Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Clinical images | Gross description | Gross images | Frozen section description | Frozen section images | Microscopic (histologic) description | Microscopic (histologic) images | Virtual slides | Cytology description | Cytology images | Positive stains | Negative stains | Molecular / cytogenetics description | Videos | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Nguyen NNJ, Downes MR. Carcinoma in situ. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/bladdercis.html. Accessed March 28th, 2025.
Definition / general
- Urothelial carcinoma in situ (CIS) is a high grade, flat, noninvasive urothelial neoplasm involving full or partial thickness of the urothelium (J Natl Compr Canc Netw 2009;7:48, Appl Immunohistochem Mol Morphol 2021;29:127)
Essential features
- CIS is defined as high grade malignant urothelial cells with severe nuclear atypia at low to intermediate power and no papillary formation
- Characterized by nuclear enlargement, hyperchromasia and loss of cellular polarity
- CK20 full thickness staining, p53 overexpression / null phenotype and loss of CD44 expression are immunohistochemical findings supporting the diagnosis of urothelial CIS
- Differential diagnoses include flat urothelial dysplasia, reactive / inflammatory urothelial dysplasia, therapy related dysplasia and shoulder lesion of a papillary urothelial carcinoma
- Treatment typically consists of Bacillus Calmette-Guérin (BCG) therapy for bladder CIS, transurethral resection for urethral CIS and nephroureterectomy for upper urinary tract CIS
Terminology
- Flat urothelial carcinoma in situ
ICD coding
- ICD-O: 8120/2 - urothelial carcinoma in situ
- ICD-11
- 2C91.0 & XH5GH8 - urothelial carcinoma of renal pelvis & urothelial carcinoma in situ
- 2C92.0 & XH5GH8 - urothelial carcinoma of ureter & urothelial carcinoma in situ
- 2C94.2 & XH5GH8 - urothelial carcinoma of bladder & urothelial carcinoma in situ
- 2C93.2 & XH5GH8 - urothelial carcinoma of urethra or paraurethral gland & urothelial carcinoma in situ
- 2C95.2 & XH5GH8 - urothelial carcinoma involving overlapping sites of urinary organs & urothelial carcinoma in situ
