Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Etiology | Clinical features | Diagnosis | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Frozen section description | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Alexiev B, Jennings L. CIC rearranged sarcoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/boneCICDUX4.html. Accessed November 27th, 2024.
Definition / general
- Undifferentiated round cell sarcoma with capicua-double homeobox 4 (CIC-DUX4) gene fusion (Am J Surg Pathol 2017;41:941)
Essential features
- Aggressive sarcoma arising predominantly in soft tissues of children and young adults (Am J Surg Pathol 2017;41:941, Histopathology 2016;69:624)
- Unique clinical presentation, morphology, immunoprofile and genetic signature that are different from Ewing sarcoma
- Round to ovoid cytomorphology with a high nuclear to cytoplasmic ratio
- Expression of CD99, WT1, DUX4, ETV4 (Am J Surg Pathol 2016;40:313, Mod Pathol 2016;29:1324, Am J Surg Pathol 2017;41:423)
- CIC-DUX4 fusion from either a t(4;19)(q35;q13) or a t(10;19)(q26;q13) translocation (Am J Surg Pathol 2017;41:941)
Terminology
- Undifferentiated round cell sarcoma with CIC-DUX4 fusion
ICD coding
- ICD-O: 8803/3 - Small cell sarcoma
Epidemiology
- Wide age range (6 - 81 years, with a mean of 32 years) (Am J Surg Pathol 2017;41:941)
- Slight predominance in males
Sites
- Trunk (Am J Surg Pathol 2017;41:941)
- Distal extremities (Am J Surg Pathol 2017;41:941)
- Other sites include viscera, head and neck, upper extremities, bone (Histopathology 2016;69:624, Am J Surg Pathol 2016;40:1298)
Etiology
- CIC-DUX4 fusion oncoprotein potentiates the transcriptional activity of CIC and activates the expression ETV1/4/5, which is a member of the E26 transformation specific (ETS) family of transcription factors (Sci Rep 2020;10:684)
- MYC amplification in majority of cases (Mod Pathol 2015;28:57)
Clinical features
- Most patients present with a rapidly growing, solitary mass in deep or superficial soft tissue (Am J Surg Pathol 2017;41:941)
- Metastatic disease may be present at initial presentation (Histopathology 2016;69:6247)
Diagnosis
- Tissue sampling is the gold standard for a definitive diagnosis
