Table of Contents
Definition / general | Essential features | Terminology | CPT coding | Sites | Diagrams / tables | Overview | Advantages of flow cytometry | Disadvantages of flow cytometry | Effectiveness | Clinical applications | Cytology images | Flow cytometry images | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Flaifel A, Wilkins R, Ward N, Brandler TC. Flow cytometry. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/cytopathologyflowcytometry.html. Accessed April 1st, 2025.
Definition / general
- Flow cytometry is a technique that measures various parameters (optical characteristics and emitted fluorescence) of cells in a flowing fluid suspension
Essential features
- Sensitive method for simultaneously obtaining information on various parameters, including optical characteristics and fluorescence of cells in suspension
- Components include fluidics, optical systems and electronic systems
- Applications are limited to cells in suspension and provide no information about cell to cell interactions
- Provides valuable clinical application in hematologic diseases and body fluids diagnostics
Terminology
- Cell sorting: separating cells, identified by specified characteristics
- Compensation: mathematical algorithm for removing bleeding / spillover of 1 fluorophore into multiple detectors
- Forward scatter: light scattered in the forward direction after interacting with a particle
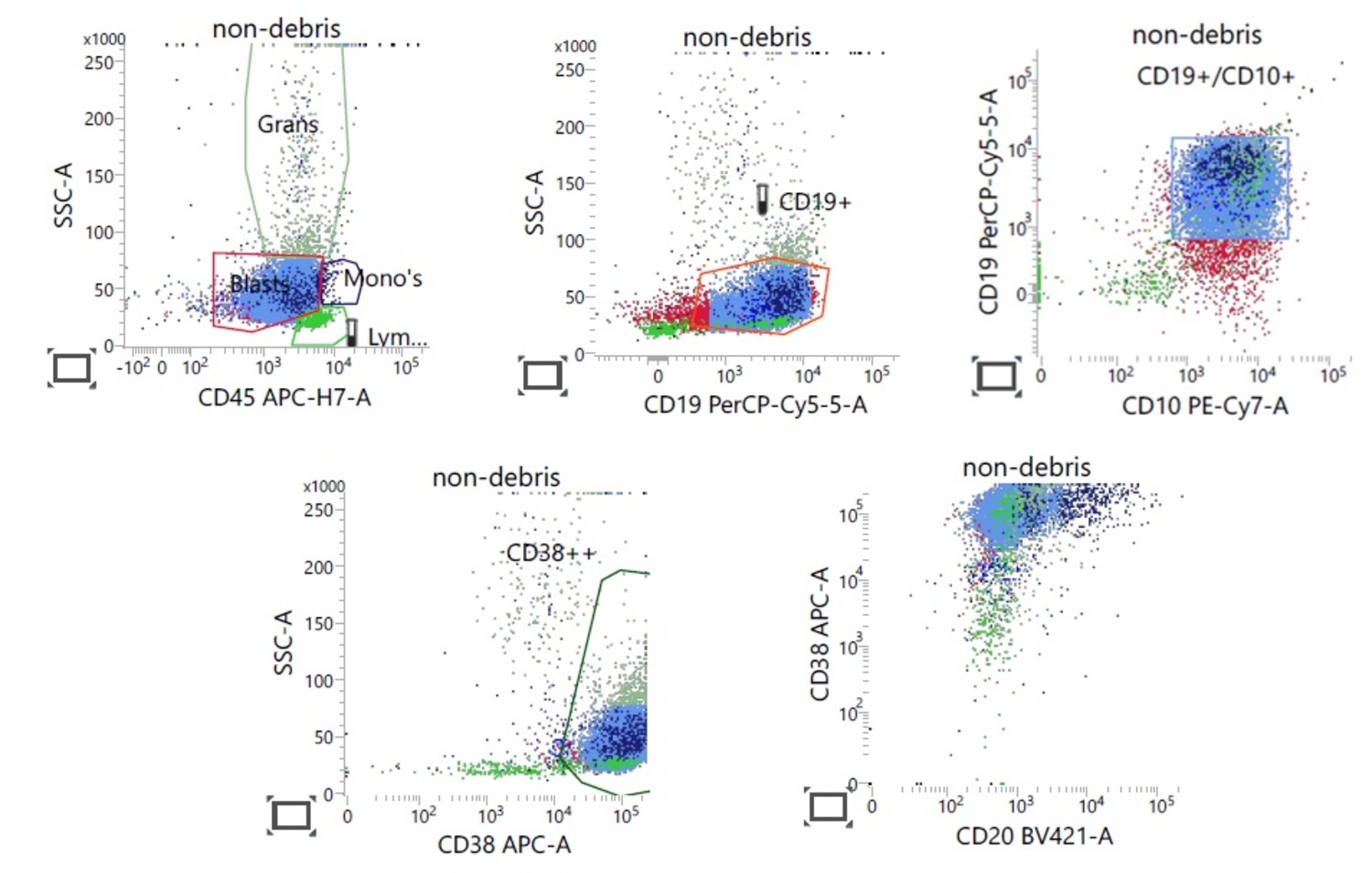
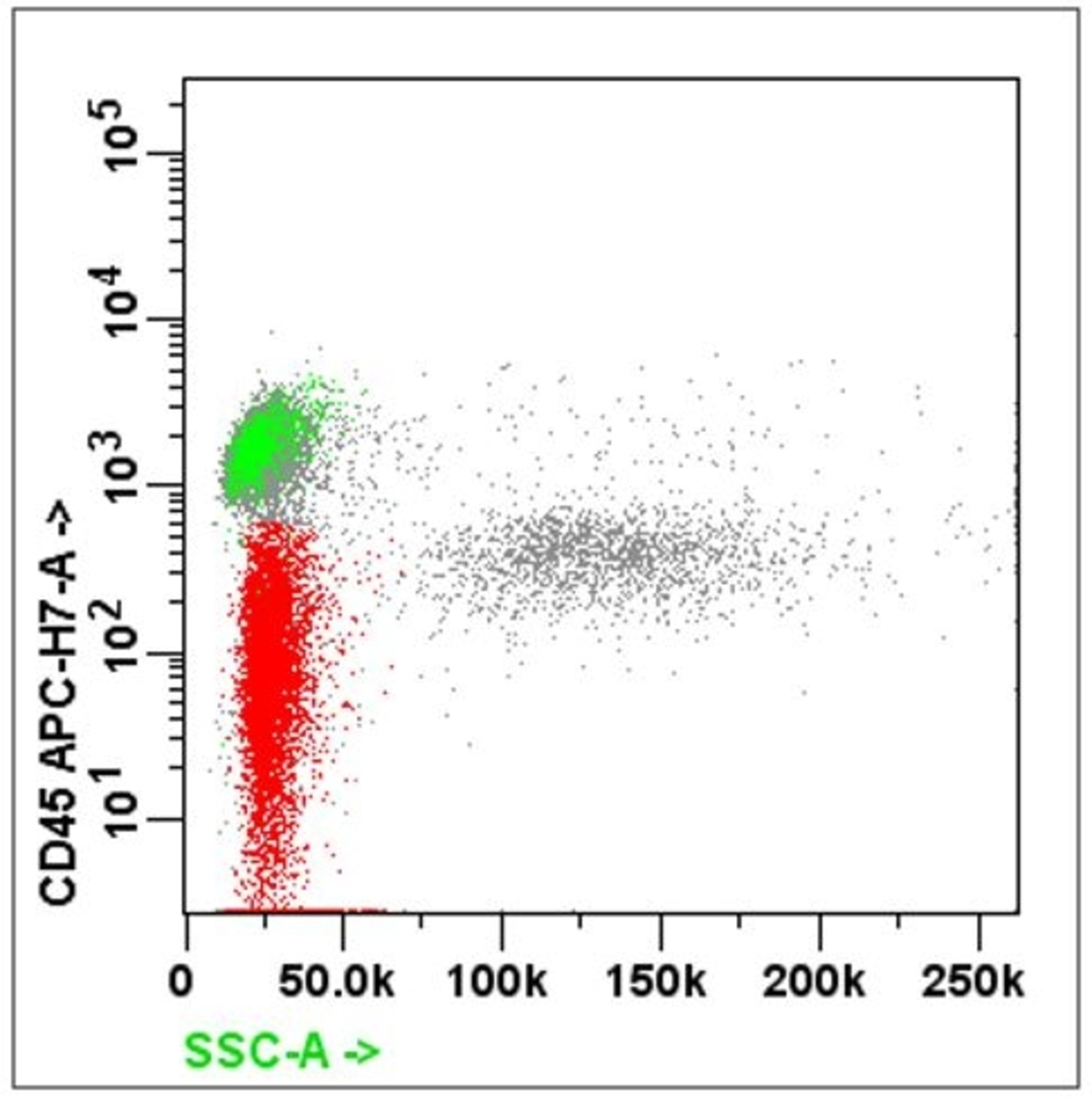
- Gating: specifying populations of cells with common characteristics to investigate further