Epidemiology
- Mean age is the seventh decade of life (Mod Pathol 2022;35:1287, Eur J Cancer Care (Engl) 2015;24:444)
- M:F ≈ 4:1 (Clin Genitourin Cancer 2022;20:e166)
- Predominantly Caucasians (Eur J Cancer Care (Engl) 2015;24:444)
- De novo, isolated CIS represents up to 3% of urothelial neoplasms (Crit Rev Oncol Hematol 2010;76:112)
- Reportedly, CIS is more frequently found concomitantly to noninvasive papillary urothelial carcinoma and invasive urothelial carcinoma or subsequently at follow up (Eur Urol 2015;67:876)
- True incidence of concomitant / secondary CIS remains uncertain considering reported numbers in the literature were part of studies that were performed before the concept of shoulder / residual lesion of papillary urothelial carcinoma was established (Urology 2013;81:1123, Surg Pathol Clin 2022;15:629)
Sites
- Urinary tract (most commonly urinary bladder) (Eur J Cancer Care (Engl) 2015;24:444, Hum Pathol 2006;37:726, Urol Oncol 2023;41:274, Int J Surg Pathol 2022;30:15)
Pathophysiology
- CCDC138 mutation, TERT promoter mutation and p53 mutation are the most common genetic findings
- DNA damage signaling related to APOBEC signature is a key signaling pathway in the progression of CIS (Nat Commun 2023;14:5670)
- At least 1 potentially actionable genomic alteration is identified among the following genes in 92% of cases
- TP53 / cell cycle pathway related genes, including TP53, MDM2 and CCND1
- Genes encoding chromatin modifying proteins, including ARID1A, KDM6A and EP300 / CREBBP
- DNA damage repair genes, including BRCA2, ATM and BRCA1
- Phosphatidylinositol 3 kinase / mitogen activated protein kinase pathway genes, including ERBB2, FGFR1 and PIK3CA (Am J Pathol 2020;190:323)
- 46 gene expression signature, including upregulation of actionable genes MTOR, TYK2, AXIN1, CPT1B, GAK and PIEZO1 and downregulation of BRD2 and NDUFB2, was found in a multiomics profiling study (iScience 2024;27:109179)
- Luminal-like phenotype (CK20+, CK5/6-), null phenotype (CK20-, CK5/6-) and mixed luminal-like / null phenotype in the majority of cases; basal-like subtype (CK20-, CK5 / 6+) in rare cases (Virchows Arch 2021;479:325, Virchows Arch 2018;472:749)
