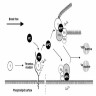
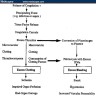
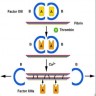
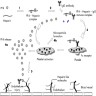
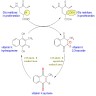
- During injury to vascular endothelium, the first response to prevent blood loss is vasoconstriction which activates endothelial cells (Ann Med 2012;44:405)
- Formation of the platelet plug in primary hemostasis is primarily orchestrated by interactions between the endothelium, von Willebrand factor (vWF) and platelets (Arterioscler Thromb Vasc Biol 2020;40:1441)
- Activated endothelial cells secrete vWF, which recruits platelets to the site of injury and results in a sequence of platelet adhesion, activation and aggregation (Int J Biochem Cell Biol 2021;131:105900, Hematol Oncol Clin North Am 2021;35:1069)
- Platelet adhesion occurs when vWF, bound to exposed subendothelial collagen, interacts with glycoprotein Ib / IX / V on platelets (Arterioscler Thromb Vasc Biol 2008;28:403)
- vWF is a plasma glycoprotein, stored in alpha granules secreted by platelets and Weibel-Palade bodies in the endothelium (Blood 2015;125:2019)
- Platelet activation results in changes in platelet and membrane morphology and secretion of microparticles from intracellular granules
- Upon activation, platelet cytoplasm and cytoskeleton rearrange to increase surface area and enable adherence to endothelium (Thromb Haemost 2002;88:186)
- Platelet aggregation is mediated by glycoprotein IIb / IIIa (GpIIb / IIIa) on the platelet surface
- During platelet activation, GpIIb / IIIa undergoes a conformational change into the active form; activated GpIIb / IIIa has high affinity for ligand binding and promotes platelet aggregation into the initial platelet plug (Blood 2007;109:5087)
Superpage
Superpage Topics
4 factor PCC
Abnormal PT and PTT - causes
Acquired dysfibrinogenemia
Acquired thrombophilia - general
Acquired von Willebrand disease (AVWD)
Activated clotting time
Activated protein C resistance / Factor V Leiden
Algorithm for workup of hereditary bleeding disorders
Amyloidosis
Anticardiolipin antibodies
Antiplasmin assay
Antiplatelet agents (pending)
Antithrombin assay
Antithrombin deficiency
Bleeding time
Clot retraction
Coagulation laboratory tests - overview
Cryoglobulin / cryofibrinogen assays
D-dimer / dimerized plasmin fragment D
Dabigatran
Direct oral anticoagulants (pending)
Disseminated intravascular coagulation (DIC)
Dysfibrinogenemia
Ecarin clotting time
Elevated coagulation factor levels in plasma
Factor assays
Factor I (fibrinogen) assay
Factor I (fibrinogen) deficiency
Factor II (prothrombin) deficiency
Factor IX deficiency (hemophilia B)
Factor V deficiency
Factor V Leiden assay
Factor VII deficiency
Factor VIII deficiency (hemophilia A)
Factor X deficiency
Factor Xa assay
Factor XI deficiency
Factor XII deficiency
Factor XIII deficiency
Heparin
Heparin - low molecular weight
Heparin cofactor II deficiency
Heparin induced thrombocytopenia
Heparin induced thrombocytopenia (HIT)
Heparinase / heparin contamination assay
Hereditary bleeding disorders - general
Hereditary bleeding disorders - testing
Hereditary thrombophilia - general
High molecular weight kininogen deficiency / assay
Homocysteine assay
Hypercoagulation panel
Hyperhomocysteinemia
Inhibitors
International sensitivity index (ISI)
Liver dysfunction
Low molecular weight heparin (LMWH)
Lupus anticoagulation & antiphospholipid antibodies
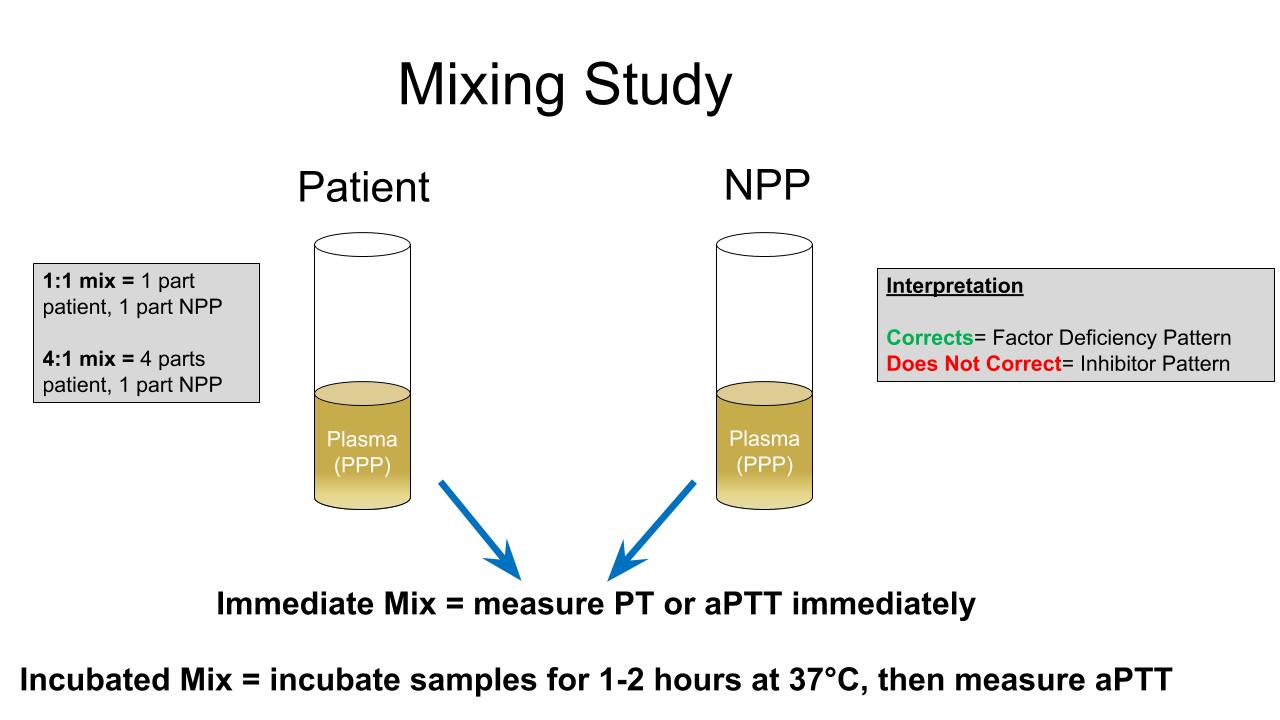
Mixing studies
Other anticoagulants & thrombolytic therapy
Physiology
Plasminogen activator antigen-1
Plasminogen assay
Platelet aggregation studies
Platelet antibodies
Platelet hyperaggregation studies
Prekallikrein assay
Prekallikrein deficiency
Procoagulants (pending)
Protein C deficiency
Protein S deficiency
Proteinuria
Prothrombin gene mutation (G20210A) / hyperprothrombinemia
PT - Prothrombin time
PT / INR and aPTT
Quality assurance
Reptilase time
Thrombin time
tPA assay
Validation of assays and instruments (pending)
Viscoelastic hemostatic assays
Vitamin K deficiency / warfarin use
von Willebrand disease and testing4 factor PCC
Table of Contents
Definition / general | Essential features | Pathophysiology | Advantage over 3 factor PCC (3F PCC) | Brand names (U.S.) | Indications | Dosing | Dosage forms considerations | Example dosing calculation | Monitoring | Contraindications | U.S. boxed warning | Board review style question #1 | Board review style answer #1Definition / general
- 4 factor prothrombin complex concentrate (4F PCC) is derived from plasma pools depleted of cryoprecipitate
- 4F PCC contains hemostatic levels of vitamin K dependent factors (II, VII, IX and X) and physiological amounts of coagulation inhibitory proteins, protein C and S
- Final formulation of 4F PCC may include heparin and antithrombin (ATIII) as specific clotting factor stabilizers to prevent activation
Essential features
- 4F PCC contains hemostatic levels of vitamin K dependent coagulation factors (II, VII, IX and X) and physiological amounts of coagulation inhibitory proteins, protein C and S
- 4F PCC is indicated for the urgent reversal of acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adult patients with
- Acute major bleeding (Kcentra only) or
- Need for an urgent surgery / invasive procedure (Kcentra and Balfaxar)
- Baseline and 30 minute postdose international normalized ratio (INR) levels, clinical response and signs of thromboembolism during and after treatment should be monitored
Pathophysiology
- 4F PCC contains the vitamin K dependent coagulation factors II, VII, IX and X, collectively referred to as the prothrombin complex, along with the antithrombotic proteins C and S
- During vitamin K antagonist therapy, a dose related deficiency of factors II, VII, IX and X may arise
- Anticoagulant action of vitamin K antagonists inhibits the carboxylation of glutamic acid residues in these factors, reducing both their synthesis and functionality
- Administration of 4F PCC elevates the levels of these coagulation factors and antithrombotic proteins in plasma (StatPearls: Prothrombin Complex Concentrate [Accessed 14 March 2024])
Advantage over 3 factor PCC (3F PCC)
- Key difference between 3F PCC and 4F PCC lies in the content of factor VII
- 3F PCC contains hemostatic levels of factor IX, varying levels of factors II and X and low levels of factor VII, whereas 4F PCC contains therapeutic levels of factors II, VII, IX, X and proteins C and S (Shock 2019;52:23)
Brand names (U.S.)
- Kcentra or Balfaxar
Indications
- U.S. Food and Drug Administration (FDA) approved use
- 4F PCC is indicated for the urgent reversal of acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adult patients with
- Acute major bleeding (Kcentra only) or
- Need for an urgent surgery / invasive procedure (Kcentra and Balfaxar)
- 4F PCC is indicated for the urgent reversal of acquired coagulation factor deficiency induced by vitamin K antagonist therapy in adult patients with
- Off label use
- Life threatening hemorrhage associated with direct factor Xa inhibitor or direct thrombin inhibitor
- Perioperative coagulopathy
- Reversal of oral direct factor Xa inhibitors in patients requiring urgent procedures, in lieu of andexanet (factor Xa inhibitor reversal agent)
- References: FDA: Package Insert - Kcentra [Accessed 19 March 2024], FDA: Package Insert - Balfaxar [Accessed 19 March 2024], Transfus Med Rev 2021;35:96, Am J Emerg Med 2022;55:38
Dosing
- Weight based dose (dose is based on body weight up to but not exceeding 100 kg; for patients weighing > 100 kg, the maximum dose should not be exceeded)
Pretreatment INR 2 - < 4 4 - 6 > 6 Dose (units of factor IX/kg body weight) 25 35 50 Maximum dose (units of factor IX) Not to exceed 2,500 Not to exceed 3,500 Not to exceed 5,000
- Fixed dose protocols have been formulated using data from Kcentra studies, suggesting an initial dose of 1,000 - 2,000 units; repeat dosing is advised in cases of modest INR reversal (Cardiovasc Drugs Ther 2022;36:533)
Dosage forms considerations
- Each vial label indicates potency in terms of factor IX nominal strength (500 units or 1,000 units); this should be taken into consideration when patient dosing calculations are performed
- Kcentra: the potency of a 500 unit vial ranges from 400 to 620 units and for a 1,000 unit vial, it ranges from 800 to 1,240 units
- Balfaxar: the potency of a 500 unit vial varies from 400 to 640 units and for a 1,000 unit vial, it ranges from 800 to 1,280 units
- References: FDA: Package Insert - Kcentra [Accessed 19 March 2024], FDA: Package Insert - Balfaxar [Accessed 19 March 2024]
Example dosing calculation
- For a 70 kg patient with a baseline INR of 7.0, the required dose of 4F PCC would be 3,500 units of factor IX
- This calculation is based on an INR range above 6, where 50 units of factor IX per kg of body weight results in 3,500 units needed
- To determine the volume to administer, the potency of the vial must be considered; in the case of a vial containing 30 units/mL of factor IX, 117 mL would be the appropriate amount (3,500 U divided by 30 U per mL equals 117 mL)
- References: FDA: Package Insert - Kcentra [Accessed 19 March 2024], FDA: Package Insert - Balfaxar [Accessed 19 March 2024]
Monitoring
- Baseline and 30 minute postdose INR levels, clinical response and signs of thromboembolism during and after treatment should be monitored
Contraindications
- Anaphylaxis or severe systemic reaction to PCC or any component of the formulation
- Known allergy to heparin or history of heparin induced thrombocytopenia
- Immunoglobulin A (IgA) deficiency, with known antibodies against IgA (Balfaxar)
- Disseminated intravascular coagulation (Kcentra)
- References: FDA: Package Insert - Kcentra [Accessed 19 March 2024], FDA: Package Insert - Balfaxar [Accessed 19 March 2024]
U.S. boxed warning
Arterial and venous thromboembolic complications
- Patients on vitamin K antagonist therapy have underlying diseases predisposing them to thromboembolic events
- Potential benefits of reversing vitamin K antagonists should be weighed against the risks of thromboembolic events, especially in patients with a history of such events
- Resumption of anticoagulation should be evaluated if the risk of thromboembolic events outweighs the risk of acute bleeding
- Both fatal and nonfatal arterial and venous thromboembolic complications have been reported with prothrombin complex concentrate in clinical trials and postmarketing surveillance
- Monitor patients receiving prothrombin complex concentrate for signs and symptoms of thromboembolic events
- Prothrombin complex concentrate was not studied in patients with a thromboembolic event, myocardial infarction, disseminated intravascular coagulation, cerebral vascular accident, transient ischemic attack, unstable angina pectoris or severe peripheral vascular disease within the prior 3 months
- Prothrombin complex concentrate may not be suitable for patients with thromboembolic events in the prior 3 months
- References: FDA: Package Insert - Kcentra [Accessed 19 March 2024], FDA: Package Insert - Balfaxar [Accessed 19 March 2024]
Board review style question #1
A 73 year old man on warfarin for atrial fibrillation presented with severe abdominal pain. Imaging studies reveal an intraperitoneal hemorrhage. The decision is made to reverse the anticoagulation urgently. The medical team debates between using a 3 factor prothrombin complex concentrate (PCC) and a 4 factor PCC.
Based on the composition of prothrombin complex concentrates, which of the following best describes the difference between 3F PCC and 4F PCC in the context of reversing warfarin induced anticoagulation?
- 3F PCC contains hemostatic levels of factor IX, varying levels of factors II and X and low levels of factor VII, whereas 4F PCC contains therapeutic levels of factors II, VII, IX, X and proteins C and S
- 3F PCC contains therapeutic levels of factors II, VII, IX and X, while 4F PCC contains hemostatic levels of factors II, IX and X, with low levels of factor VII
- 3F PCC contains therapeutic levels of factors VII, IX and X, while 4F PCC contains varying levels of factors II, IX and X, with low levels of factor VII
- Both 3F PCC and 4F PCC contain therapeutic levels of factors II, VII, IX and X but only 4 factor PCC contains proteins C and S
Board review style answer #1
A. 3F PCC contains hemostatic levels of factor IX, varying levels of factors II and X and low levels of factor VII, whereas 4F PCC contains therapeutic levels of factors II, VII, IX, X and proteins C and S. This makes 4F PCC a more suitable option for reversing warfarin induced anticoagulation due to its broader range of coagulation factors.
Answer B is incorrect because it inaccurately states that 3F PCC contains therapeutic levels of factor VII and 4F PCC contains low levels of factor VII.
Answer C is incorrect because it inaccurately describes the composition of both 3F PCC and 4F PCC. 3F PCC does not contain therapeutic levels of factor VII and 4F PCC does not contain low levels of factor VII. Instead, 4F PCC contains therapeutic levels of all 4 factors (II, VII, IX and X) and proteins C and S.
Answer D is incorrect because it states that both 3F PCC and 4F PCC contain therapeutic levels of factors II, VII, IX and X, which is inaccurate.
Comment Here
Reference: 4 factor PCC
Comment Here
Reference: 4 factor PCC
Abnormal PT and PTT - causes
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Symptoms | Laboratory | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are common initial tests in the evaluation of patients with suspected bleeding disorders
- PT evaluates extrinsic and common pathways
- aPTT measures intrinsic and common pathways
Essential features
- PT and aPTT methods measure time to fibrin clot formation
- PT and aPTT results are reported in seconds
- Common causes of prolonged PT or aPTT are factor deficiencies, inhibitors (specific or nonspecific), liver failure, disseminated intravascular coagulation (DIC), anticoagulants and preanalytic factors
- Prolonged PT and aPTT can be further evaluated with mixing studies or other coagulation tests (such as factor activities and lupus anticoagulant testing) if indicated
Terminology
- Prolonged PT or aPTT
- Abnormal coagulation screening tests
- Abnormal clotting time
Pathophysiology
Images hosted on other servers:
| Clotting time prolonged | Key differential diagnoses |
| Prothrombin time (PT) |
|
| Activated partial thromboplastin time (aPTT) |
|
| PT and aPTT |
|
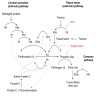
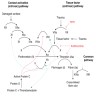
- PT evaluates clotting within the extrinsic and common coagulation pathways
- Causes of isolated prolonged PT (Clin Lab Med 2009;29:253, Lab Med 2017;48:295)
- Deficiency of or inhibitor to factor VII
- Mild decrease in common pathway factor(s)
- Medications: warfarin and other vitamin K antagonists, direct Xa inhibitors
- Liver disease (early / mild)
- DIC (early)
- Vitamin K deficiency
- aPTT evaluates clotting within the intrinsic and common coagulation pathways
- Causes of isolated prolonged aPTT (Lab Med 2017;48:295, Semin Thromb Hemost 2014;40:195, Am J Hematol 2013;88:82):
- Deficiency of or inhibitor to factors VIII, IX, or XI
- Deficiency of contact factors (factor XII, prekallikrein, high molecular weight kininogen)
- Contact factor deficiency is not associated with clinical bleeding
- Isolated aPTT prolongation rarely occurs with multiple factor deficiencies such as DIC or liver failure
- Lupus anticoagulant (nonspecific inhibitor)
- Medications: unfractionated heparin (note: aPTT is commonly used to monitor unfractionated heparin therapy), direct thrombin inhibitors
- Causes of prolonged PT and aPTT
- Deficiency of or inhibitor to common pathway factors
- Dilutional coagulopathy
- DIC
- Nonspecific inhibitor: lupus anticoagulant with hypoprothrombinemia
- Afibrinogenemia, hypofibrinogenemia or dysfibrinogenemia
- Severe liver disease
- Vitamin K deficiency (severe)
- Medications: supratherapeutic warfarin, superwarfarins, supratherapeutic unfractionated heparin, direct Xa inhibitors (high levels), direct thrombin inhibitors (high levels)
- Prolonged PT or aPTT can prompt further evaluation with a mixing study or factor assays if factor deficiency or inhibitor suspected (Lab Med 2017;48:295, Mayo Clin Proc 2007;82:864)
- A mixing study is nonspecific but patterns may support either a factor deficiency or a factor inhibitor
Clinical features
- Conditions associated with prolonged PT or aPTT: (Lab Med 2017;48:295, Mayo Clin Proc 2007;82:864, Clin Lab Med 2009;29:253, Am J Hematol 2013;88:82)
- Factor deficiency:
- Specific factor inhibitor:
- Decreased factor activity and positive Bethesda (inhibitor) assay for a specific factor
- Bleeding
- Nonspecific inhibitor (e.g. lupus anticoagulant):
- Positive lupus anticoagulant testing (e.g. dRVVT, hexagonal phospholipid neutralization assay, platelet neutralization procedure)
- No specific factor inhibitor detected with Bethesda assay
- May have thrombosis or be asymptomatic
- Vitamin K deficiency:
- Anticoagulant medications:
- Heparin - prolonged aPTT and prolonged TT that correct with heparin neutralization, normal reptilase time
- Warfarin - decreased activity of factors II, VII, IX, X
- Direct thrombin inhibitors - prolonged aPTT and prolonged TT that do not correct with heparin neutralization, normal reptilase time
- Direct Xa inhibitors - abnormal anti-Xa activity assay
- Disseminated intravascular coagulation (DIC):
- Decreased activity of multiple factors, including fibrinogen; markedly elevated D-dimer
- May have bleeding and / or thrombosis
- Liver failure:
- Decreased activity of all coagulation factors except for factor VIII which is not produced by hepatocytes
- Factor V activity is decreased in liver failure but not with vitamin K antagonists or vitamin K deficiency
- May have bleeding or be asymptomatic
- Dysfibrinogenemia, hypofibrinogenemia, afibrinogenemia:
- Dysfibrinogenemia - fibrinogen activity decreased to a greater degree than fibrinogen antigen
- Hypofibrinogenemia / afibrinogenemia - fibrinogen activity and antigen decreased to a similar degree
- Bleeding
Symptoms
- Abnormal PT / aPTT can be asymptomatic or associated with bleeding or clotting, depending on the underlying cause
Laboratory
- PT / aPTT testing are clotting times (Lab Med 2017;48:295)
- Current coagulation analyzers use 1 of 2 strategies for clot detection
- Electromechanical
- Optical
- Current coagulation analyzers use 1 of 2 strategies for clot detection
- Sample for testing: platelet poor plasma in 3.2% sodium citrate
- PT: thromboplastin (tissue factor combined with phospholipid) and calcium chloride are added to the sample to initiate clotting
- aPTT: a contact factor activator (e.g. kaolin, silica) combined with phospholipid and calcium chloride are added to the sample to initiate clotting
- Reagents used for PT and aPTT testing vary between labs and show different factor sensitivities as well as different sensitivity to lupus anticoagulants and anticoagulant medications
- Each laboratory must understand the performance characteristics of the reagent it uses
- PT and aPTT results are reported in seconds
Differential diagnosis
- Preanalytic variables that may falsely prolong PT and aPTT (Lab Med 2017;48:295, Mayo Clin Proc 2007;82:864)
- Sample drawn in EDTA instead of citrate
- EDTA strongly chelates calcium, falsely prolonging PT and aPTT, among other abnormalities (Lab Med 2012;43:1)
- Delay in specimen processing
- Loss of labile factors (V, VIII) may falsely prolong PT and aPTT
- Underfilling blood collection tube
- Causes relative citrate excess and false prolongation of PT and aPTT
- High hematocrit
- Increased RBC and decreased plasma in tube causes relative citrate excess and false prolongation of PT and aPTT
Board review style question #1
Which of the following is the most likely possible cause of combined PT and aPTT prolongation?
- Factor VII deficiency
- Low hematocrit
- Lupus anticoagulant
- Underfilled collection tube
Board review style answer #1
D. Underfilled collection tube. Underfilling the blood collection tube will result in a prolongation of aPTT and PT due to the increased concentration of citrate. High hematocrit (not low hematocrit) can also result in a relative excess of citrate due to increased red blood cells and a relatively smaller volume of plasma in the tube. Lupus anticoagulant affects aPTT to a greater degree than PT due to the high concentration of phospholipids in PT reagents. Factor VII deficiency would be expected to prolong the PT but not the aPTT.
Comment Here
Reference: Abnormal PT and PTT - causes
Comment Here
Reference: Abnormal PT and PTT - causes
Board review style question #2
A 68 year old man has an aPTT of > 150 s (reference interval 24 - 35 s) and PT of 12 s (reference interval 12 - 15.5 s). A 1:1 aPTT mixing study result is 33 s. He has no personal or family history of a bleeding disorder. What is the most likely cause of his aPTT prolongation?
- Factor IX deficiency
- Factor XIII deficiency
- Prekallikrein deficiency
- Von Willebrand factor deficiency
Board review style answer #2
C. Prekallikrein deficiency. Prekallikrein deficiency causes prolonged aPTT that corrects in a 1:1 mixing study and is not associated with clinical bleeding. The other contact factors (factor XII and high molecular weight kininogen) give a similar picture. Factor IX deficiency can also cause a prolonged aPTT that corrects in a mixing study but it is associated with a bleeding diathesis (hemophilia B). Factor XIII deficiency does not prolong the PT or aPTT (recall that factor XIII crosslinks fibrin but the PT and aPTT reaction endpoint is the formation of fibrin and these tests do not measure the effect of factor XIII). Some types of von Willebrand disease may be associated with prolonged aPTT (e.g. type 2N, type 3) due to significantly decreased factor VIII activity but the degree of aPTT prolongation is typically less than was seen in the case in this question.
Comment Here
Reference: Abnormal PT and PTT - causes
Comment Here
Reference: Abnormal PT and PTT - causes
Acquired dysfibrinogenemia
Table of Contents
Definition / general | Epidemiology | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Abnormal fibrinogen molecule that causes a decrease in the rate of fibrin polymerization
- Rarely causes bleeding or thrombosis
Epidemiology
- 80% prevalence in patients with liver disease
- 8% prevalence in patients with obstructive jaundice
Etiology
- Usually caused by liver or biliary tract disease or acute phase reaction
- Also monoclonal immunoglobulin that binds to fibrinogen
- Abnormal fibrinogen has increased sialic acid residues, which increases the net negative charge of the molecule, promoting charge repulsion between fibrin monomers, leading to decreased fibrin polymerization
- In cancer-associated dysfibrinogenemia (hepatocellular carcinoma, cervical carcinoma, breast carcinoma, renal cell carcinoma), tumor cells may secrete abnormal fibrinogen
- Usually does not cause bleeding or thrombosis, but may in alcoholic liver disease
Clinical features
- Patients are usually asymptomatic
- Rarely bleeding or thromboses
Laboratory
- Screening tests include reptilase time and thrombin time
- Fibrinogen clotting activity / antigen ratio is confirmatory test
- Patients usually have abnormal liver function tests
- Should rule out dysfibrinogenemia in family members (i.e. rule out congenital form)
- Dysfibrinogenemia typically resolves if underlying disease improves (i.e. liver disease improves or cancer undergoes remission)
Prognostic factors
- Difficult to assess as patients with liver disease often have coagulation defects that could contribute to bleeding or thrombosis
Case reports
- 63 year old man with monoclonal light chain that binds fibrinogen (Haematologica 2007;92:e111)
- 72 year old man with myeloma paraprotein that interacts with fibrinogen (Acta Haematol 2008;120:75)
Treatment
- Treat clinical findings (i.e. if patient is bleeding, can give cryoprecipitate
- If patient has thrombosis, can give heparin followed by oral anticoagulants)
Differential diagnosis
- Autoantibodies against fibrinogen
- Congenital dysfibrinogenemia
Additional references
Acquired thrombophilia - general
Definition / general
- Thrombophilia: any disorder associated with increased risk of venous thromboembolic disease; more appropriately called "hypercoagulable state" if not genetic, although terms often used interchangeably
- Common risk factors are: antiphospholipid antibodies, chronic DIC, essential thrombocythemia, heparin induced thrombocytopenia, hyperhomocysteinemia, immobility, increasing age, malignancy, nephrotic syndrome, obesity, oral contraceptives, paroxysmal nocturnal hemoglobinuria, polycythemia vera, postoperative state, pregnancy, prior thromboembolism, systemic lupus erythematosus, trauma
- Presence of more than one risk factor further increases risk (Arch Pathol Lab Med 2002;126:295)
Case reports
- 33 year old man with fatal pulmonary emboli associated with hypereosinophilia (J Clin Path 2004;57:541)
- 52 year old woman with portal vein thrombosis associated with myeloproliferative disorder (Arch Pathol Lab Med 2003;127:e385)
Additional references
Acquired von Willebrand disease (AVWD)
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- A rare bleeding disorder of primary hemostasis that appears either spontaneously or associated with lymphoproliferative or myeloproliferative diseases, solid tumors, autoimmune disorders, cardiovascular disorders, drugs or other miscellaneous causes (Acta Haematol 2009;121:177)
- Mimics congenital von Willebrand disease in terms of clinical presentation and laboratory findings
Terminology
- Also called acquired von Willebrand syndrome (AVWS)
Epidemiology
- Usually older adults, but can occur in children
Sites
- Typically mucocutaneous or gastrointestinal bleeding
Etiology
- Not completely understood and most likely multifactorial; underlying mechanisms include:
- Autoantibodies to vWF or Factor VIII causing inhibition or increased clearance
- Cell-mediated or drug-induced proteolysis of vWF
- Abnormal vWF binding to tumor cells causing increased clearance of Factor VIII-vWF complex
- Decreased synthesis
Clinical features
- Mild to moderately severe mucocutaneous or gastrointestinal bleeding in a patient with previously normal coagulation and no family history of coagulopathy (Am J Hematol 2007;82:368)
- May be underlying cause of bleeding tendency in hypothyroid patients (Haemophilia 2008;14:423)
- Many patients present with normal or increased test results, emphasizing the importance of multimer analysis in all patients with suspected disease (J Thromb Haemost 2008;6:569)
- Algorithm for clinical evaluation: Mayo Medical Laboratories
Laboratory
- Patients may have any of the following:
- Prolonged bleeding time
- Platelet assay showing prolonged closure times (PFA-100)
- Reduced vWF activity
- Reduced vWF antigen
- Reduced factor VIII activity
- Prolonged aPTT
- Most patients exhibit type II pattern on multimer electrophoresis, but can see type I as well as type III
- Use of VWF multimer assay recommended only when initial VWD testing identifies an abnormal result, or clinical information suggests a high likelihood of abnormal VWF multimer analysis (Mayo Medical Laboratories)
Prognostic factors
- Depends on underlying disorder and other comorbidities
Case reports
- 55 year-old man with gastrointestinal angiodysplasia (Haemophilia 2006;12:452)
- Transient neonatal acquired von Willebrand syndrome due to transplacental transfer of maternal monoclonal antibodies (Pediatr Blood Cancer 2009;53:655)
Treatment
- Aimed at both control of the acute bleeding episode and of the underlying disorder (i.e. hypothyroidism)
- DDAVP is usually initiated first, followed by replacement therapy with plasma derived Factor VIII-vWF concentrates
- If there is no success, then IVIG (immunoglobulin) may be tried, especially if the underlying cause is thought to be autoimmune in nature
- There is little published data on the effectiveness of plasma exchange
- Immunosuppressive agents and corticosteroids have been used, but are reported to be less effective
Differential diagnosis
- Bernard-Soulier Syndrome (Arch Pathol Lab Med 2007;131:1834)
- Congenital von Willebrand disease
Additional references
Activated clotting time
Table of Contents
Definition / generalDefinition / general
- Whole blood clotting assay, performed at point of case, including operating room or catheterization lab (Am J Clin Pathol 2011;135:741), to monitor high - dose heparin anticoagulation (cardiopulmonary bypass surgery) or to immediately measure heparin (ECMO, hemodialysis, cardiac catheterization)
- Note: test is necessary for high - dose heparin monitoring because PTT is often unclottable at very high heparin levels
- Whole blood is collected into tube with celite (diatomaceous earth), kaolin, glass particles or other activator of intrinsic pathway
- Tube may need to be shaken to disperse the activator
- Tube is monitored by instrument that records time until clot is formed
- Do not collect blood from line containing heparin
- Target reference range depends on the method: usually 70 - 180 seconds, 400+ seconds for cardiopulmonary bypass operations
- Does not correlate well with PTT but heparin level can be measured using an anti - Xa assay
- Affected by platelet count and function, lupus anticoagulant, factor deficiencies, patient and ambient temperature, hemodilution, aprotinin (reversible platelet inhibitor that prolongs elite - based tests)
Activated protein C resistance / Factor V Leiden
Table of Contents
Definition / general | Etiology | Diagrams / tables | Laboratory | Case reports | Treatment | Additional referencesDefinition / general
- Most common hereditary predisposition to venous thrombosis (20% of first episodes of thrombosis, 50% of familial thrombosis)
- Normally, activated protein C degrades activated factors V and VIII by cleaving specific arginine residues
- Almost all patients with activated protein C resistance have Factor V Leiden mutation that causes resistance to degradation by activated protein C
- Approximately 64% of people with venous thrombosis have activated protein C deficiency
- Does not appear to reduce life expectancy
- Acquired forms of activated protein C deficiency can lead to elevated factor VIII levels
Etiology
- Factor V Leiden mutations:
- 95% with activated protein C resistance have point mutation at an arginine cleavage site (Arg506Gln, 1691 G to A) called R506Q or Factor V Leiden
- Mutation causes delayed inactivation by activated protein C, prolonging its life span and procoagulant activity
- 3 - 5% frequency in heterozygous form in general white population
- Rare in African blacks and Asians
- Heterozygotes have 5 - 10x increased risk for venous thrombosis
- Homozygotes have 80x increased risk for venous thrombosis; risk occurs later in life
- Homozygosity or heterozygosity without symptoms may not require treatment
- Presence of second risk factor (genetic or acquired) is often necessary to produce thrombosis
- Acquired risk factors are smoking, malignancy, trauma, surgery, oral contraceptive use, estrogen replacement therapy, antiphospholipid antibody, heterozygosity for prothrombin G20210A, elevated serum homocysteine
- Other low frequency factor V mutations, which have unclear association with venous thrombosis:
- Factor V Hong Kong (Arg306Gly)
- HR2 haplotype with mutation 4070A to G (His199Arg) in exon 13 of Factor V gene (associated with other polymorphisms)
Laboratory
- Testing recommended if venous thromboemboli occur with these features:
- Recurrent
- Before age 50 years
- Unprovoked at any age
- At unusual anatomic sites (cerebral, mesenteric, portal or hepatic veins)
- In patient with first degree relative with venous thromboemboli before age 50 years
- Related to pregnancy or estrogen use or unexplained pregnancy loss in second or third trimesters
- May be recommended in family members (with family history), female family members who are pregnant or considering oral contraceptives
- Testing NOT recommended:
- General population screen
- Routine test during pregnancy
- Routine test before or during oral contraceptive use or hormone replacement therapy in patients without a family history of thrombosis
- As newborn initial test
- As initial test in patients with arterial thrombotic events
Case reports
- 51 year old woman with heterozygous factor V Leiden and dural sinus thrombosis (Arch Pathol Lab Med 2003;127:1359)
Treatment
- Treat venous thromboemboli similarly regardless of the presence of factor V Leiden
Additional references
Algorithm for workup of hereditary bleeding disorders
Table of Contents
Definition / general | Etiology | Diagrams / tables | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Hereditary bleeding disorders are a diverse group of diseases that include abnormalities of primary and secondary hemostasis (see etiology)
- Evaluation of a patient for a hereditary bleeding disorder is a multistep process that involves a complete and accurate history followed by laboratory evaluation
- Important considerations are whether the bleeding is truly congenital vs. a possible acquired coagulopathy, and whether the clinical symptoms suggest a disorder of primary vs. secondary hemostasis (see clinical features)
- Causes of acquired bleeding include liver disease, renal disease, vitamin K related factor deficiency (warfarin, prolonged antibiotic use, malabsorption syndromes, nutritional deficiency), DIC and dilutional coagulopathy in massive transfusion
- A complete history should include:
- Surgical history: dental procedures, bleeding during surgery vs. postoperative bleeding
- Nature of bleeding: habitual epistaxis, menorrhagia, hemarthrosis, postpartum hemorrhage, petechiae, purpura, etc.