- Side scatter: light scattered at 90 degrees after interacting with a particle
CPT coding
- Fresh tissue or body fluids
Sites
- Peripheral blood
- Bone marrow
- Body cavity fluids (pleural, peritoneal, pericardial, joints)
- Cerebrospinal fluids
- Tissue (e.g., lymph nodes)
Diagrams / tables
Overview
- Basic principles of flow cytometry (Crit Rev Biotechnol 2017;37:163)
- Single cells are dissociated in a liquid medium
- Cells are then stained with 1 or more fluorochrome tagged markers
- Laser beams with specific wavelengths strike the cells
- Cells tagged with fluorochrome dye absorb photons and emit fluorescence
- Light scatter and fluorescence from individual cells are detected by photomultiplier tubes
- Electronic impulse is converted to digital data (analog to digital) and is recorded by the computer
- Data acquisition software stores digital data as flow cytometry standard file
- Software packages display data as uni / bivariate histograms, scatter / dot plots, contour plots or density plots
- Components (Curr Protoc Immunol 2018;120:5.1.1, Crit Rev Biotechnol 2017;37:163)
- Fluidics
- Composed of a coaxial system: inner (sample) and outer (sheath)
- The aim of the system is to maintain a stable flow of cells
- Sheath fluid protects the sample from turbulence caused by resistance to the flow by the tube walls
- Air pressure of the sample controls the rate of flow of cells
- Increasing sample fluid pressure increases the rate of flow
- Lowering the sample fluid pressure decreases rate of flow
