Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Positive stains | Negative stains | Flow cytometry description | Molecular / cytogenetics description | Molecular / cytogenetics images | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Smith MC, Schwartz JR, Mason E. Myeloid neoplasms with germline SAMD9 mutation. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticmyeloidgermlineSAMD9.html. Accessed January 10th, 2025.
Definition / general
- Germline mutation in SAMD9, a regulator of cell growth
- Leading to defective hematopoiesis and a predisposition for myeloid neoplasms
- Associated with heterogenous clinical phenotypes, with MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy) syndrome being most severe
- Per the International Consensus Classification, classified under hematologic neoplasms with germline predisposition associated with a constitutional disorder affecting multiple organ systems
- Per the WHO 5th edition, classified under myeloid neoplasms with germline predisposition and potential organ dysfunction
Essential features
- Germline SAMD9 mutations associated with MIRAGE syndrome and familial predisposition to myelodysplastic syndromes (MDS) and more rarely, acute myeloid leukemia (AML)
- Germline variants are typically missense gain of function variants that lead to suppression of cell growth, including impaired hematopoiesis
- MDS presents most commonly as refractory cytopenia of childhood (RCC)
- Somatic germline rescue is common; mechanisms include acquisition of somatic loss of function SAMD9 mutations, deletion of 7q and monosomy 7
Terminology
- MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy) syndrome (Nat Genet 2016;48:792)
- Myeloid neoplasms with germline SAMD9 mutations
- Myeloid neoplasms with familial predisposition
- Myelodysplasia and leukemia syndrome with monosomy 7 (JCI Insight 2018;3:e121086)
ICD coding
- Specific coding not available for this entity
Epidemiology
- Germline SAMD9 mutations detected in 5 - 7% of pediatric MDS and in 3.4% of patients in a cohort of pediatric patients with suspected non-Fanconi anemia inherited bone marrow failure (Nat Med 2021;27:1806, Nat Commun 2017;8:1557, Blood 2018;131:717)
- Prevalence of germline SAMD9 mutations increases to 14% of patients with MDS with monosomy 7 (Nat Med 2021;27:1806)
- Median age at onset of hematologic malignancy for patients with germline SAMD9 or SAMD9L mutations is 10 years (range: 0.2 - 17.6 years) (Nat Med 2021;27:1806)
- As of 2022, SAMD9 germline variants reported in 116 individuals: 64 with MDS / monosomy 7 and 52 with MIRAGE syndrome (Front Endocrinol (Lausanne) 2022;13:953707)
Sites
- Bone marrow failure or myeloid neoplasms primarily involve the bone marrow and peripheral blood
- May present as MIRAGE syndrome
Pathophysiology
- SAMD9 (sterile alpha motif domain containing protein) gene and the homologous SAMD9L (SAMD9-like) gene on chromosome 7q are involved in growth suppression and regulation of cell proliferation (Leukemia 2018;32:1106)
- Overexpression of SAMD9 inhibits protein translation and results in cell cycle arrest (Exp Hematol 2024 Jun 6 [Epub ahead of print], Leukemia 2021;35:3232)
- SAMD9 is thought to be an anticodon nuclease that specifically cleaves and depletes phenylalanine transfer RNA, causing ribosomal pausing (Sci Adv 2023;9:eadh8502)
- Germline mutations are typically gain of function, leading to impaired hematopoiesis (among other affected organ systems), manifesting clinically as cytopenias, immunodeficiency or bone marrow failure
- Mutations are typically missense mutations in the C terminus, within or near the P loop nucleotide triphosphatase domain (Best Pract Res Clin Haematol 2024;37:101537)
- Germline gain of function mutations that suppress cell growth create selective pressure for cells to neutralize these mutations
- Somatic germline rescue (SGR) is common; multiple mechanisms have been described (Nat Med 2021;27:1806)
- Acquistion of somatic loss of function SAMD9 mutation in cis with the germline mutation, which ameliorates the growth suppressive effect of the germline mutation
- Selective / nonrandom loss of the mutant allele via deletion 7q or monosomy 7
- Considered a deleterious adaptation, as del(7q) / monosomy 7 is a known contributor to myeloid malignancy (see Diagrams / tables) (Leukemia 2018;32:1106)
- Acquisition of uniparental disomy / copy neutral loss of heterozygosity of 7q, with selective / nonrandom deletion of the mutated allele
