Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Diagnosis | Radiology description | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Positive stains | Negative stains | Sample pathology report | Differential diagnosis | Additional references | Board review style question #1 | Board review style answer #1Cite this page: Allard FD. Intra-ampullary papillary tubular neoplasm (IAPN). PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/ampullaiapn.html. Accessed March 31st, 2025.
Definition / general
- Intra-ampullary papillary tubular neoplasm (IAPN) is the name proposed in 2010 to unify the nomenclature for mass forming preinvasive (dysplastic) lesions that arise within the ampulla of Vater (Am J Surg Pathol 2010;34:1731)
Essential features
- The precursor lesion of intra-ampullary adenocarcinomas (Am J Surg Pathol 2012;36:1592)
- Majority (78%) of IAPNs in one large series harbored an invasive component (Am J Surg Pathol 2010;34:1731)
- Analogous to pancreatic or biliary intraductal papillary and tubular neoplasms (Am J Surg Pathol 2010;34:1731)
- Must be localized within (or predominantly within) the ampulla (Am J Surg Pathol 2010;34:1731)
Terminology
- Other / previous terms include intestinal type adenoma, noninvasive pancreatobiliary type neoplasm
ICD coding
- ICD-O
- ICD-10: C24.1 - malignant neoplasm of ampulla of Vater
