Table of Contents
Definition / general | Essential features | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Peripheral smear description | Peripheral smear images | Positive stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Corean J, Karner K. AML with CEBPA mutation (WHO-HAEM5-biallelic or ICC bzip). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticAMLbiallelicCEBPA.html. Accessed December 19th, 2024.
Definition / general
- Subtype of acute myeloid leukemia (AML) with recurrent genetic abnormality
- Required for diagnosis, the CEBPA mutations must be biallelic
- Associated with more favorable prognosis
Essential features
- CEBPA mutations must be biallelic, not just a single mutation, for diagnosis
- May represent a germline predisposition syndrome and germline testing may be considered in patients with persistent CEBPA mutations following morphologic remission or in patients with family history of leukemia
ICD coding
- ICD-10: C92.0 - acute myeloblastic leukemia
Epidemiology
- Accounts for 4 - 9% of AML diagnoses in children and young adults
- Less common in older patients
- Reference: Blood 2009;113:6558
Sites
- Peripheral blood and bone marrow
Pathophysiology
- Hematopoietic progenitor cells require biallelic mutations of CEBPA
- Possible continued clonal evolution
- Reference: Sci Adv 2019;5:eaaw4304
Etiology
- A subset of patients carry an underlying germline mutation, leading to predisposition to develop AML
Clinical features
- Associated with lower frequency of lymphadenopathy and myeloid sarcoma
Diagnosis
- Routine CBC with differential, bone marrow biopsy with flow cytometry, chromosome analysis and either targeted CEBPA molecular testing or next generation sequencing (massively parallel sequencing) for myeloid mutations
