Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Peripheral smear description | Peripheral smear images | Positive stains | Negative stains | Flow cytometry description | Flow cytometry images | Molecular / cytogenetics description | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Elhodaky M, Aqil B. AML with KAT6A::CREBBP. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bonemarrowneoplasticAMLKAT6ACREBBP.html. Accessed December 22nd, 2024.
Definition / general
- Acute myeloid leukemia (AML) with t(8;16)(p11.2;p13.3) / KAT6A::CREBBP is a rare AML with recurrent cytogenetic abnormality
Essential features
- Detection of KAT6A::CREBBP or t(8;16)(p11.2;p13.3) is required by cytogenetic or molecular studies for diagnosis
- Should not fulfill the diagnostic criteria for AML with defining genetic abnormalities, AML myelodysplasia related (AML MR), AML postcytotoxic therapy (AML pCT) or mixed phenotype acute leukemia (MPAL)
- Morphologically, blasts often show myelomonocytic or monocytic differentiation with some demonstrating erythrophagocytosis
- Blasts have characteristic immunophenotype as detected by flow cytometry
Terminology
- Acute myeloid leukemia with t(8;16)(p11;p13)
ICD coding
Epidemiology
- Incidence is ~0.2% of AMLs
- AML with t(8;16)(p11;p13) is a rare entity that may arise as a congenital, de novo or therapy related neoplasm, status postchemotherapy or radiation therapy (RT) (Leukemia 2008;22:1567, Leukemia 2009;23:934)
- Presents in all the age groups ranging from neonatal period in children to adults, with a median age of 60 years
Sites
- Peripheral blood, bone marrow and occasionally extramedullary disease (leukemia cutis) (Leukemia 2009;23:934, Ann Hematol 2019;98:1149, Leuk Res 2013;37:32)
Pathophysiology
- t(8;16) results in a fusion of the KAT6A gene located on chromosome 8p11 with the CREBBP gene located on chromosome 16p13 (Nat Genet 1996;14:33)
Etiology
- Adult cases are usually therapy related whereas pediatric cases are often de novo (Leukemia 2009;23:934, Leuk Res 2013;37:32, Blood 2013;122:2704, Am J Clin Pathol 2022;157:701)
