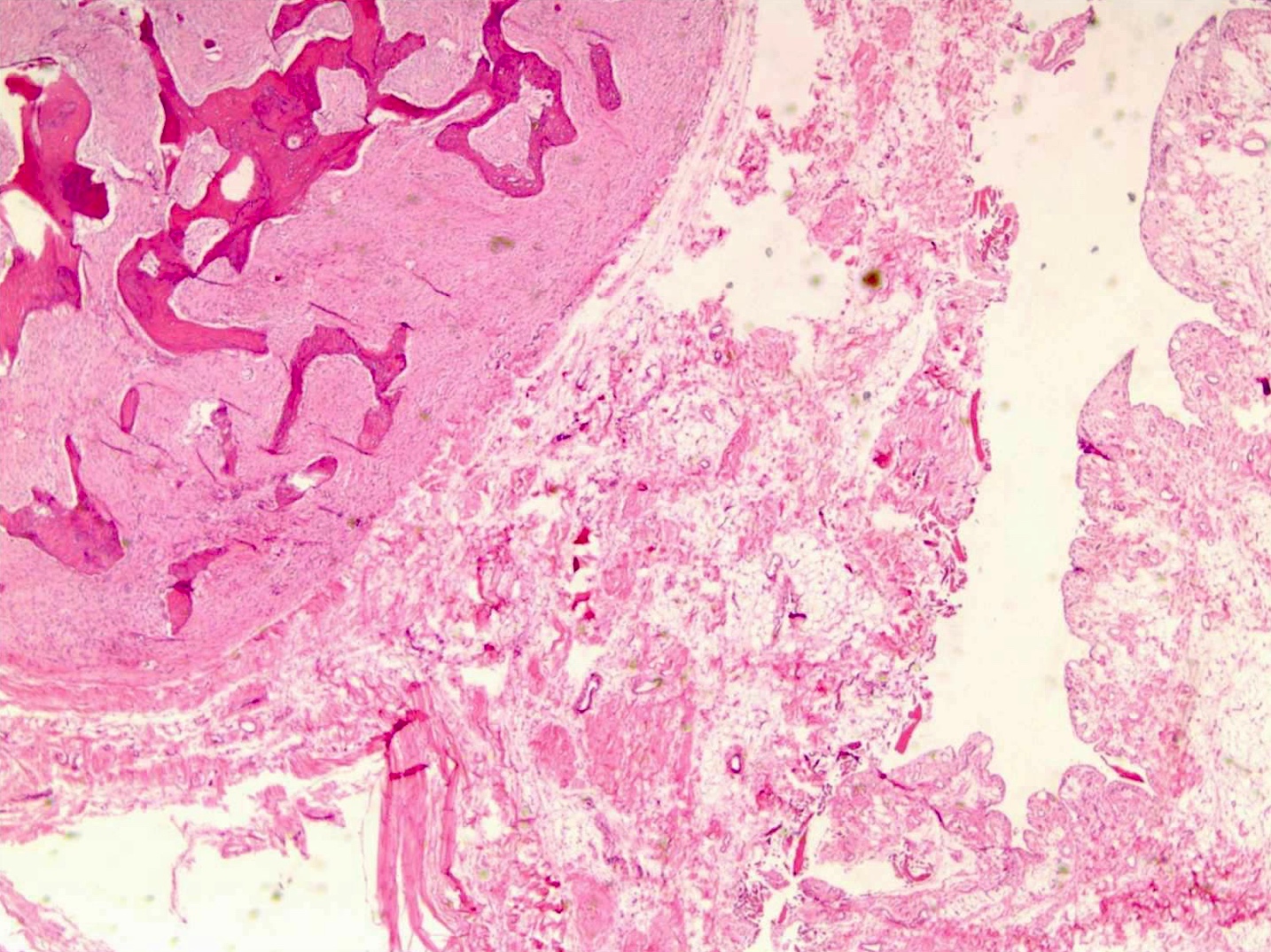
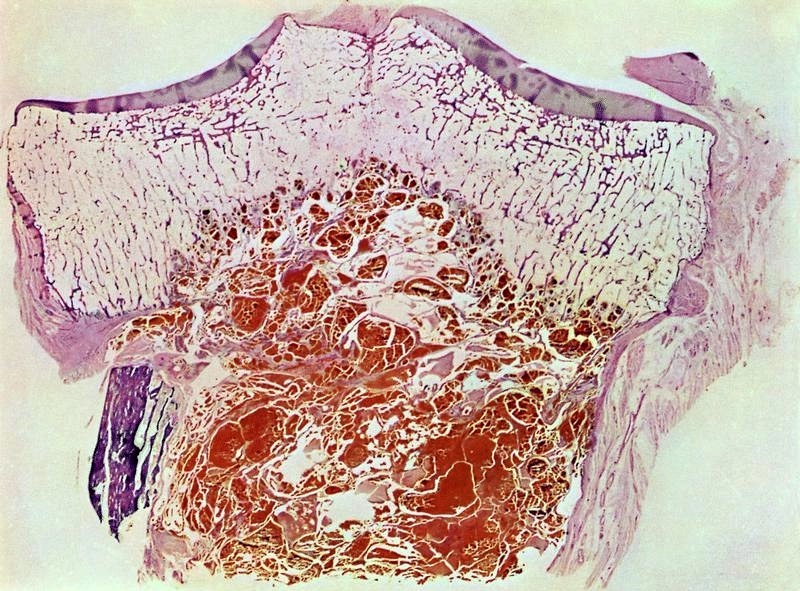
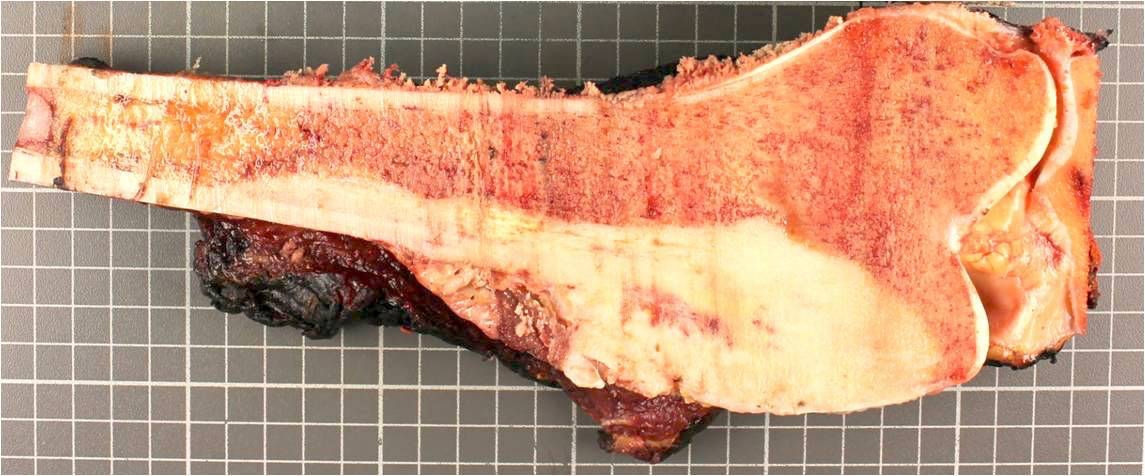
Gross description
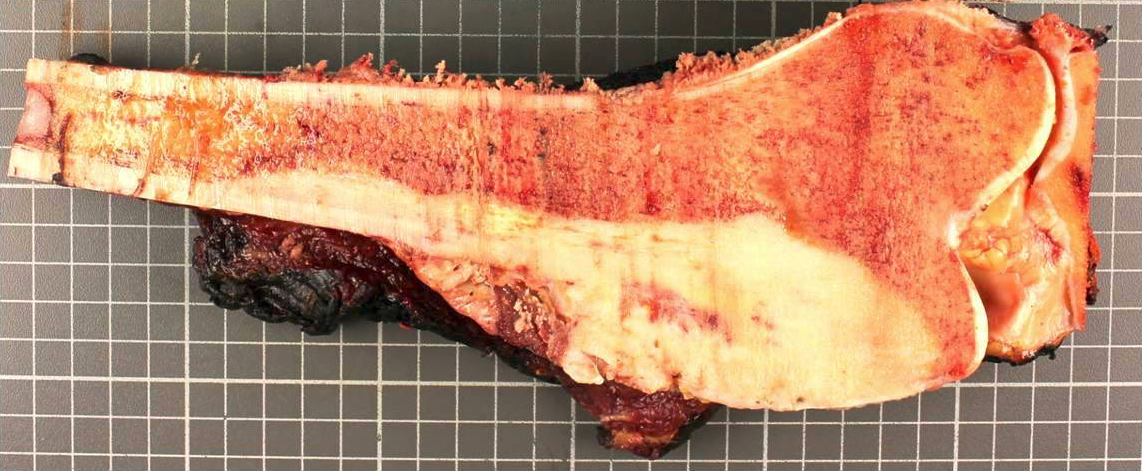
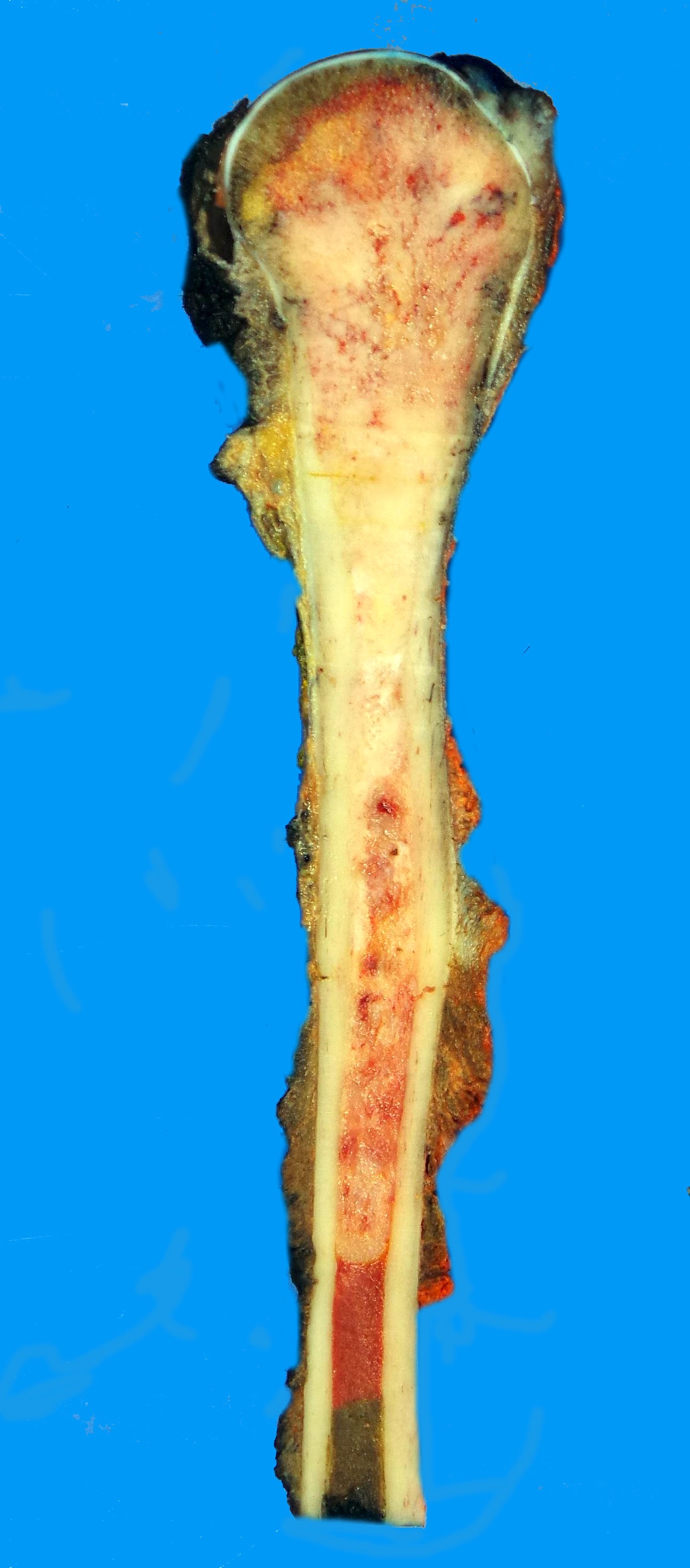
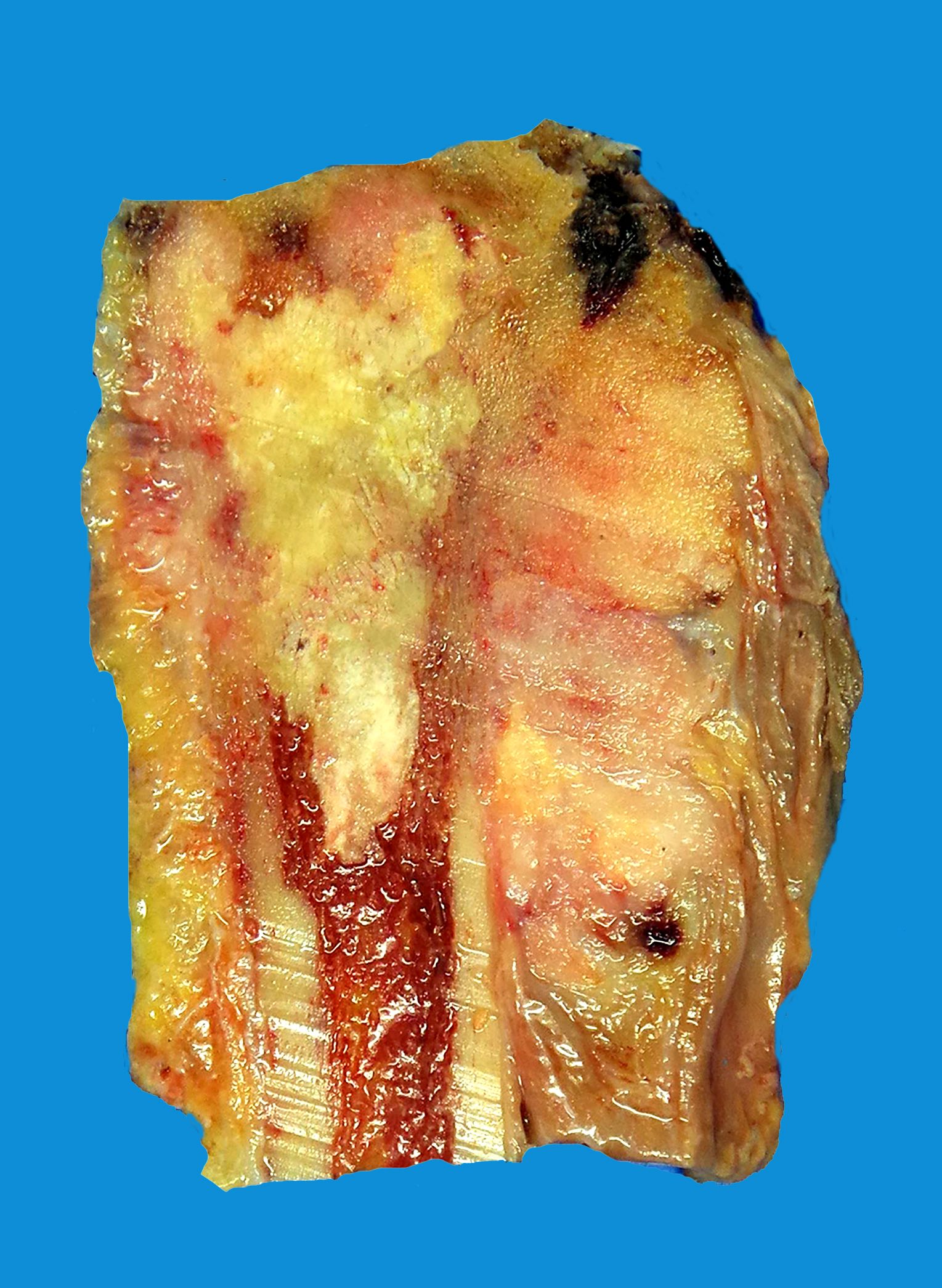
- Conventional (high grade intramedullary) osteosarcoma:
- Intramedullary mass: usually a metaphyseal epicenter with cortical permeation and a soft tissue component that raises the periosteum
- Size (mean): 5 - 10 cm
- Cut surface: gritty and mineralized (hard); may have cartilaginous areas (chondroblastic osteosarcoma), hemorrhage, necrosis and cystic change
- Low grade central osteosarcoma:
- Often fills entire metaphysis and diaphysis of involved bone
- Firm, gritty cut surface
- May demonstrate cortical destruction, periosteal reaction and soft tissue invasion
- Parosteal osteosarcoma:
- Hard lobulated mass: attached to cortex
- Variable: nodules of cartilage partially capping tumor surface
- Variable: dedifferentiated soft fleshy areas
- Periosteal osteosarcoma:
- Broad based (sessile) tumor arising from the cortical surface (may circumferentially involve bone)
- Cortex is thickened with scalloping of external surface
- Heavily ossified base of tumor
- External aspect of tumor (often the majority of the tumor) is cartilaginous
- Calcified spicules may extend perpendicularly from the cortex within the mass and intermix with the cartilage
- Rare to see cortical permeation and medullary extension
- High grade surface osteosarcoma:
- Tumor arises from the cortical surface and erodes / invades the cortex
- Cut surface may be osteoblastic, chondroblastic or fibroblastic; areas of necrosis are present
- Status postneoadjuvant chemotherapy:
- To gross: cut along long axis of tumor and map
- References: Cancer 1982;49:1679, Onco Targets Ther 2013;6:593
Gross images
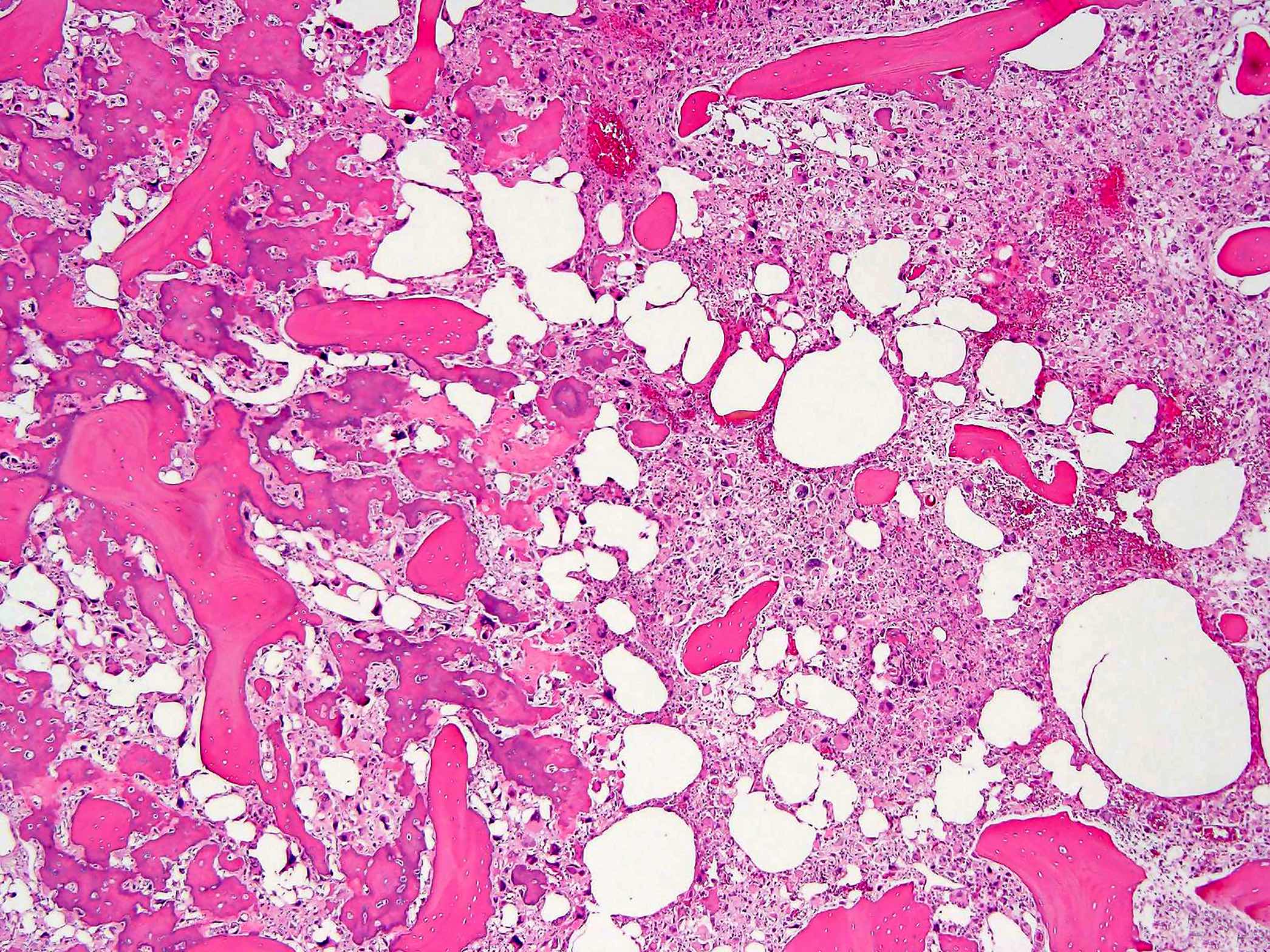
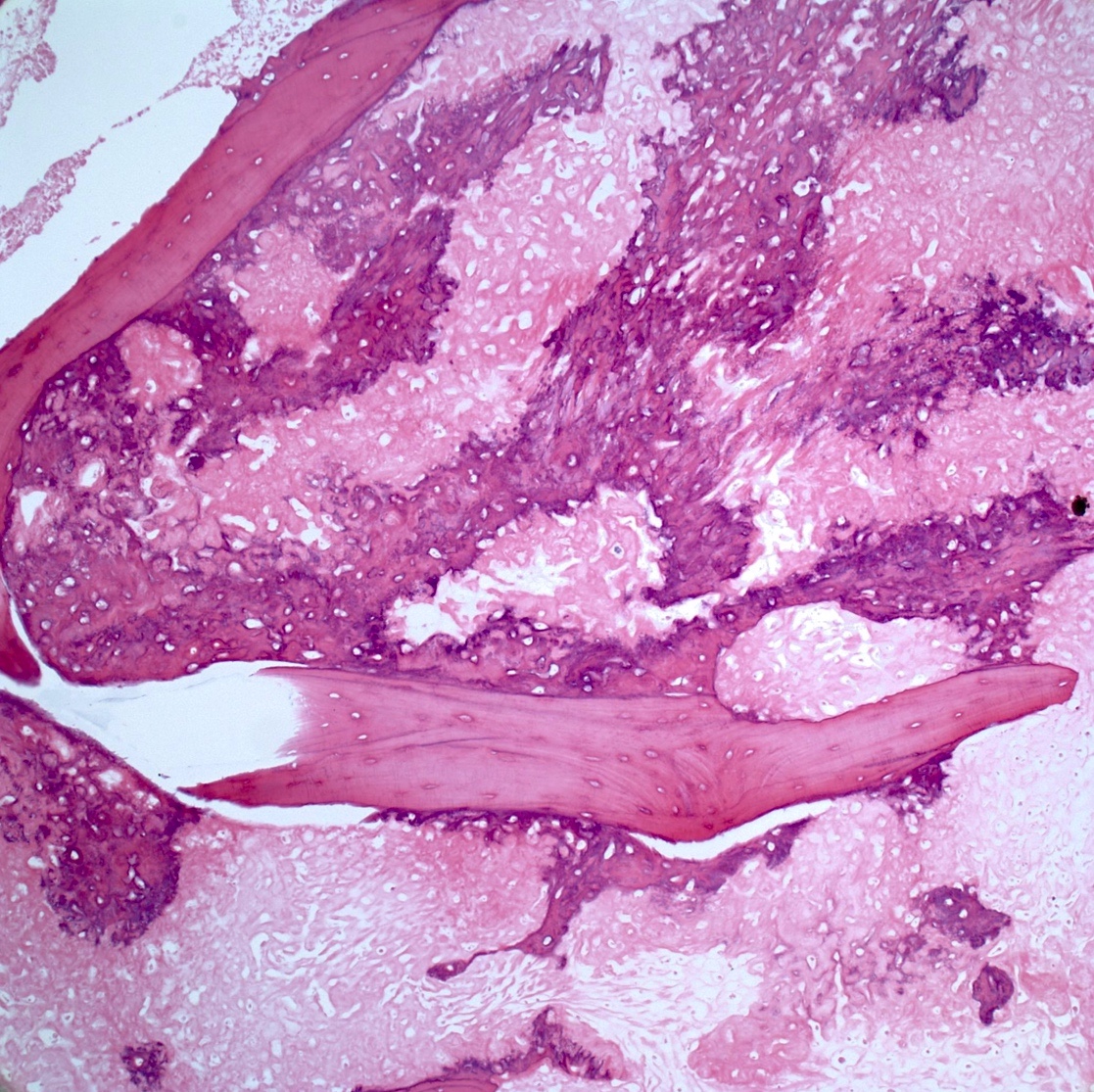
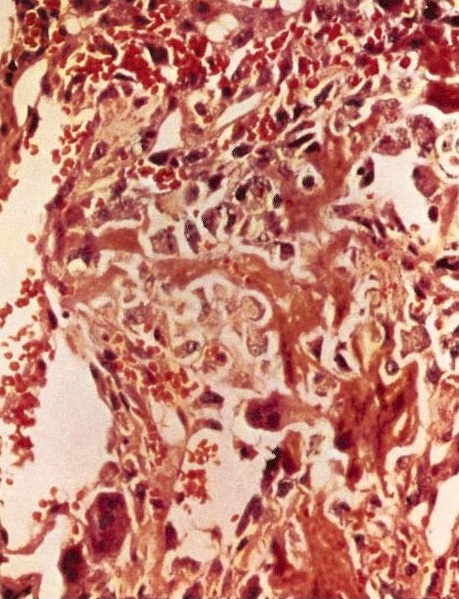
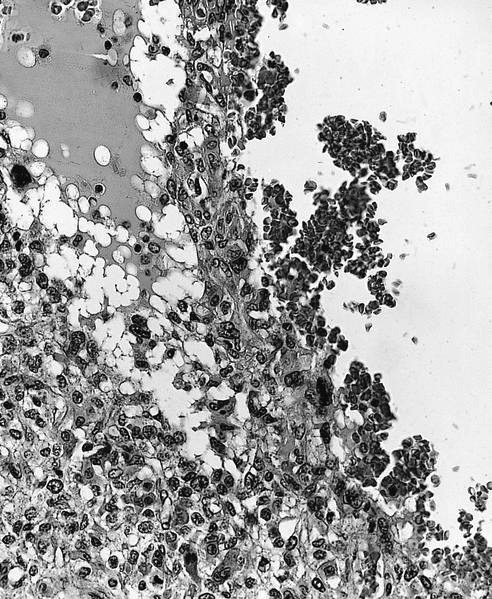
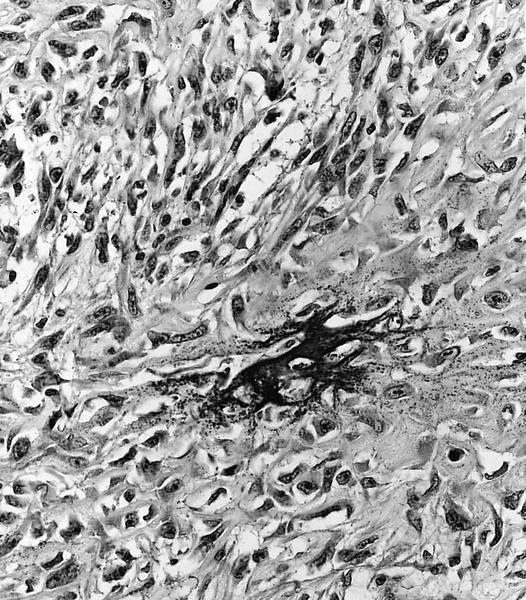
Microscopic (histologic) description
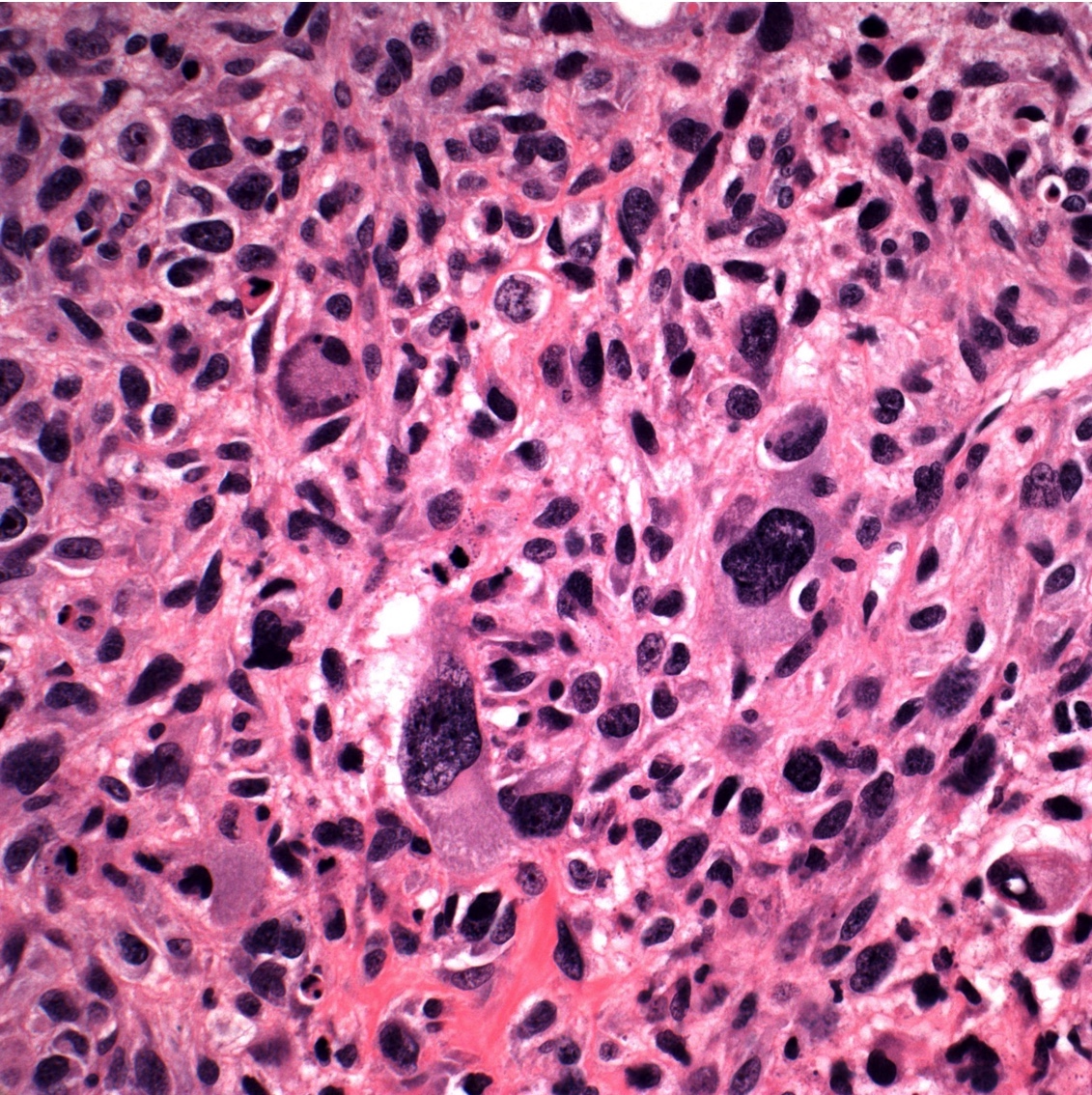
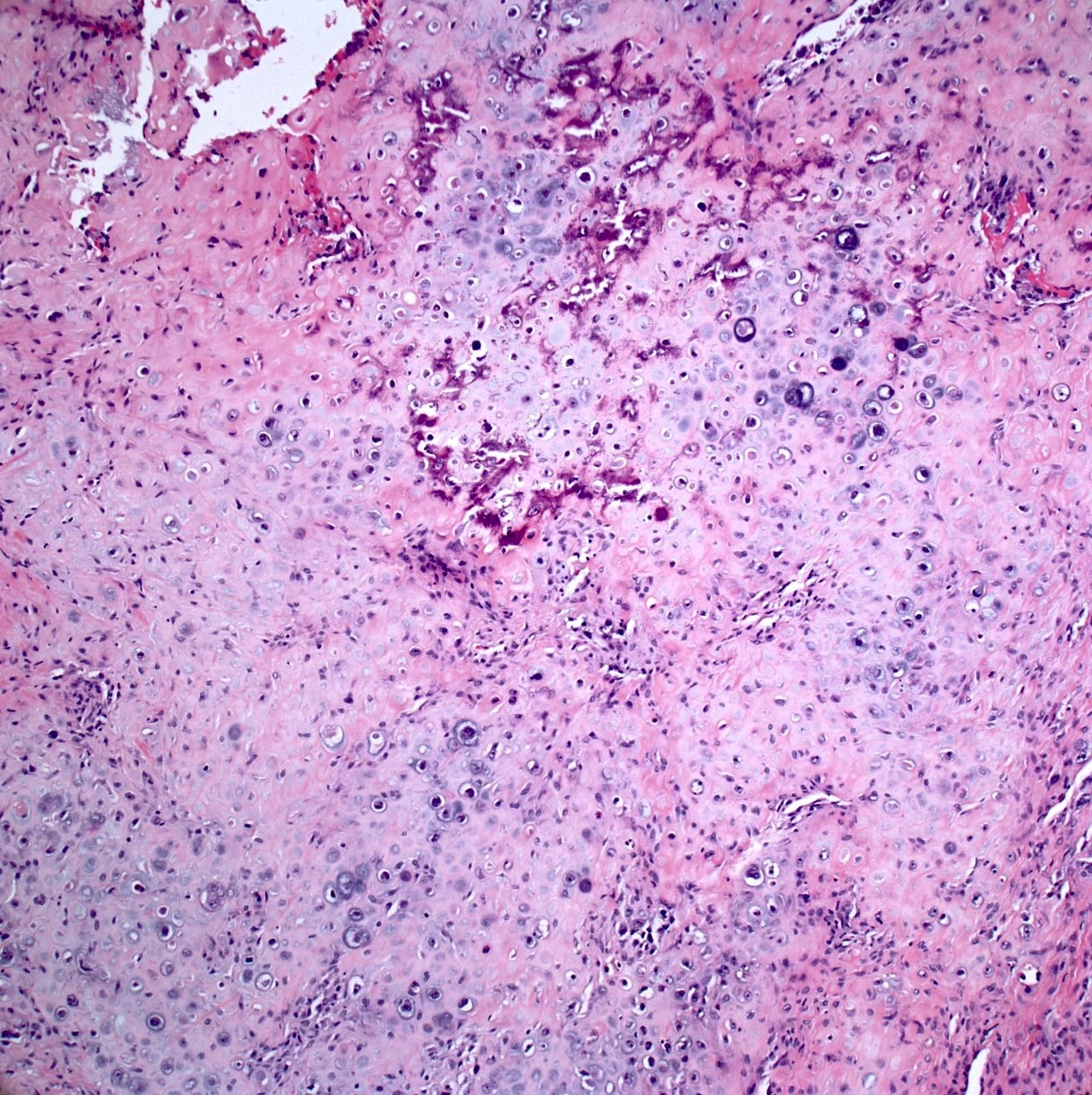
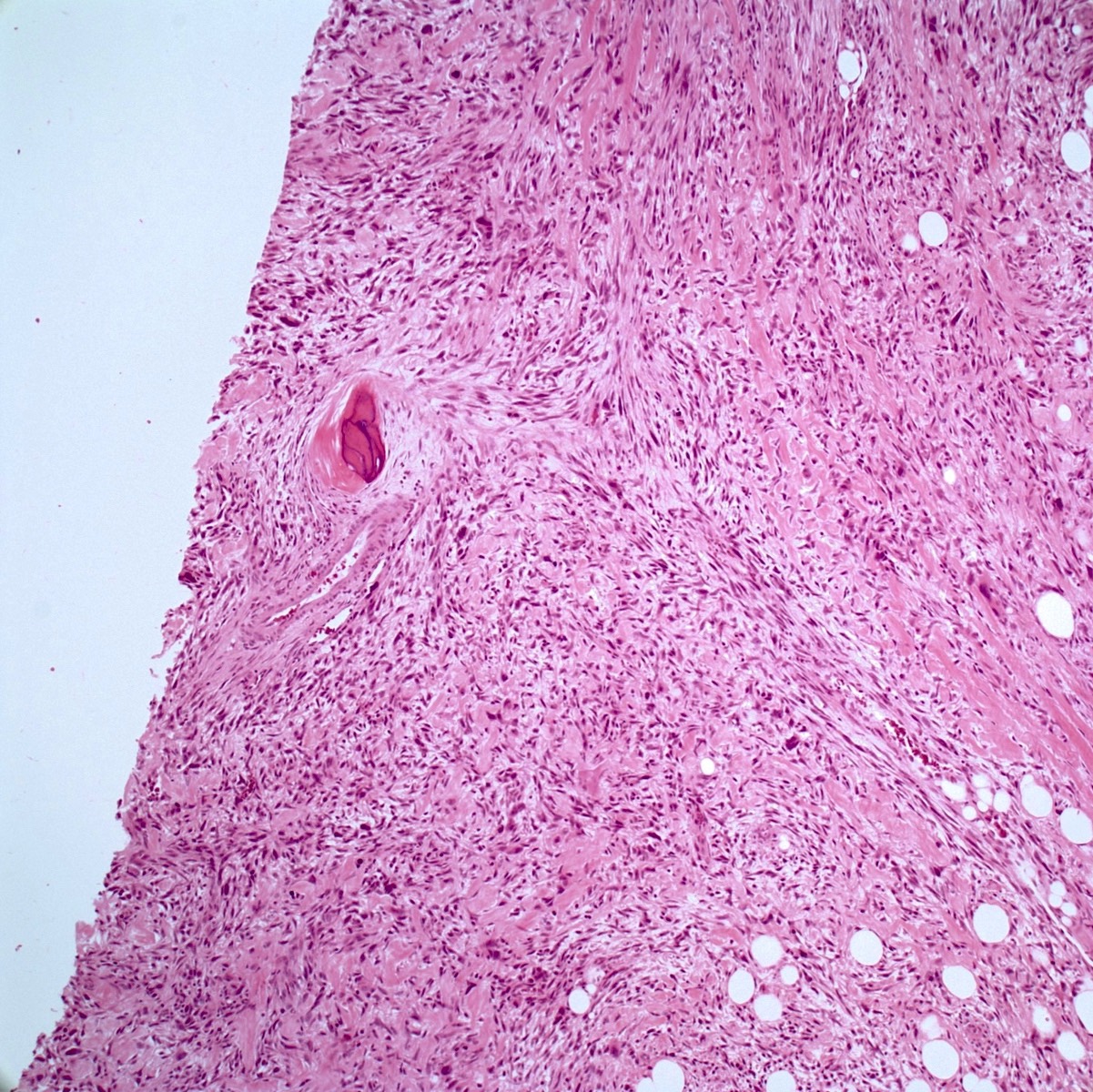
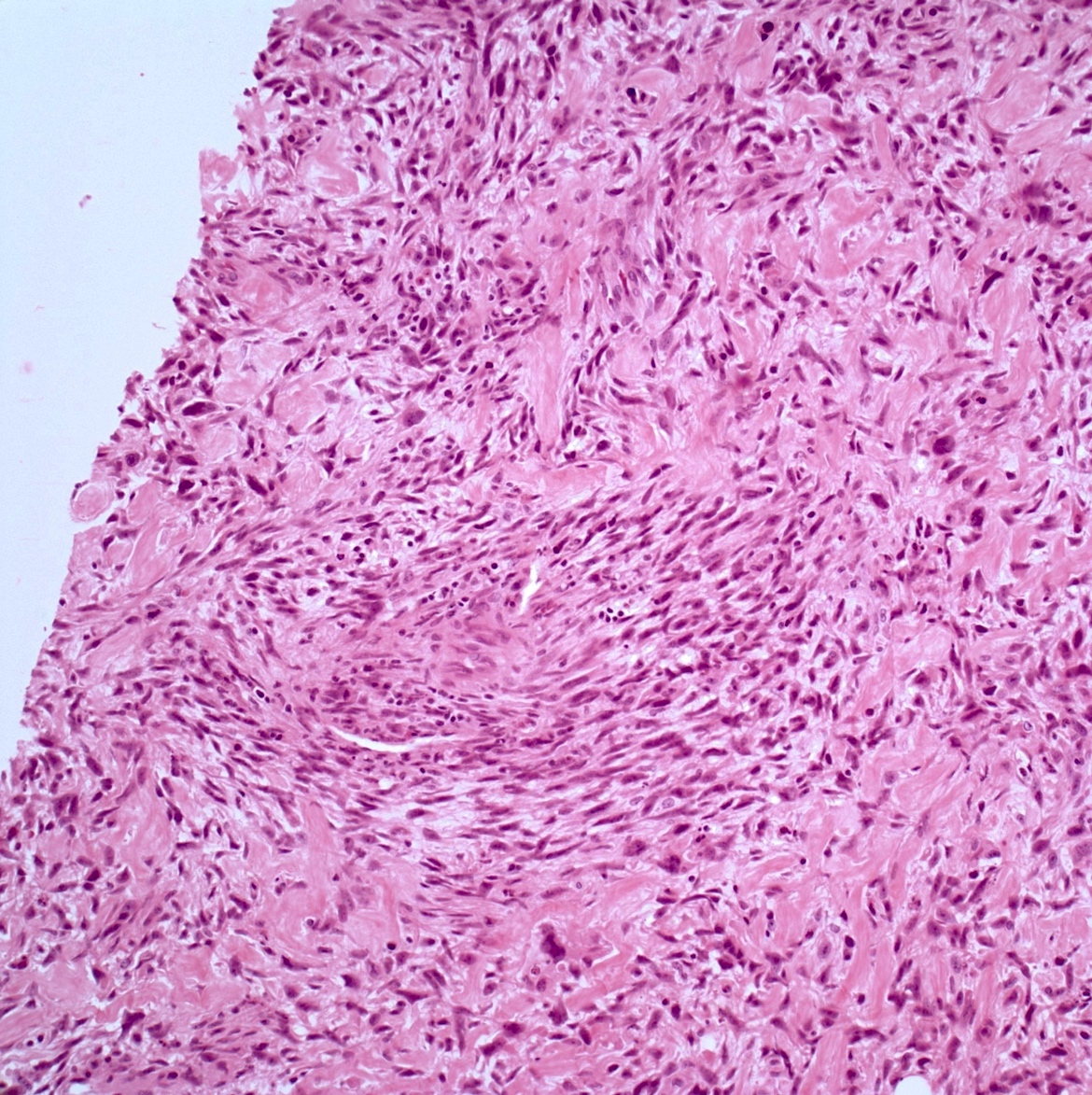
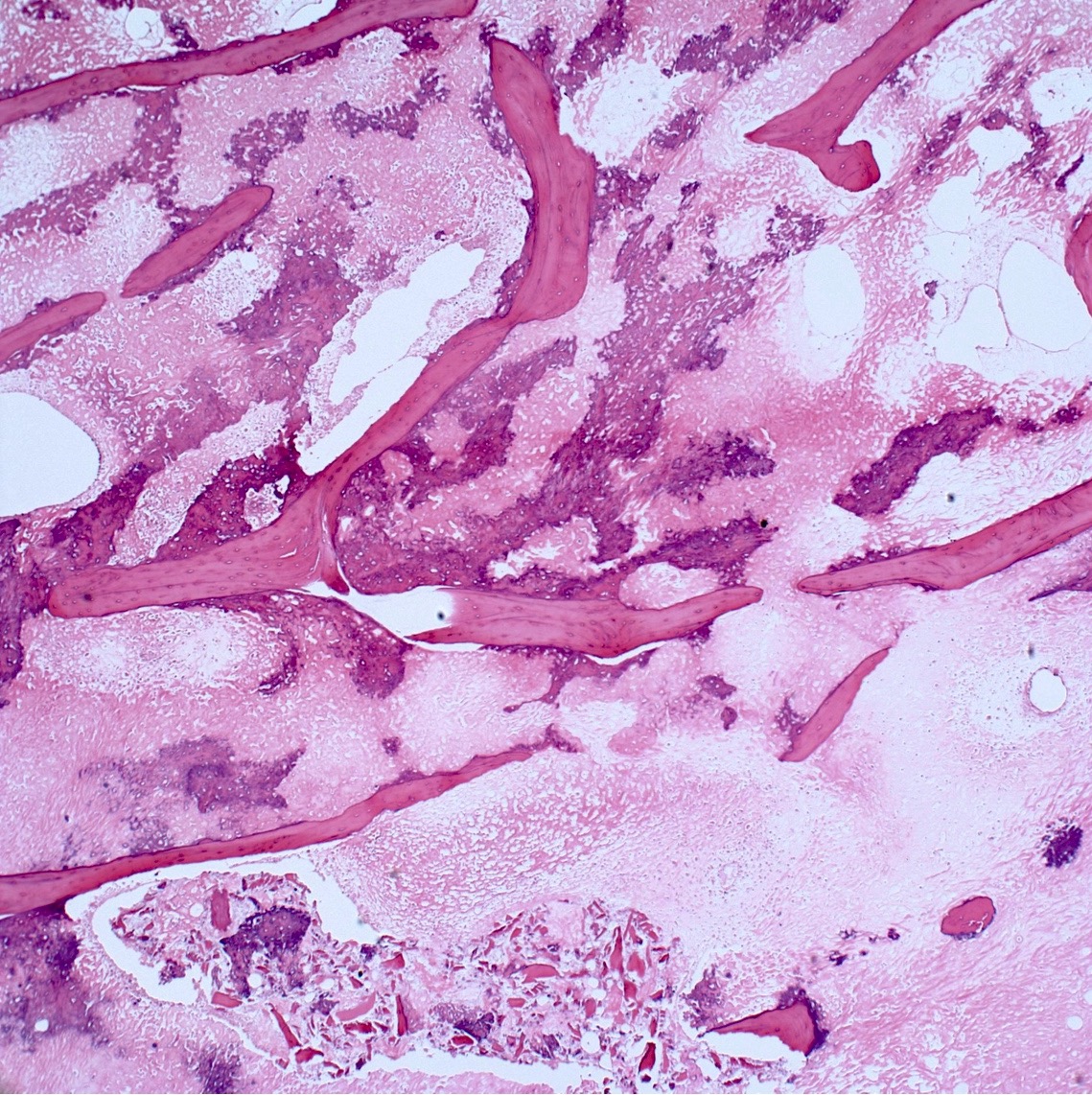
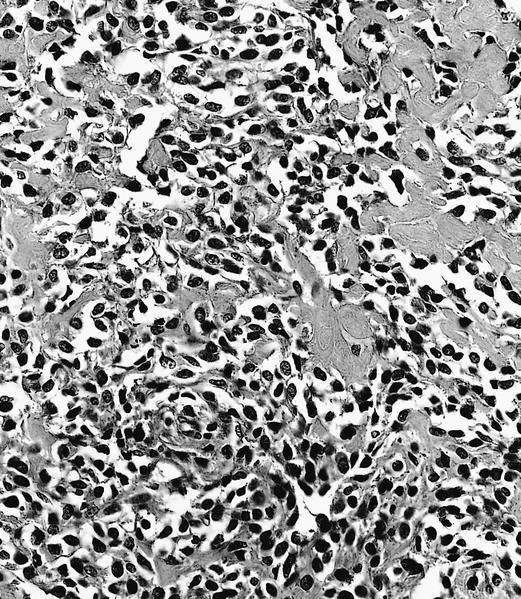
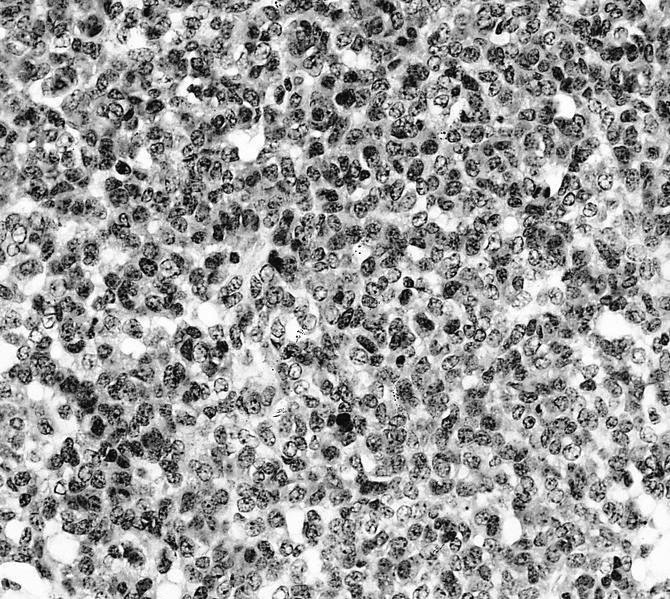
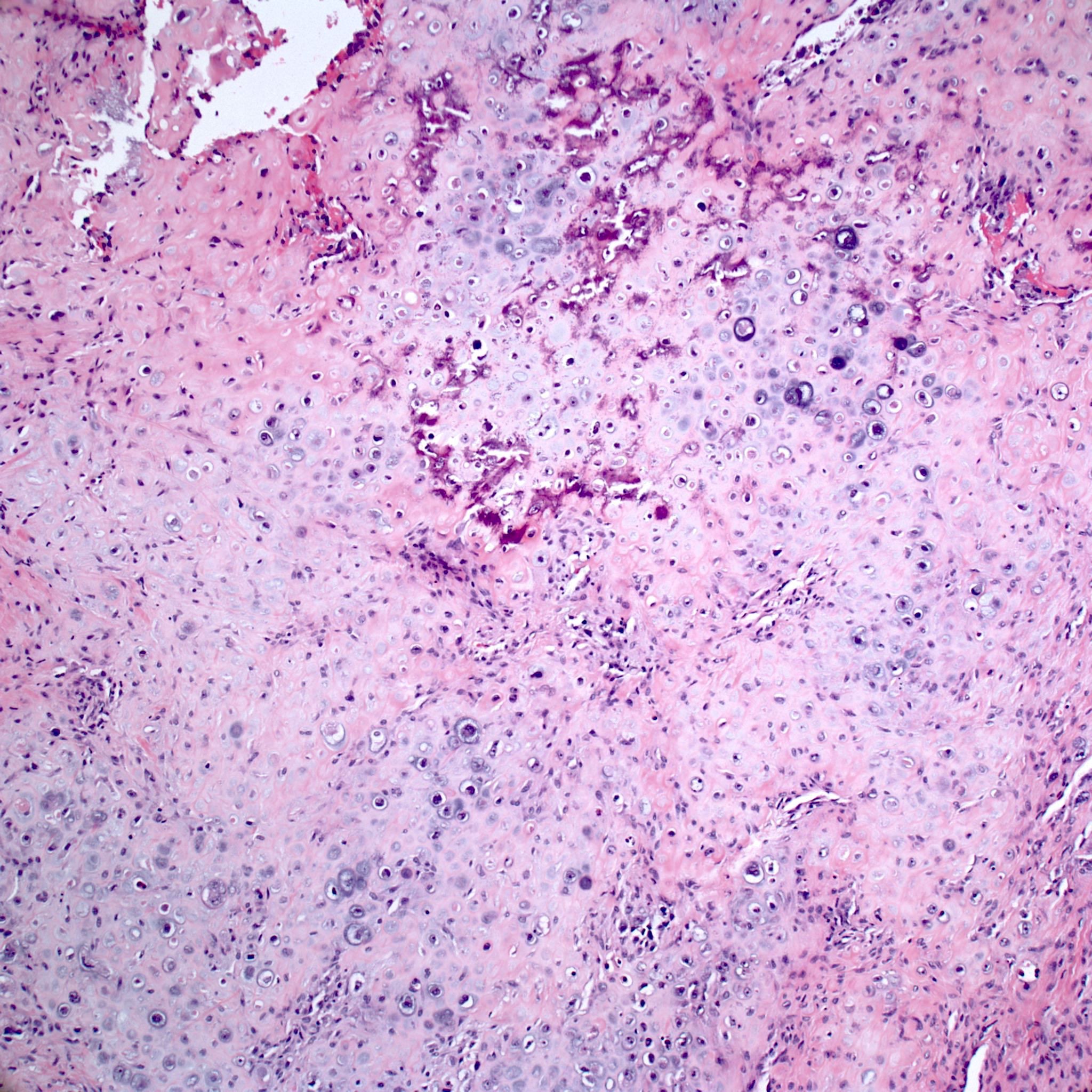
- Conventional (high grade intramedullary) osteosarcoma (Eur J Cancer 2002;38:1218, Am J Clin Pathol 2006;125:555):
- Permeative growth: intramedullary permeative growth (replacement of medullary space, surrounds and erodes native trabeculae, fills Haversian systems) and cortical destruction with soft tissue invasion
- Neoplastic cells: marked atypia (pleomorphic, hyperchromatic)
- Multiple cell morphologies often present in 1 tumor (epithelioid, plasmacytoid, spindled, small round cells, clear cells, giant tumor cells)
- Mitotic figures are easily demonstrable and atypical mitotic figures may also be identified