- For patients with menorrhagia: history of severe iron deficiency anemia, multiple red blood cell transfusions, procedures such as D&C or hysterectomy for excessive bleeding
- Family history of bleeding (but a negative family history of bleeding does not rule out a congenital bleeding disorder)
- Medication use: warfarin, heparin, aspirin or other NSAIDs, antibiotics (affecting vitamin K dependent clotting factors) and herbal medications
- Medical problems: renal failure, severe liver disease, malabsorption syndromes
Etiology
- Primary hemostasis involves formation of the platelet plug, which involves platelets, the blood vessel wall and von Willebrand factor
- Abnormalities can include problems in platelet number, adhesion or aggregation
- Secondary hemostasis involves the formation of fibrin through the humoral coagulation cascade
- Abnormalities include deficiencies of coagulation factors or contact factors, deficiencies or abnormalities of fibrinogen or connective tissue diseases
- Mutations can be inherited in an autosomal dominant, recessive or X-linked pattern
Diagrams / tables
| Bleeding disorder | Prevalence | Inheritance pattern | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Factor I (fibrinogen) deficiency
| More than 200 cases reported
|
| Autosomal recessive Autosomal dominant or recessive Autosomal dominant or recessive Factor II (prothrombin) deficiency
| Less than 100 cases reported
| Autosomal recessive
| Factor V deficiency
| Less than 1 in 1,000,000
| Autosomal recessive
| Factor VII deficiency
| 1 in 500,000
| Autosomal recessive
| Factor VIII deficiency
| 1 in 5000 male births
| X-linked recessive
| Factor IX deficiency
| 1 in 30,000 male births
| X-linked recessive
| Factor X deficiency
| 1 in 500,000
| Autosomal recessive
| Factor XI deficiency
| 4% in Ashkenazi Jews, otherwise rare
| Autosomal recessive
| Factor XIII deficiency
| More than 200 cases reported
| Autosomal recessive
| Combined factor deficiencies
|
| > 30 families reported Autosomal recessive Autosomal recessive
a2-antiplasmin deficiency
| > 10 families reported
| Autosomal recessive
| a1-antitrypsin Pittsburgh deficiency
| Only 3 cases reported
| Autosomal dominant
| von Willebrand Disease (VWD)
|
1 in 100
|
| Autosomal dominant Autosomal dominant Autosomal recessive Glanzmann thrombasthenia
| 1 in 1,000,000
| Autosomal recessive
| Bernard-Soulier syndrome
| Autosomal recessive
| Gray platelet syndrome
| Rare
| Autosomal dominant, recessive or X-linked recessive
| Wiskott-Aldrich syndrome
| 1 in 1,000,000
| X-linked recessive
| |
| Disorders of Primary Hemostasis |
|---|
| von Willebrand disease |
| Glanzmann thrombasthenia |
| Bernard-Soulier syndrome |
| Platelet storage pool disease |
| Gray platelet syndrome |
| Wiskott-Aldrich syndrome |
| Disorders of Secondary Hemostasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Factor I (fibrinogen) abnormalities
| Factor II (prothrombin) deficiency
| Factor V deficiency
| Factor VII deficiency
| Factor VIII deficiency (Hemophilia A)
| Factor IX deficiency (Hemophilia B)
| Factor X deficiency
| Factor XI deficiency
| Factor XIII deficiency
| Combined factor deficiencies
| a2-antiplasmin deficiency
| a1-antitrypsin deficiency
| Ehlers-Danlos syndrome
| Osler-Weber-Rendu syndrome
| Scurvy (vitamin C deficiency) | |
Clinical features
- Symptoms: bleeding associated with surgery, trauma, dental extractions, postpartum, circumcision or umbilical stumps; GI bleeding, intracranial hemorrhage, hemarthrosis or soft tissue hematomas, easy bruising, epistaxis, menorrhagia, hematuria
- Note: pregnant women with mild to moderate vWD (von Willebrand disease) or hemophilia A carriers typically are asymptomatic due to elevated vWF and Factor VIII during pregnancy
- Some symptoms are more suggestive of either a primary or secondary hemostatic defect and can help narrow the differential diagnosis:
- Epistaxis, menorrhagia, melena, intraoperative or immediate postoperative bleeding and petechiae suggest a platelet disorder or vWD
- Delayed postoperative bleeding is usually due to a coagulation factor deficiency, fibrinogen abnormalities or a collagen disorder
- Spontaneous hemarthrosis and intramuscular bleeds suggest coagulation factor deficiencies
- Note: hemarthrosis is common in classic hemophilias but also can occur in acquired hemophilias
Laboratory
- Basic screening tests include CBC, PT/PTT, bleeding time or platelet function assay (e.g. PFA-100), thrombin time, peripheral blood smear review (for platelet and erythrocyte morphology), fibrinogen
- Testing for vWD includes Factor VIII activity, vWF antigen, vWF activity (often done by the "ristocetin cofactor" method)
- These results may lead to ordering vWF multimer assays and blood type determination (type O patients have reduced vWF activity)
- For suspected coagulation factor abnormalities: mixing studies, factor levels, Bethesda assay (to detect coagulation factor inhibitors); can confirm hereditary deficiency by determining factor levels in relatives
- For suspected platelet disorders: platelet aggregation studies, bone marrow aspirate and biopsy, platelet-associated immunoglobulin levels
- Perform Factor XIII assay if delayed bleeding is present (often done by "urea clot lysis" method)
- More esoteric assays include PAI-1 activity and antiplasmin
- Note: lupus anticoagulants can cause prolongation of PTT, but are associated with thrombosis, not bleeding
- Deficiencies of Factor XII, prekallikrein or high molecular weight kininogen do not cause bleeding but cause prolongation of PTT
Prognostic factors
- Heterozygous patients have 30 - 60% of normal values of affected factors, usually with no or minor bleeding disorder
- Homozygous deficient patients have In hemophilia A and B, small differences in factor levels (i.e. 1% vs. 3% vs. 10%) may markedly affect the clinical presentation and course
Case reports
- Bleeding due to familial platelet cyclo-oxygenase deficiency (Thromb Res 2005;116:483)
Treatment
- Specific treatment recommendations depend on the type and severity of bleeding disorder, but in general, factor replacement therapy for factor deficiencies is the mainstay of treatment, with the exception of factor II, factor V and factor deficiencies; FFP and cryoprecipitate are also common (Haemophilia 2008;14:671)
- For von Willebrand disease: DDAVP (desmopressin), vWF concentrates, antifibrinolytic agents
- For platelet-related bleeding disorders: platelet transfusion, recombinant factor VIIa
Differential diagnosis
- Acquired factor deficiencies: due to liver disease, DIC, lupus anticoagulants, heparin, warfarin or other anticoagulants are more common than hereditary factor deficiencies, and should be ruled out first
- Acquired platelet defects due to anti-platelet medications: aspirin, glycoprotein IIB/IIIA inhibitors, clopidogrel, ticlopidine are much more common than inherited platelet abnormalities
Additional references
Amyloidosis
Table of Contents
Definition / general | Epidemiology | Sites | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Amyloidosis is a generic term for multiple disorders with extracellular tissue deposition of insoluble low molecular weight fibrils
- May be inherited or acquired
- May cause acquired Factor X deficiency due to Factor X binding to amyloid, causing a shortened half time
- Bleeding usually does not occur unless Factor X levels fall below 10% (Am J Hematol 2010;85:171)
- Some patients have normal levels of coagulation factors, but severe hemorrhage can result from amyloid deposition in small to medium sized vessel walls, leading to wall damage
- For example, cerebral amyloid angiopathy is usually asymptomatic, but can cause primary lobar intracerebral hemorrhage in the elderly
Epidemiology
- Amyloidosis is rare, but is found in 15% with multiple myeloma
Sites
- Amyloid deposits between the endothelium and the basement membrane of vessel walls
- Generally involves the microvasculature of any organ but can involve the great vessels of the brain
- Amyloid deposits in spleen can cause splenic rupture and severe hemorrhage (Amyloid 2009;16:47)
- Deposition in the liver can cause decreased synthesis of coagulation factors in patients with advanced liver disease
Etiology
- Factor X deficiency is due to:
- Binding to amyloid fibrils primarily in the liver and spleen
- Decreased synthesis of coagulation factors in patients with advanced liver disease
- AL ("amyloid light chain") amyloidosis (primary):
- AL amyloidosis is caused by deposition of immunoglobulin light chain fragments
- Usually due to a plasma cell dyscrasia; most patients have monoclonal immunoglobulin light chains detectable in the serum or urine
- 9% have Factor X levels that are ← 50% of normal (Blood 2001;97:1885)
- AA ("amyloid associated") amyloidosis (secondary):
- Composed of fragments of amyloid A
- Seen with chronic diseases associated with ongoing inflammation: rheumatoid arthritis, chronic infection, inflammatory bowel disease
- Beta-2 microglobulin amyloid:
- Amyloid composed of fibrils from beta-2-microglobulin
- Seen with long term hemodialysis
- Transthyretin (prealbumin) amyloid:
- Amyloid composed of a mutant form of transthyretin (prealbumin)
- This type of amyloid is deposited in familial amyloid polyneuropathies and in the heart in senile systemic amyloidosis
- Beta amyloid:
- Found in Alzheimer disease brain lesions
- Amylin (islet amyloid polypeptide) amyloid:
- Found in pancreas of patients with type 2 diabetes
Clinical features
- Factor X levels in amyloidosis typically range from 2% to 50%
- Bleeding symptoms usually do not arise unless Factor X levels fall below 10%
- Frequently presents as purpuric bleeding, typically occurs at pressure points
- Purpura may occur in periorbital regions (raccoon eyes) after minor trauma or valsalva maneuver
- Some patients with abnormal bleeding have no abnormality in any coagulation test (JAMA 1983;249:1322)
- Amyloid infiltration of blood vessels may contribute to the bleeding diathesis
- Bleeding due to acquired von Willebrand disease has been described in AL amyloidosis (Am J Hematol 2007;82:363)
- An interaction between amyloid beta-peptide and tissue-type plasminogen activator also may contribute to the tendency to hemorrhage in cerebral amyloid angiopathy, as in vitro studies have shown that amyloid beta-peptide analogues markedly stimulate plasminogen activation by tissue-type plasminogen activator
Laboratory
- Diagnosis of amyloidosis requires confirmation by tissue biopsy
- Fat pad aspiration biopsy has a low sensitivity for amyloidosis in patients with single organ involvement, but higher sensitivity in those with multiorgan involvement
- Because fat pad aspiration biopsy is less likely than liver, renal, or rectal biopsy to be complicated by serious bleeding, it is suggested for initial biopsy in patients with other than single organ involvement
Prognostic factors
- Prognosis varies by etiology
Case reports
- 20 year old woman with corpus luteum hemorrhage and hemoperitoneum (The Internet Journal of Anesthesiology 2007;13(2))
Treatment
- Treatment is directed toward the underlying condition
- For dialysis related amyloidosis, altering the mode of dialysis or considering renal transplantation is recommended
- For the hereditary amyloidoses in which the mutant amyloid precursor protein is produced by the liver (eg: transthyretin, apolipoprotein A-I, and fibrinogen Aa), liver transplantation may prevent further deposition of amyloid and lead to regression of established deposits
Differential diagnosis
- Factitious purpura
- Hemophilias
- Heparin
- Scurvy
- Thrombocytopenia (platelet count Vitamin K deficiency
- Warfarin ingestion
Additional references
Anticardiolipin antibodies
Antiplasmin assay
Definition / general
- Also called anti - alpha2 - antiplasmin, plasmin inhibitor
- An uncommon assay usually sent to reference laboratories
- Indications:
- Familial bleeding disorder, after ruling out more common bleeding disorders such as von Willebrand disease
- True alpha2 - antiplasmin deficiency is a rare condition ( Most cases are caused by inhibitors (antibodies)
- Specimen:
- Plasma in citrate tube, without epsilon - aminocaproic acid, aprotinin, heparin or other fibrinolysis inhibitors
- Reference range:
- Approximately 48 - 80 mg / dL, lower during first 5 days of life
- Functional assays:
- Add specific amount of excess plasmin to patient’s plasma, measure plasmin that is unbound to antiplasmin in patient’s serum by detecting color change spectrophotometrically
- Amount of unbound plasmin detected is inversely proportional to patient’s antiplasmin level
- Antigenic (immunologic) assay:
- Patient’s plasma in placed in the cylindrical well of an agarose gel containing antiplasmin antibody, which defuses into the well and forms an antigen - antibody complex and precipitin ring
- The size of the ring is proportional to the patient’s antiplasmin
- Acquired causes of decreased antiplasmin:
- Liver disease, thrombolytic therapy, DIC
Additional references
Antiplatelet agents (pending)
[Pending]
Antithrombin assay
Definition / general
- Assays detect antigenic (type I, reduced normal protein, quantitative) or functional (type II, normal amount of defective protein, qualitative) deficiencies of antithrombin (formerly called antithrombin III)
- Perform functional assay first - if decreased, perform antigenic assay on fresh specimen
- Family studies may be helpful
- Functional assays:
- Are chromogenic, use predominantly amidolytic methods (i.e. through cleavage of an amide bond), employing a synthetic peptide that mimics the natural target of the enzyme
- Patient plasma is incubated with excess thrombin and heparin
- Antithrombin neutralizes thrombin, and remaining thrombin is then quantitated with a chromogenic substance
- The amount detected is inversely proportional to the patients antithrombin
- Limitations:
- False levels may be produced if high levels of heparin cofactor II are present; this is eliminated by assays that use inhibition of factor Xa rather than thrombin
- Newer assays have protease inhibitors to minimize nonspecific substrate cleavage and bovine thrombin
- Hirudin or argatroban anticoagulation may interfere with thrombin based assays
- Antigenic assays:
- Quantification is usually via radial immunodiffusion techniques, although they have coefficients of variation of 40 - 50%
- Amidolytic assays have CV of only 9 - 14%
- Also used are latex particles coated with antithrombin antibodies (e.g. LIA)
- Light absorbance is related to the amount of antithrombin in the specimen
- Also family studies (first degree relatives)
- Limitations:
- Does not detect functional deficiencies by itself
- If initial antithrombin result is low, should do confirmatory test on repeat specimen
- Must also exclude acquired causes
Etiology
- Acquired causes of low antithrombin levels:
- Clot formation
- Surgical procedures
- Liver disease
- Nephrotic syndrome
- DIC
- Heparin (full dose therapy decreases levels by up to 30%)
- L - asparaginase therapy
- Possibly pregnancy or oral contraceptives
- Acquired causes of high antithrombin levels:
- Warfarin therapy
Laboratory
- Specimen / reference ranges:
- Plasma in sodium citrate tube
- Levels are lower in newborns; rise to adult levels (112 - 140 mg / liter) by age 6 - 12 months
- Mildly decreased values (70 - 80%) are unlikely to be associated with thrombosis
- Indications:
- Evaluation of individuals with thrombophilia (strong family history or young patient)
- Also analyze for factor V Leiden and prothrombin G20210A
- Preferable to not test during the acute phase of a thrombotic event (normal antithrombin value makes antithrombin deficiency unlikely, although cannot interpret mildly abnormal values)
Additional references
Antithrombin deficiency
Table of Contents
Definition / general | Etiology | Treatment | Molecular / cytogenetics description | Additional referencesDefinition / general
- Hereditary deficiencies occur in 0.07 - 0.17% of general population
- Present in 1 - 9% of patients with venous thrombosis
- Higher risk for venous and arterial thrombosis than protein C or S deficiency or activated protein C resistance; overall 50% have thrombosis
- First thrombotic event occurs between ages 10 - 50 years
- Often occurs with other genetic or acquired risk factors
- Heterozygotes have levels 35 - 75% of normal
Etiology
- Acute hemolytic transfusion reaction
- Acute thrombotic episodes
- Burns (extensive)
- Heparin therapy
- Inflammatory bowel disease
- L-asparaginase therapy
- Liver disease
- Malignancy
- Malnutrition
- Nephrotic syndrome
- Plasmapheresis
- Preeclampsia
- Protein poor diet
- Thrombosis-recent or active (including DIC)
Treatment
- Heparin (unfractionated or low molecular weight), followed by warfarin
- May need increased doses of heparin or antithrombin concentrates / fresh frozen plasma if resistant to heparin
- Should monitor antithrombin levels (should be 80 - 120%)
Molecular / cytogenetics description
- Many mutations exist (qualitative or quantitative)
- Usually autosomal dominant
- Homozygosity is very rare, usually incompatible with life due to neonatal thrombosis, except for those with a heparin-binding mutation subtype, who have severe thrombosis but may survive
- Type I mutations: quantitative deficiency with 50% of normal levels; due to any of 80 point mutations
- Type II mutations: dysfunctional protein; often asymptomatic
- IIa: mutations affect reactive site of target protease and heparin binding site
- IIb: mutations affect reactive site of target protease
- IIc: isolated decreased heparin binding
Additional references
Bleeding time
Definition / general
- A relatively nonspecific and nonsensitive test of platelet function, whose use is declining
- This test should usually be avoided, particularly if definitive testing, such as a von Willebrand panel is available
- Preoperative bleeding time does NOT predict surgical bleeding
- Test is affected by use of aspirin or other NSAIDs, patients should abstain from their use for 1 week prior to testing
- Test is also affected by how incision is made (very difficult to standardize)
- Procedure:
- Place blood pressure cuff on arm at 40 mm Hg
- Then trained technologist makes a small incision on patients arm, blots the blood gently every 30 seconds with filter paper, without touching the clot, to see if bleeding has stopped and records the time when it stops
- Then apply bandage
- Duke bleeding time:
- Uses earlobe or fingertip pierced with lancet
- Ivy bleeding time:
- Blood pressure cuff at 40 mm Hg on arm, and forearm cut by lancet
- Mielke (template) bleeding time:
- Template placed on skin with spring loaded blade that cuts through template, to standardize the size and depth of cut
- More reproducible than standard bleeding time but still quite variable
- Reference range:
- Varies, sample range is 1.5 - 9.5 minutes (less in newborns)
- Prolonged values:
- Platelet count less than 100K, low hemoglobin, use of aspirin or other platelet inhibitors
- Also von Willebrands and other hereditary platelet disorders, uremia
Additional references
Clot retraction
Table of Contents
Definition / generalDefinition / general
- Obsolete test
- Evaluates how well platelets keep the clot adhered to the sides of specimen tube
- Uses whole blood in red top tube
- Examine clot at 1, 2, 4 and 24 hours for clot retraction
- After clot forms, remaining 40 - 60% consists of serum and red blood cell "fall - out" from clot
- Must have normal fibrinogen and hematocrit for test be accurate
- Reduced clot formation:
- Glanzmann thrombasthenia: reduced glycoprotein IIb / IIIa causes reduced platelet aggregation and clot retraction
- DIC, hypofibrinogenemia, dysfibrinogenemia (small clot with increased red blood cell "fall - out")
Coagulation laboratory tests - overview
Table of Contents
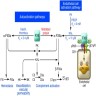
Definition / general | Laboratory | General algorithm | Bleeding tests | Clotting tests | Additional referencesDefinition / general
- Performed in almost all hospitals in US
- Necessary for diagnosis, treatment and management of bleeding and hypercoagulation disorders, to screen for coagulation disorders and to monitor anticoagulant therapy
- CAP requires laboratories to notify medical staff immediately if a critical value is obtained
- For critical values, CLIA requires laboratory to immediately alert individual or entity that requested the test, or if applicable, the individual responsible for using the test results
Laboratory
- Tubes with 3.2% citrate are preferred over 3.8% citrate (higher concentration prolongs PT and PTT if tube not filled to the recommended level)
- Do not draw specimens from indwelling catheters (which contain anticoagulants)
- If multiple tubes are drawn, draw coagulation tube after the red top and before the EDTA, heparin or oxalate / fluoride tubes
- Try to fill the sample tube completely
- Notify laboratory if patient is on anticoagulants and specify which ones
- Do not delay transport of tubes to laboratory; if delay cannot be avoided, separate plasma or serum from cells as soon as possible; store plasma (or serum) on ice for up to 4 hours, or store frozen
General algorithm
Bleeding tests
Clotting tests
Additional references
Cryoglobulin / cryofibrinogen assays
Table of Contents
Definition / generalDefinition / general
- Either asymptomatic or causes cutaneous symptoms at cold - exposed areas
- Cryofibrinogen consists of fibrinogen and other substances that precipitate at cold temperatures (cryoglobulins are immunoglobulins that precipitate at cold temperatures)
- Either primary, or associated with malignancy, infection (especially hepatitis C), inflammatory conditions, diabetes, pregnancy, oral contraceptives
- May exhibit leukocytoclastic vasculitis in skin biopsies
- Specimen:
- Two sodium citrate or EDTA tubes plus one red top tube for cryoglobulin
- Place immediately in warm water (or use warmer for heal sticks or other warming method) and transport to laboratory within 2 hours
- Dont use heparin - containing specimens (heparin precipitates fibrinogen in this assay)
- Indication:
- For patients with unexplained cutaneous ulcers or ischemia on cold - exposed areas
- Procedure:
- Centrifuge at 37C, refrigerate plasma, centrifuge at 4C
- Each mm of visible precipitate represents 1% of cryofibrinogen
- Cyrocrit is %volume of precipitate compared to total plasma
- Also perform cryoglobulin test to ensure that plasma precipitate is not a cryoglobulin
- If the cryoglobulin test is positive, serum protein electorpheresis with immune fixation should be run to determine what type of cryoglobulin is present
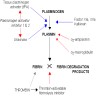
D-dimer / dimerized plasmin fragment D
Definition / general
- Marker of ongoing procoagulant activity
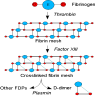
- Fibrin degradation products (fibrin split products) that are formed only by plasmin degradation of fibrin, not by plasmin degradation of intact fibrinogen, thus indicating that fibrin has been formed
- D-regions of fibrinogen are crosslinked by factor XIII after the fibrin clot is formed
- Plasmin cannot cleave the bond between the D-regions, so dimers are also found when a clot is broken down
- Normal plasma level is probably due to physiologic clotting activity
- Specimen:
- Usually plasma with citrate anticoagulant
- Values Other values are not predictive (Arch Pathol Lab Med 2004;128:519)
- Suggested guidelines for D-dimer testing to rule out pulmonary emboli in patients with low clinical suspicion (if moderate or high clinical suspicion, should do imaging studies):
- Age Acad Emerg Med 2005;12:20)
- Elevated levels are sensitive but not specific for DIC
- Elevated levels after completion of oral anticoagulation are associated with venous thromboemboli
- LIA assay:
- Mix patient plasma with latex particles coated with monoclonal anti-D-dimers or fibrin degradation product antibodies
- Detect agglutination with coagulation analyzer and semiquantitate with dilutions
- Although this is called a Latex ImmunoAssay, it differs from the qualitative latex agglutination assay that is NOT predictive of pulmonary emboli
- ELISA method:
- Also available
- False positives:
- Recent surgery
- HIV+ Castlemans disease due to interference from monoclonal gammopathy (Arch Pathol Lab Med 2004;128:328)
- High rheumatoid factor
- Liver disease
- Cancer patients
- Pregnancy
- Note: Some platforms use fibrinogen equivalent units, which are 50% the numerical value of D-dimer units
Additional references
Dabigatran
Definition / general
- Oral anti - coagulant (also rivaroxaban, apixaban) with minimal food and drug interactions that does not require coagulation monitoring
- Drug is very expensive, and is affected by humidity (Wikipedia)
- Dabigatran etexilate is prodrug of dabigatran, a direct thrombin inhibitor
- Alternative to warfarin for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (J Clin Pharmacol 2012;52:119S, J Med Econ 2012;15:695) or venous thromboembolism (N Engl J Med 2009;361:2342, Vasc Health Risk Manag 2012;8:45)
- Good patient compliance (Orthop Traumatol Surg Res 2012;98:186)
- INR levels are not necessary, and are substantially higher using Hemochron Jr. point-of-care device compared with laboratory values (Am J Med 2012;125:417)
- Can use HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran (Blood Coagul Fibrinolysis 2012;23:138)
- Contraindications: nonvalvular atrial fibrillation patients with renal insufficiency (Rinsho Shinkeigaku 2011;51:1004), INR 2.0 or greater, age 80 years or greater (N Engl J Med 2012;366:864)
Diagrams / tables
Direct oral anticoagulants (pending)
[Pending]
Disseminated intravascular coagulation (DIC)
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Epidemiology | Pathophysiology | Diagrams / tables | Clinical features | Diagnosis | Laboratory | Prognostic factors | Case reports | Treatment | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3 | Board review style question #4 | Board review style answer #4Definition / general
- First descriptions of DIC appeared in the 19th century (Semin Thromb Hemost 2014;40:874)
- DIC is a systemic activation of the coagulation system, which results in microvascular thrombosis and simultaneously potentially life threatening hemorrhage attributed to consumption of platelets and coagulation factors
- DIC is a complication to various underlying clinical conditions, including infection, malignancies, obstetrical complications, trauma (especially head trauma) and vascular disorders (e.g. Kasaback-Merritt syndrome, aortic aneurysms, etc.); of these conditions, infection related DIC is most common
- Based on the severity and stage, DIC can be categorized as nonovert (early) and overt (decompensated); DIC can present either acutely or chronically and can be subclinical
- In general, DIC patients can suffer from both bleeding and thrombosis, although thrombosis may not be readily apparent
Essential features
- Essential features of DIC can be classified as clinical and laboratory features
Terminology
- Most widely used definition of DIC was issued by a subcommittee of the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Hemostasis in 2001 (Thromb Haemost 2001;86:1327)
ICD coding
- ICD-10: D65 - Disseminated intravascular coagulation (defibrination syndrome)
Epidemiology
- Prevalence of DIC in patients with various medical conditions:
- 1% of hospitalized patients are estimated to develop DIC
- 10 - 30% of patients in the intensive care unit (ICU)
- 20% of patients with acute respiratory distress syndrome (ARDS)
- 30 - 40% of patients with severe head trauma
- 30 - 60% of patients with severe sepsis
- See Table 1 for secondary causes
- Reference: Br J Haematol 2021;192:803
Pathophysiology
- Increased tissue factor activity and thrombin generation
- Tissue factor is expressed on circulating activated monocytes in sepsis related DIC and on the surface of the malignant cells or circulating tumor derived microparticles in cancer related DIC
- In obstetric DIC, placental abruption and amniotic fluid embolism expose the circulating blood to tissue factor
- Through activation of coagulation factor (F) VII and FX, increased tissue factor activity leads to thrombin generation
- Increased platelet activation in DIC occurs through interaction with activated endothelium and the direct action of thrombin on platelets; in sepsis related DIC, inflammatory cells, cytokines and pathogens interact directly with platelets and contribute to their activation
- Levels of antithrombin, protein C and protein S decrease due to consumption, decreased synthesis in the liver and degradation by neutrophil elastase
- Fibrinolysis can be either impaired (hypofibrinolysis) or enhanced (hyperfibrinolysis) according to the pathophysiology of the underlying disease
- Both tissue plasminogen activator (tPA) and plasminogen activator inhibitor 1 (PAI1) are released from the activated endothelium, such as in patients with vascular malformations (see Table 1)
- In sepsis, the net effect is impaired fibrinolysis in most cases; in contrast, cancer is often associated with increased fibrinolytic activity
- Primary hyperfibrinolysis is a common feature of acute promyelocytic leukemia; these patients often present with severe hyperfibrinolysis and bleeding due to increased plasminogen activation on the malignant cell surface
- Reference: Br J Haematol 2021;192:803
Diagrams / tables
Images hosted on other servers:
Table 1: secondary causes
| Severe infectious diseases | Gram positive or negative organisms, malaria, hemorrhagic fevers |
| Malignancy | Solid tumors (e.g. adenocarcinomas), acute promyelocytic leukemia or monocytic leukemia |
| Trauma | Multitrauma, brain injury, burns |
| Obstetrical complications | Abruptio placentae, amniotic fluid embolism |
| Vascular malformations | Kasabach-Merrit syndrome, giant hemangiomas Other vascular malformations, large aortic aneurysms |
| Severe immunologic reactions | Transfusion reaction |
| Heat stroke | |
| Postcardiopulmonary resuscitation |
Table 2: DIC scoring systems by the JAAM and the ISTH (AMIA Annu Symp Proc 2015;2015:804)
| SIRS* criteria | |
| ≥ 3 | +1 |
| 0 to 2 | 0 |
| Platelet count | |
| < 80 × 109/L or > 50% decrease within 24 hours | +3 |
| ≥ 80 < 120 × 109/L or > 30% decrease within 24 hours | +1 |
| ≥ 120 × 109/L | 0 |
| Prothrombin time (value of patient / normal value) | |
| ≥ 1.2 | +1 |
| < 1.2 | 0 |
| Fibrin / fibrinogen degradation products | |
| ≥ 25 mg/L | +3 |
| ≥ 10 < 25 mg/L | +1 |
| < 10 mg/L | 0 |
| Diagnosis: if ≥ 4, there is positive diagnosis of DIC | |
| *Systemic inflammatory response syndrome | |
| Platelet count | |
| < 50 × 109/L | +2 |
| ≥ 50 < 100 × 109/L | +1 |
| ≥ 100 × 109/L | 0 |
| Elevated fibrin related marker | |
| Strong increase | +3 |
| Moderate increase | +2 |
| No increase | 0 |
| Prolonged prothrombin time | |
| ≥ 6 seconds | +2 |
| ≥ 3 < 6 seconds | +1 |
| < 3 seconds | 0 |
| Fibrinogen level | |
| < 100 g/mL | +1 |
| ≥ 100 g/mL | 0 |
| Diagnosis: if > 5, there is positive diagnosis of overt DIC; if < 5, suggestive (not affirmative) of nonovert DIC | |
Clinical features
- Without adequate treatment, DIC can eventually lead to multiorgan dysfunction / failure
- Patients can present with bleeding, thrombosis or both
- Septic patients are more likely to have thrombosis than bleeding
- Bleeding can present as surgical site, venipuncture site or mucocutaneous bleeding (most common)
- Gastrointestinal bleeding, CNS bleeding, hematuria or ecchymoses
- Thrombosis can present as purpura fulminans (manifestation of subdermal microthrombi with skin necrosis)
- Cold, pulseless limb
- Sudden loss of vision
- Oliguria
- Mental status changes, seizures, behavioral changes or adrenal insufficiency
- Causes of DIC can be acute (meningococcemia), chronic (retained dead fetus), localized (abdominal aortic aneurysm) or systemic (acute promyelocytic leukemia)
- Chronic causes of DIC are typically malignancy, liver disease, retained dead fetus syndrome, abdominal aortic aneurysm, giant hemangioma and head trauma
- Without adequate treatment, DIC can eventually lead to multiorgan dysfunction / failure
- Reference: Br J Haematol 2021;192:803
Diagnosis
- Most widely used DIC diagnostic scoring systems are the JAAM and ISTH (Crit Care 2016;20:287, Thromb Haemost 2001;86:1327)
- See Table 2 for a comparison of both scoring systems
Laboratory
- Prolonged prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT)
- Elevated D dimers and other fibrin degradation products (but D dimer may be falsely positive in HIV+ Castleman disease due to interference from monoclonal gammopathy (Am J Clin Pathol 2004;122:178, Arch Pathol Lab Med 2004;128:328)
- Fall in platelet count (usually not lower than 30,000 - 40,000 x 109/L)
- Consumptive deficiency of fibrinogen, antithrombin, alpha 2 antiplasmin and plasminogen
- Presence of schistocytes and microspherocytes on peripheral blood smear
- With chronic causes, fibrinogen and platelets may actually be elevated as acute phase reactants
- All coagulation factors may be variably decreased due to factor activation and consumption
- Multiorgan dysfunction may manifest as elevated cardiac enzymes or elevated BUN / creatinine
- Baseline coagulation studies and serial follow up are needed to follow the trends
Prognostic factors
- DIC is a devastating condition with a poor prognosis; the clinical course is primarily determined by the age of the patient, presence of comorbidities, identification and treatment of underlying etiologies, initial treatment response and severity of organ dysfunction, including the degree of hemostatic abnormalities
- A multicenter study of critically ill patients with DIC found that the 28 day mortality was 21.9%, which was significantly higher than non-DIC patients (11.2%) (Crit Care Med 2008;36:145)
- Another study found the mortality rate was significantly higher in sepsis patients than trauma patients (Thromb Haemost 2008;100:1099)
Case reports
- 30 year old woman with DIC due to amniotic fluid embolism (Arch Pathol Lab Med 2002;126:869)
- 48 year old woman, 63 year old man and 68 year old man with DIC (Blood 2018;131:845)
- 66 year old woman with DIC due to underlying lymphoma (Chest 2017;151:e41)
Treatment
- Treat underlying disease
- Keep fibrinogen levels above 100 mg/dL with cryoprecipitate or fresh frozen plasma
- Monitor PT, PTT, platelet count, fibrinogen and possibly antithrombin levels
- If bleeding predominates, replace coagulation factors and fibrinogen with fresh frozen plasma (FFP) and cryoprecipitate
- Consider plasmapheresis (ASFA Category III indication), platelet transfusions and immunoabsorption
- Because prothrombin concentrates (PCC) may increase the risk for thromboembolism, they are not recommended for patients with DIC, with an only rare exception in patients with potentially life threatening coagulation factor deficiency when no other alternative treatment is available
- If platelet count is lower than 50,000 x 109/L with active bleeding or lower than 10,000 x 109/L, give platelet transfusion
- If thrombosis predominates (chronic DIC), heparinization should be considered
- See Diagram 1 for diagnosis and treatment strategies
- Reference: Br J Haematol 2021;192:803
Board review style question #1
Tissue factor can be released from which of the following blood cells during infection?