Clinical features
- Presents with bleeding tendency and disseminated intravascular coagulopathy (Leukemia 2009;23:934, Blood 2013;122:2704, Leuk Res 2013;37:32, Leukemia 2008;22:1567, Am J Clin Pathol 2022;157:701)
Diagnosis
- Presence of ≥ 20% myeloid blasts in the bone marrow or peripheral blood per WHO 2022 but the blast requirement for the diagnosis is > 10% according to the 2022 International Consensus Classification (Virchows Arch 2023;482:27)
- Detection of KAT6A::CREBBP / t(8;16)(p11.2;p13.3)
- Not meeting the diagnostic criteria for AML with other defining genetic abnormalities, AML MR, AML pCT or MPAL
Laboratory
- Elevated white blood cell count with increased blast percentages (≥ 10% per ICC; ≥ 20% per WHO 5th edition) (Virchows Arch 2023;482:27, Am J Clin Pathol 2022;157:701)
- Disseminated intravascular coagulation
Prognostic factors
- Overall poor prognosis in adults
- Spontaneous remission has been reported in children diagnosed in the early neonatal period; some cases with spontaneous remission though subsequently relapse (Blood 2013;122:2704)
- Allogeneic stem cell transplantation has shown a significantly better outcome in adults with KAT6A::CREBBP without prior cytotoxic therapy or myeloid neoplasm (Br J Haematol 2021;192:832)
Case reports
- Full term female infant was noted at birth to have numerous blue maculopapular skin lesions over the body and found to have occasional circulating blasts (Pediatr Blood Cancer 2017;64:e26450)
- 23 year old man presented with pain and night sweats; circulating blasts were noted along with thrombocytopenia (Blood 2016;128:314)
- Woman in mid 40s with a history of celiac disease presented with shortness of breath and was found to have 16% circulating blasts (BMJ Case Rep 2023;16:e253812)
Treatment
- Initial studies had reported median overall survival (OS) of 4.7 - 8.5 months in AML with t(8;16) on chemotherapy (Leuk Res 2013;37:32)