- Neoplastic bone (necessary for diagnosis): no minimum quantity necessary
- Most common: filigree / lace-like disorganized woven bone (intimately associated with neoplastic cells)
- Broad sheets of bone
- Normalization: decreased cytologic atypia of neoplastic cells entrapped in the bone matrix
- Scaffolding (appositional neoplastic osteoid deposition): deposition of neoplastic osteoid on native trabeculae
- Nonneoplastic giant cells: ~25% of cases
- Histologic subtypes of conventional osteosarcoma: no prognostic significance
- Osteoblastic, chondroblastic and fibroblastic are based on the prominent matrix they secrete (often admixed in 1 tumor)
- Osteoblastic osteosarcoma: the predominant matrix is neoplastic bone (as described above)
- Chondroblastic osteosarcoma: the predominant matrix is high grade cartilage (never has low grade cartilage)
- Fibroblastic osteosarcoma: spindled to epithelioid cells, often with severe atypia, which may secrete extracellular collagen (may be extensive)
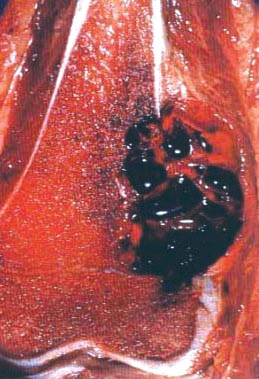
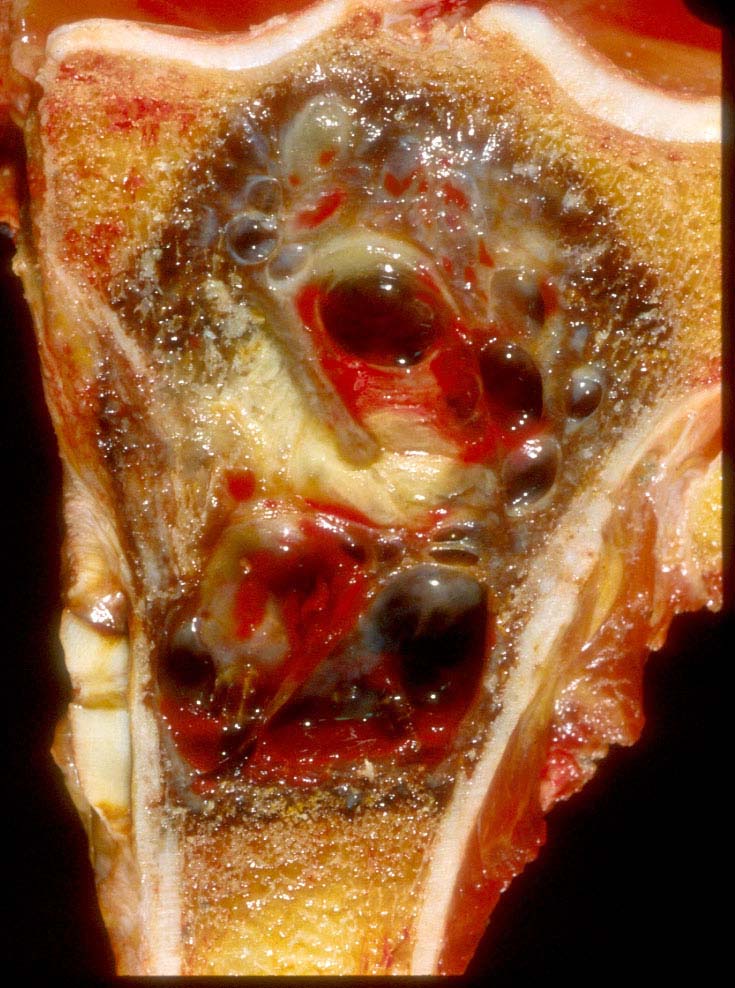
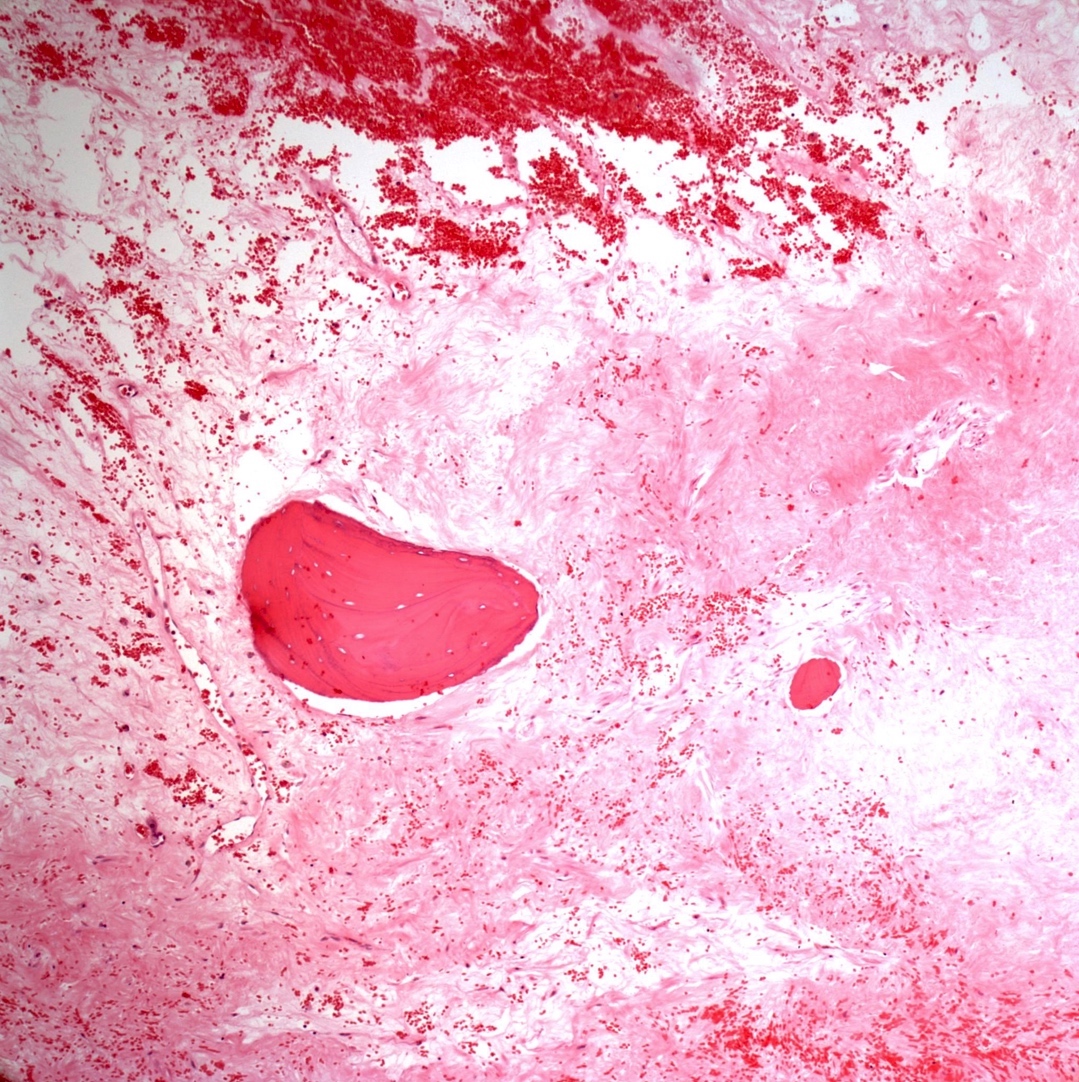
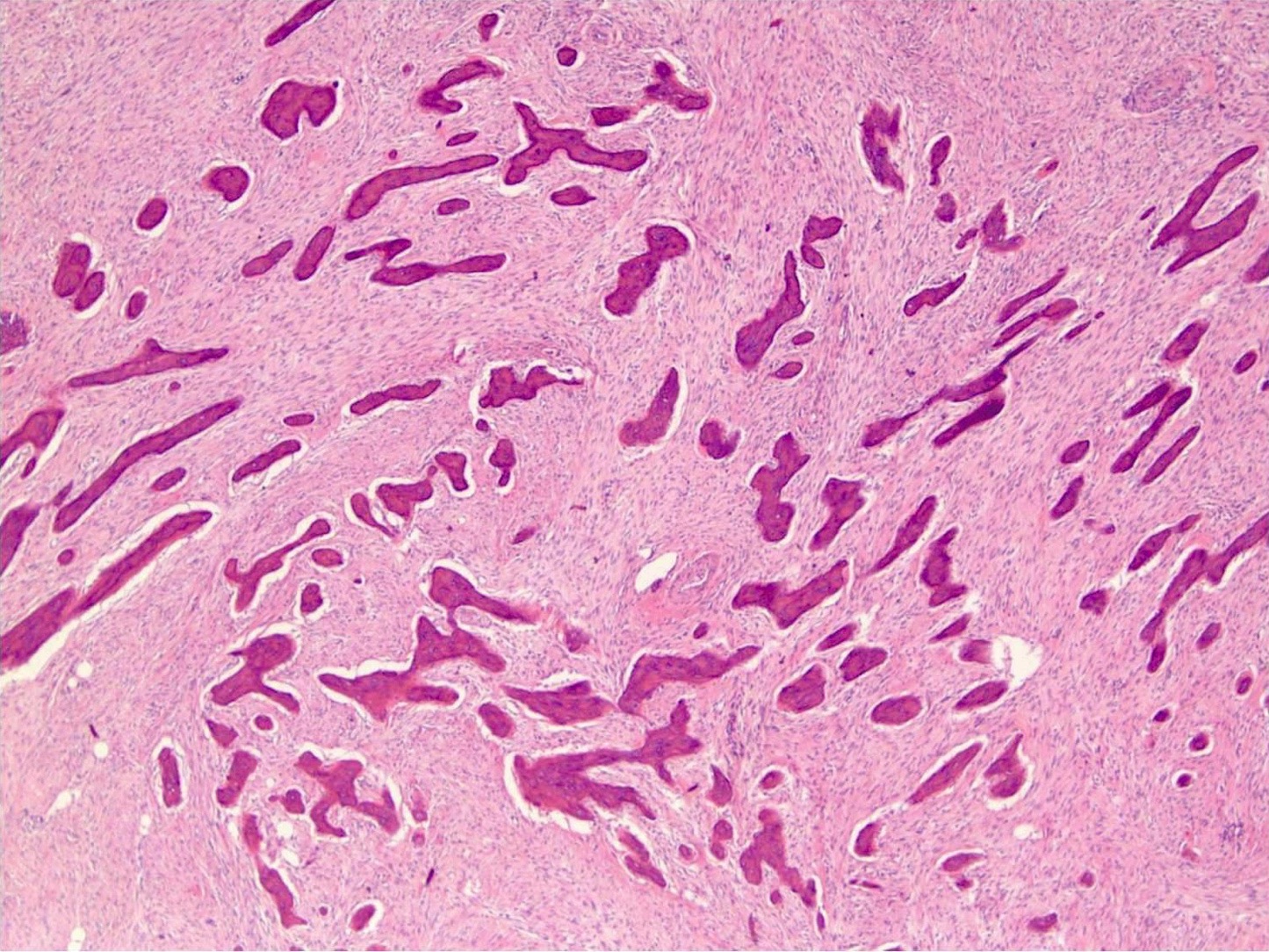
- Telangiectatic osteosarcoma: the tumor is multiloculated with large blood filled spaces; high grade malignant cells and neoplastic bone in septa (the imaging differential diagnosis is with aneurysmal bone cyst)
- Other morphologic variants: giant cell rich variant (numerous osteoclast-like giant cells), epithelioid variant, osteoblastoma-like variant, chondroblastoma-like variant, chondromyxoid fibroma-like osteosarcoma, clear cell variant, small cell variant
