Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Radiology description | Radiology images | Prognostic factors | Case reports | Treatment | Gross description | Gross images | Microscopic (histologic) description | Microscopic (histologic) images | Cytology description | Cytology images | Positive stains | Negative stains | Electron microscopy description | Molecular / cytogenetics description | Molecular / cytogenetics images | Sample pathology report | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Cite this page: Tretiakova M. Adrenal cortical carcinoma. PathologyOutlines.com website. https://www.pathologyoutlines.com/topic/adrenocorticalcarcinoma.html. Accessed March 31st, 2025.
Definition / general
- Malignant epithelial tumor of adrenal cortical cells
Essential features
- Malignant epithelial tumor of adrenal cortex
- Adrenocortical carcinoma (ACC) is a rare endocrine tumor with high mortality
- IGF2 overexpression is a major event in adrenal cortical carcinoma tumorigenesis
- Vast majority are sporadic but could present in different syndromic settings
- Heterogeneous group of tumors with prognosis dependent on patient age, clinical presentation, stage, histologic variant and molecular / genomic characteristics
- Positive for SF1, inhibin, calretinin, MelanA, synaptophysin
Terminology
- Adrenal cortical carcinoma (ACC)
- Adrenocortical carcinoma, conventional type
- Adrenal cortical adenocarcinoma
- Adrenocortical carcinoma
- Adrenal cortical tumor, malignant
ICD coding
- ICD-O: 8370/3 - adrenal cortical carcinoma
Epidemiology
- Annual incidence is stable, 0.5 - 2 cases per 1 million population (Endocr Rev 2014;35:282, World J Surg 2006;30:872)
- Accounts for 6.8% of all primary adrenal tumors and 1.4% of incidental masses (Biomedicines 2021;9:175)
- Accounts for 1.3% of all pediatric cancers (Ther Adv Chronic Dis 2021;12:20406223211033103)
- Median age 55 - 56 but can occur at any age (Ther Adv Chronic Dis 2021;12:20406223211033103)
- Some series show bimodal age distribution with peaks in the first and fifth decades (Cancer 1993;72:3145, World J Surg Oncol 2015;13:117, J Clin Med Res 2018;10:636)
- More common in females and whites; M:F = 1:1.5 - 2.5 (World J Surg Oncol 2015;13:117, Cancers (Basel) 2020;12:2720, J Clin Oncol 2004;22:838, Ther Adv Chronic Dis 2021;12:20406223211033103)
- Conventional adrenal cortical carcinoma comprises 70 - 90% of cases (Biomedicines 2021;9:175, Hum Pathol 2015;46:1799)
- Vast majority sporadic but 5 - 10% could occur in syndromic settings
Sites
- More often involves left adrenal: left to right ratio = 1.2:1 (Nat Rev Endocrinol 2011;7:323)
- Bilateral involvement uncommon, ~1% of cases (J Clin Med Res 2018;10:636)
- Rare cases reported in ectopic adrenal rests of retroperitoneum, ovary, spinal region, abdominal wall (Nat Rev Endocrinol 2011;7:323, Ann Endocrinol (Paris) 2020;81:516, Tohoku J Exp Med 2013;229:267, Endocr Pathol 2011;22:112)
Pathophysiology
- Molecular evidence for an adenoma to carcinoma progression (Virchows Arch 2012;460:9, PLoS One 2013;8:e73959)
- Insulin-like growth factor 2 (IGF2) proposed as the main oncogene in adrenal cortical carcinoma tumorigenesis with 10 - 80x fold increased mRNA and protein expression compared to normal adrenal cortex or adenoma (Endocrinol Metab Clin North Am 2015;44:399, Ther Adv Chronic Dis 2021;12:20406223211033103)
Etiology
- 90% are sporadic, with no established risk factors other than smoking in a subset of cases that harbor mutation signatures typical of smoking and mismatch repair deficiency (J Intern Med 2002;252:206, Cancer Cell 2016;29:723)
- Sporadic cases arise through acquired genetic mutations of several driver genes (i.e. IGF2, CTNNB1, TP53, ATM, TERT, RB, ZNRF3, PRKAR1A), activation of cellular signaling pathways and accumulation of massive chromosomal alterations (Cancer Cell 2016;29:723, Endocrinol Metab Clin North Am 2015;44:399, Biomedicines 2021;9:174)
- Genetic susceptibility in 5 - 10% of cases:
- Adrenal cortical carcinoma could occur in a wide variety of disorders, including multiple endocrine neoplasia type 1 (MEN1), Lynch, Li-Fraumeni, Beckwith-Wiedemann, adenomatous polyposis coli (APC) and neurofibromatosis type 1 (NF1) syndromes, Carney complex and congenital adrenal hyperplasia, thus advocating for genetic screening (Eur J Endocrinol 2012;166:269, Fam Cancer 2011;10:265, Best Pract Res Clin Endocrinol Metab 2020;34:101428, Biomedicines 2021;9:175, J Clin Endocrinol Metab 2012;97:387, Horm Metab Res 2015;47:497 Endocrinol Diabetes Metab Case Rep 2014;2014:140074, Endocr Rev 2014;35:282, J Clin Oncol 2013;31:3012)
- Li-Fraumeni syndrome accounts for most pediatric cases, especially in Brazil, where 0.3% of the population carries the founder germline TP53 R337H mutation (J Natl Cancer Inst 1994;86:1707, J Clin Oncol 2015;33:602, PLoS One 2014;9:e99893)
Clinical features
- ~50% are functional and ~50% are nonfunctional (J Clin Endocrinol Metab 2006;91:2027, N Engl J Med 1990;322:1195)
- Functional adrenal cortical carcinomas have the following symptoms related to hormone production:
- 50% cortisol excess (Cushing syndrome, rapid onset)
- 20% sex hormone secretion (mainly androgens causing hirsutism, virilization and menstrual irregularities)
- 8% aldosterone (hypertension, hypokalemia)
- 15 - 25% mixed hormone production
- Patients are younger, more likely females and present with metastatic disease
- Nonfunctional adrenal cortical carcinomas could present in 2 ways:
- 80% create a mass effect (gastrointestinal symptoms, organ compression or back pain) or mimic an infectious process (localized pain, fever due to cytokines from highly necrotic tumors)
- 20% discovered incidentally during unrelated imaging procedures
- Tumors often displace and invade adjacent organs (kidneys, liver, pancreas), adrenal vein, vena cava and retroperitoneum
- Common metastatic sites are liver (60%), lymph nodes (40%), lungs (40%), peritoneal and pleural surfaces, bone, skin (anaplastic tumors) or retroperitoneum (Endocr Rev 2014;35:282)
Diagnosis
- Only definitive criteria for malignancy are distant metastasis or local invasion
- Imaging can usually distinguish cortical adenomas from carcinomas with reasonable accuracy, except benign tumors with hemorrhage and revascularization
- Adrenal cortical carcinoma is commonly characterized by its large size, irregular invasive borders and signal heterogeneity on computed tomography (CT) and magnetic resonance imaging (MRI)
- Functional fluorodeoxyglucose PET imaging helps detect distant metastases and can improve diagnosis (Radiographics 2009;29:1319, Radiographics 2009;29:1333, Hum Pathol 2016;53:63, Hell J Nucl Med 2015;18:97)
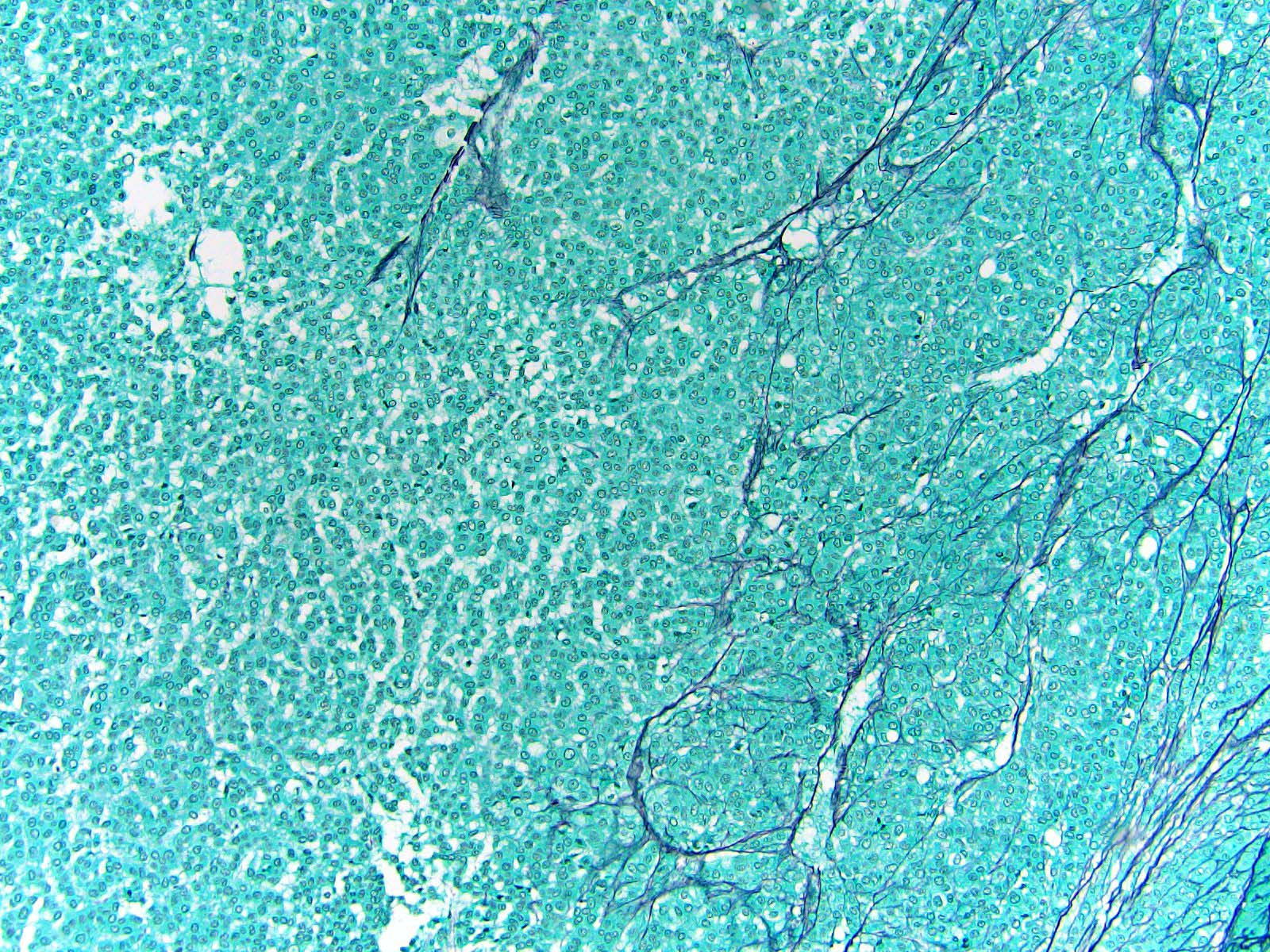