Laboratory
- Typically present with relatively higher hemoglobin levels (still anemic), lower platelet counts and lower lactate dehydrogenase than CEBPA wildtype AML
Radiology description
- PET scan identified hypermetabolic bone marrow
Prognostic factors
- Favorable prognosis similar to AML with inv(16)(p13.1q22) or t(8;21)(q22;q22.1)
- FLT3-ITD and GATA2 mutation status is of uncertain prognostic significance
- Reference: Eur J Haematol 2015;94:439
Case reports
- 18 year old woman and 30 year old man (siblings) diagnosed with AML within 2 weeks of each other; father has a history of AML (N Engl J Med 2004;351:2403)
- 19 year old man presenting with leukocytosis, bruising and petechiae is diagnosed with AML with biallelic CEBPA (SH/EAHP Workshop 2017: Familial Acute Myeloid Leukemia with Germline CEBPA Mutation [Accessed 15 September 2021])
- 33 year old man with relapsed AML following stem cell transplant (ASH Image Bank: AML with Biallelic CEBPA Mutation [Accessed 15 September 2021])
- 36 year old woman at 35 weeks gestation presents with pancytopenia and is diagnosed with AML with biallelic CEBPA mutations (Blood Adv 2017;1:500)
- 52 year old woman with 18% blasts on peripheral blood smear with cytogenetic findings of biallelic CEBPA mutations (Annals of Case Reports ReDelve 2018;2018:1)
Treatment
- Treated with similar induction and consolidation methods as other AMLs: 7+3 (cytarabine and anthracycline) and consolidation with cytarabine or azacitidine
- May benefit from stem cell transplant (cannot use family member with CEBPA mutation if germline)