- Osteoblastic, chondroblastic and fibroblastic are based on the prominent matrix they secrete (often admixed in 1 tumor)
- Osteosarcoma secondary to Paget disease:
- High grade intramedullary osteosarcoma
- Any histologic variant
- Often contains numerous osteoclast-like giant cells
- Status postneoadjuvant chemotherapy:
- Report treatment response as a percent tumor necrosis (really an assessment of tumor drop out)
- Edematous scar: loose edematous to myxoid granulation tissue, fibrosis, mild chronic inflammation
- Bony matrix remains
- Residual tumor cells: nests of tumor cells in retraction clefts are common
- Grading response to chemotherapy (same cutoffs as Ewing sarcoma) (Cancer 1993;72:3227, J Clin Oncol 1988;6:329):
- Good response is > 90% tumor necrosis
- Report treatment response as a percent tumor necrosis (really an assessment of tumor drop out)
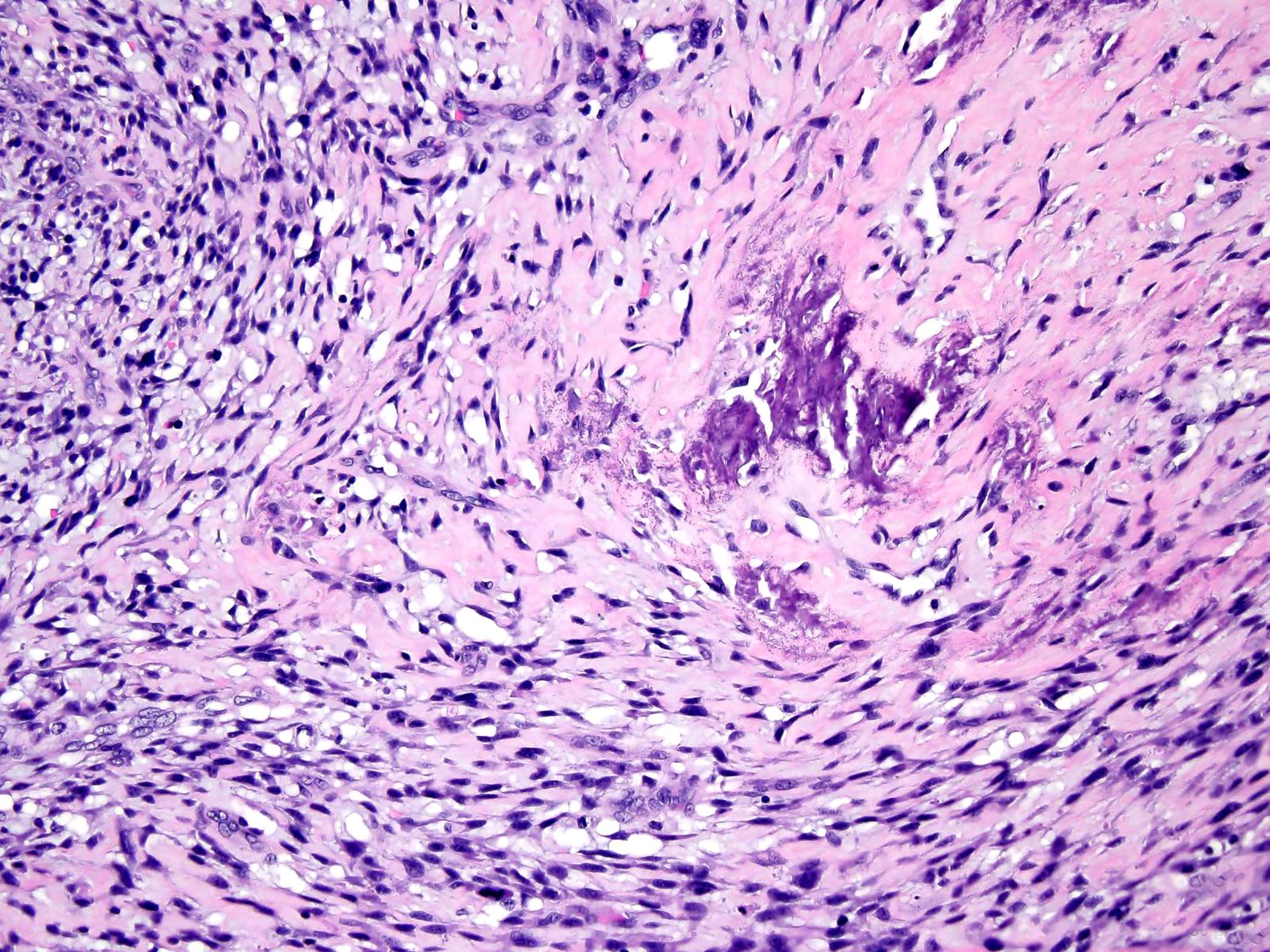
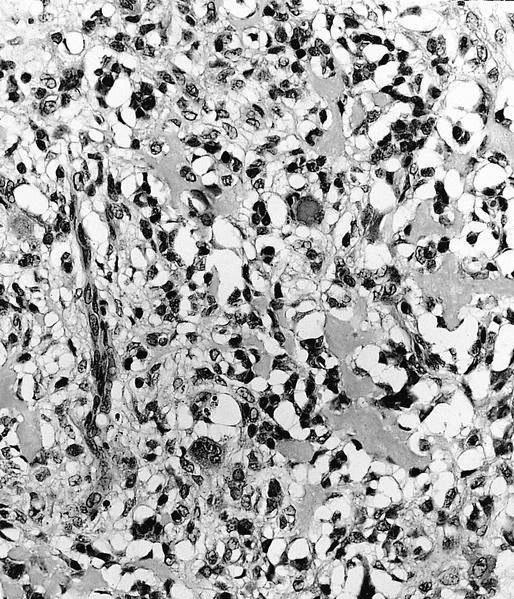
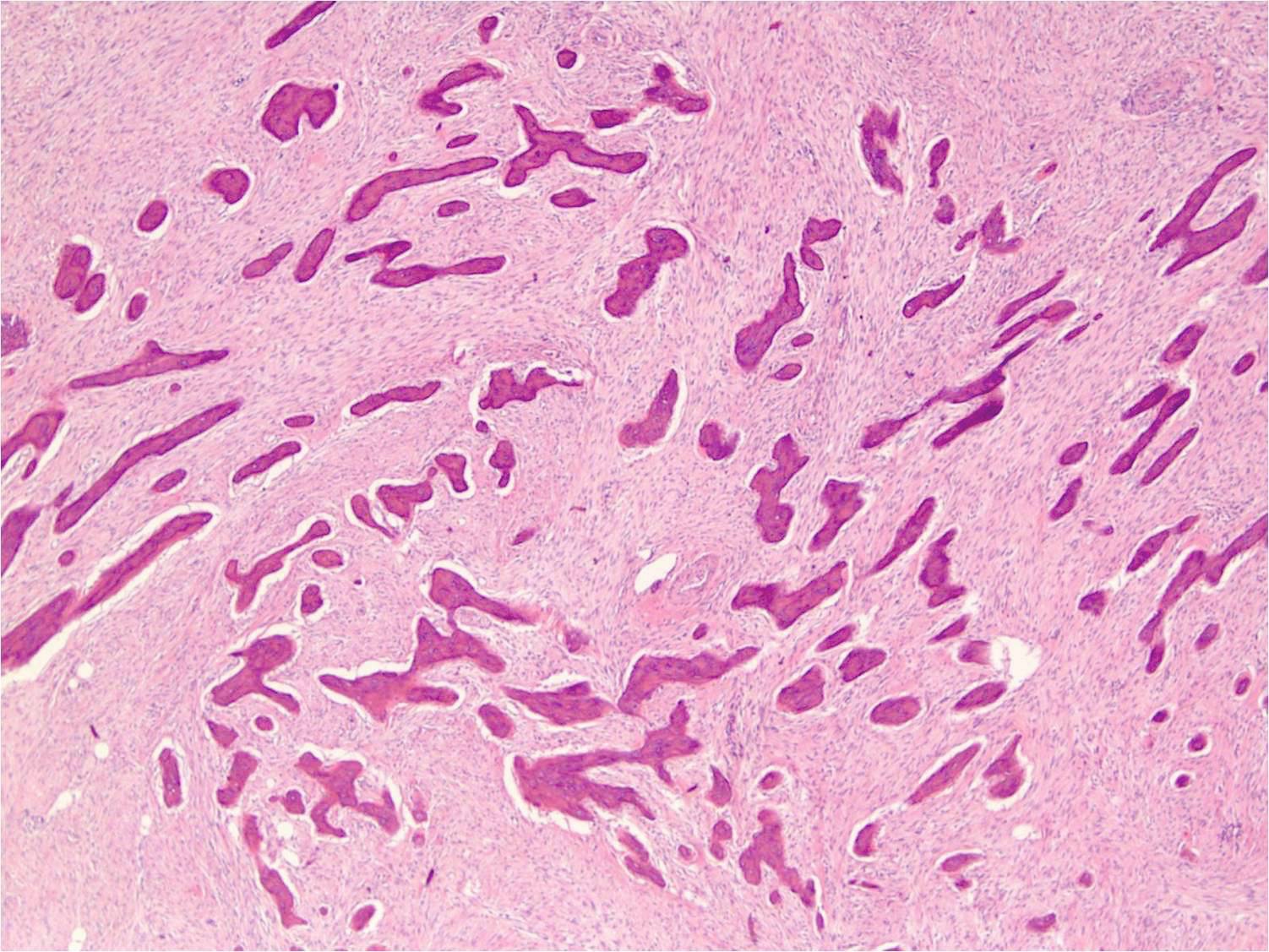
- Low grade central osteosarcoma:
- Permeative growth:
- Intramedullary (surrounds and erodes native trabeculae, fills Haversian systems)
- Cortical destruction and soft tissue invasion
- Neoplastic cells: fibroblast-like spindle cells (minimal atypia); hypocellular to moderately cellular
- Scattered mitoses may be seen
- Rare, scattered higher grade areas may be present
- Arranged in fascicles or interlacing bundles