- Positivity for predictive markers HER2 or ERβ in 91% of cases (Virchows Arch 2018;472:749)
- Comparison with papillary urothelial carcinoma
- Differences: most commonly luminal with overexpression of FOXA1 and downregulation of basal (CD44 and KRT14), immune (CXCL11) and neuronal (APLP1, MS1) markers (iScience 2024;27:109179)
- Similarities: TERT, TP53, ERBB2 and APOBEC related pathway (UROMOL class 2a) and high immune cell infiltration (UROMOL class 2b) but with higher PD-1 levels
Etiology
- Same etiologic factors as invasive urothelial carcinoma
- Mostly sporadic due to environmental factors, notably smoking (JAMA 2011;306:737)
- Can rarely present in the hereditary form in the context of Lynch syndrome or BRCA1 / BRCA2 germline mutation (Future Oncol 2018;14:277, J Med Genet 2010;47:464, J Clin Oncol 2020;38:406)
Clinical features
- Urothelial CIS is usually detected in the context of hematuria or voiding symptoms (Eur Urol 2022;81:75)
- Upper tract lesions can cause hydronephrosis (Eur Radiol 2022;32:3269)
Diagnosis
- Gross hematuria or persistent microhematuria in older patients prompts the exclusion of a bladder neoplasm via urine cytology and cystoscopic investigation (J Urol 2020;204:778, Eur Urol Focus 2024;10:115)
- Urine cytology from a voided, catheterized, washing or brushing specimen can evaluate for the presence of high grade urothelial carcinoma but cannot distinguish urothelial CIS from papillary urothelial carcinoma
- Sensitivity and specificity of urine cytology is high for high grade urothelial carcinoma but may be affected by interobserver variability (Cytojournal 2017;14:17)
- UroVysion fluorescence in situ hybridization assay, which has been approved by the Food and Drug Administration, can increase the diagnostic accuracy of high grade urothelial carcinoma on urine samples compared with urine cytology alone given its higher sensitivity, although it has lower specificity (Am J Clin Pathol 2019;151:469, Transl Androl Urol 2021;10:1908)