Diagrams / tables
Clinical features
- Highly variable clinical course, ranging from isolated hematopoietic manifestations (including mild transient cytopenias, bone marrow failure, MDS and rarely AML) to severe multisystem disease (e.g., MIRAGE syndrome)
- Rare unaffected carriers have been described (Nat Commun 2017;8:1557)
- MIRAGE syndrome (GeneReviews: MIRAGE Syndrome [Accessed 15 July 2024])
- Myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy
- Variable phenotype, with different features present in different patients
- Additional described features include prematurity, chronic lung disease, developmental delay, dysmorphology, central nervous system abnormalities, dysautonomia and hearing loss (Am J Med Genet A 2018;176:415)
- Most common hematologic manifestations include early onset, sometimes transient thrombocytopenia, with anemia or pancytopenia seen in some patients
- Subsequent progression to MDS / AML in a subset of patients
- Other disorders associated with SAMD9 variants
- Normophosphatemic familial tumoral calcinosis: biallelic germline loss of function SAMD9 variants (Am J Hum Genet 2006;79:759)
- Germline SAMD9 loss of function variants have been reported in adult MDS (Blood 2018;132:2309)
Diagnosis
- SAMD9 variants identified by sequence analysis
- Variant allele frequency of the SAMD9 mutation may be lower than expected for a germline mutation due to somatic monosomy 7 or uniparental disomy (Blood 2018;131:717)
- Bone marrow biopsies in patients with germline SAMD9 mutation may show a hypocellular marrow with megakaryocytic atypia at baseline (Semin Diagn Pathol 2023;40:429)
- Bone marrow interpretation requires caution to avoid overinterpretation and a premature diagnosis of MDS (Virchows Arch 2023;482:113)
- Evolution to MDS typically presents as refractory cytopenia of childhood (Nat Med 2021;27:1806)
- Features associated with progression to MDS include multilineage dysplasia with acquisition of monosomy 7 or del(7q), acquisition of somatic variants in known driver genes and more rarely, increased blasts (GeneReviews: MIRAGE Syndrome [Accessed 15 July 2024], JCI Insight 2018;3:e121086)
- However, spontaneous improvement in peripheral counts and resolution of monosomy 7 via uniparental disomy has been reported (Blood 2018;131:717)
Laboratory
- Dependent on clinical phenotype
- Hematologic laboratory abnormalities range from single lineage cytopenia (most commonly thrombocytopenia, which may be transient) to pancytopenia
- In MIRAGE patients, severe cytopenias may occur in acute episodes in the setting of myelosuppressive infections or may be chronic (GeneReviews: MIRAGE Syndrome [Accessed 15 July 2024])
- Cytopenias may improve with age in some MIRAGE patients
- Additional abnormalities in MIRAGE syndrome include features of adrenal insufficiency and immunodeficiency (decreased peripheral B / NK cells and low IgG and IgM have been described) (Best Pract Res Clin Haematol 2020;33:101197)
Radiology description
- Ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) may show adrenal hypoplasia in patients with MIRAGE syndrome
Prognostic factors
- Clinical phenotype (and therefore prognosis) is variable
- Somatic germline rescue, via uniparental disomy or somatic loss of function mutations in SAMD9 can lead to stable disease and remission
- 5 year overall survival in a cohort of 26 SAMD9 mutant MDS: 84% (Nat Med 2021;27:1806)
- In patients with refractory cytopenia of childhood, the presence of monosomy 7 associated with less favorable outcomes
- Acquisition of somatic mutations in cancer related genes may also be associated with disease progression
- Uniparental disomy of 7q associated with long lasting clinical remission
- In patients with MIRAGE syndrome, the median age of death is 3 years, most commonly due to infection (GeneReviews: MIRAGE Syndrome [Accessed 15 July 2024])
Case reports
- Preterm baby girl with intrauterine growth restriction, genitourinary abnormalities and transient thrombocytopenia (Front Endocrinol (Lausanne) 2019;10:625)
- 3 month old boy with neutropenia, recurrent infections and gastrointestinal symptoms (Front Immunol 2019;10:2194)
- 14 month old boy, 3 year old girl and 4 year old boy who are siblings with megakaryocytic dysplasia and monosomy 7 (Leukemia 2017;31:1827)
- 2.5 year old boy with MIRAGE syndrome demonstrating complete cytogenetic remission of monosomy 7 (Pediatr Blood Cancer 2019;66:e27589)