- Neoplastic bone:
- Bone trabeculae (fibrous dysplasia-like): curved, branching or interanastomosing
- Longitudinal lamellar bone: like parosteal osteosarcoma
- Benign multinucleated giant cells: present in 33% of cases
- With or without scattered foci of atypical cartilage
- Dedifferentiation / high grade transformation (10 - 35% of cases):
- High grade osteosarcoma
- High grade undifferentiated pleomorphic sarcoma
- Fibrosarcoma
- Most common in recurrent tumors (2 - 3 years after resection) but may be seen in the primary tumor
- Permeative growth:
- Parosteal osteosarcoma:
- Invasion: tumor invades soft tissue; 25% invade bone (cortex / medullary)
- Neoplastic cells: fibroblast-like spindle cells (minimal atypia); between bony trabeculae (may be hypocellular)
- Scattered mitoses may be seen
- Neoplastic bone: parallel bony trabeculae (osteoblastic rimming may be present)
- Cartilage (present in ~50% of cases):
- Nodules within lesion (hypercellular)
- Cartilage cap: partially overlays tumor (moderate cellularity, chondrocytes are not arranged in columns, mild to moderate atypia)
- Dedifferentiation (15 - 25% of cases): abrupt transition to high grade sarcoma
- High grade osteosarcoma
- High grade undifferentiated pleomorphic sarcoma
- Fibrosarcoma
- Most common in recurrent tumors but may be seen in the primary tumor
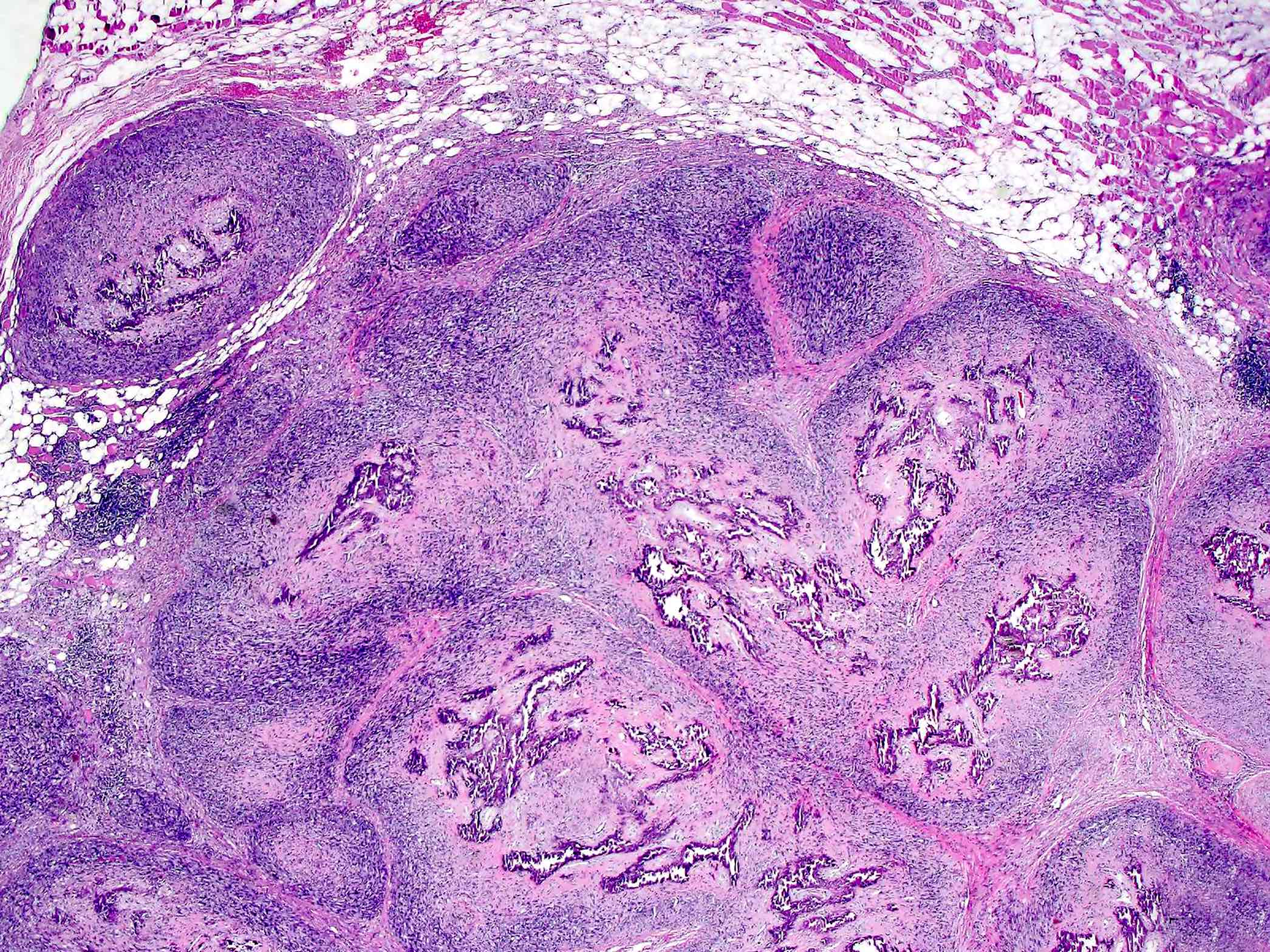
- Periosteal osteosarcoma:
- Ossified mass: intimately attached to native cortex (secondary to endochondral ossification)
- Pericortical bone: dense, mature bone