- ICD-11
- 2E92.2 & XH3DV3 - benign neoplasm of duodenum & adenoma, NOS
- 2E92.2 & XH8MU5 - benign neoplasm of duodenum & adenomatous polyp, NOS
Epidemiology
- Mean age: 64 - 67 years; range: 27 - 85 years (Am J Surg Pathol 2010;34:1731, Med Sci Monit 2019;25:7332)
- M:F = 2.4 in one surgical series and 42.5% men / 57.5% women in SEER analysis (Am J Surg Pathol 2010;34:1731, Med Sci Monit 2019;25:7332)
- Estimated prevalence based on autopsy studies is 0.04 - 0.12% (World J Gastrointest Endosc 2011;3:241)
- Incidence from SEER database study was 0.025 in 2014 (Med Sci Monit 2019;25:7332)
- Incidence has been decreasing annually since 2016 (Med Sci Monit 2019;25:7332)
Sites
- Ampulla of Vater: within the ampullary channel (common channel) or the intra-ampullary portions of the common bile duct or main pancreatic duct
Pathophysiology
- Unknown
Etiology
- Given the prevalence of neoplasia in and around the ampulla, authors have speculated that bile acids may be carcinogenic (Mutat Res 2005;589:47)
Clinical features
- Patients may be asymptomatic or present with obstructive symptoms: jaundice, weight loss, abdominal pain, nausea / vomiting, pruritus, dark urine / light stools or tarry stools (Am J Surg Pathol 2010;34:1731)
- Rarely, patients present with cholangitis or pancreatitis
Diagnosis