- Loss / disruption of reticulin framework is a distinction between adrenal cortical carcinoma and adenoma (Am J Surg Pathol 2013;37:1433, Am J Surg Pathol 2018;42:201)
- Multiple systems have been proposed for pathologic distinction between benign and malignant adrenal cortical tumors (Weiss, Wieneke, Hough, von Slooten, Lin-Weiss-Bisceglia, Helsinki) (Horm Cancer 2011;2:333, Am J Surg Pathol 1984;8:163, Am J Surg Pathol 2002;26:1612, Horm Metab Res 2021;53:709, Hum Pathol 2017;62:1)
- Weiss score is most commonly accepted in reporting, including AJCC 8th edition
| Weiss criteria: ≥ 3 for adrenal cortical carcinoma (Am J Surg Pathol 1984;8:163, Am J Surg Pathol 2002;26:1612) | |
| 1 | Nuclear grade III or IV (Fuhrman) |
| 2 | > 5 mitotic figures/50 high power fields* |
| 3 | Atypical mitotic figures* |
| 4 | Clear or vacuolated cells ≤ 25% tumor |
| 5 | Diffuse architecture (> 33% of tumor) |
| 6 | Necrosis |
| 7 | Venous invasion* (of smooth muscle walled vessels) |
| 8 | Sinusoidal invasion |
| 9 | Capsular invasion |
Laboratory
- Hormone quantification in serum or urine (free cortisol, ACTH, DHEA sulfate, 17-OH, 17-hydroxy, testosterone, 17-beta-estradiol, 17-deoxycortison)
- Dexamethasone suppression test
- Potassium
- Steroid metabolome profiling by mass spectrometry (Biomedicines 2021;9:174)
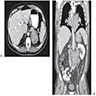
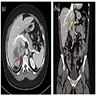
Radiology description
- Abdominal CT and MRI
- Decreased fat content compared to adenomas
- Heterogeneous enhancing large mass (> 5 cm); CT cutoff for malignancy is 46 mm and 20 Hounsfield units (HU, measurement of radiodensity) (Ann Surg 2022;275:e238)
- Irregular borders, necrosis, calcifications
- Invasion or displacement of adjacent organs, vena cava extension (Ther Adv Chronic Dis 2021;12:20406223211033103)
- PET scan is useful in diagnosing metastatic disease (Ther Adv Chronic Dis 2021;12:20406223211033103)
Radiology images
Prognostic factors
- Overall poor prognosis with 5 year mortality 50 - 90% (Endocr Rev 2014;35:282, Endocrinol Metab Clin North Am 2015;44:399, Nat Rev Endocrinol 2011;7:323, Cancers (Basel) 2020;12:1218)
- Tumor stage is the best prognostic factor (AJCC, 8th edition) (Ann Oncol 2015;26:2119, Diagn Pathol 2015;10:14, Hormones (Athens) 2020;19:197):
- Size of organ in confined tumor (rare presentation)
- pT1 (≤ 5 cm), 5 year survival 82%
- pT2 (> 5 cm), 5 year survival 58 - 61%
- Invasion (majority of tumors):
- pT3: local invasion, 5 year survival 47 - 56%
- pT4: adjacent organs (kidney, diaphragm, pancreas, liver, spleen), 5 year survival 37%
- Distant metastases at diagnosis (~40% of cases): 5 year survival 0 - 18%
- Size of organ in confined tumor (rare presentation)
- Hormone production:
- Hypercortisolism is an adverse prognostic factor in patients with completely resected disease (Eur Urol 2014;65:832)
- Age as prognostic marker:
- In children, particularly if < 5 years old at diagnosis, complete resection, tumor < 400 g, < 15 mitotic figures/20 high power fields and minimal tumor necrosis, often has good prognosis (Ann Oncol 2015;26:2119)
- In adults, age > 50 years is independently associated with an increased risk of death (Ann Oncol 2015;26:2119)
- Microscopic prognostic parameters:
- Mitotic activity defines tumor grade for low grade carcinomas (< 20 mitoses per 50 high power fields) and high grade carcinomas (≥ 20 mitoses) (Am J Surg Pathol 1989;13:202)
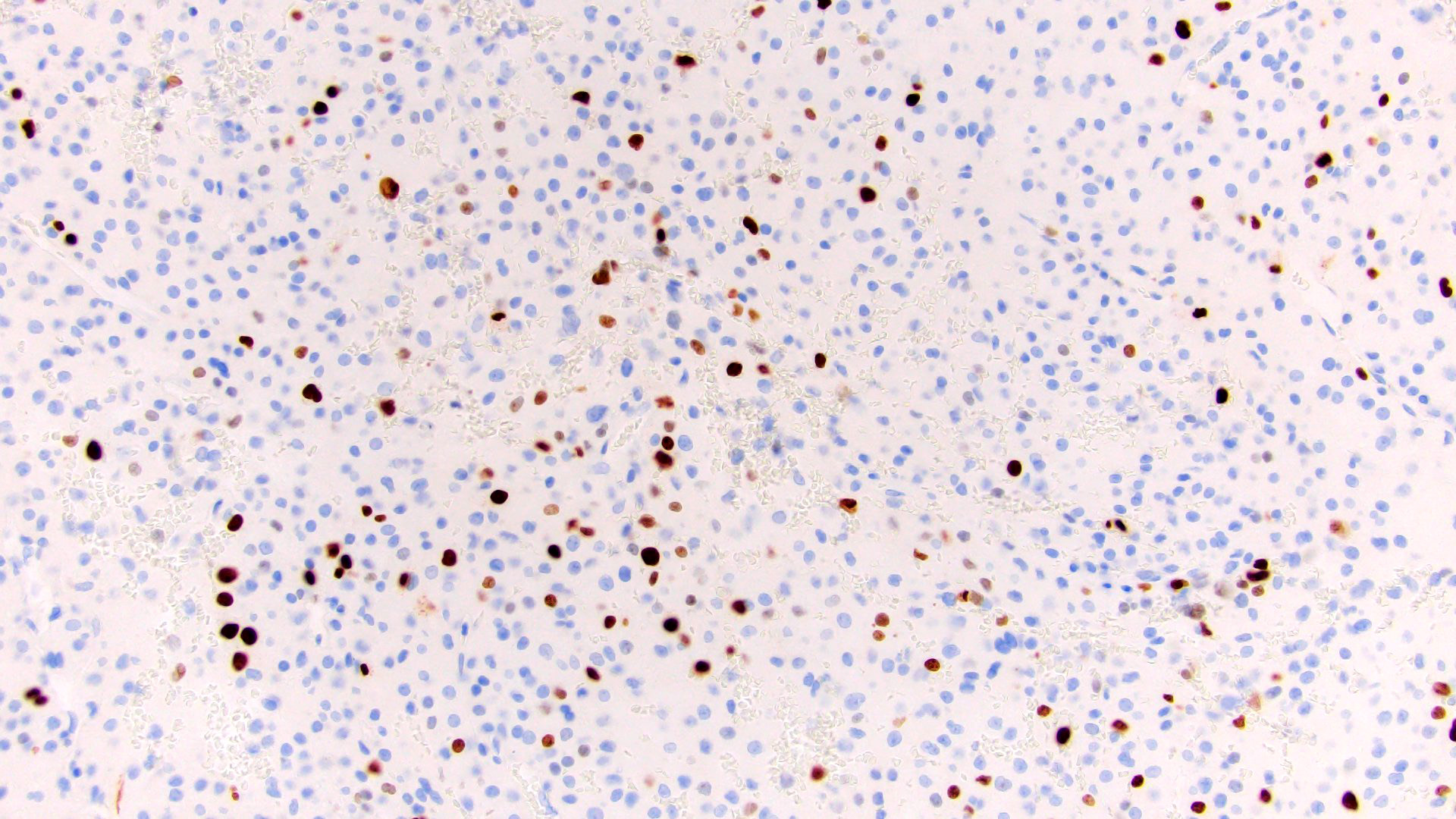
- Proliferation rate: assessment of Ki67 has demonstrated significant prognostic and predictive power in both 2 grade and 3 grade schemes (J Clin Endocrinol Metab 2015;100:841, Endocr J 2008;55:49, Am J Surg Pathol 2016;40:569, Biomedicines 2021;9:174)
- Angioinvasion is the best microscopic predictor of adverse outcome (Am J Surg Pathol 2018;42:201)
- Oncocytic, myxoid and sarcomatoid variants have different prognosis (Biomedicines 2021;9:175)
- Prognostic biomarkers:
- Correlated with poor clinical outcomes:
- Overexpression of IGF2, TOP2A, EZH2, MCM2, SOAT1, mTOR, BARD1, BUB1B, PINK1, etc. (Am J Surg Pathol 2018;42:201, Hum Mol Genet 2016;25:2789, Oncologist 2015;20:247, Biomedicines 2021;9:174)
- Loss of DAXX expression (Endocr Pathol 2018;29:137, Endocr Pathol 2021;32:102, Am J Surg Pathol 2018;42:201)
- High SF1 expression (Hum Pathol 2013;44:822, J Clin Endocrinol Metab 2010;95:E161)
- High expression of miR-483-5p, miR-450a-5p, miR-210, miR421 and low expression of miR-195 (Clin Cancer Res 2009;15:7684, Endocr Relat Cancer 2013;20:579, Biomedicines 2021;9:174)
- Low CXCR4 / CXCR7, PDL1 / PDL2, CD276 (Biomedicines 2021;9:174)
- Transcriptome profiling with different survival rates in 2 groups (J Clin Oncol 2009;27:1108, Clin Cancer Res 2009;15:668)
- 3 molecular subtypes based on DNA methylation signatures show distinct clinical outcomes with disease progression rates 7%, 56% and 96% (Cancer Cell 2016;29:723)
- Correlated with poor clinical outcomes:
Case reports
- 18 month old boy with oncocytic adrenal cortical carcinoma and rhabdomyosarcoma (J Clin Res Pediatr Endocrinol 2021;13:225)
- 32 year old woman with adrenal incidentaloma becoming an aggressive adrenal cortical carcinoma with germline APC variant (Endocrine 2020;68:203)
- 44 - 55 year old women with primary rhabdoid adrenal cortical carcinoma (Histopathology 2013;62:771)
- 65 year old woman with adrenal cortical carcinoma masquerading as pheochromocytoma (Pak J Med Sci 2021;37:1241)
- 66 year old woman with giant, androgen producing adrenal cortical carcinoma with atrial flutter (World J Clin Cases 2021;9:5575)
- 68 year old woman with estrogen secreting adrenal cortical carcinoma (IJU Case Rep 2021;4:318)
- 70 year old woman with adrenal cortical carcinoma evolving from a small adrenal incidentaloma after 9 years of latency (Cureus 2021;13:e16851)
Treatment
- Surgical (Ann Surg 2012;255:363, Ther Adv Chronic Dis 2021;12:20406223211033103):
- Radical resection is the treatment mainstay
- Lymph node dissection for localized tumors
- Excision of solitary lung metastases, metachronous metastasectomy
- Radiation:
- Adjuvant (positive margins, ruptured capsule)
- For unresectable or oligometastatic tumors, particularly in bone
- Mitotane (adrenolytic drug, derivative of insecticide dichlorodiphenyltrichloroethane [DDT]):
- Most commonly used drug in adjuvant setting after incomplete resection, in patients not eligible for surgery and for metastatic disease (Biomedicines 2021;9:174, Ther Adv Chronic Dis 2021;12:20406223211033103)
- Severe toxicity and side effects; several biomarkers (RRM1, CYP2W1) used to predict mitotane response (Clin Cancer Res 2012;18:3452, PLoS One 2014;9:e105855)
- Other drugs:
- Systemic standard chemotherapy with etoposide, doxorubicin, cisplatin and mitotane (EDP-M)
- Immunotherapy: checkpoint inhibitors (nivolumab, pembrolizumab, etc.)