- With the introduction of stem cell transplant (SCT), OS relatively improved to 18.2 months and was described even better in patients with de novo AML or noncomplex karyotype (Ann Hematol 2019;98:1149, Br J Haematol 2021;192:832)
- Cases with de novo AML and t(8;16) who received allo-SCT in first clinical remission (CR) achieved improved rates, suggesting that an early transplant in first CR is likely beneficial in these patients (Ann Hematol 2019;98:1149, Br J Haematol 2021;192:832)
- Venetoclax in combination with hypomethyling agents has shown a good response (Blood 2019;133:7)
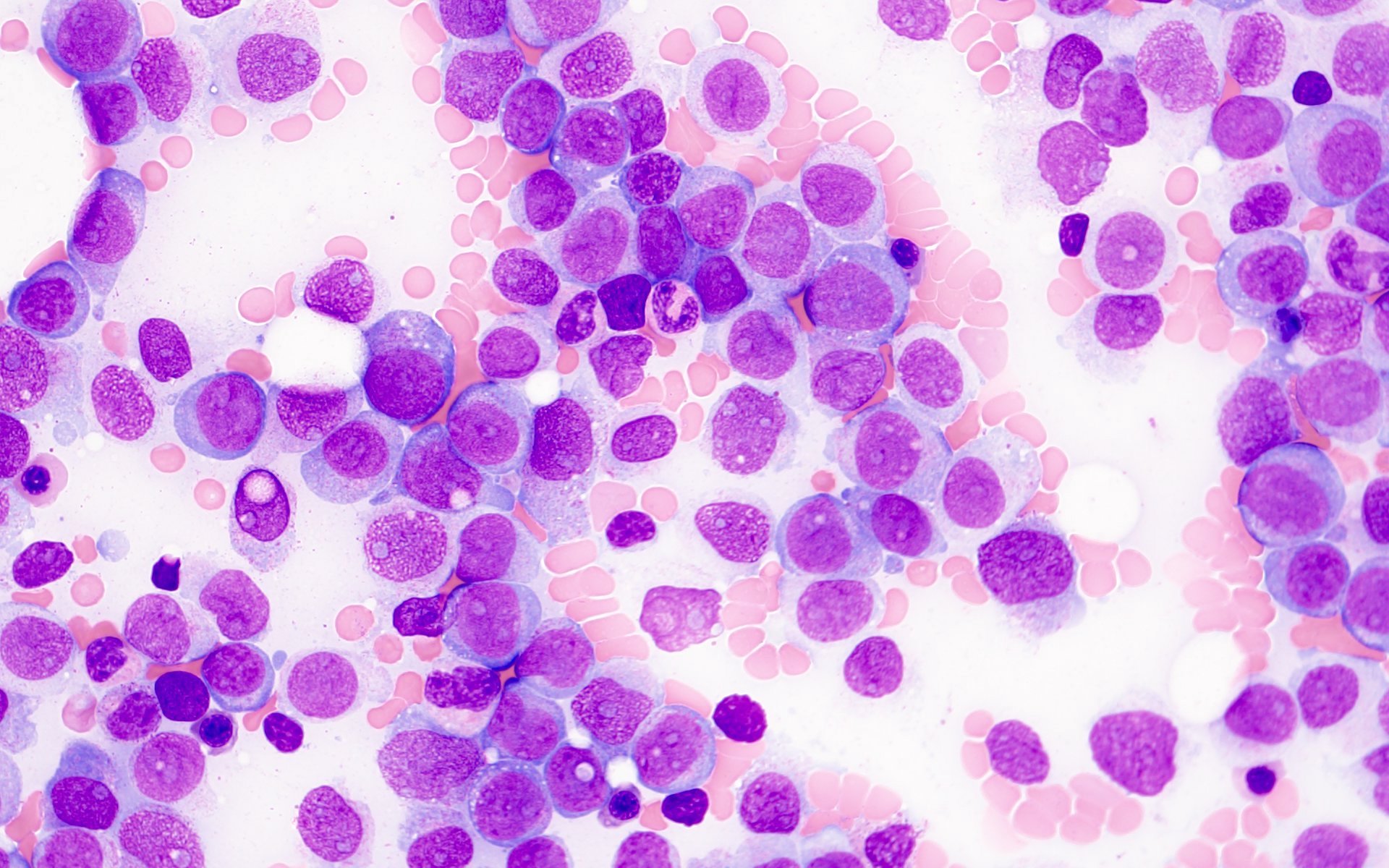
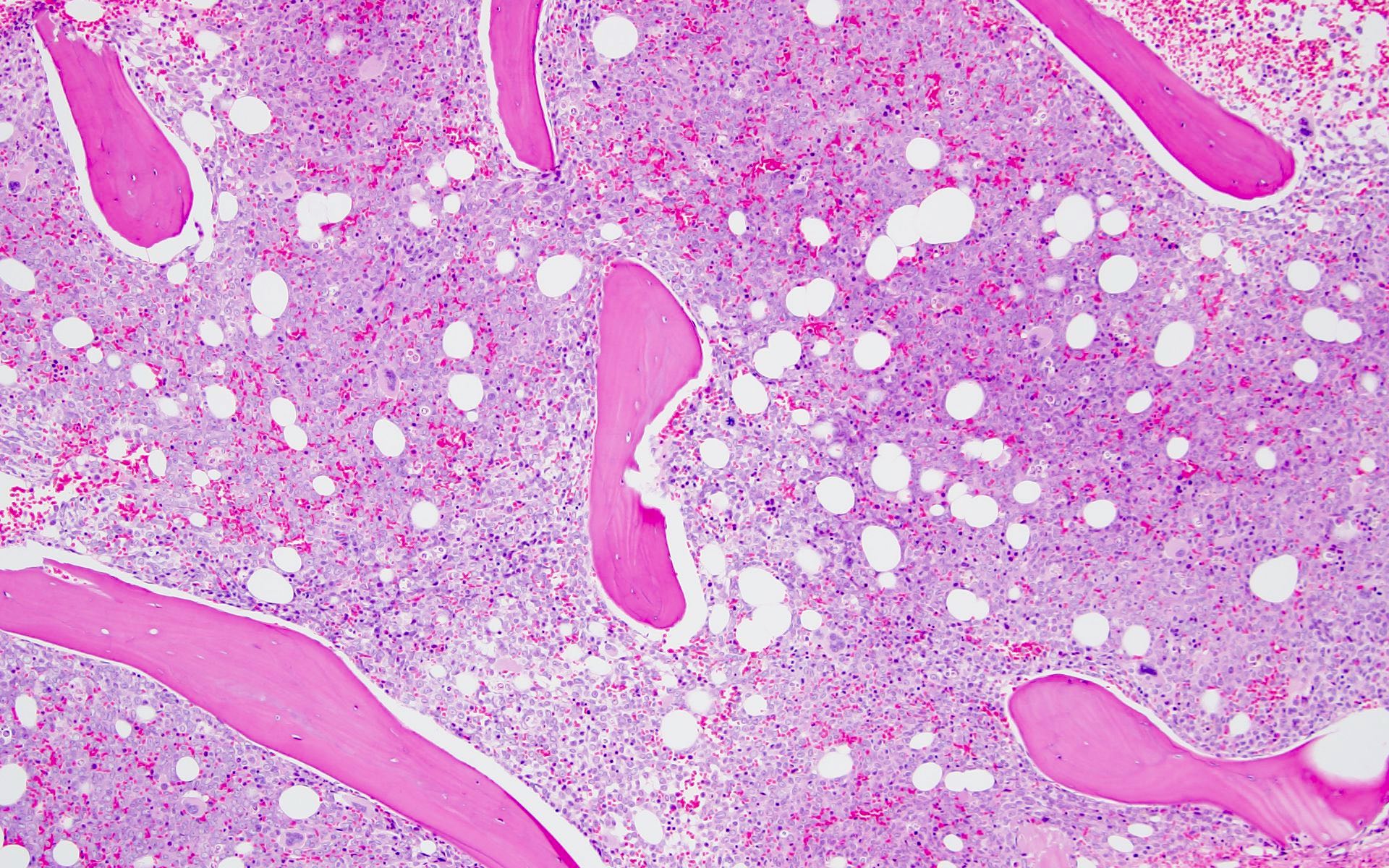
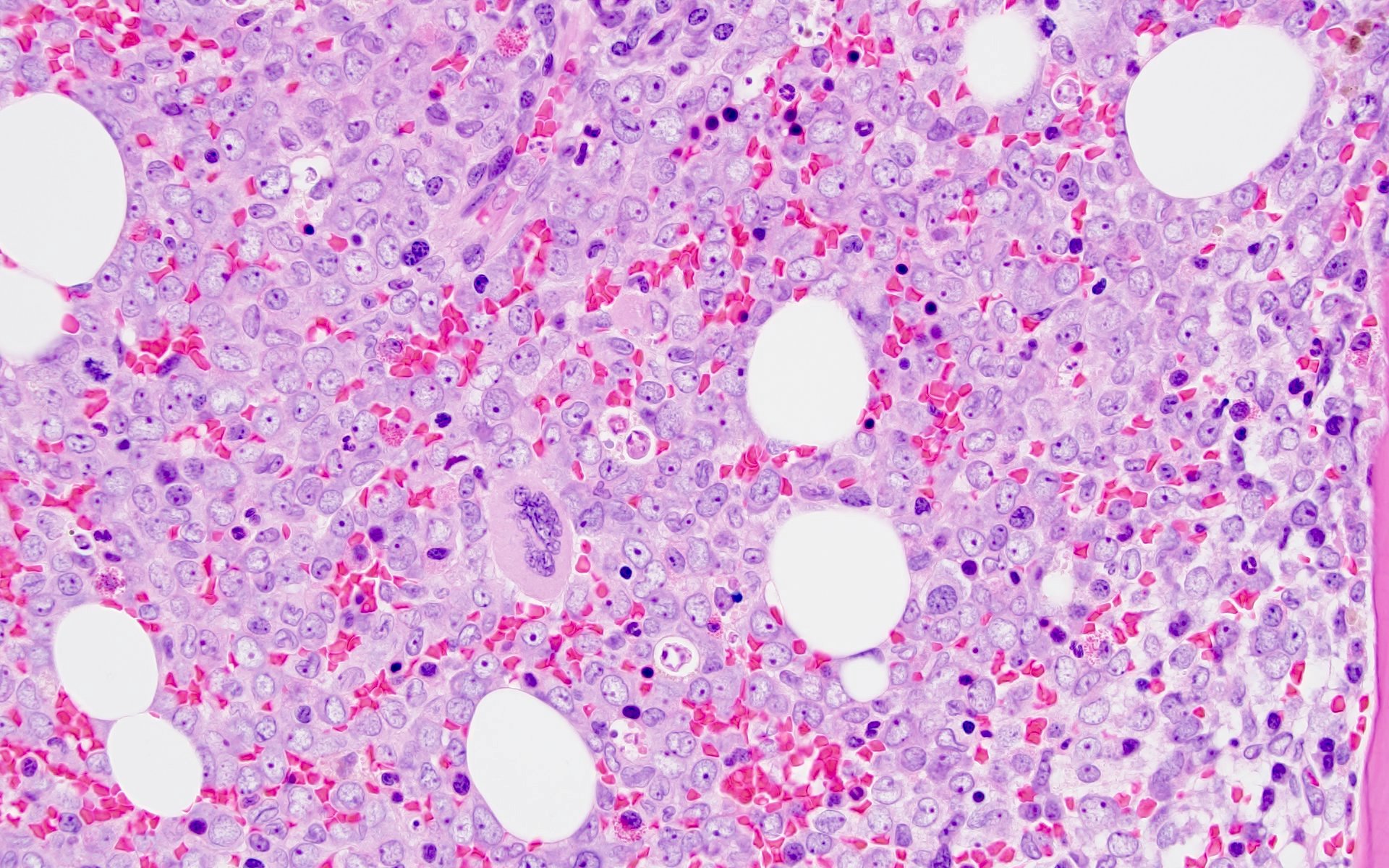
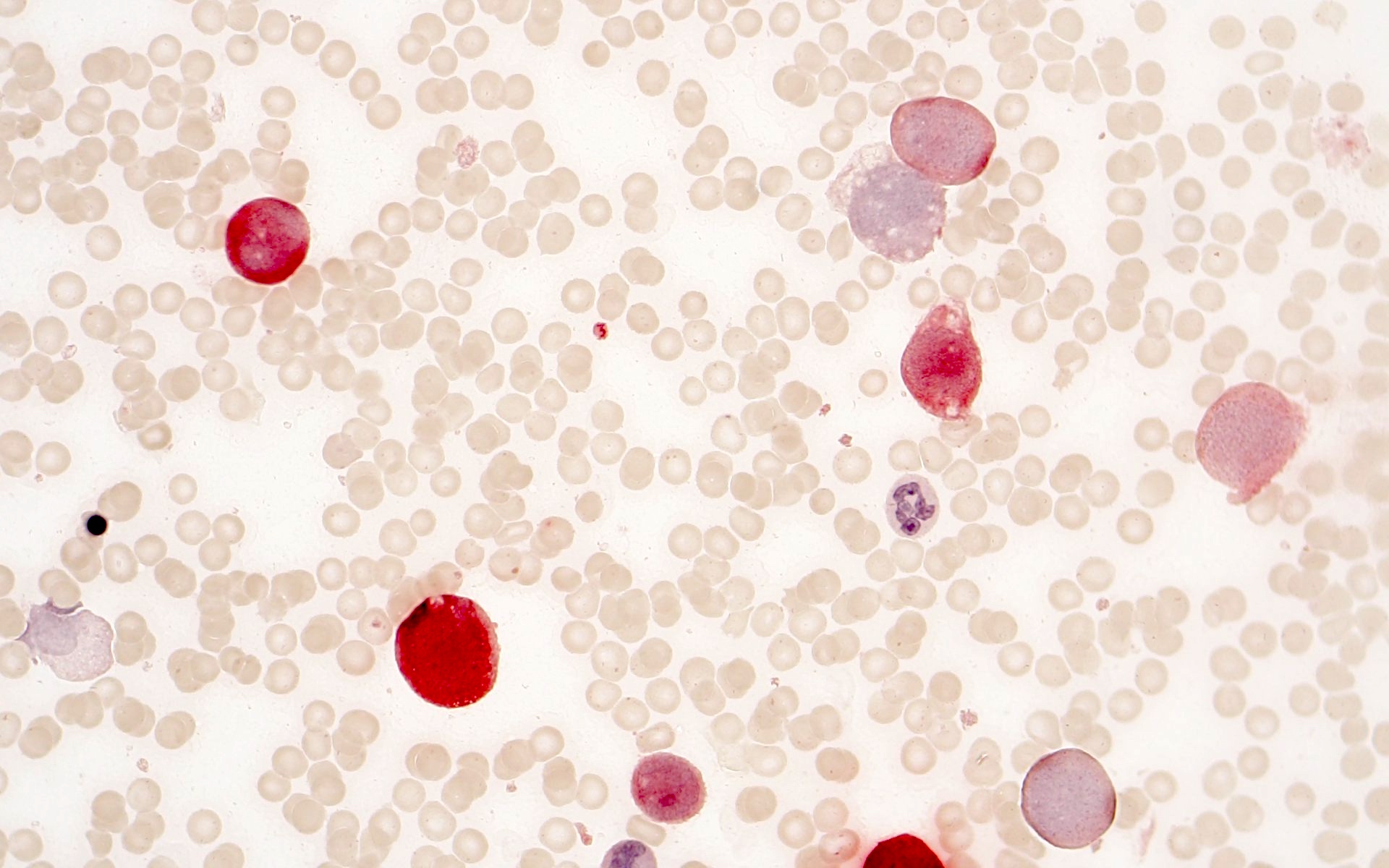
Microscopic (histologic) description
- Morphologically, blasts often show myelomonocytic or monocytic differentiation (Clin Case Rep 2014;2:333)
- Blasts are positive for myeloperoxidase (MPO) and alpha naphthyl butyrate esterase (ANB)