- Composed of a coaxial system: inner (sample) and outer (sheath)
- Optical system
- Light source
- Laser (light amplification by stimulated emission of radiation)
- Emits light with a specific wavelength
- Primary laser (488 nm wavelength) or secondary red diode laser (635 nm wavelength) may be utilized
- Excitation of the fluorochrome from laser beam results in fluorescence; the selection of fluorochrome should be appropriately matched with the wavelength of the laser used
- Laser (light amplification by stimulated emission of radiation)
- Light scattering
- Forward scatter (FSC): directly proportional to the size and surface area of the cell; it is collected by the FSC detector located on the same axis as the laser beam
- Side scatter (SSC): directly proportional to the granularity and internal complexity of the cell
- Fluorescence emission
- Fluorochrome dye can be used to characterize various properties in cells
- Fluorochrome is commonly tagged with an antibody
- Fluorochrome will absorb and emit light at a specific wavelength
- Light collection: via a set of special filters and optical mirrors
- Light source
- Electronic system (Methods Mol Biol 2011;699:1)
- Photons of light, generated by light scatter and fluorophore emission, hit the photodetectors
- Photon signal is converted into an electrical current called a photocurrent
- Photocurrent is then converted into a voltage pulse
- Voltage pulse is directly proportional to forward / side scatter and the number / brightness of fluorophores