- Mass involving the ampullary region may be found on cross sectional imaging
- Tissue diagnosis is often made via endoscopic retrograde cholangiopancreatography (ERCP) biopsy
Radiology description
- Imaging modalities used to examine the ampulla include computed tomography (CT) and magnetic resonance imaging (MRI) (Am J Surg Pathol 2010;34:1731, Radiographics 2022;42:1320)
- Imaging generally shows a dilated common bile duct, intra / extrahepatic ducts; an ampullary, duodenal or pancreatic mass; or enlarged or irregular papillae (Am J Surg Pathol 2010;34:1731)
- Double duct sign is present in 52% of cases, with both biliary and pancreatic duct dilatation (Radiographics 2022;42:1320)
- It may be challenging to identify lesions < 1 cm due to the duodenal folds (Radiographics 2022;42:1320)
Prognostic factors
- Tumor size and degree of dysplasia correlate with risk of invasive component (Am J Surg Pathol 2010;34:1731)
- Overall 3 and 5 year survival rates in patients with tumors lacking an identified invasive component is 100% (Am J Surg Pathol 2010;34:1731)
- Overall 5 year survival rates in patients with tumors containing an invasive component is 45% (Am J Surg Pathol 2010;34:1731)
- Cases showing high grade dysplasia are more likely to be associated with an invasive carcinoma (Am J Surg Pathol 2010;34:1731)
Case reports
