Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear images | Positive stains | Negative stains | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Walters-Smith M, Kaseb H. AML with t(1;3)(p36;q21); RDM16::RPN1. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticAMLt13p36q21.html. Accessed January 10th, 2025.
Definition / general
- Rare type of acute myeloid leukemia (AML)
- AML with myelodysplastic syndrome (MDS) related changes (WHO, 2017)
- Balanced translocation meeting criteria for AML with MDS related changes
- Blasts > 20% and no prior therapy
- Described as an entity under the category of AML with other rare recurring translocations in International Consensus Classification (ICC) (Blood 2022;140:1200)
- AML with fusion of PRDM16 and RPN1
Essential features
- Rare subtype of AML with rare genetic abnormalities and differentiation
- Defined by t(1;3)(p36;q21) (PRDM16::RPN1)
- Associated with dysplasia, especially dysmegakaryopoiesis (Cancer Genet Cytogenet 2010;203:187, Genes Chromosomes Cancer 2003;36:313)
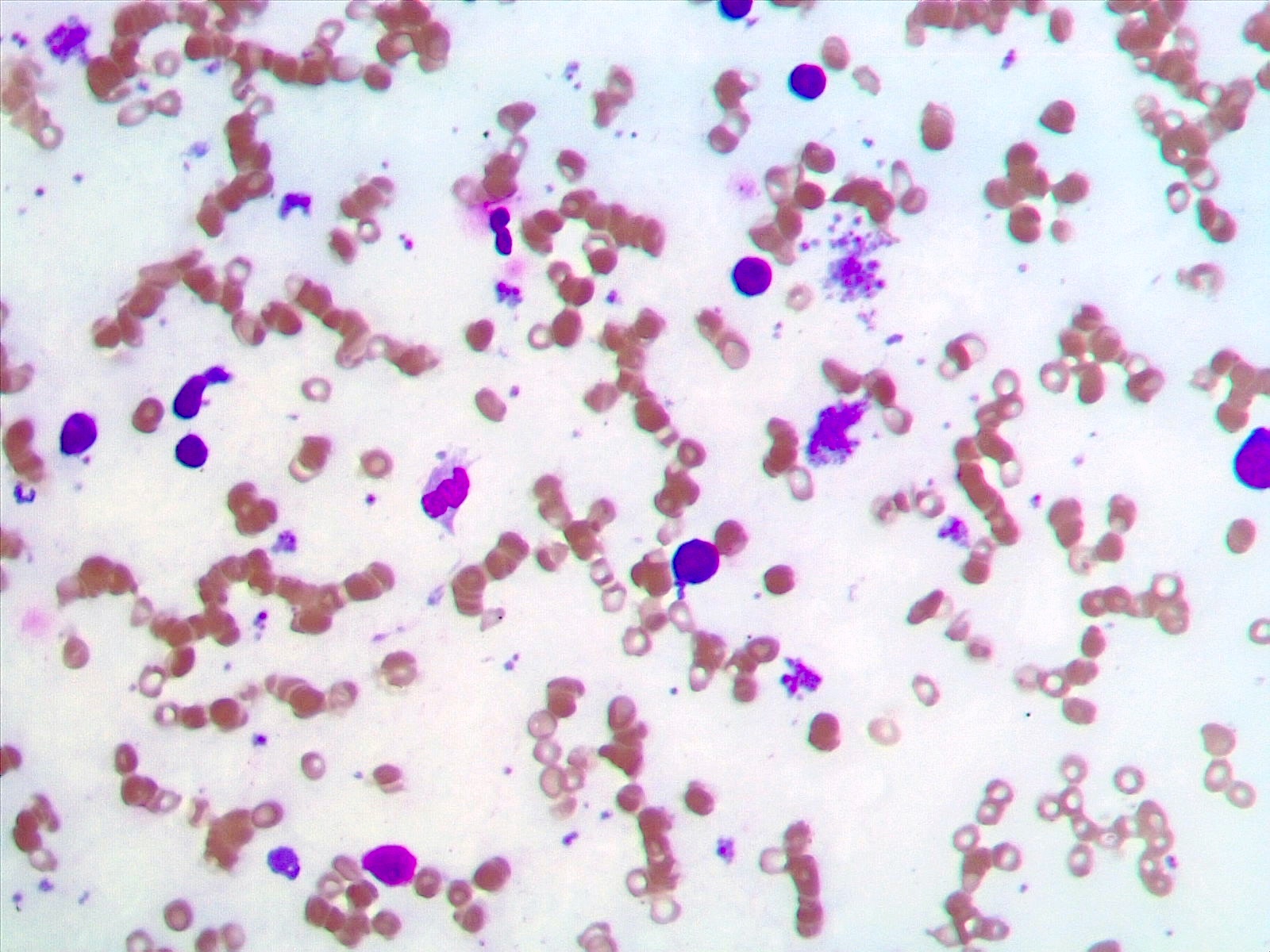
- Associated with peripheral thrombocytosis
- Associated with poor prognosis
Terminology
- Acute myeloid leukemia with t(1;3)(p36;q21) (PRDM16::RPN1)
- AML with t(1;3)(p36;q21) (PRDM16::RPN1)
ICD coding
- ICD-O: 9895/3 - acute myeloid leukemia with myelodysplasia related changes
Epidemiology
- Rare, occurs mainly in elderly patients (WHO, 2017) (Cancer Genet Cytogenet 2010;203:187)
- Wide age range (30 - 80 years); M = F (Cancer Genet Cytogenet 2010;203:187)
- t(1;3)(p36;q21) feature constitutes ≤ 1% of overall MDS cases (WHO, 2017)
Sites
- Bone marrow, spleen and liver
- Lymph node involvement can also occur
Pathophysiology
- Genes at break point of translocation include RPN1 at 3q21.3 and group of genes at PRDM16 at 1p36.3 (Br J Haematol 2012;156:76)