- Targeted therapy: sunitinib, cMET inhibitors, cabozantinib, IGF1 inhibitors, mTOR inhibitors, VEGF inhibitors (limited data) (Biomedicines 2021;9:174, Ther Adv Chronic Dis 2021;12:20406223211033103)
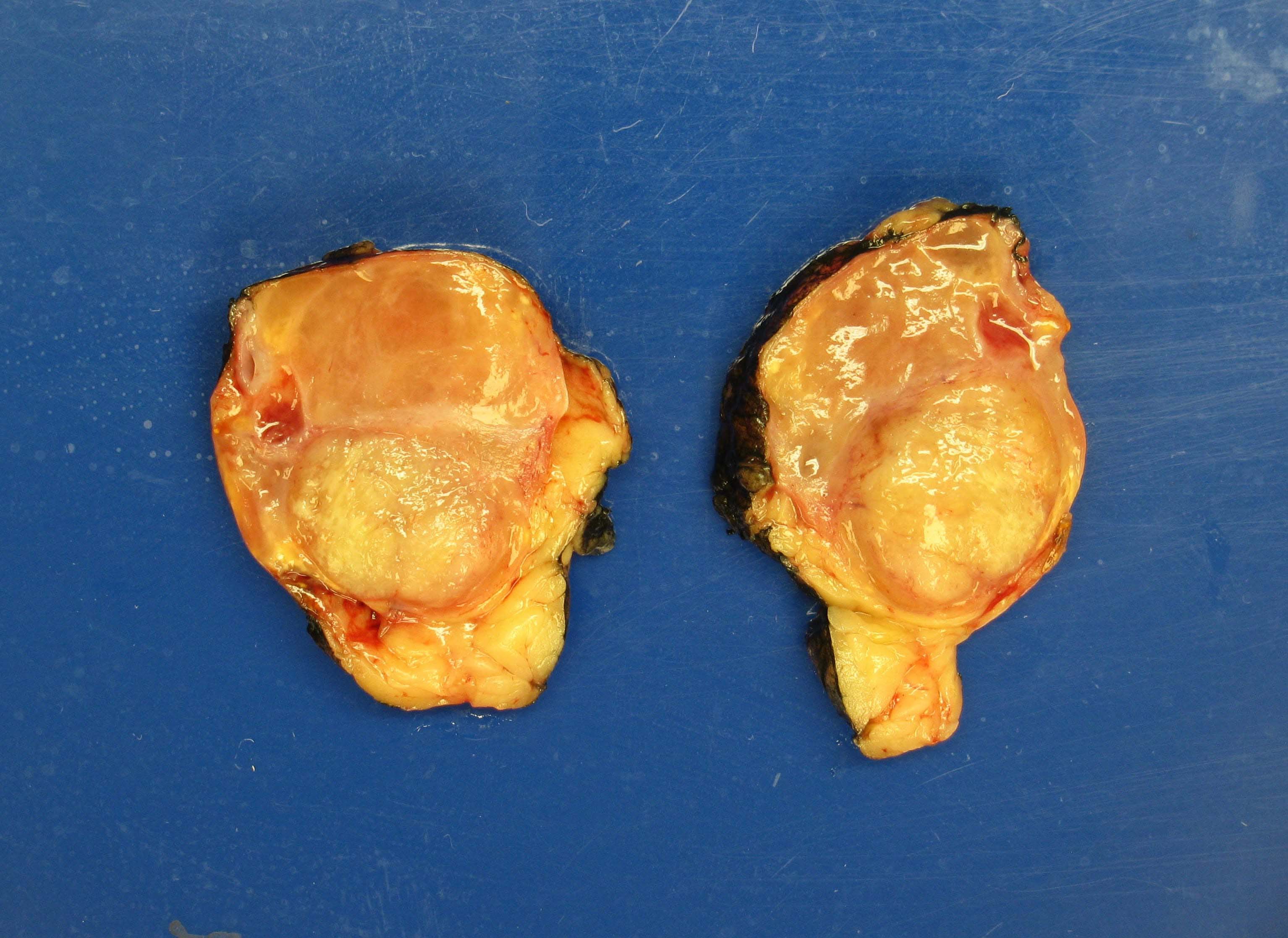
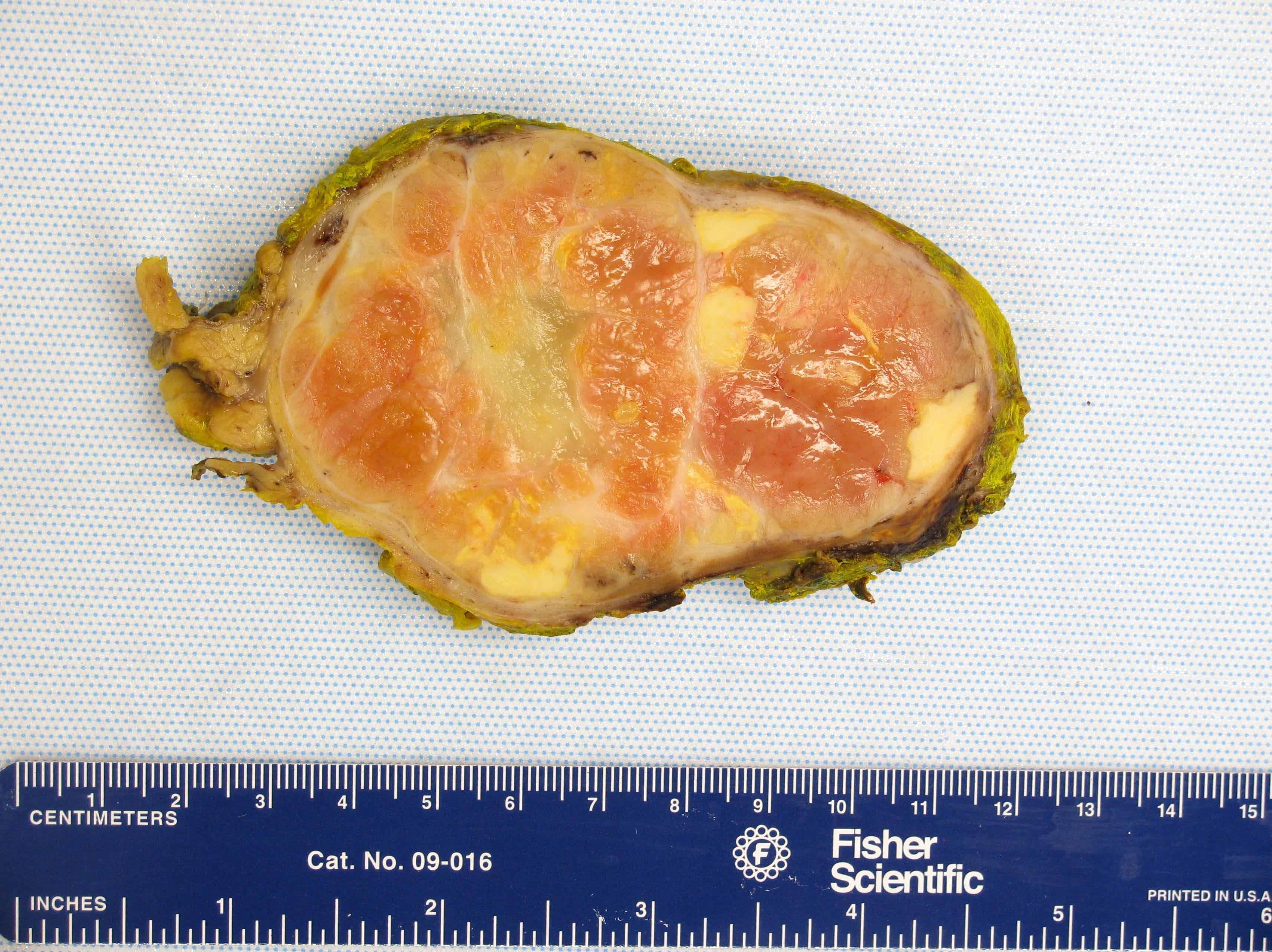
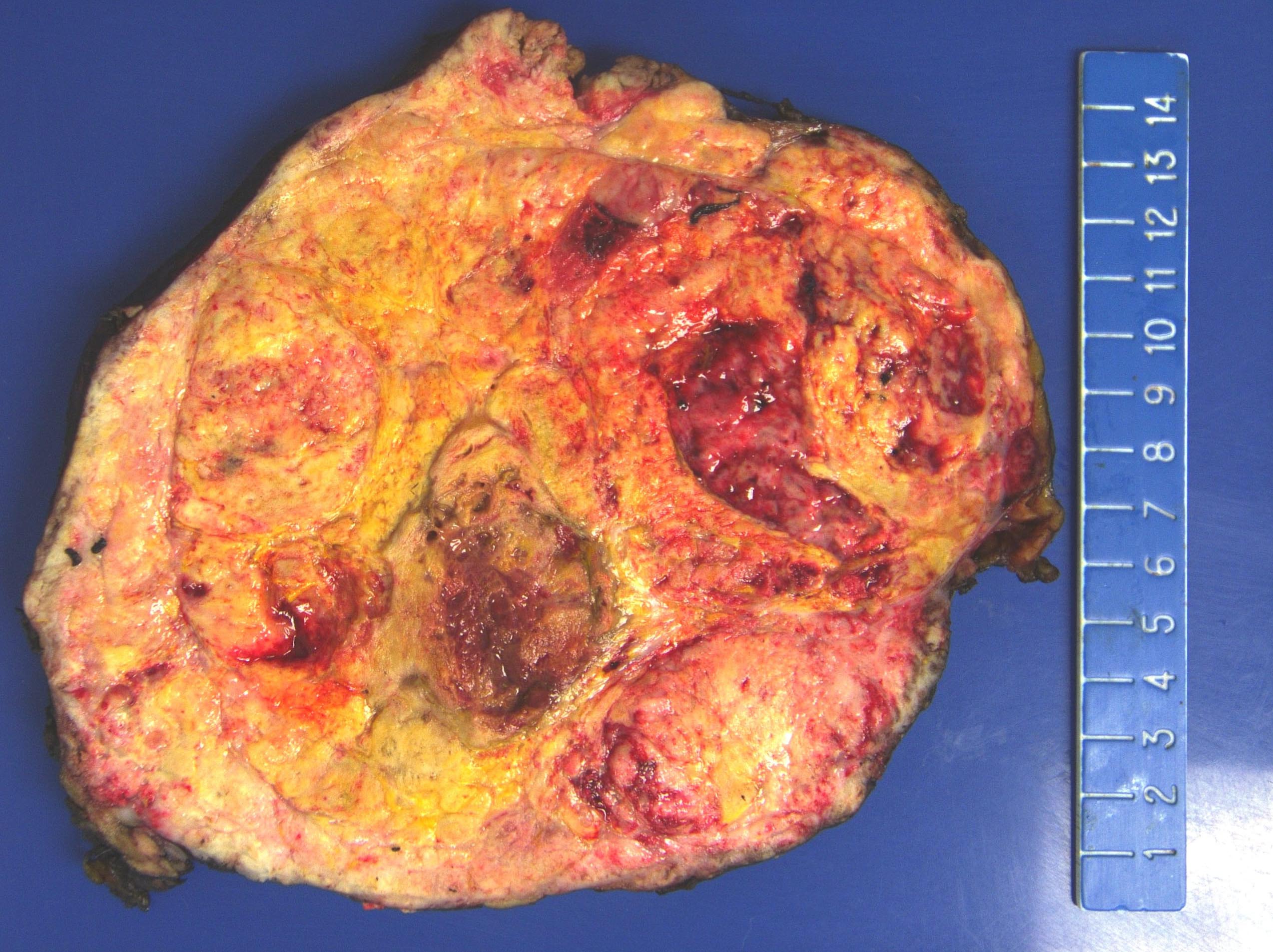
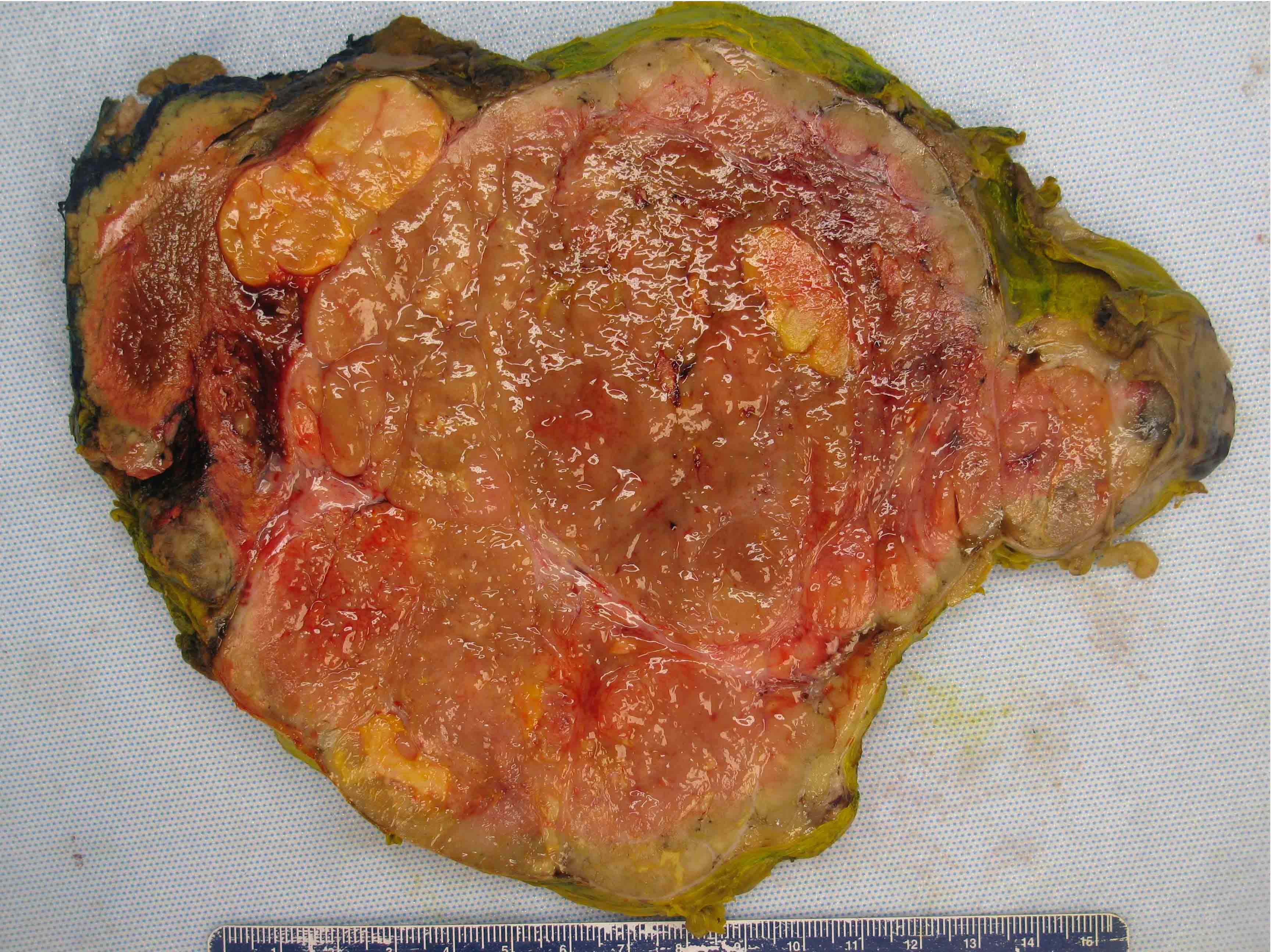
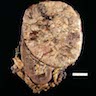
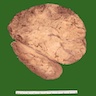