- Blue light cystoscopy combined with instillation of a photosensitizing drug such as hexaminoevulinate for optimal tumor visibility (Nat Rev Urol 2014;11:589, Tran: Blue Light Cystoscopy in Patients with Suspected Non-Muscle Invasive Bladder Carcinoma - A Review of Clinical Utility, 2017, Curr Urol 2022;16:121, Hum Pathol 2019;90:1)
- Biopsy or transurethral resection of lower urinary tract lesions identified on cystoscopy
- Biopsy of upper urinary tract lesions detected by imaging and ureteroscopy
- Random biopsies of the urinary bladder in the absence of cystoscopic findings for patients with positive cytology or for monitoring the recurrence of nonmuscle invasive bladder cancer (Eur Urol 2003;44:47, Scand J Urol Nephrol 2010;44:11)
- World Health Organization (WHO) essential diagnostic criteria
- High grade malignant urothelial cells with severe nuclear atypia at low to intermediate power
- Absence of papillary formation
- Immunohistochemistry can aid with the histologic diagnosis of urothelial CIS in challenging cases (Diagn Pathol 2019;14:91, Mod Pathol 2022;35:1287, Medicina (Kaunas) 2023;59:1609, Appl Immunohistochem Mol Morphol 2018;26:180, Hum Pathol 2024;146:43)
- Caution must be made when assessing a high grade flat lesion adjacent to papillary urothelial carcinoma or sampled at the site of prior papillary urothelial carcinoma; in these cases, morphologic features alone cannot distinguish CIS from the shoulder lesion of a high grade papillary urothelial carcinoma (Urology 2013;81:1123, Surg Pathol Clin 2022;15:629)
Laboratory
- Elevated red blood cells (≥ 3 per high power field) on urinalysis (J Urol 2020;204:778)
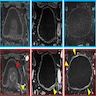
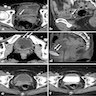
Radiology description
- Wall thickening of the urinary tract (Eur Radiol 2022;32:3269)
- Mass forming lesions can be seen in pure urothelial CIS located in the renal collecting system
Prognostic factors
- There is conflicting data whether concomitant CIS on cystectomy is an independent prognostic factor of worse pathological and clinical outcomes (World J Urol 2019;37:165, Urol Oncol 2020;38:574, World J Urol 2014;32:1295)
- CIS of the upper urinary tract has lower progression rates than bladder CIS (J Urol 2017;197:287)