- 11 year old boy with MIRAGE syndrome (Pediatr Blood Cancer 2019;66:e27726)
Treatment
- Patients with refractory cytopenia of childhood without severe neutropenia or transfusion dependency may be followed expectantly
- Transfusion support for symptomatic cytopenias
- For young children with MDS with monosomy 7 with clinically stable disease, may consider careful watching, with interval molecular / genetic studies to evaluate for loss of the monosomy 7 clone and the emergence of revertant 7q uniparental disomy (Best Pract Res Clin Haematol 2020;33:101197)
- Promising outcomes have been reported with stem cell transplants for patients with MDS
- In one cohort, 10 of 12 patients (including 6 with SAMD9 and 6 with SAMD9L mutations) were alive at median follow up of 3.1 years (Biol Blood Marrow Transplant 2019;25:2186)
- All surviving patients showed resolution of hematologic disorders, although other features of MIRAGE syndrome persist
- Another cohort of high risk / progressed SAMD9 / SAMD9L mutated MDS showed 17/21 patients alive at last follow up following stem cell transplant (Nat Med 2021;27:1806)
- In one cohort, 10 of 12 patients (including 6 with SAMD9 and 6 with SAMD9L mutations) were alive at median follow up of 3.1 years (Biol Blood Marrow Transplant 2019;25:2186)
- Given reports of familial cases, genetic screening of related donors is required to ensure donor does not carry the mutated allele
Microscopic (histologic) description
- Bone marrow is typically hypocellular for age, with or without dysplastic features (Virchows Arch 2023;482:113)
- Baseline megakaryocytic atypia (small hypolobated forms, separated nuclear lobes) with or without erythroid atypia is common (Semin Diagn Pathol 2023;40:429)
- Morphologically abnormal megakaryocytes with monosomy 7 can be seen in patients with normal, stable platelet counts and may not be indicative of progression to MDS
- Progression to MDS should be considered in the context of new or worsening cytopenias, multilineage dysplasia, increased blasts or genetic results showing monosomy 7 or acquisition of somatic mutations in other cancer related genes
Microscopic (histologic) images
Peripheral smear description
- May range from normal to single lineage cytopenia or pancytopenia with or without dysplasia
Positive stains
- Variable depending on the type of myeloid neoplasm
- No specific associations with SAMD9 mutation
Negative stains
- Variable depending on the type of myeloid neoplasm
- No specific associations with SAMD9 mutation
Flow cytometry description
- Immunophenotype is variable depending on the type of underlying myeloid neoplasm
- No specific immunophenotypic abnormalities have been associated with SAMD9 variants
Molecular / cytogenetics description
- Pathogenic SAMD9 germline variants are predominantly heterozygous, gain of function variants
- Variant allele frequency of the SAMD9 mutation may be lower than expected for a germline mutation due to somatic monosomy 7 or uniparental disomy (Blood 2018;131:717)
- In silico predictors cannot reliably distinguish pathogenic from benign mutations (Nat Med 2021;27:1806, Best Pract Res Clin Haematol 2020;33:101197)
- Additional somatic mutations in RUNX1, ASXL1, SETBP1, PTPN11, KRAS, CBL, EZH2, ETV6, RAD21 and BRAF have been described (Semin Diagn Pathol 2023;40:429, Best Pract Res Clin Haematol 2020;33:101197)
- Somatic germline rescue results in deletion 7q, monosomy 7 or uniparental disomy / copy neutral loss of heterozygosity at 7q
- Multiple reports of complete regression of clones with monosomy 7, primarily in younger patients (Best Pract Res Clin Haematol 2020;33:101197)
Videos
Dr. Marcin Wlodarski discusses germline predispositions to myeloid neoplasms, including SAMD9 / SAMD9L mutations
Sample pathology report
- Bone marrow, peripheral blood smear, aspirate smear, particle preparation, biopsy and flow cytometry:
- Moderately hypocellular marrow with maturing trilineage hematopoiesis, megakaryocytic atypia and no increase in blasts (see comment)
- Comment: This is a moderately hypocellular marrow for age (40 - 50% cellular) with maturing trilineage hematopoiesis, megakaryocytic atypia and no increase in blasts. The patient's previous sample is concurrently reviewed and demonstrates similar morphologic findings. The overall findings are consistent with the patient's known history of a germline SAMD9 variant and monosomy 7. Correlation with clinical and laboratory findings, including pending additional testing, is recommended.