- Eosinophils
- Lymphocytes
- Monocytes
- Neutrophils
- Red cells
Board review style answer #1
Board review style question #2
Which of the following laboratory findings is typical in a patient with DIC?
- Decreased fibrinogen
- Elevated plasminogen
- Elevated protein S and C
- Normal clotting times (PT, APTT and TT)
- Thrombocytosis
Board review style answer #2
Board review style question #3
Which of the following is true of disseminated intravascular coagulation?
- Clotting times are usually shortened
- DIC rarely happens in patients with cancer or a hematologic malignancy
- Patient’s fibrinogen activity is usually elevated
- Patients with promyelocytic leukemia may have markedly elevated D dimer levels
- Treatment strategy should focus on stopping bleeding for virtually all cases of DIC
Board review style answer #3
D. Patients with promyelocytic leukemia have an increased risk of DIC and may show marked elevated D dimer levels
Comment Here
Reference: Disseminated intravascular coagulation (DIC)
Comment Here
Reference: Disseminated intravascular coagulation (DIC)
Board review style question #4
Which of the following is the most common underlying condition or mechanism that leads to DIC?
- Patient with breast cancer
- Pregnant woman (30 weeks) with severe hypertension
- Patient with end stage liver disease due to hepatitis C
- Patient was involved in an automobile accident with fractures of his lower extremities
- Leukemic patient who received chemotherapy and developed a high fever and hypotension
Board review style answer #4
E. Leukemic patient who received chemotherapy and developed a high fever and hypotension
Comment Here
Reference: Disseminated intravascular coagulation (DIC)
Comment Here
Reference: Disseminated intravascular coagulation (DIC)
Dysfibrinogenemia
Definition / general
- Disorders of fibrinogen structure (over 350 described)
- Have variable effects on function (25% associated with bleeding, 20% associated with thrombosis, 55% have no symptoms or prolonged thrombin time)
- Bleeding due to defective fibrin clot formation (impaired release of fibrinopeptides A or B and impaired fibrin monomer polymerization)
- Thrombosis due to:
- defective thrombin binding to fibrin, causing increased thrombin in circulation and more thrombosis
- defective binding of tPA or plasminogen to fibrin or fibrin resistance to plasmin; includes Dusart (Paris V) and Chappel Hill III dysfibrinogens that are resistant to degradation by plasmin
- Congenital (hereditary) dysfibrinogenemia is a rare cause of hypercoagulability (350 reported cases, 0.8% of patients with venous thrombosis); usually due to single amino acid substitutions in fibrinogen Aalpha, Bbeta or gamma genes
- Recommended to use only as a second-line test in patients with thrombosis since dysfibrinogenemia is so rare
- Autosomal dominant inheritance, but higher incidence in women due to pregnancy related thrombosis, particularly post-partum and in venous lower extremities, at mean age 27 years
- Also associated with spontaneous abortions
Laboratory
- Primary screening test is thrombin time (prolonged except for fibrinogens Oslo I and Valhalla - shortened)
- Prolongation may also be due to heparin, heparin - like inhibitors, fibrin degradation products, hypofibrinogenemia, excess fibrinogen, paraproteins, excess protamine, anti - fibrinogen antibodies, anti - bovine thrombin antibodies, systemic amyloidosis, acquired dysfibrinogenemia
- The sensitivity of thrombin time assays varies for dysfibrinogenemia because many assays are designed primarily to detect heparin contamination
- Some labs use the reptilase time, which is not affected by heparin
- Confirmatory test (if thrombin time or reptilase time is prolonged):
- Fibrinogen activity-antigen ratio below reference range
- Activity measured by Clauss method (rate of clot formation after adding high concentration of thrombin to citrated plasma
- Use standard curve relating clotting time to plasma of known fibrinogen activity)
- Antigen concentration determined by ELISA, radial immunodiffusion, precipitation or thrombin clotting methods
- Perform both tests on same sample in same laboratory and using method-specific reference ranges
- Diagnosis:
- Similar laboratory test abnormalities in family members
- If necessary, demonstrate abnormal structure or function of fibrinogen
- Diagnosis of acquired dysfibrinogenemia:
- Abnormal liver function tests, no dysfibrinogenemia in family members
Additional references
Ecarin clotting time
Table of Contents
Definition / generalDefinition / general
- Measures activity of hirudin in plasma, important since severe bleeding can occur with hirudin overdose and no antidote is known
- Also measures activity of lepirudin, a recombinant form of hirudin (J Extra Corpor Technol 2001;33:117)
- Ecarin catalyzes prothrombin to meizothrombin, an active form that is inhibited by hirudin (Pathophysiol Haemost Thromb 2004;33:173)
- Test measures ability of hirudin to complex with / inhibit meizothrombin as ecarin produces it from prothrombin
- Ecarin comes from the venon of the saw - scaled viper
- Specimen:
- Sodium citrate tube filled to top
- Freeze immediately until testing occurs
- Specimens with clots or hemolysis are unacceptable, unless hemolysis is due to cardiopulmonary bypass and is produced in vivo
- Do not draw from heparinized catheter
- Reference range:
- 22.6 - 29.0 seconds
- Prolonged if hirudin or argatroban (direct thrombin inhibitors) present; also hypofibrinogenemia, dysfibrinogenemia
- False positive if prothrombin deficiency is present
Elevated coagulation factor levels in plasma
Definition / general
- May predispose to thrombosis
- Factor I (fibrinogen):
- High levels associated with increased risk for myocardial infarction and arterial thrombosis
- Often elevated in hospitalized patients since it is an acute phase reactant
- Not recommended to measure to assess thrombotic risk, since assay has not been standardized
- Independent effect appears to be modest
- Insufficient clinical data to demonstrate that lowering fibrinogen will prevent ischemic heart disease
- Factor II (prothrombin):
- High levels associated with increased risk of venous thrombosis
- See also prothrombin G20210A mutation
- Standard activity assays are not useful in assessing individual patients for hyperprothrombinemia - use the genetic assay
- Factor V:
- High levels associated with increased risk of arterial thrombosis
- Not recommended to measure to assess thrombotic risk, since not associated with venous thrombosis
- Only one study relates factor V activity and ischemic heart disease
- Methodology is inadequate to assess individual levels; there are no studies showing that reduction of factor V activity will reduce risk for ischemic heart disease or stroke
- Factor VII:
- High levels or certain genetic polymorphisms are associated with increased risk for myocardial infarction, but not an independent risk factor
- Levels are associated with triglyceride and cholesterol levels
- Factor VIII:
- High levels associated with increased risk of venous thrombosis and arterial thrombosis
- However normal range varies about 3 fold, levels vary with ABO blood type (15% lower with type 0) and is acute phase reactant
- If no acute stress reaction, no aerobic exercise in past 24 hours, no estrogenic effects, then baseline value > 150% is a risk factor
- Not recommended to assess individual risk because no established standard for elevated levels, tremendous interlaboratory variation
- Factor IX:
- High levels associated with increased risk of venous thrombosis
- Not recommended to assess individual venous thrombosis risk due to limited amount of clinical data and limited availability of factor IX ELISA
- Factor XI:
- High levels (> 121%) associated with increased risk of venous thrombosis
- Not recommended to assess individual venous thrombosis risk because:
- factor XI assays are not generally available
- one step clotting assay and PTT based assays may be too variable
- may be affected by other variables
- Factor XIII:
- Polymorphism Val34Leu may protect against venous thrombosis (Arch Pathol Lab Med 2002;126:1391)
- von Willebrand factor:
- High levels associated with increased risk of arterial thrombosis
- Not recommended to assess individual thrombotic risk because it is not an independent risk factor, and no studies show that reduction of vWF levels reduces risk of ischemic heart disease, stroke or peripheral arterial disease
Additional references
Factor assays
Definition / general
- PT (factors II, V, VII, X) or PTT (factors V, VIII, IX, XI, XII) based reactions, performed by mixing patient plasma with plasma that is deficient in the factor being measured
- The rate limiting reactant is the deficient factor which must be supplied by the patient
- PT or PTT is compared to standard curve, to determine amount of factor present in patients plasma
- Used to determine the etiology of a prolonged PT or PTT
- Factor levels are expressed as a percentage of normal plasma concentration, or units per mL of normal plasma
- Reference range is often 60 - 140% (should be determined based on the laboratorys patient population)
- Perform at multiple dilutions to rule out an inhibitor - at higher dilutions, inhibitor interference should decrease due to dilution of the inhibitor (this gives a nonlinear curve)
- Can also use chromogenic assays to quantitate
- Levels at birth of factors other than factor VIII are 10 - 100% of adult levels, but reach adult levels at 6 months
Additional references
Factor I (fibrinogen) assay
Definition / general
- Major plasma protein that is synthesized by the liver and is converted to fibrin by thrombin
- Levels can be reduced in disseminated intravascular coagulation (DIC), liver disease, inherited and acquired fibrinogen disorders, thrombolytic therapy, dilutional coagulopathy following massive transfusion and in L-asparaginase therapy
- Levels can be increased in an acute phase reaction, malignancy, females (particularly post-menopausal), pregnancy, oral contraceptive use
- Reference range is usually 1.5 - 4.0 g/L
- Purpose of fibrinogen assay: Fibrinogen assays are typically used to investigate the following:
- Investigation of abnormal coagulation tests (e.g. PT / PTT)
- Investigation of unexplained bleeding
- Investigation of suspected inherited or acquired disorders of fibrinogen (afibrinogenemia, hypofibrinogenemia and dysfibrinogenemia)
- Evaluation of DIC
- Establishment and monitoring of thrombolytic therapy
- Assessment of cardiovascular risk
- Types of fibrinogen assays:
- There are different assays that measure fibrinogen levels and are selected based upon the clinical scenario
- Clauss assay: A functional assay which is based upon the time for fibrin clot formation
- Diluted patient plasma is clotted using a high concentration of thrombin and then the clotting time is measured
- The high concentration of thrombin ensures that the clotting times are independent of the amount of thrombin present
- A calibration curve is generated by clotting times of dilutions of reference plasma samples of known fibrinogen concentrations
- The clotting time is inversely proportional to the amount of fibrinogen in the sample
- Most laboratories use an automated method based on optical density in which fibrin formation decreases light transmission and increases scattered light
- Values can be affected by turbid specimens (e.g. hyperbilirubinemia, hyperlipidemia)
- Values are falsely decreased by heparin > 0.6 units/mL or fibrin degradation products
- The most widely used laboratory method
- Typically used in investigation of bleeding diathesis, congenital and acquired fibrinogen abnormalities, DIC or thrombolytic therapy
- Immunological assays: An immunological method based on measuring fibrinogen antigen rather than functional fibrinogen
- Uses antibodies directed against fibrinogen
- Techniques include enzyme linked immunoabsorbent assays (ELISA), radial immunodiffusion or electrophoresis
- Typically used in investigation of congenital fibrinogen disorders where there is a discrepancy between functional activity and antigen level
- Clottable protein assay: Method based upon clot weight
- Thrombin is added to patient plasma in the absence of calcium ions and then the clot is washed and dissolved by alkaline urea or other reagents
- Spectrophotometry is performed, and since the majority of the protein present in the clot is fibrin, the protein concentration will be equivalent to the fibrinogen concentration
- Very labor intensive and time consuming to perform
- Occasionally used in evaluation of congenital fibrinogen disorders
- PT-based assay: Fibrinogen levels are indirectly measured (derived) and are calculated based on the prothrombin time (PT)
- A calibration curve is generated using prothrombin times and changes in optical density of a series of plasma dilutions of known fibrinogen concentration
- A PT is performed on the patient sample and the fibrinogen level is calculated based on the change in optical density
- Simple and inexpensive test
- Not recommended for routine laboratory use since values can vary based on type of reagents and analyzers used and therefore results are not interchangeable between laboratories or hospitals. Also, when compared to results given by the Clauss assay, the PT-based method has been shown to produce higher values in certain conditions such as liver disease, DIC, renal disease, dysfibrinogenemia, and in those receiving anticoagulants or thrombolytic therapy
- Note: Assays should not be performed on samples collected within 4 hours of heparin administration or on samples collected from heparin-contaminated arterial or venous lines, as heparin may lead to falsely low fibrinogen levels
- There are different assays that measure fibrinogen levels and are selected based upon the clinical scenario
Additional references
Factor I (fibrinogen) deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Clinical features | Laboratory | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor I (fibrinogen) deficiency is an inherited bleeding disorder that is characterized by a variable bleeding diathesis or paradoxically by thrombosis
- Deficiencies are classified as quantitative (afibrinogenemia and hypofibrinogenemia) or qualitative (dysfibrinogenemia) defects of the fibrinogen molecule, or both (hypodysfibrinogenemia)
- Fibrinogen is a soluble glycoprotein that is converted to fibrin by thrombin; it is synthesized mainly in the liver as a hexamer comprised of two sets of three different chains
- Normal fibrinogen levels are 1.5 - 3.5 g/L, with a half-life of 2 - 4 days
Terminology
- Afibrinogenemia is the most severe form of fibrinogen deficiency; patients have no detectable circulating fibrinogen in the plasma or platelets
- Hypofibrinogenemia is a milder form of fibrinogen deficiency; patients have fibrinogen levels Dysfibrinogenemia is the synthesis of abnormal fibrinogen molecules
- Hypodysfibrinogenemia is characterized by both low levels of fibrinogen and abnormal fibrinogen molecules
Epidemiology
- Estimated prevalence of afibrinogenemia is 1 in 1,000,000
- More than 400 cases of dysfibrinogenemia have been reported
- Hypofibrinogenemia and dysfibrinogenemia are more frequent than afibrinogenemia, but prevalence is difficult to establish due to the large number of asymptomatic patients
Sites
- Bleeding of skin and mucosa, joint and muscle, genitourinary tract, gastrointestinal tract, CNS (see clinical features below)
Etiology
- Encoded by FGA, FGB and FGG on chromosome 4 (Wikipedia: Fibrinogen [Accessed 18 June 2020])
- Afibrinogenemia is inherited in an autosomal recessive pattern
- Hypofibrinogenemia is inherited in an autosomal dominant or recessive pattern; mutations in all three genes have been described, and include deletions, frameshift, nonsense or splicing mutations; these mutations can lead to problems that affect fibrinogen synthesis, assembly, intracellular processing (with or without endoplasmic retention), domain stability and protein secretion
- Dysfibrinogenemia is inherited in an autosomal dominant or autosomal recessive pattern; most patients are heterozygous for missense mutations in one of the three fibrinogen genes, which can cause absent or delayed release of Aa and Bb, delayed or enhanced polymerization, defective cross-linking, decreased thrombin binding and defective assembly of the fibrinolytic system
Clinical features
- Afibrinogenemia is typically diagnosed in the neonatal period due to umbilical cord stump bleeding or bleeding after circumcision; patients can have bleeding or thrombosis
- Hypofibrinogenemia is associated with milder bleeding episodes, usually due to trauma or surgical challenge; patients are usually asymptomatic
- Dysfibrinogenemia is usually diagnosed in adulthood; patients can be asymptomatic, have bleeding or thrombosis or both
- Bleeding symptoms can include umbilical cord stump bleeding, bleeding after circumcision, easy bruising, mucosal bleeding, GI / GU bleeding, hemarthrosis, intracranial hemorrhage, recurrent fetal loss, menorrhagia, menometrorrhagia, placental abruption or postpartum hemorrhage; rarely patients can have hemopericardium, hemoperitoneum or spontaneous splenic rupture
- Arterial and venous thrombosis may occur
Laboratory
- Prolonged PT / PTT that corrects with mixing study (may not correct with mixing study in patients with dysfibrinogenemia)
- Prolonged thrombin time
- Low or absent fibrinogen; levels may be normal in dysfibrinogenemia
- Prolonged reptilase time
- Specific fibrinogen assays including clotting and immunologic assays
- Mild thrombocytopenia has been reported in 25% of patients with afibrinogenemia
Case reports
- Patient with congenital afibrinogenemia with retrochorionic hematoma during pregnancy (Am J Hematol 2007;82:317)
Treatment
- Use cryoprecipiate to keep level at 50 - 100 mg/dL in acute bleeding episodes, preoperative state or during pregnancy
- FFP or fibrinogen concentrates may also be used
Differential diagnosis
- Acquired fibrinogen deficiency: autoimmune disease, DIC, liver disease, medications
Additional references
Factor II (prothrombin) deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Etiology | Diagrams / tables | Clinical features | Laboratory | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Congenital deficiency of prothrombin (factor II) that results in reduced prothrombin activity and normal or reduced prothrombin antigen levels
- Prothrombin (factor II) is a vitamin-K dependent cofactor which is activated by factor Xa to form thrombin, which then converts fibrinogen to fibrin
Terminology
- Hypoprothrombinemia (type I deficiency) is a decrease in the overall synthesis of prothrombin and is characterized by reduced prothrombin activity and antigen levels
- Dysprothrombinemia (type II deficiency) is the synthesis of dysfunctional prothrombin molecules and is characterized by reduced activity and normal antigen levels
Epidemiology
- Rare; fewer than 100 cases have been reported
- Prevalence rate of approximately 1:2,000,000 in the general population
Sites
- Mucocutaneous and soft tissue bleeding (see below)
Etiology
- 40 different mutations in the prothrombin gene have been identified, the majority of which are missense mutations (~80%). However, insertion or deletion mutations (~10%) and nonsense mutations (~4%) have also been identified.
- The gene is transmitted in an autosomal recessive pattern with homozygous, heterozygous or compound heterozygous genotypes
- Homozygous individuals have functional prothrombin levels of 2 - 25%
- Heterozygous patients have prothrombin levels of 40 - 60%
- Biologic half life is 48 - 120 hours
Clinical features
- Complete deficiency is incompatible with life
- Severe hemorrhage typically occurs when levels are Need levels of 10 - 40% for surgical hemostasis
- Patients have lifelong history or family history of bleeding; varies from asymptomatic to minor bleeding to severe bleeding (depending on the mutation)
- Also easy bruising, postoperative bleeding, epistaxis, menorrhagia, miscarriage, postpartum hemorrhage, hemarthroses and intracranial bleeding (if severe deficiency)
Laboratory
- Prolonged PT and PTT that correct with mixing study (1:1 mixture of patient and normal pooled plasma)
- Type I (true deficiency) patients will have both activity and antigen levels Type II patients will have normal antigen levels and low activity
Treatment
- Recombinant factor VIIa, alternatively 10 - 20 ml fresh frozen plasma/kg, then 3 ml/kg every 12 - 24 hours as necessary
- Plasma infusion for recurrent bleeding episodes every 3 - 5 weeks
- Prothrombin complex concentrates may be used for serious bleeding
Differential diagnosis
- Acquired prothrombin deficiency due to severe liver disease, vitamin K deficiency, or anti-prothrombin antibodies (antiphospholipid syndrome)
- Must rule out other coagulation factor deficiencies that cause prolonged PT and PTT : e.g. factor V deficiency or factor X deficiency
Additional references
Factor IX deficiency (hemophilia B)
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor IX deficiency (hemophilia B) is the second most common congenital bleeding disorder that is inherited as an X-linked recessive trait
- Characterized by mild, moderate or severe bleeding episodes
Terminology
- Also known as Christmas disease
Epidemiology
- 1 in 30,000 male births
- Almost exclusively affects males
- Rarely affects females (see etiology)
- Female carriers are unaffected
Sites
- Bleeding into muscle, soft tissue or joints (hemarthrosis), GI / GU tract bleeding, easy bruising, excessive bleeding after surgery, trauma, dental procedures or circumcision; epistaxis, poor wound healing, intracranial hemorrhage, scalp hematoma, development of pseudotumors with repetitive hematoma formation, menorrhagia
- Incidence of sites of bleeding:
- Hemarthrosis: 70 - 80%
- Muscle/soft tissue: 10 - 20%
- Other major bleeds: 5 - 10%
- Central nervous system:
- Incidence of bleeding into joints:
- Knee: 45%
- Elbow: 30%
- Ankle: 15%
- Shoulder: 3%
- Wrist: 3%
- Hip: 2%
- Other: 2%
Pathophysiology
- Factor IX is a vitamin K-dependent serine protease produced in the liver
- It circulates in the plasma in its inactive form
- It is activated by factor VIIIa, and catalyzes the conversion of factor X to Xa
- It can also be activated directly by the Tissue Factor-Factor VIIa complex in the extrinsic pathway
- Factor IX has normal plasma activity of 50% - 150% (0.5 - 1.5 IU/mL)
- Its biologic half life is 18 - 24 hrs
Etiology
- Factor IX deficiency is inherited as an X-linked recessive trait, but 30% of cases are due to spontaneous mutations
- The gene for factor IX is located on a fragile region of the X chromosome
- More than 300 mutations have been identified; the most common are single point mutations; numerous point and deletion mutations produce defective, nonfunctional but immunologically detectable factor IX
- Large gene deletions and nonsense mutations are most susceptible to formation of factor IX alloantibodies
- Hemophilic females develop disease due to:
- High degree of X-inactivation in carriers
- Hemizygosity of the X chromosome in females with Turner syndrome (XO karyotype)
- Homozygosity in female progeny of a hemophilia B carrier and an affected hemophilic male
Clinical features
- Clinical severity is dependent on factor levels:
- Mild (> 5% activity; > 0.05 IU/mL); occurs in 30% - 40%; presents with bleeding after surgery or trauma
- Moderate (1%-5% activity; 0.01-0.05 IU/mL); occurs in 10%; presents with bleeding after surgery or trauma and less commonly with spontaneous bleeding
- Severe ( 30% of cases are due to spontaneous mutations and have no family history of bleeding
- 1% - 4% of patients with hemophilia B will develop alloantibody inhibitors after replacement therapy
Laboratory
- Prolonged PTT with correction after mixing study (at 0 and 2 hr)
- Normal PT and bleeding time
- Measure both factor VIII and IX activity by functional plasma clot-based assay or chromogenic substrate-based assay
- Note: diagnosis is confounded in neonates since factor IX levels are significantly reduced at birth and up to 6 months post-partum
- Rule out vWD by vWF antigen and ristocetin cofactor activity
- Bethesda assay for quantitation of inhibitor
- Candidates for genetic testing include patients who have a diagnosis of hemophilia A or B, at-risk women who are related to an affected man (proband) who has a known mutation, and female carriers of hemophilia A or B seeking prenatal diagnosis
- Genetic testing uses RFLP analysis
Prognostic factors
- Chronic complications of hemophilia include musculoskeletal problems (e.g. chronic synovitis, arthropathy, fractures, contractures), inhibitor formation (which complicates treatment), and transfusion-related infections (e.g. HIV, HBV, HCV, etc.)
Case reports
- Spontaneous hemopericardium in a patient with hemophilia B (J Invasive Cardiol 2008;20:E296)
Treatment
- Need 50 - 80% of normal levels for surgical hemostasis with major surgery or major bleeding, 40% postoperatively, 30 - 50% to prevent minor bleeding
- Plasma-derived or recombinant factor IX concentrates (1 unit/kg raises levels in vivo by 1%):
- Major surgery/bleeding - 50 - 80 units factor IX concentrate/kg every 12 - 24 hours as necessary, usually for 7 - 10 days
- Postoperatively - 40 units/kg every 12 - 24 hours, usually for 7 days
- Minor bleeding - postoperatively; 30 - 40 units/kg every 12 - 24 hours as necessary
- Prophylaxis in severe hemophilia B - 25 - 40 units/kg two times weekly
- Treatment of acute bleeding episodes in patients with inhibitors:
- For low-titer inhibitors: high dose factor IX (to overwhelm inhibitor), porcine factor IX (if no cross reactivity with inhibitor)
- For high-titer inhibitors: factor IX bypassing agents (prothrombin complex concentrates, FEIBA, recombinant factor VIIa
Differential diagnosis
- Acquired hemophilia B: autoantibody against factor IX
- Factor VIII deficiency (hemophilia A)
- Other factor deficiencies: XI, XII
- von Willebrand Disease: particularly type 2N or type 3)
Additional references
Factor V deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor V deficiency is a rare congenital bleeding disorder that is inherited as an autosomal recessive trait and is characterized by decreased or absent factor V activity
Terminology
- Also called autoprothrombin I deficiency, Owrens disease, labile factor deficiency or parahemophilia (severe deficiency)
- Deficiency classified as either:
- Type I: both reduced antigen level and activity (quantitative defect)
- Type II: normal or mildly reduced antigen level and reduced activity (qualitative defect)
Epidemiology
- Rare, incidence of less than 1 per 1,000,000 in the homozygous form
- Over 200 cases have been described in the literature
Sites
- Autosomal recessive trait with variable heterozygote expressivity
- There have been 56 mutations described including insertion/deletion, missense, splice site and nonsense mutations (in order of decreasing frequency)
- Parental consanguinity is often present, especially in countries (Muslim or southern India) where consanguineous marriages are common
Pathophysiology
- Factor V is a plasma glycoprotein that is synthesized in the liver and is also present in platelet a-granules
- Platelet factor V accounts for approximately 20% of the total body pool of factor V
- Factor V is a plasma cofactor for the prothrombinase complex that converts prothrombin to thrombin
- Deficiency leads to predisposition for hemorrhage, while some mutations (most notably factor V Leiden) predispose to thrombosis
Clinical features
- May be associated with bruising, epistaxis, menorrhagia, GI / GU bleeding, umbilical stump bleeding or bleeding after surgery, trauma, dental procedures, pregnancy or circumcision
- Severe deficiencies may resemble hemophilia A or B, and are associated with intracranial hemorrhage; however hemarthroses are not as common
- There are mild, moderate and severe forms
- Patients with mild to moderate deficiency (> 20 - 30%) have a heterozygous genotype and are usually asymptomatic and may not be diagnosed until adulthood
- Patients with severe deficiency ( Levels dont always correlate with severity of symptoms
- Complications of treatment include the development of factor V alloantibodies (inhibitors)
Laboratory
- Prolonged PT / PTT with correction after mixing study with normal pooled plasma
- Normal thrombin time
- Specific clot-based factor V assay for diagnosis
Prognostic factors
- Prognosis is good for most patients with factor V deficiency
- Most severe cases have been in patients who present in the perinatal period with intracranial bleeding
Case reports
- Severe gestational factor V deficiency presenting with intracranial hemorrhage detected by ultrasound (Haemophilia 2007;13:432)
Treatment
- Since there are no factor V concentrates available, fresh frozen plasma (FFP) is the mainstay of treatment (15 to 20 mL/kg followed by smaller amounts, such as 5 mL/kg every 12 hours, adjusting the dosage on the basis of Factor V levels, PT and APTT)
- Cryoprecipitate and prothrombin complex concentrates do not contain factor V
- For refractory patients or patients with inhibitors, prothrombin complex concentrates, recombinant activated FVIIa and platelet transfusions have been successfully used
- Patients with inhibitors may need immunosuppression
Differential diagnosis
- Acquired factor V deficiency: seen in liver disease or patients with DIC
- Acquired factor V inhibitors
- Combined factor VIII/factor V deficiency
Additional references
Factor V Leiden assay
Definition / general
- Dilute patient plasma 1 : 5 with factor V deficient plasma (dilutes the effect of other factor deficiencies or elevations) and add polybrene (neutralizes unfractionated heparin or low molecular weight heparin)
- If lupus anticoagulant is present, must perform DNA based test for Factor V Leiden (or perhaps 1 : 40 dilution of plasma or add phospholipids to neutralize lupus anticoagulant)
- Calculate ratio of PTT with versus without exogenous activated protein C; normal is 2.0 or more, factor V Leiden usually
- Many feel that positive results should be confirmed with a genetic assay
- Other assays:
- Prothrombin - based factor V assay with factor V deficient plasma (no interference from lupus anticoagulant)
- Modified Russell viper venom time test (high phospholipids neutralizes lupus anticoagulant)
- Factor Xa - based assay with factor V deficient plasma
- DNA based tests such as PCR (using whole blood, not plasma)
- Absence of MnlI cleavage at mutation site, guanine to adenine at #1691, or arginine to glutamine at amino acid #506 indicates factor V Leiden mutation
Additional references
Factor VII deficiency
Table of Contents
Definition / general | Epidemiology | Terminology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor VII deficiency is a rare congenital bleeding disorder that is inherited as an autosomal recessive trait, and is characterized by variable bleeding symptoms, ranging from asymptomatic to life threatening hemorrhage
Epidemiology
- Factor VII deficiency is the most common of the rare congenital coagulation disorders
- Incidence is 1 in 500,000
Terminology
- Also known as proconvertin deficiency
- The deficiency is classified as:
- Type I: decreased synthesis or increased clearance (quantitative defect)
- Type II: dysfunctional molecule (qualitative defect)
- Type I: decreased synthesis or increased clearance (quantitative defect)
Sites
- Bleeding into skin and mucosa, joint and muscle, genitourinary tract, gastrointestinal tract, CNS (see clinical features below)
Pathophysiology
- Factor VII is a vitamin K-dependent coagulation factor that is synthesized in the liver (Wikipedia)
- Circulating factor VII forms a complex with exposed tissue factor (from injured vascular endothelium), and is activated by proteases to initiate the extrinsic coagulation cascade to form a fibrin clot
- Biologic half-life is 3.5 hours
Images hosted on other servers:
Etiology
- Autosomal recessive inheritance
- More than 130 mutations have been described, predominantly missense and splice-site mutations and less commonly small deletion and nonsense mutations
- Other environmental and genetic factors influence phenotype since patients with identical factor VII gene mutations have been shown to have discordant bleeding severity
Clinical features
- Patients with a homozygous or compound heterozygous genotype develop bleeding symptoms while heterozygotes are typically asymptomatic
- There is little correlation of factor levels and bleeding symptoms
- Patients exhibit easy bruising, epistaxis, soft tissue hematoma, menorrhagia, menometrorrhagia, postpartum bleeding, postoperative bleeding, hemarthrosis, retroperitoneal bleeding, gastrointestinal bleeding, intracranial hemorrhage
- Paradoxically, thrombotic episodes have been reported (e.g. deep venous thrombosis) in 3 - 4%, but in most cases, thrombotic risk factors were identified (Haemophilia 2008;14:564)
- Few cases have been reported of inhibitor development after replacement therapy
Laboratory
- Prolonged PT
- Normal PTT (although may be prolonged)
- Obtain specific factor VII activity assay to confirm
Prognostic factors
- Patients with severe deficiency typically have life-threatening bleeds (e.g. intracranial, gastrointestinal) within the first 6 months of life
Case reports
- Multiple cerebral aneurysms in congenital factor VII deficiency (AJNR Am J Neuroradiol 2004;25:784)
Treatment
- For mild hemorrhage, recommended to maintain factor levels of 5% - 10% of normal to stop bleeding; for surgical procedures, recommended to maintain levels of 15% - 20% of normal
- Recombinant factor VIIa is the treatment of choice; single dose for mild to moderate bleeding, or every 4 - 6 hours for severe bleeding episodes
- Due to the short half-life of factor VII (3.5 hr), it is difficult to give fresh frozen plasma (FFP) every 4 - 6 hours to maintain levels without producing volume overload
- Plasma derived factor VII concentrates and prothrombin complex concentrates are associated with post-treatment thrombosis
Differential diagnosis
- Acquired factor VII deficiency: due to warfarin, vitamin K deficiency, liver disease
- Acquired factor VII inhibitors
- Familial combined factor deficiencies: e.g. factor VI/factor VIII or factor II, VII, IX and X
Additional references
Factor VIII deficiency (hemophilia A)
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor VIII deficiency (hemophilia A) is the most common congenital bleeding disorder that is inherited as an X-linked recessive trait
- It is characterized by mild, moderate or severe bleeding episodes
Terminology
- Factor VIII is also known as the anti-hemophilic factor
Epidemiology
- Almost exclusively affects males
- 1 in every 10,000 births or 1 in 5000 male births
- Rarely affects females (see etiology)
- Female carriers are unaffected
Sites
- Bleeding into muscle, soft tissue or joints (hemarthrosis), GI / GU tract bleeding, easy bruising, excessive bleeding after surgery, trauma, dental procedures or circumcision; epistaxis, poor wound healing, intracranial hemorrhage, scalp hematoma, development of pseudotumors with repetitive hematoma formation, menorrhagia
- Incidence of sites of bleeding:
- Hemarthrosis: 70 - 80%
- Muscle/soft tissue: 10 - 20%
- Other major bleeds: 5 - 10%
- Central nervous system:
- Incidence of bleeding into joints:
- Knee: 45%
- Elbow: 30%
- Ankle: 15%
- Shoulder: 3%
- Wrist: 3%
- Hip: 2%
- Other: 2%
Pathophysiology
- Factor VIII is a plasma protein produced in the liver and by the reticuloendothelial system (Wikipedia)
- It circulates mainly bound to von Willebrand factor protein
- It functions as a cofactor along with activated factor IX to activate factor X, which in turn with its cofactor, factor Va, activates thrombin
- Normal plasma activity is 50 - 150% (0.5-1.5 IU/mL)
- Biologic half life is 8 - 12 hours
Etiology
- Inherited as an X-linked recessive trait, however 30% are due to spontaneous mutations
- The gene for factor VIII is a large gene located on a fragile region of the X chromosome
- The most common mutation involves inversion of intron 22; less common mutations include missense, large deletions, small point mutations and insertions/deletions
- Hemophilic females develop disease due to:
- High degree of X-inactivation in carriers
- Hemizygosity of the X chromosome in females with Turner syndrome (XO karyotype)
- Homozygosity in female progeny of a hemophilia A carrier and an affected hemophilic male
Clinical features
- Clinical severity is dependent on factor levels:
- Mild (> 5% activity; > 0.05 IU/mL); occurs in 30 - 40% and typically presents with bleeding after surgery or trauma
- Moderate (1 - 5% activity; 0.01 - 0.05 IU/mL); occurs in 10% and presents with bleeding after surgery or trauma, less commonly with spontaneous bleeding
- Severe (
- 30% of cases are due to spontaneous mutations, and have no family history of bleeding
- 35% of patients with severe hemophilia A will develop alloantibody inhibitors to factor VIII after replacement therapy (Factor VIII inhibitor)
- Formation of factor VIII alloantibodies is highest in individuals with intron 22 inversions, large deletions and nonsense mutations (Blood Coagul Fibrinolysis 2003;14:S17)
Laboratory
- Prolonged PTT with correction after mixing study (at 0 and 2 hr)
- Normal PT and bleeding time
- Measure both factor VIII and IX activity by functional plasma clot-based assay or chromogenic substrate-based assay
- Rule out vWD by vWF antigen and ristocetin cofactor activity
- Bethesda assay for quantitation of inhibitor
- Candidates for genetic testing include patients who have a diagnosis of hemophilia A or B, at-risk women who are related to an affected man (proband) who has a known mutation, and female carriers of hemophilia A or B seeking prenatal diagnosis
- First-line testing involves identification of the inversion of intron 22
- Obtain linkage analysis using restriction fragment length polymorphism (RFLP) if no inversion is detected or family members are available for testing
- Cord blood sampling for measurement of factor VIII activity in male fetus of known carrier
Prognostic factors
- Chronic complications of hemophilia include musculoskeletal problems (e.g.chronic synovitis, arthropathy, fractures, contractures), inhibitor formation (which complicates treatment), and transfusion-related infections (e.g. HIV, HBV, HCV, etc.)