Case reports
- 57 year old man with carcinoma in situ involving retrocaval ureter (IJU Case Rep 2020;3:128)
- 70 year old man with carcinoma in situ of the bladder invading the prostate and seminal vesicle (Cancer Res Treat 2020;52:1283)
- 85 year old man with extramammary Paget disease of the penis associated with urothelial carcinoma in situ of the bladder (J Cutan Pathol 2022;49:663)
Treatment
- BCG is the preferred first line treatment for urothelial carcinoma of the bladder; radical cystectomy may be performed in BCG refractory cases (Eur Urol 2022;81:75)
- Transurethral resection is the primary treatment of urothelial CIS of the prostatic urethra
- Radical nephroureterectomy with cuff of bladder for upper tract lesions
- Reference: NCCN Guidelines Version 4.2024 - Bladder [Accessed 28 June 2024]
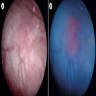
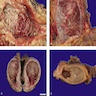
Gross description
- Erythematous patch, with or without a velvety or granular appearance (Hum Pathol 2019;90:1)
- Can be grossly unremarkable (Crit Rev Oncol Hematol 2010;76:112)
Frozen section description
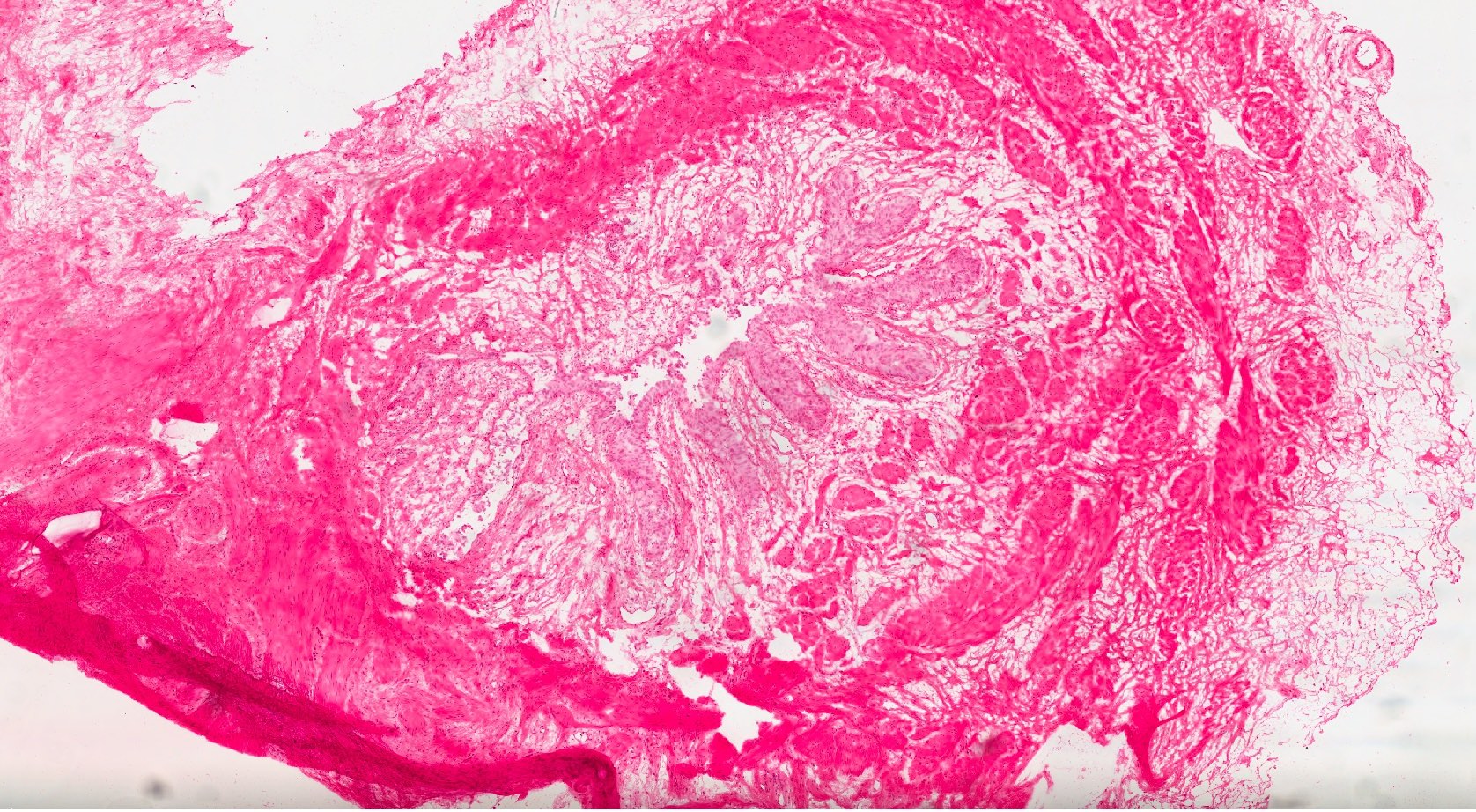
- Marked nuclear enlargement (5 - 6 times the size of an adjacent lymphocyte), hyperchromasia and loss of polarity (Am J Clin Exp Urol 2014;2:156, Appl Immunohistochem Mol Morphol 2021;29:127)
Frozen section images
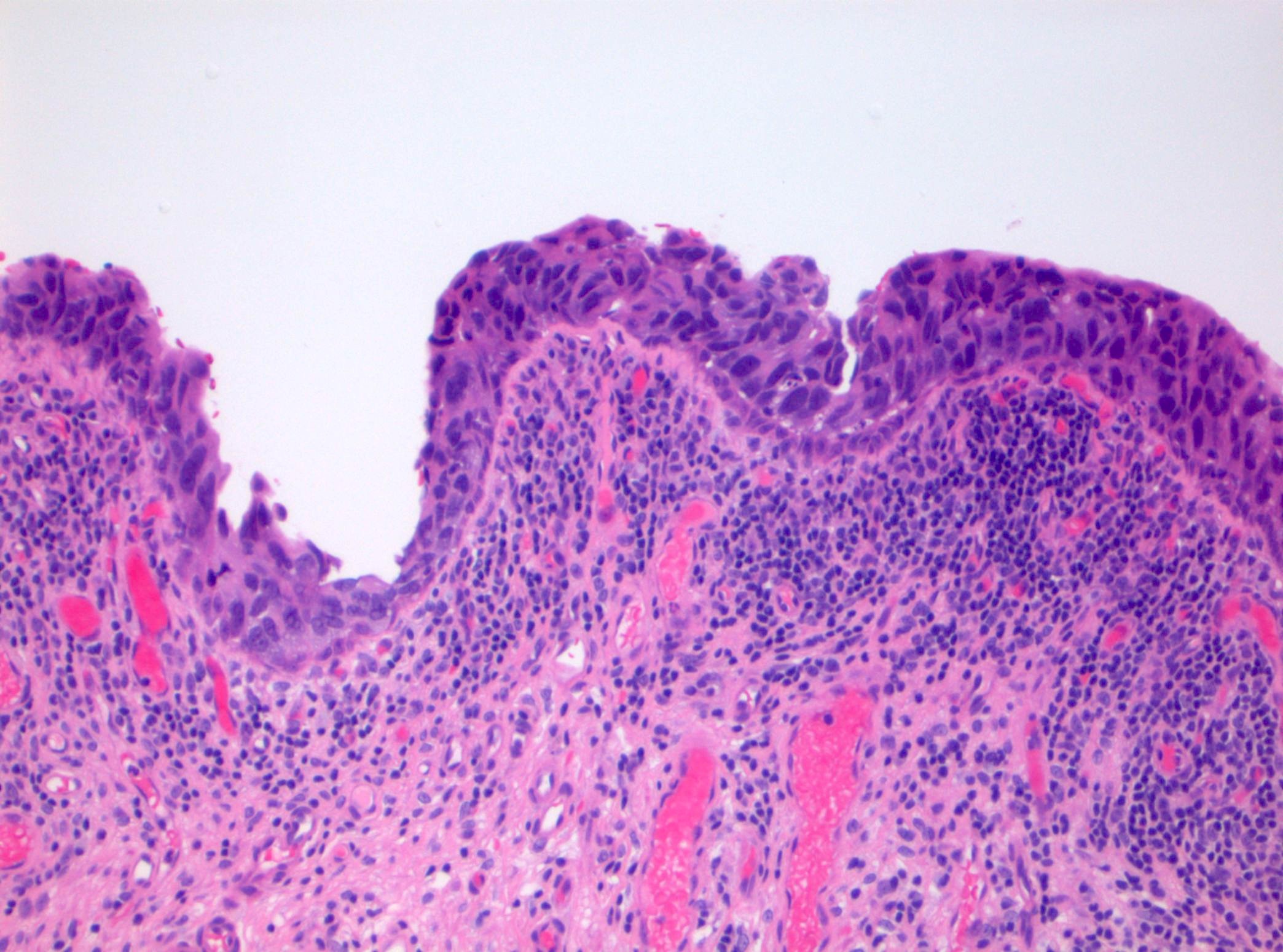
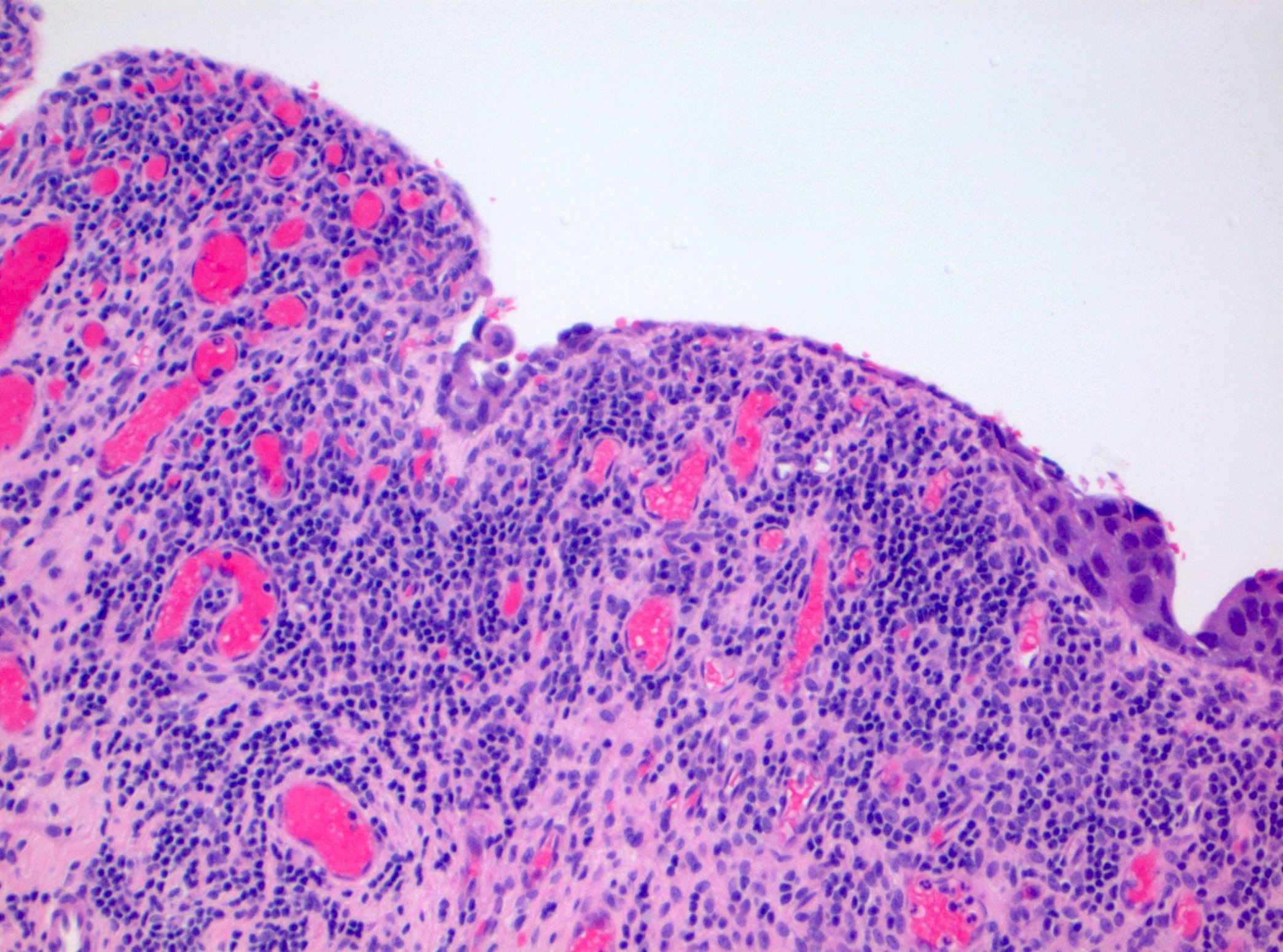
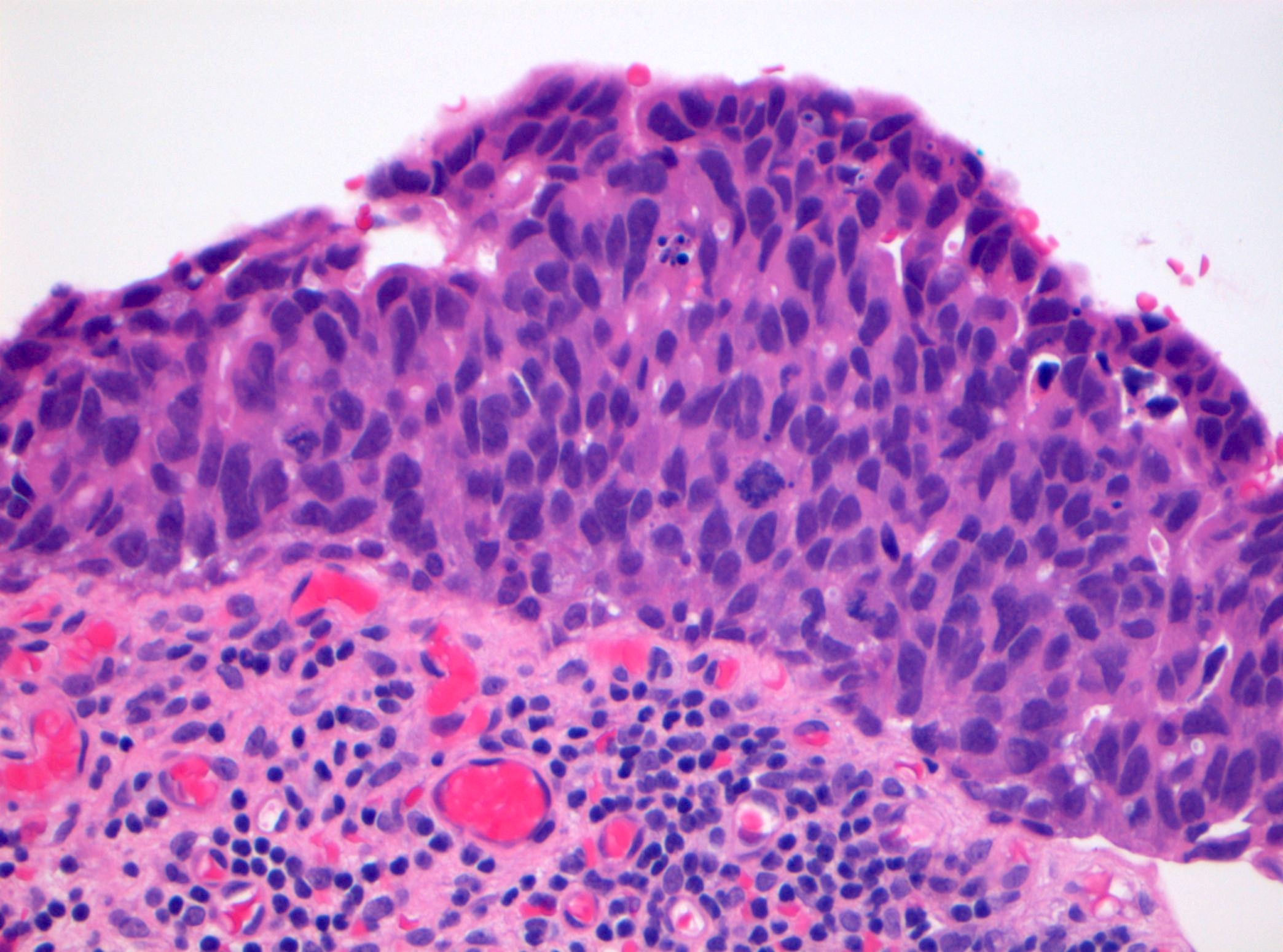
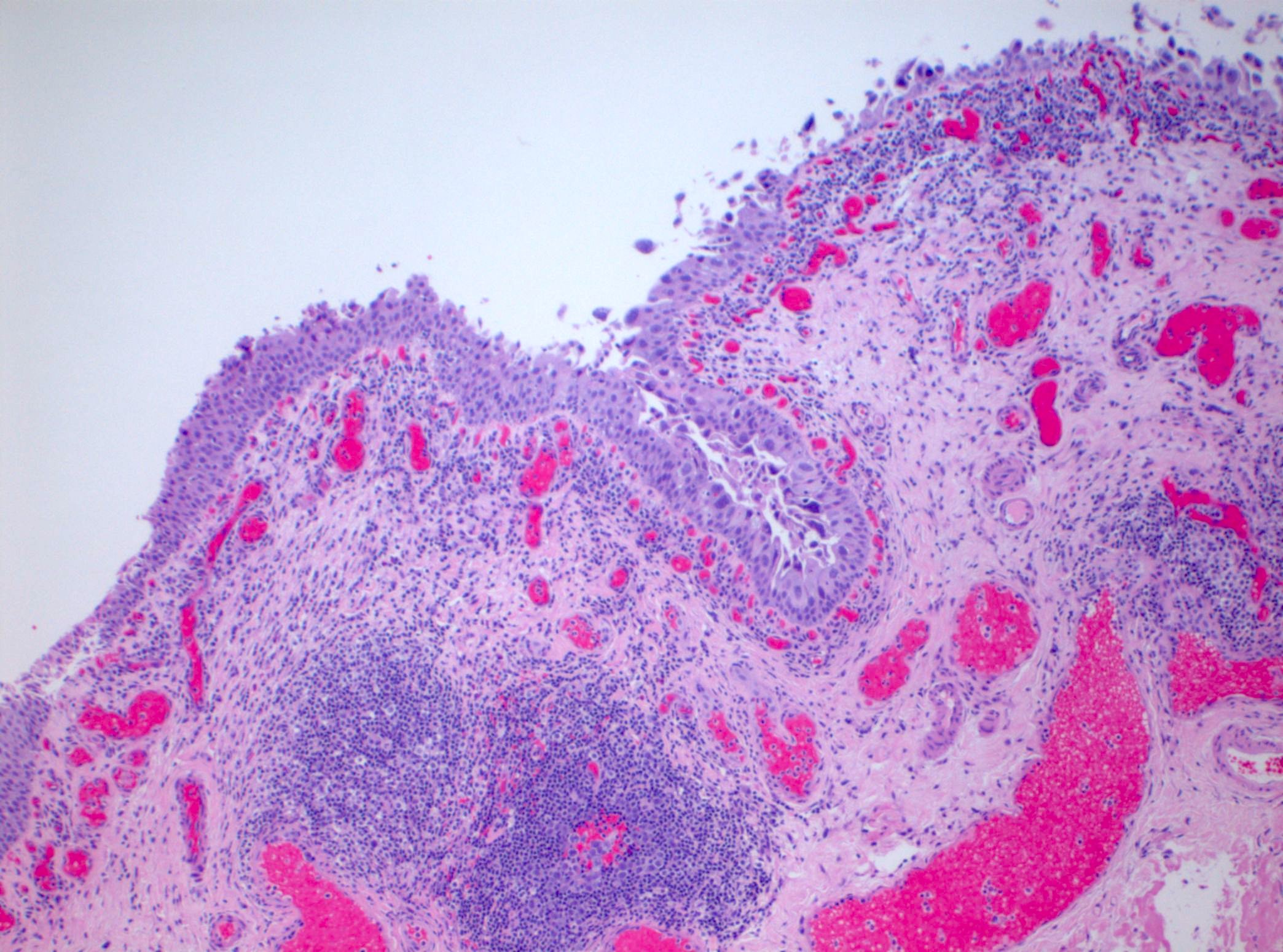
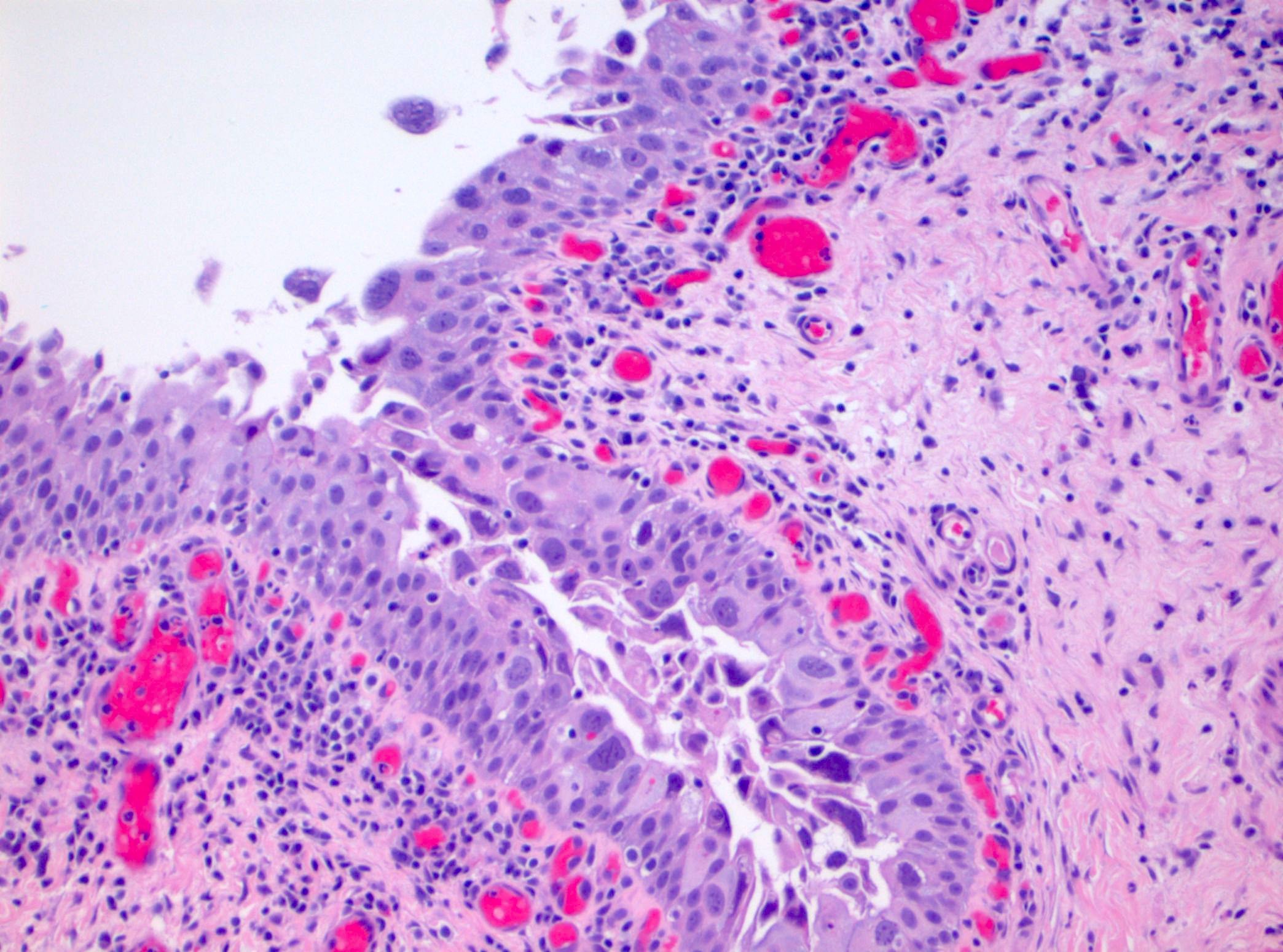
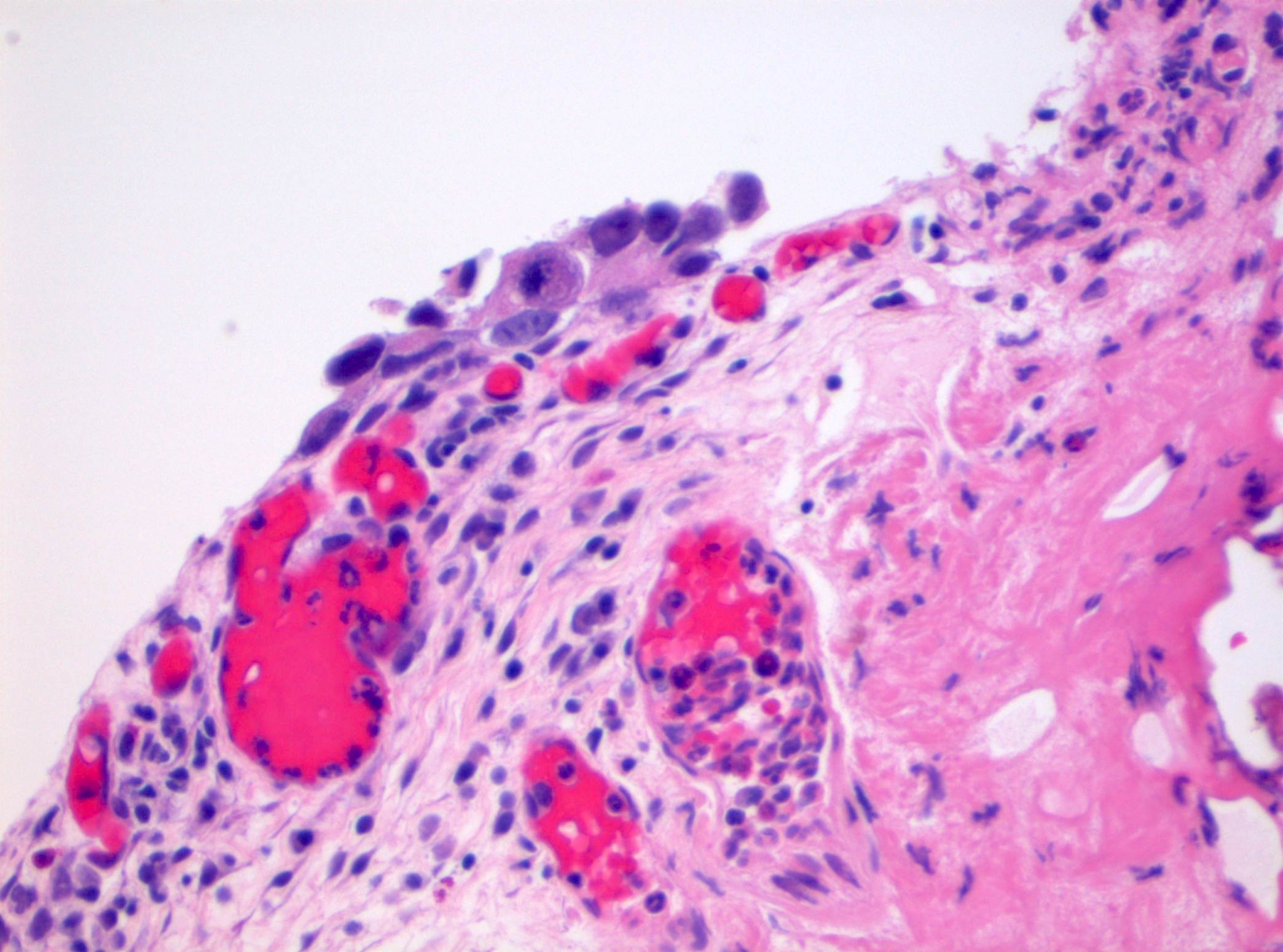
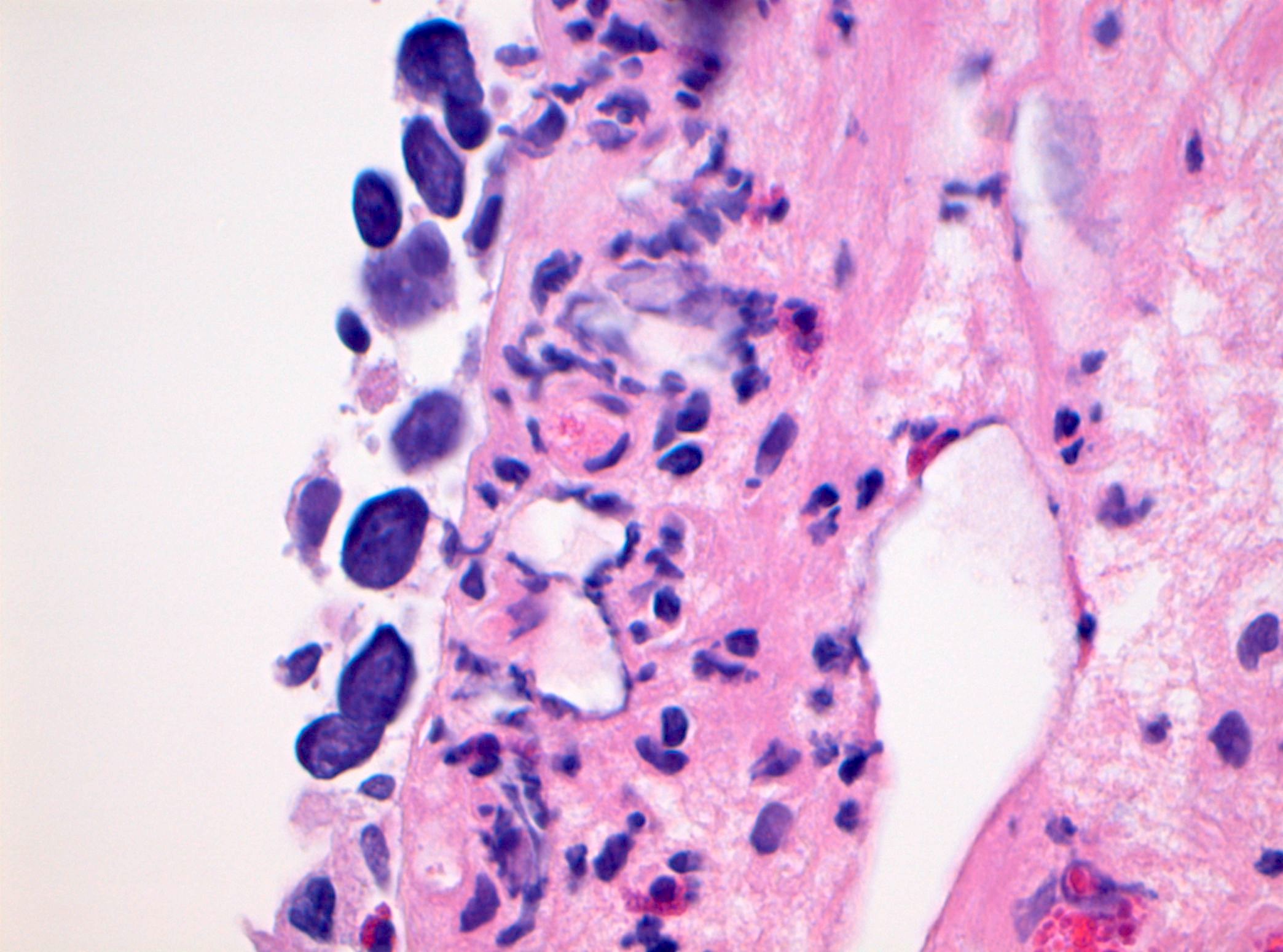
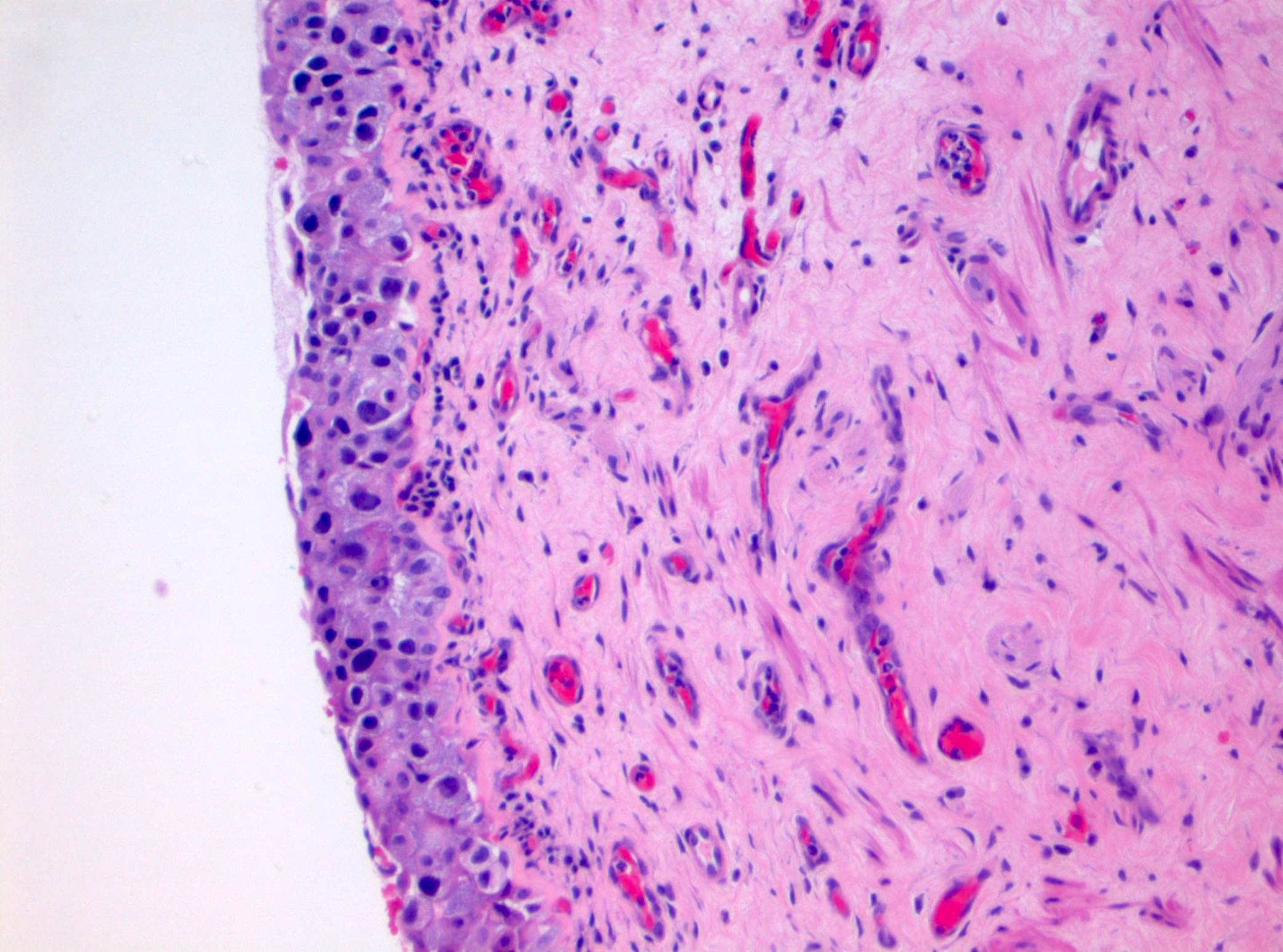
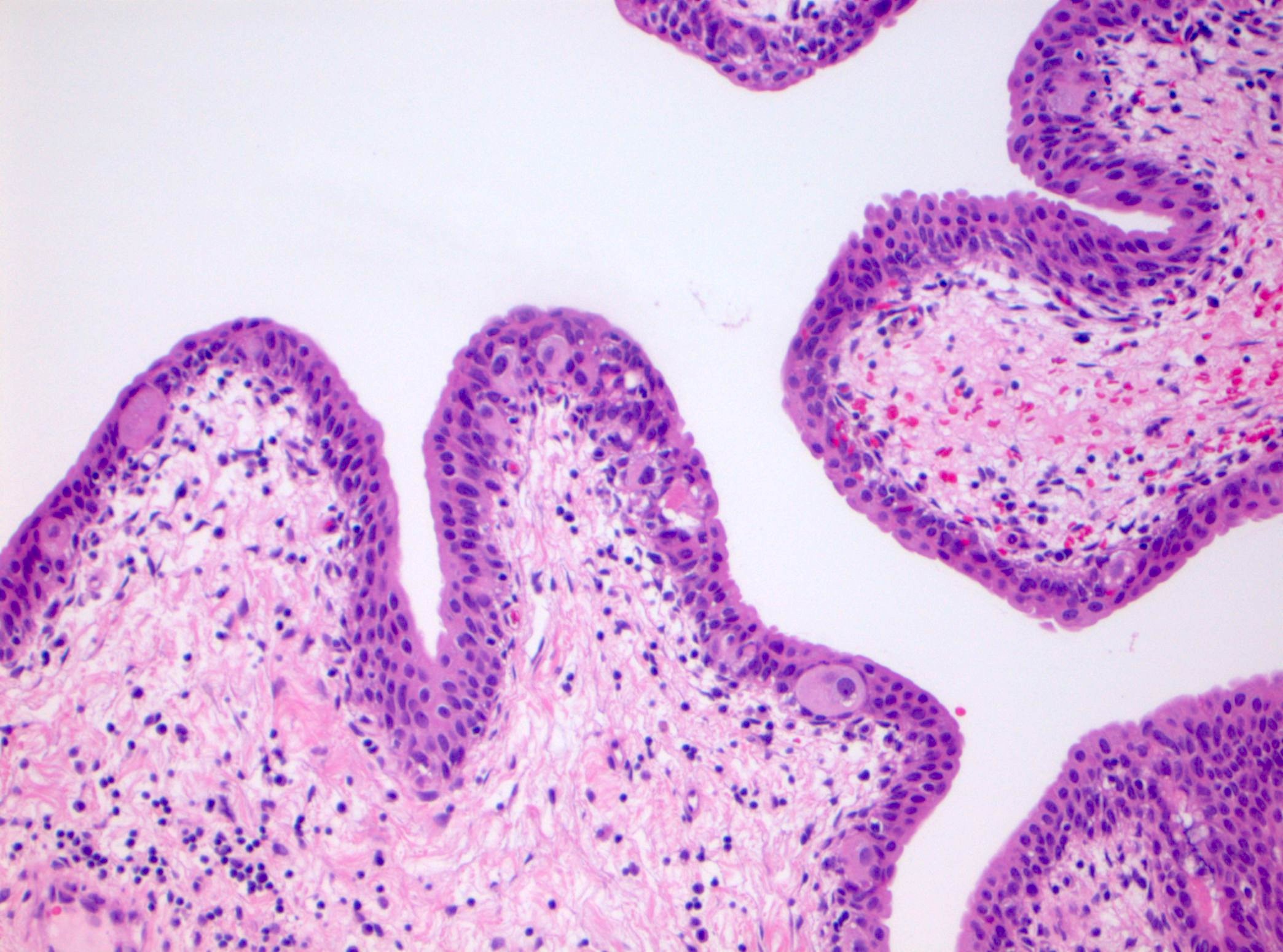
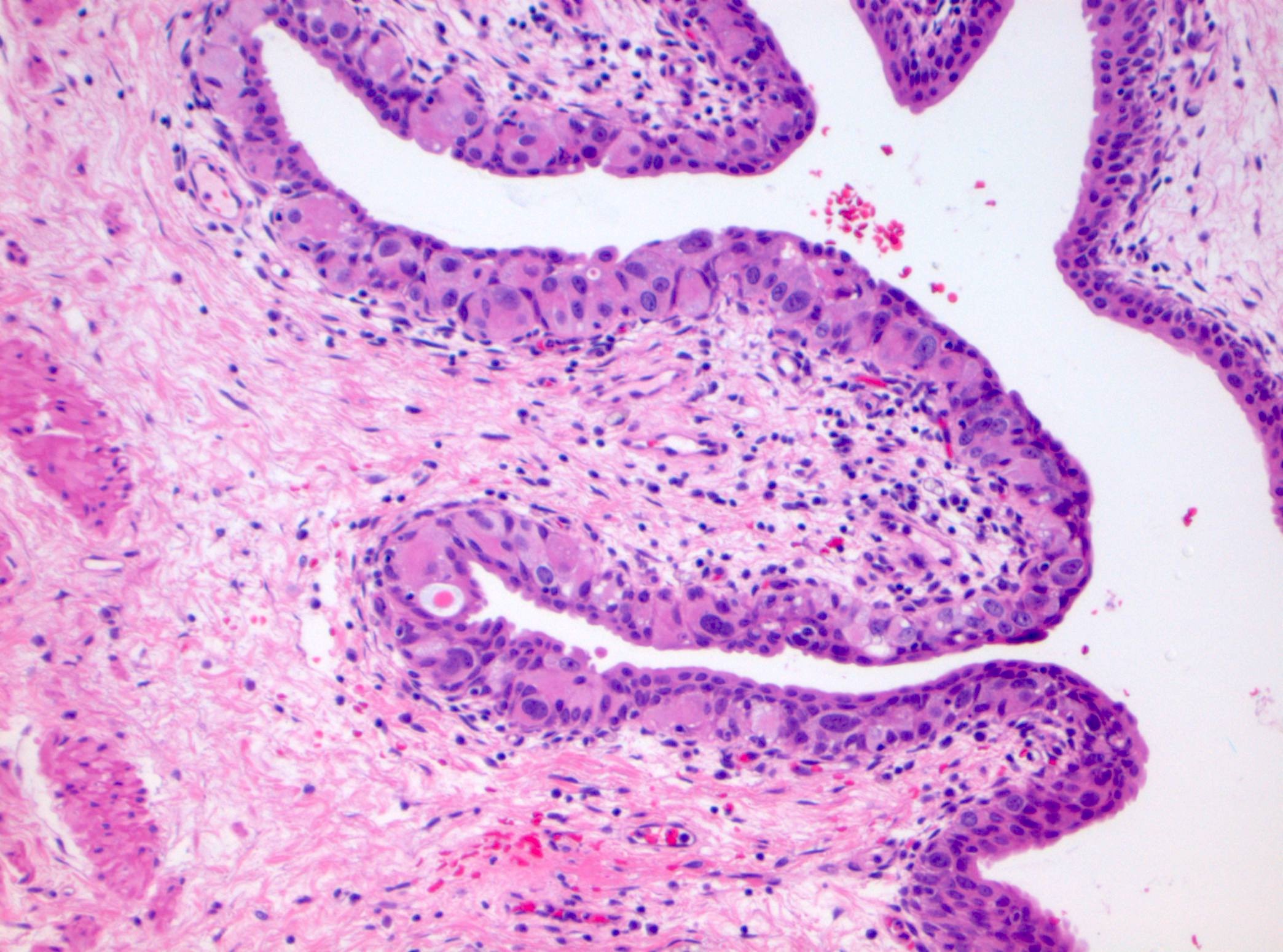
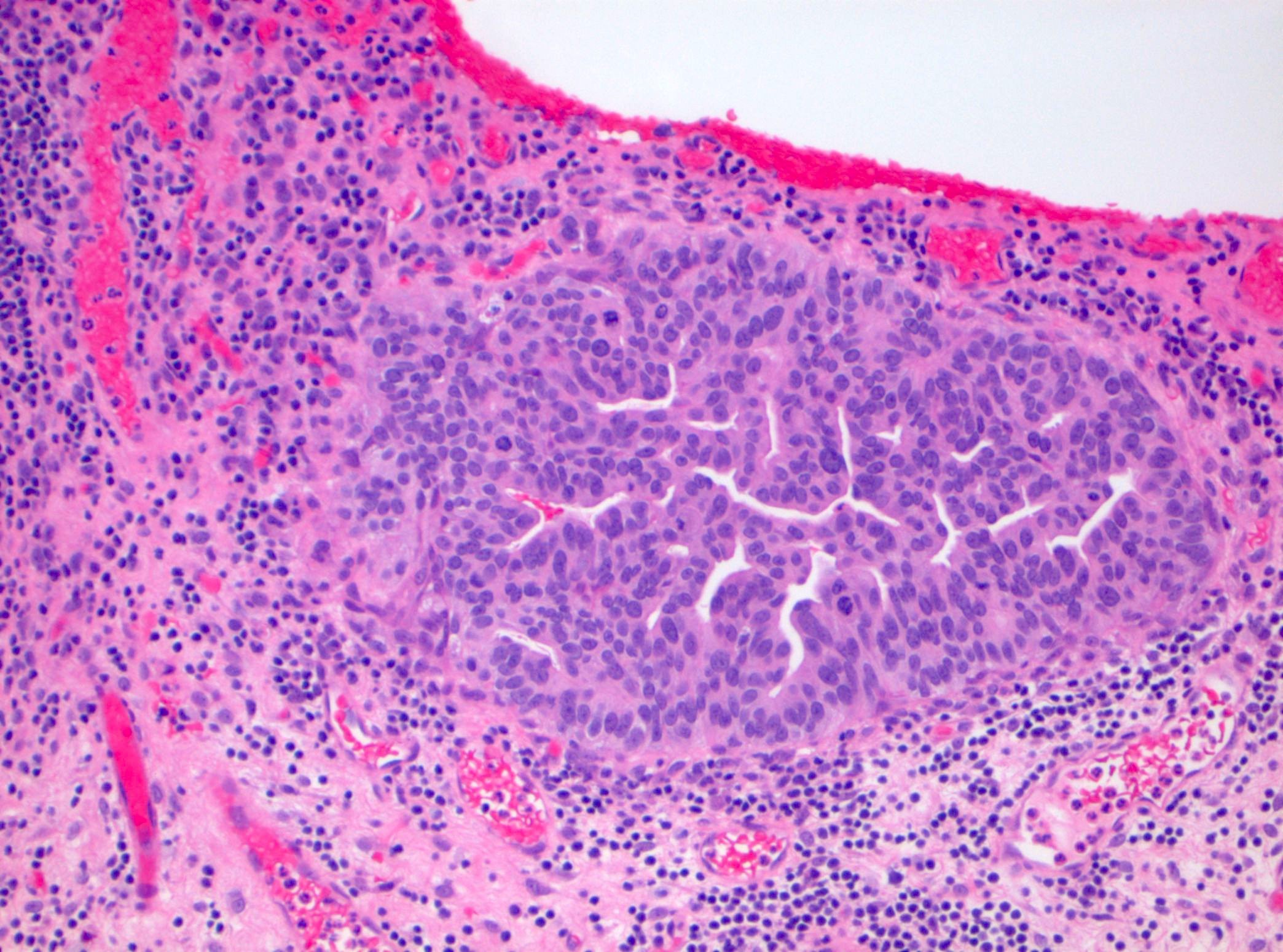
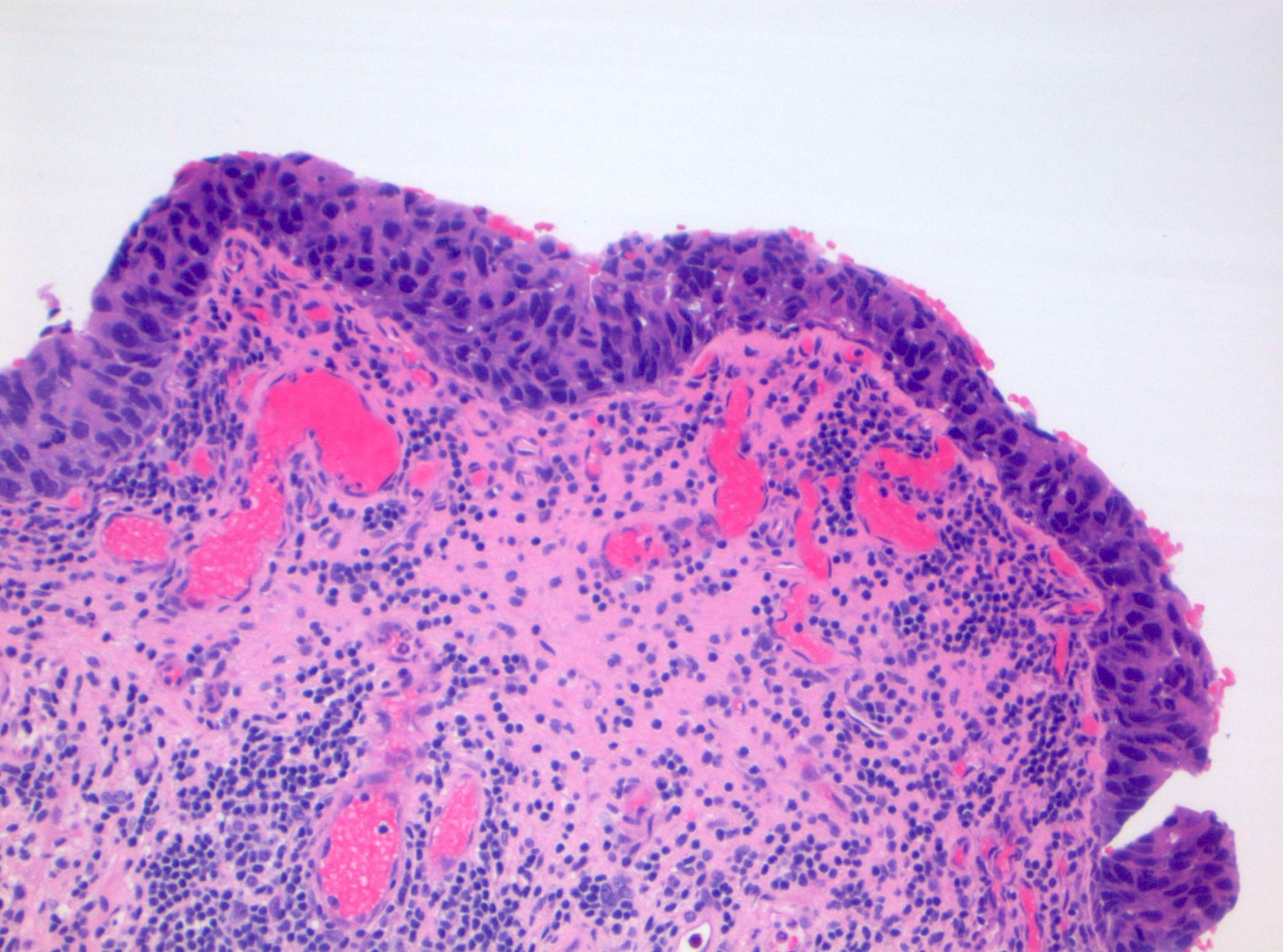
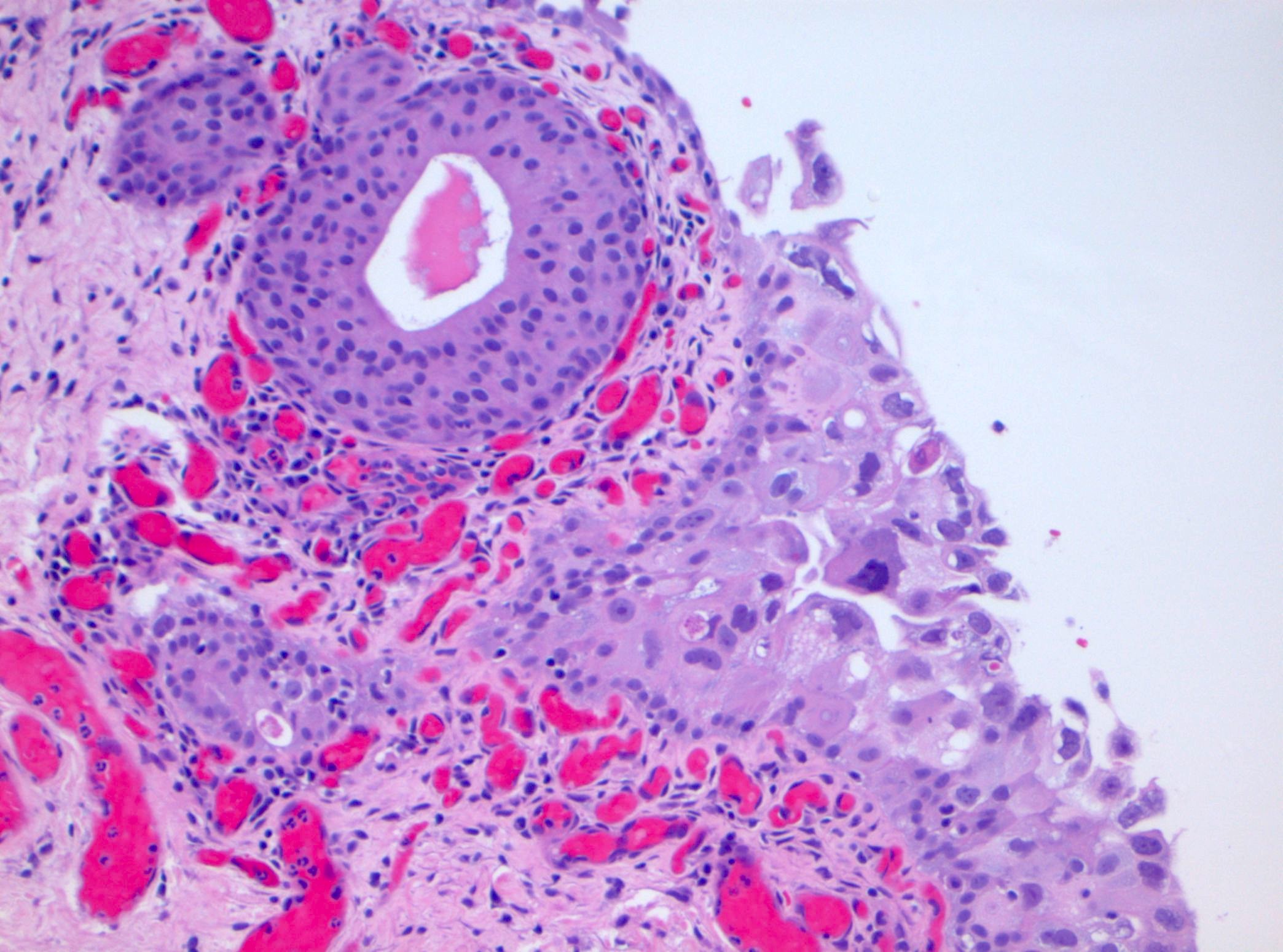
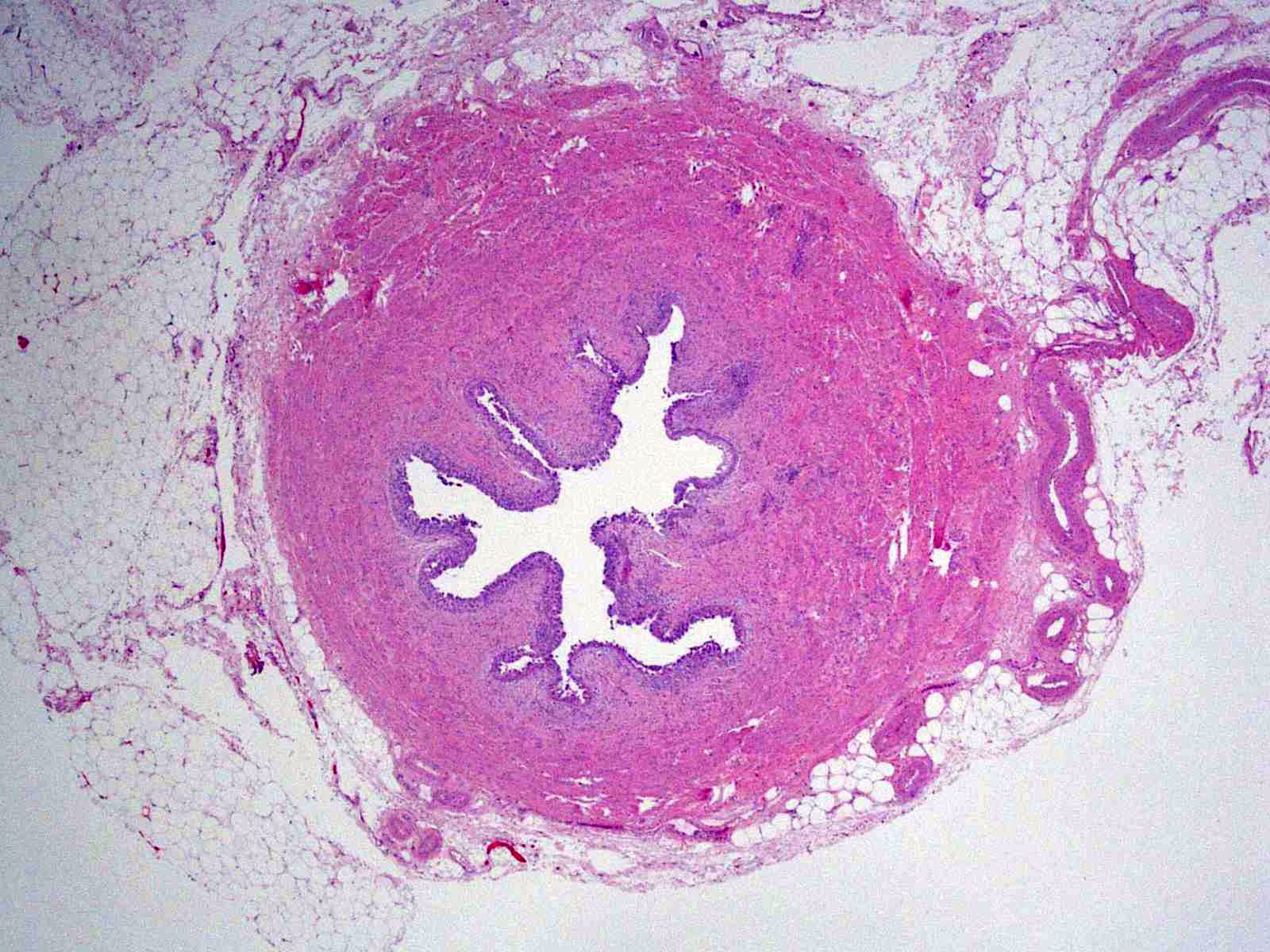
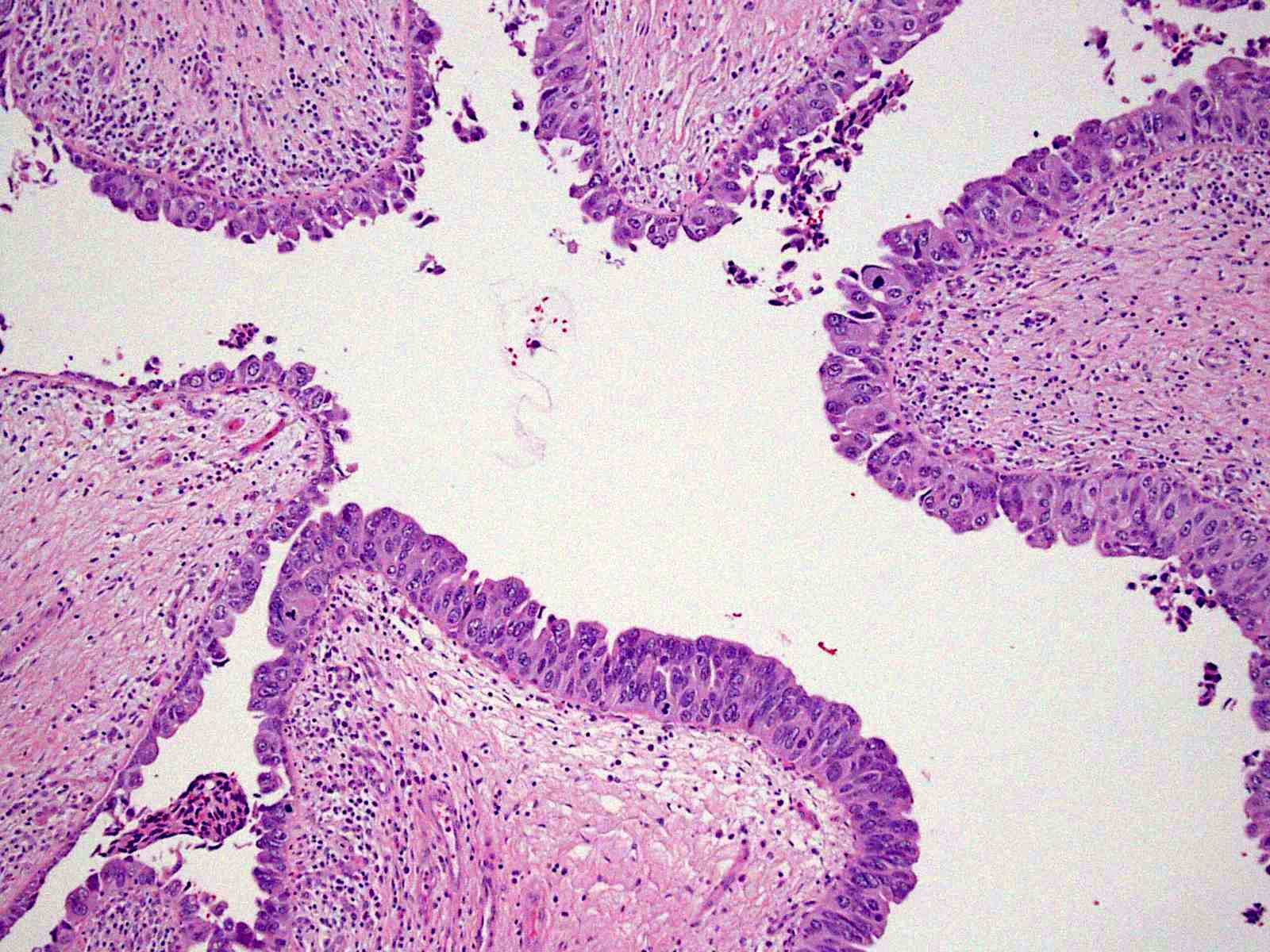
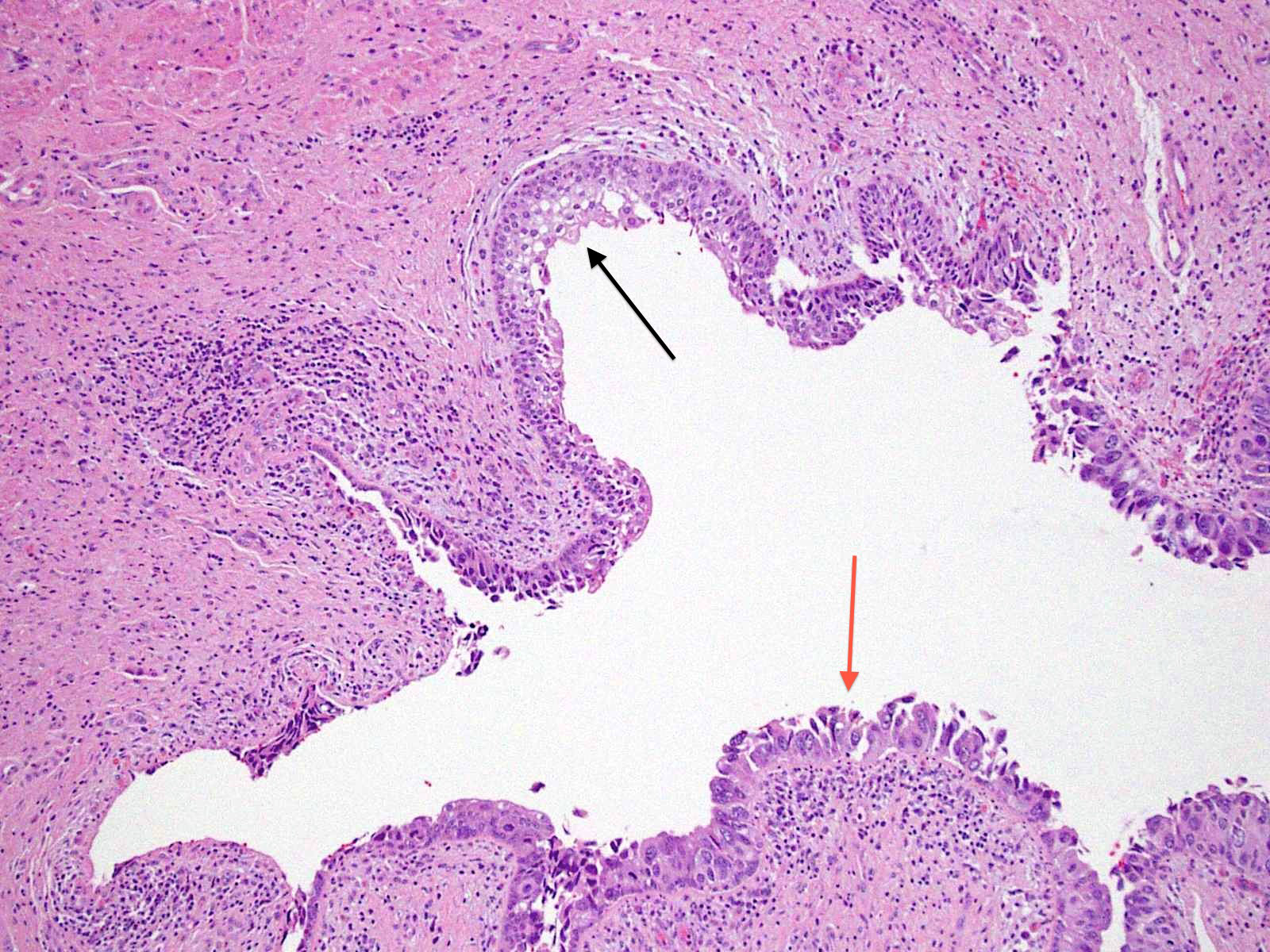
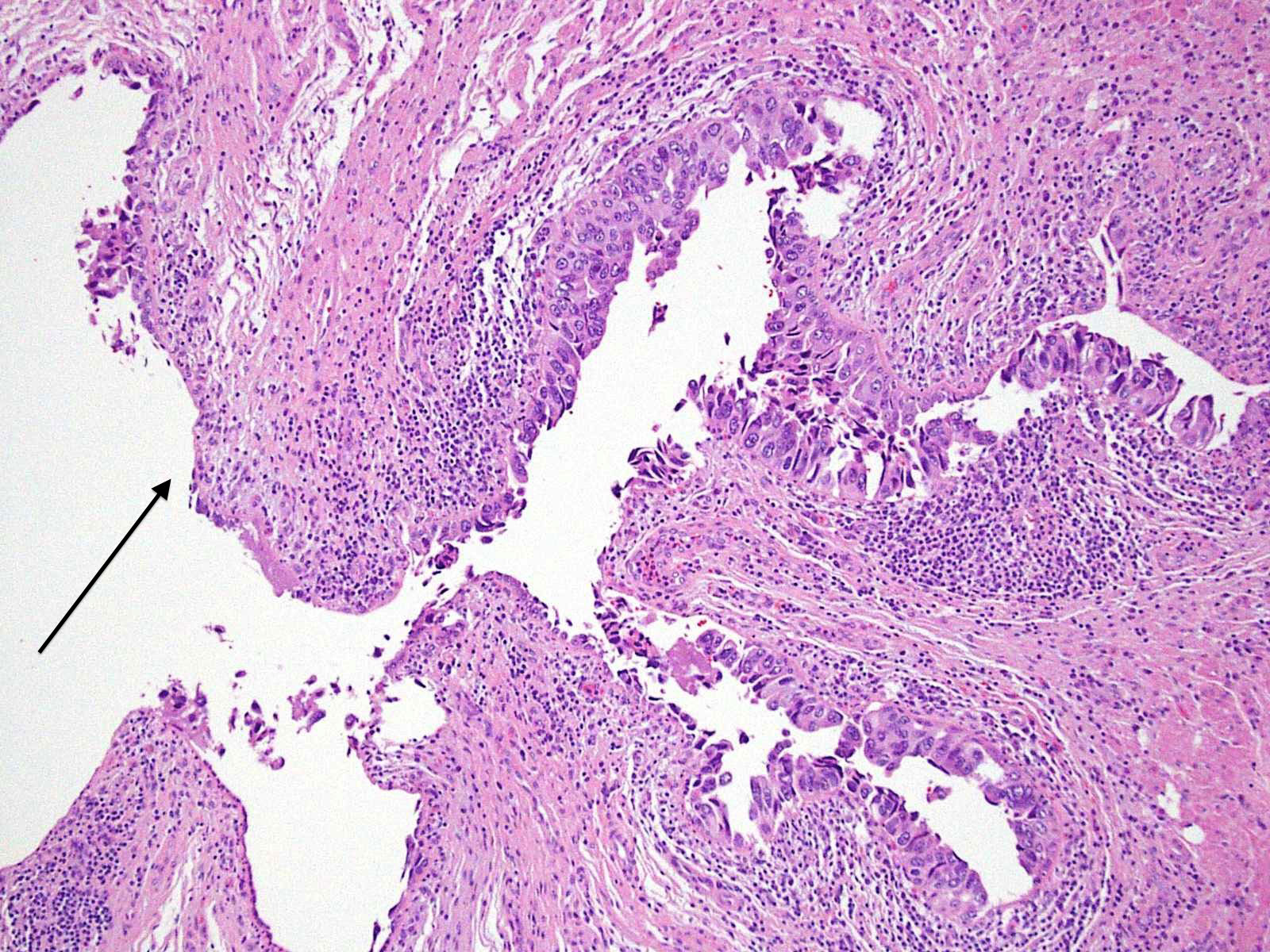
Microscopic (histologic) description
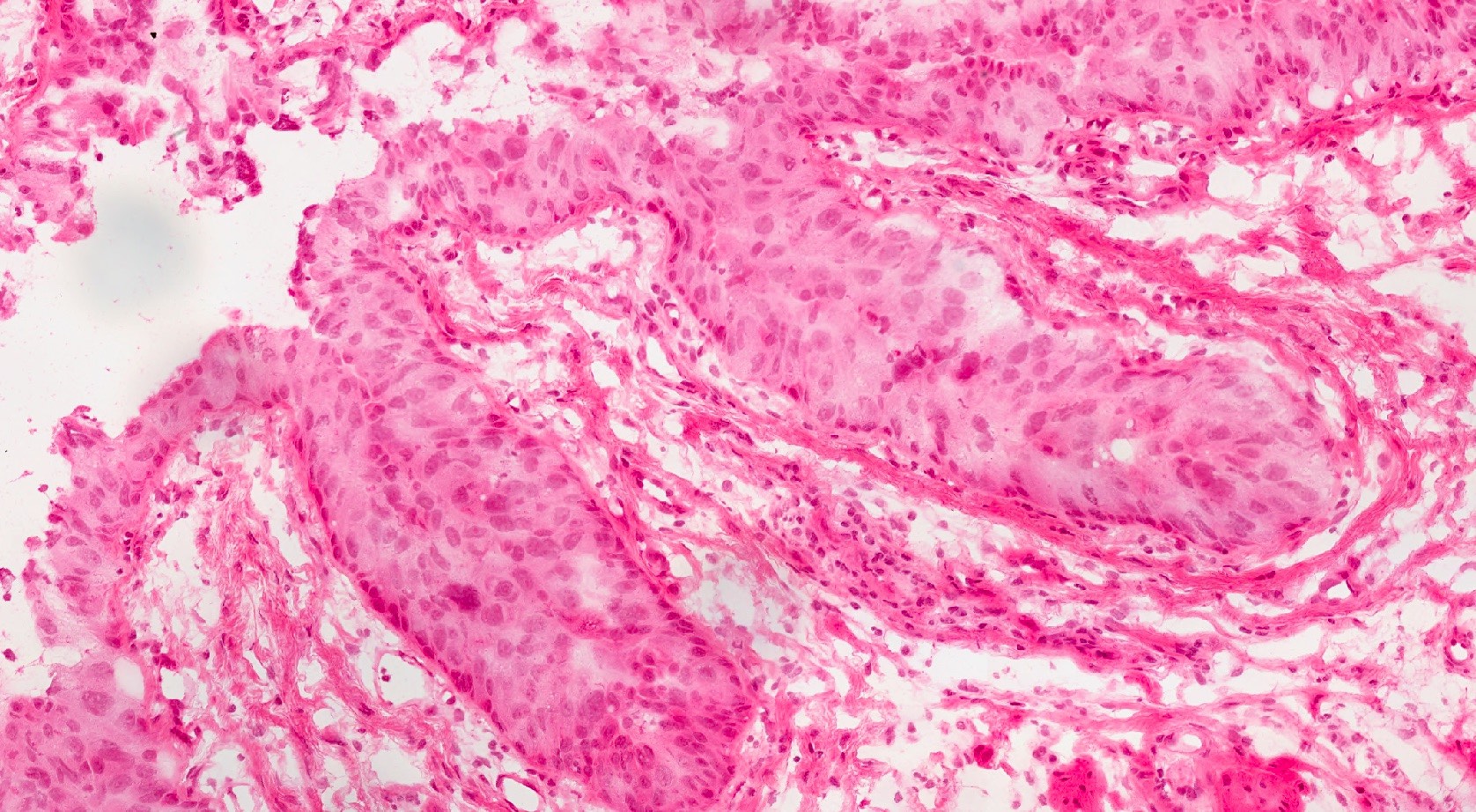
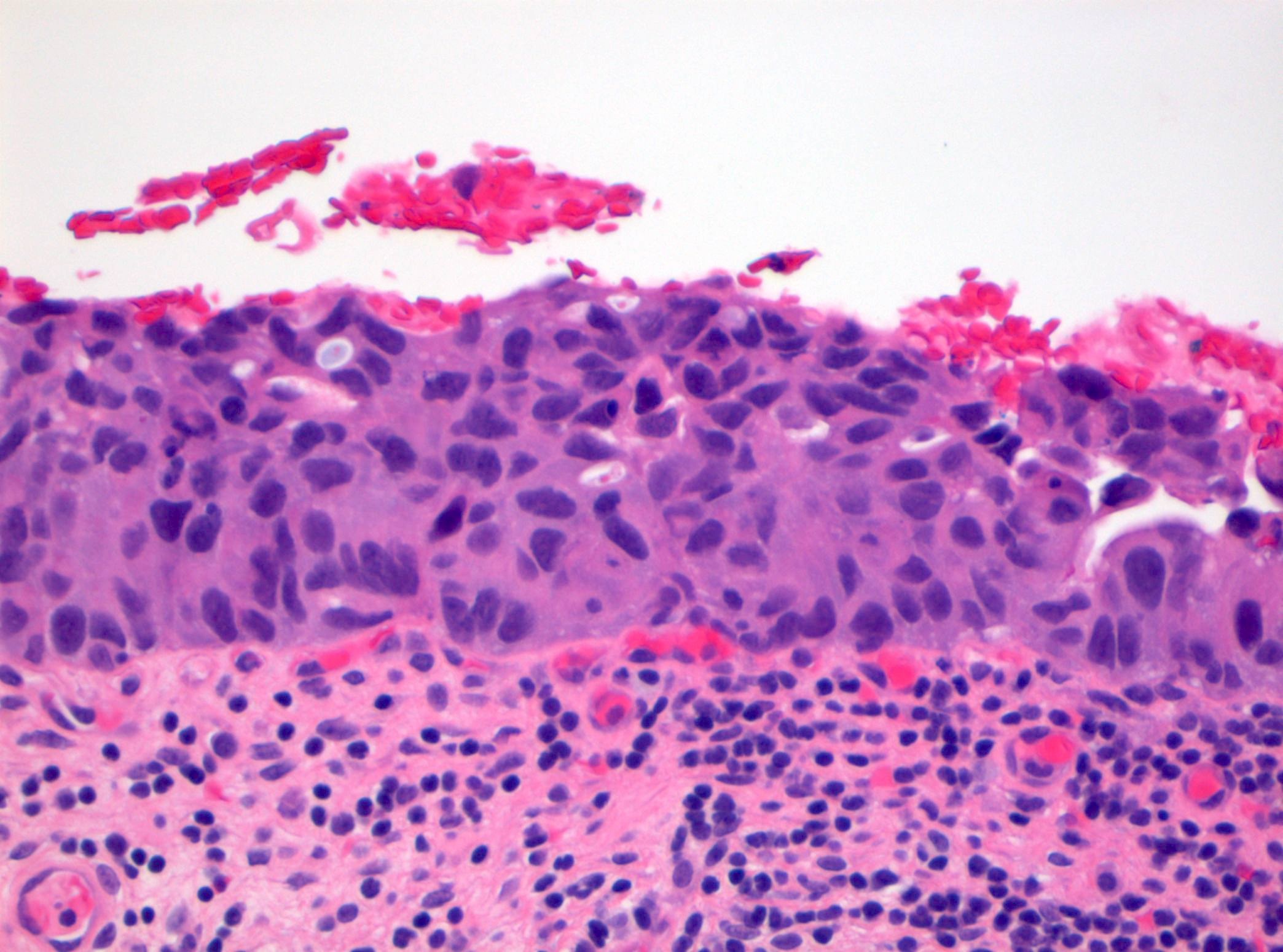