Gross description
- Solitary, bulky and yellow, tan or red-brown mass; usually > 200 g
- Tumor size: median 10 - 12 cm; largest reported case is 28 cm (J Clin Med Res 2018;10:636, Am J Surg Pathol 1989;13:202)
- Heterogeneous cut surface with areas of necrosis and hemorrhage
Gross images
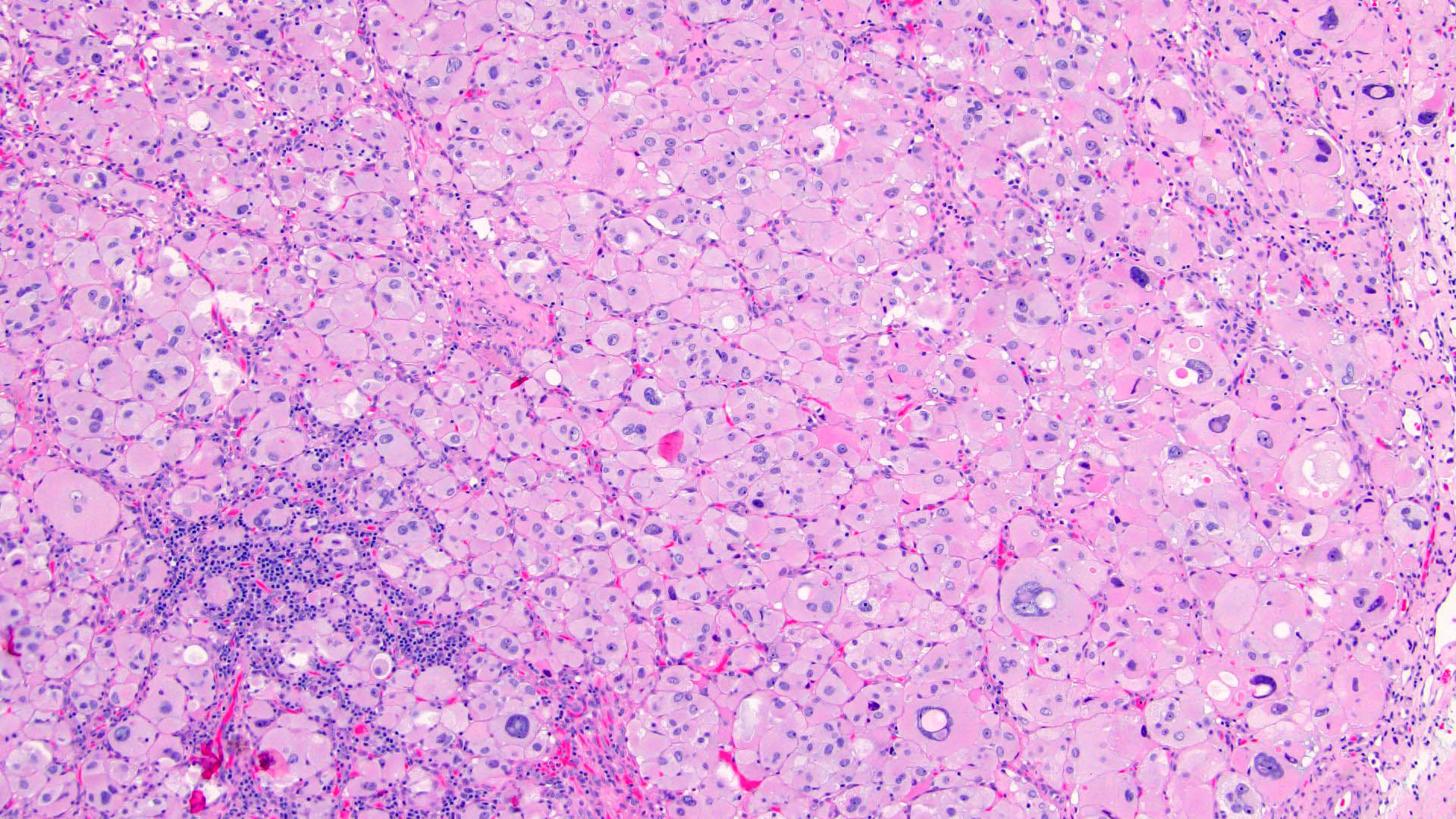
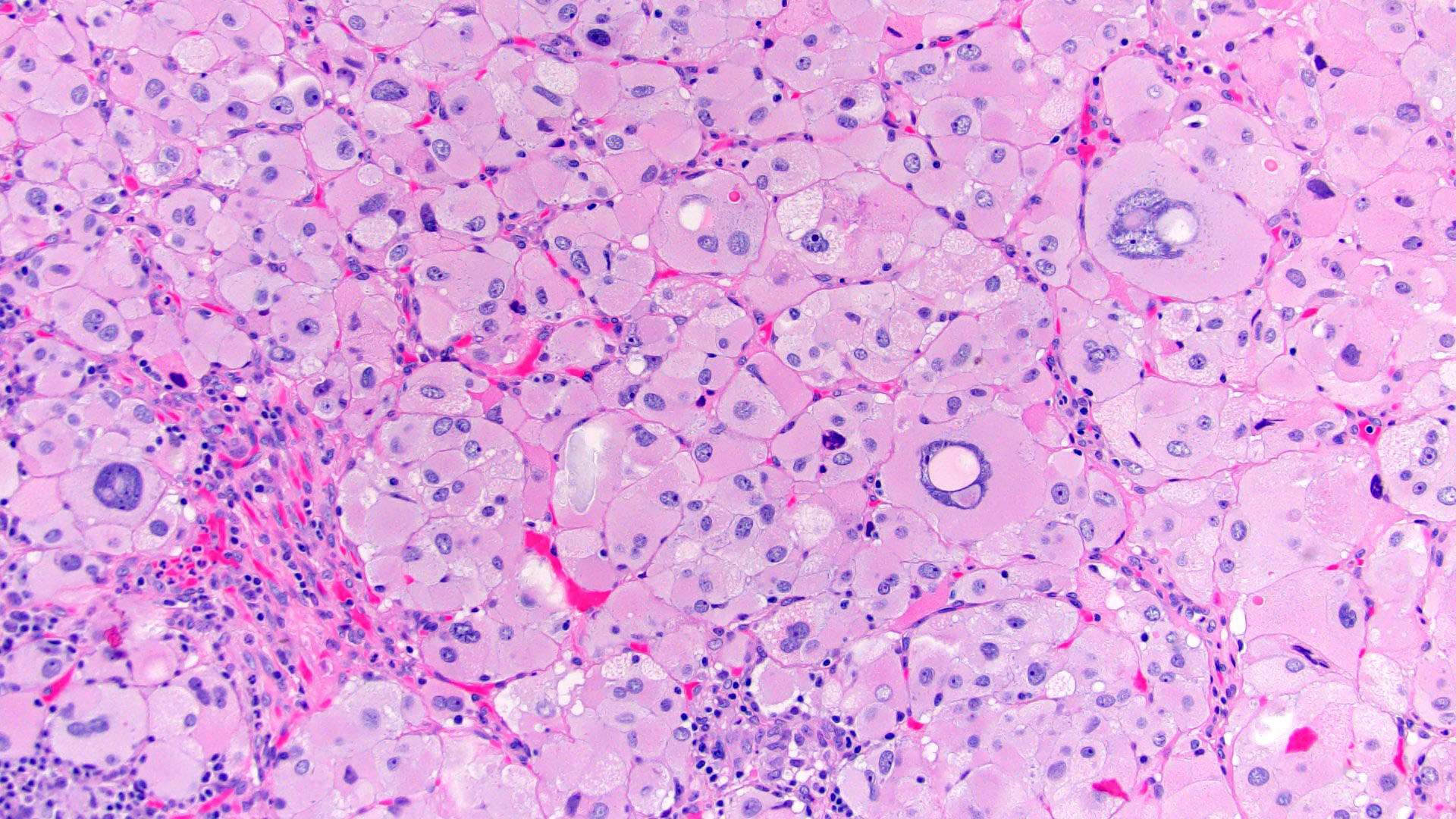
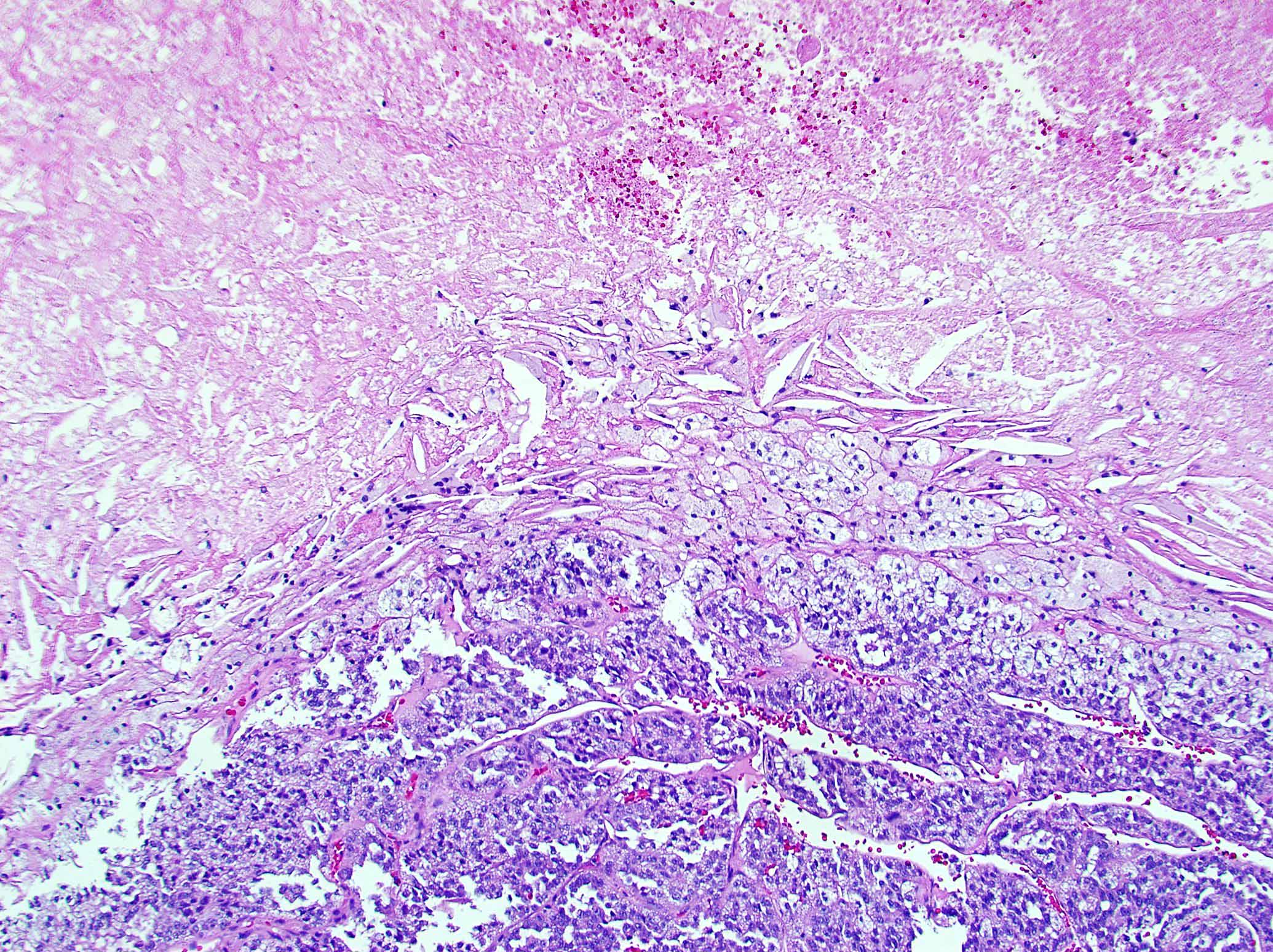
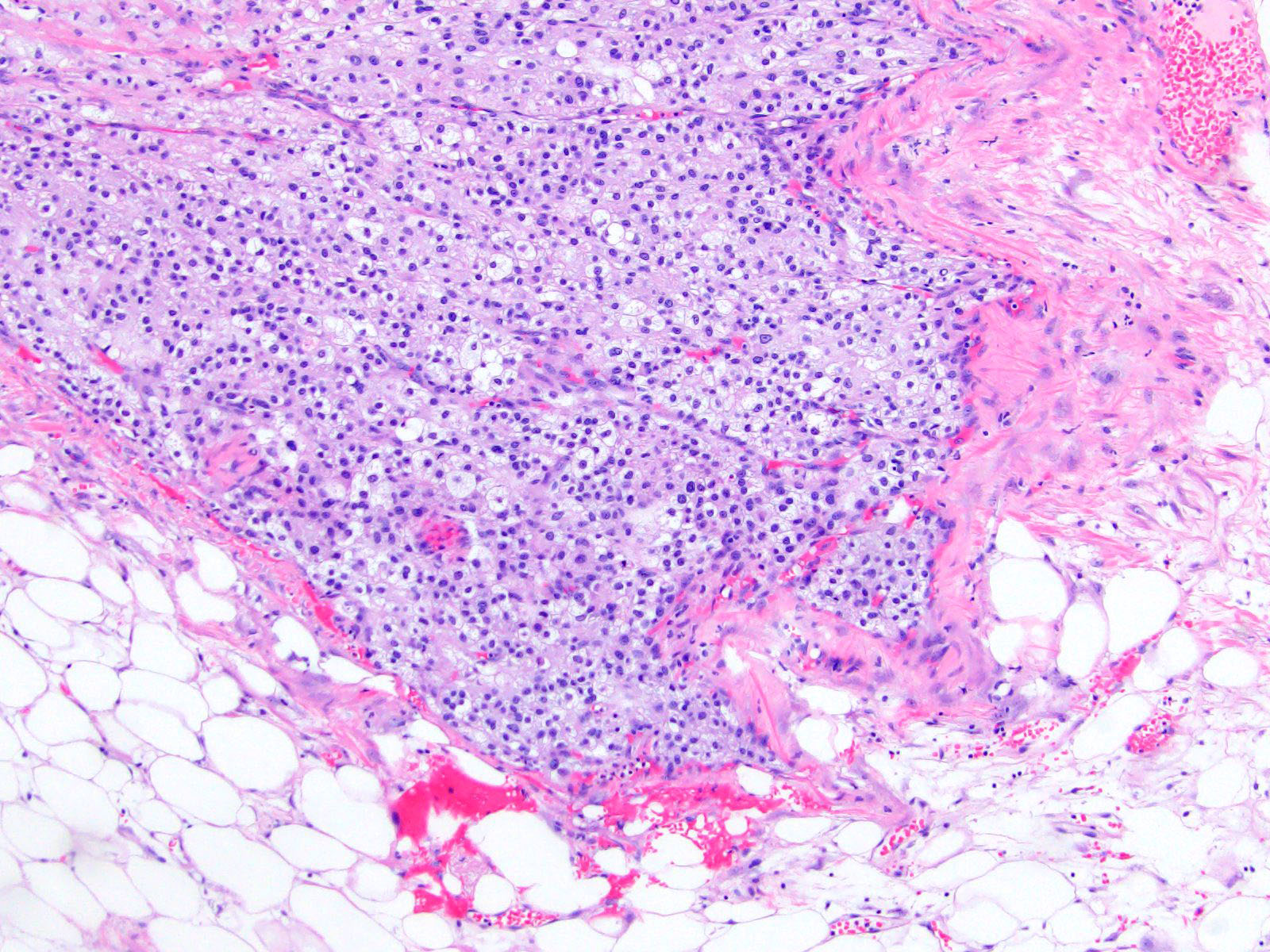