Case reports
- Intraosseous hematoma in a newborn (AJNR Am J Neuroradiol 2000;21:308)
- Intracranial pseudotumor (Br J Neurosurg 2009;23:455))
Treatment
- Need 80 - 100% of normal factor VIII levels for surgical hemostasis with major surgery or major bleeding, 30 - 50% postoperatively or to prevent minor bleeding
- Use plasma-derived or recombinant factor VIII concentrates (1 unit/kg raises levels in vivo by 2%):
- Major surgery/bleeding - 40 - 50 units factor VIII concentrate/kg every 12 hours as necessary, usually for 7 - 10 days
- Postoperatively - 15 - 25 units/kg every 12 hours as necessary, usually for 7 - 10 days
- Minor bleeding - 15 - 20 units/kg every 12 - 24 hours as necessary
- Mild / moderate bleeding - DDAVP (if patients respond to DAVP)
- Prophylaxis in severe hemophilia A 25 - 40 units/kg three times weekly
- Antifibrinolytic and topical agents (e.g. epsilon-aminocaproic acid, tranexamic acid, fibrin sealants) as adjuvant therapy
- Treatment of acute bleeding episodes in patients with inhibitors:
- For low-titer inhibitors: high dose factor VIII (to overwhelm inhibitor), porcine factor VIII (if no cross reactivity with inhibitor)
- For high-titer inhibitors: factor VIII bypassing agents (prothrombin complex concentrates, FEIBA, recombinant factor VIIa)
Differential diagnosis
- Acquired hemophilia A: autoantibody against factor VIII
- Factor IX deficiency (hemophilia B)
- Familial combined factor deficiencies: typically have an autosomal recessive pattern
- Other factor deficiencies: XI, XII
- von Willebrand Disease: particularly type 2N or type 3
Additional references
Factor X deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor X deficiency is a rare congenital bleeding disorder that is inherited as an autosomal recessive trait, and is characterized by a variable bleeding tendency
Terminology
- Also known as Stuart-Prower Factor Deficiency
- Deficiency classified as either:
- Type I: decreased functional activity and antigen level (quantitative defect)
- Type II: decreased functional activity and near normal antigen level (qualitative defect)
Epidemiology
- Estimated incidence of 1 in 500,000 to 1,000,000; however, in countries where consanguinity is more common (e.g. Iran), incidence is reported to be 1 in 200,000
- Estimated carrier incidence of 1 in 500
Sites
- Bleeding into skin and mucosa, joint and muscle, genitourinary tract, gastrointestinal tract, CNS (see clinical features below)
Pathophysiology
- Factor X is a vitamin K-dependent serine protease produced in the liver
- It is the first enzyme in the common pathway to form a fibrin clot
- Its activated form (in complex with factor Va, Ca++ and phospholipid) cleaves prothrombin to thrombin
- It has a long half-life of 20 - 40 hours
Images hosted on other servers:
Etiology
- Inherited as an autosomal recessive trait
- More than 80 mutations have been identified, which include small deletions, missense and frameshift mutations
Clinical features
- May be associated with bruising, epistaxis, menorrhagia, GI / GU bleeding, umbilical stump bleeding or bleeding after surgery, trauma, dental procedures, pregnancy or circumcision, recurrent fetal loss
- Bleeding symptoms in severe deficiency are similar to that seen in patients with factor VIII (hemophilia A) and factor IX (hemophilia B) deficiency
- Heterozygotes are usually asymptomatic but may have mild mucocutaneous bleeds
- Patients with severe deficiency are either homozygous or compound heterozygous
- Bleeding symptoms tend to correlate with factor X activity levels: mild (> 6% - 10%), moderate (1% - 5%) or severe ( A small percentage of patients develop factor X inhibitors with factor replacement therapy
Laboratory
- Prolonged PT and PTT that correct with mixing study
- Prolonged Russells viper venom time (measures the direct activation of factor X)
- Normal thrombin and bleeding time
- Factor X specific functional and immunologic assays for confirmation
Prognostic factors
- Particular genotypes [Gly(- 20)Arg, Gly94Arg and Gly380Arg mutations] are associated with higher rates of hemarthrosis and intracranial bleeding
Case reports
- Factor X deficiency presenting as chronic bilateral subdural hematomas in a 7 month old infant (Pediatr Neurosurg 2010;46:54)
Treatment
- For minor bleeding episodes, maintain factor X levels at 10% - 15% of normal using FFP (15 - 20 mL/kg followed by 3 - 6 mL/kg every 24 hours)
- For major bleeding episodes, trauma or surgical procedures, factor X rich prothrombin complex concentrates can be used to maintain factor X levels at 50% of normal
Differential diagnosis
- Acquired factor X deficiency (liver disease, vitamin K deficiency): also exhibits reduced levels of other coagulation factors; isolated factor X deficiency is associated with respiratory infection, AML and other malignancies, amyloidosis
- Acquired factor X inhibitors in patients without congenital factor X deficiency are rare but have been reported in leprosy and chemical exposure
- Other factor deficiencies: e.g. factor V, prothrombin
Additional references
Factor Xa assay
Definition / general
- Used to determine levels of heparin, low molecular weight heparin, danaparoid, etc.
- Measures ability of heparin or other drugs in patients plasma to inhibit known amount of factor Xa
- Usually reported out based on standard curve for the drug in question
- Note: The term anti-Xa level should be avoided because this suggests measuring a factor X inhibitor, but the factor Xa assay is really a drug level using chromogenic Xa inhibition as the methodology; as the reference range and standard curve vary with the drug tested, the clinician should indicate the drug (heparin, danaparoid, etc.)
- Used to monitor heparin, particularly if PTT has baseline prolongation due to lupus anticoagulant or factor XII deficiency
- Note: Can cautiously use PTT to monitor heparin, even if lupus anticoagulant present, if factor Xa assay demonstrates that it is not affected by the lupus anticoagulant
- Also used to monitor low molecular weight heparin and danaparoid, which dont prolong PTT; these drugs usually do not need to be monitored except if renal failure, pregnancy (increased dosage needed in third trimester), newborns (increased dosage needed), over- or underweight patients, prolonged use or high risk for bleeding/thrombosis
- Chromogenic factor X assays:
- Used to monitor warfarin in the presence of a lupus anticoagulant, hirudin or argatroban (which prolong the PT and increase the INR), because warfarin decreases factor X (also factors II, VII, IX), and the chromogenic assay has no interference from lupus anticoagulant, hirudin or argatroban
- Patient plasma is added to a known amount of excess factor Xa with excess antithrombin
- Anticoagulant binds to antithrombin and inhibits factor Xa
- Residual factor Xa is inversely proportional to anticoagulant in plasma, cleaves a chromogenic substrate, and colored compound is detected by spectrophotometer
- Results reported in antifactor Xa units/mL
Laboratory
- Draw specimen 4 hours after subcutaneous injection of low molecular weight heparin or 6 hours after subcutaneous injection of danaparoid to avoid falsely low values
- Must deliver to laboratory immediately (or separate plasma from cells within 1 hour), because platelets release platelet factor 4, which neutralizes heparin
- Delays may cause falsely low values
- Approximate therapeutic range for treatment of existing deep venous thrombosis:
- Heparin 0.3 to 0.7 anti-Xa international units/mL
- Low molecular weight heparin either 0.4 to 1.1 units/mL for twice a day dosing or 1 to 2 units/mL for once daily dosing
- Danaparoid 0.5 to 0.8 units/mL
Interpretation
- Low levels of factor Xa are due to:
- Not collecting specimen at right time or delayed transportation to lab (see Laboratory above)
- Higher therapeutic dose needed
- High levels of factor Xa are due to:
- Renal failure
- Heparin contamination (specimen drawn from indwelling line containing heparin)
- Lower therapeutic dose needed
Additional references
Factor XI deficiency
Table of Contents
Definition / general | Sites | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Laboratory | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor XI deficiency is a rare bleeding disorder that is inherited most commonly as an autosomal recessive trait, and is characterized by variable bleeding episodes
Sites
- Bleeding into skin and mucosa, genitourinary tract, gastrointestinal tract (see clinical features below)
Pathophysiology
- Factor XI is a serine protease that is produced in the liver, and is involved in the contact (intrinsic) pathway
- It is activated by factor XIIa, thrombin or autoactivated, and activates factor IX, which in turn activates factor X to convert prothrombin to thrombin
- Its biologic half-life is 60 - 80 hrs
Etiology
- Most commonly inherited as an autosomal recessive trait
- An autosomal dominant pattern of inheritance is possible, as the dimeric structure of factor XI may result in a dominant negative effect through intracellular heterodimer formation (Blood 2004;104:128)
- The gene for factor XI is located on the long arm of chromosome 4
- To date, over 180 mutations have been described with three main genotypes: type I are splice site mutations, type II are nonsense mutations and type III are missense mutations
- Type II and III mutations are predominantly seen in Ashkenazi Jews
- Type II mutations are also seen in Iraqi Jews and Israeli Arabs
Clinical features
- Deficiency can occur in homozygous, heterozygous or a combined heterozygous form
- Bleeding manifestations do not correlate with factor XI levels
- Most bleeding episodes in patients with severe deficiency are injury-related
- Spontaneous bleeding is rare
- May be associated with bruising, epistaxis, menorrhagia, GI / GU bleeding, umbilical stump bleeding or bleeding after surgery, trauma, dental procedures, pregnancy or circumcision
- Up to 33% of patients with severe deficiency develop inhibitors after replacement therapy
Laboratory
- Prolonged PTT that corrects with mixing study
- Normal PT, thrombin time
- Specific factor XI assay
Case reports
- Cerebellar hemorrhage due to factor XI deficiency (Cerebrovasc Dis 2005;19:138)
Treatment
- 10 - 20 ml fresh frozen plasma/kg, then 5 - 10 ml/kg every 24 hours as necessary
- Antifibrinolytic therapy has been used in women with factor XI deficiency and menorrhagia
- Patients with inhibitors have been treated successfully with plasma, prothrombin complex concentrates, and recombinant activated factor VII
- Note: factor XI concentrates may promote thromboembolic complications
Differential diagnosis
- Acquired factor XI deficiency associated with systemic lupus erythematous
- Combined familial factor deficiency
- Heparin contamination
- Liver dysfunction
- Other factor deficiency causing prolonged PTT: factor VIII, IX, XII, but factor XII deficiency is not associated with bleeding manifestations
- von Willebrand Disease
Additional references
Factor XII deficiency
Table of Contents
Definition / general | Epidemiology | Pathophysiology | Etiology | Diagrams / tables | Clinical features | Laboratory | Case reports | Differential diagnosis | Additional referencesDefinition / general
- Factor XII deficiency is a congenital disorder that is most commonly inherited as an autosomal recessive trait and is not associated with a bleeding diathesis
- It is typically discovered in individuals with an isolated prolonged PTT
- Homozygous individuals have undetectable factor XII levels
- Heterozygous individuals have factor XII levels between 20 - 60%
Epidemiology
- Actual prevalence difficult to determine since individuals are asymptomatic
- One study of 300 healthy blood donors reported a prevalence of 2.3% (Thromb Haemost 1994;71:68)
Pathophysiology
- Factor XII is a coagulation protein which is either autoactivated by contact with a number of artificial or biologic negatively charged surfaces (contact activation) or by proteolytic activation on the surface of endothelial cells by prekallikrein/kallikrein and high molecular weight kininogen
- Activated factor XII converts prekallikrein to kallikrein (which activates more factor XII, liberates bradykinin from high molecular weight kininogen, and activates complement components C3 and C5), activates factor XI which eventually leads to thrombin generation via the intrinsic pathway, and also activates C1 esterase, thereby activating the complement system
Etiology
- Most commonly inherited in an autosomal recessive pattern
- Autosomal dominant inheritance has been described in one family (Blood 1972;40:412)
Diagrams / tables
Clinical features
- Not associated with bleeding episodes, even after major surgical procedures or trauma
- Most patients are detected by routine preoperative coagulation studies (isolated prolonged PTT)
- Very rarely may manifest with epistaxis or easy bruising
- There is some debate about the association of factor XII deficiency and an increased risk of arterial and venous thrombosis, myocardial infarction and pulmonary embolism
Laboratory
- Prolonged PTT that corrects with mixing study
- Normal PT, thrombin time and bleeding time
- Specific factor XII assay is diagnostic
Case reports
- Combined factor VIII and factor XII deficiency (Am J Hematol 1992;39:137)
- Acquired factor XII deficiency by orthotopic liver transplant (Am J Transplant 2006;6:1743)
Differential diagnosis
- Acquired factor XII deficiency: associated with nephrotic syndrome, liver transplantation, autoimmune disease
- Factor XII deficiency in association with von Willebrand disease, factor VIII or factor IX deficiency
- Heparin contamination
- High molecular weight kininogen deficiency
- Liver disease
- Lupus anticoagulants
- Prekallikrein deficiency
Additional references
Factor XIII deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Sites | Pathophysiology | Diagrams / tables | Etiology | Clinical features | Laboratory | Prognostic factors | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Factor XIII deficiency is a congenital disorder that is inherited as an autosomal recessive trait and is associated with a variable bleeding tendency
- Acquired factor XIII deficiency is associated with liver failure, inflammatory bowel disease, leukemia, disseminated intravascular coagulation, Henoch-Schonlein purpura, systemic lupus erythematosus and exposure to certain drugs (phenytoin, isoniazid, valproate)
Terminology
- Factor XIII is also known as fibrin stabilizing factor
Epidemiology
- Estimated at 1 in 2 to 5 million births
- More frequent in regions where consanguineous marriages are more common
Sites
- Skin and mucosa, joints, soft tissue, gastrointestinal tract, genitourinary tract, CNS (see clinical features below)
Pathophysiology
- Factor XIII is a transglutaminase that circulates as a zymogen comprised of 2 catalytic A subunits and 2 carrier B subunits
- The A subunit is synthesized in platelets, monocytes and macrophages while the B subunit is synthesized in the liver; the A and B dimers then assemble in the plasma to form a heterotetramer
- Factor XIII is activated by thrombin and is responsible for catalyzing the final step in the coagulation cascade by cross-linking fibrin (in the presence of calcium)
- Deficiency is due to a defect in either the A gene (type 2) or B gene (type 1)
Etiology
- Inherited as an autosomal recessive trait
- Most cases are due to mutations in A subunit gene on chromosome 6
- More than 70 mutations have been identified, most of which are missense and nonsense mutations
- Only 5 mutations in FXIII B deficient patients have been identified; gene is on chromosome 1
Clinical features
- Variable bleeding tendency, from mild to severe depending on factor levels
- Umbilical cord stump bleeding, intracranial hemorrhage, soft tissue hematoma, bleeding after circumcision, gastrointestinal bleeding, gingival bleeding, epistaxis, hematuria, surgical site bleeding, menorrhagia, joint bleeding, delayed healing, spontaneous abortion, recurrent miscarriage
- Plasma half life is 9 - 12 days
- Factor XIII levels above 3 - 5% are usually sufficient to prevent spontaneous bleeding
- Severe bleeding typically occurs in individuals with Compound heterozygotes are usually asymptomatic
Laboratory
- Normal PT, PTT, thrombin time, fibrinogen
- Screening test for factor XIII deficiency uses the clot solubility test in which patient plasma is incubated with thrombin and calcium; deficiency will cause the clot to dissolve in the presence of urea or acid
- A standard mixing test using patient plasma and normal pooled plasma is usually performed to rule out the presence of an inhibitor
- Confirmatory testing uses a quantitative factor XIII activity assay
Prognostic factors
- Although there is a life-long risk of bleeding, prognosis is excellent due to good response to treatment; subsequent risk of development of inhibitors is low
Case reports
- Spontaneous splenic rupture in a patient with factor XIII deficiency (Pediatr Blood Cancer 2008;50:113)
Treatment
- Factor XIII concentrate, FFP or cryoprecipitate for replacement therapy or for treatment of acute bleeding episodes:
- Factor XIII concentrate: 10 - 20 U/kg at 4 - 6 week intervals
- FFP: 10 mL/kg every 4 - 6 weeks
- Cryoprecipitate: 1 bag per 10 - 20 kg every 3 - 4 weeks
- To prevent miscarriage, maintain factor XIII levels > 10% in early gestation and > 30% at time of delivery to prevent significant bleeds
Differential diagnosis
- Acquired factor XIII deficiency
Additional references
Heparin
Table of Contents
Definition / general | Pathophysiology | Diagrams / tables | Clinical features | Laboratory | TreatmentDefinition / general
- Also called unfractionated heparin
- Short acting anticoagulant with half life of approximately 90 minutes
Pathophysiology
- Derived from porcine intestinal mucosa or bovine lung, which contain heparin-rich mast cells
- Markedly enhances activity of antithrombin, which inhibits activated factors II, IX, X, XI, XII, kallikrein and probably VII, but doesnt cause a true decrease in factor levels
- Most heparin preparations are heterogeneous, with a molecular weight between 7 - 25K daltons
- Anticoagulant activity is variable, since only 1 / 3 of heparin molecules have the pentasaccharide sequence necessary for antithrombin mediated anticoagulant activity
Clinical features
- Used as initial anticoagulant therapy, to treat deep venous thrombosis, post-operatively and for other short-term indications
- Decreases morbidity and mortality from acute thrombotic disease
- Complications include hemorrhage (overcoagulation) and heparin-induced thrombocytopenia (up to 3% of patients)
- Recommended that 90% of patients should achieve therapeutic anticoagulation within 24 hours
Laboratory
- Recommended to monitor with PTT assay (that has been standardized using determination of heparin levels), activated clotting time (if high heparin levels present, as during cardiopulmonary bypass surgery, since no clotting occurs at these levels with PTT) or heparin levels assayed by measuring activity against factor Xa (therapeutic range is 0.3 to 0.7 anti-Xa units/ml) within 12 hours (Clin Lab Med 2009;29:283)
- Also monitor platelet count within 72 hours, with platelet monitoring to continue periodically for 20 days; PT is usually normal
- Note: often the cause of prolonged PTT is heparin in sample collected through indwelling line; identify by treating with heparinase
Treatment
- Protamine sulfate (for emergency reversal of heparin)
Heparin - low molecular weight
Table of Contents
Definition / general | Diagrams / tables | Clinical features | Laboratory | Additional referencesDefinition / general
- Can be used instead of standard heparin for many patients, with similar efficacy and safety
- Produced by breaking heparin into shorter polysaccharide chains
- Molecular weight is approximately 5,000 daltons
Clinical features
- Less likely to bind to acute phase reactant proteins, platelets, platelet factor 4, macrophages and other sites, due to its shorter length
- Has more predictable anticoagulant effect than standard heparin, less need for laboratory monitoring, lower incident of heparin induced thrombocytopenia, greater bioavailability
- Longer half life than standard heparin (4 vs. 1.5 hours), which is prolonged in renal failure
- Inhibits factor Xa by 2 to 4x more than factor IIa, so does not substantially prolong PT and PTT
- Unlike regular heparin, does not as readily inhibit thrombin or factor IXa (this also contributes to a lower number of bleeding side effects)
Laboratory
- Typically do not monitor except for periodic platelet counts
- Indications for monitoring include pregnancy, renal failure, obesity, prolonged use, infants and children, patients at high risk for bleeding / thrombosis
- Monitor by measuring anti-factor Xa activity, drawn 4 hours after injection
- Typical therapeutic range is 0.6 to 1.0 U/ml for twice a day dosing, higher for once a day dosing, 1.0 to 2.0 U/ml for prophylactic dosing
- Effects are reversed with protamine sulfate
Additional references
Heparin cofactor II deficiency
Definition / general
- Very rare hypercoagulable condition, either hereditary (autosomal dominant, 15 families documented through 2002) or acquired (liver disease)
- Associated with thrombosis, but not a strong risk factor by itself
- Testing patients with thromboembolic disease for heparin cofactor II deficiency is not recommended as a first or second line test (Arch Pathol Lab Med 2002;126:1394)
Case reports
- Family with homozygous deficiency, but no significant symptoms (Circulation 2004;110:1303)
Heparin induced thrombocytopenia
Definition / general
- Determine if thrombosis or thrombocytopenia in a patient exposed to heparin is due to anti-heparin antibody (actually antibody to heparin bound to platelet factor 4 on platelet surface)
- Heparin exposure may be minimal (heparin - coated catheter)
- Note: up to 8% of heparinized patients have antibody without symptoms, 1 - 5% have thrombocytopenia, 1 / 3 of these develop arterial or venous thrombosis, 20 - 30% of these die and 20 - 30% become disabled
- Affected patients usually have reduction in platelet count within 4 - 20 days after heparin exposure for the first time, 1 - 3 days after reexposure to heparin; platelet count typically decreases 50% or more to under 100K
- Starts to rise 2 - 3 days after ceasing heparin with normal levels at 4 - 10 days after heparin cessation; however, thrombosis may occur for several weeks after heparin is stopped
- Antibody binds to heparin - platelet factor 4 complex, antibody then binds to platelet Fc receptor, which activates the platelet, causing thrombocytopenia and thrombosis
- Test should be performed in acute setting, before antibody disappears
- Note: initial test may be negative and need to be repeated after several days; a negative test by itself has very poor predictive value
Diagnosis
- Methodology:
- Either ELISA (90% sensitive; heparin complexed to platelet factor 4 as antigen), platelet aggregation (add patient plasma / serum to donor platelets and heparin, check for platelet aggregation) or serotonin release assays (add patient plasma / serum and heparin to donor platelets with radiolabeled serotonin, check for release of serotonin from platelets activated by the antibody)
- 4-T score:
- Developed to determine pre-test probability of HIT by assigning scores of 0, 1 or 2 in 4 clinical categories:
- Thrombocytopenia severity
- Timing of symptoms in relation to heparin exposure
- Presence or absence of thrombosis
- Possible alternatives to the symptoms
- Score 0 - 3: HIT very unlikely; 4 - 5: intermediate probability; 6 - 8: high likelihood (Postgrad Med J 2007;83:575)
- Developed to determine pre-test probability of HIT by assigning scores of 0, 1 or 2 in 4 clinical categories:
Heparin induced thrombocytopenia (HIT)
Definition / general
- Common complication of heparin therapy, may cause life threatening venous or arterial thrombosis (Postgrad Med J 2007;83:575)
- Prevent by monitoring platelet count for at least 20 days after initiation of heparin therapy
- Caused by formation of antibodies against heparin and platelet factor 4 complex
- Testing: ELISA assay screen followed by serotonin release assay for conformation
- Use 4T score (Online 4T Score Calculator) to evaluate pre-test probability of HIT
Clinical features
- Clinical note: platelet counts often fall slightly during the first 24 hours of heparin treatment, unrelated to heparin induced thrombocytopenia
Treatment
- Permanently discontinue heparin, avoid platelet transfusions
- Recommended to not use low molecular weight heparin (may cross react) or warfarin (may cause venous limb gangrene)
- Instead use danaparoid, hirudin or argatroban
Heparinase / heparin contamination assay
Definition / general
- To detect heparin contamination of specimens, which may cause a prolonged PTT
- Also used to remove heparin from specimens so coagulation tests can be performed without interference
- Heparinase degrades unfractionated and low molecular weight heparin at multiple sites, including the antithrombin binding site (pentasaccharide sequence), producing fragments up to 1000 daltons, which lack anticoagulant activity
Diagnosis
- Measure PTT before and after heparinase (add 1 mL of patient plasma to one vial of heparinase, keep at room temperature for 15 minutes)
- Alternative is to add heparin - binding cellulose to specimens, which binds to heparin, then centrifuge and use supernatant plasma (free of heparin)
- Note:
- Normal thrombin time rules out heparin prolonging the PTT
- May have coagulation abnormality in addition to heparin contamination
- Marked reduction of PTT, but with elevated value, may indicate residual heparin
- The PTT may never totally correct in normal patients with large amounts of heparin contamination
Hereditary bleeding disorders - general
Table of Contents
Definition / general | Essential features | Overview | Epidemiology | Clinical features | Laboratory | Treatment | Hereditary thrombocytopenia | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hereditary bleeding disorders are a diverse group of diseases that occur due to platelet dysfunction or absence / deficiency of specific clotting proteins and result in abnormalities of primary or secondary hemostasis
- Most common hereditary bleeding disorders
- Von Willebrand disease
- Hemophilia A (factor VIII deficiency)
- Hemophilia B (factor IX deficiency)
- Less common hereditary bleeding disorders
- Factor I (fibrinogen) deficiency / dysfunction
- Factor II (prothrombin) deficiency
- Factor V deficiency
- Factor VII deficiency
- Factor X deficiency
- Factor XI deficiency (also known as hemophilia C)
- Factor XIII deficiency
- Rare disorders
- α2 antiplasmin deficiency
- Combined factor deficiencies: combined factor V and VIII, combined vitamin K dependent clotting factors deficiency
- Glanzmann thrombasthenia
- Bernard-Soulier syndrome
- Gray platelet syndrome
Essential features
- Hereditary bleeding disorders could occur due to platelet dysfunction (i.e., primary hemostasis disorder) or absence / deficiency of specific clotting proteins (i.e., secondary hemostasis disorder)
- Primary hemostasis: formation of a platelet plug that involves the endothelium, platelets and von Willebrand factor (vWF)
- Secondary hemostasis: formation of fibrin plug by activated coagulation factors
- Von Willebrand disease is the most common hereditary bleeding disorder (M = F), followed by hemophilia A and B (M > F)
- Patients with abnormalities in primary hemostasis are more likely to manifest mucocutaneous bleeding, while those with abnormalities in secondary hemostasis tend to have muscle and joint bleeds
Overview
- Hemostasis means stopping bleeding
- Vasoconstriction is the initial response following vessel injury (Ann Med 2012;44:405)
- Primary hemostasis involves the formation of a weak platelet plug, where damaged endothelium exposes the procoagulant subendothelial matrix, leading to platelet adhesion by von Willebrand factor (Ann Med 2012;44:405)
- Normal platelet function can be summarized with the triple A mnemonic: adhesion, activation and aggregation (Hematol Oncol Clin North Am 2021;35:1069)
- Secondary hemostasis involves the formation of cross linked fibrin by activated coagulation factors (Ann Med 2012;44:405)
- Inheritance pattern can be autosomal dominant, recessive or X linked
- In many patients, there is a family history of bleeding (Int J Lab Hematol 2018;40:6)
Epidemiology
| Disorders of primary hemostasis | ||
|---|---|---|
| Bleeding disorder | Prevalence | Inheritance pattern / genetic mutation |
Von Willebrand disease (vWD)
| 1 in 100 (based on abnormal laboratory results); 1 in 1,000 (symptomatic patients) (Hematol Oncol Clin North Am 2021;35:1085) | Chr 12p, VWF gene, multiple mutations identified
|
| Glanzmann thrombasthenia | 1 in 1,000,000 | Autosomal recessive / chr 17q21, ITGA2B or ITGB3 genes (StatPearls: Glanzmann Thrombasthenia [Accessed 1 November 2024]) |
| Bernard-Soulier syndrome | < 1 in 1,000,000 | Autosomal recessive; rare cases of autosomal dominant inheritance / GPIbα, GPIbβ or GPIX genes on chromosomes 17p12, 22q11.2 and 3q21, respectively (Orphanet J Rare Dis 2006;1:46) |
| Gray platelet syndrome | Rare (Autosomal dominant, recessive or X linked recessive / chr 3p21.31, NBEAL2 gene (J Blood Med 2021;12:719) | |
| Quebec platelet disorder | 1 in 300,000 in Quebec, Canada | Autosomal dominant / chr 10q22, PLAU gene (Expert Rev Hematol 2011;4:137) |
| Wiskott-Aldrich syndrome | 1 - 10 in 1,000,000 | X linked recessive / Xp 11.22-23, WAS gene (StatPearls: Wiskott-Aldrich Syndrome [Accessed 1 November 2024]) |
| Congenital amegakaryocytic thrombocytopenia | Rare (Autosomal recessive / chr 1p34.2, MPL gene (Haematologica 2021;106:2439) | |
| Hermansky-Pudlak syndrome | 1 - 9 in 1,000,000; 1 in 1,800 in Puerto Rico (Semin Respir Crit Care Med 2020;41:238) | Autosomal recessive / mutations in 1 of 10 genes (HPS1, AP3B1, HPS3, HPS4, HPS5, HPS6, DTNBP1, BLOC1S3, PLDN and AP3D1) |
| Chédiak-Higashi syndrome | Rare (Autosomal recessive / chr 1q42.1-q42.2, LYST gene (StatPearls: Chediak-Higashi Syndrome [Accessed 1 November 2024]) | |
Clinical features
- Symptoms: bleeding associated with surgery, trauma, dental extractions, postpartum, circumcision or umbilical stumps, easy bruising, epistaxis, menorrhagia, gastrointestinal bleeding, hemarthrosis, soft tissue hematomas, hematuria, intracranial bleeding
- Patients with primary hemostasis abnormalities are more likely to manifest mucocutaneous bleeding (Eur J Pediatr 2012;171:1)
- Reasons to suspect hereditary platelet disorder (Int J Mol Sci 2021;22:4521)
- Persistence of neonatal thrombocytopenia or onset of bleeding symptoms in childhood
- Family history of thrombocytopenia or mucocutaneous bleeding
- Association with other diseases (check for unique physical characteristics and findings on peripheral blood smear)
- Bleeding out of proportion to the platelet count
- Patients with secondary hemostasis abnormalities tend to have muscle and joint bleeds (Eur J Pediatr 2012;171:207)
- Heterozygous patients have 30 - 60% of normal values of affected factors, usually with no or minor bleeding disorder (UpToDate: Rare Inherited Coagulation Disorders [Accessed 1 November 2024])
- Factor I, X, XI or XIII deficient heterozygotes may have bleeding symptoms (UpToDate: Rare Inherited Coagulation Disorders [Accessed 1 November 2024])
- Homozygous deficient patients have UpToDate: Rare Inherited Coagulation Disorders [Accessed 1 November 2024])
- In hemophilia A and B, small differences in factor levels (i.e., 5%) may markedly affect the clinical presentation, disease course and management (UpToDate: Clinical Manifestations and Diagnosis of Hemophilia A and B [Accessed 1 November 2024])
Laboratory
- Basic screening tests include complete blood count (CBC), prothrombin time (PT) / activated partial thromboplastin time (aPTT), platelet function assay (e.g., PFA 100 / PFA 200), thrombin time, peripheral blood smear review (for platelet and erythrocyte morphology) and fibrinogen (Int J Lab Hematol 2018;40:6)
- For suspected platelet disorders: platelet aggregation studies, bone marrow aspirate and biopsy, flow cytometry, electron microscopy, next generation sequencing (Hematol Oncol Clin North Am 2021;35:1069)
- Testing for von Willebrand disease includes factor VIII activity, vWF antigen and vWF activity (measured by ristocetin cofactor activity or vWF GPIbM activity assay) (Pediatr Clin North Am 2013;60:1419)
- These results may lead to obtaining vWF multimer analysis and blood type (group O patients have reduced vWF antigen and activity) (Pediatr Clin North Am 2013;60:1419)
- For suspected coagulation factor abnormalities: mixing studies, factor levels, Bethesda assay (to detect coagulation factor inhibitor titers) (Int J Lab Hematol 2018;40:6)
- Factor XIII assay if delayed bleeding is present with normal PT and aPTT (measured by factor XIII activity assay or urea clot lysis method) (Hematol Oncol Clin North Am 2021;35:1171)
Treatment
- Bleeding in patients with hereditary platelet disorders is managed with platelet transfusions, antifibrinolytic agents, DDAVP (desmopressin) or recombinant factor VIIa, depending on the defect (Hematol Oncol Clin North Am 2021;35:1069)
- Specific treatment recommendations are dependent on the type and severity of bleeding disorder but generally factor replacement therapy for factor deficiencies is the mainstay of treatment, with the exceptions of factor V (which is treated with plasma), factor II and factor X deficiencies (which are treated with prothrombin complex concentrates) (Haemophilia 2008;14:671)
- For von Willebrand disease, use vWF concentrates, DDAVP (desmopressin; contraindicated in vWD type 2B) or antifibrinolytic agents (Hematology Am Soc Hematol Educ Program 2016;2016:683)
Hereditary thrombocytopenia
- Hereditary thrombocytopenia (inherited thrombocytopenia) encompasses a group of rare genetic disorders characterized by a bleeding tendency
- Most individuals with hereditary thrombocytopenia present with mild thrombocytopenia and a minimal or no risk of spontaneous bleeding
- Diagnosing hereditary thrombocytopenia can be challenging, requiring the exclusion of other causes of low platelet counts, such as infections or immune disorders
- Over the past decade, the advent of high throughput sequencing technologies, including whole exome and whole genome sequencing, has significantly advanced our understanding of these conditions
- Currently, more than 40 genes have been implicated in various forms of hereditary thrombocytopenia
- Recent discoveries have also highlighted that certain genetic mutations associated with hereditary thrombocytopenia may predispose individuals to additional congenital abnormalities, hematological malignancies or bone marrow failure
- Given that the most common forms of hereditary thrombocytopenia pose little to no risk of spontaneous bleeding, most patients require only regular monitoring and in some cases, intervention during hemostatic challenges
- Historically, platelet transfusion was the only treatment option for hereditary thrombocytopenia; however, thrombopoietin receptor agonists (TPO RAs) have emerged as a promising therapy, reducing the need for platelet transfusions in preparation for surgical or hemostatic challenges and offering longterm management for patients with significant spontaneous bleeding
- For the most severe forms, such as Wiskott-Aldrich syndrome and congenital amegakaryocytic thrombocytopenia, hematopoietic stem cell transplantation remains the treatment of choice
Differential diagnosis
- Antiplatelet medications, such as aspirin, glycoprotein IIB / IIIA inhibitors, clopidogrel, ticlopidine
- Anticoagulants (heparin, warfarin or other anticoagulants) (Wikipedia: Anticoagulant [Accessed 1 November 2024])
- Liver disease:
- Patients with liver disease (e.g., liver dysfunction, acute liver failure, cirrhosis) may have varying degrees of thrombocytopenia
- Mechanism can be due to impaired platelet production from decreased synthesis of thrombopoietin, platelet sequestration in the spleen in the setting of portal hypertension, bone marrow suppression from infection (e.g., hepatitis C virus), alcohol use or antibiotic and antiviral treatment
- Disseminated intravascular coagulation (DIC):
- Can be caused by sepsis, malignancy, trauma, obstetrical complications, among others
- Complete blood count may show increased white blood cell count and low platelet count
- Prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), low fibrinogen, increased D-dimer
- Peripheral smear may show evidence of microangiopathic hemolytic anemia (MAHA), with schistocytes and helmet cells
- Vitamin K deficiency:
- Results in deficiency in vitamin K dependent coagulation factors: factors II, VII, IX, X, protein C and S
- Certain patients are at increased risk: on warfarin, taking antibiotics, have fat malabsorption (celiac disease, cystic fibrosis, short bowel syndrome, on a diet lacking in vitamin K)
- Acquired von Willebrand syndrome:
- Due to any qualitative, structural or functional disorder of vWF
- Negative family history
- Lack of previous bleeding
- Decreased vWF activity to antigen ratio, normal vWF propeptide antigen, loss of high molecular weight multimers
- Often associated lymphoproliferative, myeloproliferative, cardiovascular and immunological disorders
Board review style question #1
What is the most common hereditary bleeding disorder?