- Cytological features
- Nuclear enlargement (often 5 - 6 times the size of a lymphocyte), with or without pleomorphism
- May have prominent nucleoli, irregular nuclear contour or uneven chromatin distribution
- Frequent mitotic figures seen in the mid to upper layers of the urothelium; atypical figures may be present (Am J Surg Pathol 2001;25:356, Pathology 2021;53:86, J Natl Compr Canc Netw 2009;7:48, Adv Anat Pathol 2021;28:179)
- Architectural features
- Loss of cellular polarity (urothelial cells lose their perpendicular orientation to the basement membrane)
- Nuclear crowding with or without nuclear overlapping
- Irregular thickness characterized by hyperplasia, attenuation or denudation
- Patterns of urothelial CIS
- Large cell pleomorphic: abundant eosinophilic cytoplasm and nuclear pleomorphism
- Large cell nonpleomorphic: abundant eosinophilic cytoplasm without nuclear pleomorphism
- Small cell: scant eosinophilic cytoplasm without nuclear pleomorphism
- Clinging: variable denudation of the urothelium with a patchy monolayer of atypical cells
- Pagetoid: clusters or isolated atypical cells within the urothelium
- Undermining / overriding: presence of atypical cells that undermine / override normal urothelium adjacent to another pattern of urothelial CIS
- Micropapillary: slender tufts of CIS morphologically similar to serous borderline tumor of the ovary; absence of fibrovascular core (Hum Pathol 2012;43:2124)
- Plasmacytoid: round nuclear shape with eccentric, enlarged nuclei shape and globular eosinophilic cytoplasm (Am J Surg Pathol 2019;43:1638)
- With glandular differentiation: atypical urothelial cells characterized by columnar epithelium with apical cytoplasm (Am J Surg Pathol 2018;42:971)
- Urothelial CIS may be seen in association with inflammation and vascular congestion of the underlying lamina propria (J Natl Compr Canc Netw 2009;7:48)
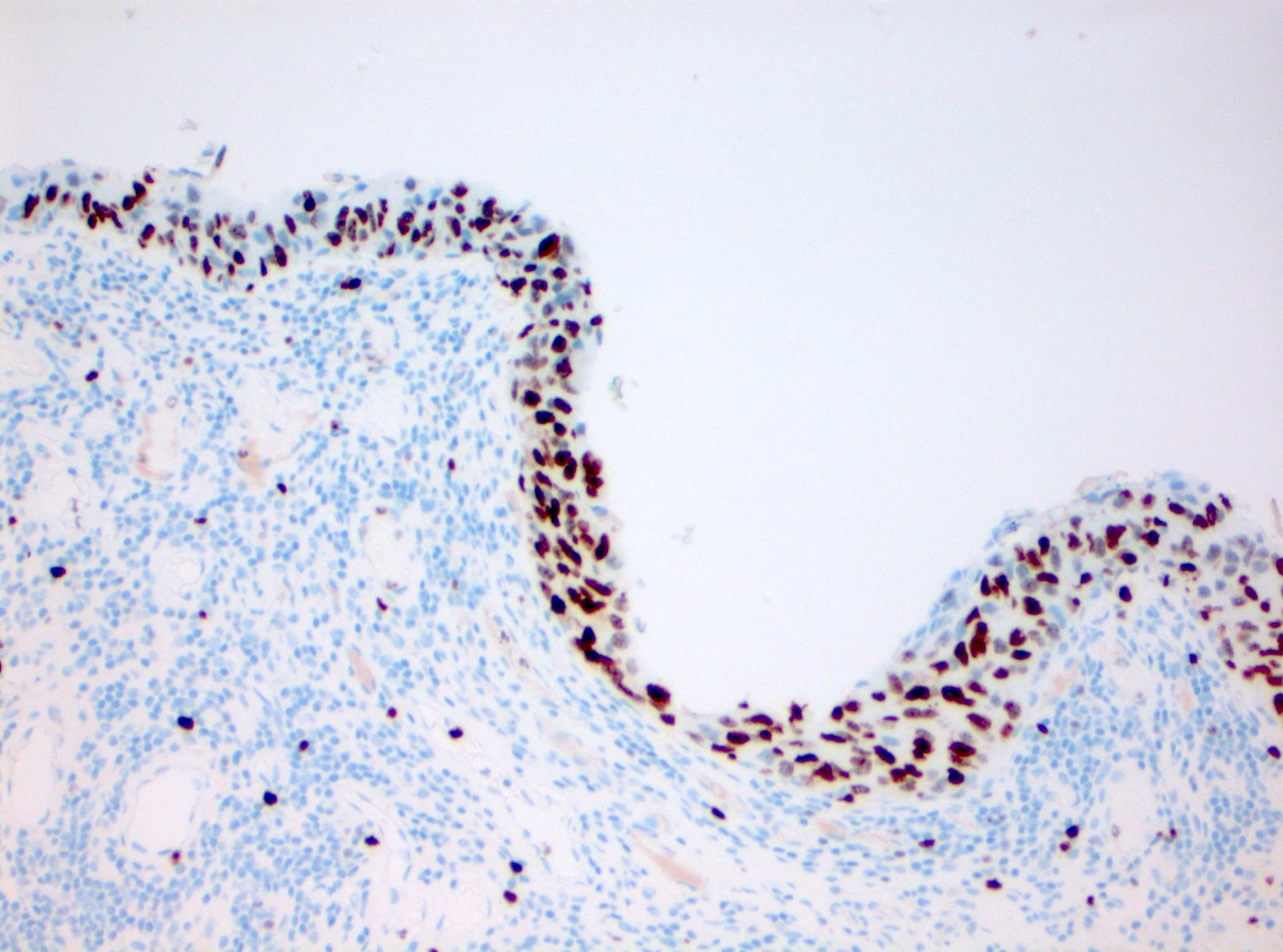
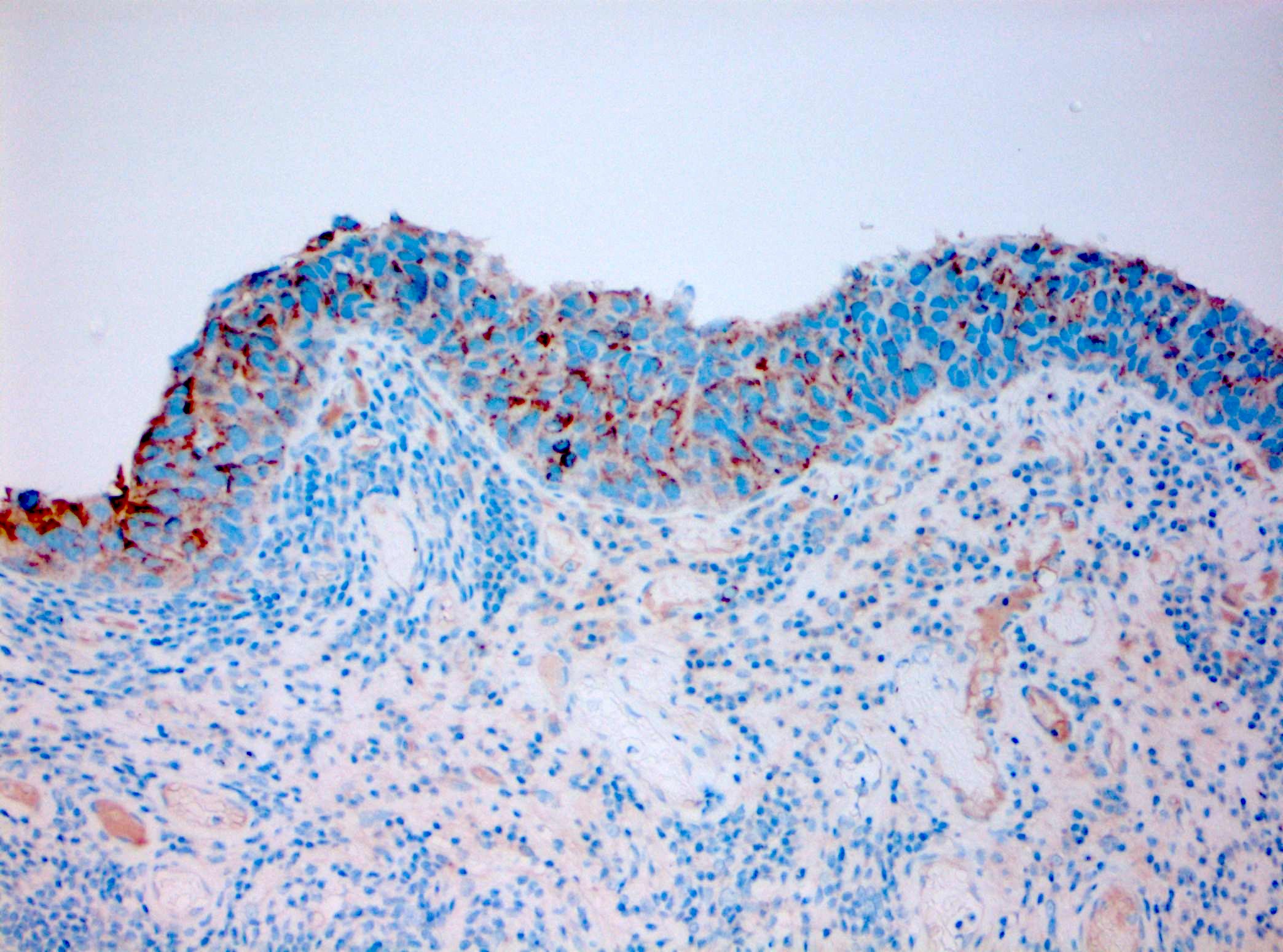
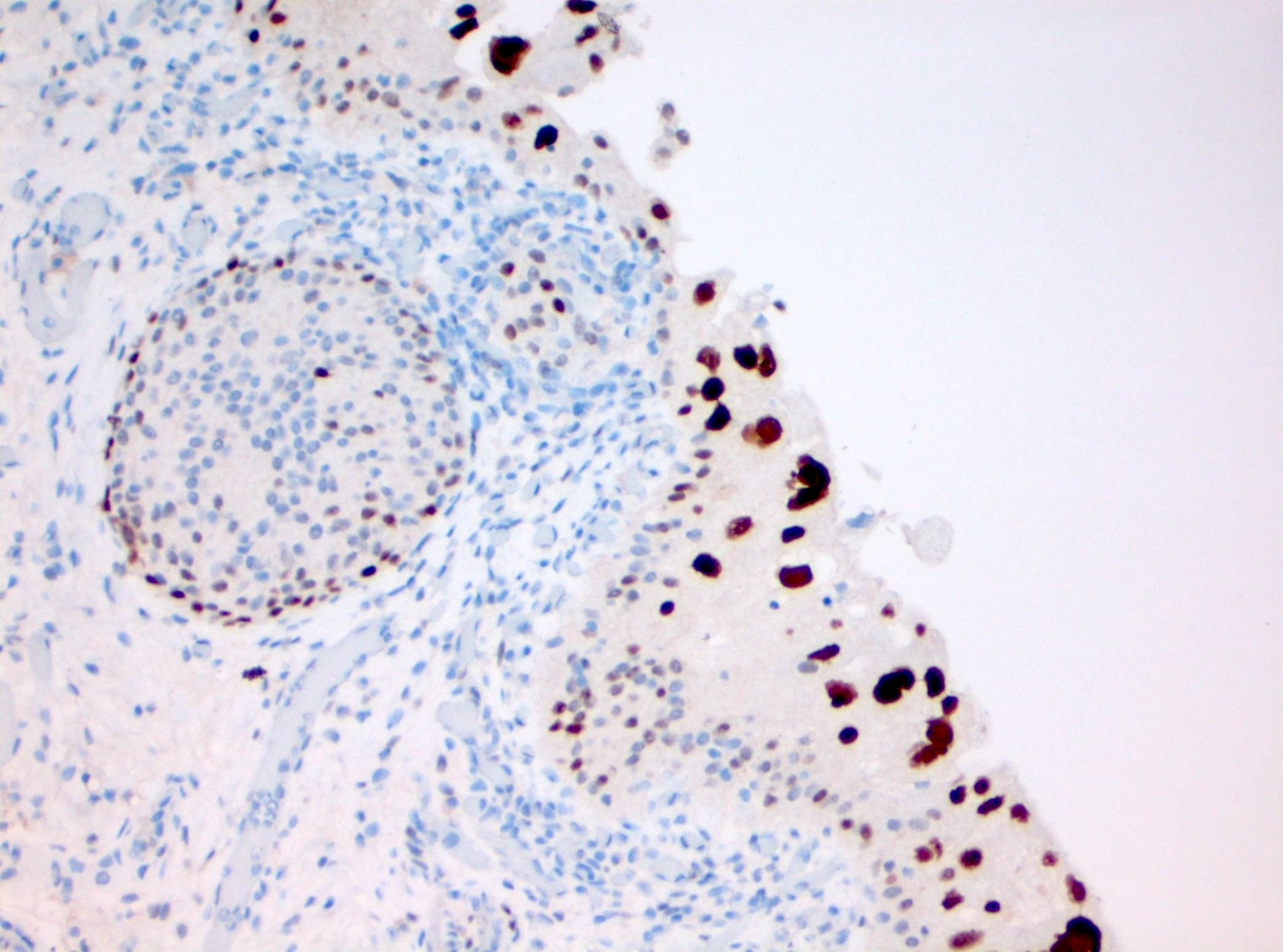
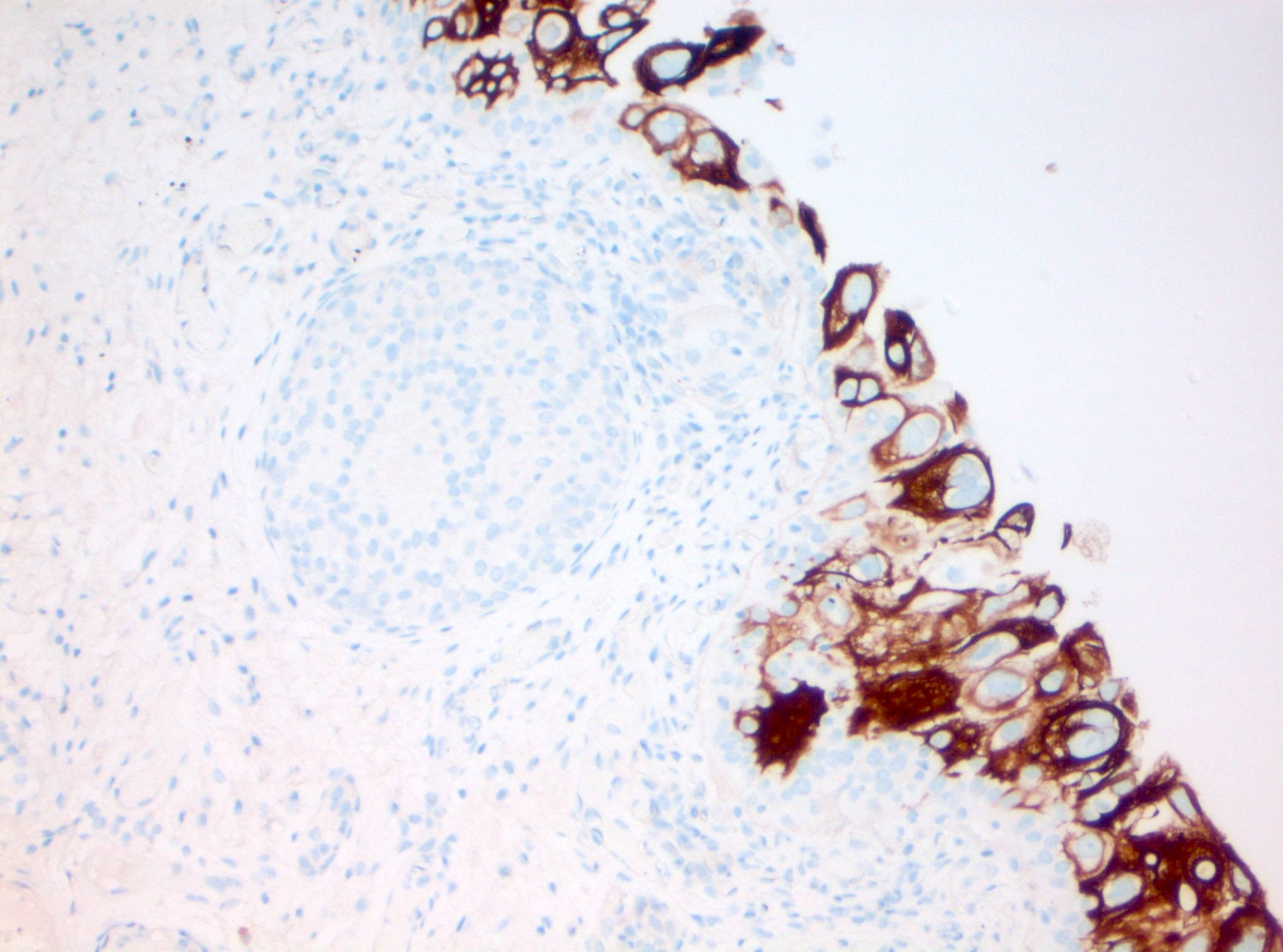
Microscopic (histologic) images
Contributed by Michelle R. Downes and Sean R. Williamson, M.D.