- 61 year old woman presented with combined IAPN and ampullary neuroendocrine carcinoma (World J Clin Cases 2022;10:8045)
- 66 year old man presented with mucinous adenocarcinoma of the ampulla arising within an IAPN (Surg Case Rep 2021;7:25)
- 80 year old man presented with micropapillary adenocarcinoma of the ampulla arising within an IAPN (World J Clin Cases 2021;9:2671)
Treatment
- Surgery is the mainstay of treatment: transduodenal ampullectomy or pancreatoduodenectomy (Whipple procedure)
- Endoscopic resections are increasingly being used (J Clin Gastroenterol 2012;46:8, World J Gastrointest Endosc 2011;3:241, Gastrointest Endosc 2015;82:773)
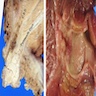
Gross description
- Intraluminal polypoid or papillary mass forming exophytic lesion within a dilated ampullary duct (Am J Surg Pathol 2010;34:1731)
- Lesion fills and may obstruct the ampullary channel (Am J Surg Pathol 2010;34:1731)
- Duodenal orifice is often widened, irregular and may be ulcerated (Am J Surg Pathol 2010;34:1731)
- Average tumor size was 2.7 cm in one large series (Am J Surg Pathol 2010;34:1731)
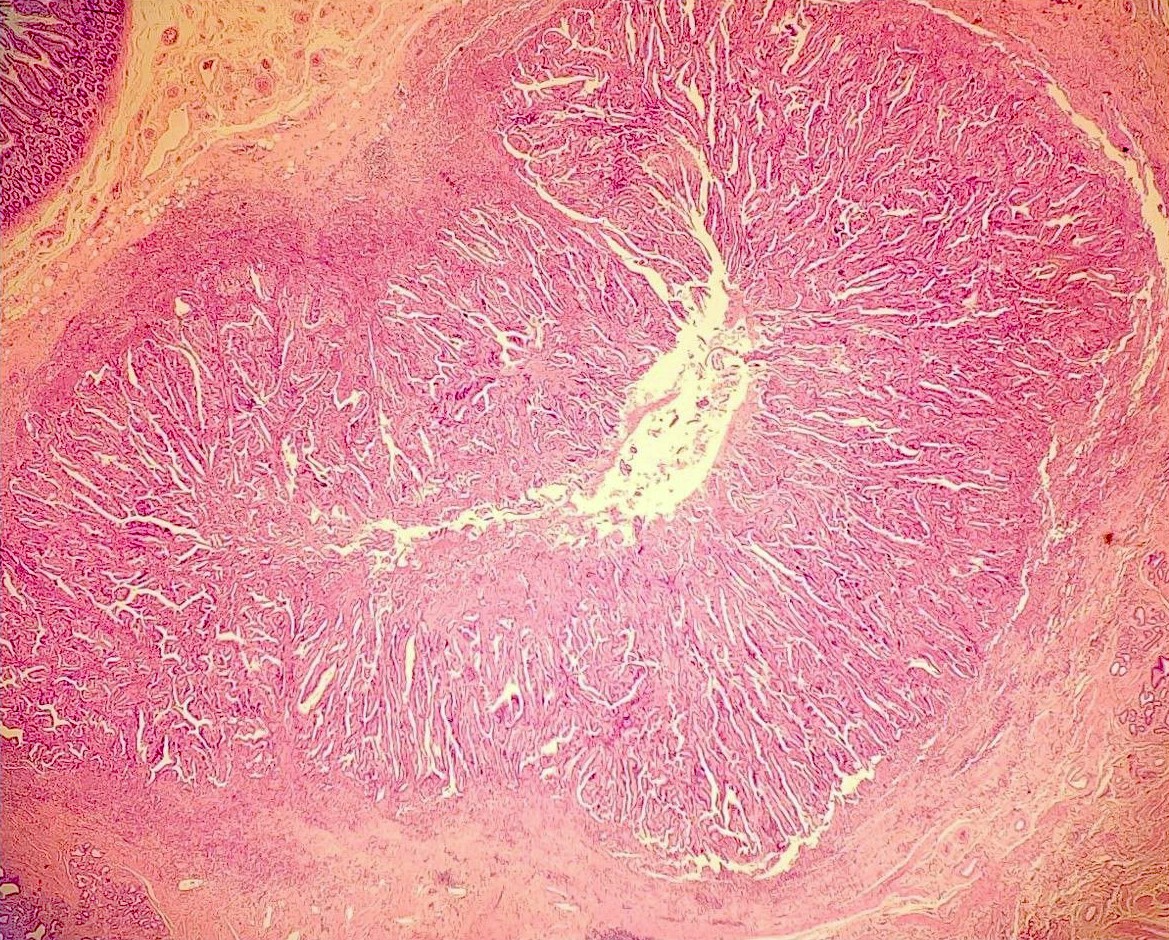
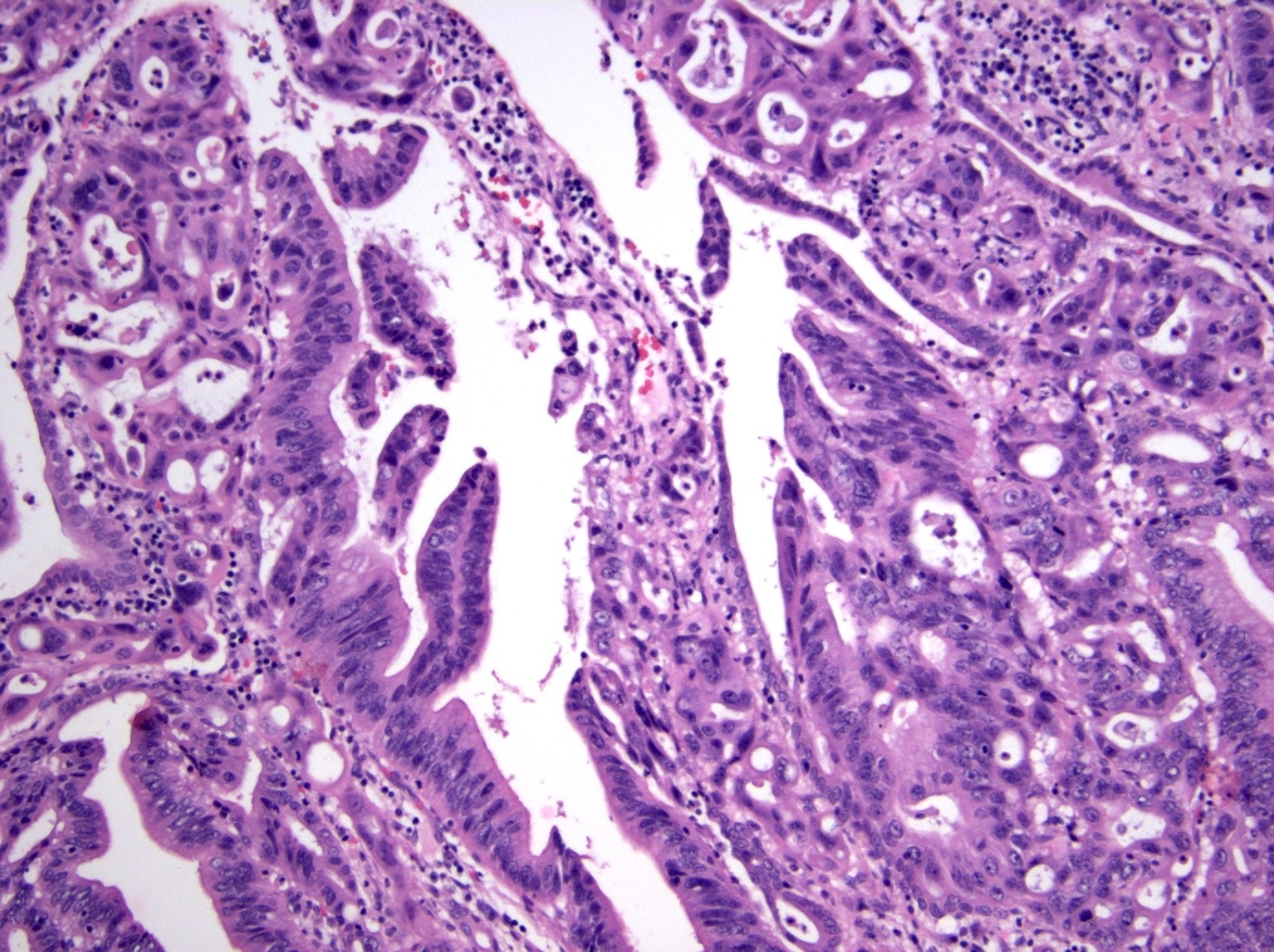
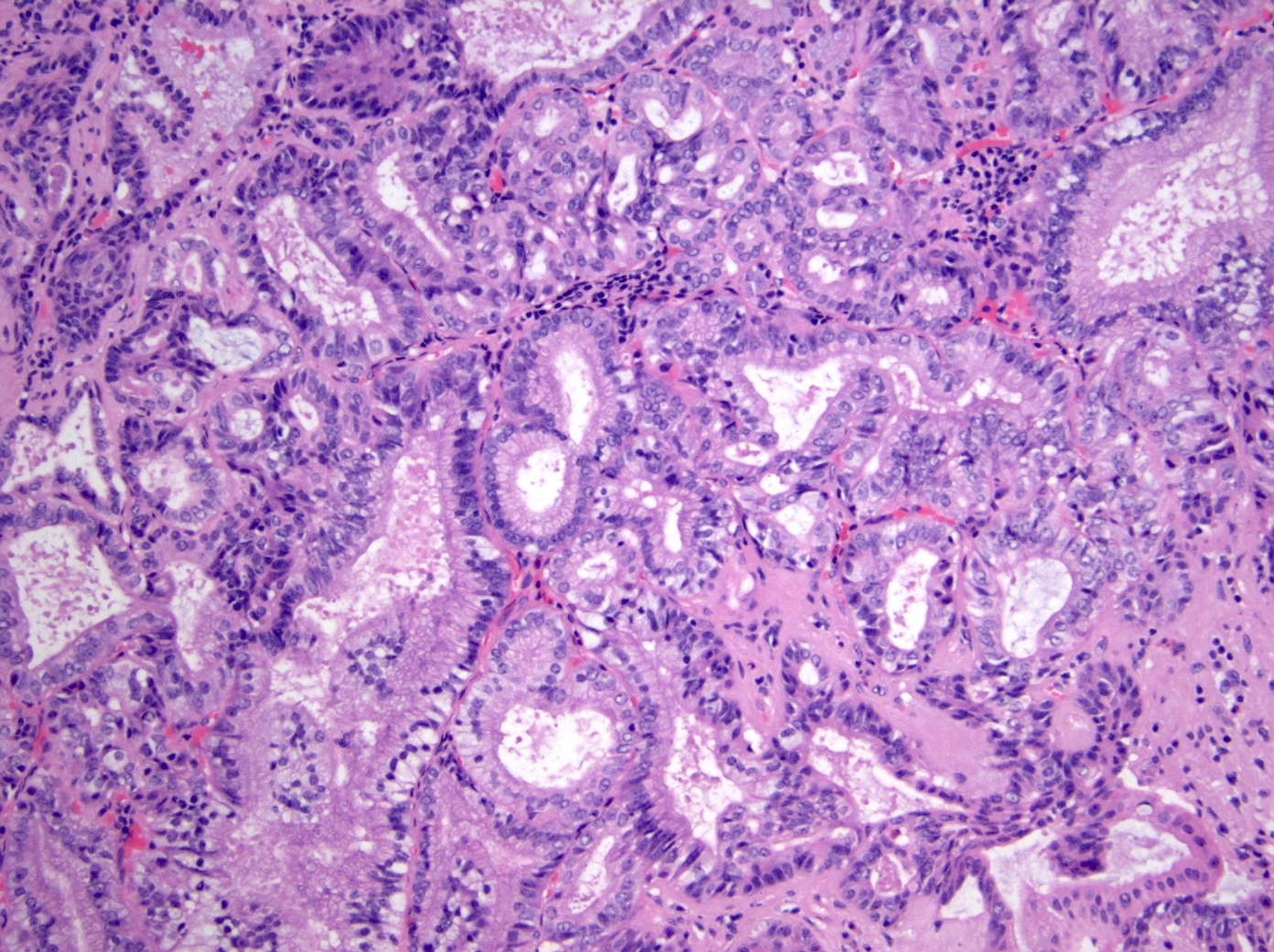
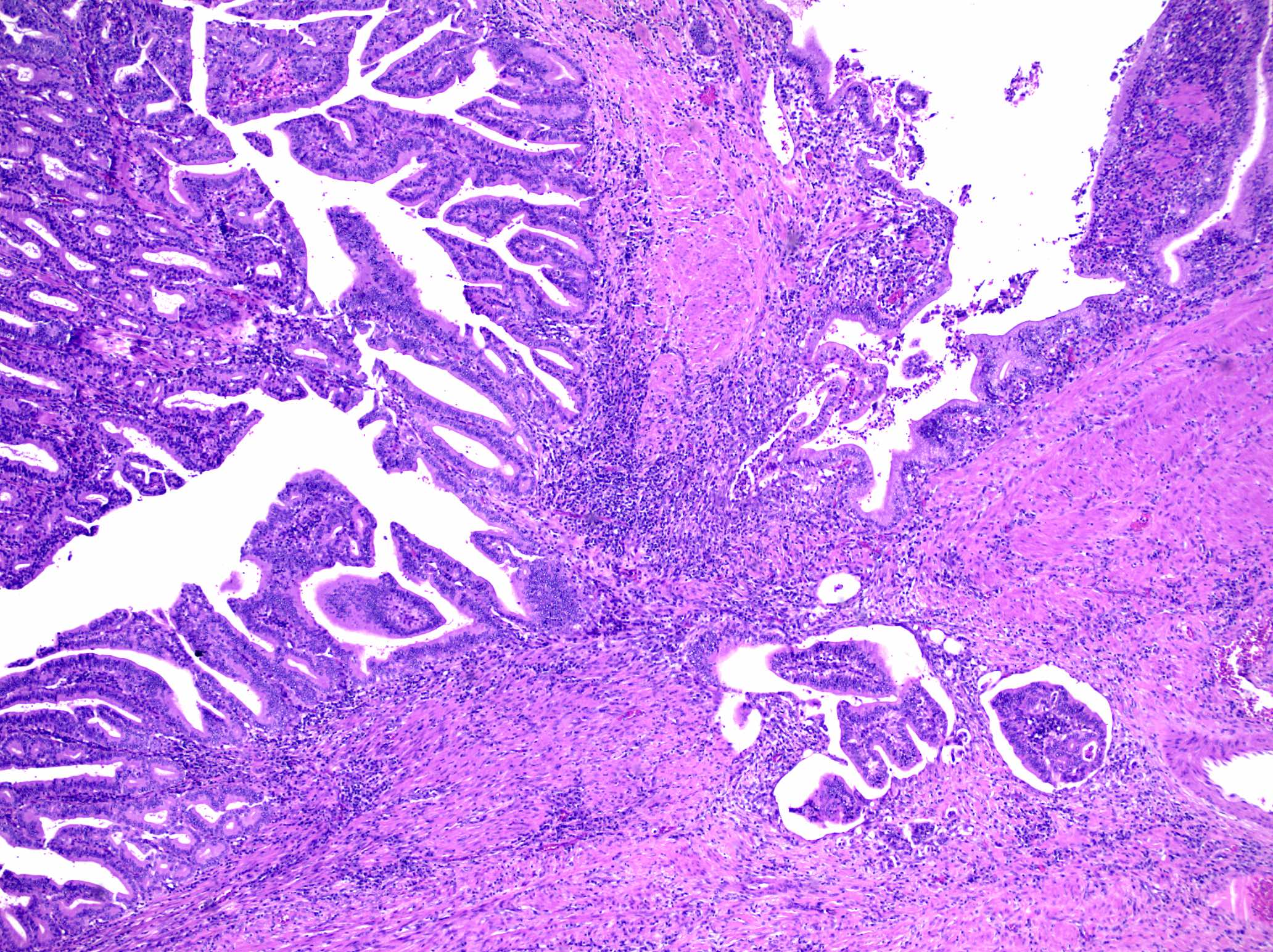
Microscopic (histologic) description
- Noninvasive exophytic tumor