- Fibrinogen deficiency
- Glanzmann thrombasthenia
- Hemophilia A
- Hemophilia B
- Von Willebrand disease
Board review style answer #1
E. Von Willebrand disease has a prevalence of 1 in 1,000 - 1 in 100. Answer A is incorrect because fibrinogen deficiency is a rare inherited bleeding disorder with a prevalence of 1 in 1,000,000. Answer B is incorrect because Glanzmann thrombasthenia is a rare disorder with a prevalence of 1 in 1,000,000. Answer C is incorrect because hemophilia A is diagnosed in 1 of 5,000 male births. Answer D is incorrect because hemophilia B is diagnosed in 1 of 30,000 male births.
Comment Here
Reference: Hereditary bleeding disorders - general
Comment Here
Reference: Hereditary bleeding disorders - general
Board review style question #2
Which of the following findings is suggestive of a primary hemostasis disorder?
- Bleeding into joints
- Mucosal bleeding
- Negative family history of bleeding
- Prolonged prothrombin time or activated partial thromboplastin time
Board review style answer #2
B. Mucosal bleeding. Patients with primary hemostasis abnormalities are more likely to manifest mucocutaneous bleeding. Answer A is incorrect because bleeding into joints manifests in patients with secondary hemostasis. Answer C is incorrect because primary hemostasis disorders are inherited and can run in families. Answer D is incorrect because prolonged prothrombin time or activated partial thromboplastin time are often seen in secondary hemostasis disorders.
Comment Here
Reference: Hereditary bleeding disorders - general
Comment Here
Reference: Hereditary bleeding disorders - general
Hereditary bleeding disorders - testing
Table of Contents
Factor VII assay | Factor VIII assay | Factor VIII inhibitor assay / Bethesda assay | Factor IX assay | Factor XI assay | Factor XIII assay | Protein C assays | Protein S assays | Prothrombin gene 20210A testingFactor VII assay
- Usually a one stage, prothrombin based assay
- Results distorted by cold activation of factor VII, by variable sensitivity of thromboplastins to activity of factor VII versus VIIa
- Can also use a chromogenic assay (Haemostasis 1983;13:161)
Factor VIII assay
- Assay is usually a PTT based clotting assay that measures factor VIII activity
- Use severely deficient factor VIII plasma as substrate for one stage clotting assay, although there is tremendous interlaboratory variability
- Can also use chromogenic assay, particularly when assessing B chain deleted recombinant Factor VIII (i.e., refacto)
- Low levels: hemophilia, vWF disease with decreased vWF antigen levels, delay in transporting specimen to lab (Goldman: Cecil Medicine: Expert Consult, 23rd Edition, 2007)
- Levels are not greatly decreased at birth or throughout childhood
- Levels may increase during pregnancy
Factor VIII inhibitor assay / Bethesda assay
- Methodology (Am J Clin Pathol 2009;131:552):
- Prepare serial dilutions of patient plasma in citrated saline from 1:1 to 1:160 (or higher)
- Mix each dilution with an equal volume of normal plasma containing a normal amount of coagulation factors, incubate for 2 hours, then perform factor VIII assay
- Titer of inhibitor is dilution that inhibits 50% of factor VIII in assay
- Can use porcine Factor VIII to assess cross reactivity and assist in therapeutic decisions
Factor IX assay
- To detect congenital or acquired factor IX deficiency
- Congenital factor IX deficiency (hemophilia B) is inherited as a sex linked recessive in 1 per 30,000 males
- Acquired factor IX deficiency is seen in liver disease, vitamin K deficiency, Coumadin therapy
- A linear, dose dependent, false decrease is caused by bivalirudin, lepirudin, argatroban and fondaparinux (Arch Pathol Lab Med 2004;128:1142, Goldman: Cecil Medicine: Expert Consult, 23rd Edition, 2007)
Factor XI assay
- Determine by one step clotting activity assay, comparing dilutions of patient plasma to clotting times of dilutions of pooled plasma from normals
- Use plasma from individuals with < 1% activity of immunodepleted normal plasma
- Can also use chromogenic substrate after add inhibitors to factor XIIa and kallikrein
- Levels may decrease during pregnancy (J Clin Pathol 1975;28:332)
Factor XIII assay
- Indications:
- Patients with familial bleeding disorder but normal PT and PTT and normal von Willebrand panel
- Factor XIII deficiency causes delayed bleeding after clot formation due to deficient crosslinking of the fibrin clot
- Screening assay:
- Evaluates clot stability in 5M urea
- Add calcium to patient plasma to make it clot, incubate for 30 minutes at 37°C, then place clot in 5M urea for 24 hours at room temperature
- Normal patients have stable cots but patients with factor XIII deficiency of 1 - 2% of normal have clots that dissolve in urea
- Screening assay does not detect heterozygotes
- 2% acetic acid can also be used as a clot stability screening reagent but will only detect patients down to 4% deficiency
- Quantitative assay:
- Reference range is 70 - 140% of normal
- Detects values of 50% of normal (heterozygous deficiencies)
- Expensive and not readily available, factor XIII is activated by thrombin, attaches glycine ethyl ester to a peptide substrate, releasing ammonia detected by photometer
- High serum ammonia levels falsely decrease the result
- Newborns may have lower levels than adults
Protein C assays
Definition / general
- Deficiencies are either quantitative (type I: reduced amount of normal protein) or qualitative (type II: normal amount of defective protein) (Thromb Haemost 1984;51:1)
- Assays are either functional (measure protein activity) or antigenic (immunoassays that measure quantity, not function)
- Perform functional assay first; if decreased, perform antigenic assay; must exclude acquired causes (Arch Pathol Lab Med 2002;126:1337)
- Low values should be confirmed on a new specimen
- Assays should be performed with platelet poor plasma, using sodium citrate collection tubes
- Functional assays are clot based or chromogenic
- Clot based functional assays:
- Detect all known type I and II variants; patients protein C is activated by Southern Copperhead venom (Agkistrodon contortrix contrortrix), which degrades synthetic substrate, factor Va or factor VIIIa with clot based PTT assay; the prolongation of clotting time is proportional to the amount of factor activity
- PT based assay or amidolytic assays are affected by lupus anticoagulants (raises protein C result), elevations of factor VIII > 200% (decreases the result), acute phase reactions or factor V Leiden mutation (decrease the result); cannot perform on patients taking hirudin or argatroban
- Chromogenic functional assays:
- Not affected by lupus anticoagulants, factor VIII levels, factor V Leiden or other coagulation abnormalities that interfere with clot based functional assays; may not detect qualitative deficiencies detected by clot based assays; patients protein C is activated by snake venom, which cleaves a synthetic substrate, which releases a chromogenic that is measured spectrophotometrically
- Other assays:
- Antigenic assays: either ELISA, electroimmunoassay (Laurell rocket method) or radioimmunoassay; variable levels, sso use 3 standard deviations as cutoff
- ELISA: uses antibody to protein C immobilized to microtiter place; add plasma; add secondary antiprotein C antibody coupled to an enzyme for colorimetric detection; use standard curve to determine plasma protein C
- Laurell rocket antigenic assay: agarose gel has antibody to protein C; plasma samples are put into wells and electrophoresed; antigen antibody complexes precipitate during electrophoresis and height of precipitin arc is proportional to plasma protein C, which is compared to standard curve using pooled normal plasma; may be unable to detect protein C levels < 5%
- Radioimmunoassay: similar to ELISA but uses single, radiolabeled antibody
Etiology
- Acquired causes of low protein C levels: more common than hereditary deficiencies; clot formation, surgery, liver disease, warfarin (should be discontinued at least 10 - 30 days prior to testing), DIC, vitamin K deficiency, vitamin K antagonist therapy and L-asparaginase therapy; repeat protein C test once these conditions are no longer present
- Acquired causes of increased protein C (may mask protein C deficiency): ischemic heart disease, pregnancy, postmenopausal women, hormone replacement therapy and oral contraceptives
Diagnosis
- Necrotic skin in newborns days 1 - 3 of life (purpura fulminas neonatorum, can also test parents also for heterozygosity); evaluation of cause of venous thromboembolism (recommended to use chromogenic protein C assays initially)
- Nonindications: screening before oral contraceptives or oral anticoagulants (discontinue for 10 days or test family members)
Interpretation
- Values falsely increased by bivalirudin, lepirudin, argatroban and fondaparinux, lowered by warfarin (must discontinue for 10 days prior to testing) (Arch Pathol Lab Med 2004;128:1142)
- Reference range: 70 - 140% of normal; newborns levels are 20 - 30% of adult values; usually rise to near adult levels by age 6 months but may remain below adult normal levels until age 10 years
Protein S assays
Definition / general
- Deficiencies are either quantitative (type I: reduced normal protein) or qualitative (type II: normal amount of defective protein)
- Assays are either functional (measure protein activity) or antigenic (immunoassays that measure quantity, not function)
- Gold standard to measure free protein S or APC cofactor activity of protein S is considered the polyclonal ELISA with or without polyethylene glycol precipitation, although this procedure has poor reproducibility
- Perform functional assay first (detects all types of deficiencies)
- Functional assays are clot based, cannot be performed in patients taking hirudin or argatroban
- Antigenic assays measure free protein S (functionally active form) or total (bound plus free) protein S; usually 60% of protein S is bound to C4b binding protein
- Free protein S levels in protein S deficient patients are very sensitive to timing, temperature and dilutional conditions of assays compared to normal individuals
Etiology
- More common than hereditary deficiencies; clot formation, surgery, liver disease, warfarin (should be discontinued at least 10 days prior to testing), nephrotic syndrome, DIC, L-asparaginase therapy, any stimulus to acute phase response (increases C4b binding protein, decreases free protein S), newborns (12 - 60% of adult levels, rise to adult levels by 6 months), women (lower than men before menopause, while taking oral contraceptives, during pregnancy or with hormone replacement therapy), vitamin K antagonist drugs, vitamin K deficiency, elevated factor VIII levels (> 200%) in PTT based functional assays or thrombosis; also nephrotic syndrome, varicella infection and HIV infection
- Classification of deficiencies: all have low functional protein S; I: also low free and total protein S; II / IIb: also normal free and total protein S; III / IIa: low free but normal total protein S
Laboratory
- Methodology:
- Clot based protein S method is based on the addition of activated protein C, which in the presence of protein S, accelerates the inhibition of thrombin activated factors VIII and V
- The prolongation of clotting time is proportional to the amount of factor S activity
- Interference may occur with elevated factor VIII (acute phase reactions or otherwise) (Thromb Res 1995;77:375)
- Values falsely increased by bivalirudin, lepirudin, argatroban and fondaparinux, lupus anticoagulants (Arch Pathol Lab Med 2004;128:1142)
- Reference ranges (nmol/L)
- Each lab should establish its own, values in acute phase plasma are higher:
- Total protein S: - 65% of value in pooled normal human plasma (289 - 397)
- Free protein S: 71 - 115
- C4 binding protein beta+: 228 - 310
- Total C4 binding protein: 257 - 423
Prothrombin gene 20210A testing
Definition / general
- Mutation in G to A transition at nucleotide 20210 in 3 untranslated portion of prothrombin gene, which introduces a new Hind III restriction site
- Can identify heterozygotes and homozygotes
- G20210A mutation in heterozygotes is associated with increased risk of first venous thromboembolic episode (Br J Haematol 2001;113:630)
- Multiplexed arrays test for factor V Leiden, MTHFR C677T and other sequences
- Specimen is whole blood
Laboratory
- Usually PCR amplification of 3 untranslated region of prothrombin gene surrounding the 20210 polymorphism, then either gel electrophoresis, radioisotopic probing or restriction endonuclease digestion with Hind III to detect the nucleotide sequence
Hereditary thrombophilia - general
Table of Contents
Definition / generalDefinition / general
- Thrombotic disorders due in part to deficiencies in natural anticoagulant or fibrinolytic systems
- Acquired risk factors, such as oral contraceptive use, may synergistically increase the risk for thrombosis
- Disorders often occur at a young age; usually affects venous system
- Workup includes documenting all acquired risk factors for thrombosis
High molecular weight kininogen deficiency / assay
Table of Contents
Definition / general | Terminology | Pathophysiology | Clinical features | Laboratory | Case reports | Differential diagnosisDefinition / general
- High molecular weight kininogen deficiency is a rare congenital disorder inherited as an autosomal recessive trait, not associated with a bleeding diathesis
- Typically discovered in individuals with an isolated prolonged PTT
Terminology
- High molecular weight kininogen is also known as Fitzgerald factor, Williams factor and Flaujeac factor
Pathophysiology
- High molecular weight kininogen is a protein produced by the liver (with no inherent catalytic activity) that is involved in the early steps of the intrinsic coagulation pathway; it functions as a cofactor and binds with prekallikrein and factor XI to help facilitate their activation by factor XIIa
- The kininogens (low and high molecular weight) are also involved in the kinin-kallikrein system and function to inhibit thrombin activation of platelets and stimulate liberation of nitric oxide, prostacyclin and tissue plasminogen activator
Clinical features
- Not associated with bleeding
Laboratory
- Isolated prolonged PTT
- Negative lupus anticoagulant
- Specific assay to test for deficiency uses high molecular weight kininogen deficient plasma mixed with patient plasma; a PTT is performed and is compared to a standard curve of high molecular weight kininogen vs. PTT
- Interference occurs in these assays if patient is on heparin, hirudin or argatroban, possibly danaparoid
- Lower levels in newborns, increase to adult levels by age 6 months
Case reports
- 66 year old man evaluated for cardiac surgery (Thromb Haemost 2001;85:195)
Differential diagnosis
Homocysteine assay
Definition / general
- Suspected risk factor for arerial or venous thrombosis, although evidence is weak (Arch Pathol Lab Med 2002;126:1367)
- 70% of homocysteine is bound to albumin, 30% is oxidized to disulfides, 2% is free
- Reference range is 5 - 15 micromolar (reflects free, non-bound form)
- Gender and local population specific reference ranges are strongly recommended, because levels are affected by dietary intake of methionine and vitamins, gender and age (lower in premenopausal women)
- High levels may also be due to vitamin B12 deficiency, post-myocardial infarction or stroke
- Usually recommended to measure after 10 hour fast, although this may not be necessary
- Increase test specificity by measuring 3 - 6 hours after methionine load of 0.1 g of L-methionine/kg
- Must put specimen on ice if plasma separation cannot be performed within 30 minutes, because homocysteine is produced and exported by red blood cells and levels rise after collection in EDTA-anticoagulated tubes
- Alternatively can use acid citrate tubes and hold for up to 6 hours
Diagnosis
- Reduce all forms of homocysteine to free homocysteine, then quantify using either:
- High performance liquid chromatography is standard (Am J Clin Pathol 2008;130:969)
- Fluorescence based immunoassay (Abbott’s IMx analyzer) - reduce using dithiothreitol, then convert to S-adenosyl-L-homocysteine (SAH) via SAH hydrolase; SAH is measured with monoclonal antibody and fluorescent tracer
- Conventional amino acid analyzer with separation column (slow, but can also detect related amino acids, such as methionine, cystathionine and cysteine)
Hypercoagulation panel
Table of Contents
Definition / generalDefinition / general
- Panels are useful to identify all factors predisposing to thrombosis; vary by institution
- Laboratory must be notified if patient is receiving therapeutic anticoagulants (heparin, warfarin, danaparoid, hirudin, argatroban)
- Venous thrombosis panel typically includes assays for activated protein C resistance (factor V Leiden), protein C, protein S, antithrombin, prothrombin G20210A mutation assay, antiphospholipid antibodies and homocysteine
- Less common are assays for plasminogen, dysfibrinogenemia (e.g. reptilase time), heparin cofactor II or platelet hyperaggregability
- Arterial thrombosis panel may include antiphospholipid antibodies, homocysteine levels, lipoprotein (a) (if arterial thrombosis occurs with coronary artery disease, myocardial infarction or stroke)
- In special circumstances, arterial thrombosis may be due to thrombotic diatheses tested on the venous thrombosis panel
- Note: clinicians often confuse Factor V Leiden (to workup thrombosis) with Factor V assay (not the correct test); prevent with use of hypercoagulation panel
Hyperhomocysteinemia
Definition / general
- Homocysteine is an amino acid, derived from methionine, may be converted to cysteine
- Its metabolic pathways require vitamins B12, B6 and folate
- Elevated levels may be hereditary (due to mutations in these pathways) or acquired (due to deficiencies of vitamins B12, B6 or folate, renal failure, carcinoma, hypothyroidism or medications)
- Elevations in homocysteine are associated with increased risk of arterial and venous thrombosis and atherosclerosis, based on retrospective case control studies
- Prospective studies show a weak positive association with arterial thrombosis, and no definite association for venous thrombosis
- Homozygosity or heterozygosity for the C677T mutation in MTHFR gene (methylene tetrahydrofolate reductase), which is involved in homocysteine metabolic pathway, does not appear to be a risk factor for thrombosis, but may be significant in folate - deficient patients
- Although MTHFR was previously thought to be associated with thrombosis, newer data suggests this test is not useful in the first - line evaluation of thrombosis
- Mutation in the methioninesynthetase gene (MTR) also can lead to increased homocystein levels but, as with MTHFR mutations, are not thought to be associated with thrombosis
- Consider testing patients with documented coronary artery disease, cerebrovascular disease or peripheral vascular disease for homocysteine
- High levels can be treated with vitamins B6, B12, folic acid, trimethylglycine, although they may not reduce the risk of future cardiovascular events
- Homocysteinemia is usually associated with a moderately elevated plasma homocysteine, while homocysteinuria is a specific genetic entity with very high plasma homocysteine levels
Additional references
Inhibitors
Table of Contents
Autoimmune based inhibitors in nonhemophiliac patients | Bovine coagulation factor inhibitors | Factor V inhibitor | Factor VIII inhibitor | Factor IX inhibitorAutoimmune based inhibitors in nonhemophiliac patients
Definition / general
Epidemiology
Etiology
Clinical features
Laboratory
Treatment
Additional references
- An acquired hemophilia that occurs after development of an autoantibody directed against a specific coagulation protein in patients with no prior coagulation defect
Epidemiology
- Incidence of 0.2 to 1.0 per million per year
- Median age at presentation is 60 - 70 years
- M = F
Etiology
- Associated with autoimmune disorders, solid tumors, hematologic malignancies, dermatologic disorders, inflammatory bowel disease, respiratory diseases, diabetes, acute hepatitis (B and C), severe drug reactions, post childbirth
- 50% occur in patients with no known medical problems
Clinical features
- Most common inhibitor is antifactor VIII (Semin Thromb Hemost 2009;35:760)
- Mortality rate of 8 - 22% with severe bleeding in up to 90% of affected individuals
- Patients present with soft tissue bleeding such as gastrointestinal, urinary tract or intramuscular (versus intra-articular bleeding in hereditary hemophilia)
- An acquired hemophilia should be suspected in patients with a new onset bleeding disorder accompanied by an isolated prolonged PTT (Curr Gerontol Geriatr Res 2010;2010:927503)
- Patients should be referred to a hemophilia center with expertise in managing inhibitors (Haematologica 2009;94:566)
- Poor prognostic factors are advanced age and lack of treatment (Semin Thromb Hemost 2009;35:769)
Laboratory
- Prolonged PTT that does not correct with mixing studies
- Note: PTT may initially be normal and then increases after 1 - 2 hours incubation
- Normal PT
- A nonlinear curve in a factor assay is often a clue to the presence of an inhibitor
- The Bethesda assay is performed to detect and quantitate presence of inhibitor by diluting inhibitor patient plasma with pooled normal plasma
- Each Bethesda unit of inhibitor indicates a decrease of factor VIII concentration in assay by 50% (1 unit → a reduction from 100% to 50%, 2 units → to 25%, 3 units → 12.5%, etc.)
Treatment
- Prothrombin complex concentrates, recombinant factor VIIa, DDAVP, factor VIII concentrates, immunosuppressive agents, plasmapheresis (variable success) (BMC Res Notes 2010;3:161)
Additional references
Bovine coagulation factor inhibitors
Definition / general
Laboratory
Case reports
Treatment
Differential diagnosis
- Antibodies that develop against bovine proteins after exposure to topical bovine thrombin preparations which can potentially crossreact with corresponding human coagulation factors
- Antibodies can form against bovine thrombin, factor V / Va and fibrinogen and less commonly against bovine factors VII and X
- Some fibrin glues contain bovine thrombin and cryoprecipitate (containing human fibrinogen)
- After use of bovine fibrin glue to achieve hemostasis, 1.7% develop a clinically significant inhibitor
Laboratory
- Prolonged PT and PTT that does not correct with mixing studies
Case reports
- 3 month old and 11 year old children who developed antifactor V antibodies following bovine thrombin exposure (Pediatr Blood Cancer 2007;49:1025)
Treatment
- Steroids, cyclophosphamide, cyclosporine A, IV immunoglobulin
- Management of acute bleeding includes plasmapheresis, platelet transfusions and immunoabsorption
Differential diagnosis
Factor V inhibitor
Definition / general
Epidemiology
Sites
Etiology
Clinical features
Laboratory
Prognostic factors
Case reports
Treatment
Differential diagnosis
Additional references
- An acquired alloantibody that develops against factor V that promotes either its increased clearance from the circulation or interference with its coagulation function
- Most are polyclonal IgG (which may also be associated with IgM or IgA)
- May behave like factor VIII inhibitor in mixing studies, with increasing PTT or PT after 1 - 2 hours
Epidemiology
- 20% of cases are idiopathic
- In one study, 42% of cardiac surgery patients and 20% of neurosurgery patients developed factor V inhibitors after bovine thrombin exposure (Transfusion 2002;42:18)
Sites
- Mucocutaneous and surgical site bleeding are the most common
- Hematuria, gastrointestinal bleeding, intracranial bleeding and hemospermia have also been observed
Etiology
- Most cases of acquired factor V inhibitors are iatrogenic and are caused by exposure to bovine protein (i.e., bovine thrombin preparations)
- Antibodies are generated as an immune response to bovine factor V which then crossreacts with endogenous factor V
- Other risk factors associated with factor V inhibitor development include recent surgery, drug exposure (e.g., aminoglycosides, B lactam antibiotics), infections, blood transfusions, autoimmune disorders, malignancy and pregnancy
Clinical features
- Highly variable
- Bleeding is typically severe and frequently fatal, however, in some instances there may be little to no bleeding
- Inhibitors usually manifest 7 - 10 days postoperatively and then disappear within 8 - 10 weeks
- Noniatrogenic inhibitors may persist for significantly longer periods
- The titer of the inhibitor correlates with clinical severity
- Bovine thrombin associated factor V inhibitors are often accompanied by antibodies to other coagulation proteins such as fibrinogen, prothrombin and thrombin
Laboratory
- Prolongation of PT and PTT with failure to correct with mixing studies
- A nonlinear curve in a factor assay is often a clue to the presence of an inhibitor
- Thrombin times may be prolonged if thrombin inhibitor is also present
- The Bethesda assay is performed to detect and quantitate the presence of inhibitor by diluting inhibitor patient plasma with pooled normal plasma
- Each Bethesda unit indicates a decrease of factor V concentration in the assay by 50% (1 unit → reduction from 100% to 50%, 2 units → to 25%, 3 units → to 12.5%, etc.)
- The Nijmegen assay is also used (Thromb Haemost 1995;73:247)
Prognostic factors
- Noniatrogenic inhibitors are associated with more severe bleeding and fatal outcomes
Case reports
- Factor V inhibitor associated with cold agglutinin disease (Ann Hematol 1998;76:49)
Treatment
- Steroids, cyclophosphamide, cyclosporine A, IV immunoglobulin
- Management of acute bleeding includes plasmapheresis, platelet transfusions and immunoabsorption
Differential diagnosis
- May behave like factor VIII inhibitor in mixing studies, with increasing PTT or PT after 1 - 2 hours
- Combined factor V / factor VIII deficiency
Additional references
Factor VIII inhibitor
Definition / general
Terminology
Epidemiology
Sites
Etiology
Clinical features
Laboratory
Prognostic factors
Case reports
Treatment
Differential diagnosis
Additional references
- An acquired antifactor VIII alloantibody, "antibody produced by one individual that reacts with alloantigens of another individual of the same species," that develops following infusion of factor VIII concentrates (plasma derived and recombinant) causing either increased clearance from the circulation or interference with coagulation function (e.g., inhibition of interaction of factor VIII with phospholipid, etc.)
Terminology
- When challenged with factor VIII concentrate, inhibitor patients are either low or high responders:
- Low responders are patients who develop a low titer of inhibitor (< 5 Bethesda Units [BU])
- 50% of inhibitors are low titer and transient
- High responders are patients who develop a high titer of inhibitor (> 5 BU)
- Low responders are patients who develop a low titer of inhibitor (< 5 Bethesda Units [BU])
- Bypassing agents are coagulation factor treatment products that do not contain factor VIII
Epidemiology
- Develops in 10 - 20% of patients with severe hemophilia A after infusion of factor VIII containing products, less often with mild / moderate disease
- Rarely occurs de novo in patients without hereditary hemophilia, causing acquired hemophilia A (Autoimmune based inhibitors)
- Risk factors for development of inhibitors:
- Specific factor VIII genotype (i.e., major deletions or rearrangements have higher risk than those with small deletions or missense mutations)
- Increased severity of hemophilia A (most likely due to more aggressive treatment)
- Younger age
- Race (blacks and Hispanics more affected than whites)
- Family history of factor VIII inhibitors
Sites
- Typically associated with intra-articular and soft tissue bleeding, similar to hemophilia B
Etiology
- Multiple genetic and environmental factors (see Epidemiology)
Clinical features
- Occurs after at least one infusion of factor concentrate, at a median of 9 - 12 exposures
- Are predominantly polyclonal IgG but isolated instances of IgM and IgA have been reported
- Titer of inhibitor often increases after treatment with factor VIII containing products
- This does not happen with autoimmune factor VIII inhibitor
- The presence of an inhibitor should be suspected when a hemophilia patient shows a decreased response to replacement therapy (i.e., when a sufficient dose of factor concentrate does not control an acute bleeding episode)
Laboratory
- Prolonged PTT that does not correct with mixing studies
- Note: PTT may initially be normal and then increase after 1 - 2 hours incubation
- Normal PT
- A nonlinear curve in a factor assay is often a clue to the presence of an inhibitor
- The Bethesda assay is performed to detect and quantitate the presence of inhibitor by diluting inhibitor patient plasma with pooled normal plasma
- Each Bethesda unit indicates a decrease of factor VIII concentration in assay by 50% (1 unit reduction from 100% to 50%, 2 units → to 25%, 3 units → 12.5%, etc.)
- The Nijmegen assay is also used (Thromb Haemost 1995;73:247)
Prognostic factors
- Patients with high titer inhibitor (> 5 BU) are less likely to respond to treatment
- Factor VIII genotypes that includes large gene deletions, inversions, nonsense and splice site mutations show less response to treatment (J Thromb Haemost 2009;7:1809)
- Data is inconclusive regarding relative inhibitor risk of plasma derived versus recombinant factor concentrates: both can lead to formation
Case reports
- Acquired factor VIII inhibitor in association with myelodysplastic syndrome (Intern Med J 2009;39:e7)
Treatment
- Acute bleeding episodes can be treated with high dose human factor VIII for low titer patients (to overwhelm inhibitor) or porcine factor VIII (if no cross reactivity with inhibitor)
- For high-titer inhibitors, factor VIII bypassing agents (prothrombin complex concentrates, FEIBA or recombinant factor VIIa (J Thromb Haemost 2004;2:899)
- FEIBA can also be used for minor or major surgical procedures (Haemophilia 2009;15:1300)
- Immunosuppression for autoimmune based inhibitors, with a possible role for plasmapheresis
Differential diagnosis
- Lupus-like anticoagulant:
- Occasionally can cause a false positive inhibitor screen by prolonging the PTT and leading to a nonlinear curve in a factor assay
Additional references
Factor IX inhibitor
Definition / general
Terminology
Epidemiology
Sites
Etiology
Clinical features
Laboratory
Prognostic factors
Case reports
Treatment
Differential diagnosis
Additional references
- An acquired antifactor IX alloantibody ("antibody produced by one individual that reacts with alloantigens of another individual of the same species") that develops following infusion of factor IX concentrates (plasma derived and recombinant) causing either increased clearance from the circulation or interference with coagulation function (e.g., inhibition of interaction of factor IX with phospholipid, etc.)
Terminology
- When challenged with factor IX concentrate, inhibitor patients are either low or high responders
- Low responders are patients who develop a low titer of inhibitor (< 5 Bethesda Units [BU])
- 50% of inhibitors are low titer and transient
- Bypassing agents are coagulation factor treatment products that do not contain factor IX
Epidemiology
- Develops in 1.5 - 3% of patients with severe hemophilia B after transfusion of factor IX containing products, less commonly with mild / moderate disease
- The lower inhibitor rate in hemophilia B is due to the lower proportion of severe cases of hemophilia B (~30%) compared with hemophilia A (~60%)
- Rarely arises in nonhemophilia patients with autoimmune disorders causing acquired hemophilia B
- Risk factors for development of inhibitors:
- Specific factor IX genotype (i.e., major deletions or nonsense mutations have higher risk than those with small deletions or missense mutations)
- Increased severity of hemophilia B (most likely due to more aggressive treatment)
- Younger age
- Race (particularly individuals of Scandinavian descent)
- Family history of factor IX inhibitors
Sites
- Typically associated with intra-articular and soft tissue bleeding, similar to hemophilia A
Etiology
- Multiple genetic and environmental factors (see Epidemiology)
Clinical features
- Occurs after at least one infusion of factor concentrate, at a median of 9 - 12 exposures
- Antibodies are predominantly polyclonal IgG
- Titer of inhibitor often increases after treatment with factor IX containing products
- The presence of an inhibitor should be suspected when a hemophilia patient shows a decreased response to replacement therapy (i.e., when a sufficient dose of factor concentrate does not control an acute bleeding episode)
- With the development of inhibitors, some patients may experience allergic or anaphylactic reactions following exposure to concentrate
Laboratory
- Prolonged PTT that does not correct with mixing studies
- Note:
- With factor VIII inhibitors, the PTT may initially be normal and then increase after 1 - 2 hours incubation
- In contrast, factor IX inhibitors immediately inactivate factor IX activity and therefore do not require prolonged incubation
- Normal PT
- A nonlinear curve in a factor assay is often a clue to the presence of an inhibitor
- The Bethesda assay is performed to detect and quantitate the presence of inhibitor by diluting inhibitor patient plasma with pooled normal plasma
- Each Bethesda unit indicates a decrease of factor IX concentration in assay by 50% (1 unit reduction from 100% to 50%, 2 units → 25%, 3 units → 12.5%, etc.)