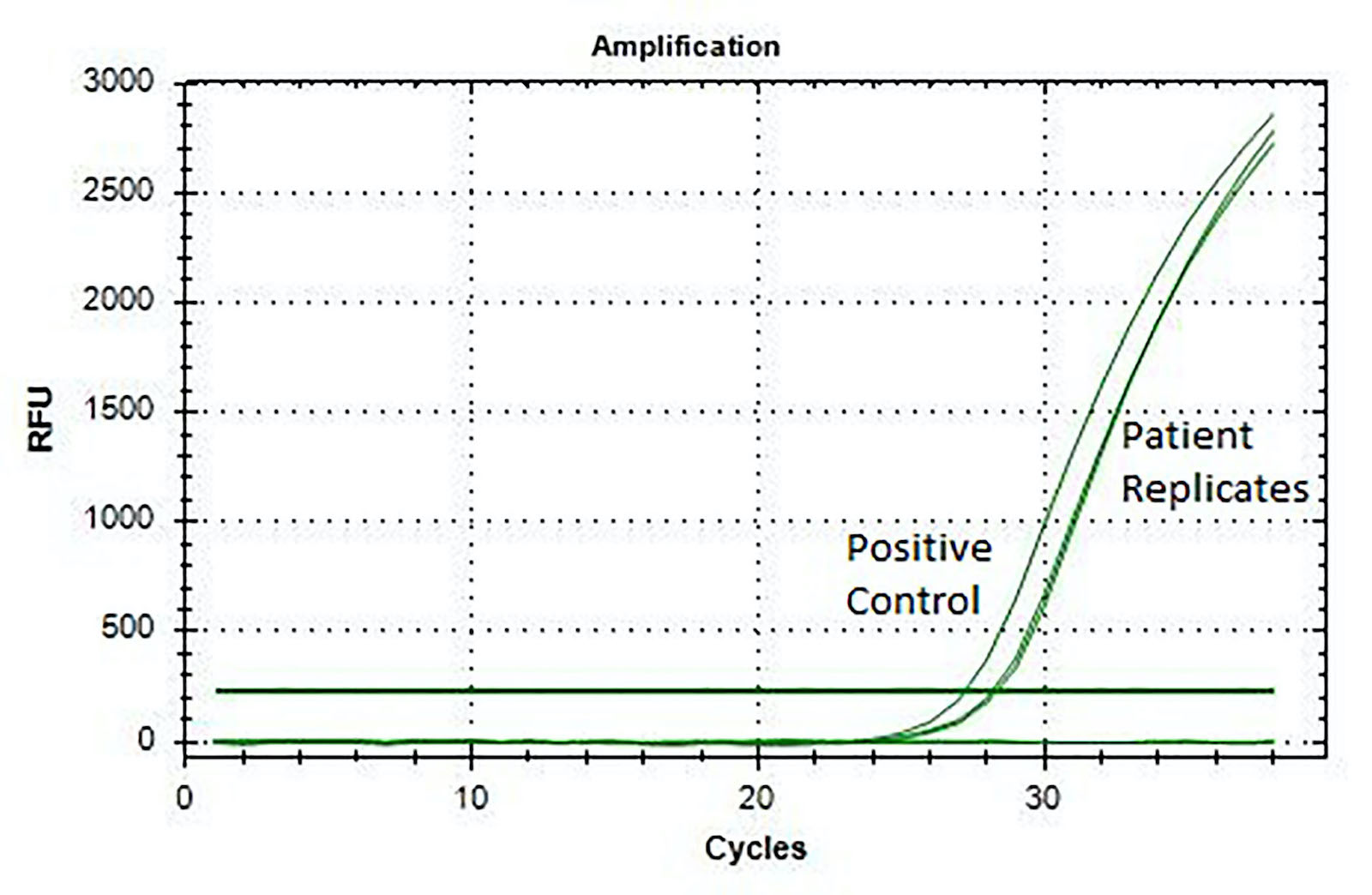
- Fluorescence in situ hybridization (FISH) (Histopathology 2017;71:461)
- Reverse transcriptase polymerase chain reaction (RT-PCR) (J Clin Pathol 2017;70:697, Histopathology 2017;71:461)
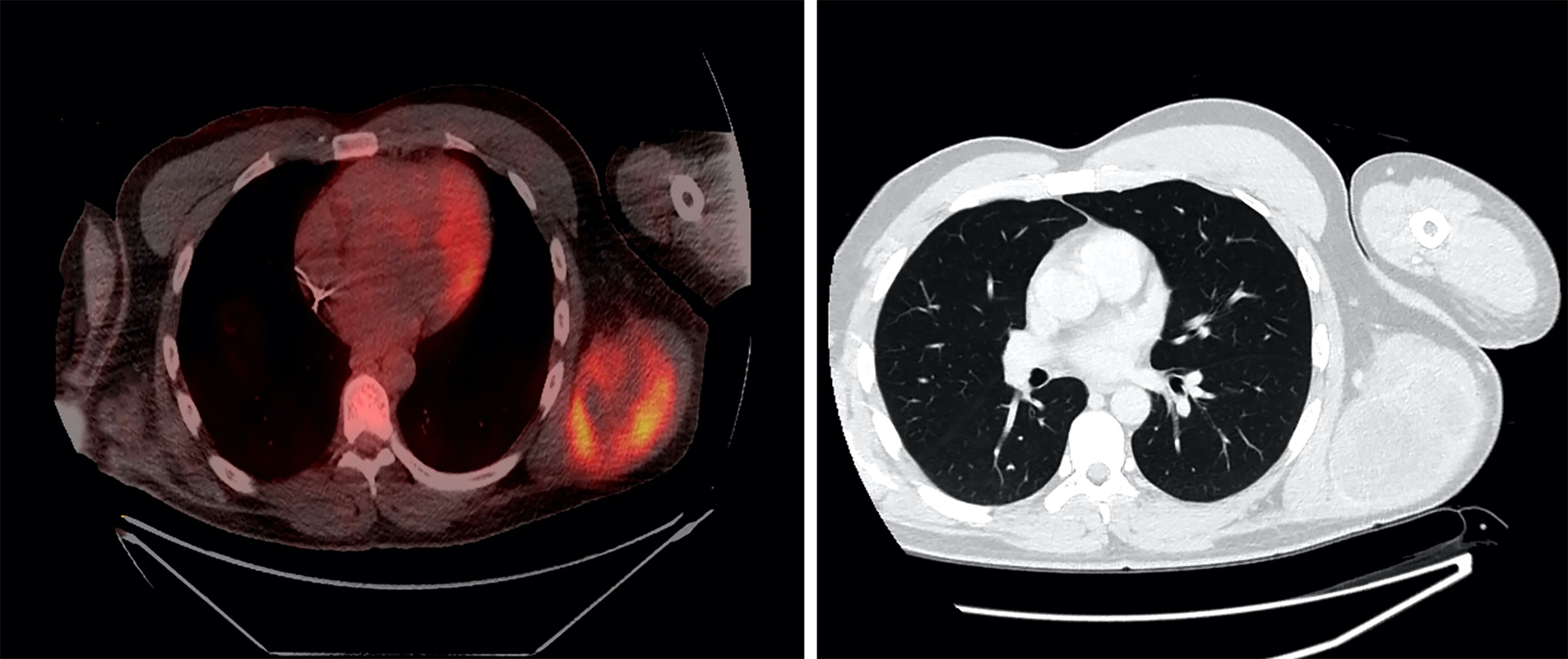
Radiology description
- Large heterogeneous appearing hypermetabolic mass on PET / CT
Prognostic factors
- CIC-DUX4 fusion positive sarcomas have a significantly unfavorable outcome compared to Ewing sarcoma (Hum Pathol 2019;86:57)
- High metastatic rate, mainly to the lung (Histopathology 2016;69:624)
- 43% 5 year overall survival (Am J Surg Pathol 2017;41:941)
Case reports
- 12 year old boy with kidney mass (Pediatr Dev Pathol 2018;21:406)
- 13 year old girl with thigh mass (Diagn Cytopathol 2018;46:958)
- 24 year old man with a posterior mediastinal mass and a 69 year old woman with a gluteal mass (Cancer Cytopathol 2016;124:350)
- 38 year old man with deep abdominal wall mass (Pathol Res Pract 2017;213:1315)
- 40 year old man with mass in deep soft tissues in his thigh (Pathol Res Pract 2015;211:877)
Treatment