- CBC
- White blood cell (WBC): 1.8 k/microL
- Hemoglobin (Hgb): 10.0 g/dL
- Platelet count (plt ct): 256 k/microL
- Mean corpuscular volume (MCV): 101 fL
- Red cell distribution width (RDW): 15.0%
- A Wright stained peripheral smear shows macrocytic anemia with mild, nonspecific anisopoikilocytosis. There is neutropenia with a relative monocytosis, with no significant dysplasia. There are no circulating blasts, plasma cells or abnormal lymphocytes. Platelets are adequate in number without significant morphologic abnormalities.
- Wright stained aspirate smears are cellular and particulate. Myeloid and erythroid elements show no significant dysplasia. Megakaryocytes demonstrate atypia (small hypolobated forms, separated nuclear lobes). The myeloid:erythroid ratio is 1.1:1. There is no increase in blasts, plasma cells or lymphocytes. No ring sideroblasts are present on a Prussian blue iron stain.
- H&E and PAS stained bone marrow biopsy and particle preparation sections are reviewed. The material is adequate and moderately hypocellular for age (40 - 50% cellular). There is trilineage hematopoiesis with a full range of maturation and relative erythroid hyperplasia. Megakaryocytes appear appropriate in number and include atypical forms (small hypolobated forms, separated nuclear lobes). Blasts appear to comprise less than 5% of marrow cellularity. There is no increase in or abnormal distribution of plasma cells or lymphocytes.
- Flow cytometry: Myeloblasts are not increased (0.7% of total cells), with the following immunophenotype: positive for CD33 (moderate), CD34 and CD45 (dim) and negative for CD19. B cells are not increased (4.3% of total cells), with the following immunophenotype: positive for CD19 (bright) and CD45 (bright) and negative for CD34. A subset of B cells has an immunophenotype consistent with hematogones. Presumed T / NK cells are present (34% of total cells), with the following immunophenotype: positive for CD45 (bright) and negative for CD19 and CD34.
Differential diagnosis
- Germline SAMD9L mutation:
- SAMD9L gene is related to but distinct from SAMD9
- Germline mutations cause ataxia - pancytopenia syndrome: neurologic symptoms, cytopenias, MDS / AML with monosomy 7 and immunodeficiency (Blood 2017;129:2266)
- Similar to patients with germline SAMD9 mutations, patients with germline SAMD9L mutations may present with bone marrow failure or MDS / AML without other syndromic features