- Erythrophagocytosis by the leukemic blasts is seen in some cases (Leukemia 2009;23:934, Clin Case Rep 2014;2:333)
Microscopic (histologic) images
Cytology description
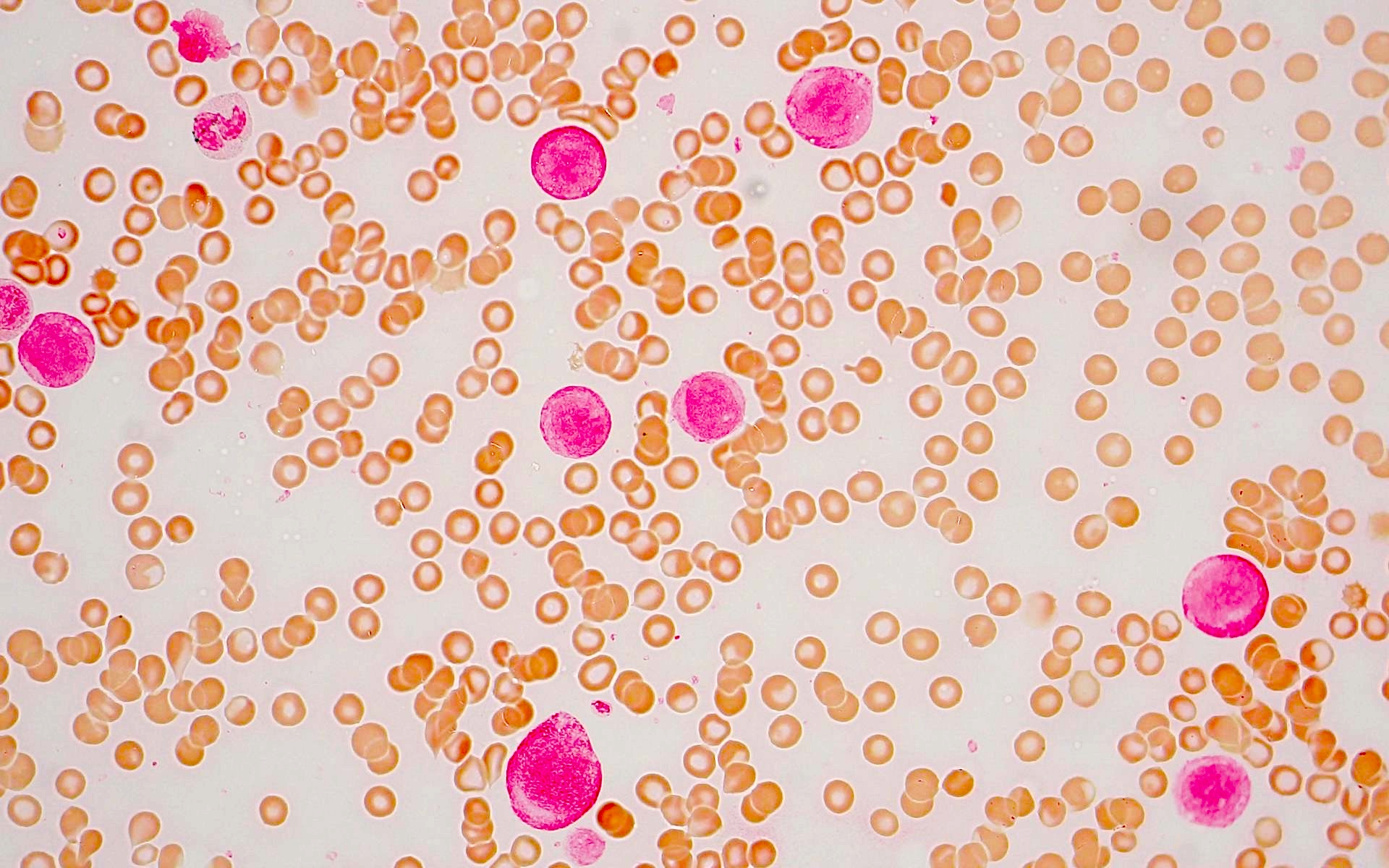
- Blasts are medium to large in size with irregular / folded nuclei, fine chromatin, prominent nucleoli and moderate to abundant cytoplasm
- Some blasts show cytoplasmic granules or vacuolization
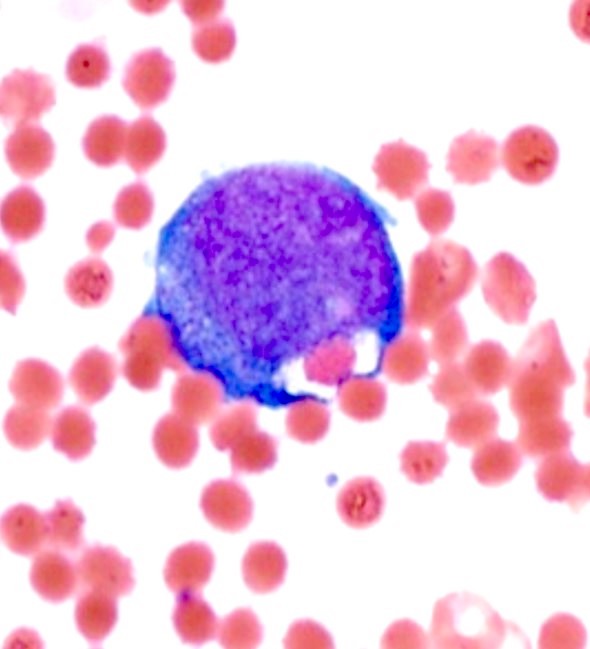
Peripheral smear description
- Blasts often show myelomonocytic or monocytic differentiation with prominent cytoplasmic granulation with or without Auer rods
Peripheral smear images
Positive stains
Negative stains
Flow cytometry description
- Distinct phenotypic profile characterized by
- Distinguishing from maturing myeloid elements is possible by the brighter expression of CD33 on the leukemic blasts (Am J Clin Pathol 2022;157:701)
Molecular / cytogenetics description
- t(8;16) leads to a fusion product involving KAT6A and CREBBP (Br J Haematol 2020;190:133)
- Recurrent mutations are identified in ASXL1, FLT3, TP53, RUNX1, EZH2, SRSF2, CBL and TET2 (Br J Haematol 2021;192:832, Am J Clin Pathol 2022;157:701)
- Concomitant cytogenetic abnormalities and even complex karyotypes can be seen (Ann Hematol 2019;98:1149)
Sample pathology report
- Peripheral blood, bone marrow aspirate and left posterior iliac crest bone marrow core biopsy:
- Acute myeloid leukemia with KAT6A::CREBBP / t(8;16)(p11;p13) extensively involving a hypercellular bone marrow (see comment)
- Comment: The blasts are positive for MPO and CD68 indicating a myelomonocytic lineage. Some of the blasts show erythrophagocytosis.
- Peripheral blood smear:
- Peripheral blood smear shows normochromic normocytic anemia with mild anisopoikilocytosis. Neutrophils are decreased with unremarkable morphology. There are occasional medium to large blasts with irregular / folded nuclei, fine chromatin, prominent nucleoli and moderate to abundant granular cytoplasm. Rare Auer rods are seen. The platelets are markedly decreased.