- Growth pattern often shows a mixture of papillary and tubular architecture (Am J Surg Pathol 2010;34:1731)
- In one large series, cases showing predominantly papillary architecture were more likely to contain high grade dysplasia (Am J Surg Pathol 2010;34:1731)
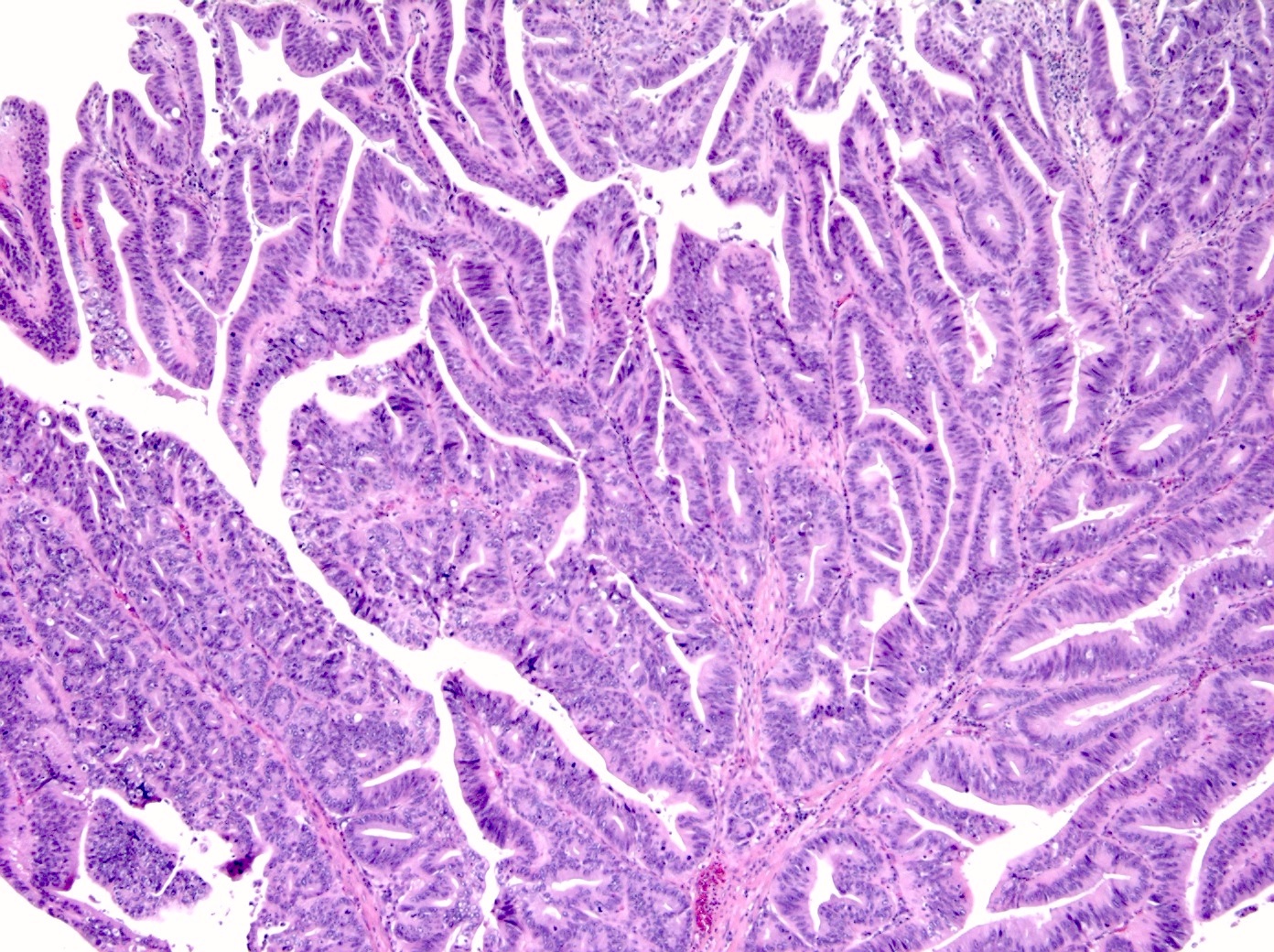
- When considering the predominant phenotype, the majority show an intestinal phenotype, with a minority showing a gastric / pancreatobiliary phenotype (74% versus 26%, respectively) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Intestinal type
- Tall columnar cells with elongated nuclei and inconspicuous nucleoli
- Paneth, goblet or endocrine cells may be present
- Appears similar to conventional colonic adenomas
- Pancreatobiliary type
- Cuboidal cells with round nuclei arranged predominantly in a single layer
- Some resemble a pyloric gland adenoma histologically
- Intestinal type
- When taking into account all morphology present, 45% of cases show a mixed phenotype (Am J Surg Pathol 2010;34:1731)
- Many to most do not show significant mucin production (mucin hypersecretion is seen in 8.5%) (Ann Surg 2016;263:162, Am J Surg Pathol 2010;34:1731)
- At least focal high grade dysplasia is present in most cases (66.7 - 94%) (Am J Surg Pathol 2010;34:1731, Hum Pathol 2014;45:1910)
- Increased degree of architectural complexity and nuclear atypia