- The Nijmegen assay is also used (Thromb Haemost 1995;73:247)
Prognostic factors
- Patients with a high titer of inhibitor (> 5 BU) are less likely to respond to treatment
- Data is inconclusive regarding the relative inhibitor risk of plasma derived versus recombinant factor concentrates
- Both can lead to formation of inhibitors
Case reports
- Development of an IgA factor IX inhibitor (Am J Hematol 1984;17:321)
Treatment
- Treatment is primarily supportive
- Aggressive treatment should be aimed at correcting the underlying cause
- Acute bleeding episodes can be treated with high dose human factor IX for low titer patients (to overwhelm the inhibitor) or porcine factor IX (if no cross reactivity with inhibitor)
- For high titer inhibitors, factor IX bypassing agents (prothrombin complex concentrates, FEIBA or recombinant factor VIIa (J Thromb Haemost 2004;2:899)
- FEIBA can also be used for minor or major surgical procedures (Haemophilia 2009;15:1300)
- For autoimmune based inhibitors, use immunosuppression and possibly plasmapheresis
Differential diagnosis
- Lupus-like anticoagulant:
- Occasionally can cause a false positive inhibitor screen by prolonging the PTT and leading to a nonlinear curve in a factor assay
Additional references
International sensitivity index (ISI)
Table of Contents
Definition / generalDefinition / general
- Measure of sensitivity of particular PT reagent - determined by manufacturer
- Used to resolve interlaboratory variations in PT (Arch Pathol Lab Med 2004;128:308, J Clin Pathol 2003;56:114)
- Different PT reagents have different sensitivities to factor deficiencies
- High ISI (3.0) means insensitive reagent vs. low ISI (1.0) means sensitive reagent
- Labs should use reagents with an ISI of 1.0 to 1.5, if possible
Liver dysfunction
Table of Contents
Definition / generalDefinition / general
- Liver is site of production of most coagulation factors, but response of each factor to liver disease is variable due to differences in biologic half lives and acute phase reactions
- PT usually prolonged first, then PTT
- Factor VII: shortest biologic half life, often affected earliest with largest decrease in serum level
- Factor VII also decreases earliest with warfarin treatment
- Factor VIII: may be normal or elevated due to acute phase reaction
- Factors XI and XII: long biologic half lives, may be normal until liver disease is advanced
Low molecular weight heparin (LMWH)
Table of Contents
Definition / generalDefinition / general
- Recommended to monitor using chromogenic antifactor Xa assay on specimens obtained up to 4 hours after subcutaneous injection of LMWH (Chest 2001;119:64S)
- Recommended to use different calibrations for LMWH and unfractionated heparin, and to establish calibration curves for each lot and type of LMWH
- Don’t use PTT to monitor because LMWH doesn’t affect thrombin or factor IXa
Lupus anticoagulation & antiphospholipid antibodies
Definition / general
- Also called lupus inhibitor
- One of the 2 main types of antiphospholipid antibodies (other is anticardiolipin antibodies)
- Common in patients with systemic lupus erythematosus but most cases occur in patients without SLE (Arch Pathol Lab Med 2002;126:1424, Firestein: Kelley's Textbook of Rheumatology, 9th Edition, 2012)
- May cause increased PTT (not time dependent), increased or normal PT
- Prolongs clotting times by binding to phospholipid cofactors in coagulation cascade; often not true for HIV+ patients (Arch Pathol Lab Med 1993;117:595)
- Indications:
- Patients with venous thromboembolism (particularly if no family history or associated with autoimmune disease)
- Unexplained stroke (young person or autoimmune disease), cerebral venous thrombosis, recurrent or late pregnancy loss
- May be considered for arterial thrombosis (particularly in young patient or no documented atherosclerosis)
- Specimen: plasma (citrate tube)
Diagnosis
- An algorithm combining several tests is necessary
- All are clotting time based:
- Russell viper venom time (sensitive to abnormalities in factors X and V, diluted for screening)
- Kaolin clotting time
- Dilute PT (tissue thromboplastin inhibition test)
- PTT based assays (should have low concentration of phospholipids to enhance sensitivity)
- Less commonly Textarin (obtained from venomous Australian snake, not sensitive to abnormalities of factor X but sensitive to abnormalities of factor V
- Less commonly Taipan venom (insensitive to abnormalities of factors X or V)
- Note: all venom assays are sensitive to abnormalities in factor II, calcium and platelets
- Use of commercially available integrated test systems is recommended:
- Staclot procedure: add diluent to tube 1 and egg phosphatidylethanolamine to tube 2
- Add platelet poor plasma with polybrene (neutralizes heparin) to both tubes, incubate and add PTT reagent
- PTT in tube 2 should be 12+ seconds shorter than tube 1 to be a positive test for lupus anticoagulant
- To demonstrate persistence, positive test must be confirmed by repeat testing after 6 - 12 weeks
- Screening assay has low concentration of phospholipids to enhance sensitivity
- Should have platelet count less than 10K
- Abnormal (prolonged) PTT results may be repeated after mixing with equal amount of normal platelet poor plasma
- Continued prolongation of clotting time indicates an inhibitor (not a factor deficiency)
- Confirmed by adding excess phospholipids, which should shorten clotting time towards normal
- Must also rule out factor VIII inhibitors, heparin and other coagulopathies
- Values prolonged by bivalirudin, lepirudin, argatroban and fondaparinux (Arch Pathol Lab Med 2004;128:1142)
- Results vary based on dilutions in factor XII, XI, IX and VIII assays
- May be mistaken for a factor VIII inhibitor if dilutions to abnormal factor assays are not done
- Don’t test patients being treated with anticoagulants (or interpret with caution)
Antiphospholipid antibodies
- Acquired antibodies against phospholipid protein complexes
- Occurs in 3 - 5% of general population; most common cause of acquired thrombophilia
- Rate of thrombosis per year is 1% if no history of thrombosis, 4% in systemic lupus erythematosus (SLE) patients, 5.5% in patients with a history of thrombosis, 6% if high titer of IgG anticardiolipin
- Includes lupus anticoagulant (most patients do not have SLE), anticardiolipin antibody and anti β2 glycoprotein antibodies
- Antibodies are against phospholipids (usually transient, secondary to infection) or various plasma protein antigenic targets (β2-glycoprotein I, protein C, protein S, annexin V, high and low molecular weight kininogens, thrombomodulin, prothrombin, factors XI and XII, complement factor H)
- First described by Wassermann in 1906 (Wasserman test was complement fixation procedure using saline liver extracts from fetuses with congenital syphilis)
- Associated with an increased risk of arterial or venous thrombosis, thrombocytopenia, recurrent miscarriages; causes 1 / 3 of strokes in patients younger than age 50 years (often due to mitral or aortic valve emboli), 15% of deep venous thromboses, 5 - 15% of recurrent spontaneous abortions, eclampsia, maternal DVT's; also multi infarct dementia, chorea, migraine, livedo reticularis in skin
- Catastrophic antiphospholipid syndrome resembles TTP-HUS
- Antiphospholipid antibody syndrome: diagnosis requires a positive lupus anticoagulant or anticardiolipin antibody on 2 separate occasions, at least 6 - 12 weeks apart and either venous or arterial thrombosis, thrombocytopenia or recurrent fetal loss
- References: Arch Pathol Lab Med 2002;126:1424, J Thromb Haemost 2006;4:295
Mixing studies
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Diagrams / tables | Specimen | Principles | Laboratory | Interpretation of results | Pitfalls and preanalytic variables | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Mixing studies are typically used to investigate abnormal clotting time results
- Mixing studies help distinguish clotting time prolongation due to a coagulation factor deficiency or an inhibitor (specific or nonspecific)
- Mixing study may direct further coagulation testing but it is not by itself diagnostic
Essential features
- Mixing studies help distinguish clotting time prolongation due to a coagulation factor deficiency or an inhibitor, e.g. lupus anticoagulant
- To perform a mixing study, mix patient plasma and normal pooled plasma and measure the clotting time that was initially prolonged
- If the clotting time corrects, this suggests a factor deficiency; if the clotting time does not correct, this suggests presence of a circulating inhibitor
- Mixing study may direct further coagulation testing but it is not by itself diagnostic, e.g. may aid in selecting factor assays
Terminology
- Mixing test
- Inhibitor screen
- Inhibitor assay
- Circulating anticoagulant
- Prothrombin time (PT) 1:1 mix
- Partial thromboplastin time (PTT) 1:1 mix
- Partial thromboplastin time (PTT) correction
ICD coding
Diagrams / tables
Contributed by Tori Seasor, M.D. and Karen A. Moser, M.D.
| Differential diagnosis of prolonged PT or aPTT incorporating mixing study results (correction versus noncorrection) | ||
| Clotting time prolonged | Mixing study result | Key differential diagnoses |
| Prothrombin time (PT) | Corrects |
|
| Does not correct |
| |
| Activated partial thromboplastin time (aPTT) | Corrects |
|
| Does not correct |
| |
| PT and aPTT | Corrects |
|
| Does not correct |
| |
Specimen
- Platelet poor plasma in 3.2% sodium citrate
Principles
- To perform a mixing study, mix patient plasma and normal pooled plasma (NPP) and measure the clotting time that was initially prolonged
- In factor deficiency: NPP adds sufficient clotting factors to overcome the deficiency and correct the clotting time
- If an inhibitor is present, it typically inhibits the clotting factors in patient plasma and NPP so the clotting time remains prolonged
- Inhibitors can be specific inhibitors (antibodies directed against a specific coagulation factor), nonspecific inhibitors (e.g. lupus anticoagulant) or medications such as heparin, direct thrombin inhibitors or direct Xa inhibitors
- Mixing study results aid in selection of further coagulation testing, such as assays for specific factor deficiencies or inhibitors
Laboratory
- CLSI H47-A2 includes general guidance for the performance of PT and aPTT mixing studies (CLSI: H47-A2 - One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test, 2nd Edition, 2008)
- Mixing study is performed for the prolonged clotting time only (e.g. if the PT is prolonged, a PT mixing study will be performed)
- Mixing studies vary in the ratio of patient plasma to normal pooled plasma (NPP) and in incubation time
- Patient plasma to NPP ratio
- 1:1 mixing study (equal parts patient plasma and NPP) is most commonly used
- 4:1 mixing study (4 parts patient plasma and 1 part NPP) is sometimes used to evaluate minimally prolonged aPTT as it may be more sensitive in this scenario (Am J Clin Pathol 2002;117:62)
- Incubation time
- Immediate: clotting time is evaluated immediately after mixing patient plasma and NPP
- Incubated: mixed patient plasma and NPP is incubated at 37 °C for 1 - 2 hours, depending on local laboratory protocols
- Incubation control consists of patient plasma and NPP incubated separately at 37 °C for same amount of time as mix; at end of incubation, patient plasma and NPP are mixed and clotting time is measured
- Incubation control accounts for loss of labile factors during incubation
- Incubation performed if immediate mixing study does not show an inhibitor pattern
- Incubated aPTT mixing studies are useful to identify time dependent inhibitors (such as factor VIII inhibitors and some lupus anticoagulants)
- Incubated PT mixing studies are not typically useful
- Incubation control consists of patient plasma and NPP incubated separately at 37 °C for same amount of time as mix; at end of incubation, patient plasma and NPP are mixed and clotting time is measured
- Patient plasma to NPP ratio
- Definition of normal pooled plasma is also not standardized
- NPP can be purchased from a vendor
- Making NPP locally is not preferred but if necessary it is recommended to use platelet poor plasma from a minimum of 20 - 30 donors with coagulation factor levels of approximately 100% for NPP (Semin Thromb Hemost 2014;40:195)
Interpretation of results
- If a clotting time corrects in the immediate (PT) or immediate and incubated mixing study (aPTT), it is interpreted as a factor deficiency pattern
- Principle: at least 50% activity of a given factor is sufficient to produce a normal clotting time
- Example:
Patient factor activity NPP factor activity 1:1 mix factor activity Mixing study interpretation 2% 100% 51% Correction
- If a clotting time does not correct in the immediate (PT) or immediate or incubated mixing study (aPTT), it is interpreted as an inhibitor pattern
- Principle: the inhibitor binds all available factor, from both the patient and NPP and the clotting time remains prolonged
- There is no standard definition of correction of aPTT or PT mixing studies
- CLSI H60-A (CLSI: H60-A - Laboratory Testing for the Lupus Anticoagulant, 1st Edition, 2014) recommends interpreting mixing studies in the setting of lupus anticoagulant testing by using either
- 1:1 mix normalized ratio (ratio = 1:1 mix result(s) / 1:1 mix mean of reference interval(s))
- Rosner index (see below)
- CLSI H60-A (CLSI: H60-A - Laboratory Testing for the Lupus Anticoagulant, 1st Edition, 2014) recommends interpreting mixing studies in the setting of lupus anticoagulant testing by using either
- Strategy for interpretation should be determined and validated locally by individual laboratories
- Example strategies for determining mixing study correction (Semin Thromb Hemost 2013;39:283)
- Clotting time of mix returns to within laboratory's reference interval
- Clotting time of mix falls within a 2 - 3 standard deviation range of the mean normal clotting time
- Compare the clotting time of mix to the NPP clotting time (ratio)
- Estimated factor correction method takes into account effects of single versus multiple factor deficiencies (Blood Coagul Fibrinolysis 2016;27:90)
- Rosner index and Chang percentage are calculations created to assist in interpretation of mixing study results
- Rosner index is used in conjunction with a 1:1 mixing study; originally described for use in lupus anticoagulant testing
- Rosner index > 15 suggests presence of an inhibitor (Thromb Haemost 1987;57:144)
Rosner index = Clotting time of 1:1 mix − clotting time of NPP × 100 Clotting time of patient sample
- Rosner index > 15 suggests presence of an inhibitor (Thromb Haemost 1987;57:144)
- Chang percentage is used in conjunction with a 4:1 mixing study (optimal sensitivity) (Am J Clin Pathol 2002;117:62)
- Chang percentage result of < 50% suggests the presence of an inhibitor
Chang percentage = Initial patient clotting time − clotting time 4:1 mix × 100 Initial patient clotting time − clotting time NPP
- Chang percentage result of < 50% suggests the presence of an inhibitor
- Rosner index is used in conjunction with a 1:1 mixing study; originally described for use in lupus anticoagulant testing
Pitfalls and preanalytic variables
- Multiple factor deficiencies may cause mixing study noncorrection, which could be incorrectly interpreted as an inhibitor (Semin Thromb Hemost 2014;40:195)
- High hematocrit or underfilled collection tubes can cause prolonged aPTT and PT due to excess citrate anticoagulant in the sample
- Anticoagulant effect that appears as an inhibitor should be taken into account when interpreting mixing studies
- Heparin: affects aPTT mixing studies
- May be detected with a thrombin time or anti-Xa activity assay and corrected by a neutralization procedure
- Direct thrombin inhibitor (DTI) may affect aPTT > PT mixing studies
- Direct Xa inhibitors may affect PT > aPTT mixing studies
- Heparin: affects aPTT mixing studies
- If a specimen is not properly centrifuged to ensure removal of platelets, platelets can interfere with clotting time results by neutralization of lupus anticoagulant effect (if present) by phospholipids present in platelet cell membranes (J Thromb Haemost 2009;7:1737)
- Rare occurrence of increased prolongation of aPTT (rather than shortening) when a patient's sample containing lupus anticoagulant is mixed with NPP (lupus cofactor effect) (Semin Thromb Hemost 2012;38:385)
- Lupus cofactor effect is reagent dependent and may represent a prozone effect (Am J Clin Pathol 2016;146:262)
- Decreased factor II in patient plasma may account for lupus cofactor effect in some cases (Am J Clin Pathol 2020;153:229, TH Open 2020;4:e40)
Additional references
Board review style question #1
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are performed on a 64 year old man. The patient is taking no anticoagulants or other medications. The results of a 1:1 mixing study are
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 36 s
Which of the following is the next best test to order?
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 36 s
Which of the following is the next best test to order?
- 4:1 mixing study
- Factor VIII activity
- Factor X activity
- von Willebrand factor activity
Board review style answer #1
B. Factor VIII activity. The mixing study indicates a corrected result which suggests a factor deficiency as a cause of the prolonged aPTT. The next step would be to investigate which factor deficiency is causing this, which can be accomplished with factor VIII, IX and XI activity testing. Factors VIII, IX and XI are factors in the intrinsic coagulation pathway that can prolong the aPTT and are associated with clinical bleeding. Lupus anticoagulant testing could also be considered, as some weak lupus anticoagulants may show apparent correction in mixing studies.
Comment Here
Reference: Mixing studies
Comment Here
Reference: Mixing studies
Board review style question #2
Coagulation laboratory tests are drawn on a 35 year old woman with the recent diagnosis of an autoimmune disease. The physician ordered a 1:1 mixing study after the original results revealed a prolonged activated partial thromboplastin time (aPTT).
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 50 s
Which of the following do these laboratory results suggest?
Patient aPTT: 51 s (reference range 38 - 42 s)
Immediate 1:1 aPTT mixing study: 50 s
Which of the following do these laboratory results suggest?
- Factor V Leiden mutation
- Factor VII deficiency
- Lupus anticoagulant
- Vitamin K deficiency
Board review style answer #2
C. Lupus anticoagulant. The aPTT was prolonged in the patient sample and the addition of NPP in the immediate mixing study did not correct the aPTT to the normal reference range. This result suggests an inhibitor that overcomes the normal coagulation factors in the patient sample as well as the NPP. Lupus anticoagulants behave as nonspecific inhibitors in aPTT mixing studies and are a common cause of unexpected aPTT prolongation. The recent history of an autoimmune disorder diagnosed in a young woman would also raise concern for a lupus anticoagulant as an explanation for her prolonged aPTT.
Comment Here
Reference: Mixing studies
Comment Here
Reference: Mixing studies
Other anticoagulants & thrombolytic therapy
Danaparoid (Orgaran)
Definition / general
- Approved to prevent deep venous thromboses
- Also an alternative to heparin for patients with heparin induced thrombocytopenia (Crit Care 2007;11:R102)
- Use is decreasing due to newer anticoagulants
- Composed of low molecular weight glycosaminoglycans (mixture of heparan sulfate, dermatan sulfate and chondroitin sulfate) that primarily inhibit factor Xa, factor IIa to a much lesser extent
Clinical features
- Similar to low molecular weight heparin in having a more predictable anticoagulant effect, with less need for laboratory monitoring
- Monitor, if desired, by measuring inhibitor of factor Xa using standard curve; draw 6 hours after subcutaneous injection; PT and PTT are unaffected
- Therapeutic levels to treat DVT are 0.5 - 0.8 anti-factor Xa units/ml, lower for DVT prophylaxis
- Long half-life, is prolonged with renal failure
- No reversal agent is known but incomplete reversal is shown with protamine sulfate
- Not marketed in the US since 2004, still available in some countries.
Hirudin
Definition / general
- Anticoagulant approved by FDA to treat thrombosis in patients with heparin induced thrombocytopenia (does not itself cause this type of syndrome)
- Derivatives include lepirudin, refludan
- Recombinant protein, cloned from a leech, which directly inhibits factor IIa (thrombin) (Wikipedia: Hirudin [Accessed 19 April 2021])
- Also has fibrinolytic properties
- More predictable anticoagulant effect than standard heparin, less need for laboratory monitoring, although close monitoring is still advised with PTT, though this assay has limitations (ecarin clotting time is preferable)
- Prolongs PT, PTT, thrombin time, ACT and interferes with most clotting based assays
- Therapeutic range to treat DVT is PTT that is 1.5 - 2.5 x normal but more specific assays are also used to monitor, such as ecarin clotting time
- Half-life ~1 hour, may be prolonged if antibodies develop, dramatically prolonged in renal failure
- No reversal agent
- Reference: Thromb Haemost 2008;99:819
Thrombolytic therapy
Definition / general
- Used to treat myocardial infarction, pulmonary embolism, arterial or venous thrombosis, thrombotic stroke
- Thrombolytic agents include recombinant t-PA, urokinase, streptokinase (only tPA is widely available in the USA)
- Presence of fibrin degradation products and D-dimers, decreased fibrinogen and plasminogen, prolonged thrombin time, PT and PTT
- References: Clin Lab Med 2009;29:159
Diagrams / tables
Images hosted on other servers:
Warfarin (Coumadin)
Definition / general
- Therapeutic anticoagulant to prevent thromboembolism by impairing regeneration of active vitamin K (warfarin is a synthetic derivation based on coumarin)
- Name incorporates the acronym for the organization which funded the key research (WARF) and the ending -arin, indicating its link with coumarin (Wikipedia: Wisconsin Alumni Research Foundation [Accessed 19 April 2021])
Clinical features
- Takes 4 - 5 days for complete therapeutic effect due to long half-life of factors II and X
- Must be supplemented with another anticoagulant such as heparin (bridged) until INR is in therapeutic range for 2 consecutive days to prevent Warfarin Skin Necrosis in those with low Protein C
- Therapeutic effect is measured by INR; goal is often INR between 2 and 3
- INR may be elevated by lupus anticoagulants or use of hirudin with warfarin
- Warfarin should not be used alone for acute heparin induced thrombocytopenia because it causes paradoxical thrombosis; must add a rapid acting anticoagulant (hirudin, danaparoid, argatroban) until INR is therapeutic
- PTT may be normal even if low warfarin levels
Treatment of bleeding / overdose
- Vitamin K, fresh frozen plasma
- Also prothrombin complex concentrates (J Clin Pathol 2004;57:1132, J Thromb Haemost 2006;4:1853)
Diagrams / tables
Images hosted on other servers:
Physiology
Table of Contents
Definition / general | Essential features | Primary hemostasis | Secondary hemostasis | Intrinsic pathway | Extrinsic pathway | Common pathway | Tertiary hemostasis | Diagrams / tables | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Hemostasis is a highly complex process of clot formation in response to blood vessel injury (Indian J Anaesth 2014;58:515)
- Disorders in the hemostatic pathway can lead to either hypercoagulability (in which excessive clot formation occurs) or bleeding disorders (in which clot formation does not adequately stop bleeding)
Essential features
- Hemostasis can be categorized into 3 stages (Curr Med Chem 2004;11:2245):
- Primary hemostasis is the formation of a weak platelet plug at the site of vascular injury
- Secondary hemostasis is the formation of a fibrin plug by coagulation factors; coagulation factor activation occurs via the intrinsic and extrinsic pathways, which merge to form the common pathway
- Tertiary hemostasis describes the anticoagulation and fibrinolytic balance to promote healing and prevent excessive clotting
- The 3 major constituents of the hemostatic system are platelets, coagulation proteins and endothelium (Cardiovasc Diagn Ther 2018;8:568)
Primary hemostasis
Secondary hemostasis
- In secondary hemostasis, the fibrin plug is formed by the activated coagulation factors (Ann Med 2012;44:405)
- Fibrinogen is cleaved into fibrin by factor II (thrombin) at the end of the common pathway (Arterioscler Thromb Vasc Biol 2011;31:494)
- Insoluble fibrin is crosslinked by factor XIII, which strengthens the fibrin plug
- Coagulation factors are synthesized in the liver, except factor VIII and vWF (Blood Coagul Fibrinolysis 2000;11:S69)
- Coagulation proteins circulate as inactive zymogens; factors must undergo enzymatic cleavage to function (Hemodial Int 2006;10:S2)
- Factors II, VII, IX and X and protein C and S are synthesized in a vitamin K dependent manner; vitamin K epoxide reductase is a key enzyme in the vitamin K cycle (Vitam Horm 2008;78:23)
- Warfarin is an inhibitor of vitamin K epoxide reductase and suppresses the synthesis of active factors II, VII, IX and X and protein C and S
- Protein C and S are anticoagulant proteins that degrade factor Va and VIIIa (Adv Clin Exp Med 2013;22:459)
- The model of secondary hemostasis is comprised of 2 separate pathways, the intrinsic and extrinsic pathway, that merge to form a common pathway; in vivo, it appears that these pathways may not be so distinctly separated
Intrinsic pathway
- Intrinsic pathway is comprised of factors VIII, IX, XI, XII, prekallikrein and high molecular weight kininogen (Indian J Anaesth 2014;58:515)
- The pathway is initiated when factor XII (Hageman factor) is activated by exposure to negatively charged molecules such as inorganic polyphosphate released by platelets (Cell 2009;139:1143)
- Factor XIIa initiates a chain of activation involving factor XI, IX, VIII and ultimately factor X, initiating the common pathway with the activation of thrombin (factor II) (Arterioscler Thromb Vasc Biol 2019;39:331)
- Factor VIIIa increases the ability of factor IXa to activate factor X (Thromb Res 2007;119:1)
- Hemophilia A, B and C are inherited deficiencies of factor VIII, IX and XI, respectively; hemophilia A and B are X linked recessive disorders (Acta Neuropathol Commun 2021;9:111)
- Factor XIIa additionally converts prekallikrein to kallikrein; the functions of kallikrein include converting prorenin to renin, kininogen to bradykinin and further activating factor XII (J Clin Invest 2002;109:1007)
- The function of the intrinsic and common pathways of coagulation is measured with activated partial thromboplastin time (aPTT); aPTT measures the time it takes plasma to form a clot when exposed to factor XII activator (Methods Mol Biol 2013;992:111)
- Prolonged aPTT can be indicative of a factor deficiency, such as hemophilia or an inhibitor to the intrinsic or common pathway; an aPTT mixing study is used to differentiate between these causes (Eur J Haematol 2020;104:519)
Extrinsic pathway
- Extrinsic pathway is comprised of tissue factor and factor VII (Indian J Anaesth 2014;58:515)
- Tissue factor (TF) is a glycosylated protein expressed on subendothelial tissues; TF is activated in response to vascular wall damage (Curr Pharm Des 2015;21:1152)
- Activated TF binds to and activates factor VII
- Factor VIIa converts factor X into factor Xa, initiating the common pathway
- Clinically, the extrinsic and common coagulation pathways are assessed by prothrombin time (PT); PT is a measurement of the time it takes for clot formation when plasma is exposed to tissue factor (StatPearls: Prothrombin Time [Accessed 15 June 2022])
- Prolonged PT can reveal a clotting factor deficiency or inhibitor to the extrinsic or common pathway; a PT mixing study can differentiate between these causes (Mayo Clin Proc 2007;82:864)
- International normalized ratio (INR) is the ratio between a patient's PT and a standardized, control PT value (StatPearls: Prothrombin Time [Accessed 15 June 2022])
Common pathway
- Both the intrinsic and extrinsic pathways of coagulation lead to the activation of factor X
- Common pathway involves fibrinogen (factor I), factors II, V and X
- Prothrombinase complex is formed by factor Xa, factor V and calcium; this complex converts prothrombin (factor II) to thrombin (factor IIa) (Indian J Anaesth 2014;58:515)
- Thrombin converts fibrinogen to fibrin, further activates factors V and VIII and cleaves factor XIII (Blood 2005;106:2605)
- Factor XIII further stabilizes the clot by covalently crosslinking fibrin (Blood 2005;106:2605)
- Antithrombin is a protease inhibitor that inhibits activated coagulation factors, primarily thrombin and Xa (J Thromb Haemost 2020;18:3142)
- Heparin inhibits thrombin generation by increasing antithrombin activity (Prog Mol Biol Transl Sci 2019;163:1)
Tertiary hemostasis
- Tertiary hemostasis is the process of fibrinolysis (clot dissolution), in which fibrin is cleaved to prevent excessive clotting
- Tissue type plasminogen activator (tPA) is produced by endothelial cells to mediate clot breakdown; urokinase type plasminogen activator (uPA) also functions in fibrinolysis (Front Immunol 2019;10:1348)
- In the presence of fibrin, tPA and uPA cleave plasminogen to plasmin (J Thromb Haemost 2007;5:804)
- Plasmin is a serine protease that degrades fibrin clot to soluble fibrin and fibrinogen degradation products (Int J Mol Sci 2021;22:2758)
- D dimer is a soluble fibrin degradation product that is used as an important clinical marker for coagulation and fibrinolysis (Am J Hematol 2019;94:833)
- Fibrinolysis inhibitors, such as antiplasmin (which binds and inactivates fibrin) and plasminogen activator inhibitor 1 (PAI1; inhibitor of uPA / tPA), prevent excessive fibrinolysis (FEBS J 2005;272:4852, Transfus Apher Sci 2019;58:572)
Diagrams / tables
Board review style question #1
What are the basic components of hemostasis?
- Phospholipids, platelets and coagulation proteins
- Tissue factor, platelets and phospholipids
- Vascular endothelium, platelets and coagulation proteins
- Vascular endothelium, platelets and fibrinogen
- Vascular endothelium, tissue factor and platelets
Board review style answer #1
C. Vascular endothelium, platelets and coagulation proteins
Comment Here
Reference: Coagulation - Physiology
Comment Here
Reference: Coagulation - Physiology
Board review style question #2
Which coagulation factor deficiency has normal PT and aPTT?