- Voltage pulse may then be amplified (linear or logarithmic) or digitized by an analog digital converter (ADC)
- Newer flow cytometers (termed digital systems) digitize photocurrent early without prior amplification or processing
- Digitization involves organization of the continuous analog data into digital channels through process known as binning
- Analog digital converter outputs a data file in a standard FSC format
- Fluidics
Advantages of flow cytometry
- Sensitive method for simultaneously obtaining information on various parameters and processes, including expression of surface markers or the presence of intracellular cytokines and proteins
- High throughput system that can excel in characterizing heterogeneous cell populations
- Capable of sorting cells based on specific features
- Indispensable tool for the classification and immunophenotyping hematologic neoplasms
- Reference: J Invest Dermatol 2012;132:1
Disadvantages of flow cytometry
- Limited to cells in suspension, so information on tissue architecture and cell - cell interactions is not available
- Analyses requiring more fluorophores are subject to signal spillover
- Analysis is complicated by the amount of data generated
- Results are limited to the specific immunofluorescence panels that are being tested
- Less accurate in diagnosing certain lymphomas (i.e., Hodgkin lymphoma)
- Limited utility in evaluating infectious etiologies (e.g., EBV, HHV8)
- Reference: J Invest Dermatol 2012;132:1
Effectiveness
- Efficient flow cytometry satisfies the following
- High sensitivity