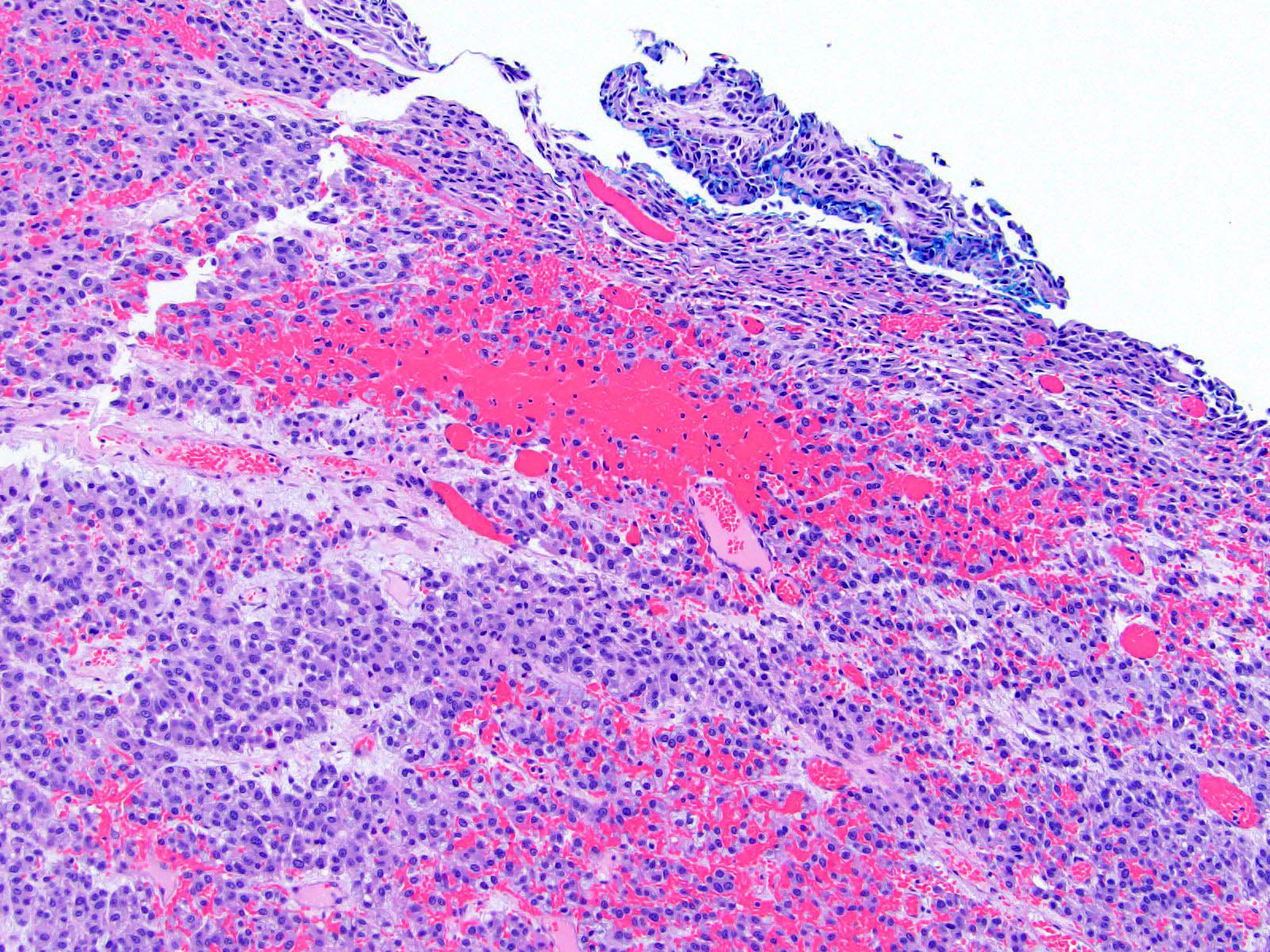
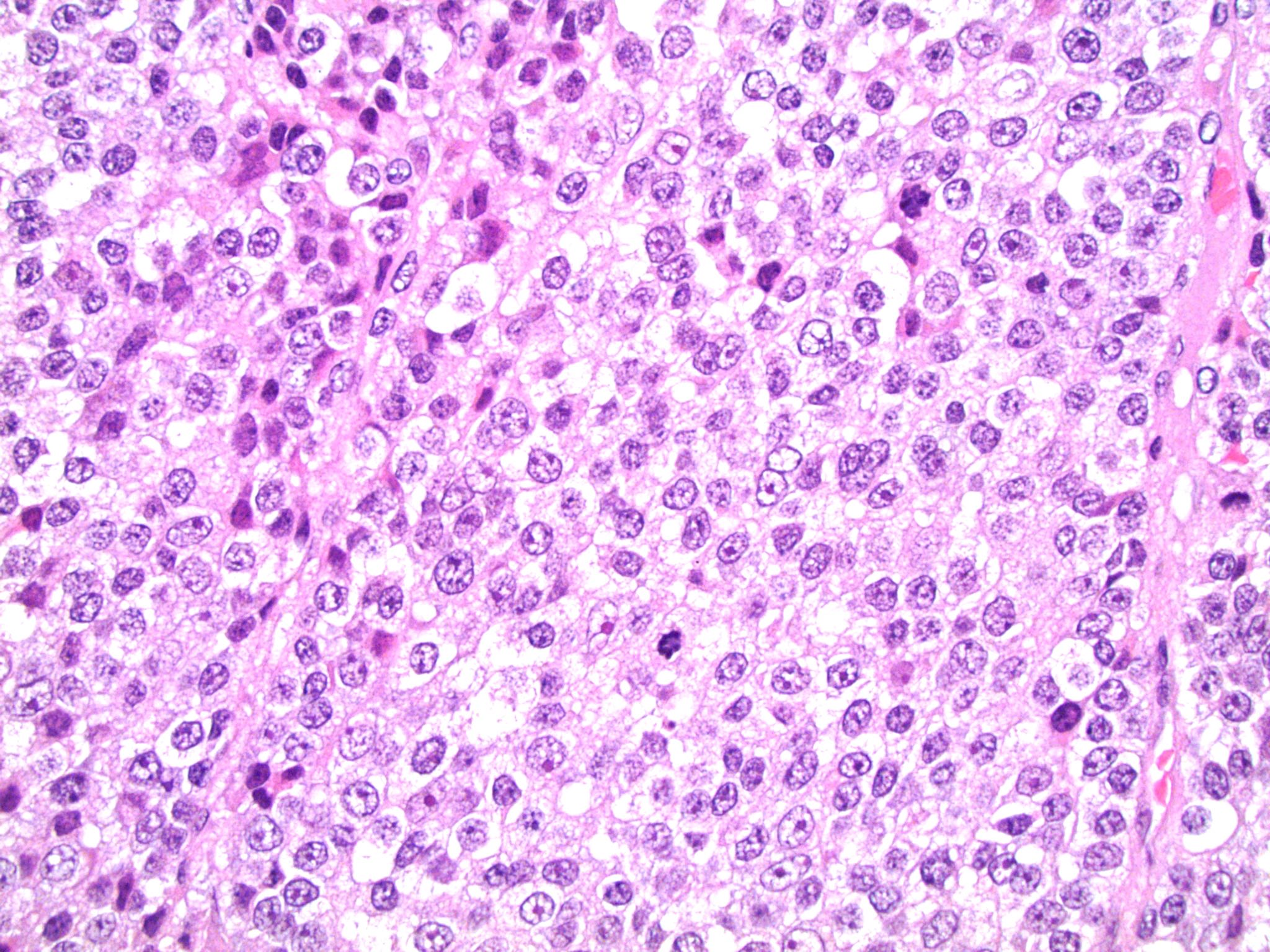
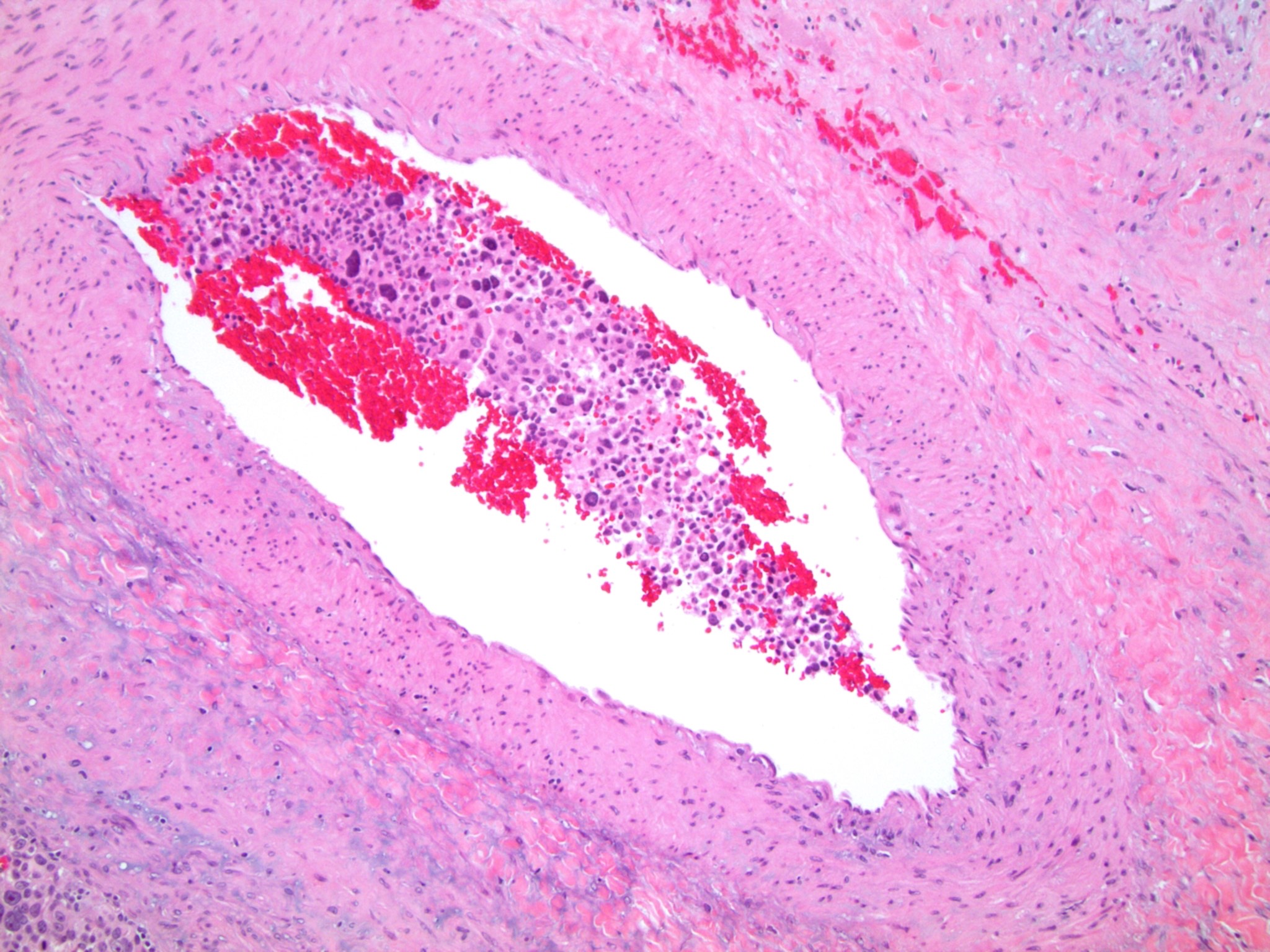
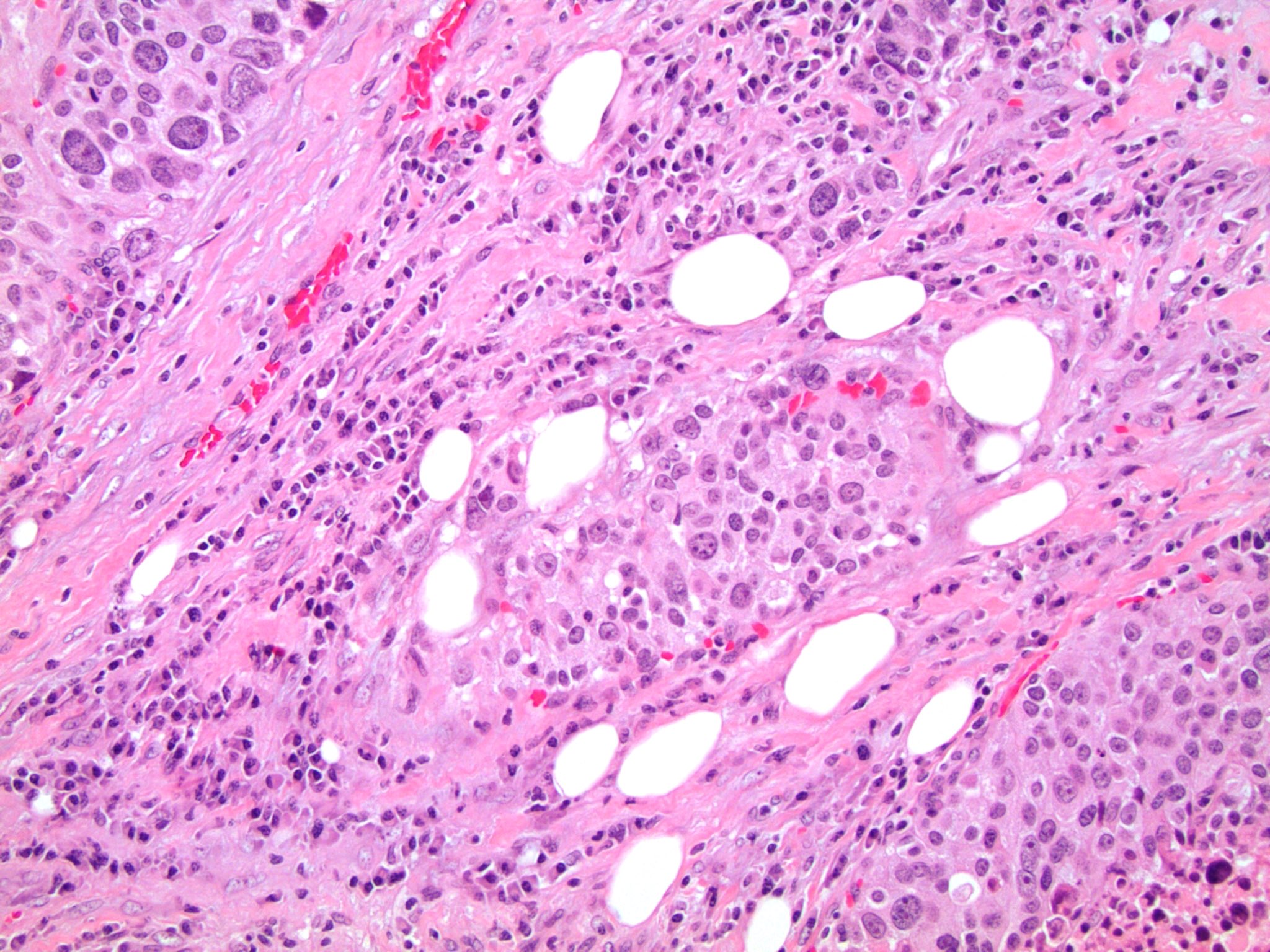
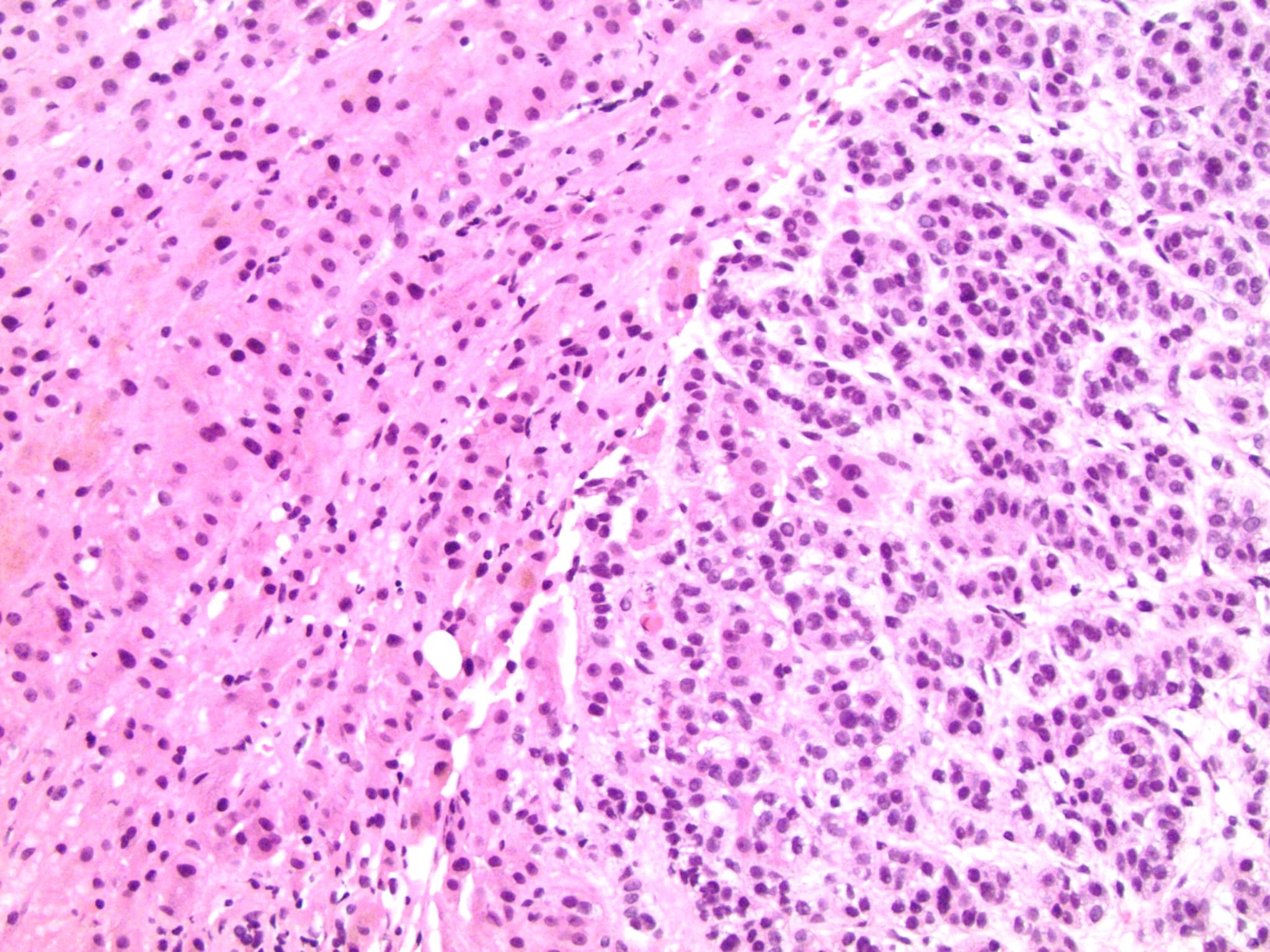
Microscopic (histologic) description
- Encapsulated tumor composed of variably sized nests, large sheets and trabeculae
- Invasion of thick fibrous capsule
- Lymphovascular invasion (venous or sinusoidal)
- Areas of necrosis, hemorrhage, degeneration are common