- Lesions with a pancreatobiliary phenotype are more likely to have more extensive high grade dysplasia (Am J Surg Pathol 2010;34:1731)
Microscopic (histologic) images
Cytology description
- Majority of IAPNs are accessible to endoscopic biopsy; however, exfoliative cytology is sometimes done and can be used to diagnose dysplasia (Cytojournal 2017;14:19)
Positive stains
- Intestinal phenotype: MUC2 (85%), CDX2 (94%), CK20 (93%), CK7 (63%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: MUC1 / EMA (89%), MUC5AC (95%), MUC6 (83%), CK7 (89%) (Am J Surg Pathol 2010;34:1731)
Negative stains
- Intestinal phenotype: MUC1 / EMA (21%), MUC5AC (31%), MUC6 (24%) (Am J Surg Pathol 2010;34:1731)
- Gastric / pancreatobiliary phenotype: CDX2 (39%), CK20 (39%), MUC2 (22%) (Am J Surg Pathol 2010;34:1731)
Sample pathology report
- Ampulla, endoscopic ampullectomy:
- Intra-ampullary papillary tubular neoplasm, pancreatobiliary type, with multifocal high grade dysplasia
- No invasive carcinoma identified
- Resection margins are free of dysplasia
Differential diagnosis
- Intraductal papillary mucinous neoplasm (IPMN):
- These lesions arise in the pancreatic ducts (main duct or side branch ducts) and generally have significant mucin production