- Threshold: the capability to distinguish dim cells from the particle free background
- Resolution: the ability to separate dim cells from unstained ones
- Relative measured values of fluorescence (which depends on the instrument’s linearity accuracy)
- Reproducibility of the results
- High sensitivity
- Quality control
- Periodically: laser time delay, laser alignment
- Every day: photomultiplier voltage settings, compensation, gating control for multicolor flow
- External quality assessment
- Reference: Clin Lab Med 2007;27:671
Clinical applications
- Hematologic diseases
- Diagnosis and sub classifying non-Hodgkin lymphoma (NHL)
- WHO approaches to classify the lymphomas based on the lineage of the cells: B cell and T / NK cell; precursor versus mature
- Useful in immunophenotyping different lymphomas
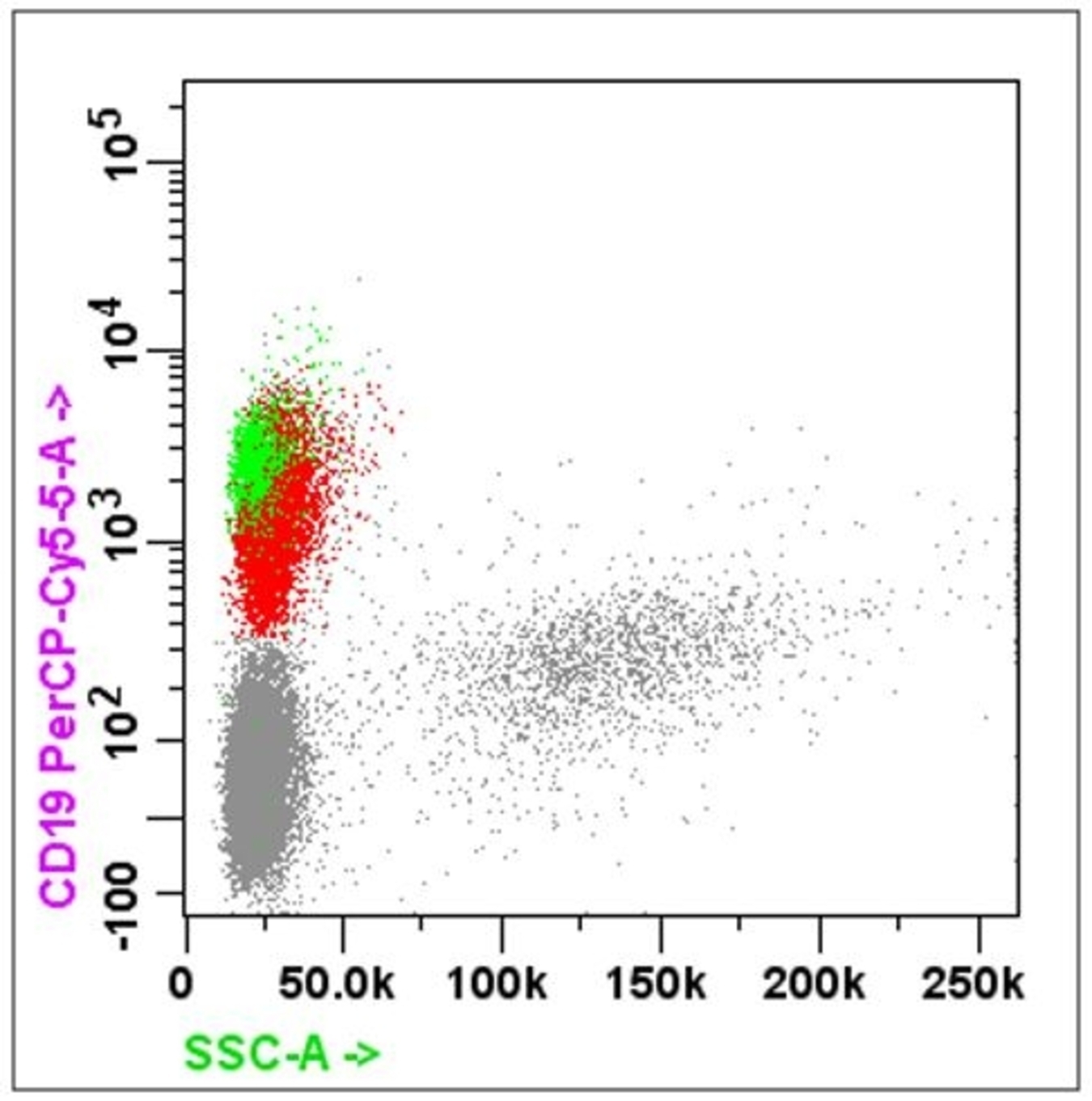
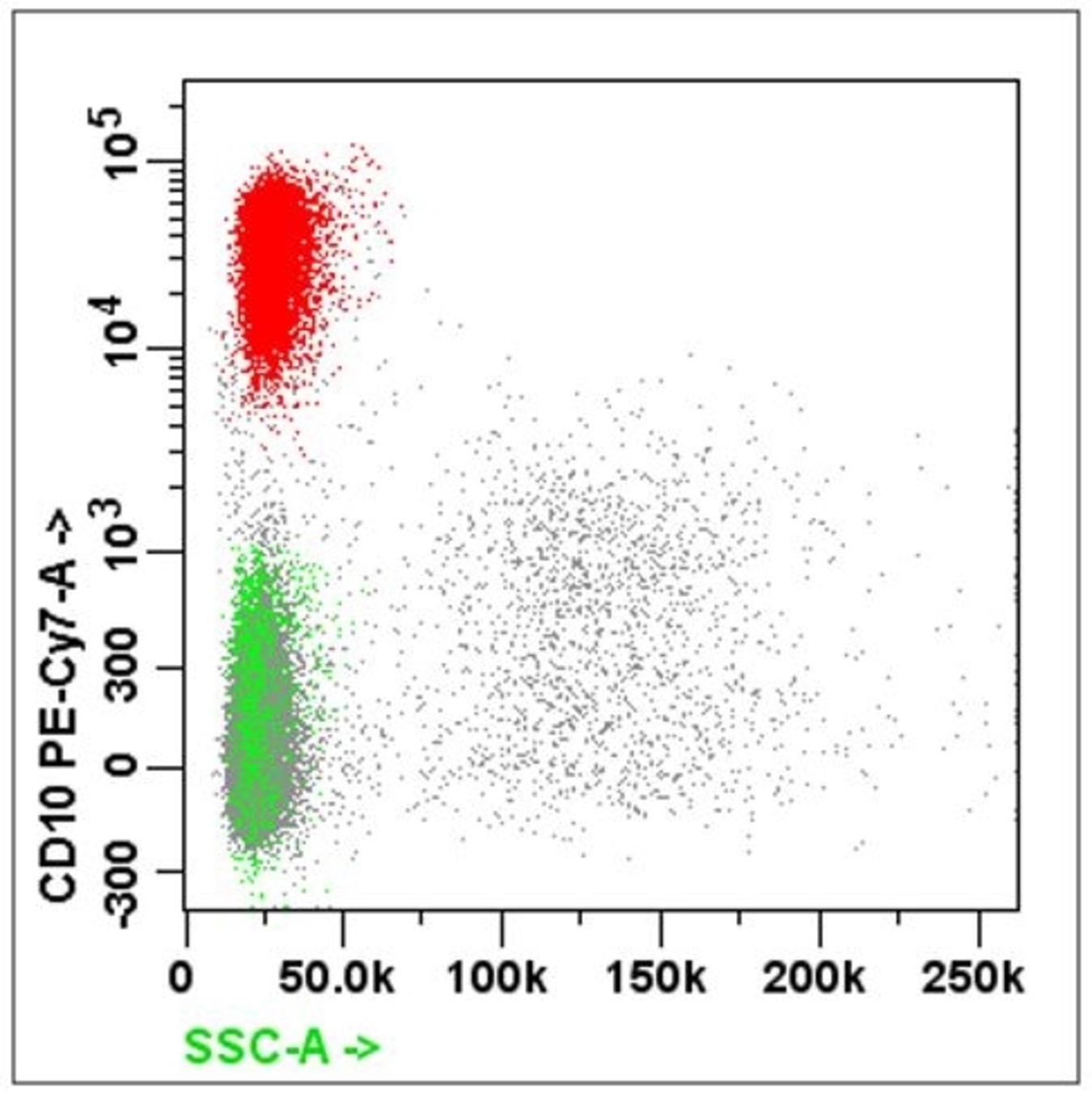
- B cell markers: CD19, CD20, CD79a, PAX5, CD10, CD23
- T cell markers: CD2, CD3, CD4, CD5, CD7, CD8
- NK cell markers: CD2, CD3, CD56
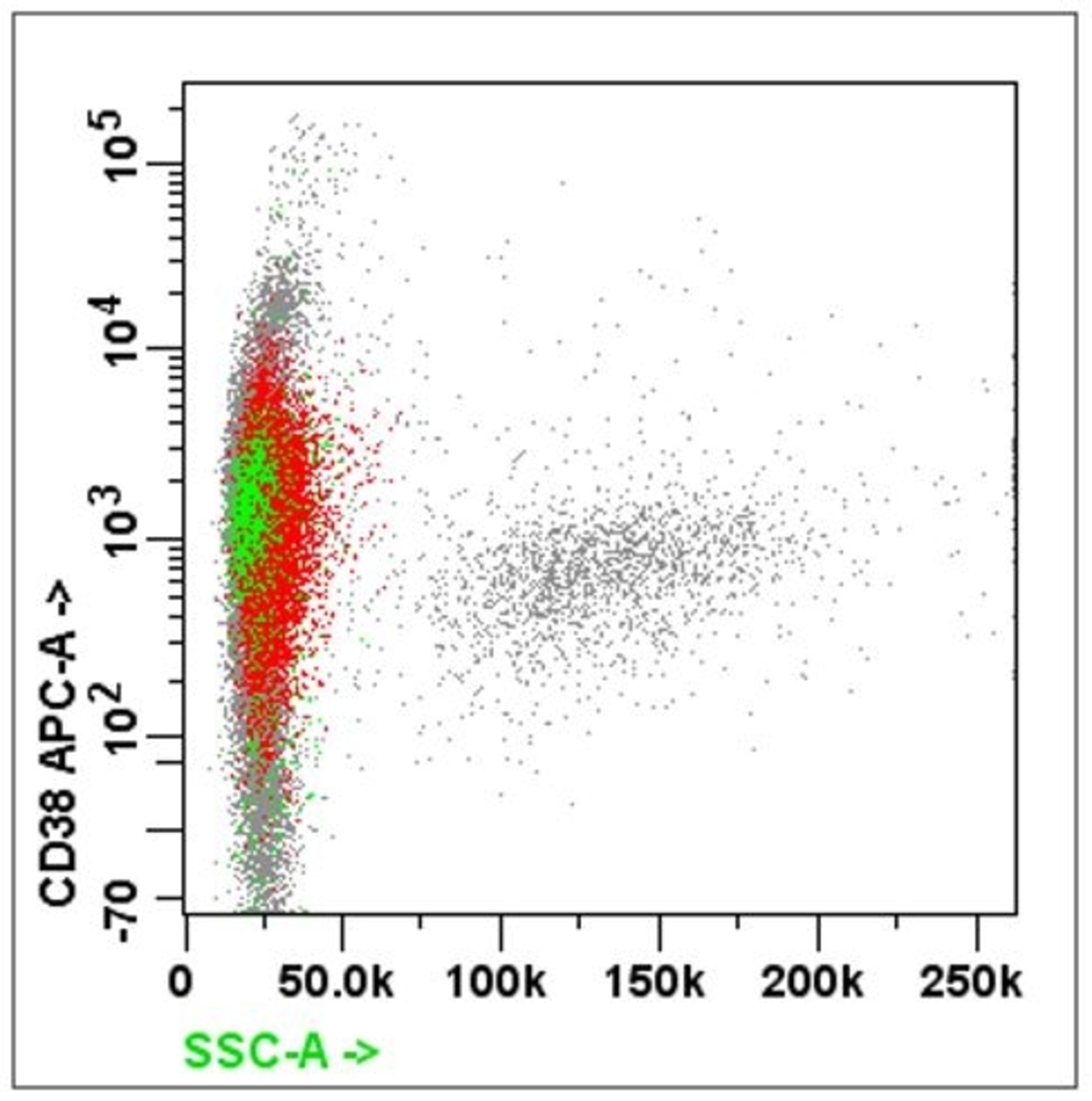
- Plasma cell markers: CD38, CD138, CD56
- Fine needle aspiration of lymph node with flow cytometry
- Rapid onsite evaluation
- Material processed for
- Routine smears
- Cell block
- Flow cytometry
- Cytogenetics
- False negative in flow cytometry after fine needle aspiration (FNA) can be due to
- Predominantly necrotic tissue or fibrosed lymph node
- Material diluted with blood
- Few and scattered atypical cells as in Hodgkin lymphoma
- Assessment of minimal residual disease (Blood Cancer J 2020;10:108)