- Bony spicules: radiate from the dense pericortical bone peripherally and admix with the hyaline cartilage component
- Large vascular cores in center of bony spicules
- Periphery of spicules is calcified / osseous or chondro-osseous and merges with (atypical) hyaline cartilage
- Periphery of mass (majority of the tumor’s volume):
- Atypical hyaline cartilage (appearance of grade 1 - 3 chondrosarcoma); may have myxoid change
- Osseous component (always present but not the dominant component): intermediate grade osteosarcoma intermixed with cartilaginous component; may have lace-like bone but large areas of conventional osteoblastic osteosarcoma are not present
- May have an admixed fibroblastic component (fascicles of mitotically active spindle cells)
- Ossified mass: intimately attached to native cortex (secondary to endochondral ossification)
- High grade surface osteosarcoma: same features as conventional (high grade, intramedullary) osteosarcoma
- May have osteoblastic, chondroblastic or fibroblastic areas
- Low grade areas are not present
- Extraskeletal osteosarcoma: may be any type of osteosarcoma
- Nearly all high grade (marked cytologic atypia, numerous mitoses)
- Bone may be lacy / filigree or trabecular / sheet-like
Microscopic (histologic) images
Contributed by Jesse Hart, D.O., Borislav A. Alexiev, M.D. and AFIP
Positive stains
- Often positive: SATB2, SMA, CD99, osteocalcin (Histopathology 2016;69:84)
- SOX9 (not reliable distinguisher with chondrosarcoma) (Hum Pathol 2022;121:56)