- Factor II
- Factor V
- Factor X
- Factor XIII
Board review style answer #2
Plasminogen activator antigen-1
Definition / general
- Uncommon test; perform if strong evidence of familial bleeding disorder, but normal results for von Willebrand disease or possibly if unexplained premature myocardial infarction
- May predict severe hepatic veno-occlusive disease after allogeneic bone marrow transplantation (Br J Haematol 2002;118:1087)
- Not a known risk factor for hypercoagulability (Arch Pathol Lab Med 2002;126:1401), although high levels are associated with arterial thrombosis; low levels are associated with rare familial bleeding disorder
- Has circadian rhythm with highest values in morning; in one study, mean level was 23 ng/mL at 9 am vs. 10 ng/mL at 4 pm; also is acute phase reactant, so don’t measure immediately following thrombosis; also elevated during pregnancy
Laboratory
- Collection: collect blood from steadily flowing venipuncture, discard first 3 - 5 mL (if this is the only test) and avoid platelet contamination of plasma (platelets contain PAI1) by separating plasma from cells or storing on ice
- Reject specimen if antifibrinolytic agent is present in specimen
- Reference range: 4 - 40 ng/mL for antigen assay, 0 - 12 units/mL for functional assay
- Functional assay: add patient plasma to known amount of urokinase / tPA, which binds to patient PAI1; residual urokinase is detected by adding plasminogen, which converts it to plasmin, which cleaves a chromogenic substrate; amount of released color is inversely proportional to patient PAI1 (inhibitors of antiplasmin and plasmin are present to prevent their interference)
- ELISA (antigen) assay: also available
Plasminogen assay
Definition / general
- Indications: patients with familial venous thrombosis but no evidence of other hypercoagulable states, occasionally used to monitor thrombolytic therapy or for patients with ligneous conjunctivitis
- Either functional (based on plasmin activity) or immunologic (based on concentration of plasminogen antigen) (Haemostasis 1988;18:47)
- Functional assays:
- Determine plasmin enzyme activity with plasmin-specific chromogenic substrate
- Add streptokinase to patient plasma, complex cleaves a chromogen releasing a colored compound
- Color is measured spectrophotometrically and is proportional to plasminogen in sample
- Expressed as percentage of normal plasma (reference range 75 - 130%)
- Immunologic assays:
- Radial immunodiffusion methods
- Used if dysplasminogenemia is being evaluated
- Ratio of functional activity to antigen is significantly decreased compared to controls
Interpretation
- Plasminogen levels are increased by oral contraceptives (which increase cholesterol levels), pregnancy and acute phase reactants
- Plasminogen levels are decreased by liver disease, thrombolytic therapy and DIC; newborns have levels that are 60% of adults, increase to adult values by age 6 months
Platelet aggregation studies
Definition / general
- Used to assess platelet function if a familiar bleeding disorder is suspected, but the PT, PTT, platelet count and von Willebrand tests are normal (which is unusual)
- May include platelet responses to adenosine diphosphate (ADP), epinephrine, collagen and arachidonic acid
- Agglutination with ristocetin may also be assessed
- Usually 60% or more platelets aggregate with the above agonists, but not spontaneously; aggregation is decreased in newborns (Br J Haematol 1988;68:53)
- Note: testing is labor intensive and must be scheduled in advance because a normal control must be drawn simultaneously
- A platelet function assay (e.g. PFA-100) may be used to assess platelet function; although easier to perform, it is not as robust as platelet aggregation and must be interpreted with caution
- Hereditary disorders:
- Consider in patients with bleeding histories, no obvious acquired cause, but abnormal platelet aggregation study repeated at least once, same abnormality in family members
- May be a platelet storage pool disorder (deficiency in alpha or dense platelet granules), Glanzmann thombasthenia (deficiency of platelet glycoprotein IIb / IIIa, reduced aggregation by all agonists except ristocetin) or Bernard-Soulier disease (deficiency of platelet glycoprotein Ib, causes decreased ristocetin-induced aggregation only)
Laboratory
- Aggregometry with platelet-rich plasma to measure optical transmission or electric impendence (J Thromb Haemost 2009;7:1029)
- Whole blood aggregation with a lumiaggregometer can measure both aggregation and ATP release
- Abnormalities are often due to medications (aspirin - affects arachidonate aggregation; other platelet-inhibiting agents); also uremia, monoclonal gammopathy and myeloproliferative disorders
Platelet antibodies
Definition / general
- Either autoimmune (idiopathic thrombocytopenic purpura), alloimmune (neonatal alloimmune thrombocytopenia, post-transfusion purpura, platelet transfusion refractoriness) or heparin-induced
- These tests must be ordered and interpreted cautiously, considering the clinical presentation (Blood 1997;89:1112)
- Platelet antibody disorders:
- Drug-induced thrombocytopenia:
- Detected by a difficult serotonin release assay (add patient plasma / serum plus drug and platelets with radiolabeled serotonin; drug antibodies, if present, stimulate platelets and radioactive serotonin is released)
- Also detected with flow cytometry
- Offending drugs include quinine and quidinine, sulfonamides, sulfonylureas, gold salts, salicylates
- Mechanism is either non-immune (marrow suppression or non-immune destruction) or immune (platelet counts due to immune causes may drop to
- Idiopathic thrombocytopenic purpura (ITP):
- Autoantibody against platelets, usually directed against GP IIb / IIIa, less commonly GP Ib / IX
- Diagnosis of exclusion; usually resolves in children but is chronic in adults
- Tests to order include peripheral blood smear, CBC, HIV, thyroid function tests, liver function tests and bone marrow biopsy
- Although not technically contraindicated, platelet transfusions tend to be futile until the offending antibody is removed via some kind of immunosuppression
- Neonatal alloimmune thrombocytopenia (NAIT):
- Incidence of 1 per 1 - 5K live births
- Father and newborn have antigen that mother lacks, mother produces antibodies to this antigen (usually PI - A1 component of GP IIb / IIIa), which crosses the placenta and destroys fetal platelets
- Newborn platelet counts are Newborn can sometimes develop intracranial hemorrhage due to extremely low platelet count
- If possible, give platelets negative for the antigen the mother lacks; otherwise, treatment of choice is washed maternal platelets
- Platelet refractoriness:
- In thrombocytopenic patients with multiple platelet transfusions, due to formation of HLA - A, HLA - B or less commonly ABO antibodies that destroy transfused platelets
- Platelet crossmatch using immobilized platelets may be performed in referral centers
- Post-transfusion purpura:
- Patient has antibody directed against transfused platelet antigen absent on patients platelets
- For unknown reasons, these antibodies also destroy platelets own antigens
- Typically antigen is PI - A1 component of GP IIb / IIIa or an HLA antigen the patient lacks
- Patients have sudden onset of severe thrombocytopenia 5 - 12 days after transfusion of platelet product, resolves 14 days after transfusion
- In thrombocytopenic phase, common anti-platelet antibodies (GPIIb - IIIa, GPIb - IX and GPIa - IIa) are present, in addition to antibodies to the specific antigen the donor platelets express that precipitated the event
- After resolution of the PTP episode, the general platelet antibodies will disappear, while the donor specific platelet antibodies will persist (Vox Sang 1999;76:120)
- Drug-induced thrombocytopenia:
Laboratory
- Types of tests:
- ELISA: test for specific antiplatelet antibodies; antigen of interest is bound to surface of microtiter plate, then add patient plasma and antibody will bind to antigen
- Antigen capture immunoassay: specific antigens are bound to solid phase, then add patient serum and patient antibodies will bind to antigens
- Platelet antigen typing by antigen capture immunoassays: patients platelet antigens are immobilized by monoclonal antibodies onto a solid phase; then add antibodies of known specificity (Am J Clin Pathol 1990;93:552)
- Flow cytometry: may be used
- Lymphocytotoxicity assay: determine percent reactive antibody (HLA antibodies in patients who are refractory to platelet transfusions)
- Polymerase chain reaction: can be used to identify patients platelet antigens
Platelet hyperaggregation studies
Definition / general
- Hyperaggregation may rarely be associated with hypercoagulability, including myocardial infarction, strokes and venous thrombosis
- Tested patients should have abstained from aspirin, NSAIDs or platelet-inhibiting drugs for 7 days prior to testing
- Indications: patients with unexplained hypercoagulability and normal values in hypercoagulation panel
Laboratory
- Similar to platelet aggregation
- Centrifuge citrated plasma gently to draw red and white blood cells into a pellet, which leaves platelets suspended in the plasma
- Then add various agonists at multiple low concentrations and a control (no agonist, to measure spontaneous aggregation), and measure platelet aggregation with an aggregometer (which measures optical density)
- Must carefully evaluate patient's use of medications (including over the counter)
- Must compare to normal control, and results can be subjective
Additional references
Prekallikrein assay
Table of Contents
Definition / generalDefinition / general
- Also known as Fletcher factor
- Screening assays: preincubate PTT sample for 10 minutes prior to adding calcium; a prolonged PTT that shortens after the 10 minute preincubation is suspicious for prekallikrein deficiency
- PTT performed with elagic acid as the intrinsic pathway activator will be normal in prekallikrein deficiency
- Specific assay: preincubate patient plasma with prekallikrein-deficient plasma for 1 minute, then add calcium and perform PTT; prekallikrein level is determined from a standard curve of prekallikrein vs. PTT (Thromb Res 2002;105:463)
- Interference is due to hirudin, argatroban, danaparoid and heparin (add heparinase)
- Reference range is 60 - 140% of normal; newborn levels are lower, but increase to near adult levels by age 6 months
- Indications: prolonged PTT corrected with mixing study, factors VIII, IX, XI and XII are normal, PT and fibrinogen are normal, lupus anticoagulant assays are negative
Prekallikrein deficiency
Table of Contents
Definition / general | Terminology | Epidemiology | Pathophysiology | Etiology | Clinical features | Laboratory | Case reports | Treatment | Differential diagnosis | Additional referencesDefinition / general
- Prekallikrein deficiency is a rare congenital disorder that causes an isolated prolonged PTT but is not associated with a bleeding tendency
Terminology
- Also known as Fletcher factor deficiency
Epidemiology
- Rare
Pathophysiology
- Prekallikrein is a contact factor that complexes with high molecular weight kininogen and is cleaved by factor XII (Hageman factor) to produce kallikrein in the initial steps of the intrinsic pathway
Etiology
- Autosomal recessive inheritance
- Homozygous individuals have Heterozygous individuals have 20% - 60% of normal activity
- There are rare variants of abnormal prekallikrein molecules
Clinical features
- No bleeding tendency
- Usually detected in asymptomatic individuals after the incidental finding of isolated prolonged PTT
- There have been anecdotal reports of prekallikrein deficiency and increased risk of arterial and venous thrombosis, but usually thrombotic risk factors were identified (Acta Haematol 2010;123:210)
Laboratory
- Prolonged PTT that corrects after mixing study
- Normal PT, thrombin time and bleeding time
- Specific functional prekallikrein assay is diagnostic
Case reports
- Prekallikrein deficiency resulting in mucosal hemorrhage (Am J Med Sci 2009;338:429)
Treatment
- Typically not required
Differential diagnosis
- Acquired prekallikrein deficiency due to DIC or liver disease, rarely due to antibodies to prekallikrein
- Factor XII deficiency
- High molecular weight kininogen deficiency
Additional references
Procoagulants (pending)
[Pending]
Protein C deficiency
Definition / general
- Hereditary deficiencies occur in 0.14 - 0.5% of general population (the clinically significant incidence is much lower)
- > 160 mutations exist, either type I (76%, usually quantitative) or type II (dysfunctional protein, normal protein levels)
- Causes 1 - 11% of cases of venous thrombosis
- These patients are also at risk for warfarin-induced skin necrosis if treated with warfarin and no heparin until warfarin levels are therapeutic; this paradoxical clotting is due to a faster fall in natural anticoagulant proteins than procoagulant proteins in these patients
- Heterozygotes have levels 35 - 65% of normal
- First thrombotic event occurs between ages 10 - 50 years
- Only 30% have thromboembolism, increasing to 75% if coexisting factor V Leiden
- Homozygotes (1 per 500 - 750K births) with severely decreased levels present as newborns with DIC and purpura fulminans neonatorum, leading to death unless anticoagulation and replacement therapy with fresh frozen plasma is started
- Homozygous protein C deficiency can be cured with liver transplant; however, this is usually too risky so replacement is preferred treatment
- Must exclude acquired causes of protein C deficiency
Etiology
- Acquired causes of low protein C levels:
- Clot formation
- Surgery
- Liver disease
- Warfarin (should be discontinued at least 10 days prior to testing) or Vitamin K antagonist therapy
- DIC
- Vitamin K deficiency
- L-asparaginase therapy
- Acquired causes of increased protein C (may mask protein C deficiency):
- Ischemic heart disease
- Pregnancy
- Postmenopausal women
- Hormone replacement therapy
- Oral contraceptives
Additional references
Protein S deficiency
Definition / general
- Hereditary deficiencies occur in 0.7% of general population
- Many mutations exist (qualitative or quantitative)
- Much lower prevalence of thrombophilia with clustering in families
- Variable penetrance may be due to coexisting risk factors, such as factor V Leiden
- Causes 1 - 9% of cases of venous thrombosis
- These patients also at risk for warfarin - induced skin necrosis if started on warfarin without the addition of heparin until warfarin levels are therapeutic
- Heterozygotes have levels 35 - 65% of normal
- First thrombotic event occurs between ages 10 - 50 years
- 50% risk by age 45
- Homozygotes with severely decreased levels present as newborns with DIC and purpura fulminans, leading to death unless anticoagulation and replacement therapy with fresh frozen plasma is started
- Type I (2 / 3):
- Low free and total protein S antigen, with decreased APC cofactor activity
- Type II (rare):
- Normal free and total protein S antigen, and decreased APC cofactor activity
- Type III (1 / 3):
- Normal to low total protein S, low free protein S antigen, and an elevated fraction of protein S bound to C4B protein
- Testing recommended:
- Individual with family history who requests testing, to confirm abnormal protein S result
- Must interpret with caution
- Testing not recommended:
- During pregnancy or postpartum, during inflammatory, thrombotic or surgical event
- Within 30 days of taking warfarin
- Must delay longer periods for vitamin K antagonists (Phenprocoumon)
- Clinical note:
- Acquired protein S deficiency is often seen during pregnancy due to increased C4b, which may reduce levels to 40% or less
Additional references
Proteinuria
Table of Contents
Definition / generalDefinition / general
- Proteinuric patients are in a more prothrombotic state than healthy controls, but antiproteinuric therapy ameliorates the prothrombotic state (J Thromb Haemost 2011;9:2416)
- Patients with nephrotic syndrome may have decreased factors XI and XII
Prothrombin gene mutation (G20210A) / hyperprothrombinemia
Definition / general
- Mutation in G to A transition at nucleotide 20210 in 3 untranslated portion of prothrombin gene, which introduces a new Hind III site
- Associated with (may not directly cause) increased prothrombin levels, 2 - 5x increased risk of venous thrombosis
- Patients with G20210A mutation have increased levels of TAFI (Thrombin Activated Fibrinolysis I, a protein that inhibits clot lysis), which makes clots exist longer, leading to increased thrombotic episodes
- Risk is multiplicative if taking oral contraceptives and have factor V Leiden gene
- Heterozygous form occurs in 1 - 2% of normal individuals, 6 - 20% of patients with venous thrombosis
- Testing via nucleic acid based assay is preferred, as the lack of linearity of the clot - based Factor II assay at the high end makes this unsuitable for diagnostic use
Laboratory
- Recommended patients to test:
- Patients with recurrent venous thromboembolic events
- First episode before age 50
- First unprovoked venous thromboemboli at any age
- Thromboses at unusual anatomic sites (cerebral, mesenteric, portal or hepatic veins)
- Venous thromboemboli in patient with first degree relative with venous thromboemboli before age 50 years
- Venous thromboemboli related to pregnancy or estrogen use, or unexplained pregnancy loss in second or third trimesters
- Young individuals with myocardial infarction and no other risk factors
- Also test factor V Leiden and other mutations (combination most clearly impacts clinical decision making)
- Testing not recommended:
- As general population screen
- Routine test during pregnancy
- Routine test before or during oral contraceptive use or hormone replacement therapy
- As newborn initial test
- As initial test in patients with arterial thrombotic events
Treatment
- Patients with thromboemboli and this mutation should receive similar treatment as other patients with venous thromboemboli
Additional references
PT - Prothrombin time
Definition / general
- Most commonly performed laboratory coagulation test
- Measures clotting time from factor VII activation through fibrin formation (i.e. extrinsic and common pathway)
- Used as screening test and to monitor warfarin anticoagulation; can only detect single factor deficiencies if level is 15 - 45% of normal
- Anticoagulant is usually 3.2% sodium citrate (recommended by Clinical and Laboratory Standards Institute; 3.8% sodium citrate causes prolonged PT if samples are Arch Pathol Lab Med 1997;121:956)
- Test should use a thromboplastin that is insensitive to heparin in therapeutic range
- PT is more sensitive to deficiencies in common pathway than aPTT
Laboratory
- Warfarin monitoring
- Warfarin is monitored using INR (international normalized ratio), which standardizes PT results for patients on oral anticoagulants
- Goal is INR of 2 - 3
- Calculated as INR = (patient PT / mean normal PT)ISI, where ISI is the International Sensitivity Index which is used to calibrate a particular batch of thromboplastin reagent to a universal standard (seebelow)
- PT / INR should be checked daily at onset of warfarin use until dose and INR are stable (usually at least a week since half life of factors II and X are long), then need to check decreases gradually to every 4 weeks
- May be improved by instrument-specific International Sensitivity Index (ISI) values, in-house calibrators or calibration curves (Arch Pathol Lab Med 2004;128:308); ISI measures sensitivity of PT reagent to factor deficiencies (1.0 is sensitive, 3.0 is insensitive, value determined by manufacturer)
- Algorithm for working up a prolonged PT
- (1) add heparinase; if PT corrects to normal, prolongation is due to presence of heparin
- (2) mixing study (determine if etiology if factor deficiency or factor inhibitor); mix patient plasma with equal amount of normal plasma and determine the PT of the mixture after incubation for 2 hours
- (a) if PT of mixture is normal, prolonged PT is due to factor deficiency; do assays for factors I, II, V, VII, X
- (b) if PT of mixture is still prolonged, suggests presence of inhibitor (rare)
- (c) if PTT of mixture is initially normal but becomes prolonged after incubation for 1 - 2 hours, may be due to factor V inhibitor (rare)
Interpretation
- Reference interval should be established using at least 120 subjects for each reference population or subclass, and verified using at least 20 subjects
- Usual reference range is 10 - 14 seconds, up to 16 seconds at birth and decreasing to adult values at age 6 months
- Limitations: lupus anticoagulants, use of hirudin or argatroban - must use alternative assays, such as chromogenic factor X assays
- Prolonged PT: usually due to deficiencies of factors I (fibrinogen), II, V, VII, X, less commonly due to an inhibitor or anticoagulant (heparin, hirudin, argatroban) and rarely lupus anticoagulant or specific factor inhibitor
- Prolonged PT with normal PTT: warfarin or vitamin K deficiency (decreases function of factors II, VII, IX, X, protein C, protein S), liver dysfunction (decreases hepatic synthesis of all coagulation factors except factor VIII) and DIC
- Markedly prolonged values may be due to long acting warfarin-like rodenticide toxicity (Arch Pathol Lab Med 2004;128:e181)
PT / INR and aPTT
Table of Contents
Definition / general | Essential features | Terminology | ICD coding | Laboratory | Clinical features | Interpretation | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are common initial tests in the evaluation of patients with suspected bleeding disorders
- PT evaluates extrinsic and common pathways
- aPTT measures intrinsic and common pathways
- International normalized ratio (INR) is a calculation allowing for the standardization and comparison of PT clotting time results between laboratories
Essential features
- PT and aPTT assays measure time to fibrin clot formation
- PT and aPTT results are reported in seconds
- INR is calculated from PT clotting time values and allows for standardization to monitor warfarin anticoagulant therapy
Terminology
- Coagulation screening tests
- Clotting times
ICD coding
Laboratory
- Clinical & Laboratory Standards Institute (CLSI) H47-A2 includes general guidance for the performance of PT and aPTT (CLSI: H47-A2 - One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test, 2nd Edition, 2008)
- PT and aPTT are clot based coagulation tests measuring time to formation of fibrin clot (Lab Med 2017;48:295)
- Coagulation analyzers use 1 of 2 strategies for clot detection
- Electromechanical
- Optical
- Coagulation analyzers use 1 of 2 strategies for clot detection
- Methods (Lab Med 2017;48:295, Semin Thromb Hemost 2014;40:195, Res Pract Thromb Haemost 2020;4:761):
- Whole blood sample for testing: platelet poor plasma in 3.2% sodium citrate (collection tube with light blue top)
- Centrifuged at 1,500 x g (3,000 RPM) for 15 minutes to separate out plasma that contains < 10,000/uL of platelets
- PT and aPTT results are reported in seconds
- PT: thromboplastin (tissue factor combined with phospholipid) and calcium chloride are added to the sample to initiate clotting
- PT reagents also contain heparin neutralizers (up to 2 U/mL) (Mayo Clin Proc 2007;82:864)
- PT measures effects of factors in extrinsic and common pathways
- INR is a calculated value to allow for comparison of PT results from different laboratories or reagent lots (Semin Thromb Hemost 2014;40:195)
- PTpatient is the measured PT of the patient in seconds
- PTnormal is the geometric mean PT (mean normal prothrombin time, MNPT) determined from a group of at least 20 normal plasmas from healthy individuals including males and females
- Geometric mean = antilog{[log(X1) + log(X2) + log(X3) + … + log(Xn)]/n}
- International sensitivity index (ISI) is an experimentally derived value that is typically determined by a manufacturer for specific instrument / reagent combinations (CLSI: H47-A2 - One-Stage Prothrombin Time (PT) Test and Activated Partial Thromboplastin Time (APTT) Test, 2nd Edition, 2008)
- International sensitivity index determination
- WHO reference method
- International sensitivity index = slope of the orthogonal regression line derived from comparison on logarithmic axes of PT results for local versus reference method on fresh plasma samples (20 normal plasmas and 60 plasmas from stably anticoagulated patients on vitamin K antagonists)
- Scope of study required not practical for clinical laboratories
- International sensitivity index reflects the sensitivity of a PT reagent to deficiencies of vitamin K dependent factors
- Lower International sensitivity index values correlate with more vitamin K antagonist sensitive reagents
- International sensitivity index determination
INR = [ PTpatient ] PTnormal ISI
- aPTT: a contact factor activator (e.g., kaolin, silica) combined with phospholipid and calcium chloride are added to the sample to initiate clotting
- aPTT measures effects of factors in intrinsic and common pathways
- Whole blood sample for testing: platelet poor plasma in 3.2% sodium citrate (collection tube with light blue top)
- Reagents (Lab Med 2017;48:295, Clin Lab Med 2009;29:253, Mayo Clin Proc 2007;82:864):
- Reagents used for PT and aPTT testing vary between labs and show different factor sensitivities
- PT reagents differ in phospholipid concentration and source as well as source of tissue factor
- aPTT reagents differ in contact activator, phospholipid type and phospholipid concentration
- Reagents used for PT and aPTT testing vary between labs and show different factor sensitivities
- Considerations when validating new reagent lots (Lab Med 2017;48:295, Clin Lab Med 2009;29:253)
- PT
- Must determine mean normal prothrombin time and input this into instrument or laboratory information system (LIS) for calculation of INR when new lot put into use
- Must input new mean normal prothrombin time into instrument or LIS for calculation of INR when new lot put into use
- APTT
- Must determine heparin therapeutic range if aPTT will be used to monitor unfractionated heparin (UFH)
- Heparin therapeutic range determined by measuring aPTT and UFH anti-Xa activity on a set of at least 20 plasmas from patients treated with UFH (Semin Thromb Haemost 2017;43:253)
- Draw line of best fit
- aPTT heparin therapeutic range represents aPTT values in seconds corresponding to UFH anti-Xa activity between 0.3 and 0.7 IU/mL
- Heparin therapeutic range determined by measuring aPTT and UFH anti-Xa activity on a set of at least 20 plasmas from patients treated with UFH (Semin Thromb Haemost 2017;43:253)
- Must determine heparin therapeutic range if aPTT will be used to monitor unfractionated heparin (UFH)
- Optional studies
- Determination of factor sensitivity of PT and aPTT reagents is considered a best practice but is not a required study under Clinical Laboratory Improvement Amendments (CLIA)
- Some reagent manufacturers provide lot level information about factor sensitivity in PT and aPTT package inserts
- Saline dilution curves help describe level of combined factor deficiencies, at which a clotting time prolongs
- Prepared by measuring clotting time of serial dilutions of normal plasma with saline
- Determination of factor sensitivity of PT and aPTT reagents is considered a best practice but is not a required study under Clinical Laboratory Improvement Amendments (CLIA)
- PT
- Preanalytic variables (Mayo Clin Proc 2007;82:864, Lab Med 2017;48:295)
- Variables that may affect either PT or aPTT
- Underfilled collection tube
- Not collecting blood to fill line
- May occur if phlebotomist fails to account for dead space volume in a butterfly collection device
- High hematocrit
- Heparin contamination from a line draw
- Tends to affect aPTT more than PT due to heparin neutralizers present in PT reagents
- May affect PT at very high levels of heparin
- Wrong anticoagulant in collection tube (e.g., ethylenediaminetetraacetic acid [EDTA], heparin)
- Underfilled collection tube
- Samples must be processed (centrifuged, plasma removed from cells and testing performed or plasma frozen for later testing) within a specified time for valid results (Semin Thromb Hemost 2019;45:443)
- PT: 24 hours
- aPTT (not for heparin monitoring): 4 hours
- aPTT (for heparin therapeutic monitoring): 1 hour
- Variables that may affect either PT or aPTT
Clinical features
- PT testing: can be used clinically for evaluation of bleeding, assessment of liver disease, screening evaluation of factors II, V, VII, X and fibrinogen (Mayo Clin Proc 2007;82:864, Lab Med 2017;48:295, Res Pract Thromb Haemost 2020;4:761)
- See Abnormal PT / aPTT values for differential diagnosis
- Therapeutic monitoring
- INR used to monitor warfarin therapy
- PT / INR not used to monitor heparin or direct oral anticoagulant therapy (DOAC)
- INR is a component of the model for end stage liver disease (MELD) score that assesses severity of end stage liver disease (MDCalc: MELD Score [Accessed 4 May 2021])
- PT / INR may also be measured on point of care (POC) instruments
- POC PT / INR are only approved for monitoring of vitamin K antagonists
- May be used in limited clinical settings
- Anticoagulation clinics
- Home use for carefully selected patients
- Comparison between POC PT / INR and central laboratory values may be challenging and varies by instrument and reagent
- Possible sources of error to consider specifically with POC PT / INR performed outside of a clinical laboratory
- Inadequate training for users
- Inattention to quality control procedures
- Inappropriate patient selection
- Failure to participate in External Quality Assessment (EQA) program
- Failure to refer unexpected or questionable results for central laboratory PT / INR
- aPTT testing: can be used clinically for monitoring heparin and certain direct thrombin inhibitor therapy, screening evaluation of factors II, V, VIII, IX, XI, XII, fibrinogen, prekallikrein and HMWK, screening for lupus anticoagulant (lupus sensitive aPTT reagents) (Mayo Clin Proc 2007;82:864, Lab Med 2017;48:295, Res Pract Thromb Haemost 2020;4:761)
- See Abnormal PT / aPTT values for differential diagnosis
- Therapeutic monitoring
- Unfractionated heparin (if aPTT has heparin therapeutic range determined by laboratory)
- Argatroban, an anticoagulant (direct thrombin inhibitor) for treating patients with thrombosis and heparin induced thrombocytopenia
- aPTT not used to monitor low molecular weight heparin or dabigatran therapy
Interpretation
- Reference intervals are dependent upon the instrument, anticoagulant, tube type, reagent and reagent lot
- Determination of a laboratory specific reference interval that is applicable to the current reagent lot and testing methods in use is crucial (Clin Lab Med 2009;29:253, Lab Med 2017;48:295)
- PT results are reported in seconds with common values of 10 - 13 seconds (exact interval determined by individual laboratory)
- aPTT results are reported in seconds with common values 25 - 40 seconds (exact interval determined by individual laboratory)
- INR
- INR improves comparison of prolonged PT values between laboratories for the purpose of monitoring warfarin therapy
- INR therapeutic range for warfarin = 2.0 to 3.0 for most indications (Blood Adv 2020;4:4693)
- Critical values
- Determined by individual laboratories but may be similar to the following recommended values from the International Council for Standardization in Haematology (ICSH) (Semin Thromb Hemost 2020;46:398)
- PT / INR: INR 4.0 - 6.0
- aPTT: should be locally determined
- Determined by individual laboratories but may be similar to the following recommended values from the International Council for Standardization in Haematology (ICSH) (Semin Thromb Hemost 2020;46:398)
Board review style question #1
The laboratory supervisor tells you that a new lot of reagent is to be started next week and the international sensitivity index (ISI) needs to be updated. If this is done incorrectly, which laboratory result(s) will be affected?
- aPTT
- INR
- PT
- PT and aPTT
- Thrombin time
Board review style answer #1
B. INR
The international normalized ratio (INR) is used to standardize PT across different laboratories to monitor warfarin therapy. The international sensitivity index (ISI) value is specific to a reagent lot and analyzer. The INR is then calculated by the following equation:
Comment Here
Reference: PT / INR and aPTT
The international normalized ratio (INR) is used to standardize PT across different laboratories to monitor warfarin therapy. The international sensitivity index (ISI) value is specific to a reagent lot and analyzer. The INR is then calculated by the following equation:
| INR = | [ | PTpatient | ] |
| PTnormal |
ISI
Comment Here
Reference: PT / INR and aPTT
Board review style question #2
A patient has an unexpectedly prolonged aPTT of > 150 seconds and a normal PT. The patient is not receiving any anticoagulants and has no history of bleeding or thrombosis. The laboratory investigates this unexpected value and identifies a prolonged thrombin time of > 150 seconds. What is the most likely preanalytic variable that would explain this pattern of results?
- EDTA anticoagulant in tube
- Heparin contamination
- High hematocrit without citrate volume correction
- Underfilled collection tube
Board review style answer #2
B. Heparin contamination
The marked prolongation of both the aPTT and thrombin time in this case is best explained by heparin contamination. Although all of the other preanalytic variables listed could cause falsely prolonged clotting times, they would be expected to also falsely prolong the PT (which was normal in this case) and may not show such extreme aPTT and thrombin time prolongation as can be seen with heparin.
Comment Here
Reference: PT / INR and aPTT
The marked prolongation of both the aPTT and thrombin time in this case is best explained by heparin contamination. Although all of the other preanalytic variables listed could cause falsely prolonged clotting times, they would be expected to also falsely prolong the PT (which was normal in this case) and may not show such extreme aPTT and thrombin time prolongation as can be seen with heparin.
Comment Here
Reference: PT / INR and aPTT
Board review style question #3
Your laboratory is provided a reagent lot with an international sensitivity index (ISI) of 1.31 on your instrument. The PT is performed with this reagent in 40 normal donors. With this information, the arithmetic mean is calculated to be 12.9 seconds and the geometric mean is calculated is 13.0 seconds. A patient sample PT is 18.1 seconds using this reagent lot in your laboratory. What is the international normalized ratio (INR) for this patient sample?