- Bone marrow aspirate smear / touch preparation:
- Bone marrow aspirate smears and touch preparations are cellular and adequate for interpretation. The myeloid series show left shifted maturation with increased blasts with similar morphology as described in the peripheral blood smear. The erythroid precursors show progressive maturation. Megakaryocytes are decreased.
- Bone marrow core biopsy (decalcified) / particle clot:
- Unilateral bone marrow core biopsy is hypercellular for age (80 - 90% cellular) with extensive replacement by blasts that are medium to large in size with fine chromatin and pinpoint nucleoli. The erythroid precursors show progressive maturation. Megakaryocytes are decreased but show unremarkable morphology.
Differential diagnosis
- AML with monocytic differentiation:
- Acute promyelocytic leukemia:
- Presentation with coagulopathy (DIC) and blasts with prominent cytoplasmic granulation can be similar to AML with KAT6A::CREBBP but phenotype and molecular studies are helpful in resolving this differential
- AML with KMT2A rearrangement:
- Extramedullary involvement is seen in 33% of adult patients
- Morphology is similar to AML with KAT6A::CREBPP with myelomonocytic or monocytic blasts
- Erythrophagocytosis is not seen
- Blasts show monocytic differentiation with expression of CD33, CD65, CD4, CD15, HLA-DR, lysozyme and variable CD34 and CD117
- Presence of KMT2A rearrangement with any one of the described > 80 fusion partners
- AML with NPM1 mutation:
- Morphology may be similar to AML with KAT6A:CREBPP with myelomonocytic or monocytic blasts
- Blast with cup shaped nuclear morphology is considered to be highly specific for this entity
- Multilineage dysplasia is commonly seen
- Immunophenotype can be quite variable; however, the majority of blasts predominantly are negative for CD34 but with expression of CD117 and CD33
- NPM1 mutation detection is an essential diagnostic criterion
- Concomitant FLT3 internal tandem duplication (ITD) mutation evaluation is important for risk assessment
- Patients with AML with NPM1 mutation along with FLT3 ITD mutation have poorer prognosis than AML with NPM1 mutation alone
Additional references
Board review style question #1
Board review style answer #1
B. CD34-, CD117-, CD13+, CD33+, CD11b+, CD64+. Acute myeloid leukemia with t(8;16)(p11.2;p13.3) / KAT6A::CREBBP shows blasts that are negative for CD34 and CD117 and positive for CD13, CD33, CD11b and CD64, among others. Answer C is incorrect because blasts are CD34- with myelomonocytic markers expression. Answer A is incorrect because blasts are usually CD34- and express monocytic markers (CD14, CD11b and CD64). Answer D is incorrect because blasts are usually CD34- and CD117-.
Comment Here
Reference: AML with KAT6A::CREBBP
Comment Here
Reference: AML with KAT6A::CREBBP
Board review style question #2
A 42 year old man with no significant past medical history presented with fatigue and weight loss for 2 months. Laboratory workup showed leukocytosis (WBC 23.0 K/uL), anemia (8.0 g/dL) and occasional circulating blasts in peripheral blood. The bone marrow biopsy showed > 20% myeloid blasts with coexpression of CD64 and partial CD11b. This finding (see image) is characteristically seen in ~75% cases of which disease?
- AML with CEBPA
- AML with DEK::NUP214
- AML with KAT6A::CREBBP
- AML with NPM1
Board review style answer #2
C. AML with KAT6A::CREBBP. AML with KAT6A::CREBBP has myelomonocytic / monocytic differentiation with ~75% cases demonstrating erythrophagocytosis. Answers A, B and D are incorrect because they do not show significant erythrophagocytosis; however, AML with t(6;9) / DEK::NUP214 is associated with basophilia and multilineage dysplasia.
Comment Here
Reference: AML with KAT6A::CREBBP
Comment Here
Reference: AML with KAT6A::CREBBP