- Ultrasensitive method of determining disease progression, relapse and response to therapy
- May be used with or without next generation sequencing to detect specific populations with a sensitivity between 10-5 and 10-6
- Usually performed on bone marrow aspirate fluid (multiple myeloma and acute leukemias) but may also use peripheral blood in certain instances (e.g., CLL or ALL)
- Limitations include hemodilution and sampling error
- One major application of flow cytometry is demonstrating the clonality of lymphoma
- Light chain restriction: monoclonal B cell proliferation has altered kappa to lambda chain ratio
- Diagnosis and sub classifying non-Hodgkin lymphoma (NHL)
- Flow cytometry of cytology samples
- Effusion
- Detection of carcinoma in fluid
- Higher synthetic phase (S phase) cells may be used as indirect evidence of malignancy
- Epithelial cell adhesion molecule (EpCAM [CD326]) or its antibody (BerEP4) can be used to identify epithelial cells (Appl Immunohistochem Mol Morphol 2009;17:202)
- Possible pitfalls
- Poorly differentiated carcinoma may not express any markers
- Benign cells may express epithelial markers, especially in peritoneal washings and instrumentalizations
- Effusion due to involvement by a lymphoreticular neoplasm
- Cytology along with flow cytometry have 100% sensitivity and 94% specificity in diagnosing lymphoma (Diagn Cytopathol 2006;34:335)
- Some panels suggested include
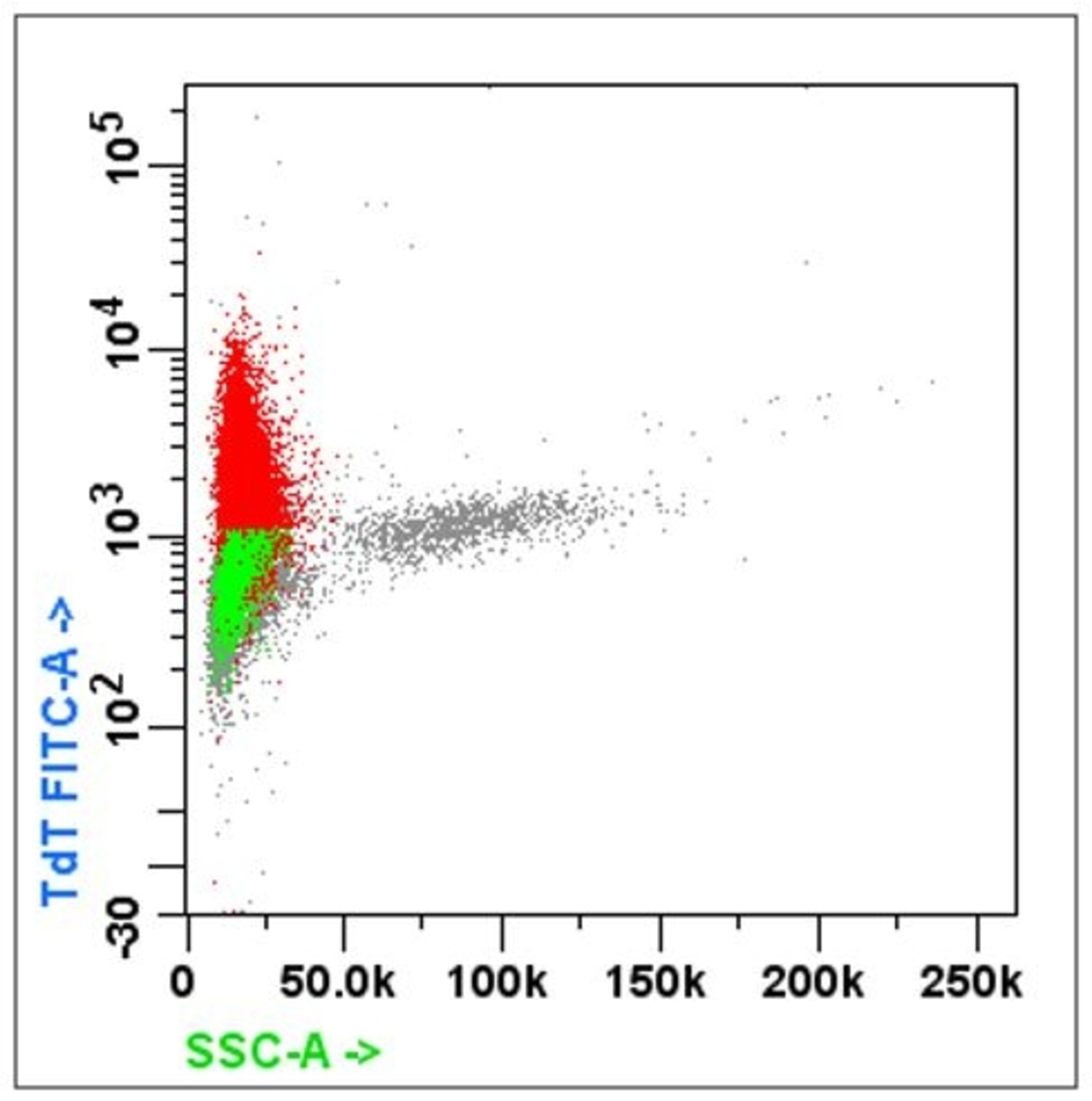
- Acute leukemia: MPO, Tdt, CD45 / CD34 / CD7 / CD13 and CD33 / CD56 / CD19