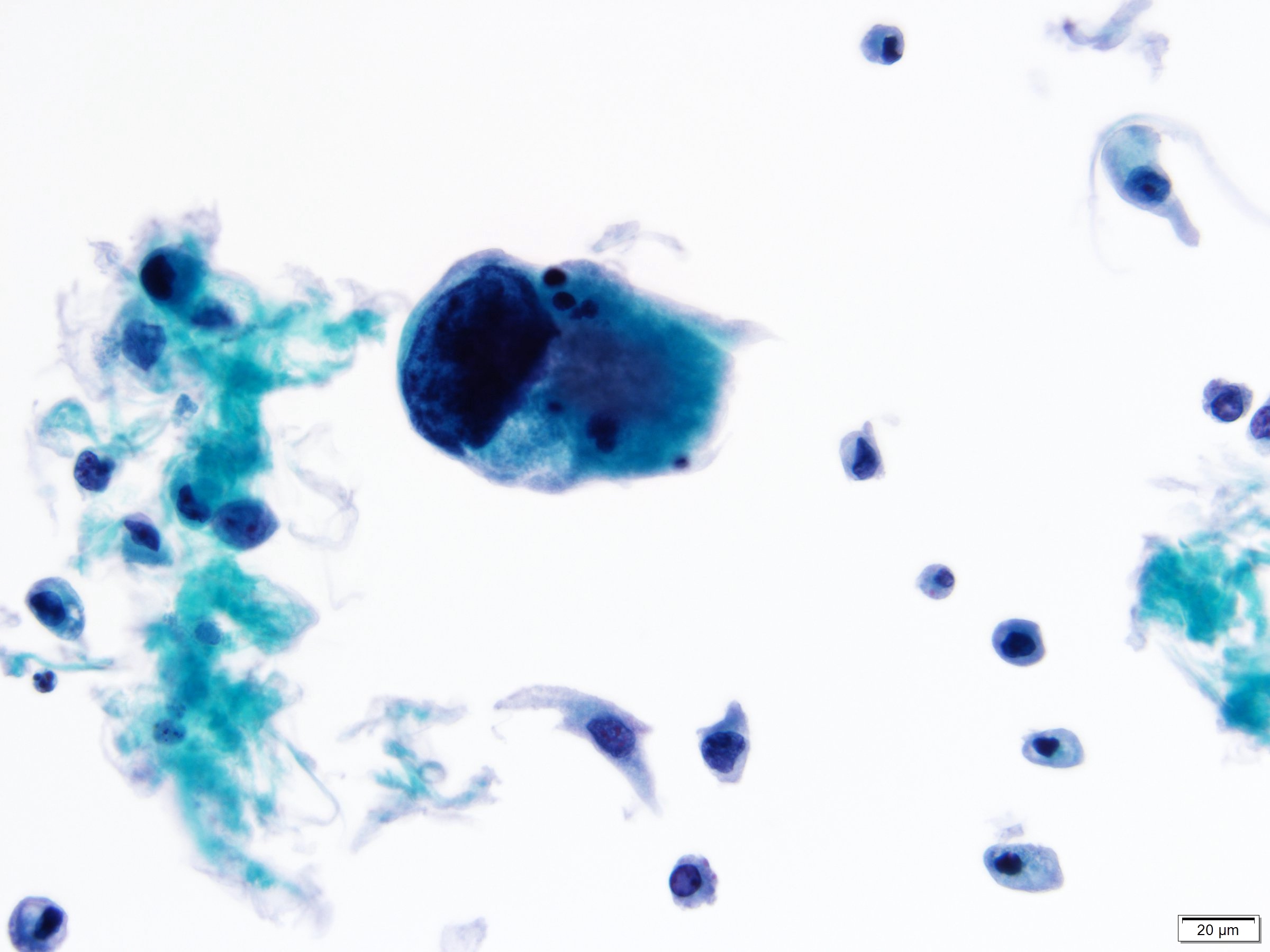
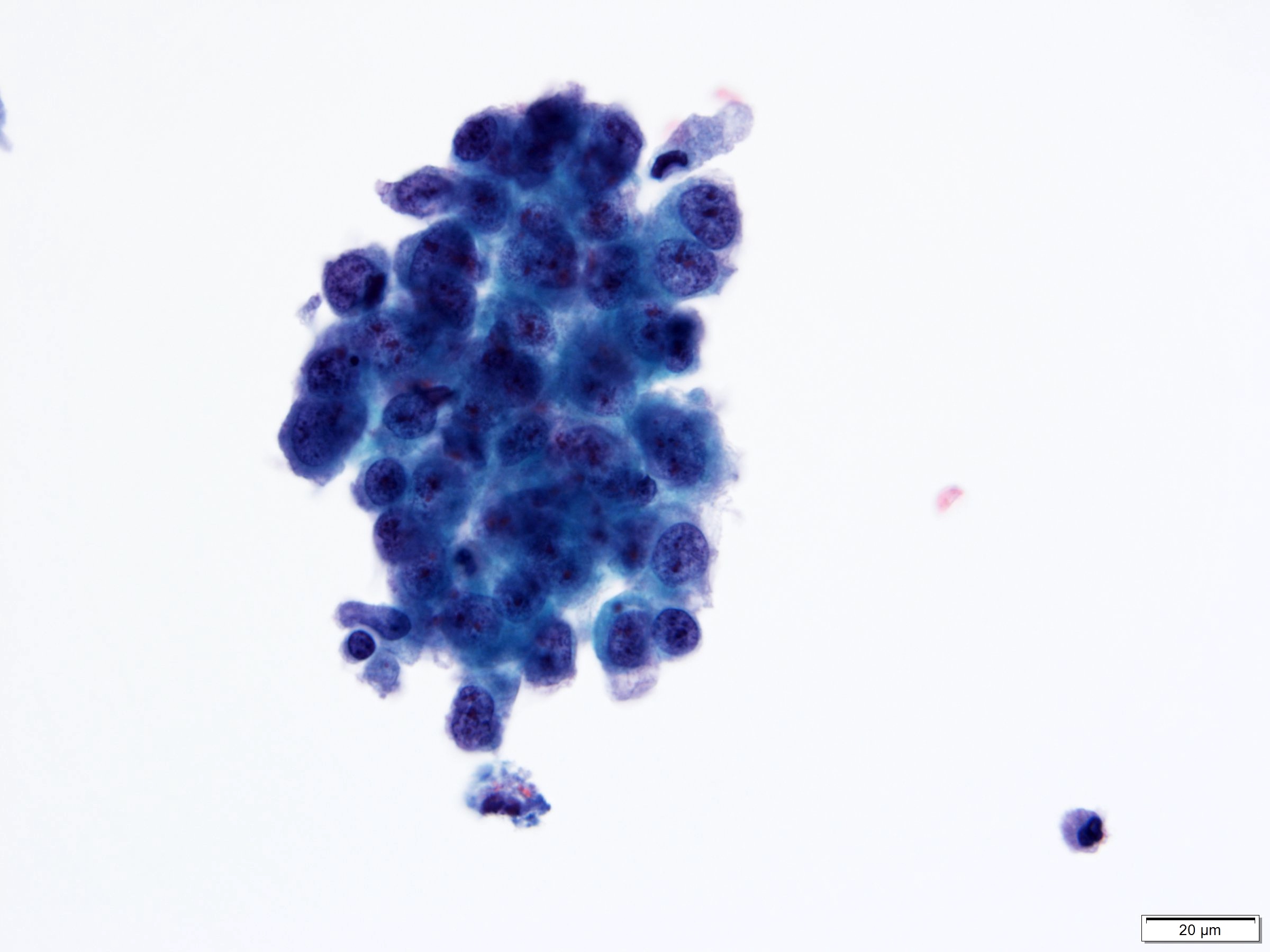
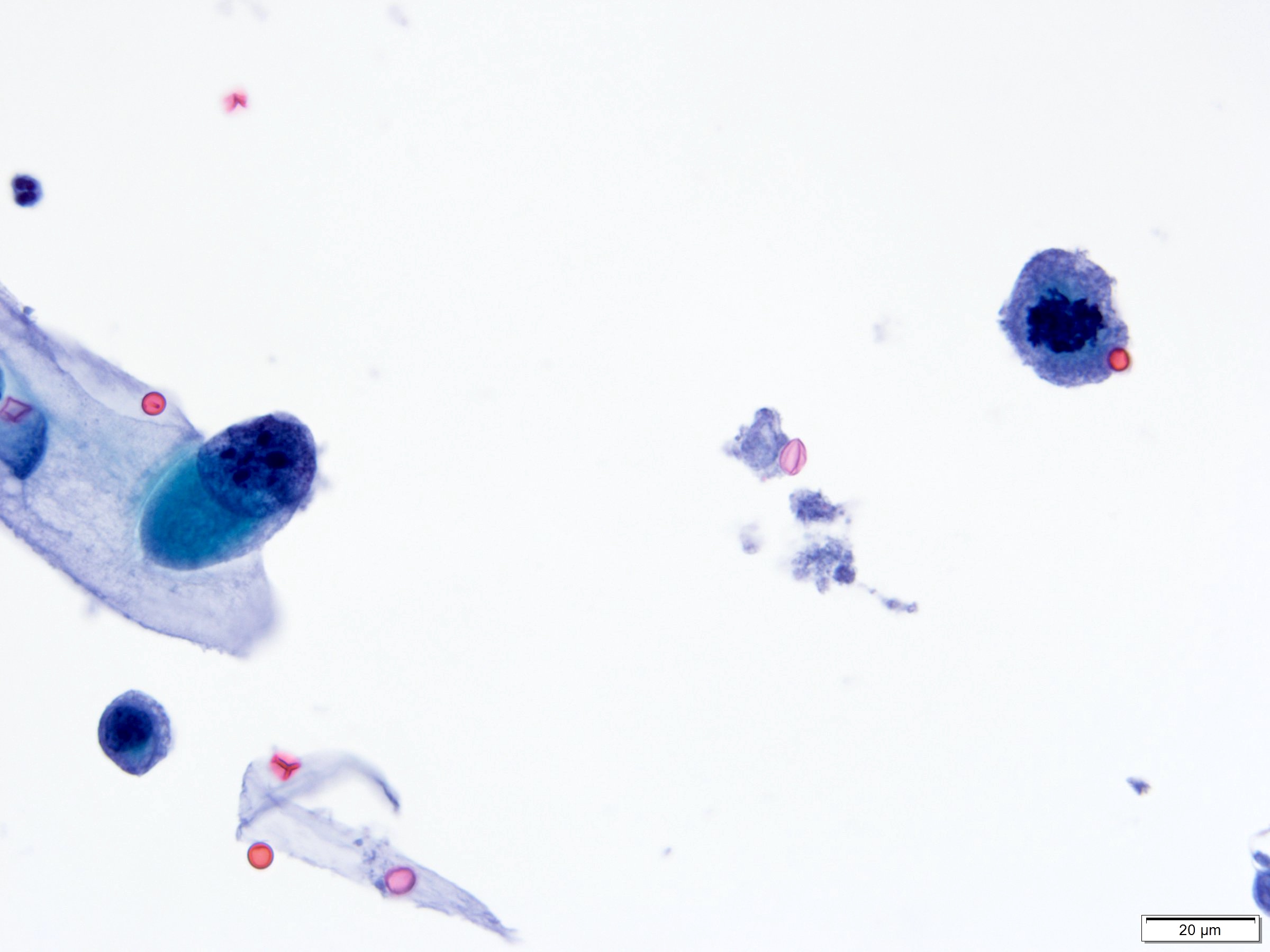
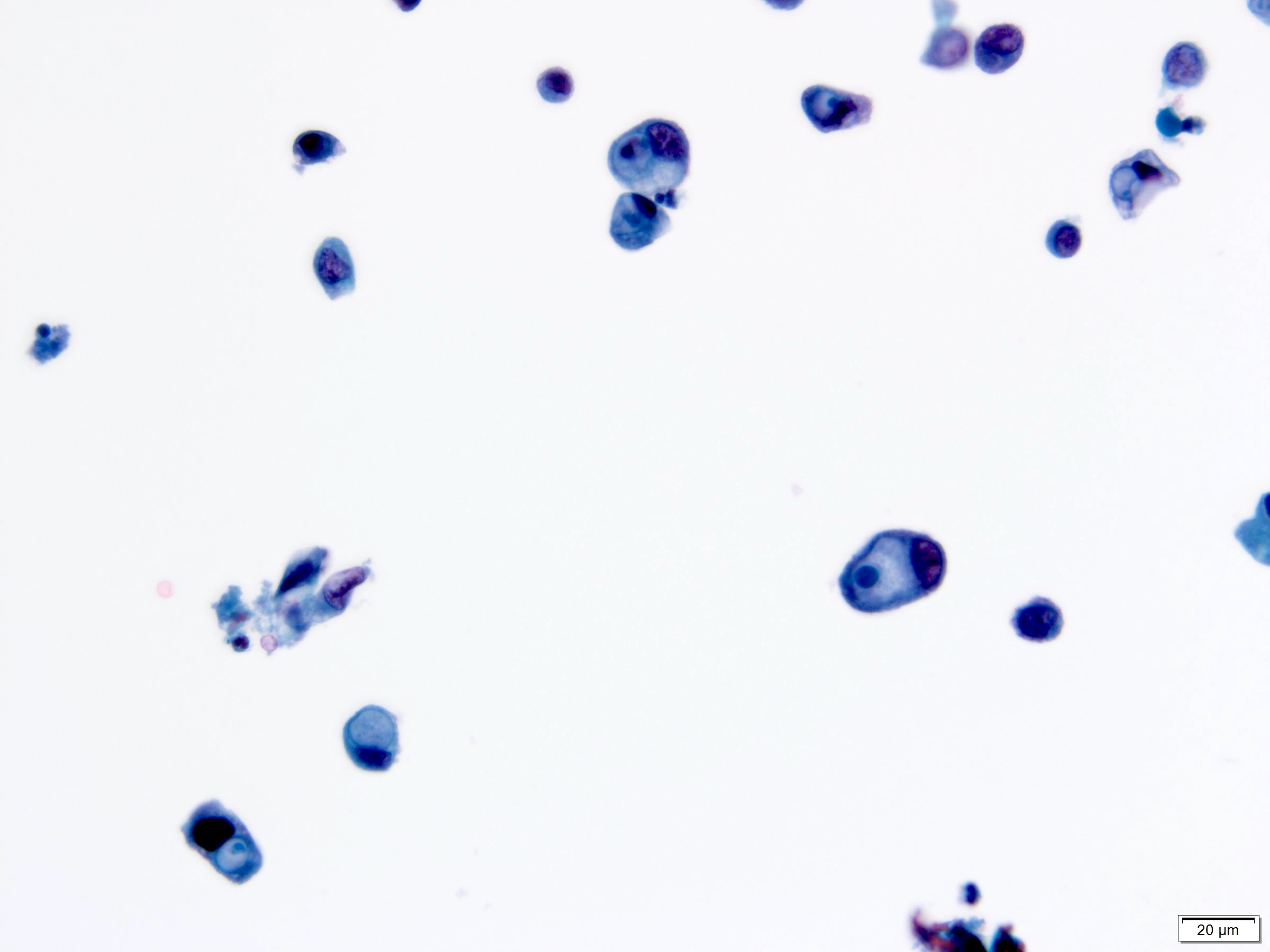
Cytology description
- Cytological findings of CIS are reported as positive for high grade urothelial carcinoma according to The Paris System (TPS)
- 5 - 10 abnormal cells (ideally > 10)
- High N:C ratio (≥ 0.7)
- Moderate to severe hyperchromatic nuclei
- Marked irregular nuclear membrane
- Coarse / clumped chromatin
Positive stains
- Immunohistochemistry can aid in the diagnosis of CIS but due to the immunophenotypic heterogeneity in urothelial lesions, morphology remains the diagnostic gold standard (Adv Anat Pathol 2021;28:179)
- Immunohistochemical findings supporting the diagnosis of CIS
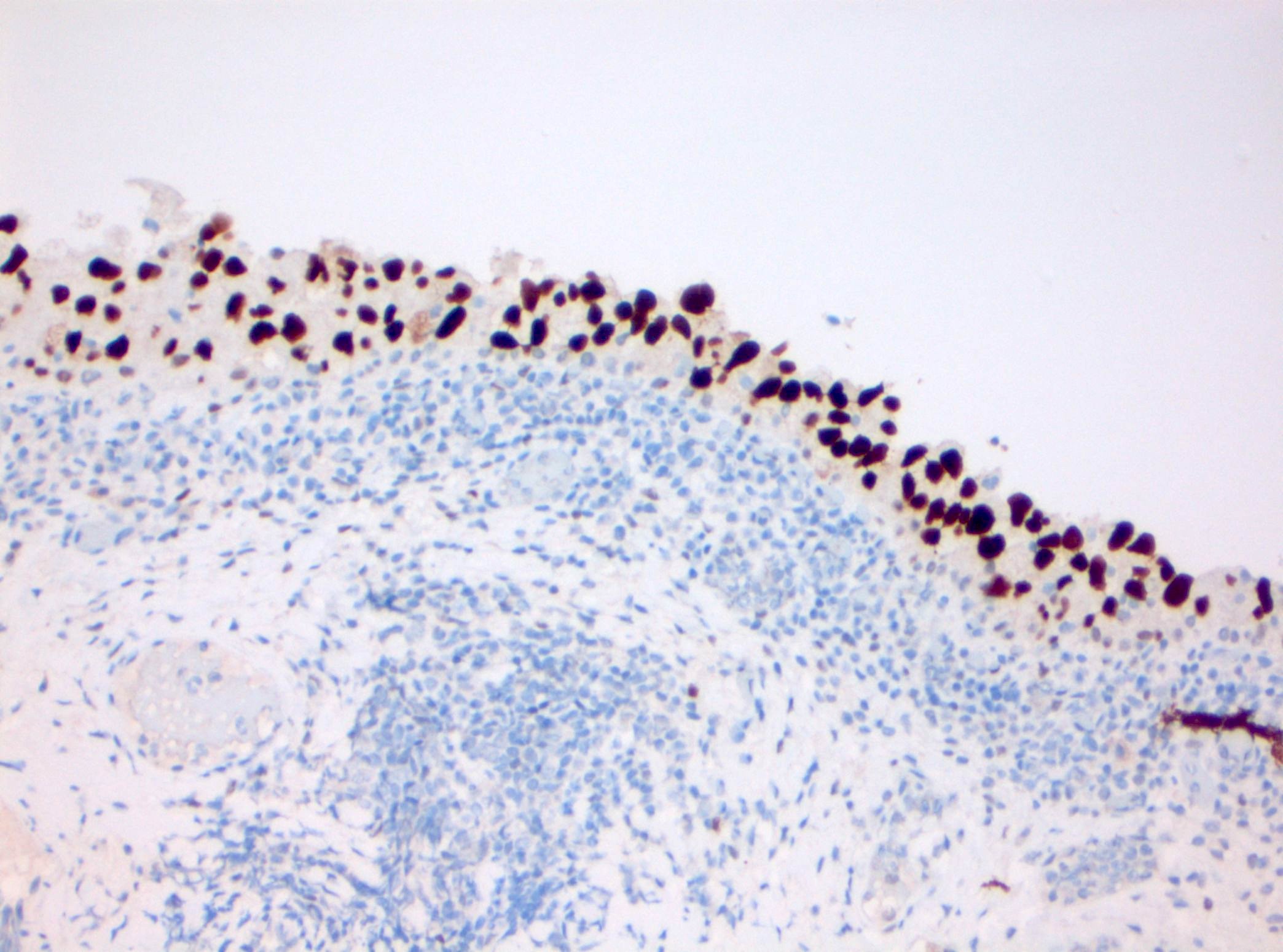
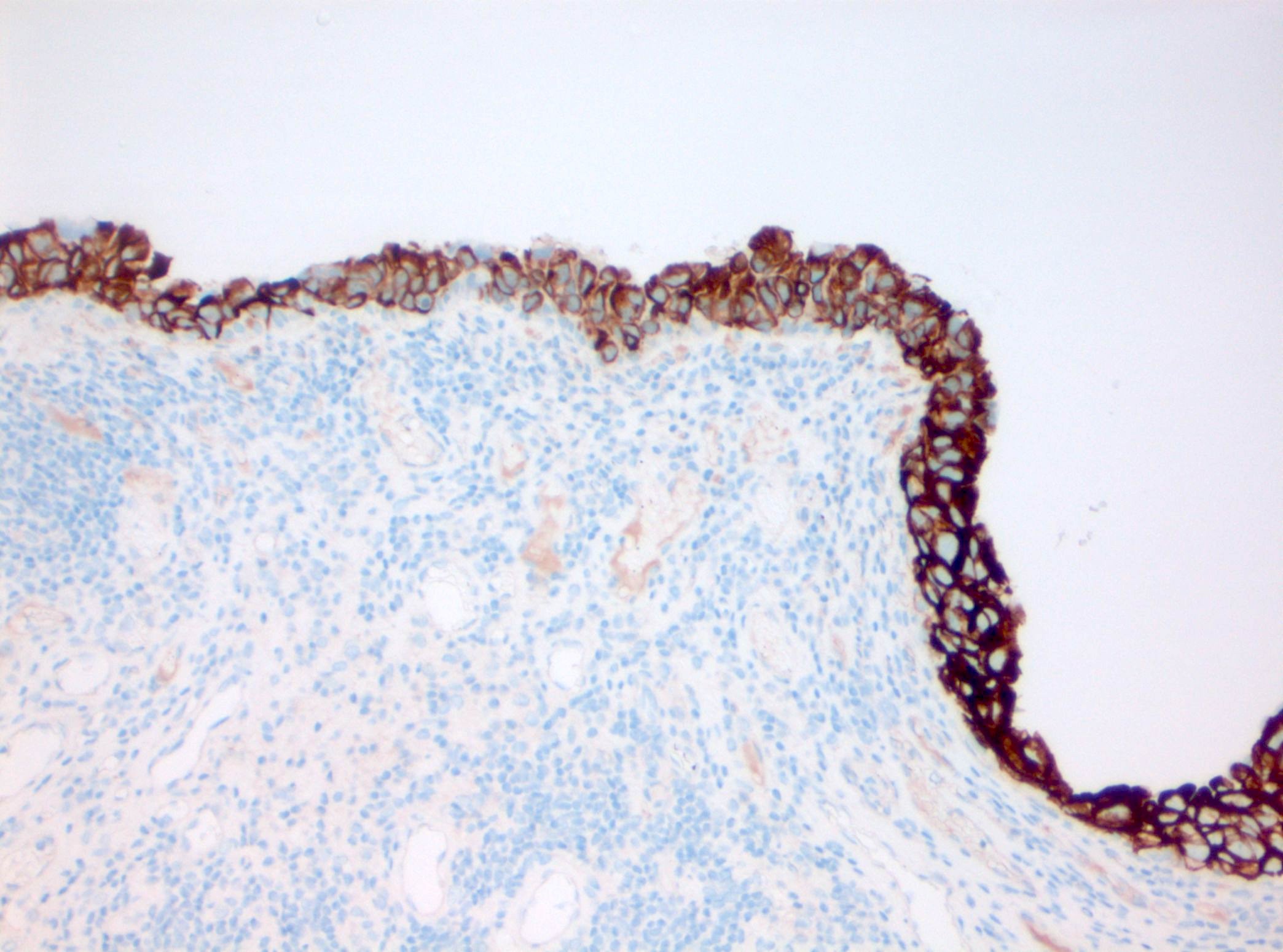
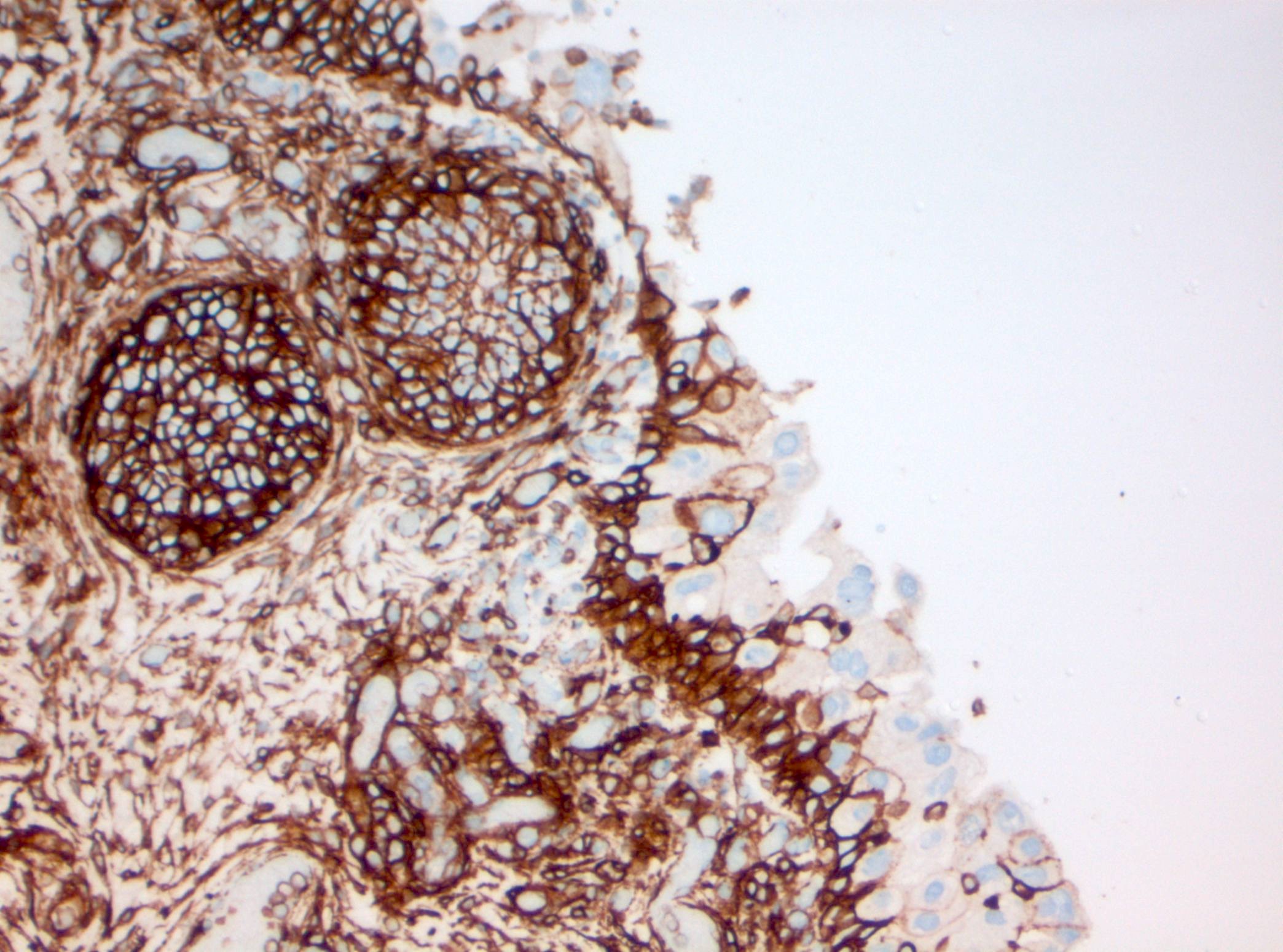
- CK20: full urothelial thickness staining (Am J Surg Pathol 2001;25:1074, Hum Pathol 2020;98:81, Diagn Pathol 2019;14:91, Am J Surg Pathol 2013;37:1815)
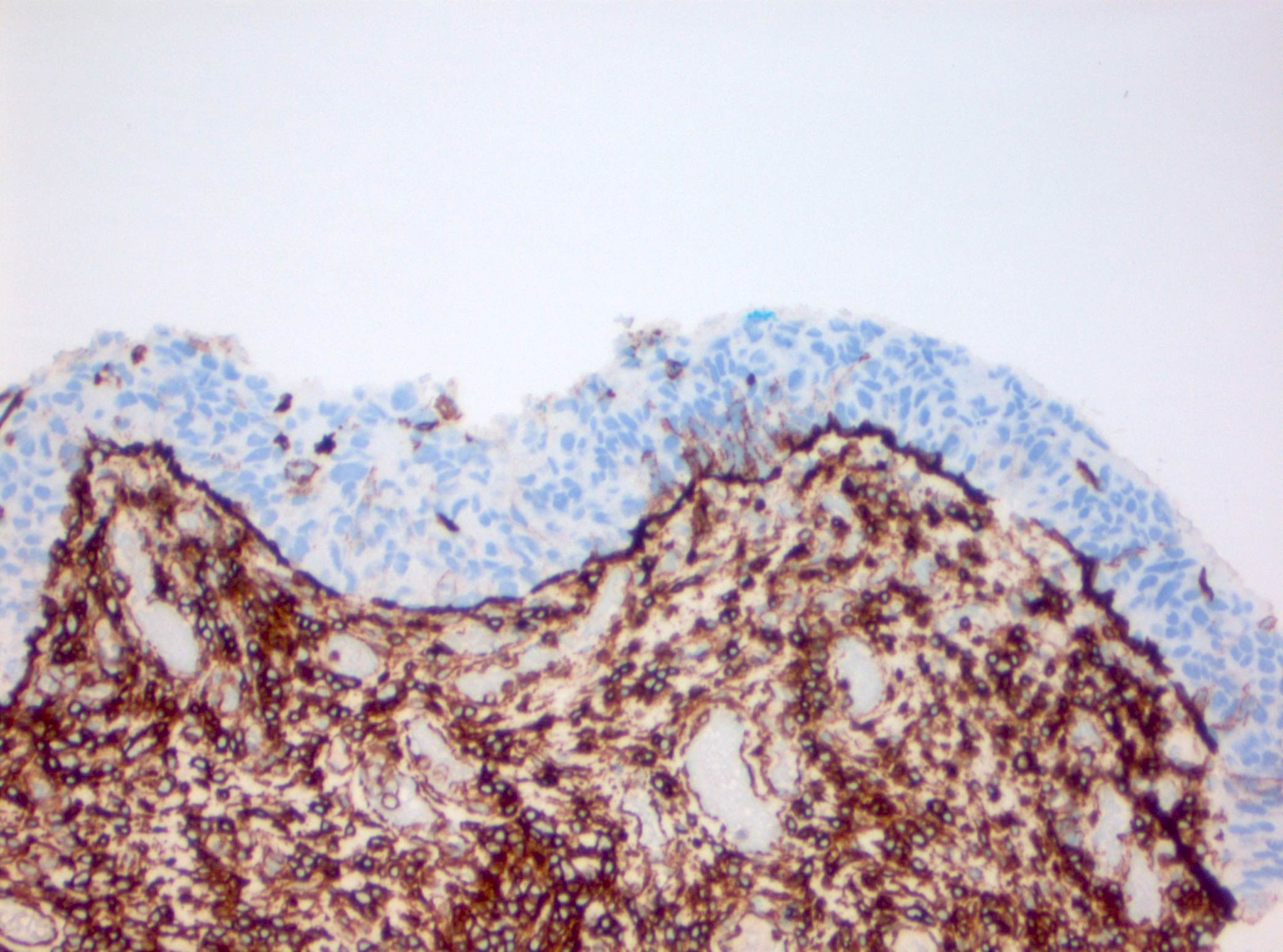
- p53: overexpression or null phenotype (Mod Pathol 2022;35:1287)
- AMACR: strong staining in at least one - third of the urothelial thickness in subset of cases (Am J Surg Pathol 2014;38:1013, Diagn Pathol 2019;14:91)
- Triple panel shows CK20 full thickness, CD44 loss and p53 overexpression or null phenotype (Appl Immunohistochem Mol Morphol 2018;26:180)
- CK17: full urothelial thickness staining in CIS of the upper urinary tract (Hum Pathol 2024;146:43)
- Ki67: elevated proliferation index (also seen in florid reactive urothelial atypia) (Pathol Int 2005;55:248, Hum Pathol 2020;98:81)
Negative stains
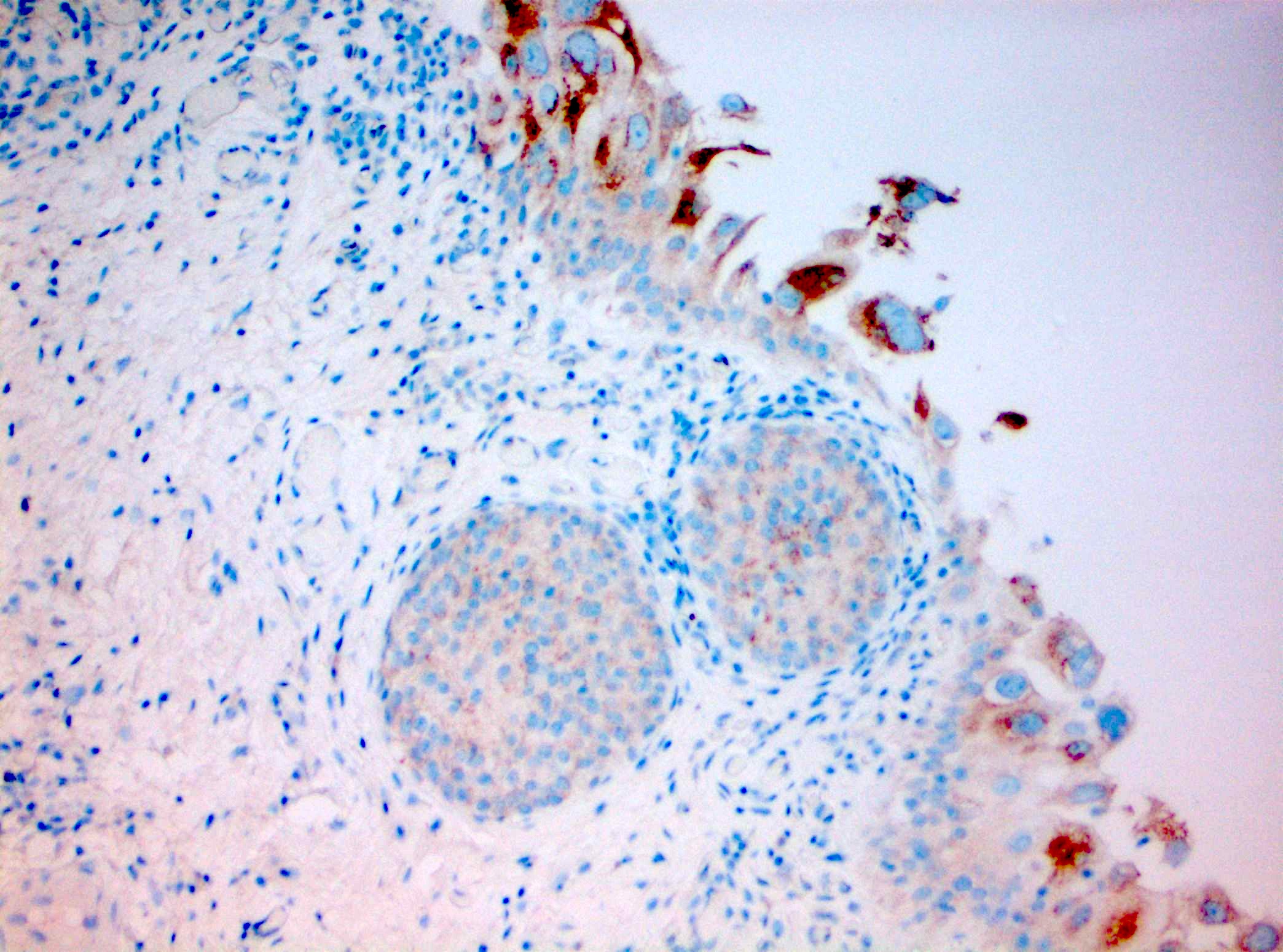
- CD44: typically absent staining or residual membranous staining of the basal cells (Am J Surg Pathol 2001;25:1074, Diagn Pathol 2019;14:91, Am J Surg Pathol 2013;37:1815)
Molecular / cytogenetics description
- See Pathophysiology
Videos
Urothelial CIS
Sample pathology report
- Bladder, transurethral resection:
- Carcinoma in situ present, Tis
- Muscularis propria sampled
- Bladder, transurethral resection:
- Papillary urothelial carcinoma, high grade (grade 3/3 WHO 1973)
- Negative for invasion, Ta
- Muscularis propria sampled
- Carcinoma in situ present (Tis) in separately submitted sample
- Bladder and prostate, cystoprostatectomy:
- Urothelial carcinoma, high grade
- Invasion of bladder neck, prostate and bilateral seminal vesicle, pT4
- Carcinoma in situ in prostatic urethra
- Positive for lymphovascular space invasion
- Margins positive: periprostatic soft tissue and en face urethral margin
- 1 node, negative for carcinoma (0/1)
- Kidney and ureter (right), nephroureterectomy:
- Urothelial carcinoma, high grade, of distal ureter; 3.0 cm in maximum gross dimension
- Invasion through ureteral wall into adipose tissue, pT3
- Extension to inked soft tissue (adipose tissue) margins
- Positive for lymphovascular space invasion
- Carcinoma in situ present
- En face ureteral margin and vasculature, negative for malignancy
- Kidney with hydronephrosis, atrophy and chronic inflammation
Differential diagnosis
- Reactive / inflammatory urothelial atypia:
- Nuclear enlargement often < 3 times the size of a lymphocyte with minimal pleomorphism (Appl Immunohistochem Mol Morphol 2021;29:127)
- Round nuclei with fine and evenly distributed chromatin and often small nucleoli
- Absence of atypical mitosis
- Inflammatory cells within the urothelium support the diagnosis of inflammatory atypia but can also be present in urothelial CIS