- Related to chromosome 3 abnormalities, such as inv(3)(q21q26), t(3;3)(q21;q26), ins(3;3)(q26;q21q26) and t(1;3)
- t(1;3) show dysmegakaryopoietic features similar to the 3q21q26 syndrome
- PRDM16 gene (MEL1, MDS1::EVI1-like gene) is activated as a result of translocation of the gene next to RPN1 gene at 3q21 (Genes Chromosomes Cancer 2003;36:313)
- PRDM16 gene at 1p36.3 (the breakpoints are located within a 90 kb region in the 5' region of PRDM16)
- RPN1 gene at 3q21 (the breakpoints are located within a 60 kb region centromeric to the breakpoint cluster region of the 3q21q26 / inv(3) syndrome)
- Aberrant expression of PRDM16 as a result of RPN1 translocation is thought to be associated with pathogenesis of this AML subtype
Etiology
- Aberrant expression of PRDM16 as a result of RPN1 translocation
Clinical features
- Majority of cases present
- Anemia (common)
- Thrombocytosis and leukocytosis
- Pancytopenia
- Some cases present as MDS and then progress to AML (WHO, 2017)
- Translocation (1;3) can be the sole chromosomal abnormality at initial diagnosis or can be associated with other chromosomal abnormalities
- Peripheral thrombocytosis is a distinct feature (Cancer Genet Cytogenet 2010;203:187)
- t(1;3) was first reported in 1984 (Blood 1984;64:553)
- Aberrant expression of PRDM16 as a result of RPN1 translocation is associated with various other hematological conditions (Cancer Genet Cytogenet 2010;203:187)
- MDS
- Myelodysplastic / myeloproliferative neoplasms (MDS / MPN)
- Myeloproliferative neoplasms (MPN)
- AML
- Acute lymphoblastic leukemia (ALL)
- Prognosis is poor; median survival is ~6 months
- WHO diagnostic criteria (WHO, 2017)
- > 20% peripheral blood or bone marrow blasts
- Any of the following:
- History of MDS or MDS / MPN
- MDS related cytogenetic abnormality
- Multilineage dysplasia
- Absence of both of the following:
- Prior cytotoxic or radiation therapy
- Recurrent cytogenetic abnormalities of AML
| WHO revised 4th edition | WHO 5th edition | ICC classification |
|
|
|
Diagnosis
- Peripheral blood
- Bone marrow biopsy
- Cytogenetics
- Karyotype analysis showing (1;3)(p36;q21) (PRDM16::RPN1)
- Molecular genetics
- Immunophenotyping
Laboratory
- Peripheral blood
- Anemia, thrombocytosis and leukocytosis
- Bone marrow
- Usually hypercellular marrow
- Blasts
- Molecular studies
- Translocation can be detected by karyotyping or AML FISH PRDM16::RPN1 t(1;3)(p36.3;q21.3) (Mayo Clinic Laboratories: AMLMF [Accessed 15 February 2023])
Prognostic factors
- Independent of t(1;3)(p36.3;q21.2) abnormality, prognosis has been shown to be worse in AML (Mod Pathol 2015;28:965, Leuk Res 1995;19:121)
- Very few known complete remission outcomes in AML with t(1;3)(p36.3;q21.2) (Cancer Genet Cytogenet 2010;203:187)
- Survival period was worse following detection of t(1;3)(p36.3;q21.2) abnormality in AML (21.3 months) than in acute lymphoblastic leukemia with t(1;3)(p36.3;q21.2) (78.6 months) (Cancer Genet Cytogenet 2010;203:187)
- High expression of PRDM16 correlated with poor prognosis and decreased 5 year survival (Genes Chromosomes Cancer 2017;56:800)
Case reports
- 6 year old girl with t(1;3)(p36;q21) following treatment for AML (Cancer Genet Cytogenet 2009;191:59)
- 59 year old woman and 66 and 82 year old men presenting with (1;3)(p36;q21) (PRDM16::RPN1) (Genes Chromosomes Cancer 2003;36:313)
- AML with t(1;3) and extreme thrombocytosis (Cancer Genet Cytogenet 2010;203:187)
Treatment
- No specific treatments for this specific translocation have been reported