- Lymphoma: CD45 / CD3 / CD4 / CD8 and CD19 / CD20 / CD10
- For suspected T cell lineage, must include: CD3 / CD2 / CD5 / CD7 / CD8, CD26
- For suspected B cell lineage, must include CD5 / CD23 / CD10 / CD38 / CD138 / FMC7 / surface kappa and lambda light chain
- For suspected myeloma: CD20 / CD19 / CD38 / CD138 / CD27 / CD45 / cytoplasmic kappa / lambda
- Primary effusion lymphoma
- Cytomorphology shows large cells with nuclear pleomorphism and nucleoli
- Flow cytometry
- Positive: CD45, CD30, CD38, CD138, CD43
- Negative for B cell markers: CD19, CD20 and no light chain restriction
- Absent or aberrant expression of T cell markers: CD2, CD3, CD5, CD7
- Detection of carcinoma in fluid
- Bronchoalveolar lavage (BAL) (Am J Respir Crit Care Med 2012;185:1004)
- Cellular analysis may provide useful adjunct information for patients who lack radiographic evidence of usual interstitial pnuemonia
- Predominant inflammatory cellular pattern could help clinicians determine etiology of interstitial lung disease
- Effusion
- Identification of therapeutic targets
- Selection of antibody based therapies
- Rituximab: anti-CD20
- Epratuzumab: anti-CD22
- Gemtuzumab: anti-CD33
- Blinatumomab: directed against CD19 and CD3
- Selection of antibody based therapies
- Nonhematologic disease
- Assessment of nonhematologic neoplasms (Am J Clin Pathol 2020;153:99)
- May utilize antibodies directed against epithelial markers such as MOC31, Ber-EP4, CK5 / 8 and MUC1