- Large cells with granular clear to eosinophilic cytoplasm, often pleomorphic
- Frequent intranuclear inclusions, mitoses, atypical mitoses
- Grade defined by mitotic frequency:
- Low grade: ≤ 20 mitoses/50 high power fields
- High grade: > 20 mitoses/50 high power fields
- Morphologic subtypes:
- Conventional (vast majority, 70 - 90% of cases)
- Oncocytic (> 90% oncocytic cells; second most common subtype)
- Myxoid (abundant extracellular mucin)
- Sarcomatoid (mesenchymal differentiation)
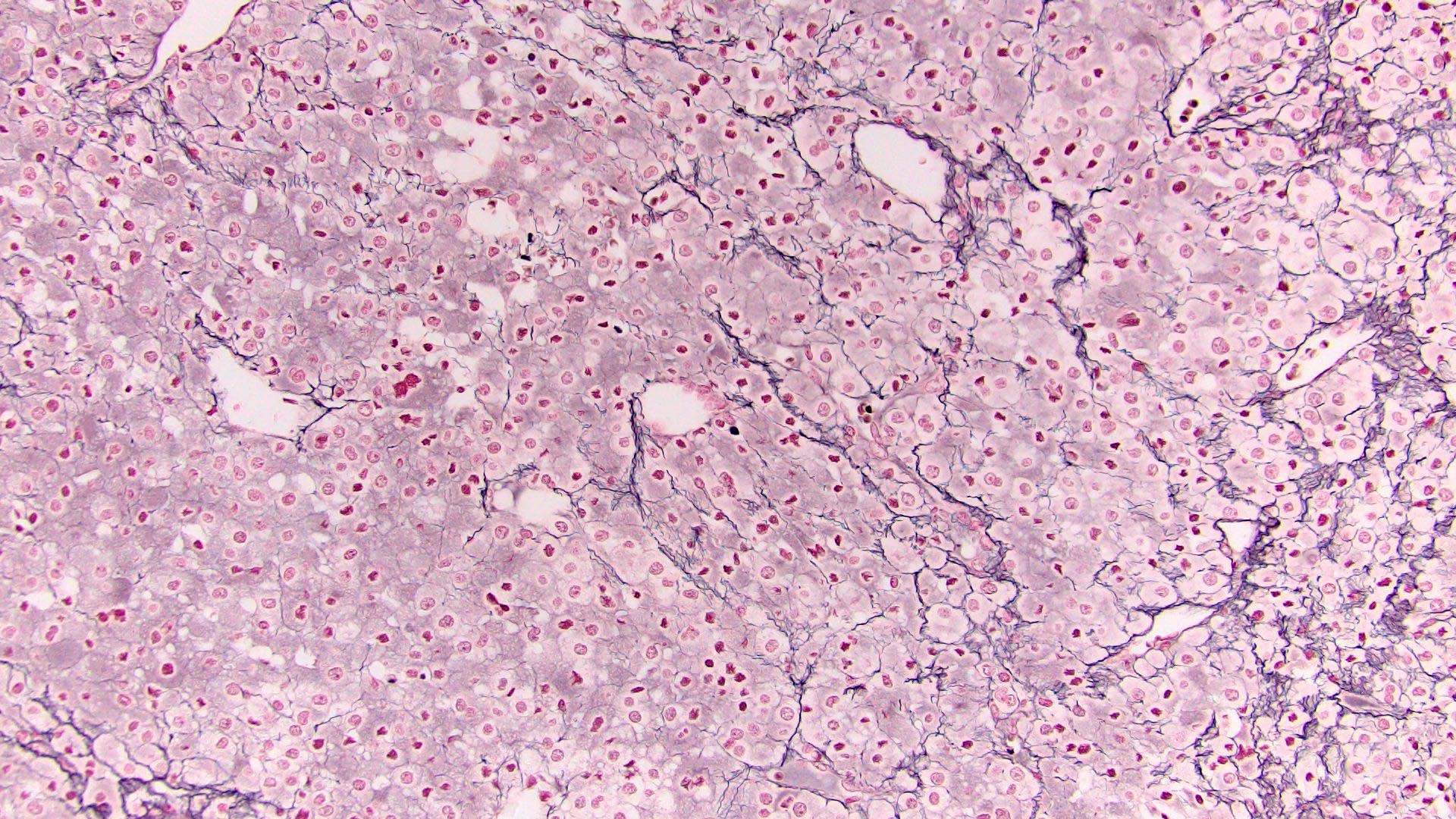
- Loss of continuity of the reticular fibers or basement membrane network (> 33% lesion)
- References: Am J Surg Pathol 1989;13:202, Am J Surg Pathol 2002;26:1612, Am J Surg Pathol 2018;42:201, Histopathology 2018;72:82
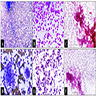
Microscopic (histologic) images
Contributed by Maria Tretiakova, M.D., Ph.D.
Contributed by Debra Zynger, M.D.