- Relapsed patients have favorable prognosis as well (Blood 2013;122:1576)
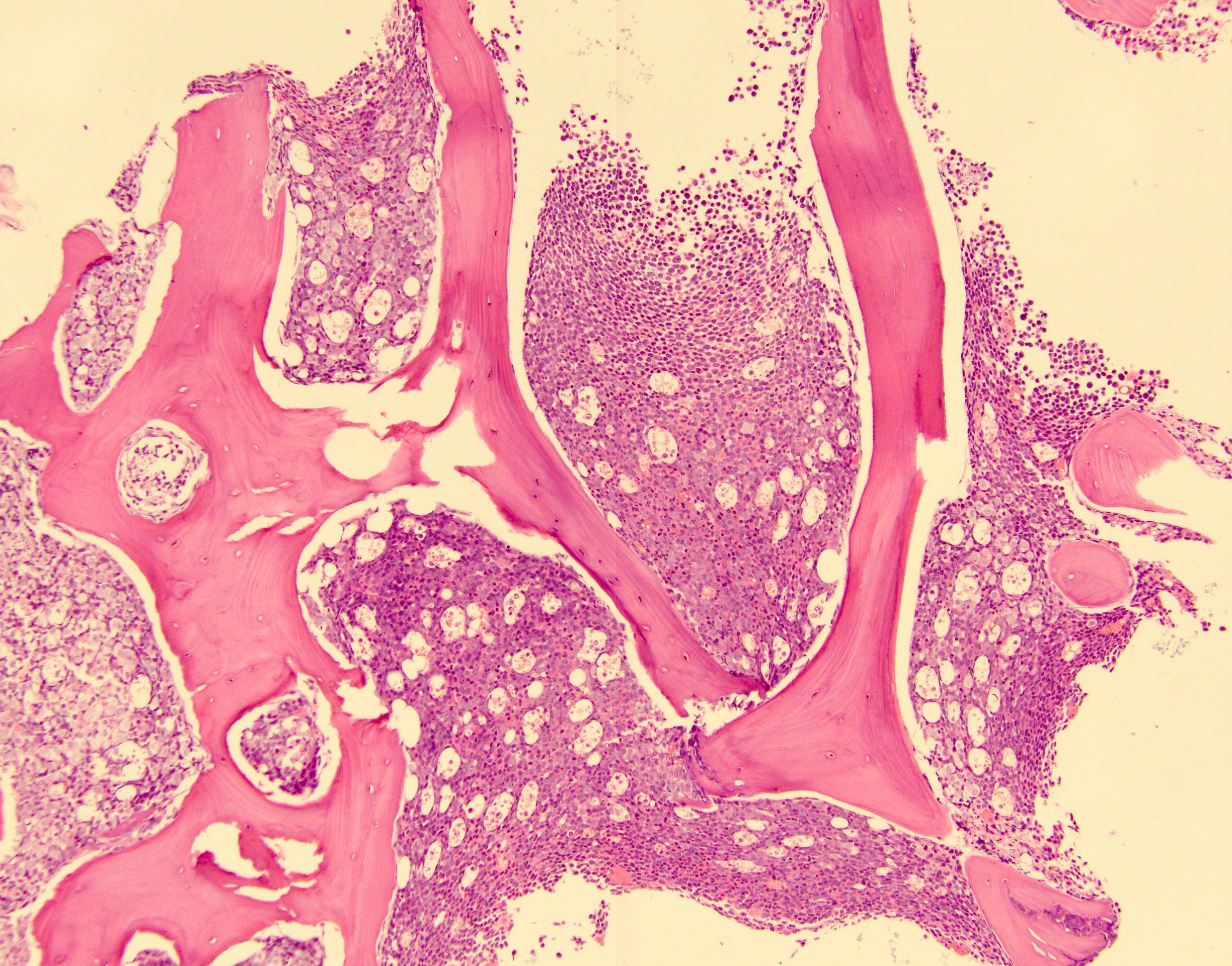
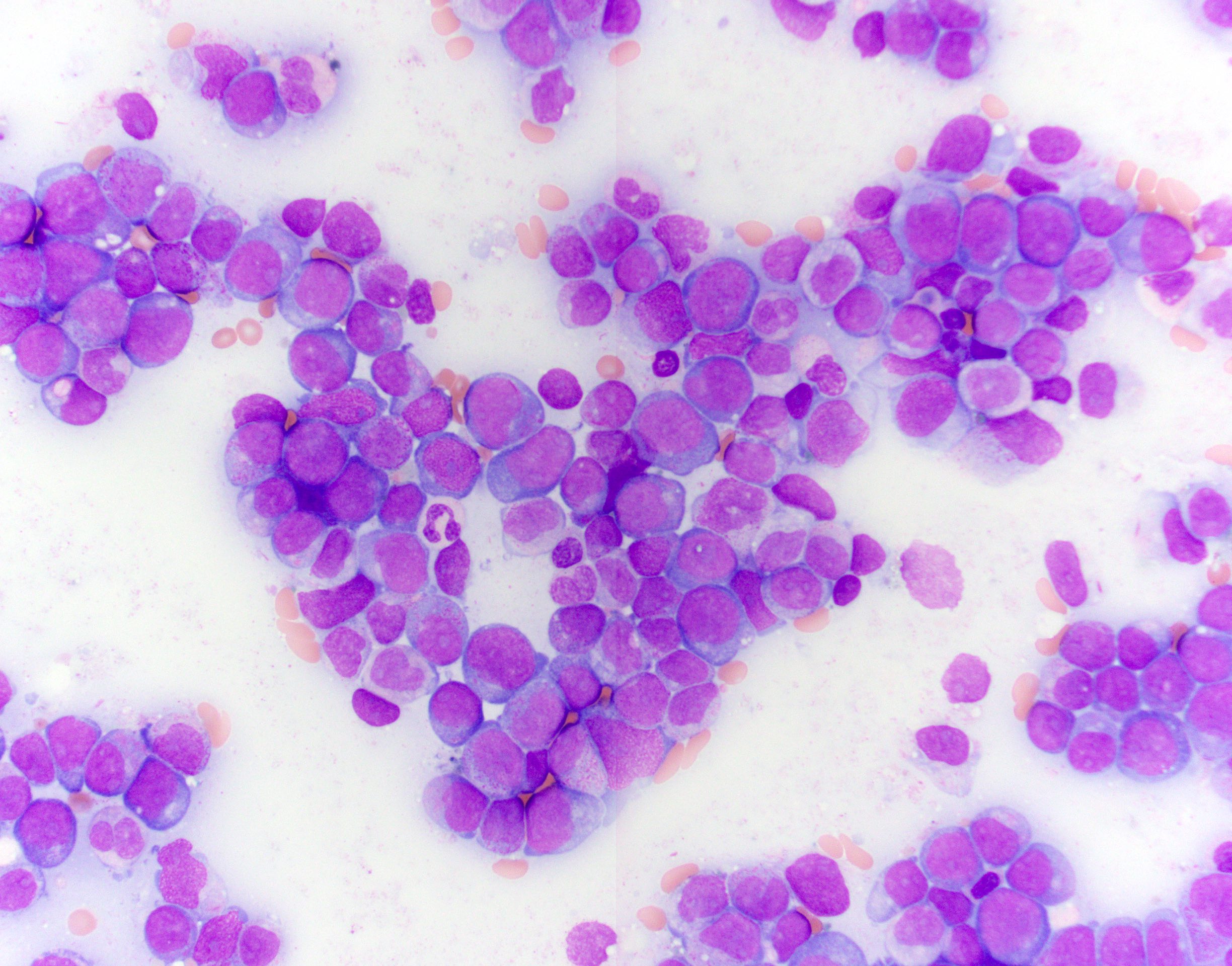
Microscopic (histologic) description
- No distinctive morphologic features
- Typically, AML with or without maturation morphology
- Multilineage dysplasia is present in 26% of cases of de novo AML with mutated CEBPA without an associated adverse prognosis; does not change classification
- Reference: Haematologica 2017;102:529
Microscopic (histologic) images
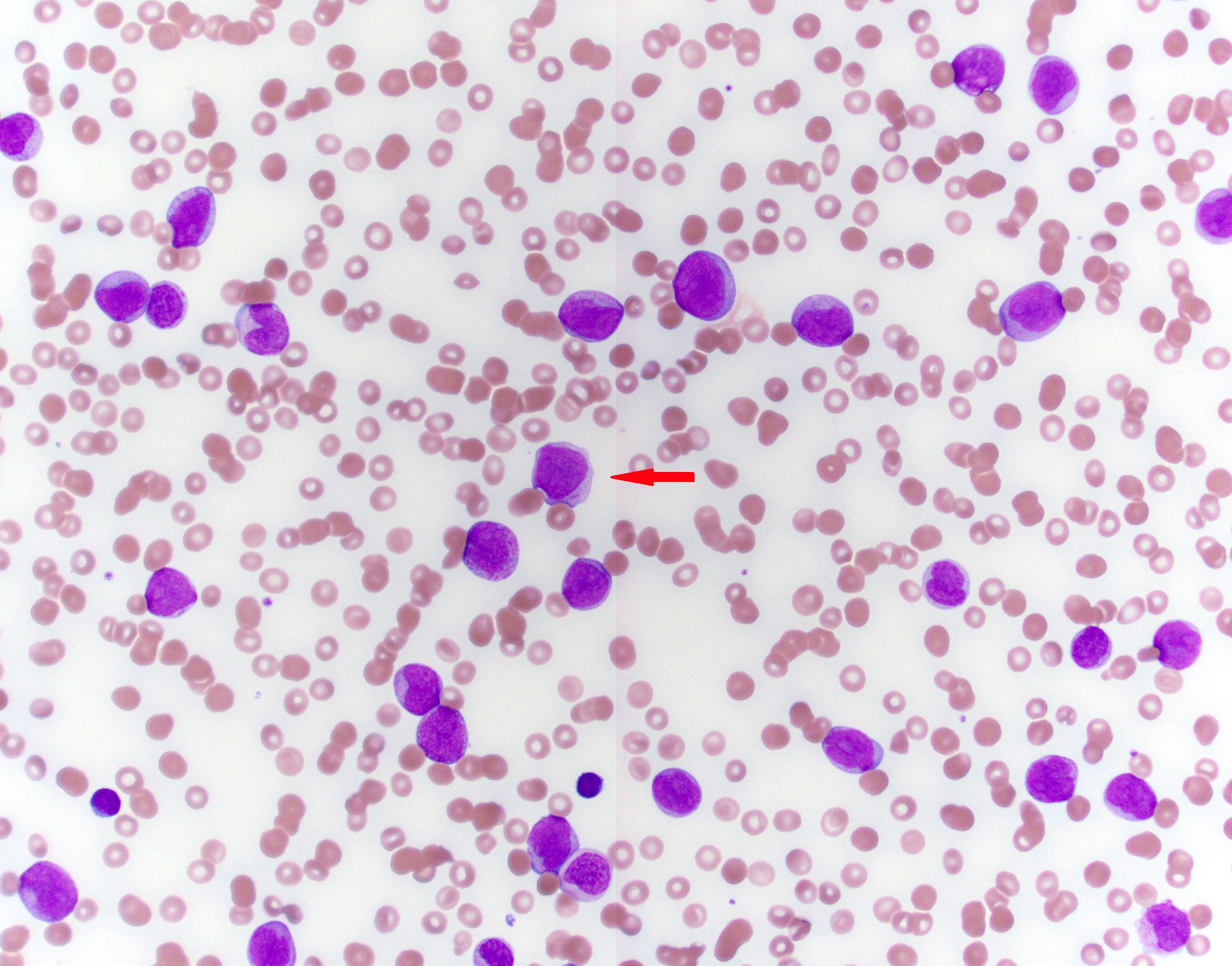
Peripheral smear description
- Varying amount of peripherally circulating blasts with or without maturation
Positive stains
- Depending on the blast immunophenotype, immunohistochemistry for CD34 is typically positive
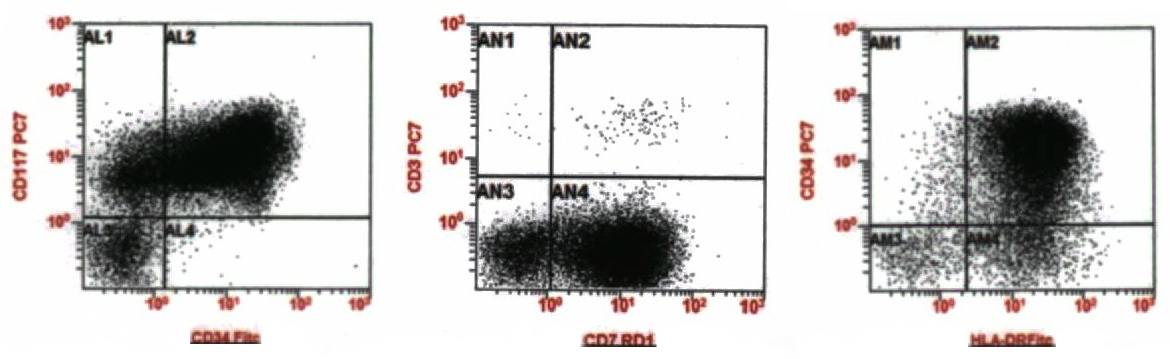
Flow cytometry description
Molecular / cytogenetics description
- Biallelic mutations of CEBPA required for diagnosis, disrupting the N and C terminus of CEBPA (J Clin Oncol 2010;28:570, Blood 2009;113:3088)
- In familial cases, typically 1 mutation is germline, the other is somatic
- CCAAT / enhancer binding protein alpha (CEBPA)
- Mutation types include mutations in the encoding gene and promoter hypermethylation (Haematologica 2011;96:384)
- Normal karyotype in 70% of cases
- FLT3-ITD seen in 5 - 9% of cases
- GATA2 seen in 39% of cases
- Subset of cases have abnormal karyotype, del(9q) is common but does not make a diagnosis of AML with myelodysplasia related changes
- Other reports of del(11q), which should be diagnosed as AML with myelodysplasia related changes
- Patients should be evaluated for familial / germline syndromes (Blood 2015;126:1214)
Sample pathology report