- Occasionally focally positive: cytokeratin, EMA
- Parosteal and low grade central osteosarcoma: may be positive for MDM2 and CDK4
Negative stains
Molecular / cytogenetics description
- Conventional (high grade, intramedullary) osteosarcoma:
- Complex karyotype (Cytogenet Genome Res 2008;122:5)
- Not present: IDH1 or IDH2 mutations (J Pathol 2011;224:334)
- TP53 inactivation: ~40% (Li-Fraumeni syndrome increases risk of osteosarcoma) (Cancer 2012;118:1387)
- Rb inactivation: ~35% (germline RB1 mutation increases risk for postradiation osteosarcoma) (Int J Oncol 2007;30:1205)
- Amplification / gain of 8q21-24 (contains c-Myc): 45 - 55% (Genes Chromosomes Cancer 2003;38:215)
- MDM2 amplification: ~10% (Genes Chromosomes Cancer 2010;49:518)
- Parosteal and low grade central osteosarcoma:
- MDM2 gene amplification (12q13-15): 85 - 90% of cases (Genes Chromosomes Cancer 2010;49:518)
- TP53 mutation: < 5% of cases
- Karyotype: not complex (very few abnormalities other than supernumerary ring chromosomes)
Sample pathology report
- Bone, right distal femur and proximal tibia, radical resection (status postneoadjuvant chemotherapy):
- Residual high grade osteosarcoma (see synoptic report, key findings below)
- Tumor size: 15.2 x 8.0 x 8.0 cm
- Tumor location: Centered in the right distal femur, involving the metaphysis, adjacent diaphysis and epiphysis. Peripherally invades through the cortical bone into the soft tissue on the medial, posterior, anterior and lateral aspects of the distal femur.