- Proper grossing and sampling with specific location information of the tissue sampled are crucial in making the distinction between these entities because, without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at the microscopic level
- Duodenal / periampullary adenoma:
- These lesions will generally show an intestinal phenotype (will look like a colonic adenoma)
- Proper grossing and sampling with specific location information of the tissue sampled are crucial in making the distinction between these entities because, without knowledge of the specific location of a given lesion, these can be histologically indistinguishable at the microscopic level
- Intra-ampullary adenocarcinoma:
- This is an adenocarcinoma that may arise from an IAPN but will show an invasive component
Additional references
Board review style question #1
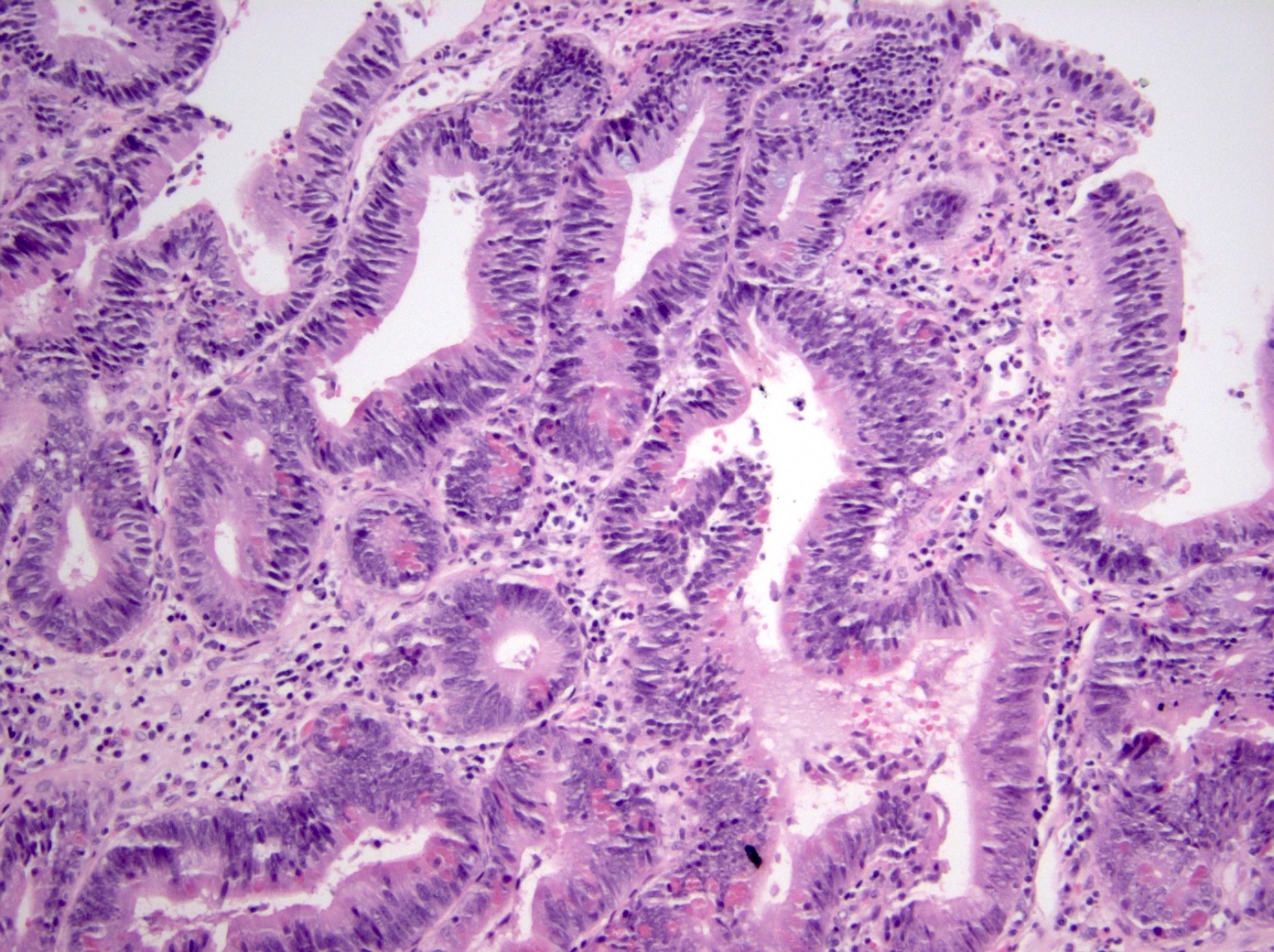
A 65 year old man presented with jaundice, weight loss and nausea. Magnetic resonance imaging (MRI) showed a dilation of the intra and extrahepatic bile ducts with an ill defined fullness in the head of the pancreas; endoscopic retrograde cholangiopancreatography (ERCP) revealed a papillary mass in the ampullary channel. Forcep biopsy demonstrates histologic findings consistent with intra-ampullary papillary tubular neoplasm (IAPN) with high grade dysplasia, including the pictured findings. What is the most likely immunoprofile for this lesion?