- Bone marrow aspirate, particle clot section and core biopsy:
- Acute myeloid leukemia with biallelic mutations of CEBPA (84.3% blasts by morphology)
- Microscopic description:
- Peripheral blood smear:
- Differential (100 cells)
- Neutrophils: 8%
- Lymphocytes: 7%
- Monocytes: 1%
- Eosinophils: 0%
- Basophils: 0%
- Metamyelocytes: 1%
- Blasts: 83%
- 1 nRBC/100 leukocytes
- Erythrocyte number: decreased
- Erythrocyte morphology: macrocytic, marked anisopoikilocytosis with ovalocytes, moderate polychromasia
- Leukocyte number: increased
- Leukocyte morphology: normal segmentation and granulation of neutrophils; blasts are small to intermediate in size with fine nuclear chromatin, nucleoli and scant basophilic cytoplasm
- Platelet number: decreased
- Platelet morphology: normal
- Differential (100 cells)
- Aspirate:
- Aspiration differential count (300 cells)
- Blasts: 84%
- Promyelocytes: 2%
- Myeloids: 6%
- Erythroids: 4%
- Lymphocytes: 4%
- Specimen quality: adequate
- Spicules: present
- Trilineage hematopoiesis: scant residual
- Erythroid maturation: decreased, full spectrum maturation
- Myeloid maturation: blast morphology is similar to that described in the peripheral blood
- Megakaryocyte morphology: 1 hyperlobated megakaryocyte identified in touch preparation
- Aspiration differential count (300 cells)
- Core biopsy:
- Bone trabeculae: normal
- Cellularity: 80%
- Trilineage hematopoiesis: scant residual
- Erythroid maturation and localization: markedly decreased
- Myeloid maturation and localization: sheets of blasts
- Megakaryocyte number: rare
- Megakaryocyte histotopography: rare hyperlobated megakaryocyte
- Peripheral blood smear:
- Flow cytometry studies:
- Interpretation: increased atypical CD34 positive myeloblasts (partial CD2, CD7 positive, CD11b positive, CD33 negative), representing approximately 89% of the leukocytes, consistent with acute myeloid leukemia
- Chromosome results: 46,XY[20]
- Myeloid mutation panel by NGS result:
- CEBPA c.332_339del, p.Ala111fs (NM_004364.4) variant frequency: 48.3%
- CEBPA c.759dup, p.Lys254fs (NM_004364.4) variant frequency: 47.3%
- TET2 c.2746C>T, p.Gln916* (NM_001127208.2) variant frequency: 48.9%
- TET2 c.4201G>T, p.Glu1401* (NM_001127208.2) variant frequency: 43.6%
- WT1 c.1410_1411ins34, p.Arg471fs (NM_024426.4) variant frequency: 1.0%
Differential diagnosis
- Acute myeloid leukemia with myelodysplasia related changes:
- Present: history of myelodysplastic syndrome or myelodysplastic / myeloproliferative neoplasm; myelodysplastic syndrome related cytogenetic abnormality or multilineage dysplasia
- Absence of prior cytotoxic or radiation therapy for an unrelated disease and recurrent cytogenetic abnormality found in AML
- Acute myeloid leukemia with (other) recurrent genetic abnormalities:
- Through FISH, chromosome analysis and mutation analysis, AML defining recurrent genetic abnormalities can be identified
- This includes t(8;21), inv(16) or t(16;16), PML-RARA, t(9;11), t(6;9), inv(3) or t(3;3), t(1;22), BCR-ABL1, mutated NPM1, mutated RUNX1
- Therapy related myeloid neoplasms:
- Onset of 2 - 10 years post cytotoxic chemotherapy or radiation administered for a previous neoplastic or nonneoplastic disease
- Acute myeloid leukemia, NOS:
- With the absence of pertinent history and recurrent cytogenetic abnormalities, AML is classified as NOS
Additional references