- Chemotherapy
- Conventional chemotherapy has had little success in achieving complete remission (Cancer Genet Cytogenet 2010;203:187)
- Stem cell transplantation
Microscopic (histologic) description
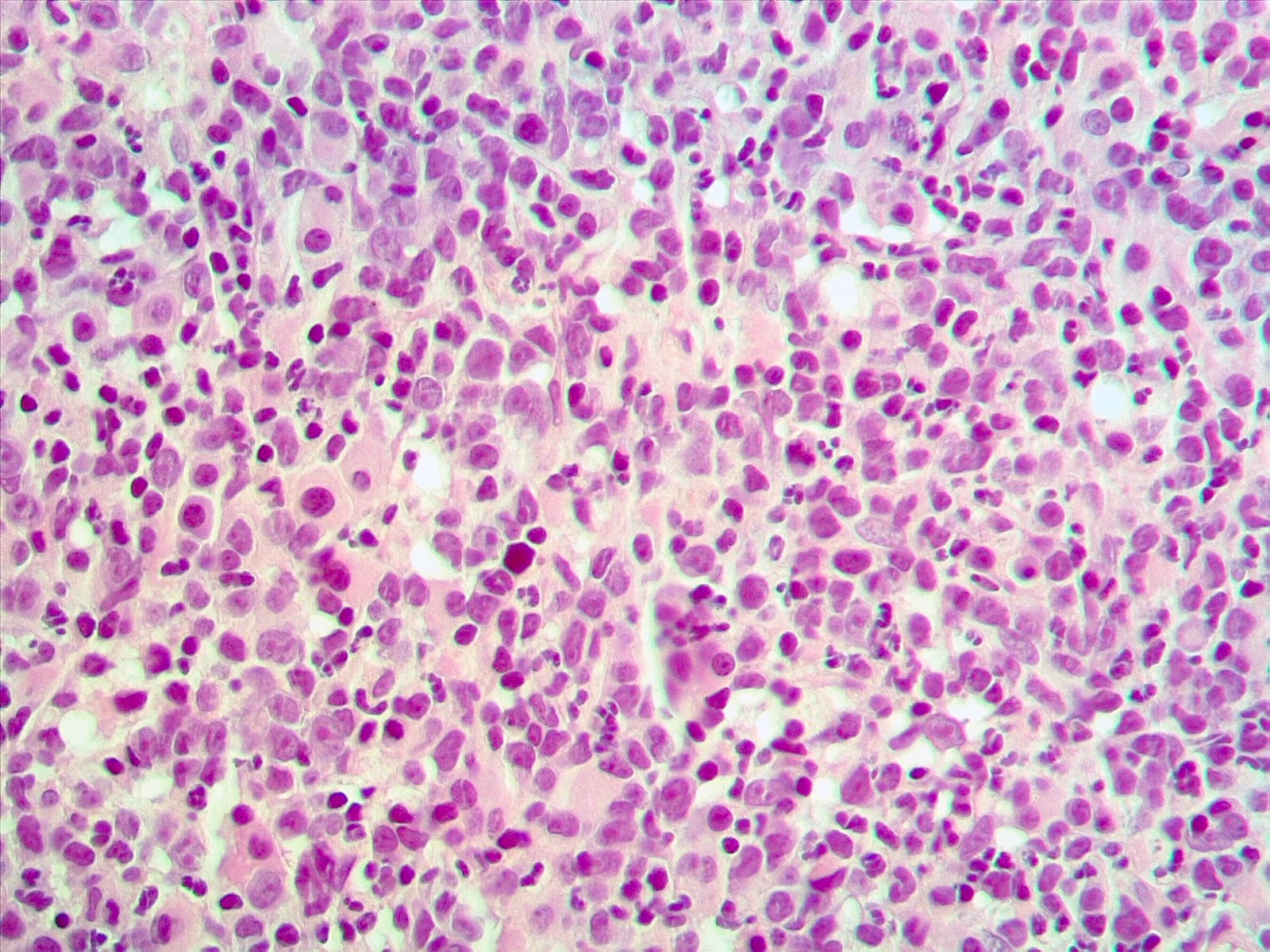
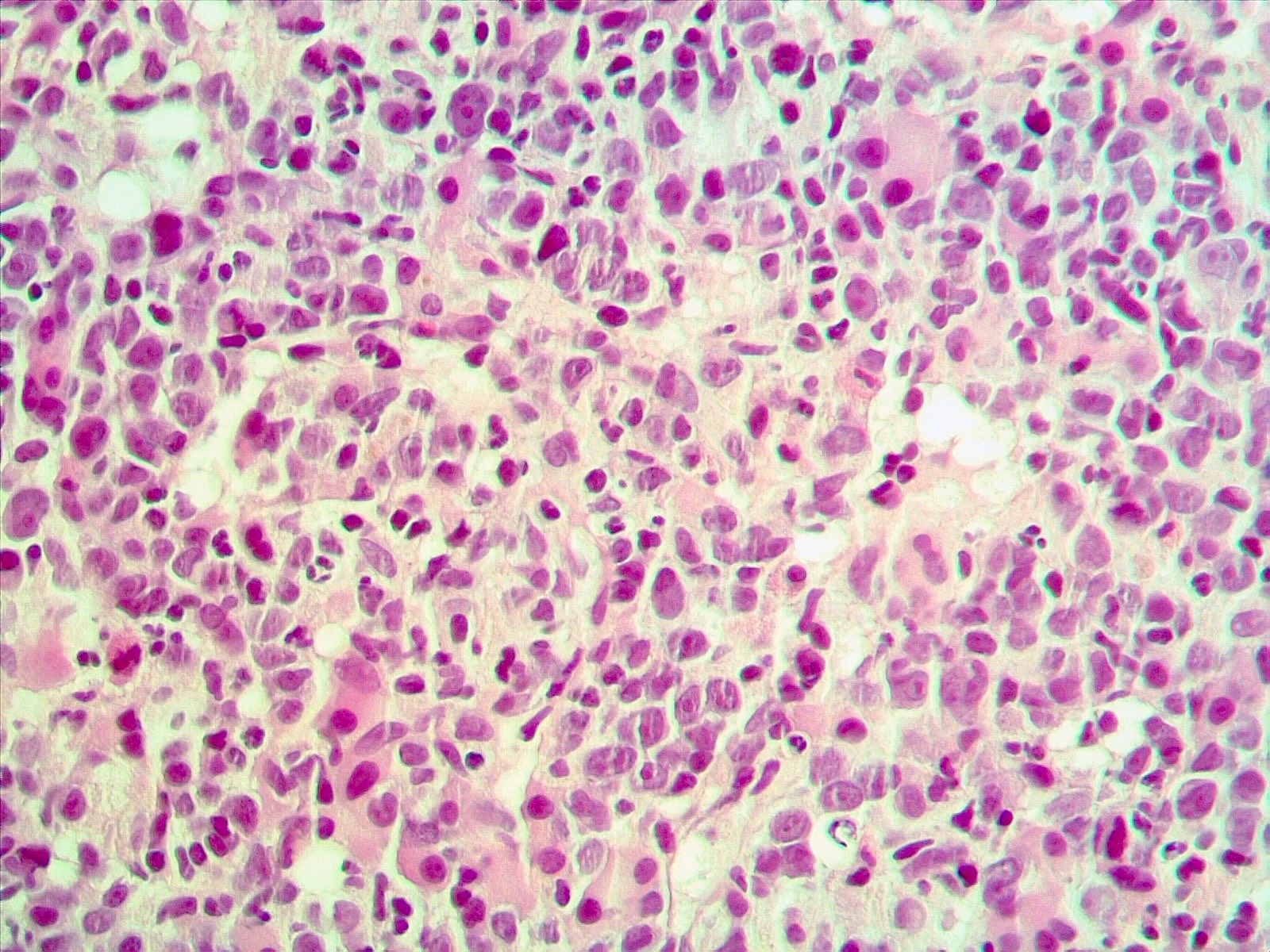
- Presentation as AML (M1 - M5) has been reported; however, presentation as AML M4 (French American British [FAB] classification) is common (Cancer Genet Cytogenet 2010;203:187)
- Prominent monocytic component has been reported in some cases
- Multilineage dysplasia especially dysmegakaryopoiesis (Cancer Genet Cytogenet 2010;203:187, Genes Chromosomes Cancer 2003;36:313)
- At least 50% dysplasia across 2 different lineages (WHO, 2017)
- Megakaryocytes
- Megakaryocytic hyperplasia and dysplasia
- Peripheral thrombocytosis is a distinct feature
Microscopic (histologic) images
Positive stains
- Variable and possibly related to the patient's distinct AML FAB type
- Usually positive
- Variable positivity
- Possible abnormal expression of CD56, CD72
- Other myeloid markers may be positive
- Reference: Cancer Genet Cytogenet 2010;203:187
Negative stains
- Variable and possibly related to the patient's distinct AML FAB type
- Usually negative
- Reference: Cancer Genet Cytogenet 2010;203:187
Molecular / cytogenetics description
- Translocation can be detected by karyotyping or AML FISH PRDM16::RPN1 t(1;3)(p36.3;q21.3) (Mayo Clinic Laboratories: AMLMF [Accessed 15 February 2023])
Sample pathology report
- Right posterior iliac crest, core biopsy, aspirate smear, touch imprint and clot particle:
- Acute myeloid leukemia with t(1;3)(p36;q21) (see comment)