- Immunoreactive for CDX2, CK20 and MUC2; negative for MUC1 and MUC5AC
- Immunoreactive for CDX2, MUC1 and MUC2; negative for CK20 and MUC5AC
- Immunoreactive for MUC1 and MUC5AC; negative for CDX2, CK20 and MUC2
- Immunoreactive for MUC1, MUC2 and MUC5AC; negative for CDX2, CK20 and CK7
Board review style answer #1
A. Immunoreactive for CDX2, CK20 and MUC2; negative for MUC1 and MUC5AC. The question discusses a typical clinical presentation for a patient who has an intra-ampullary papillary tubular neoplasm (IAPN). The histologic image demonstrates neoplastic epithelium composed of columnar cells with elongated, hyperchromatic nuclei. Paneth cells and goblet cells are present. These findings support an intestinal phenotype; answer A contains the most common immunoprofile for an intestinal type IAPN. Answer C is incorrect because it is the most common immunophenotype for a pancreatobiliary type IAPN. Answer B is incorrect because CK20 would be expected to be positive and MUC1 would be expected to be negative in an IAPN with intestinal phenotype. Likewise, answer D is incorrect because CK20 and CDX2 would be expected to be positive, while MUC1 and MUC5AC would be expected to be negative, in an IAPN with intestinal phenotype.
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)
Comment Here
Reference: Intra-ampullary papillary tubular neoplasm (IAPN)