- Percent treatment response: ~60% (~40% residual viable tumor)
- Discontinuous tumor foci: Not identified
- Lymphovascular invasion: Not identified
- Surgical margins: Negative for tumor
- Lymph nodes: 1 benign lymph node (0/1)
- Additional findings: Dermal scar / biopsy site
- Stage (AJCC, 8th edition): ypT2 ypN0
- Residual high grade osteosarcoma (see synoptic report, key findings below)
Differential diagnosis
- Chondrosarcoma:
- Older age group (> 50 years old)
- Often are low grade, predominantly cartilaginous; no production of osteoid matrix by malignant tumor cells
- May have IDH1 or IDH2 mutations
- Dedifferentiated chondrosarcoma with osteosarcomatous dedifferentiation:
- Age: > 50 years old
- Low grade cartilaginous component definitionally present (grade I or II chondrosarcomatous component)
- IDH1 or IDH2 mutation: ~50%
- Ewing sarcoma (may be in differential with small cell osteosarcoma):
- No osteoid matrix production
- Negative for SATB2
- Contains a characteristic gene rearrangement (EWSR1::ETS transcription factor)
- Osteochondroma (may be in differential with parosteal osteosarcoma):
- Bone contiguous with native marrow
- Intramedullary space filled with fat and hematopoietic elements (not spindle cells)
- Cartilage cap completely covers lesion, chondrocytes arranged in small chords and nests
- Bizarre parosteal osteochondromatous proliferation (may be in differential with parosteal osteosarcoma):
- Usually involves small tubular bones (hands and feet)
- Haphazard arrangement of hyaline cartilage (hypercellular and atypical chondrocytes), bone (disorganized blue bone and woven bone) and spindle cells
- Negative for MDM2 gene amplification
- Fibrous dysplasia (may be in differential with parosteal osteosarcoma):
- No permeative growth
- No MDM2 gene amplification
- GNAS mutation
- Osteoblastoma (Am J Clin Pathol 2006;125:555):
- The most important histologic parameters for malignancy (not seen in osteoblastoma) include permeation of architecturally normal bone at its interface with tumor and increased and atypical mitotic figures
- Chondroblastoma (Am J Clin Pathol 2006;125:555):
- No osteoid or bone formation
- No atypical mitotic figures
- No infiltration of adjacent intertrabecular spaces
Additional references
Board review style question #1
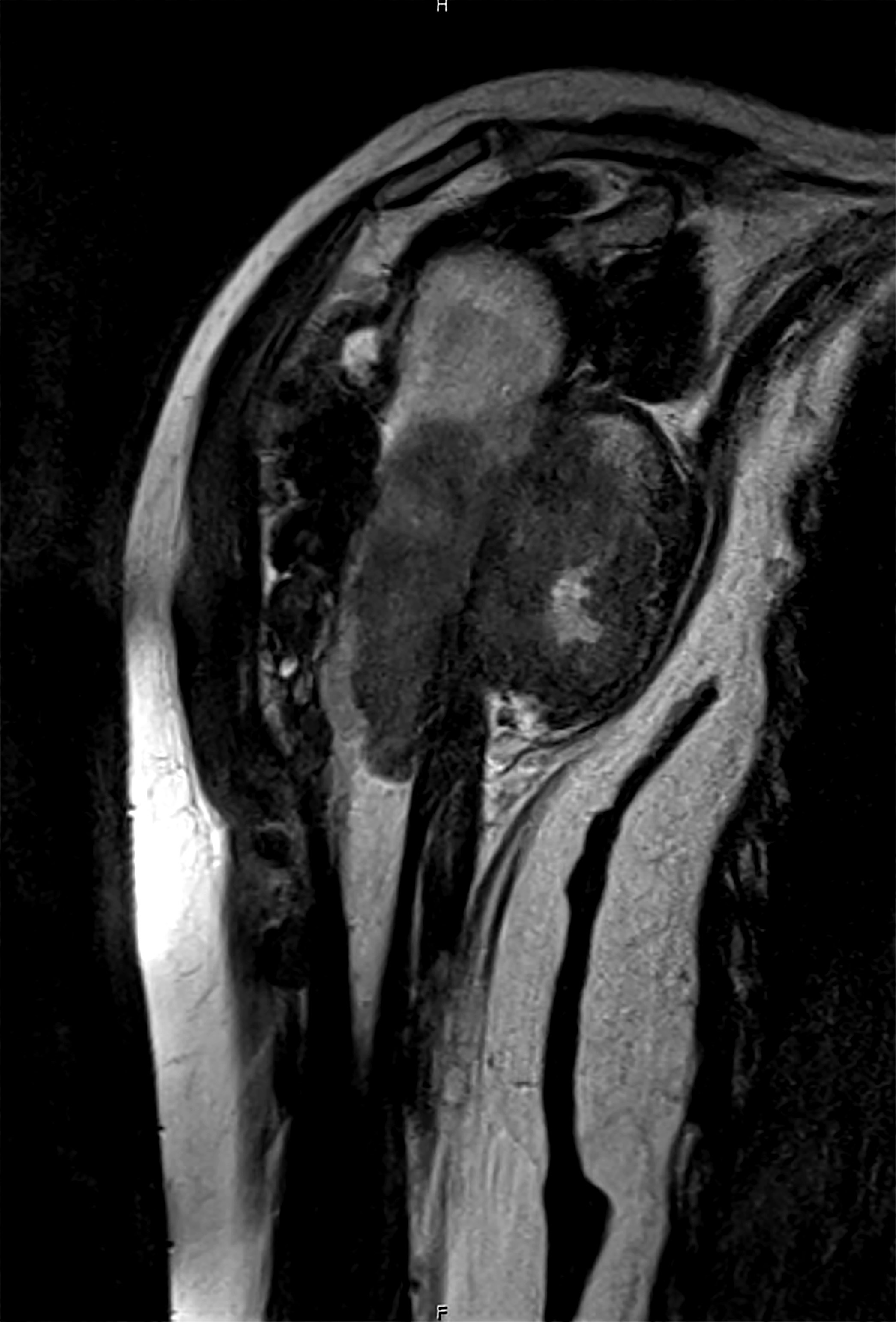
A 12 year old girl has a 10.5 cm intramedullary mass in the distal femur, which was resected (see gross and microscopic images). High power views demonstrate bland spindle cells. Which of the following is most accurate regarding this tumor?
- Most likely, genetic abnormality is amplification of the MDM2 gene
- Most likely, genetic abnormality is a mutation in TP53
- Patient likely has lung metastases
- Tumor likely has a GNAS mutation
- Unusual tumor in the pediatric population
Board review style answer #1
A. Most likely, genetic abnormality is amplification of the MDM2 gene. The tumor is a low grade central osteosarcoma.
Comment Here
Reference: Conventional osteosarcoma and osteosarcoma overview
Comment Here
Reference: Conventional osteosarcoma and osteosarcoma overview
Board review style question #2
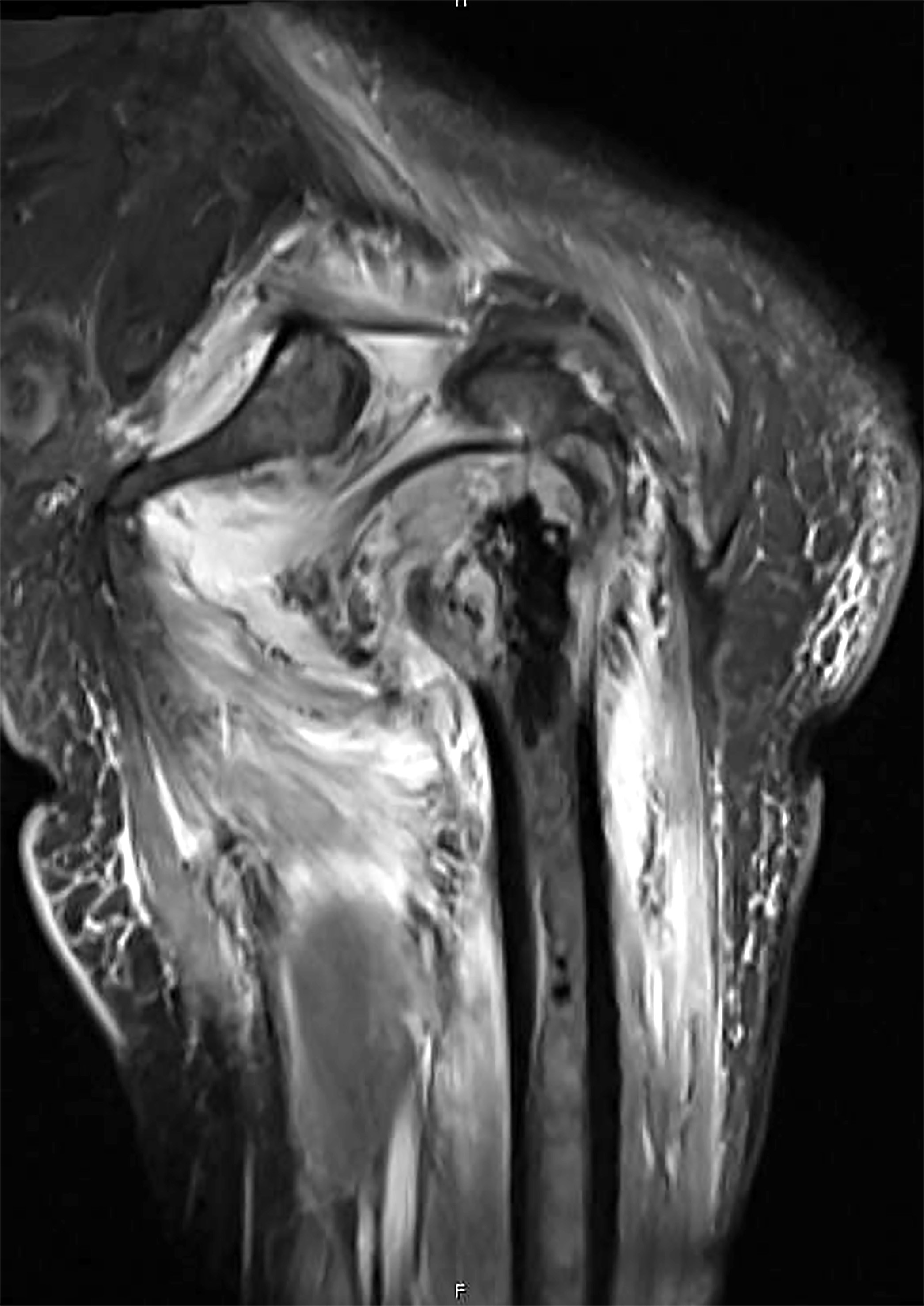
A 16 year old boy had a biopsy from a 16 cm mass in the proximal humerus (see image). Which of the following is true?
- Probability of metastasis is low
- Should recommend that the surgeon send intraoperative cultures upon resection
- Treatment will include neoadjuvant chemotherapy followed by resection
- Tumor likely has a mutation in the IDH1 or IDH2 gene
- Tumor is most often seen in older patients
Board review style answer #2
C. Treatment will include neoadjuvant chemotherapy followed by resection. This is a conventional high grade osteosarcoma.
Comment Here
Reference: Conventional osteosarcoma and osteosarcoma overview
Comment Here
Reference: Conventional osteosarcoma and osteosarcoma overview