- Patients treated with neoadjuvant chemotherapy showed an inferior survival compared to patients managed by surgery first (Am J Surg Pathol 2017;41:941)
- Further studies are needed to clarify the role of chemotherapy in CIC rearranged sarcomas (Genes Chromosomes Cancer 2012;51:207)
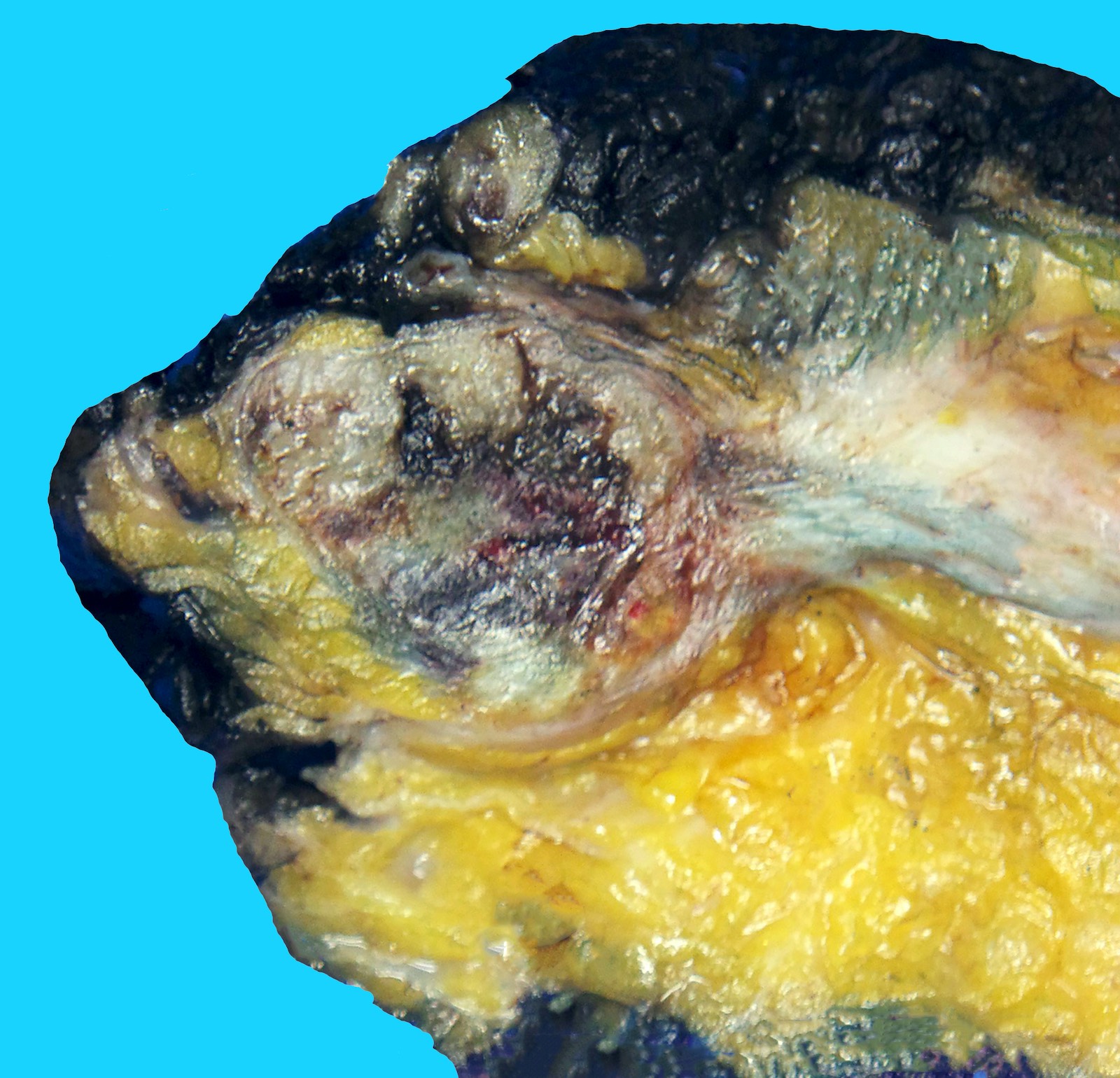
Gross description
- Large mass with fleshy cut surface (Am J Surg Pathol 2004;28:523)
- Necrosis and hemorrhage
- Cystic changes
Frozen section description
- Diagnosis of CIC-DUX4 sarcoma on a small tissue fragment without molecular studies would be challenging
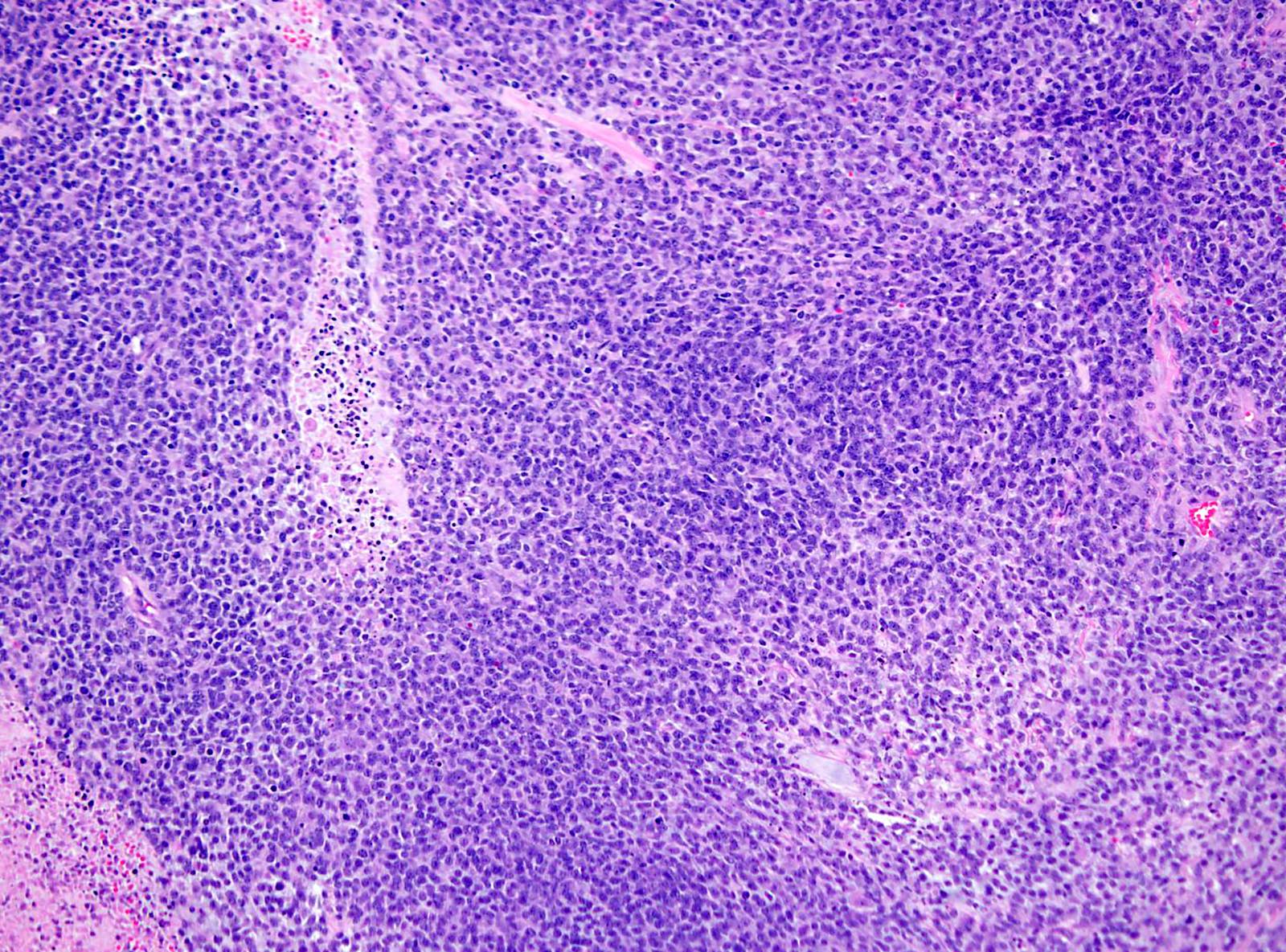
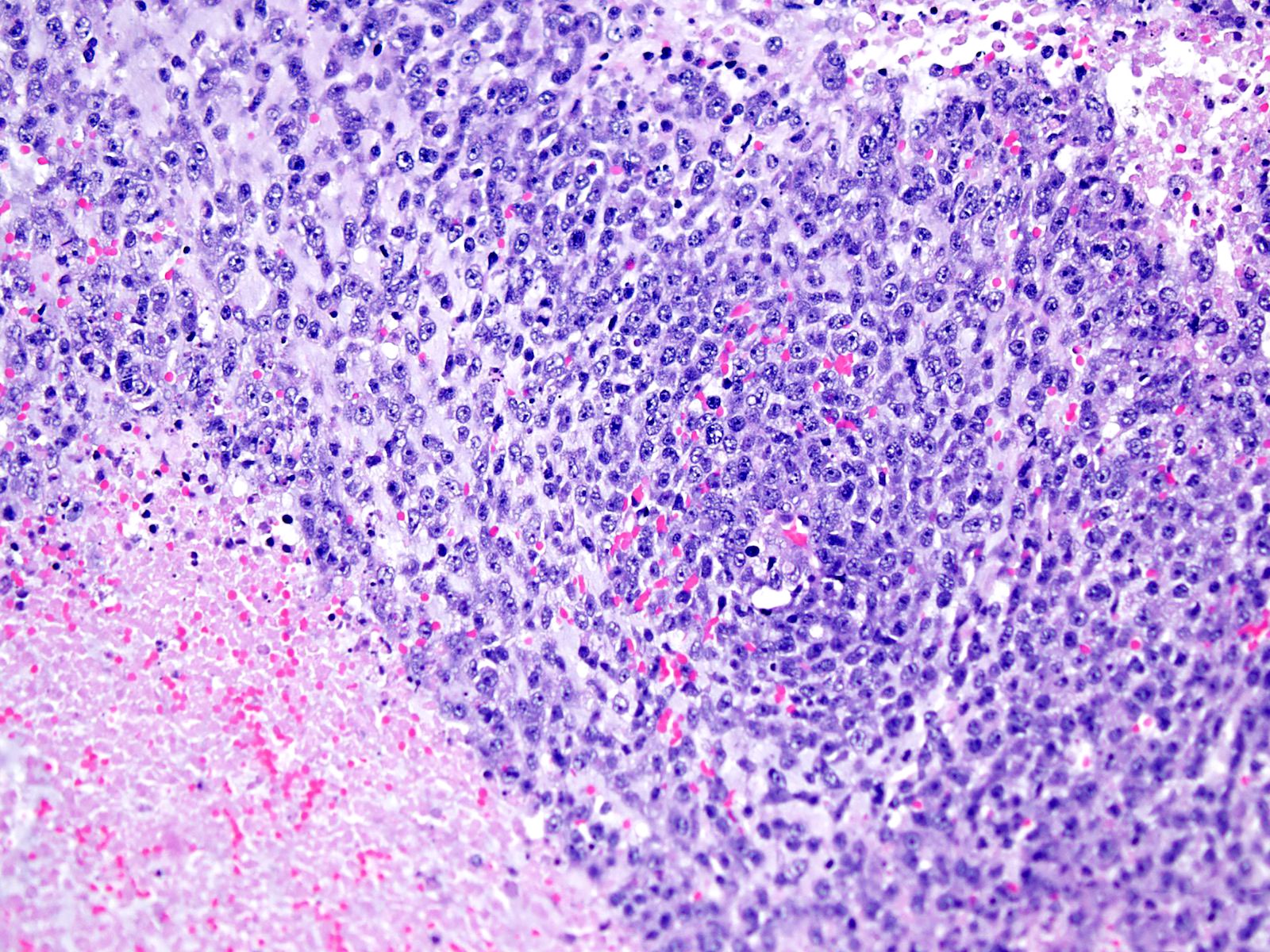
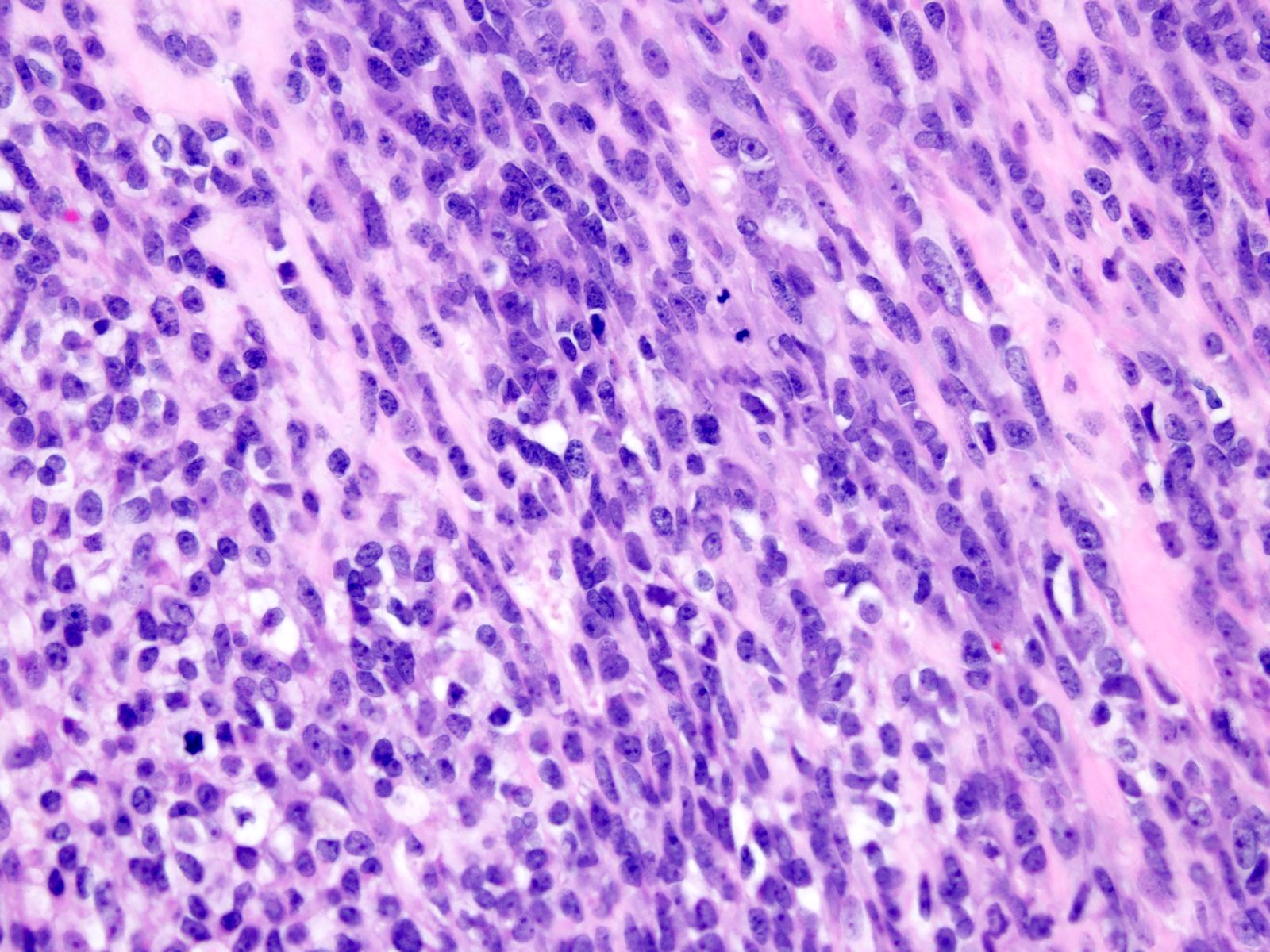
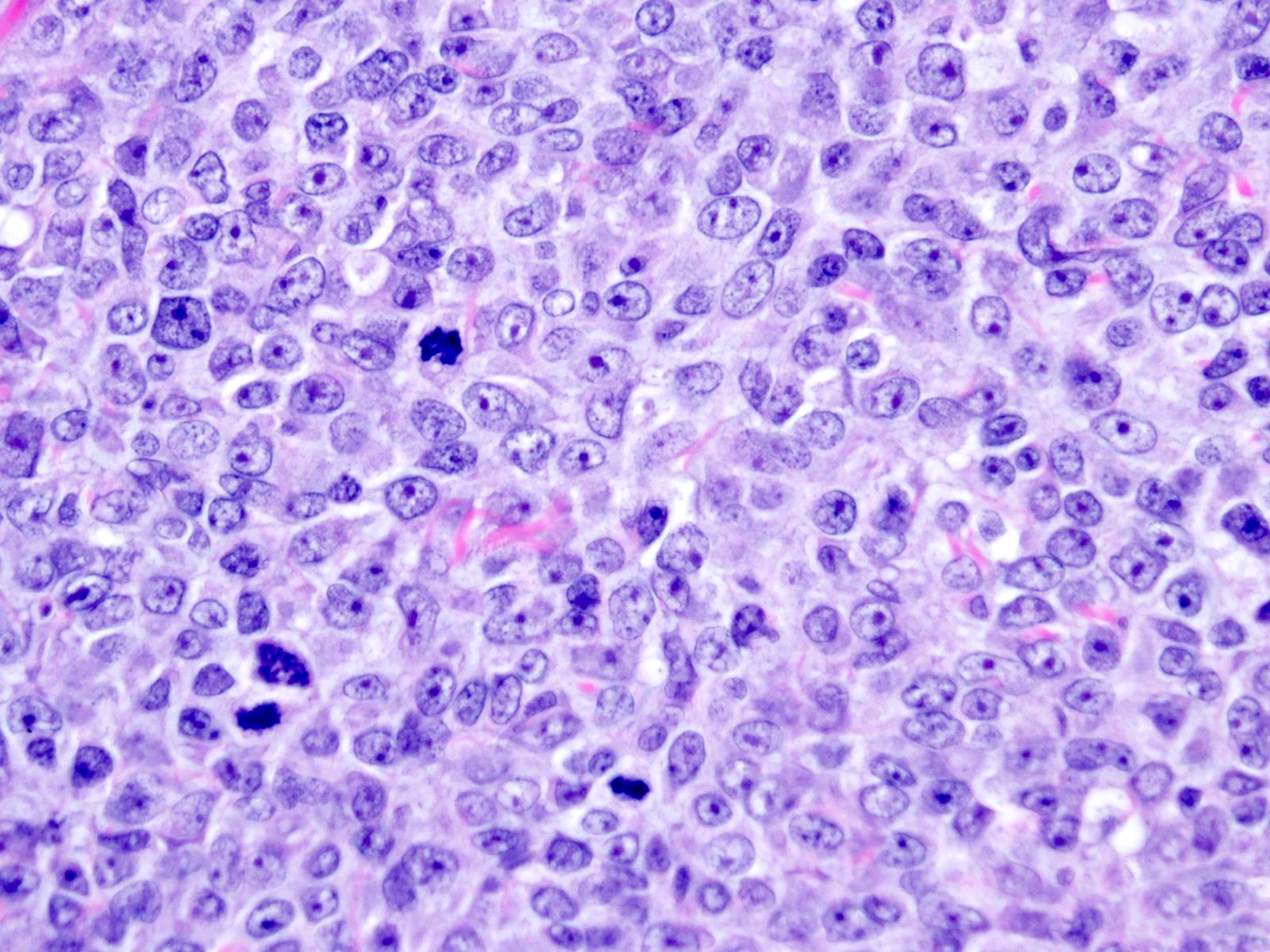
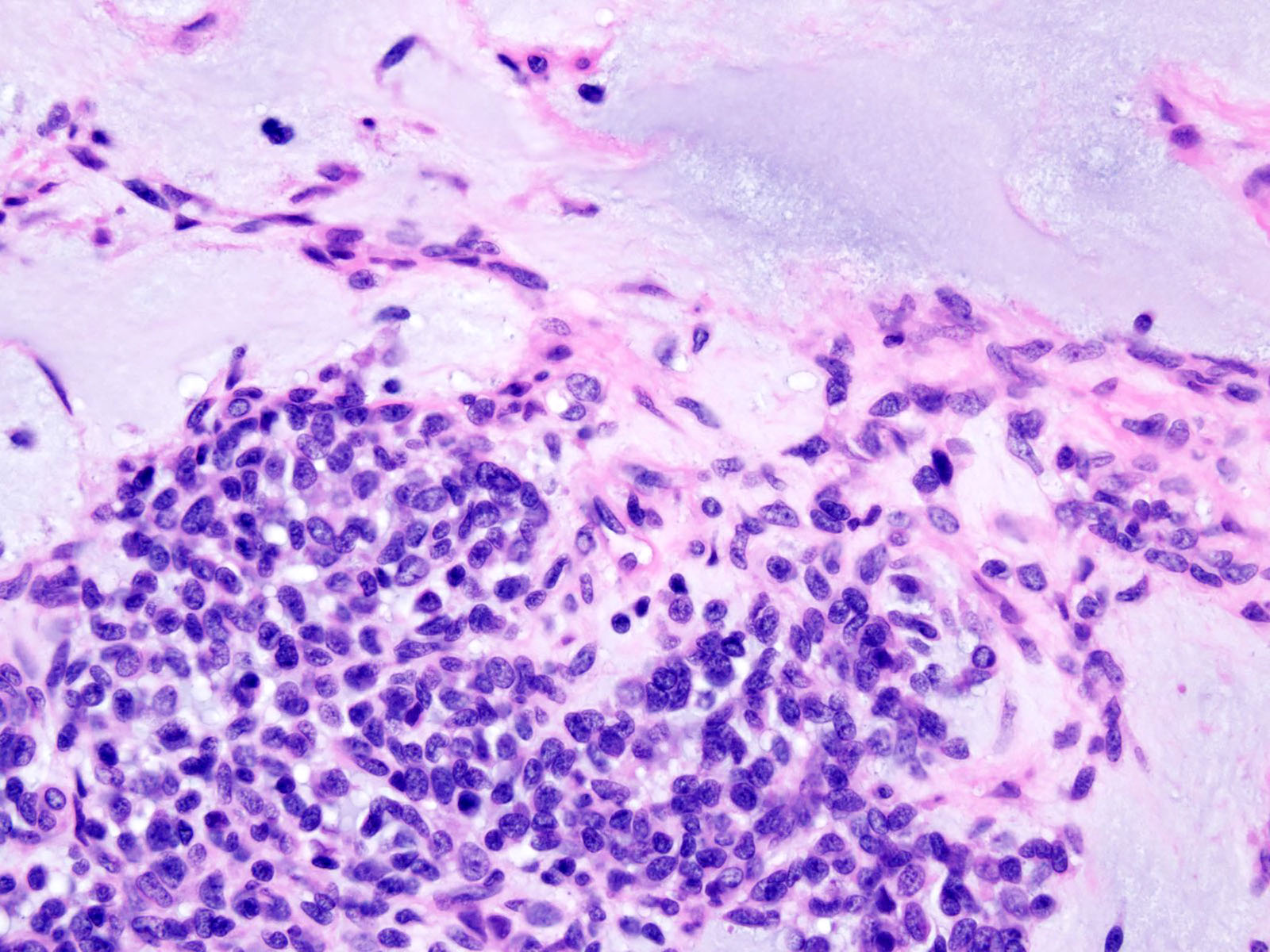
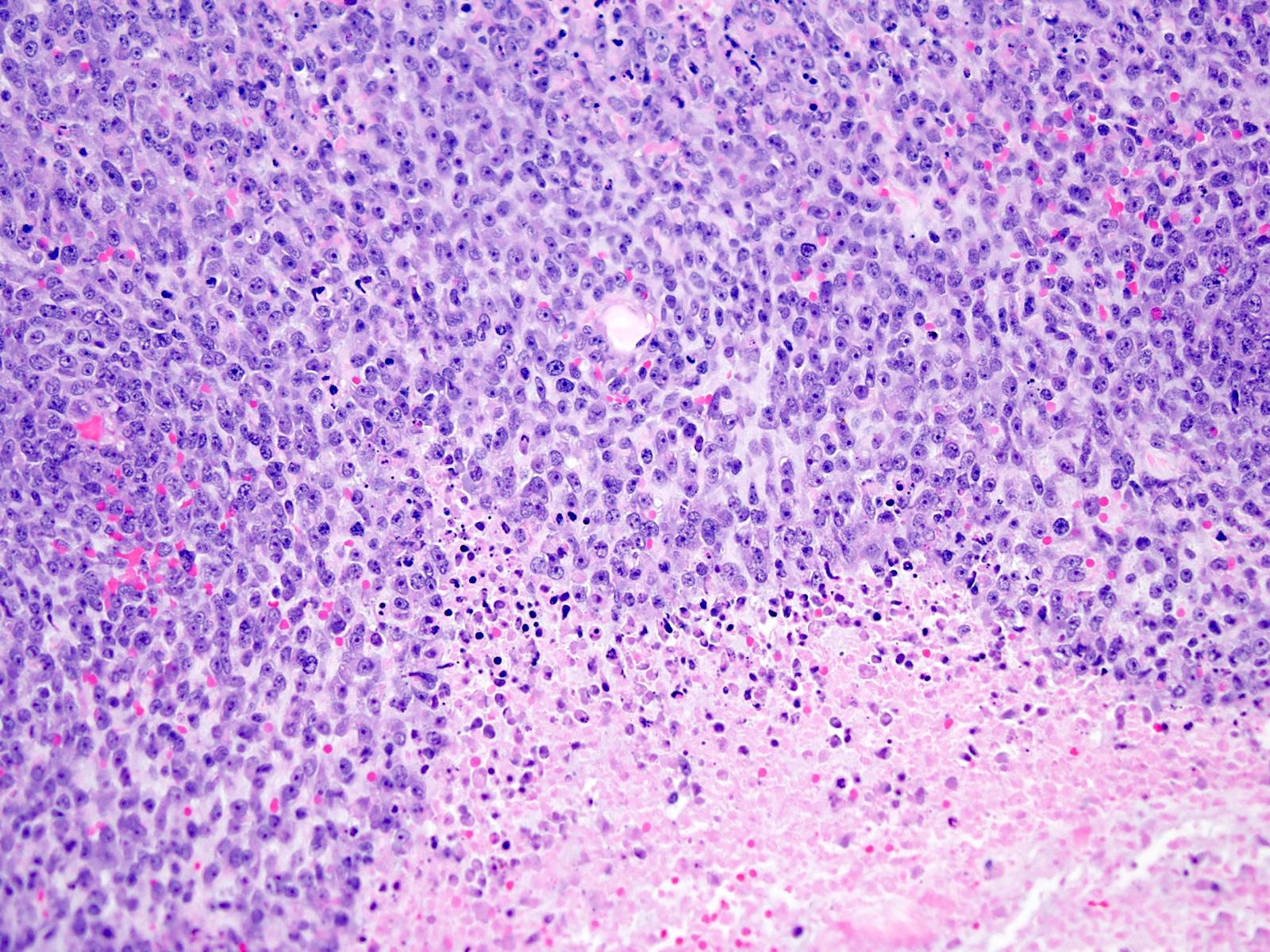
Microscopic (histologic) description
- Solid and often nodular growth pattern
- Less common architectural patterns include fascicular arrangement of neoplastic cells and reticular growth (Am J Surg Pathol 2004;28:523)