Cytology description
- Single cells, poorly cohesive cell clusters in necrotic background
- Cytoplasm is vacuolated to densely eosinophilic
- Often marked nuclear atypia and mitotic activity
- Reference: Diagn Cytopathol 2018;46:1064
Positive stains
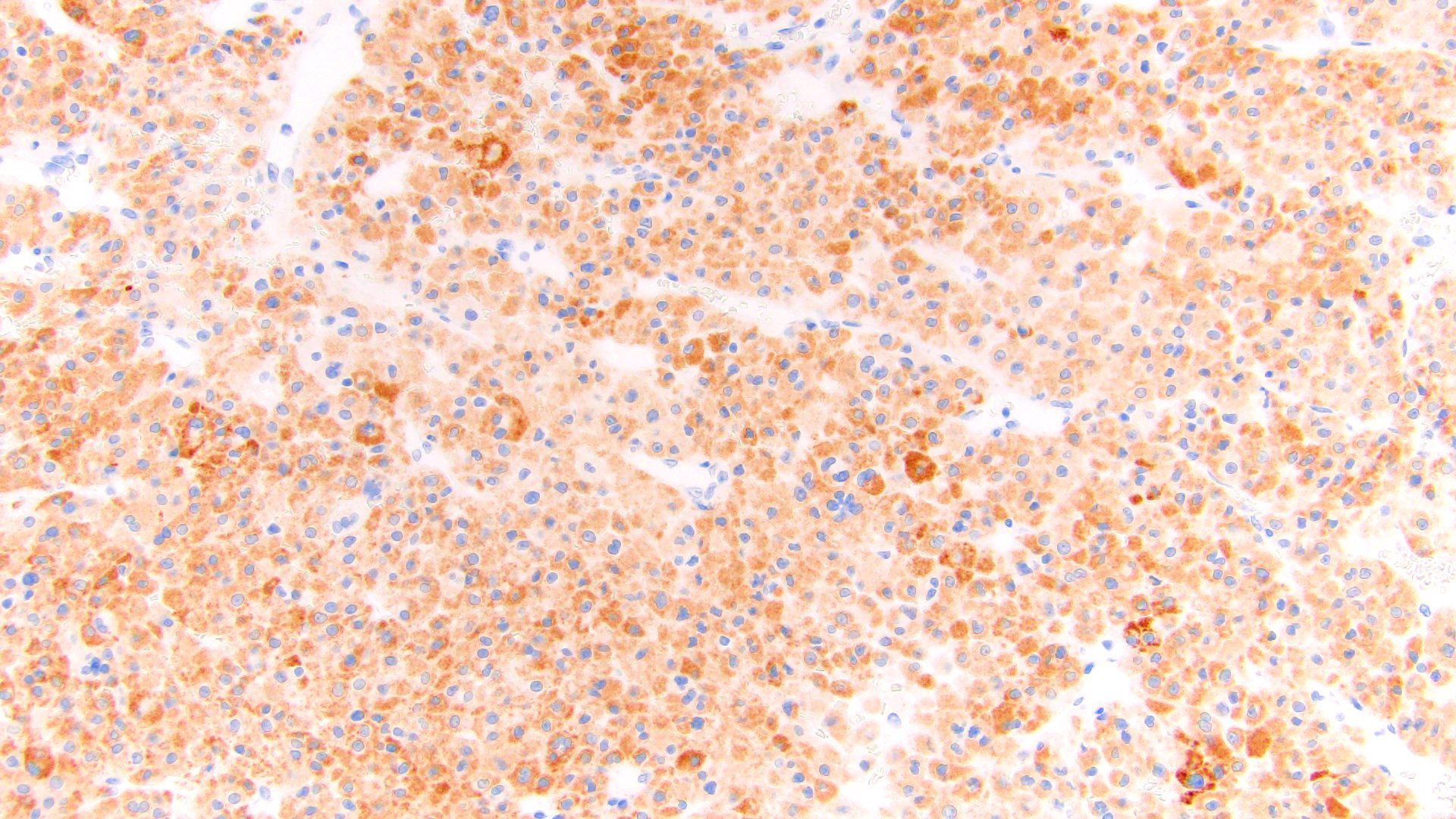
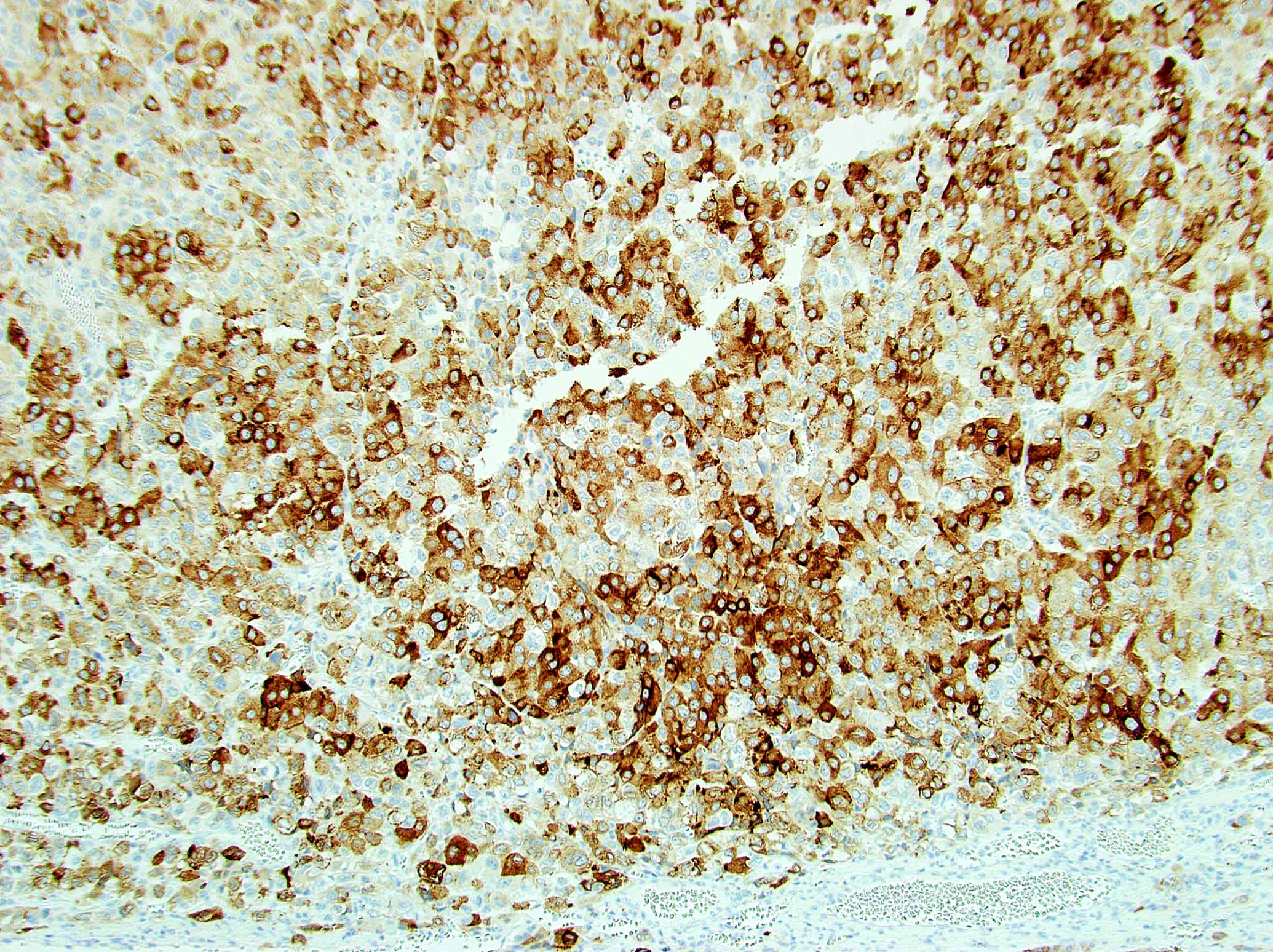
- Steroidogenic factor 1 (SF1), calretinin, inhibin, MelanA / MART1, synaptophysin, neuron specific enolase (NSE), IGF2
- Vimentin, p53, INSM1, CAM 5.2 (other keratins weak or negative)
- Reticulin to highlight disrupted network
- References: Endocr Pathol 2006;17:345, J Clin Endocrinol Metab 2010;95:E161, Appl Immunohistochem Mol Morphol 2010;18:414, Am J Surg Pathol 2010;34:423, Am J Surg Pathol 2011;35:678, Appl Immunohistochem Mol Morphol 2014;22:24, Am J Surg Pathol 2018;42:201
Negative stains
- PAX8, CAIX, CD10, CK7, CK20, EMA, CEA, GATA3, S100, arginase, HepPar1, glypican and chromogranin (Endocr Pathol 2006;17:345, J Clin Endocrinol Metab 2010;95:E161, Appl Immunohistochem Mol Morphol 2010;18:414, Am J Surg Pathol 2010;34:423, Am J Surg Pathol 2011;35:678, Appl Immunohistochem Mol Morphol 2014;22:24)
Electron microscopy description
- Abundant rough and smooth endoplasmic reticulum
- Intracytoplasmic droplets of lipids
- Many mitochondria with tubular cristae
- Occasionally dense core granules (associated with neuroendocrine immunostains)
- Reference: Int J Surg Pathol 2004;12:231
Molecular / cytogenetics description
- Most frequent significantly mutated driver genes: IGF2, CTNNB1, TP53, TERT, ZNRF3, PRKAR1A, RPL22, TERF2, CCNE1, NF1 (Cancer Cell 2016;29:723)
- Often harbors massive DNA loss, followed by whole genome duplication associated with aggressive clinical course / hallmark of disease progression (Cancer Cell 2016;29:723)
- Increased TERT expression, decreased telomere length and activation of cell cycle programs (Cancer Cell 2016;29:723)
- DNA methylation signatures identified 3 adrenocortical carcinoma molecular subtypes with distinct clinical outcomes with disease progression rates of 7%, 56% and 96% (Cancer Cell 2016;29:723)
Sample pathology report
- Adrenal gland, left, adrenalectomy:
- Adrenal cortical carcinoma with the following features:
- Tumor size: 8.5 cm x 7.5 cm x 7 cm
- Tumor (gland) weight: 228 g
- Tumor extent: tumor invades through the adrenal capsule
- Histologic type: focal myxoid and oncocytic features
- Histologic grade: low grade
- Lymphovascular invasion: small vessel (capillary lymphatic)
- Margins: involved circumferential margin (blue ink)
- pTNM, AJCC 8th edition: pT3 NX
- Ancillary studies: Ki67 mitotic rate 20%
- Reticulin stain: disruption of reticulin framework
- Adrenal cortical carcinoma with the following features:
Differential diagnosis
- Adrenal cortical adenoma:
- Pheochromocytoma:
- Renal cell carcinoma, clear cell type:
- Hepatocellular carcinoma:
- Metastatic tumors:
- Most likely to be bilateral; check clinical history
- Often glandular, squamous or small cell morphology
- Most commonly lung in origin
- Also melanoma and carcinomas from breast, colorectal and bladder
- References: Endocr Res 2000;26:861, Endocr Relat Cancer 2009;16:573, Histopathology 2014;64:567, Am J Surg Pathol 2018;42:201, J Clin Endocrinol Metab 2015;100:841, Am J Surg Pathol 2013;37:1433, Endocr Pathol 2006;17:345, J Clin Endocrinol Metab 2010;95:E161, Appl Immunohistochem Mol Morphol 2010;18:414, Am J Surg Pathol 2010;34:423, Am J Surg Pathol 2011;35:678, Appl Immunohistochem Mol Morphol 2014;22:24, Am J Surg Pathol 2021;45:1264
Board review style question #1
A 54 year old woman presents to clinic complaining of recent onset of hot flashes, headaches, weight gain and muscle weakness. On exam, she has hypertension, acne, facial plethora and mild abdominal distension. A noncontrast CT of the abdomen reveals a heterogeneous enhancing 9.3 cm mass in the left adrenal gland. An adrenalectomy was done and is shown in the photo. Which of the gross features will be most predictive of a malignant diagnosis?
- Areas of necrosis
- Fibrous capsule
- Size (9.3 cm)
- Tan-yellow color
- Weight 205 g
Board review style answer #1
A. Areas of necrosis. Macroscopically, adrenal cortical carcinomas (ACC) tend to be large, lobulated, yellow masses with heterogeneous cut surface and fibrous capsule; however, in organ confined disease, only the presence of confluent necrosis would be consistent with either borderline or malignant tumor. Further microscopic examination will be essential to further substantiate ACC diagnosis with overtly malignant features (diffuse architecture with loss of reticulin continuity, high mitotic activity, atypical mitoses or lymphovascular invasion).
Comment Here
Reference: Adrenal cortical carcinoma
Comment Here
Reference: Adrenal cortical carcinoma
Board review style question #2
Board review style answer #2
E. SF1, reticulin stain, Ki67. This is an adrenal cortical carcinoma. The clinical presentation and image are suggestive of functional (cortisol producing) adrenocortical tumor. Expression of SF1 will confirm adrenocortical origin. Reticulin will be useful to highlight disrupted reticulin framework supporting malignant diagnosis of adrenal cortical carcinoma (ACC). Adrenal cortical carcinomas can be stratified in prognostic groups based on mitotic activity and proliferation index by Ki67. Chromogranin, synaptophysin and CD56 could be useful in distinguishing pheochromocytoma from an adrenocortical tumor. AE1 / AE3, CK7 and TTF1 expression will be consistent with metastatic lung cancer. PAX8, CAIX and TFE3 expression panel will be helpful to confirm and classify renal cell carcinoma. MelanA, inhibin and calretinin are expressed in benign and malignant adrenocortical tumors, thus not ideal for precise diagnosis and prognostication of this neoplasm.
Comment Here
Reference: Adrenal cortical carcinoma
Comment Here
Reference: Adrenal cortical carcinoma