- Comment: The bone core biopsy demonstrates extensive replacement of marrow by blast cells in areas arranged into clusters and aggregates surrounded by fibrotic stroma. The marrow aspirate smears reveal an increased population of blasts. Blast phenotyping with immunohistochemical stains and flow cytometry analysis reveal positivity for myeloid markers. Cytogenetics evaluation reveals t(1;3)(p36;q21). Overall, the results support the diagnosis of AML with t(1;3)(p36;q21).
Differential diagnosis
- MDS with excess blasts:
- Blasts < 20%
- May show other MDS related cytogenetic or molecular abnormalities
- May show t(1;3)(p36;q21); these cases may progress to AML
- Myeloproliferative neoplasms:
- Blasts < 20%
- May show t(1;3)(p36;q21); these cases may progress to AML
Additional references
Board review style question #1
A 60 year old woman presented with fatigue and weight loss. The patient's initial laboratory workup showed leukocytosis, anemia and thrombocytosis. A bone marrow biopsy was performed, which showed increased blasts and multilineage dysplasia. A karyotype showed with t(1;3)(p36;q21). Which of the following is true about this entity?
- Blasts show a distinct phenotype characteristic of this entity
- Case shows overexpression of the PRDM16 gene
- Frequently associated with NPM1 mutation
- Has an intermediate prognosis
Board review style answer #1
B. Case shows overexpression of the PRDM16 gene. Overexpression of the PRDM16 gene as a result of RPN1 translocation is an important finding in in AML t(1;3)(p36;q21) patients. Answer A is incorrect because flow cytometry is usually nonspecific in AML t(1;3)(p36;q21). Answer C is incorrect because NPM1 mutation has not shown to be associated with AML t(1;3)(p36;q21). Answer D is incorrect because these cases typically show a poor prognosis.
Comment Here
Reference: AML with t(1;3)(p36;q21)
Comment Here
Reference: AML with t(1;3)(p36;q21)