- Small round or ovoid cells with amphophilic or lightly eosinophilic cytoplasm (Genes Chromosomes Cancer 2012;51:207, Mod Pathol 2016;29:1523)
- Round or oval nuclei with variable chromatin patterns and small to medium sized nucleoli
- Higher degree of heterogeneity in nuclear shape and size, compared with the rather monomorphic appearance seen in Ewing sarcoma family of tumors (Genes Chromosomes Cancer 2012;51:207)
- Some cases may display a predominant spindle cell, epithelioid or plasmacytoid / rhabdoid morphology (Am J Surg Pathol 2017;41:941)
- Mitotic figures are common (Genes Chromosomes Cancer 2012;51:207)
- Most cases show geographic necrosis (Mod Pathol 2016;29:1523)
- Some degree of moderate nuclear pleomorphism may be observed
- Myxoid stroma in a third of cases (Am J Surg Pathol 2017;41:941)
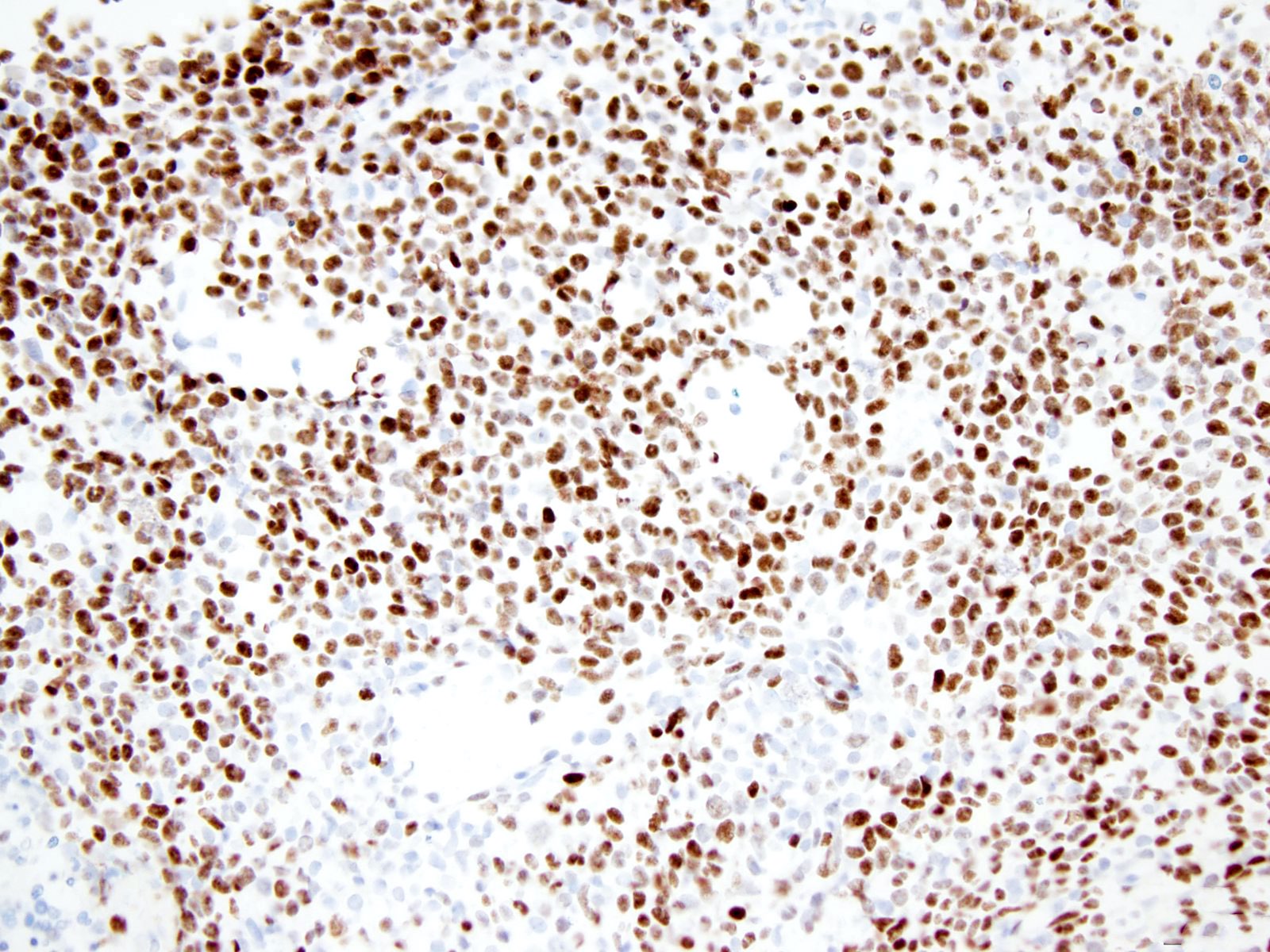
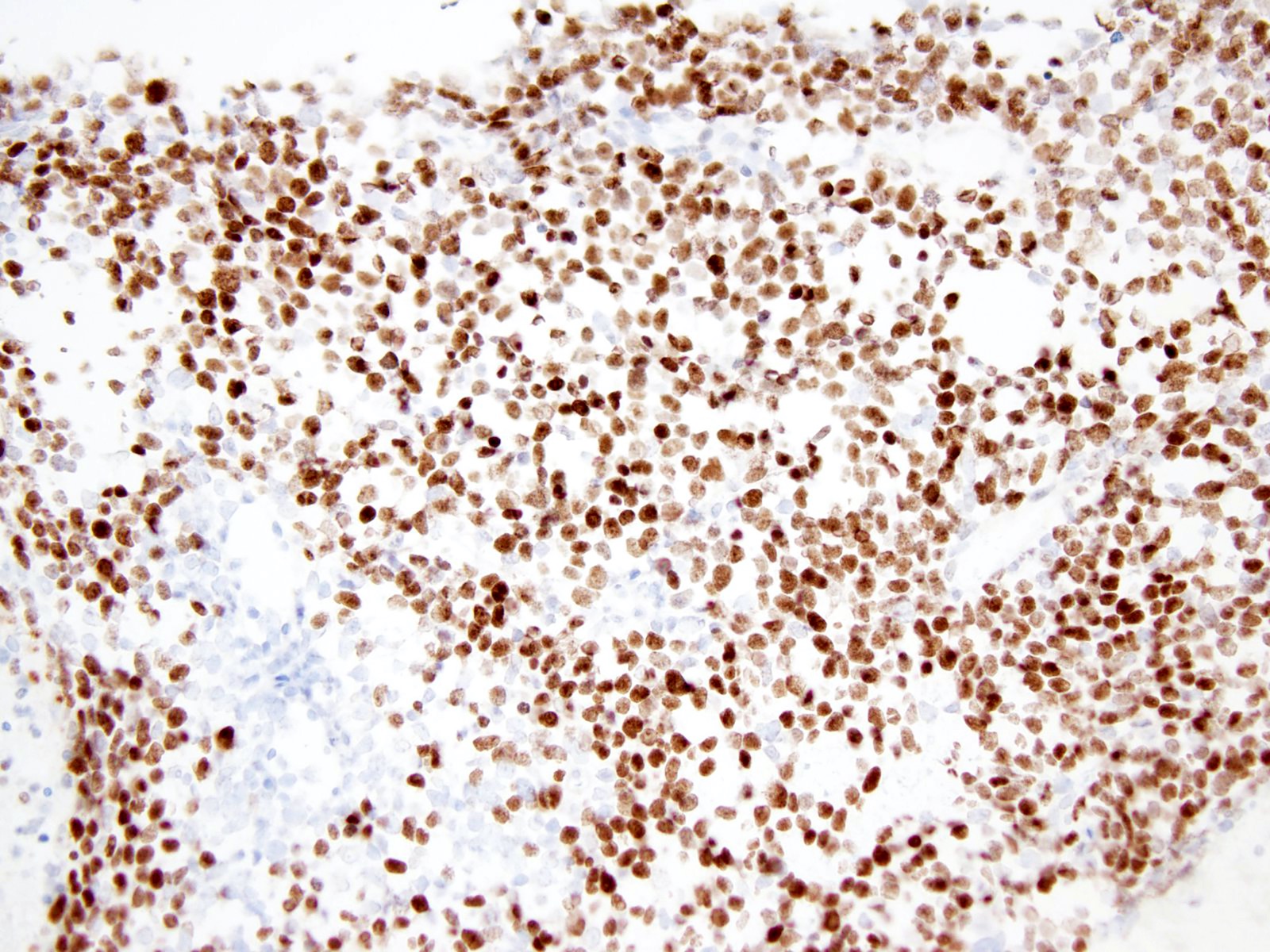
Microscopic (histologic) images
Cytology description
- Hypercellular smears, with tumor cells arranged in large groups and singly dispersed
- Individual cells with high nuclear to cytoplasmic ratio, eccentric round to ovoid nuclei, irregular nuclear contours and small nucleoli (Diagn Cytopathol 2018;46:958)