- Targeted panel of CD45, CD33 and EpCAM (CD326) has been used to distinguish monocytes / macrophages, mesothelial cells and epithelial cells
- Assessment of nonhematologic neoplasms (Am J Clin Pathol 2020;153:99)
- DNA content analysis
- Use of propidium iodide that interposes into the DNA helical structure
- Fluorescence is directly proportional to the amount of DNA
- DNA content aneuploidy can be determined in a tumor cell
- Use of propidium iodide that interposes into the DNA helical structure
Board review style question #1
In a typical flow cytometry, the side scatter (SSC) and forward scatter (FSC) provide information about which of the following?
- Architecture and granularity
- Cytoplasmic complexity and size
- Intracellular signaling and viability
- Size and surface markers
Board review style answer #1
Board review style question #2
Which of the following is a clinical application of flow cytometry?
- Demonstrating clonality of lymphoma
- Easily identifying Hodgkin lymphoma
- Identifying benign cells in instrumented effusion fluid
- Identifying relevant information about architecture of carcinoma
Board review style answer #2
A. Demonstrating clonality of lymphoma. Answer B is incorrect because Reed-Sternberg cells are admixed in a rich inflammatory background which consists mainly of T cells, B cells, eosinophils, histiocytes and plasma cells. Answer C is incorrect because benign cells are scant in instrumented fluid. Answer D is incorrect because flow cytometers analyze suspensions of single cells.
Comment Here
Reference: Flow cytometry
Comment Here
Reference: Flow cytometry