- Typically not associated with adrenal hypoplasia, enteropathy, growth restriction or genital abnormalities
- Germline GATA2 mutation:
- Associated with cytopenias, bone marrow hypocellularity and progression to MDS / AML with monosomy 7
- Additional features of GATA2 haploinsufficiency include immunodeficiency, genital abnormalities and hearing loss
- Not associated with adrenal hypoplasia, growth restriction or enteropathy
- Additional congenital bone marrow failure syndromes with a predisposition to myeloid malignancy: Fanconi anemia, Shwachman-Diamond syndrome, dyskeratosis congenita and other telomere biology disorders
- Clinical syndromes with features overlapping with MIRAGE syndrome (GeneReviews: MIRAGE Syndrome [Accessed 15 July 2024]):
- Triple A syndrome (adrenal insufficiency, achalasia and alacrima):
- Germline variants in AAAS gene
- Adrenal insufficiency but no hematologic abnormalities (Adv Exp Med Biol 2010;685:1)
- IMAGe syndrome (intrauterine growth restriction [IUGR], metaphyseal dysplasia, adrenal hypoplasia and genitourinary abnormalities):
- Germline variants in CDKN1C gene
- Adrenal hypoplasia and genital abnormalities but no hematologic abnormalities (GeneReviews: IMAGe Syndrome [Accessed 15 July 2024])
- Triple A syndrome (adrenal insufficiency, achalasia and alacrima):
Additional references
Board review style question #1
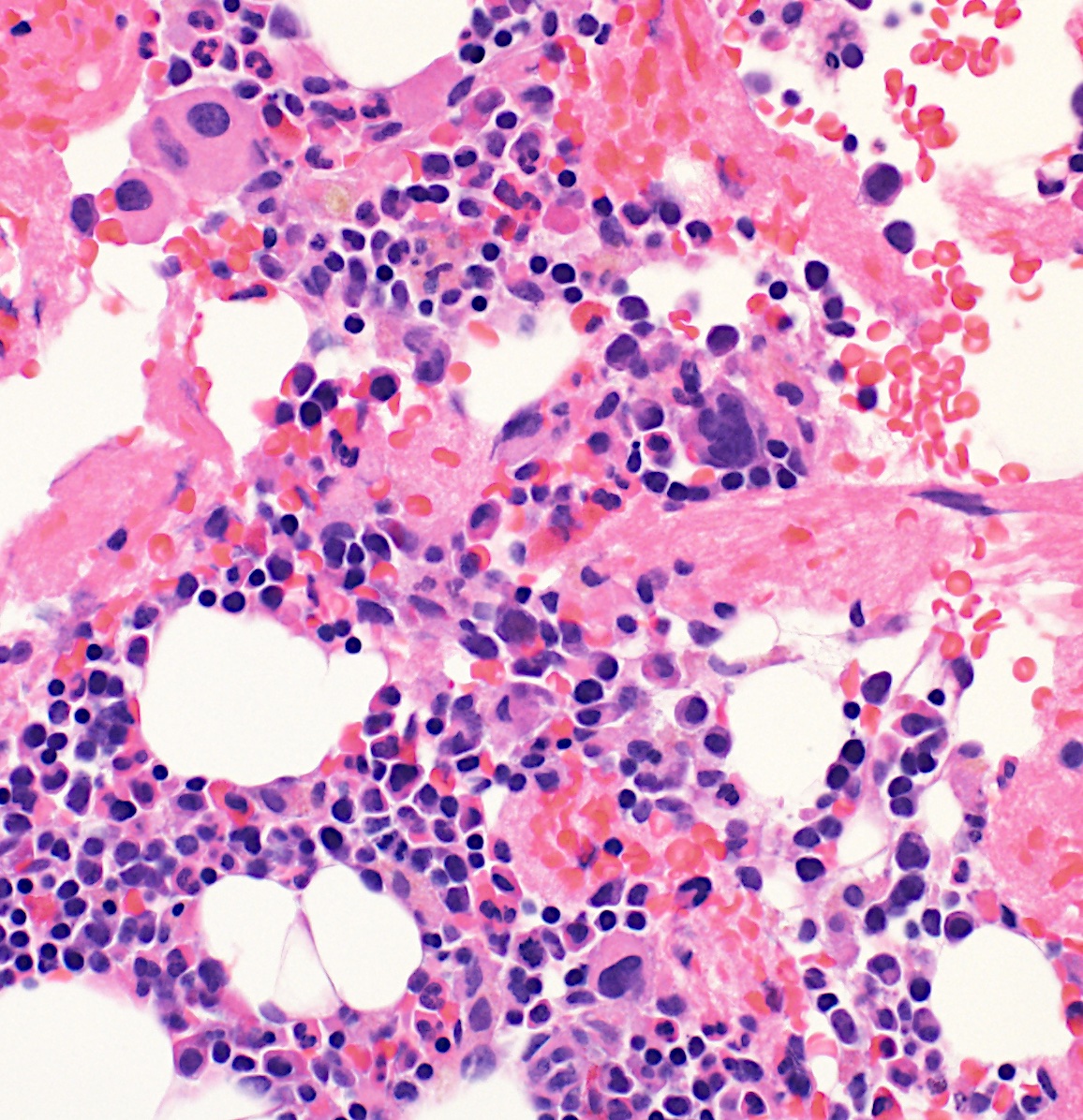
A 9 year old boy presented with macrocytic anemia and thrombocytopenia. A bone marrow biopsy showed a hypocellular marrow with megakaryocytic atypia, as shown in the image. Sequencing analysis detected a SAMD9 mutation at a variant allele frequency of 49%. Which of the following is true regarding germline mutations in the SAMD9 gene?
- Germline disease associated mutations in pediatric patients are typically loss of function mutations
- Germline SAMD9 mutations are associated with an increased risk of developing classic Hodgkin lymphoma
- Germline SAMD9 mutations are associated with ataxia - pancytopenia syndrome
- Patients with germline SAMD9 mutations may show acquisition of uniparental disomy of chromosome 7q
Board review style answer #1
D. Patients with germline SAMD9 mutations may show acquisition of uniparental disomy of chromosome 7q. Germline SAMD9 mutations are commonly associated with somatic germline rescue, which can occur via acquisition of somatic loss of function SAMD9 mutations, deletion of chromosome 7 / 7q or acquisition of uniparental disomy at chromosome 7q.