- Cytoplasmic vacuoles (Cancer Cytopathol 2016;124:350)
- Mitotic figures
- Necrosis
- Myxoid stromal component
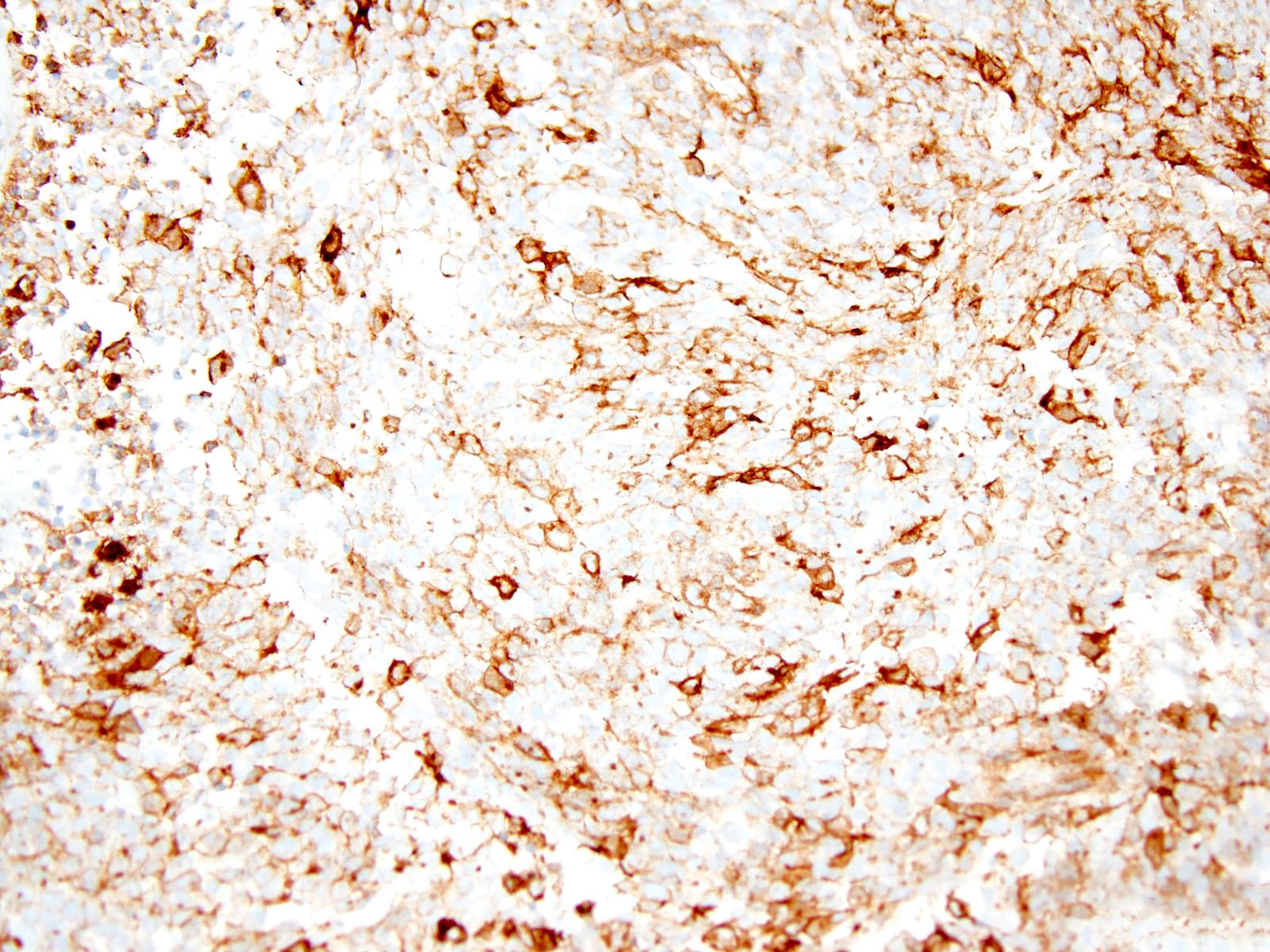
Positive stains
- CD99 (Am J Surg Pathol 2017;41:941)
- WT1 (N terminal) (Genes Chromosomes Cancer 2012;51:207)
- WT1 (C terminal) (Mod Pathol 2016;29:1523)
- DUX4 (Am J Surg Pathol 2017;41:941)
- ETV4 (Mod Pathol 2016;29:1523)
- ERG (Virchows Arch 2017;470:373)
- FLI1 (Pathol Res Pract 2017;213:1315)
- Calretinin (Pathol Res Pract 2017;213:1315)
- MYC (Mod Pathol 2015;28:57)
- INI1, retained (Pathol Res Pract 2017;213:1315)
Negative stains
- Pancytokeratin AE1 / AE3 (Virchows Arch 2017;470:373)
- Myogenin (Am J Surg Pathol 2016;40:313)
- Desmin (Virchows Arch 2017;470:373)
- CCNB3 (Virchows Arch 2017;470:373)
- S100 (Am J Surg Pathol 2016;40:313)
- NY-ESO-1 (Virchows Arch 2017;470:373)
- NKX2.2 (Histopathology 2017;71:461)
Electron microscopy description
- Heterogeneity: in cell density, from tightly packed to loosely unconnected areas (Ultrastruct Pathol 2020;44:237)
- Polygonal to pleomorphic cells with small processes
- Round, oval, polygonal or elongated nuclei
- Abundant glycogen in the cytoplasm and rare cell adhesions
- No neuroendocrine granules present
Molecular / cytogenetics description
- CIC-DUX4 gene fusion, resulting from either a t(4;19) or t(10;19) translocation, is the most common genetic abnormality (Am J Surg Pathol 2017;41:941, J Clin Pathol 2017;70:697)
- Some tumors harbor CIC rearrangements with non-DUX4 partner genes including FOXO4, LEUTX, NUTM1 and NUTM2A (Sci Rep 2020;10:684)
- Trisomy for chromosome 8 (Mod Pathol 2015;28:57)
Sample pathology report
- Chest wall mass, excision:
- CIC-DUX4 associated undifferentiated small round cell sarcoma (see comment)
- Comment: There is a subcutaneous solid nodular proliferation of small to medium sized round / ovoid and spindle cells with scant amount of amphophilic or lightly eosinophilic cytoplasm. The cells contain medium sized round to oval vesicular nuclei with small nucleoli. High mitotic activity (21 mitoses/10 high power fields) and areas of necrosis are present. Patchy myxoid or edematous stromal changes are seen.