- Triple panel shows CD44 full thickness, CK20 umbrella cells only and p53 wild type (Appl Immunohistochem Mol Morphol 2018;26:180)
- Therapy related urothelial atypia:
- Regenerative atypia (nuclear enlargement with even chromatin distribution and prominent nucleoli), granulomatous inflammation, ulceration, tumor necrosis, lamina propria edema and inflammation, umbrella cell atypia with cytoplasmic vacuolation or multinucleation can be seen as part of therapy related changes (Adv Anat Pathol 2021;28:179, Semin Diagn Pathol 2018;35:218, Histopathology 2017;70:10)
- Urothelial dysplasia:
- Histological features falling short for the diagnosis of urothelial CIS (Appl Immunohistochem Mol Morphol 2021;29:127)
- Characterized by urothelial disorder and nuclear enlargement often < 3 times the size of a lymphocyte
- Triple panel shows CK20 full thickness, CD44 loss and p53 wild type (Appl Immunohistochem Mol Morphol 2018;26:180)
- High grade papillary urothelial carcinoma:
- Presence of fibrovascular core
- High grade flat lesion adjacent to papillary urothelial carcinoma or sampled at the site of prior papillary urothelial carcinoma could represent the shoulder lesion of a papillary urothelial carcinoma (Surg Pathol Clin 2022;15:629)
Additional references
Board review style question #1
A 70 year old woman who presented with macroscopic hematuria underwent cystoscopy, which revealed a small, flat, erythematous lesion in the left lateral bladder wall. Transurethral resection of the bladder lesion was performed and showed enlarged urothelial cells with intraurothelial inflammatory cells. What is the best stain combination that can be used to distinguish urothelial carcinoma in situ from reactive atypia?
- AMACR, Ki67
- Ki67, CK20
- p53, CK20
- p63, GATA3
Board review style answer #1
C. p53, CK20. CK20 full thickness staining and aberrant p53 expression support the diagnosis of urothelial carcinoma in situ versus reactive atypia. Answer A is incorrect because AMACR lacks sensitivity and Ki67 can be elevated in both urothelial carcinoma in situ and florid reactive urothelial atypia. Answer D is incorrect because GATA3 and p63 are positive in benign and neoplastic urothelium. Answer B is incorrect because Ki67 can be elevated in both urothelial carcinoma in situ and florid reactive urothelial atypia.
Comment Here
Reference: Carcinoma in situ
Comment Here
Reference: Carcinoma in situ
Board review style question #2
What morphologic findings are the most consistent with urothelial carcinoma in situ?
- Nuclear enlargement, denudation, ulceration, granulomatous inflammation, absence of papillary formation
- Nuclear enlargement, hyperchromasia, loss of cellular polarity, papillary formation
- Nuclear enlargement, hyperchromasia, nuclear contour irregularities, loss of cellular polarity, absence of papillary formation
- Nuclear enlargement, intraurothelial inflammation, round nuclei with fine chromatin, absence of papillary formation
Board review style answer #2
C. Nuclear enlargement, hyperchromasia, nuclear contour irregularities, loss of cellular polarity, absence of papillary formation. All these findings are consistent with urothelial carcinoma in situ. Answer D is incorrect because these findings are most consistent with reactive / inflammatory atypia. Answer A is incorrect because these findings are most consistent with Bacillus Calmette-Guérin induced urothelial atypia. Answer B is incorrect because these findings are consistent with high grade papillary urothelial carcinoma.
Comment Here
Reference: Carcinoma in situ
Comment Here
Reference: Carcinoma in situ