Answer A is incorrect because germline SAMD9 mutations are typically gain of function mutations. Answer B is incorrect because germline SAMD9 mutations are associated with an increased risk of myeloid malignancies, including MDS and more rarely, AML. Answer C is incorrect because germline SAMD9 mutations are associated with MIRAGE syndrome. Ataxia - pancytopenia syndrome is associated with germline mutations in the related gene SAMD9L.
Comment Here
Reference: Myeloid neoplasms with germline SAMD9 mutation
Answer A is incorrect because germline SAMD9 mutations are typically gain of function mutations. Answer B is incorrect because germline SAMD9 mutations are associated with an increased risk of myeloid malignancies, including MDS and more rarely, AML. Answer C is incorrect because germline SAMD9 mutations are associated with MIRAGE syndrome. Ataxia - pancytopenia syndrome is associated with germline mutations in the related gene SAMD9L.
Comment Here
Reference: Myeloid neoplasms with germline SAMD9 mutation
Board review style question #2
A 5 year old boy presents with an extensive history of recurrent infections and is noted to have moderate to severe pancytopenia. Bone marrow biopsy reveals a hypocellular marrow with dyserythropoiesis and dysplastic megakaryoctyes. Hypospadias, adrenal hypoplasia, severe thrombocytopenia and anemia were noted at birth. A heterozygous germline variant in which gene is most likely to be identified in this patient?
- AAAS
- FANCA
- SAMD9
- SBDS
Board review style answer #2
C. SAMD9. The clinical features of genital abnormalities, pancytopenia, myelodysplasia and adrenal hypoplasia in a pediatric patient raise suspicion for MIRAGE syndrome. MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, enteropathy) syndrome is associated with a germline variant in the SAMD9 gene, a regulator of cell growth. In the presence of this variant, a rescue mechanism results in monosomy 7, predisposing to acute leukemia and myelodysplasia.
Answer A is incorrect because variants in this gene are associated with triple A syndrome (adrenal insufficiency, achalasia, alacrima), a rare disease with autosomal recessive inheritance. Hematologic abnormalities are not described in this syndrome (Adv Exp Med Biol 2010;685:1). Answer B is incorrect because variants in over 20 genes, including FANCA, FANCB and BRCA2, are associated with Fanconi anemia. Bone marrow failure is seen in this syndrome, while congenital abnormalities include skeletal malformations, abnormal pigmentation of skin and microcephaly. These abnormalities are not identified in this patient (GeneReviews: Fanconi Anemia [Accessed 15 July 2024). Answer D is incorrect because variants in this gene are associated with Shwachman-Diamond syndrome, which manifests as cytopenia and a predisposition to acute leukemia and myelodysplasia; however, patients with Shwachman-Diamond syndrome may also demonstrate metaphyseal dysplasia and exocrine pancreatic dysfunction. The clinical scenario as described is not compatible with this diagnosis.
Comment Here
Reference: Myeloid neoplasms with germline SAMD9 mutation
Answer A is incorrect because variants in this gene are associated with triple A syndrome (adrenal insufficiency, achalasia, alacrima), a rare disease with autosomal recessive inheritance. Hematologic abnormalities are not described in this syndrome (Adv Exp Med Biol 2010;685:1). Answer B is incorrect because variants in over 20 genes, including FANCA, FANCB and BRCA2, are associated with Fanconi anemia. Bone marrow failure is seen in this syndrome, while congenital abnormalities include skeletal malformations, abnormal pigmentation of skin and microcephaly. These abnormalities are not identified in this patient (GeneReviews: Fanconi Anemia [Accessed 15 July 2024). Answer D is incorrect because variants in this gene are associated with Shwachman-Diamond syndrome, which manifests as cytopenia and a predisposition to acute leukemia and myelodysplasia; however, patients with Shwachman-Diamond syndrome may also demonstrate metaphyseal dysplasia and exocrine pancreatic dysfunction. The clinical scenario as described is not compatible with this diagnosis.
Comment Here
Reference: Myeloid neoplasms with germline SAMD9 mutation