- Immunohistochemically the cells have strong expression of CD99, WT1 (N terminal) and MYC while are negative for pankeratin AE1 / AE3, EMA, myogenin, S100 and SOX10. INI1 is preserved. The tumor is positive for CIC-DUX4 fusion transcript.
- This constellation of morphological, immunohistochemical and molecular features strongly supports the diagnosis of CIC-DUX4 associated undifferentiated small round cell sarcoma. It is a sarcoma associated with an aggressive clinical course, with an inferior overall survival compared to Ewing sarcoma.
Differential diagnosis
- Ewing sarcoma:
- Mean age at diagnosis of 15 years versus peak incidence in the fourth decade for CIC rearranged sarcomas (Am J Surg Pathol 2017;41:941)
- Most Ewing sarcomas occur in skeletal locations (Am J Surg Pathol 2017;41:941)
- Entirely uniform nuclei are more common in Ewing sarcomas (Am J Surg Pathol 2016;40:313)
- Prominence of the nucleoli, spindle cell elements and myxoid stroma are more common in the CIC rearranged sarcomas
- NKX2.2+ (Histopathology 2017;71:461)
- DUX4- (Am J Surg Pathol 2017;41:941)
- WT1-
- Pathognomonic translocations involving the EWSR1 gene (Am J Surg Pathol 2017;41:941)
- BCOR-CCNB3 (Ewing-like) sarcoma:
- Wide morphologic spectrum ranging from round to spindle cell phenotype (Am J Surg Pathol 2018;42:604)
- Monomorphic, ovoid nuclei with similar fine chromatin pattern
- Rich capillary network
- Myxoid or collagenous stroma
- Alternating hypercellular and hypocellular areas (Am J Surg Pathol 2018;42:604)
- BCOR+ (Am J Surg Pathol 2017;41:1713)
- CCNB3+ (Pathol Int 2015;65:410)
- SATB2+ (Am J Surg Pathol 2018;42:604)
- Cyclin D1+ (Am J Surg Pathol 2017;41:1713)
- NKX2.2- (Am J Surg Pathol 2018;42:604)
- WT1- (Am J Surg Pathol 2018;42:604)
- BCOR-CCNB3 fusions (Am J Surg Pathol 2018;42:604)
- Malignant peripheral nerve sheath tumor (MPNST):
- Arising (i) from a peripheral nerve (ii) from a preexisting benign nerve sheath tumor (usually neurofibroma) or (iii) in patients with NF1 disease (Am J Surg Pathol 2016;40:896)
- In the absence of these 3 associations, according to the WHO classification, the diagnosis was based on the histologic, IHC and ultrastructural features of Schwann cell differentiation (Am J Surg Pathol 2016;40:896)
- Typical histologic findings include fascicles of alternating hypercellular and hypocellular areas, branching hemangiopericytoma-like vascular pattern, whorls, palisades or rosette-like arrangements (Am J Surg Pathol 2016;40:896)
- S100 protein / SOX10 positive Schwann cells are reduced or absent (Hum Pathol 2017;67:1)
- Complete loss of trimethylated histone 3 lysine 27 expression is detectable in about half of MPNSTs (Hum Pathol 2017;67:1)
- DUX4-
- Synovial sarcoma:
- Biphasic: well formed glandular structures admixed with a monotonous atypical spindle cell proliferation in the stroma
- Monophasic: sheets of tumor cells with oval to spindle, vesicular nuclei surrounded by an indistinct rim of amphophilic to lightly eosinophilic cytoplasm
- Alternating zones of hypercellularity and hypocellularity
- Hemangiopericytoma-like vasculature
- Poorly differentiated morphology with rounded or spindle cells showing elevated nuclear grade, rhabdoid cells and high mitotic index
- Broad spectrum cytokeratins+ and EMA+ (Am J Surg Pathol 2005;29:569)
- CD99+ and BCL2+ (Am J Surg Pathol 2005;29:569)
- TLE1+ (Am J Clin Pathol 2011;135:839)
- NY-ESO-1+ and MAGE-A4+ (Oncol Lett 2019;17:3937)
- SS18-SSX fusion oncoprotein (Cancer Cell 2018;33:1128)
- Alveolar rhabdomyosarcoma:
- Alveolar, nested to solid growth pattern (Head Neck Pathol 2018;12:181)
- Primitive, undifferentiated or round blue cell pattern (Head Neck Pathol 2018;12:181)
- Classical rhabdomyoblastic differentiation with strap cells or cross striations is seldom seen
- Crush artifact
- Multinucleation
- Lymphovascular invasion
- Expression of muscle markers: desmin, myogenin, MyoD1 (Head Neck Pathol 2018;12:181)
- Aberrant expression of epithelial and neuroendocrine markers (Adv Anat Pathol 2013;20:387, Head Neck Pathol 2018;12:181)
- DUX4-
- Unique t(2;13)(q35;q14) or t(1;13)(p36;q14) chromosomal translocations, resulting in PAX3 / FOXO1 and PAX7 / FOXO1 fusion genes (Diagn Mol Pathol 2009;18:138, Adv Anat Pathol 2013;20:387)
- Desmoplastic small round cell tumor:
- Predominantly intraabdominal, pelvic or peritoneal based mass (Am J Clin Pathol 2000;114:345)
- Islands of monotonous small cells within prominent desmoplastic stroma (Int J Surg Pathol 2017;25:158)
- Solid variant, lacking evidence of desmoplastic stroma
- Polyphenotypic multidirectional differentiation, with expression of epithelial (pankeratin AE1 / AE3), muscle (desmin) and neural markers (NSE) (Int J Surg Pathol 2016;24:672)
- WT1+ (C terminal) ( Am J Surg Pathol 2000;24:830, Am J Clin Pathol 2000;114:345)
- DUX4-
- EWSR1-WT1 fusion gene (Int J Surg Pathol 2016;24:672, Am J Surg Pathol 2000;24:830)
Board review style question #1
Which of the following is true about CIC-DUX4 rearranged sarcoma?
- High metastatic rate, brain most common
- Diagnosis always requires clinicopathological and radiological correlation
- Prognosis is poor
- Tumor is always negative for ERG
- Tumor is characterized by cords of epithelioid cells distributed in a desmoplastic stroma
Board review style answer #1
Board review style question #2
A 65 year old man presented with a left thigh mass. Hematoxylin eosin stains demonstrated proliferation of small to medium sized round / ovoid cells with medium sized round to oval vesicular nuclei and clear or pale eosinophilic cytoplasm. Increased mitotic activity, geographic necrosis and patchy myxoid stromal change were seen. Immunohistochemical stains for CD99, WT1 and DUX4 were positive in tumor cells while all of the following were negative: pankeratin AE1 / AE3, S100, SOX10, myogenin, NY-ESO-1, NKX2.2 and CCNB3. INI1 was retained. Which of the following is most likely the correct diagnosis?
- Synovial sarcoma, poorly differentiated
- Extraskeletal Ewing sarcoma
- Epithelioid sarcoma
- BCOR-CCNB3 (Ewing-like) sarcoma
- CIC-DUX4 associated undifferentiated round cell sarcoma
Board review style answer #2
E. CIC-DUX4 associated undifferentiated round cell sarcoma
Comment Here
Reference: CIC-DUX4 fusion tumor
Comment Here
Reference: CIC-DUX4 fusion tumor