Board review style question #1
Which of the following is true of AML with biallelic CEBPA mutation?
- The karyotype must not show any aberrations
- This has a poor prognosis
- This is typically a therapy related myeloid neoplasm
- This may be germline associated
Board review style answer #1
D. This entity may be germline associated and patients and their families are recommended to be tested for germline mutations. However, this is not always the case, as this leukemia typically presents de novo. The karyotype is most often normal but there are well documented karyotypic abnormalities identified, such as del(9q), which does not influence prognosis.
Comment Here
Reference: AML with biallelic mutation of CEBPA
Comment Here
Reference: AML with biallelic mutation of CEBPA
Board review style question #2
Which of the following is the most frequently encountered flow cytometry immunophenotype for AML with biallelic CEBPA mutation?
- Positive for CD33 and CD117, negative for CD34 and HLA-DR
- Positive for CD41, CD61 and CD42b
- Positive for CD33, CD13 (low expression), CD117, CD123, CD4, CD36, CD64, negative for HLA-DR
- Positive for CD13, CD7, CD34 and HLA-DR
Board review style answer #2
D. Positive for CD13, CD7, CD34 and HLA-DR. This entity typically has expression of CD7 in 50 - 73% of cases. Additionally, the blasts usually show expression for CD34 and HLA-DR. Answer A is describing the immunophenotype typical of acute promyelocytic leukemia. Answer B is describing megakaryocytic lineage markers. Answer C is describing AML with monocytic differentiation.
Comment Here
Reference: AML with biallelic mutation of CEBPA
Comment Here
Reference: AML with biallelic mutation of CEBPA