- 1.2
- 1.5
- 1.6
- 1.8
Board review style answer #3
B. 1.5
The calculated patient INR for this given ISI and PTnormal is 1.5, with the PTpatient (18.1 seconds) and the PTnormal being the PT of normal donors using the geometric mean (13.0 seconds) using the formula:
Comment Here
Reference: PT / INR and aPTT
The calculated patient INR for this given ISI and PTnormal is 1.5, with the PTpatient (18.1 seconds) and the PTnormal being the PT of normal donors using the geometric mean (13.0 seconds) using the formula:
| INR = | [ | PTpatient | ] |
| PTnormal |
ISI
Comment Here
Reference: PT / INR and aPTT
Quality assurance
Table of Contents
Definition / generalDefinition / general
- Important to prevent laboratory errors
- CLIA regulations require patient and control specimens be tested in duplicate only for manual coagulation tests, but not for automated tests
- CLIA regulations require calibration and calibration verification procedures to substantiate continued accuracy throughout the laboratorys reportable range of test results
Reptilase time
Table of Contents
Definition / generalDefinition / general
- Clotting time similar to thrombin time, but uses snake venom (reptilase) instead of thrombin
- Measure rate of fibrin clot formation after addition of reptilase to citrated plasma
- Generates a fibrin clot by cleaving fibrinopeptide A from fibrinogen
- Prolonged by decreased or dysfunctional fibrinogen, or high levels of fibrin degradation products; also amyloidosis (inhibits fibrinogen conversion to fibrin); also prolonged by increased fibrinogen associated with acute phase reaction (Am J Clin Pathol 2002;118:263)
- Used to diagnose dysfibrinogenemia (also thrombin time)
- Not prolonged by heparin or hirudin (unlike thrombin time)
Thrombin time
Table of Contents
Definition / generalDefinition / general
- Measures rate of fibrin clot formation after addition of standard concentration of thrombin to citrated plasma (Key, Makris, O'Shaughnessy and Lillicrap (Eds): Practical Hemostasis and Thrombosis, 2nd Edition, 2009 (Page 53))
- Thrombin cleaves fibrinogen, releasing fibrinopeptides A and B, converting fibrinogen to fibrin
- Useful to diagnose dysfibrinogenemia after more common disorders are excluded
- Reference range: 10 - 13 or 16 - 24 seconds, depending on reaction conditions and thrombin concentration
- Prolonged if even small amounts of heparin, hirudin or argatroban anticoagulants are present
- Also prolonged with dysfibrinogenemia, amyloidosis (inhibits fibrinogen conversion to fibrin), DIC, thrombolytic therapy or thrombin inhibitors in patients exposed to bovine thrombin
tPA assay
Definition / general
- tPA activity / antigen and PAI-1 activity / antigen are used to evaluate fibrinolysis; often PAI-1 activity and tPA antigen are ordered together
- PAI-1 rapidly forms a complex with free tPA in the specimen
- Not recommended for routine clinical laboratories due to complexity and special handling requirements
- Can also measure tPA plasma concentration by ELISA or other immunologic assays
- Resting level is usually low, not clinically significant
- Post-stimulation level (such as after venous occlusion for 10 minutes) may be more useful
- Higher levels in patients with acute paraquat intoxication (J Korean Med Sci 2011;26:474)
Laboratory
- Must first inhibit interaction of tPA with PAI1 (its functional inhibitor) by acidifying plasma
- Determine activity by measuring plasmin activity from conversion of plasminogen
Validation of assays and instruments (pending)
[Pending]
Viscoelastic hemostatic assays
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Diagrams / tables | Clinical features | Laboratory | Case reports | Treatment | Additional references | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2 | Board review style question #3 | Board review style answer #3Definition / general
- Viscoelastic hemostatic assays provide comprehensive, real time analysis of coagulation status (Diagnostics (Basel) 2020;10:118)
- Contribute to a better understanding of blood coagulation in conjunction with conventional coagulation tests, which measure the impact of individual clotting components but not interactions between them
- Different reagents allow the tests to determine the relative contribution of circulating plasma components and cellular elements on blood coagulation
- First generation devices: reagents are pipetted into the device separately from the blood sample (e.g. TEG®5000, ROTEM® Delta)
- Newer generation devices: use a single cartridge for reagents so no pipetting is required (e.g. TEG®6s, ROTEM® Sigma, Quantra®)
- Some newer generation devices also include platelet function testing (e.g. TEG®6s)
- Support rapid treatment for patients in a variety of clinical settings and allow goal directed transfusion of blood products
Essential features
- Viscoelastic hemostatic assays measure dynamics of whole blood clotting
- Provide a comprehensive real time analysis of hemostasis, from initial fibrin fiber formation to platelet interactions and fibrinolysis
- Newer generation viscoelastic hemostatic assays are designed to be fast, effective and portable
- Results are clinically relevant, allowing goal directed coagulation factor transfusions
Terminology
- Viscoelastic hemostatic assay / analyzer: an in vitro diagnostic method of global coagulation / hemostasis analysis that provides a real time, holistic view of ex vivo clotting; it allows for examination of both cellular and plasma protein contributions to clotting, including platelet number and function, fibrin(ogen) function and coagulation factor function
- Thromboelastography: a subtype of in vitro viscoelastic hemostatic diagnostic technology that monitors and analyzes the coagulation state of a whole blood sample by measuring the shear modulus / dynamics of clot formation in low shear conditions in order to assist in the assessment of a patient’s hemostatic status and its clinical implications
- Thrombelastograph (TEG®): an in vitro diagnostic hemostasis system by Haemonetics®
- Rotational thromboelastometry (ROTEM®): an in vitro diagnostic hemostasis system by Werfen®
- TEG®5000 (legacy version): a Thrombelastograph® analyzer using thromboelastography by Haemonetics®
- TEG®6s (next generation device): a Thrombelastograph® analyzer using multichannel cartridge based thromboelastography by Haemonetics®
- ROTEM® Delta (legacy version): a viscoelastic hemostasis analyzer using rotational thromboelastometry by Werfen®
- ROTEM® Sigma (next generation device, not FDA cleared as of January 2022): a viscoelastic hemostasis analyzer using multichannel cartridge based rotational thromboelastometry by Werfen®
- Sonoclot® SC1, SCP1, SCP2 (legacy versions): viscoelastic hemostatic analyzers using linear motion by Sienco
- Sonoclot® SC1P4 (next generation device): a viscoelastic hemostatic analyzer using linear motion by Sienco
- Quantra®: a viscoelastic hemostasis analyzer using sonic estimation of elasticity via resonance (SEER) sonorheometry by Hemosonics®
- ClotPro®: a viscoelastic hemostasis analyzer using elastic motion thromboelastography produced by enicor, acquired by Haemonetics®
- Emerging technologies include laser speckle rheometry, mechanical resonant frequency, ultrasonic deformation and parallel plate viscometry:
- Laser speckle rheometry is a noninvasive optical technique that can measure viscoelastic properties of blood without applying any external force or physically contacting the test sample
- Mechanical resonant frequency is an object's natural frequency of vibration, which is affected by the surrounding medium and can be used to detect changes in a detector's vibration frequency as the surrounding blood sample changes during blood clot formation
- Ultrasonic deformation measures shape deformations of suspended blood droplets, which are directly correlated to the viscoelastic properties of the sample
- Parallel plate viscometry measures the shear stress of a blood sample positioned between two parallel plates to calculate the sample's viscoelastic properties
Pathophysiology
- Abnormalities in hemostasis can result in excessive blood clotting (thrombosis) or bleeding (hemorrhage) (Physiol Rev 2013;93:327)
- Viscoelastic hemostatic assays measure the dynamics of whole blood clotting (Diagnostics (Basel) 2020;10:118)
- Viscoelasticity is characteristic of a material that behaves in both a viscous (permanent deformation) and elastic (temporary deformation) manner
- Earliest viscoelastic hemostatic assays used rotation of cup and pin to measure the viscoelasticity of whole blood
- Thromboelastography: the cup rotates and the resulting rotation in the pin is measured
- Rotational thromboelastometry: the cup is held stationary and a rotational force is applied to the pin
- Before clotting, the whole blood sample remains viscous and the pin does not move
- Response to shear stress will be permanent deformation
- As clot strength develops, the sample gradually develops an elastic element, which results in movement of the pin
- Total pin deflection is tracked and plotted
- Other viscoelastic hemostatic assays are based on linear motion of the pin rather than rotation
- For the TEG®6s analyzer, new measurement techniques include fixed vibration resonance frequency measured with LED illumination
- Assays and associated reagents have been developed to investigate different coagulation pathways, the contributions of platelets and fibrinogen to clot strength and the effect of clot lysis
- Some of the newer devices include platelet function testing, useful in measuring the impact of antiplatelet medication or platelet function post injury
- More recent technologies are available as cartridge based systems that automate all sample aliquoting, reagent mixing and testing
Diagrams / tables
Clinical features
- Viscoelastic hemostatic assays are currently used for the management of major bleeding and guiding transfusion therapy
- Most common indications are trauma hemorrhage, cardiac surgery, managing goal directed antiplatelet therapies, obstetric hemorrhage and liver disease
- Viscoelastic hemostatic assays are recommended in trauma guidelines for assessing coagulopathy and guiding blood product transfusions in acutely bleeding trauma, surgical and critically ill patients (J Trauma Acute Care Surg 2020;89:999, Crit Care 2016;20:100, American College of Surgeons: Massive Transfusion in Trauma Guidelines [Accessed 26 May 2021], Anaesth Crit Care Pain Med 2019;38:539)
- Viscoelastic monitoring is also recommended in guidelines for:
- Monitoring and managing hemostasis in cardiac surgery (NICE: Detecting, managing and monitoring haemostasis - viscoelastometric point‑of‑care testing [Accessed 26 May 2021], Eur J Anaesthesiol 2017;34:332, Anesthesiology 2015;122:241, National Blood Authority Australia: Patient Blood Management Guidelines - Module 2 Perioperative [Accessed 23 December 2021], Eur J Cardiothorac Surg 2018;53:79, Anaesth Crit Care Pain Med 2019;38:539)
- Liver transplant surgery (Anaesth Crit Care Pain Med 2019;38:539, Eur J Anaesthesiol 2017;34:332)
- Postpartum hemorrhage (Anaesth Crit Care Pain Med 2019;38:539, Eur J Anaesthesiol 2017;34:332, J Thromb Haemost 2016;14:205, Obstetric Anaesthetists' Association: OAA/AAGBI Guidelines for Obstetric Anaesthetic Services 2013 [Accessed 26 May 2021])
- Viscoelastic monitoring has potential for use in the clinical management of patients with stroke / congenital bleeding disorders and in the management of treatment with direct acting oral anticoagulants
- Platelet inhibition and aggregation assays, available with some technologies, allow for the quantification of response to aspirin and P2Y12 receptor inhibitors, with potential uses in:
- Personalized dual antiplatelet therapy for cardiology patients
- Optimizing timing for cardiac surgery in patients on antiplatelet therapy
- Monitoring hemostasis during cardiac surgery
- Reducing blood transfusions and risk of complications following cardiac surgery
- Predicting ischemic event risk following coronary interventions
- Monitoring of platelet function also has a range of potential uses in the emergency setting:
- Assessing coagulopathy profiles in trauma patients with burns and traumatic brain injury
- Guiding treatment of patients undergoing surgery and neurological treatment
- Evidence on the use of platelet function tests available on viscoelastic devices is currently limited to research studies
- Such uses do not yet reflect established evidence or clinical practice and may go beyond the intended use as indicated by the manufacturer
- Limitations of viscoelastic monitoring include (Hong Kong Med J 2015;21:45):
- User variability
- Lack of universally agreed algorithms to guide clinical use
- Relatively few large scale randomized clinical trials completed using viscoelastic testing
- Inability to measure the influence of all in vivo pathologies; in particular, the influence of epithelial cells on the coagulation process
- Interchangeability of results between viscoelastic assays because of the different coagulation activators used
- Older models are labor intensive and require skilled trained staff to operate with accuracy
Laboratory
- Variables used to assess the clotting process are based on changes in clot strength over time
- Details of those most likely to be used in clinical practice are illustrated in the example thromboelastography parameter tracing in Diagram 1
- Below is some of the terminology commonly associated with thromboelastography / rotational thromboelastometry (respectively) (Diagnostics (Basel) 2020;10:118)
- Reaction time (R) / clotting time (CT): the time taken for the amplitude of the clot to reach 2 mm
- Reflects the process of thrombin generation and the initial formation of fibrin fibers
- Increases in R time / CT reflect hypocoagulability; low R time / CT reflects hypercoagulability
- Coagulation time (K) / clot formation time (CFT) is the interval between the amplitudes of 2 - 20 mm
- Represents the process of clot strengthening, which is mainly dependent on fibrinogen availability
- Prolonged K time / CFT suggests hypocoagulability, while hypercoagulability can shorten K time / CFT
- Angle (α) is the tangent line of the tracing that starts as the tracing leaves the baseline
- Reflects the speed of clotting and depends on coagulation factors, including fibrinogen
- Angle that is smaller than the reference range suggests that the patient is in a hypocoagulable state, while a larger angle is likely to be encountered in hypercoagulable patients
- Maximum amplitude (MA) / maximum clot firmness (MCF) is the most divergent point of the tracing, representing the maximum strength of the clot
- Depends largely on the concentrations and functionality of platelets and fibrin
- Lysis 30 (LY30) / lysis index 30 (LI30) is the percentage reduction in the area under the tracing at 30 minutes after the maximum amplitude
- LY30 / clot formation (LI30)
- LY30 / LI30 is an assessment of clot stability and shows the speed and extent of fibrinolysis (high values are associated with hyperfibrinolysis while for the LI30 smaller values represent hyperfibrinolysis)
- Examples of traces from thromboelastography and rotational thromboelastometry most likely to be observed in hypocoagulable and hypercoagulable states in clinical settings are shown in Diagram 2 and Diagram 3, respectively
Case reports
- 38 year old woman with antiphospholipid syndrome who developed septic shock after labor induction (J Anesth 2020;34:781)
- 39 year old man who developed acute limb ischemia secondary to infection with COVID-19 (J Thromb Thrombolysis 2020;50:292)
- 42 year old man with ventricular assist device with intracranial hemorrhage underwent a thromboelastometry guided anticoagulation reversal (Am J Emerg Med 2021;41:265.e5)
- 45 year old woman underwent anticoagulation management during cardiopulmonary bypass complicated by antiphospholipid syndrome (J Card Surg 2020;35:1354)
- 52 year old woman with aneurysmal subarachnoid hemorrhage and hypercoagulation assessed comprehensively by thromboelastometry (Acta Neurochir Suppl 2020;127:165)
Treatment
- Utilization of viscoelastic hemostatic assays for the diagnosis and management of numerous disorders have been previously summarized in the following reference: Transfusion 2020;60:S6
Additional references
Board review style question #1
Considering the thromboelastography citrated kaolin (CK) assay tracing shown above (red line), what are the probable causes suggested by a normal reaction time (R) value, low α angle value and low maximum amplitude?
- Fibrinolytic state; abnormal fibrinolysis
- Hypercoagulable state; a tendency towards increased blood clotting
- Hypocoagulable state; excessive bleeding caused by low fibrinogen levels
- Hypocoagulable state; excessive bleeding caused by low platelet number / platelet dysfunction
Board review style answer #1
D. Hypocoagulable state; excessive bleeding caused by low platelet number / platelet dysfunction
Comment Here
Reference: Viscoelastic hemostatic assays
Comment Here
Reference: Viscoelastic hemostatic assays
Board review style question #2
Considering the thromboelastography citrated kaolin (CK) and citrated kaolin heparinase (CKH) assay tracings shown above (red and green lines, respectively), what are the probable causes suggested by a prolonged R time, low α angle value and normal maximum amplitude on the CK tracing, corresponding with a normalized reaction time on the CKH tracing?
- Fibrinolytic state; abnormal fibrinolysis
- Hypercoagulable state; a tendency towards increased blood clotting
- Hypocoagulable state; excessive bleeding caused by the presence of heparin
- Hypocoagulable state; excessive bleeding caused by low platelet number / platelet dysfunction
Board review style answer #2
C. Hypocoagulable state; excessive bleeding caused by the presence of heparin
Comment Here
Reference: Viscoelastic hemostatic assays
Comment Here
Reference: Viscoelastic hemostatic assays
Board review style question #3
Maximum amplitude represents the maximum strength of the clot and is largely dependent on
- Concentration of coagulation factors
- Concentrations and functionality of platelets and fibrin
- Lupus anticoagulation
- von Willebrand factor
Board review style answer #3
B. Concentrations and functionality of platelets and fibrin
Comment Here
Reference: Viscoelastic hemostatic assays
Comment Here
Reference: Viscoelastic hemostatic assays
Vitamin K deficiency / warfarin use
Table of Contents
Definition / general | Essential features | Terminology | Pathophysiology | Clinical features | Laboratory | Case reports | Treatment | Differential diagnosis | Board review style question #1 | Board review style answer #1 | Board review style question #2 | Board review style answer #2Definition / general
- Vitamin K plays an important role in coagulation pathways because it is a cofactor required for the activity of several key proteins (Adv Nutr 2012;3:182)
- Warfarin and other vitamin K antagonists (VKA) are employed in a wide range of clinical settings
Essential features
- Vitamin K deficiency and warfarin administration can result in prolonged prothrombin time (PT) / international normalized ratio (INR)
- Mild vitamin K deficiency affects PT due to its predominant effect on factor VII; however, severe deficiency can cause prolonged PT and partial thromboplastin time (PTT) (StatPearls: Vitamin K Deficiency [Accessed 14 March 2023])
- Warfarin anticoagulation is associated with increased risk of bleeding; therefore, appropriate dosing and monitoring are essential
- Warfarin's effect varies depending on a number of genetic, dietary and medication factors (Br J Clin Pharmacol 2021;87:1717)
Terminology
- Vitamin K antagonist (VKA)
- Vitamin K2 (menaquinone)
- International normalized ratio (INR)
- Deep vein thrombosis (DVT)
- Pulmonary embolism (PE)
- Prothrombin complex concentrate (PCC)
- Disseminated intravascular coagulopathy (DIC)
- Prothrombin time (PT)
- Partial thromboplastin time (PTT)
Pathophysiology
- Vitamin K is a fat soluble vitamin that participates as a cofactor in carboxylation of glutamic acid residues of factors II, VII, IX and X and proteins C, S and Z (Haematologia (Budap) 1985;18:71)
- Vitamin K deficiency
- Rare in general
- Common in newborns who require routine vitamin K prophylaxis at birth (Blood Rev 2009;23:49)
- Predisposing factors (J Perinatol 2016;36:S29)
- Fat malabsorption syndromes (vitamin K is a fat soluble vitamin)
- Cystic fibrosis
- Primary biliary cholangitis
- Primary sclerosing cholangitis
- Biliary atresia
- Intestinal diseases associated with malabsorption, such as inflammatory bowel disease, short bowel syndrome, active celiac disease
- Malnutrition
- Liver failure
- Medications
- Broad spectrum antibiotics
- Diminish intestinal bacteria that are responsible for synthesizing absorbable vitamin K (K2)
- Affect vitamin K activation in the liver by inhibiting the function of vitamin K epoxide reductase
- Very high doses of vitamin E
- Broad spectrum antibiotics
- Fat malabsorption syndromes (vitamin K is a fat soluble vitamin)
- Hereditary combined vitamin K dependent clotting factor deficiency (VKCFD) (Orphanet J Rare Dis 2010;5:21)
- Very rare
- Autosomal recessive
- Implicated genes are gamma glutamyl carboxylase (GGCX) and vitamin K2,3 epoxide reductase complex (VKORC)
- Activities for all vitamin K dependent factors are reduced and there could be some developmental and skeletal anomalies
- PT and PTT are prolonged
- Bleeding is often of mucocutaneous pattern
- Management
- Acute management (surgery, severe bleeding): vitamin K administration and plasma transfusion
- Prophylaxis: vitamin K daily with INR monitoring
- Warfarin (Coumadin) inhibits the function of the vitamin K epoxide reductase complex in the liver
- Resulting in an impairment of the reduced form of vitamin K
- Reduced form of vitamin K is required for the gamma carboxylation of vitamin K dependent coagulation factors (II, VII, IX and X) as well as anticoagulant proteins C and S
- Indications (Chest 2008;133:160S)
- Stroke
- Atrial fibrillation
- Heart failure
- Prosthetic heart valve
- Deep vein thrombosis
- Pulmonary embolism
- Antiphospholipid syndrome
- Contraindications
- Active clinically significant bleeding
- Severe thrombocytopenia
- Major trauma
- Invasive procedure
- Previous intracranial hemorrhage
- Uncontrolled severe hypertension
- There is significant interindividual variability in the dosing of warfarin that is a result of many variables (Br J Clin Pharmacol 2021;87:352)
- Genetic factors due to polymorphisms in the genes that encode hepatic cytochrome P450 enzyme (CYP2C9) and vitamin K epoxide reductase (VKORC1) (JAMA Intern Med 2014;174:1330)
- Drug - drug interactions
- Concomitant comorbidities
- Excessive dietary vitamin K intake
Clinical features
- Vitamin K deficiency can result in
- Easy bruising
- Ecchymoses
- Mucosal bleeding
- Gastrointestinal bleeding
- Epistaxis
- Intracranial bleeding
- Hematuria
- Hemoptysis
- Warfarin complications
- Higher rates of thromboembolic and bleeding complications in some high risk patients (South Med J 2005;98:96)
- Age > 65
- Concomitant comorbidities (liver disease, atrial fibrillation, gastrointestinal bleeding, renal insufficiency, cerebrovascular disease)
- Concomitant medications (aspirin, amiodarone)
- Skin necrosis (Arch Dermatol 1986;122:1408)
- Reported in some patients within the first few days of initiation of warfarin
- Lesions occur in areas rich in subcutaneous fat (trunk, extremities, breast, penis)
- Attributed to rapid reduction in protein C level, which induces a hypercoagulable state
- Microscopically, fibrin thrombi with cutaneous vessels and associated interstitial hemorrhage
- Higher rates of thromboembolic and bleeding complications in some high risk patients (South Med J 2005;98:96)
Laboratory
- Prothrombin time (PT) and international normalized ratio (INR) (Hematology Am Soc Hematol Educ Program 2012;2012:460)
- PT
- PT measures the time it takes citrated patient's plasma to form a fibrin clot when exposed to tissue factor (TF) in the presence of phospholipids
- PT measures the activity of the extrinsic (VII) and common pathways (I, II, V, X)
- INR
- INR is calculated as the ratio of a patient's PT to the control sample PT raised to the international sensitivity index (ISI) value of the control sample (INR = [patient PT ÷ control PT]ISI)
- Each manufacturer assigns an ISI value for any tissue factor they manufacture
- ISI value indicates how the particular batch of tissue factor compares to an internationally standardized sample
- INR was developed to standardize results due to differences in tissue factor between manufacturers that can lead to differences in PT values
- PT
- Point of care (POC) testing (Br J Haematol 2010;150:501)
- POC testing can be performed using a device located at or near the location of the patient rather than in a central laboratory
- Clotting times are among the tests that can be performed at POC
- This can aid in home monitoring of warfarin therapy
- Causes for prolonged PT / INR and a normal PTT
- Mild vitamin K deficiency
- Warfarin administration
- Factor VII deficiency
- Early disseminated intravascular coagulation
- Mild liver disease
Case reports
- 5 week old boy with COVID-19 infection and intracranial hemorrhage (J Neurosurg Case Lessons 2021;1:CASE20163)
- 2 month old boy with bleeding from injection sites (Ital J Pediatr 2018;44:36)
- 65 year old man with warfarin resistance (J Int Med Res 2022;50:3000605221103959)
Treatment
- Vitamin K deficiency and coagulopathy
- Single dose of vitamin K followed by PT / INR after 12 - 24 hours
- If coagulopathy persists, a second dose is administered in 48 - 72 hours
- Warfarin associated bleeding or supratherapeutic INR (see Table 1)
- Management is determined by whether or not the patient is bleeding and the degree of INR elevation
- If not associated with bleeding, hold warfarin dose(s)
- If associated with bleeding, discontinue warfarin and administer vitamin K
- Prothrombin complex concentrate (PCC) can be given for serious bleeding to provide rapid and full reversal
- If PCC is not available, donor plasma can be administered
- Management is determined by whether or not the patient is bleeding and the degree of INR elevation
| Table 1: management of warfarin (Chest 2012;141:e152S, Blood Adv 2018;2:3257) | |
| Clinical setting | Management |
|
|
|
|
|
|
Differential diagnosis
- Disseminated intravascular coagulation (DIC):
- In DIC, coagulation factors are depleted and consumed
- If DIC is mild, only PT is prolonged
- As DIC progresses in severity, PTT is prolonged along with PT
- Liver disease:
- Because all coagulation factors are synthesized in the liver, with the exception of factor VIII, liver disease will also affect vitamin K dependent factors
- Measurement of factors V and VII can help distinguish between liver disease and vitamin K deficiency
- Vitamin C deficiency:
- Among the clinical presentation of vitamin C deficiency (scurvy) is mucocutaneous bleeding (perifollicular and gingival hemorrhage)
- Vitamin C plays a role in collagen synthesis, especially type IV, which is the main component of blood vessel walls and skin
- Malabsorption:
- Maldigestion of nutrients in the intestinal lumen
- There is usually a widespread mucosal involvement or a reduced absorptive surface
- Classic presentation includes chronic diarrhea, unintentional weight loss and signs and symptoms related to nutrient deficiencies
Board review style question #1
A 7 day old boy presents with agitation, lethargy and poor feeding. He was born at term via spontaneous vaginal delivery. All postnatal supplements and vaccines were not administered due to parental refusal. The newborn was breastfed exclusively. His body is covered with ecchymoses. Complete blood count showed normocytic normochromic anemia, normal white blood cell count and mild thrombocytopenia. A transcranial ultrasound reveals hemorrhage in the left cerebral hemisphere. Which of the following abnormalities is likely to be seen in this patient?
- Congenital protein C deficiency
- Decreased factor VIII level
- Factor V Leiden mutation
- Prolonged partial thromboplastin time (PTT)
- Prolonged prothrombin time (PT)
Board review style answer #1
E. Prolonged prothrombin time (PT).
The lack of postnatal vitamin K supplementation and exclusive breastfeeding increases risk of vitamin K deficiency, which usually results in prolonged PT. Answer A is incorrect because congenital protein C deficiency leads to an inability to inactivate clotting factors and control thrombin production and can be associated with thrombosis rather than bleeding. Answer B is incorrect because while decreased factor VIII level or hemophilia A can present with spontaneous intracranial hemorrhage in neonates, the lack of vitamin K supplementation and exclusive breast feeding are more likely to be associated with deficiency of vitamin K dependent coagulation factors. Answer C is incorrect because factor V Leiden mutation increases the likelihood of thrombosis, not bleeding. Answer D is incorrect because deficiency of vitamin K will impact its dependent factors II, VII, IX and X, with factor VII affected early due to short half life. In more advanced stages, PTT is expected to be prolonged as well.
Comment Here
Reference: Vitamin K deficiency / warfarin use
Comment Here
Reference: Vitamin K deficiency / warfarin use
Board review style question #2
A 68 year old woman with alcoholic hepatitis and advanced cirrhosis presents with ecchymoses. Partial thromboplastin time (PTT) is normal while prothrombin time (PT) is elevated. Complete blood count showed normocytic normochromic anemia, normal white blood cell count and mild thrombocytopenia. Which deficiency is the most probable cause of this clinical scenario?
- Fibrinogen
- Riboflavin
- Vitamin A
- Vitamin C
- Vitamin K
Board review style answer #2
E. Vitamin K. Vitamin K is essential for the synthesis of coagulation factors II, VII, IX and X, as well as the natural anticoagulants protein C and S. Patients with alcohol use disorders often have low dietary intake of vitamin K and may also have malabsorption. Vitamin K deficiency leads to prolonged PT, which measures the activity of the extrinsic and common pathways. Answers A - D are incorrect because hypofibrinogenemia, deficiency of riboflavin, vitamin A and vitamin C do not typically result in normal PTT and prolonged PT.
Comment Here
Reference: Vitamin K deficiency / warfarin use
Comment Here
Reference: Vitamin K deficiency / warfarin use
von Willebrand disease and testing
Table of Contents
Definition / general | Essential features | Diagrams / tables | Clinical features | Diagnosis | Case reports | Molecular / cytogenetics images | Sample pathology report | Board review style question #1 | Board review style answer #1Definition / general
- Most common inherited bleeding disorder, affecting 1 - 2% of the total population with no gender preference (eMedicine: Pediatric Von Willebrand Disease [Accessed 1 August 2022])
- Some patients with von Willebrand disease (vWD) remain asymptomatic; prevalence of symptomatic vWD is very low at 0.01% of the total population (Paediatr Child Health 2002;7:245)
Essential features
- Gene for von Willebrand factor (vWF) is located on chromosome 12
- vWF is synthesized by endothelial cells and megakaryocytes
- vWF is a large polypeptide that polymerizes to form multimers of up to 100 subunits
- Roles in hemostasis:
- Adheres to platelet plug and fibrin clot, both essential to hemostasis at site of endothelial injury, particularly in high flow vessels
- vWF mediates platelet adhesion to endothelium and formation of platelet plug by serving as a bridge between them; binds to glycoprotein Ib (GPIb) on platelets surfaces and to exposed subendothelium at site of endothelial injury (Best Pract Res Clin Haematol 2001;14:257, Thromb Haemost 1998;79:456)
- vWF supports fibrin clot by serving as protective carrier protein for factor VIII; without vWF, factor VIII has shorter half life and its plasma levels are lower
- Adheres to platelet plug and fibrin clot, both essential to hemostasis at site of endothelial injury, particularly in high flow vessels
Diagrams / tables
Table 1: Clinical characteristics of vWD subtypes (Clin Appl Thromb Hemost 2017;23:900)
| Condition | Inheritance pattern | Common mutation domain | Defect | Clinical presentation |
| Type 1 | Autosomal dominant | Throughout vWF gene (OMIM: Von Willebrand Disease, Type 1 [Accessed 3 August 2022]) | Decreased concentration of functionally normal vWF | Mild to moderate mucocutaneous bleeding |
| Type 2A | Autosomal dominant (OMIM: Von Willebrand Disease, Type 2 [Accessed 3 August 2022]) | A1, A2 | Enhanced susceptibility to cleavage by ADAMTS13 | Moderate to severe mucocutaneous bleeding |
| CK | Impaired dimer assembly | |||
| D1, D2 | Impaired multimer assembly | |||
| A1, A2, D3 | Impaired secretion of vWF, with enhanced intracellular retention | |||
| Type 2B | Autosomal dominant (OMIM: Von Willebrand Disease, Type 2 [Accessed 3 August 2022]) | A1 | Increased affinity of GPIb binding site on vWF for the GPIb receptor | Moderate to severe mucocutaneous bleeding |
| Type 2M | Autosomal dominant (OMIM: Von Willebrand Disease, Type 2 [Accessed 3 August 2022]) | A1 | Reduced affinity of GPIbα binding site on vWF for the GPIb receptor | Moderate to severe mucocutaneous bleeding |
| A3 | Reduced affinity of vWF for collagen | |||
| Type 2N | Autosomal recessive (OMIM: Von Willebrand Disease, Type 2 [Accessed 3 August 2022]) | D' - D3 | Reduced affinity of vWF for FVIII | Moderate to severe hemophilia-like bleeding |
| Type 3 | Autosomal recessive (OMIM: Von Willebrand Disease, Type 3 [Accessed 3 August 2022]) | Throughout vWF gene | Null alleles result in virtual absence of vWF | Severe mucocutaneous bleeding and hemophilia-like bleeding |
| Rare: D1 - D2 | Impaired dimer formation and intracellular retention |
Table 2: Laboratory characteristics of subtypes of vWD (Clin Appl Thromb Hemost 2017;23:900, Teruya: Management of Bleeding Patients, 2nd Edition, 2021)
| Condition | vWF antigen level (vWF:Ag) in % | vWF activity (vWF:Act) in % | vWF:Act / vWF:Ag | Factor VIII (FVIII) | Multimers |
| Type 1 | < 30 | < 30 | > 0.7 | Low, normal | All low |
| Type 2A | Mildly low | Low | < 0.7 | Normal, mildly low | Absent high and intermediate multimers |
| Type 2B | Normal, low | Low | < 0.7 | Normal, mildly low | Absent high multimers |
| Type 2M | Normal, low | Low | < 0.7 | Normal, mildly low | Normal |
| Type 2N | Normal, low | Normal, low | > 0.7 | Low | Normal |
| Type 3 | < 3 | < 3 | Not applicable | Low | Absent |
| Low vWF | 30 - 50 | Normal, low | > 0.7 | Normal | Normal |
| Normal | 50 - 200 | 50 - 200 | > 0.7 | Normal | Normal |
Clinical features
- Symptoms are similar to a platelet function defect (epistaxis, easy bruising, bleeding, menorrhagia)
- vWD can be due to deficient (quantitative) or defective (qualitative) vWF (see Table 1)
- Type 1 (70 - 80% of vWD):
- Most common, partial quantitative deficiency of vWF but normal function
- Normal platelet count
- Mildest form of disease
- Type 2 (15 - 20%):
- Qualitative deficiency of vWF, variable quantitative deficiency, heterogeneous clinical presentation
- Type 2A:
- Most common type 2 subtype
- Relative reduction of intermediate and high molecular weight multimers due to in vivo proteolytic degradation or defective multimer assembly and secretion
- Normal platelet count
- Type 2B:
- Hemostatic defect due to intermittent thrombocytopenia and qualitatively abnormal vWF, with increased binding of vWF to GPIb (platelet vWF receptor), causing faster clearing of vWF coated platelets from the bloodstream
- Platelet count drops further during pregnancy, surgery, 1-deamino-8-D-arginine vasopressin (DDAVP) therapy
- Rapid clearance of platelets can be a problem following DDAVP therapy in type 2B vWD; if a previous patient trial of DDAVP has not been performed, then it is unwise to use DDAVP in an urgent bleeding situation as the resulting thrombocytopenia often complicates the problem
- Reduction of high molecular weight multimers but an increase in low molecular weight fragments
- Reduced ristocetin cofactor but increased ristocetin induced platelet aggregation (RIPA) (Blood 2018;131:1292)
- Type 2A:
- Type 2M:
- Rare
- Due to mutation that impairs vWF and GPIb binding
- Type 2N:
- Rare, sometimes referred to as the Normandy variant
- Markedly reduced affinity of vWF for factor VIII, causes reduction of factor VIII level
- Other vWF lab tests are normal
- Often misdiagnosed as hemophilia A (X linked recessive) but males and females in type 2N are equally affected
- Qualitative deficiency of vWF, variable quantitative deficiency, heterogeneous clinical presentation
- Type 3:
- Very rare, often associated with consanguinity
- Most severe clinical bleeding
- Homozygous patients have marked deficiencies of plasma vWF and factor VIII activity, no vWF in platelets and endothelial cells, no secondary transfusion response, no response to DDAVP
- Platelet type or pseudo vWD:
- Rare disorder of mutation in GP1BA gene (not vWF gene), causing increased binding of vWF to GPIb (platelet surface membrane glycoprotein 1b) with gain of function and similar clinical findings as type 2B
- Loss of higher vWF multimers
- Enhanced ristocetin induced platelet aggregation (RIPA)
- Variable thrombocytopenia
- Type 1 (70 - 80% of vWD):
Diagnosis
- Preliminary testing is recommended as starting points; these include complete blood count (CBC), prothrombin time (PT) and activated partial thromboplastin time aPTT
- Specific testing for diagnosis: vWF antigen level (vWF:Ag), vWF activity (vWF:Act), factor VIII level (FVIII:C) (see Table 2)
- vWF:Ag
- Determines the amount of vWF in plasma
- Enzyme linked immunosorbent assay (ELISA) or latex immunoassay (LIA) (Can J Vet Res 2008;72:420)
- Levels can increase with injury, infection or other acute phase reactant stimuli
- Type O patients have lower vWF antigen levels compared with other blood types; although bleeding symptoms may depend on vWF antigen levels, regardless of ABO type (Blood 1987;69:1691)
- vWF:Act
- Mostly commonly accomplished by ristocetin cofactor activity assay (vWF:RCo)
- Ristocetin is an antibiotic that mimics the subendothelium, causing immobilization of vWF and inducing GPIb binding
- Determines the ability of ristocetin to agglutinate exogenous platelets in the presence of vWF
- Aggregation of platelets implies linkage via fibrinogen and GPIIb / IIIa; ristocetin links platelets through vWF and GPIb; appropriate term is agglutination
- Rate of agglutination is related to the concentration of functionally normal vWF
- vWF:Act / vWF:Ag ratio
- Ratio of vWF activity to vWF antigen level in plasma
- < 0.7 is generally indicative of a qualitative vWD (Blood Adv 2021;5:280)
- FVIII:C
- Measures FVIII concentration in plasma
- Usually a clot based assay such as aPTT, ability of plasma to shorten aPTT of FVIII deficient control plasma
- vWF:Ag
- Additional testing for subtype classification:
- RIPA
- Patient's platelets are mixed with standard concentrations of ristocetin and patient's plasma is added to cause platelet agglutination (measured by aggregometer)
- Increased aggregation in type 2B vWD in response to low concentration of ristocetin due to GPIb mutation, which increases affinity for vWF
- vWF collagen binding assay (vWF:CB)
- Functional vWF binds to collagen and is detected (Br J Haematol 2002;116:187)
- ELISA based
- vWF multimer analysis (see Molecular / cytogenetics images)
- Detects the size distribution of multimers using radiolabeled or enzyme linked anti-vWF antibody
- Involves separation of multimers by size using agarose gel electrophoresis of patient's plasma (Thromb Res 2010;126:543)
- Normal in types 1, 2N or 2M (type 1 has reduced quantity of all sizes but difficult to identify on gel)
- DDAVP challenge
- Recommended in patients with mild to moderate types 1 and 2
- vWF:Ag, vWF:Ag, FVIII:C are measured at standard intervals after DDAVP administration
- Response is adequate if 2 - 4 fold increase in antigen and activity levels is observed and at least 30% increase in factor VIII level for at least 12 hours
- May achieve hemostasis in type 1
- Contraindicated in type 2B
- Gene sequencing with mutation analysis
- To detect mutations in the vWF gene
- Not widely used due to genetic complexity and phenotypic variability
- Can help subclassify type 2 vWD
- RIPA
- Repeat testing is often recommended because vWF and factor VIII become elevated during minor illnesses, injury, stress, pregnancy, estrogen use, other acute phase reactions or in newborns
Case reports
- 2 year old girl presented with type 3 vWD (Blood 2018;132:5031)
- 10 year old boy presented with types 1 and 2A vWD (Pediatr Blood Cancer 2018;65:e27279)
- 24 year old woman presented with type 2M vWD (Cureus 2018;10:e3112)
Sample pathology report
- History: A 14 year old girl presented to the emergency department with fatigue and heavy menses. She was referred to hematology for evaluation. Platelet count, PT/ INR and PTT are all within normal limits.
- Results: The von Willebrand factor (vWF) antigen level is normal at 87% (normal adult range 55 - 200%). The vWF functional antibody (vWF GPIb binding activity) level is normal at 87% (normal adult range 50 - 155%). The vWF collagen binding activity is normal at 89% (normal adult range 46 - 159%). The ratio of each of the cofactor functional assays to von Willebrand antigen is normal. The factor VIII level is normal at 80% (normal range 57 - 152%). Blood group is A.
- Conclusion: The current results provide no definitive evidence for von Willebrand disease.
Board review style question #1
A 17 year old girl with a history of von Willebrand disease (vWD) of unknown type is scheduled to undergo wisdom tooth extraction. The hematologist considered the 1-deamino-8-D-arginine vasopressin (DDAVP) challenge test. In which one of the following types of vWD has DDAVP been known to cause thrombocytopenia?
- Type 1
- Type 2A
- Type 2B
- Type 2N
- Type 3
Board review style answer #1
C. Type 2B. The von Willebrand factor (vWF) gain of function mutation increases platelet aggregation leading to thrombocytopenia.
Comment Here
Reference: von Willebrand disease and testing
Comment Here
Reference: von Willebrand disease and testing
Recent Coagulation Pathology books
Find related Pathology books: transfusion, lab medicine, hematopathology, management, other